Abstract
Background
Curcumin is a major constituent of rhizomes of Curcuma longa that elicits beneficial effects for oxidative damage. The aim of this study was to investigate whether curcumin could attenuate hydrogen peroxide (H2O2)-induced apoptosis in H9c2 cardiomyoblasts and the underlying mechanisms.
Results
The present study showed that exposure of H9c2 cells to H2O2 caused a significant increase in apoptosis as evaluated by flow cytometry analysis and the pretreatment of curcumin protected against H2O2-induced apoptosis. Exposure of cells with curcumin caused a dose-dependent induction of heme oxygenase-1 (HO-1) protein expression. Curcumin also decreased the cleaved caspase-3 (CC3) protein expression level and increased the Bcl-2/Bax ratio in H2O2-stimulated H9c2 cells. ZnPP-IX, a HO-1 inhibitor, partly reversed the anti-apoptotic effect of curcumin. Further, LY294002, an inhibitor of PI3K, partially reversed the effect of curcumin on HO-1 protein induction, leading to the attenuation of curcumin-mediated apoptosis resistance.
Conclusion
These results demonstrated that the anti-apoptotic function of curcumin required the upregulation of HO-1 protein through the PI3K/Akt signaling pathway. Curcumin might be used as a preventive and therapeutic agent for treatment of cardiovascular diseases associated with oxidative stress.
Keywords: Curcumin, Cardiomyocyte apoptosis, Oxidative stress, Heme oxygenase-1, PI3K/Akt
Background
Oxidative stress-induced apoptosis has long been implicated in the pathogenesis of cardiovascular diseases such as myocardial ischemic injury and infarction [1, 2]. Oxidative damage, mediated by reactive oxygen species (ROS) which can be generated following cell lysis, oxidative burst, or the presence of an excess of free transition metals, can attack proteins, DNA, and membrane lipids, thereby leading to the loss of cell integrity, enzyme function, and genomic stability [3, 4]. Therefore, therapeutic intervention targeting the apoptosis is a reasonable strategy for the treatment of cardiovascular diseases.
Curcumin, a major component of turmeric powder extracted from the rhizomes of the plant Curcuma longa, has been applied for centuries in indigenous medicine to treat various diseases [5]. This bioactive phytochemical is a potent inhibitor of tumor promotion and possesses anti-inflammatory and anti-oxidative activities [6]. In addition, curcumin seems to be, even at relatively low concentrations, an effective anti-apoptotic agent [7]. One study reported that curcumin attenuated peroxynitrite-induced apoptosis in primary cultured rat spiral ganglion neurons [8], and another study demonstrated that curcumin had the potential to protect experimental autoimmune myocarditis [9]. Nevertheless, the possible protective effect of curcumin on the toxicity in cardiomyoblasts has not been tested in vitro. Furthermore, the precise mechanism underlying this response is still unclear.
Heme oxygenase-1 (HO-1), which is the rate-limiting enzyme responsible for the degradation of heme into free ferrous iron, carbon monoxide (CO) and bilirubin, exerts cytoprotective effects in various diseases [10, 11]. Recent experimental evidence indicated that increased HO-1 production provided cellular protection against oxidative injury induced by ischemia/reperfusion [12] or the use of hydrogen peroxide (H2O2) [13, 14]. Protein kinase B (PKB, Akt), one of the most important downstream target kinases of phosphoinositide 3-kinase (PI3K), is an important signaling molecule activated by anti-apoptotic agents [15], while extracellular signal-regulated kinases (ERKs) mediate another important signaling pathway involved in anti-apoptotic effects [16]. Several studies reported that HO-1 provided protection against various forms of stress through the activation of the PI3K/Akt or ERK1/2 signaling pathways [17, 18].
The H9c2 cell line, derived from the embryonic BDIX rat heart ventricle, is considered a close surrogate for cardiomyocytes and has been proven to be ideal for signal transduction studies [19]. H2O2, as one of the main ROS, could cause DNA damage and lipid peroxidation and has been widely used to induce apoptosis in various cell types [20]. In this study, H2O2 was used to induce apoptosis in H9c2 cells, as it is a well-established model to study oxidative stress-induced cardiomyocyte apoptosis [21, 22]. Here we aimed to investigate the anti-apoptotic effect of curcumin in H2O2-stimulated H9c2 cells and to explore the role of HO-1 and its associated signaling pathways.
Methods
Chemicals and reagents
Curcumin, Zine protoporphyrin-IX (ZnPP-IX, a HO-1 inhibitor), dimethyl sulfoxide (DMSO), H2O2 and methyl thiazolyl tetrazolium (MTT) were from Sigma Chemical. LY294002 (a PI3K inhibitor) and rabbit polyclonal antibodies specific for total ERK1/2 (t-ERK1/2), phospho-ERK1/2 (p-ERK1/2), total Akt (t-Akt), phospho-Akt (p-Akt, serine 473), Bcl-2, Bax, cleaved caspase-3 (CC3) and GAPDH were from cell signaling. Rabbit polyclonal antibodies specific for HO-1 were obtained from Stressgen Bioreagents.
Cell culture
H9c2 cardiomyoblasts from the American Type Culture Collection (ATCC, CRL-1446) were maintained in DMEM supplemented with 10% heat-inactivated fetal bovine serum, 100 U/mL penicillin and 100 μg/mL streptomycin in a humid atmosphere of 5% CO2 and 95% air at 37 °C. Cells were regularly passaged and subcultured to 90% confluence before experimental procedures. Curcumin dissolved in DMSO was diluted with low-serum medium (1% FBS/DMEM) to the final concentrations before use. The final concentration of DMSO in the incubation mixture was not more than 0.1% (v/v).
Cell viability assay
Cell viability was assessed by MTT assay. Briefly, the H9c2 cells subcultured in 96-well plates at 1 × 104 cells/well were incubated with the test chemicals for indicated time period. Then 5 mg/mL MTT was added to the culture media and cells were incubated further for an additional 4 h. After this incubation, the formed formazan was solubilized by adding DMSO, and optical density of the solubilized cell extract was measured at 490 nm using a microplate reader. The reduction in optical density was considered being the decrease in cell viability.
Annexin-V FITC/PI assay
Apoptosis was detected using an Annexin-V FITC/PI detection kit according to the manufacturer’s directions (KeyGEN, Nanjing, China). The cells were digested with 0.25% trypsin, washed with ice-cold PBS and resuspended in binding buffer (5 × 105 cells/mL). Then, the cells were centrifuged at 1000g for 5 min at 4 °C. After the supernatant had been discarded, 500 μL of binding buffer, 5 μL of annexin-V-FITC and 5 μL of propidium iodide were added to the cell suspension. After mixing gently, the suspensions were incubated for 15 min at room temperature without light. Finally, the cells were analyzed by flow cytometry (BD LSRII; BD Biosciences).
Western blot analysis
Cells were lysed in ice-cold cell lysis buffer. The protein concentration was determined using BCA method. Protein was separated by SDS-PAGE, and then transferred onto polyvinylidene difluoride membrane. The membranes were blocked in TBS-T with 5% (w/v) skim milk at room temperature for 2 h, followed by overnight incubation at 4 °C with primary antibodies diluted in TBS-T. After washing in TBS-T, the membranes were incubated for 1 h with a horseradish peroxidase-conjugated secondary antibody diluted in TBS-T. After washing once more in TBS-T, the labeled protein was detected using enhanced chemiluminescence reagents and exposed to film. The intensity of the bands was analyzed with Alpha Ease FC image software.
Statistical analysis
All data represented the mean of samples from three independent experiments. Results were presented as mean and standard deviation (mean ± SD). Statistical significance was determined by one-way ANOVA followed by Student–Newman–Keuls test for comparison of several groups. A p value less than 0.05 was considered being statistically significant.
Results
Curcumin reduced H2O2-induced cell toxicity
As shown in Fig. 1a, 200–600 μM H2O2 reduced the cell viability in a dose-dependent manner. In the presence of 400 and 600 μM of H2O2, the percentage of viable cells was reduced to 58.92 ± 8.02 and 37.76 ± 8.54% of the control, respectively (p < 0.05). Then we evaluated whether curcumin was cytotoxic to H9c2 cells. As shown in Fig. 1b, cell viability was not significantly affected by treatment with increasing doses of curcumin up to 15 μM compared to that of the control group. However, a significant decrease in cell viability was observed in cells treated with 20 and 25 μM curcumin (87.88 ± 9.85 and 65.3 ± 10.94% of the control, p < 0.05). Next, we tested whether the pretreatment with curcumin was able to protect against H2O2-induced cytotoxicity. As shown in Fig. 1c, pretreatment with 10 and 15 μM of curcumin significantly increased the cell viability to 73.61 ± 8.14 and 84.93 ± 8.41% of the control, respectively. Our results indicate that curcumin may have protective role against H2O2-induced cell death.
Fig. 1.
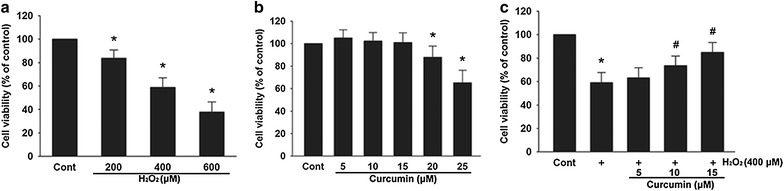
Curcumin reduced H2O2-induced cell toxicity. Cell viability was examined using the MTT assay. a Effect of H2O2 on cell viability. Cells were treated with 200–600 μM of H2O2 for 3 h. b Effect of curcumin on cell viability. Cells were treated with 5–25 μM of curcumin for 24 h. c Curcumin protected the cells from H2O2-induced cytotoxicity in a dose-dependent manner. After pretreated with 5–15 μM of curcumin for 12 h, the cells were washed and incubated with 400 μM H2O2 for 3 h. Data were presented as mean ± SD (n = 3). *p < 0.05 vs. Cont (control), # p < 0.05 vs. H2O2
Curcumin increased HO-1 protein expression
Curcumin treatment for 12 h increased HO-1 protein expression in a dose-dependent manner (Fig. 2a). Curcumin (15 μM) induced a significant increase of HO-1 protein expression for the 3-time points tested, with a maximum of 3.06 ± 0.31-fold increase after the 12 h treatment (Fig. 2b).
Fig. 2.
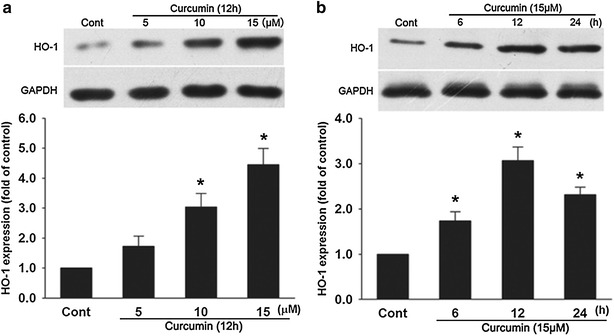
Curcumin increased HO-1 protein expression. a Cells were incubated with 5–15 μM of curcumin for 12 h as indicated. b Cells were incubated with 15 μM of curcumin for the indicated amounts of time. HO-1 protein expression was determined by western blot analysis. Data were presented as mean ± SD (n = 3). *p < 0.05 vs. Cont
The anti-apoptotic effect of curcumin was reversed by ZnPP-IX
As shown in Fig. 3a, b, 400 μM of H2O2 led to a significant increase in apoptosis in H9c2 cells compared with the control group, and apoptosis was decreased markedly by curcumin. The anti-apoptotic effect of curcumin was notably reversed by ZnPP-IX. We next showed that H9c2 cells subjected to H2O2 had decreased Bcl-2/Bax ratio compared with the control group, while curcumin pretreatment increased the Bcl-2/Bax ratio compared with the H2O2 group. Again, this effect of curcumin was partly blocked by ZnPP-IX (Fig. 3c). Furthermore, western blot analysis also showed that H2O2 caused a significant increase in CC3 levels compared with the control which is reduced by the pretreatment with curcumin. Co-incubation with ZnPP-IX partly negated this effect of curcumin (Fig. 3d).
Fig. 3.
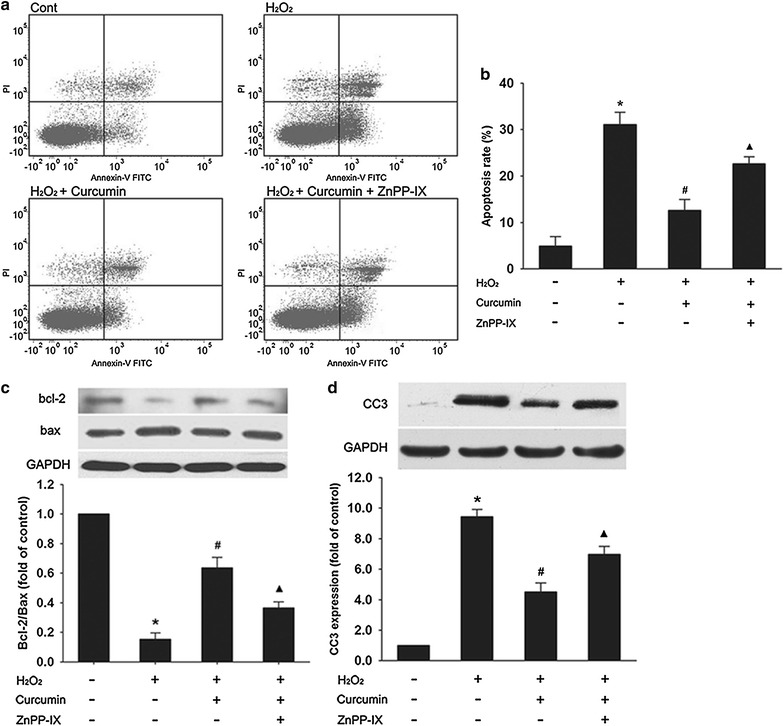
The anti-apoptotic effect of curcumin was partly reversed by ZnPP-IX. After pretreated with 15 μM of curcumin for 12 h in the absence or presence of 10 μM ZnPP-IX, the cells were washed and incubated with 400 μM H2O2 for 3 h. a, b Percentage of apoptotic cells was detected by flow cytometry analysis using Annexin-V FITC/PI staining. Apoptotic cells included Annexin V (+)/PI (−) and Annexin V (+)/PI (+) cells. c, d The Bcl-2, Bax, and CC3 protein expression were determined by western blot analysis. The Bcl-2/Bax ratio was calculated. Data were presented as mean ± SD (n = 3). *p < 0.05 vs. control, # p < 0.05 vs. H2O2, ▲ p < 0.05 vs. H2O2 + curcumin
Curcumin enhanced phosphorylation of Akt but had no influence on ERK1/2 phosphorylation
As shown in Fig. 4, the levels of p-Akt increased remarkably in the first 30 min and then began to decrease continuously in the following hours, while curcumin had no significant influence on p-ERK1/2 at any time point tested. In addition, total levels of Akt and ERK1/2 did not change significantly among these treatments.
Fig. 4.
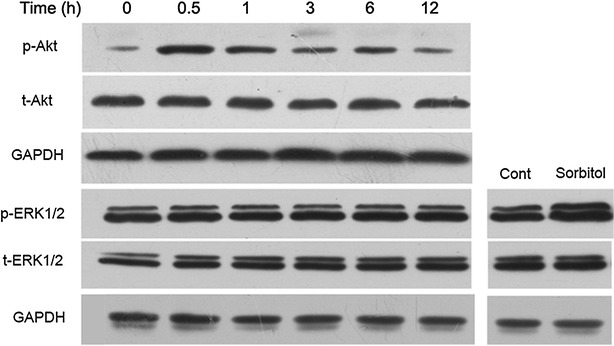
Curcumin enhanced phosphorylation of Akt but had no influence on ERK1/2 phosphorylation. Cells were treated with 15 μM curcumin for the indicated times. The expression levels of p-Akt, t-Akt, p-ERK1/2 and t-ERK1/2 were measured by western blot analysis. Cell extracts from H9c2 cells stimulated by osmotic shock (0.5 M sorbitol, 30 min) served as a positive control. Representative blots of three independent experiments were shown
Influence of LY294002 on apoptosis and HO-1 expression
To determine whether the activation of the PI3K/Akt pathway by curcumin is instrumental to the survival of H9c2 cells by modulating HO-1 expression, we tested the effects of LY294002 (an inhibitor of PI3K) on the protein expression of CC3 and HO-1. As shown in Fig. 5a, curcumin decreased the CC-3 protein expression levels compared with the H2O2 group, but this effect was largely negated by LY294002. The increase of HO-1 protein expression induced by curcumin was also partly abolished by LY294002. Furthermore, curcumin increased the Bcl-2/Bax ratio compared with the H2O2 group. And this effect of curcumin was also partially blocked by LY294002 (Fig. 5b). As expected, Akt phosphorylation enhanced by curcumin was completely reduced by LY294002 (Fig. 5c). Co-incubation with LY294002 partly negated the increase of HO-1 induced by curcumin (Fig. 5d).
Fig. 5.
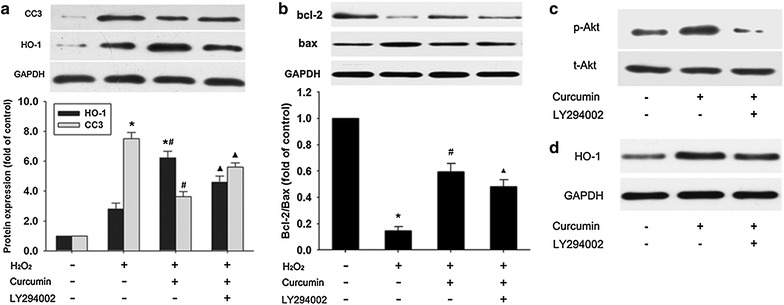
Influence of LY294002 on apoptosis and HO-1 expression. a, b After pretreated with 15 μM of curcumin for 12 h in the absence or presence of 50 μM of LY294002, the cells were washed and incubated with 400 μM H2O2 for 3 h. c Cells were treated with 15 μM curcumin for 30 min in the absence or presence of 50 μM LY294002, which was added 1 h before curcumin. d Cells were incubated with 15 μM of curcumin for 12 h in the absence or presence of 50 μM of LY294002. The CC3, Bcl-2, Bax, HO-1, p-Akt and t-Akt protein expression were determined by western blot analysis. The Bcl-2/Bax ratio was calculated. Data were presented as mean ± SD (n = 3). *p < 0.05 vs. control, # p < 0.05 vs. H2O2, ▲ p < 0.05 vs. H2O2 + curcumin
Discussion
H2O2 is a strong oxidant that can cause a marked decrease in cell viability. The present study confirmed that treating H9c2 cells with H2O2 resulted in a dose-dependent viability loss. Curcumin is a hormetic compound, at higher doses it is cytotoxic, but at lower doses, it is implicated in cellular adaptive stress responses [23]. Our study showed that administration of curcumin at higher doses (20 and 25 μM) for 24 h induced cell death, whereas curcumin lower than 15 μM (including 15 μM) were nontoxic to H9c2 cells. We then investigated the protective effect of curcumin against H2O2-induced cell toxicity by MTT assay. The results showed that curcumin protected H9c2 cells from H2O2-induced cytotoxicity in a dose-dependent manner.
We have shown that H9c2 cells incubation with 400 μM of H2O2 decreased the cell viability about 40% in comparison to the control. Moreover, typical features of apoptosis such as an increase of phosphatidylserines externalization, an elevated CC3 expression [24] and a decreased Bcl-2/Bax ratio [25] indicate that the cell death observed in the cell viability assay is mainly of apoptotic nature. Recently, curcumin was shown to be implicated in the suppression of apoptosis in various cell types such as vascular smooth muscle cells [26] and renal proximal tubular cells [27]. Our study demonstrated for the first time that H2O2-induced apoptosis of H9c2 cells was significantly inhibited by curcumin pretreatment.
Pharmacological and genetic induction of HO-1 has been shown to exert an anti-apoptotic effect in various cardiovascular diseases [28, 29]. A previous study demonstrated that HO-1 was upregulated in endothelial cells [30] and skin fibroblast cells [31] by curcumin in vitro, and here we showed that curcumin induced HO-1 protein expression in a dose-dependent manner in H9c2 cells. In addition, the anti-apoptotic effect of curcumin was demonstrated to be partly attributed to the induction of HO-1 because the inhibitor of HO-1 (ZnPPIX) markedly reversed the protection of curcumin, as revealed by a decrease of Bcl-2/Bax ratio, and an increase of CC3 protein expression and apoptotic cells. These results suggest that the induction of HO-1 may play a significant role in mediating the anti-apoptotic effect of curcumin in H2O2-stimulated H9c2 cells.
Since PI3K/Akt and ERK1/2 are the common signaling pathways for the modulation of HO-1 expression [17, 18], the influence of curcumin on the phosphorylation of Akt and ERK1/2 was measured. In our study, curcumin activated the PI3K/Akt pathway, but not the ERK1/2 pathway. These effects of curcumin are consistent with previous evidence using rat aortic vascular smooth muscle cells [26]. This means that it is the phosphorylation of Akt but not ERK1/2 involved in curcumin-mediated protection. Although most studies showed the PI3K/Akt pathway participated in the regulation of HO-1 expression, the role of Akt phosphorylation in HO-1 activation still remained controversial. For instance, in agreement with our data, pharmacological activation of the PI3K/Akt pathway by carnosol (a constituent of the herb of rosemary), which led to the induction of HO-1 protein, efficiently protected rat pheochromocytoma PC12 cells against oxidative stress [32]. However, piceatannol which is an anti-inflammatory and anti-proliferative plant-derived stilbene elevated HO-1 protein levels in bovine aortic endothelial cells via PKC and tyrosine kinase pathways, but not the PI3K/Akt pathway [33]. A possible explanation for these different findings could be that the mechanisms of HO-1 activation induced by various chemicals may differ significantly in different cell types. Our results are consistent with the requirement of Akt phosphorylation for the upregulation of HO-1 by curcumin because upregulation of HO-1 expression induced by curcumin was partly blocked by LY294002. In addition, LY294002 also partially reversed the anti-apoptotic effect of curcumin. These results suggested that the induction of HO-1 through the PI3K/Akt pathway was critically involved in curcumin-mediated apoptosis resistance.
ZnPP-IX, significantly, but not completely, suppressed the anti-apoptotic effect of curcumin against H2O2. This data suggested that the anti-apoptotic effect of curcumin was probably attributed not only to the involvement of HO-1 but also to other elements. In addition, inhibition of PI3K/Akt pathway did not entirely reverse the curcumin-induced increase in HO-1 protein levels, suggesting that other PI3K/Akt-independent pathways are also involved in the effect of curcumin on HO-1. Moreover, a previous study showed that CO and bilirubin, products of heme metabolism by HO-1, exhibited a potent anti-apoptotic effect in doxorubicin-stimulated H9c2 cells [34], however, whether CO or bilirubin is involved in the cytoprotection afforded by curcumin is still unknown. Thus, experiments aimed at broadening our understanding of the more detailed mechanisms will be the subject of interest in future studies.
Conclusions
Our results demonstrated that curcumin can protect H9c2 cells from H2O2-induced apoptosis and that such anti-apoptotic effect largely depends on the upregulation of HO-1 protein expression through the PI3K/Akt pathway. As a consequence, we speculate that curcumin, which exerts potential protection against oxidative stress-mediated apoptosis, might be used as a preventive and therapeutic agent for treatment of cardiovascular diseases associated with oxidative stress.
Authors’ contributions
YS drafted the manuscript. XY participated in the design of the study and performed the statistical analysis. HJ conceived of the study, and participated in its design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
Acknowledgements
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
Funding
This research is supported in part by the National Science Foundation of China (No. 30973228).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- H2O2
hydrogen peroxide
- HO-1
heme oxygenase-1
- CC3
cleaved caspase-3
- ROS
reactive oxygen species
- CO
carbon monoxide
- PKB
protein kinase B
- PI3 K
phosphoinositide 3-kinase
- ERKs
extracellular signal-regulated kinases
- MTT
methyl thiazolyl tetrazolium
- ZnPP-IX
Zine protoporphyrin-IX
- DMSO
dimethyl sulfoxide
- ATCC
American Type Culture Collection
Contributor Information
Xiaobo Yang, Email: t_t_l081@cntv.cn.
Hong Jiang, Email: q_y_h081@cntv.cn.
Yao Shi, Email: y_l_h726@sina.com.
References
- 1.Abbate A, Bussani R, Amin MS, Vetrovec GW, Baldi A. Acute myocardial infarction and heart failure: role of apoptosis. Int J Biochem Cell Biol. 2006;38:1834–1840. doi: 10.1016/j.biocel.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 2.Buja LM. Myocardial ischemia and reperfusion injury. Cardiovasc Pathol. 2005;14:170–175. doi: 10.1016/j.carpath.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 3.Halliwell B, Aruoma OI. DNA damage by oxygen-derived species Its mechanism and measurement in mammalian systems. FEBS Lett. 1991;281:9–19. doi: 10.1016/0014-5793(91)80347-6. [DOI] [PubMed] [Google Scholar]
- 4.Hensley K, Robinson KA, Gabbita SP, Salsman S, Floyd RA. Reactive oxygen species, cell signaling, and cell injury. Free Radic Biol Med. 2000;28:1456–1462. doi: 10.1016/S0891-5849(00)00252-5. [DOI] [PubMed] [Google Scholar]
- 5.Ammon HP, Wahl MA. Pharmacology of Curcuma longa. Planta Med. 1991;57:1–7. doi: 10.1055/s-2006-960004. [DOI] [PubMed] [Google Scholar]
- 6.Shishodia S, Sethi G, Aggarwal BB. Curcumin: getting back to the roots. Ann NY Acad Sci. 2005;1056:206–217. doi: 10.1196/annals.1352.010. [DOI] [PubMed] [Google Scholar]
- 7.Scharstuhl A, Mutsaers HA, Pennings SW, Szarek WA, Russel FG, Wagener FA. Curcumin-induced fibroblast apoptosis and in vitro wound contraction are regulated by antioxidants and heme oxygenase: implications for scar formation. J Cell Mol Med. 2009;13:712–725. doi: 10.1111/j.1582-4934.2008.00339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu W, Fan Z, Han Y, Lu S, Zhang D, Bai X, Xu W, Li J, Wang H. Curcumin attenuates peroxynitrite-induced neurotoxicity in spiral ganglion neurons. Neurotoxicology. 2011;32:150–157. doi: 10.1016/j.neuro.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 9.Mito S, Thandavarayan RA, Ma M, Lakshmanan A, Suzuki K, Kodama M, Watanabe K. Inhibition of cardiac oxidative and endoplasmic reticulum stress-mediated apoptosis by curcumin treatment contributes to protection against acute myocarditis. Free Radic Res. 2011;45:1223–1231. doi: 10.3109/10715762.2011.607252. [DOI] [PubMed] [Google Scholar]
- 10.Bae JW, Kim MJ, Jang CG, Lee SY. Protective effects of heme oxygenase-1 against MPP(+)-induced cytotoxicity in PC-12 cells. Neurol Sci. 2010;31:307–313. doi: 10.1007/s10072-010-0216-6. [DOI] [PubMed] [Google Scholar]
- 11.Bao W, Li K, Rong S, Yao P, Hao L, Ying C, Zhang X, Nussler A, Liu L. Curcumin alleviates ethanol-induced hepatocytes oxidative damage involving heme oxygenase-1 induction. J Ethnopharmacol. 2010;128:549–553. doi: 10.1016/j.jep.2010.01.029. [DOI] [PubMed] [Google Scholar]
- 12.Katori M, Buelow R, Ke B, Ma J, Coito AJ, Iyer S, Southard D, Busuttil RW, Kupiec-Weglinski JW. Heme oxygenase-1 overexpression protects rat hearts from cold ischemia/reperfusion injury via an antiapoptotic pathway. Transplantation. 2002;73:287–292. doi: 10.1097/00007890-200201270-00023. [DOI] [PubMed] [Google Scholar]
- 13.Xu JJ, Wang YL. Propofol attenuation of hydrogen peroxide-mediated oxidative stress and apoptosis in cultured cardiomyocytes involves haeme oxygenase-1. Eur J Anaesthesiol. 2008;25:395–402. doi: 10.1017/S0265021508003542. [DOI] [PubMed] [Google Scholar]
- 14.Zheng Y, Liu Y, Ge J, Wang X, Liu L, Bu Z, Liu P. Resveratrol protects human lens epithelial cells against H2O2-induced oxidative stress by increasing catalase, SOD-1, and HO-1 expression. Mol Vis. 2010;16:1467–1474. [PMC free article] [PubMed] [Google Scholar]
- 15.Franke TF, Hornik CP, Segev L, Shostak GA, Sugimoto C. PI3K/Akt and apoptosis: size matters. Oncogene. 2003;22:8983–8998. doi: 10.1038/sj.onc.1207115. [DOI] [PubMed] [Google Scholar]
- 16.Zhuang S, Schnellmann RG. A death-promoting role for extracellular signal-regulated kinase. J Pharmacol Exp Ther. 2006;319:991–997. doi: 10.1124/jpet.106.107367. [DOI] [PubMed] [Google Scholar]
- 17.Hsu CL, Wu YL, Tang GJ, Lee TS, Kou YR. Ginkgo biloba extract confers protection from cigarette smoke extract-induced apoptosis in human lung endothelial cells: role of heme oxygenase-1. Pulm Pharmacol Ther. 2009;22:286–296. doi: 10.1016/j.pupt.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 18.Kim JW, Li MH, Jang JH, Na HK, Song NY, Lee C, Johnson JA, Surh YJ. 15-Deoxy-Delta(12,14)-prostaglandin J(2) rescues PC12 cells from H2O2-induced apoptosis through Nrf2-mediated upregulation of heme oxygenase-1: potential roles of Akt and ERK1/2. Biochem Pharmacol. 2008;76:1577–1589. doi: 10.1016/j.bcp.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 19.Turner NA, Xia F, Azhar G, Zhang X, Liu L, Wei JY. Oxidative stress induces DNA fragmentation and caspase activation via the c-Jun NH2-terminal kinase pathway in H9c2 cardiac muscle cells. J Mol Cell Cardiol. 1998;30:1789–1801. doi: 10.1006/jmcc.1998.0743. [DOI] [PubMed] [Google Scholar]
- 20.Gardner AM, Xu FH, Fady C, Jacoby FJ, Duffey DC, Tu Y, Lichtenstein A. Apoptotic vs. nonapoptotic cytotoxicity induced by hydrogen peroxide. Free Radic Biol Med. 1997;22:73–83. doi: 10.1016/S0891-5849(96)00235-3. [DOI] [PubMed] [Google Scholar]
- 21.Wang B, Shravah J, Luo H, Raedschelders K, Chen DD, Ansley DM. Propofol protects against hydrogen peroxide-induced injury in cardiac H9c2 cells via Akt activation and Bcl-2 up-regulation. Biochem Biophys Res Commun. 2009;389:105–111. doi: 10.1016/j.bbrc.2009.08.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu W, Fu YC, Zhou XH, Chen CJ, Wang X, Lin RB, Wang W. Effects of resveratrol on H2O2-induced apoptosis and expression of SIRTs in H9c2 cells. J Cell Biochem. 2009;107:741–747. doi: 10.1002/jcb.22169. [DOI] [PubMed] [Google Scholar]
- 23.Mattson MP, Cheng A. Neurohormetic phytochemicals: low-dose toxins that induce adaptive neuronal stress responses. Trends Neurosci. 2006;29:632–639. doi: 10.1016/j.tins.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 24.Zhang X, Zhao M, Chen L, Jiao H, Liu H, Wang L, Ma S. A triterpenoid from Thalictrum fortunei induces apoptosis in BEL-7402 cells through the P53-induced apoptosis pathway. Molecules. 2011;16:9505–9519. doi: 10.3390/molecules16119505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim IS, Choi DK, Jung HJ. Neuroprotective effects of vanillyl alcohol in Gastrodia elata Blume through suppression of oxidative stress and anti-apoptotic activity in toxin-induced dopaminergic MN9D cells. Molecules. 2011;16:5349–5361. doi: 10.3390/molecules16075349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kang ES, Woo IS, Kim HJ, Eun SY, Paek KS, Chang KC, Lee JH, Lee HT, Kim JH, Nishinaka T, Yabe-Nishimura C, Seo HG. Up-regulation of aldose reductase expression mediated by phosphatidylinositol 3-kinase/Akt and Nrf2 is involved in the protective effect of curcumin against oxidative damage. Free Radic Biol Med. 2007;43:535–545. doi: 10.1016/j.freeradbiomed.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 27.Bhaskaran M, Reddy K, Radhakrishanan N, Franki N, Ding G, Singhal PC. Angiotensin II induces apoptosis in renal proximal tubular cells. Am J Physiol Renal Physiol. 2003;284:F955–F965. doi: 10.1152/ajprenal.00246.2002. [DOI] [PubMed] [Google Scholar]
- 28.Wang G, Hamid T, Keith RJ, Zhou G, Partridge CR, Xiang X, Kingery JR, Lewis RK, Li Q, Rokosh DG, Ford R, Spinale FG, Riggs DW, Srivastava S, Bhatnagar A, Bolli R, Prabhu SD. Cardioprotective and antiapoptotic effects of heme oxygenase-1 in the failing heart. Circulation. 2010;121:1912–1925. doi: 10.1161/CIRCULATIONAHA.109.905471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yeh CH, Chen TP, Wang YC, Lin YM, Lin PJ. HO-1 activation can attenuate cardiomyocytic apoptosis via inhibition of NF-kappaB and AP-1 translocation following cardiac global ischemia and reperfusion. J Surg Res. 2009;155:147–156. doi: 10.1016/j.jss.2008.07.044. [DOI] [PubMed] [Google Scholar]
- 30.Motterlini R, Foresti R, Bassi R, Green CJ. Curcumin, an antioxidant and anti-inflammatory agent, induces heme oxygenase-1 and protects endothelial cells against oxidative stress. Free Radic Biol Med. 2000;28:1303–1312. doi: 10.1016/S0891-5849(00)00294-X. [DOI] [PubMed] [Google Scholar]
- 31.Lima CF, Pereira-Wilson C, Rattan SI. Curcumin induces heme oxygenase-1 in normal human skin fibroblasts through redox signaling: relevance for anti-aging intervention. Mol Nutr Food Res. 2011;55:430–442. doi: 10.1002/mnfr.201000221. [DOI] [PubMed] [Google Scholar]
- 32.Martin D, Rojo AI, Salinas M, Diaz R, Gallardo G, Alam J, De Galarreta CM, Cuadrado A. Regulation of heme oxygenase-1 expression through the phosphatidylinositol 3-kinase/Akt pathway and the Nrf2 transcription factor in response to the antioxidant phytochemical carnosol. J Biol Chem. 2004;279:8919–8929. doi: 10.1074/jbc.M309660200. [DOI] [PubMed] [Google Scholar]
- 33.Wung BS, Hsu MC, Wu CC, Hsieh CW. Piceatannol upregulates endothelial heme oxygenase-1 expression via novel protein kinase C and tyrosine kinase pathways. Pharmacol Res. 2006;53:113–122. doi: 10.1016/j.phrs.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 34.Kim DS, Chae SW, Kim HR, Chae HJ. CO and bilirubin inhibit doxorubicin-induced cardiac cell death. Immunopharmacol Immunotoxicol. 2009;31:64–70. doi: 10.1080/08923970802354762. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.


