Abstract
A yeast causing widespread infection of laboratory mice was identified from 26S rRNA gene sequences as Candida pintolopesii. To determine the relationship of C. pintolopesii with other members of the Kazachstania (Arxiozyma) telluris species complex, nucleotide sequences from domains 1 and 2 of the 26S rRNA gene, the mitochondrial small-subunit rRNA gene, and the RNA polymerase II gene were phylogenetically analyzed. That analysis resolved the 48 strains examined into five closely related species: K. telluris, Candida bovina, C. pintolopesii, Candida slooffiae, and a previously unknown species. One or more strains of each of the last four species formed an ascosporic state much like that of K. telluris. To place these ascosporogenous strains taxonomically, it is proposed that they be assigned to the teleomorphic genus Kazachstania as K. bovina (type strain NRRL Y-7283, CBS 9732, from the nasal passage of a pigeon), K. heterogenica (type strain NRRL Y-27499, CBS 2675, from rodent feces), K. pintolopesii (type strain NRRL Y-27500, CBS 2985, from the peritoneal fluid of a dead guinea pig), and K. slooffiae (type strain NRRL YB-4349, CBS 9733, from the cecum of a horse). On the basis of multigene sequence analyses, K. heterogenica appears to be a hybrid of K. pintolopesii and a presently unknown species. With the exception of K. bovina, the phylogenetically defined species show a moderate degree of host specificity.
During 2002, laboratory mice received by the National Institutes of Health from several different suppliers were infected with a yeast, which rendered the mice unsuitable for laboratory use. Domains 1 and 2 (D1 and D2, respectively) of the large-subunit (26S) rRNA genes of isolates from seven mice were sequenced in our laboratory, and the sequences were identical to one another and to that of the type strain of Candida pintolopesii. Strains of a species generally have the same D1 and D2 rRNA gene sequences, or the sequences may sometimes vary by 1 to 3 nucleotides (10). Consequently, from these sequence comparisons, we concluded that the mice were infected with C. pintolopesii.
C. pintolopesii is a member of the Kazachstania (Arxiozyma) telluris species complex. Arxiozyma telluris was first described as Saccharomyces tellustris by van der Walt (17). Because the ascospore walls of this species are roughened as a result of surface ornamentation (5), van der Walt and Yarrow (18) concluded that S. tellustris was not typical of the smooth-spored Saccharomyces sensu stricto species and redescribed it as A. telluris. Candida bovina, C. pintolopesii, and Candida slooffiae are phenotypically much like A. telluris and were believed to represent the anamorphic states of this ascosporogenous species (4, 19, 20). Mendonça-Hagler and Phaff (12) tested this possibility by measuring the extent of nuclear DNA complementarity among the four taxa. All four were reported to share 80% or greater nuclear DNA relatedness, leading Mendonça-Hagler and Phaff (12) to propose that the four taxa represent the same species. Hurt (2) reexamined the nuclear DNA relatedness among the four taxa using a free-solution reassociation method rather than the filter-binding technique of Mendonça-Hagler and Phaff (12). In contrast to the earlier results, Hurt (2) found only 20 to 56% nuclear DNA relatedness among the various pairings, which would suggest that the taxa represent four separate, but closely related, species. James et al. (3) compared the four taxa by phylogenetic analysis of the nucleotide sequences of the 18S rRNA gene, 26S D1 and D2 rRNA genes, and the mitochondrially encoded cytochrome oxidase II gene. These results again suggested that the taxa represent four separate but closely related species, and in view of this genetic divergence, James et al. (3) proposed reinstatement of C. pintolopesii and C. slooffiae as distinct species.
More recently, Kurtzman and Robnett (11) examined genus boundaries in the Saccharomyces complex by multigene sequence analysis. The ca. 80 species resolved into 14 clades, which were interpreted as genera, resulting in redescriptions of some previously accepted genera and the proposal of five new genera (8). Clade 2 from the analysis included several species of Saccharomyces and Kluyveromyces that were phylogenetically unrelated to the type species of their respective genera, as well as species of the monotypic genera Arxiozyma, Kazachstania, and Pachytichospora. Of the last three genera, Kazachstania has taxonomic priority, resulting in the transfer of A. telluris and other clade 2 species to Kazachstania.
In the present study, we compared the mouse isolates with other strains of the K. telluris complex and resolved five clades by D1 and D2 26S rRNA gene sequence analysis, consistent with the report of James et al. (3). We further compared these isolates by analysis of the mitochondrial small-subunit rRNA gene and RNA polymerase II gene sequences. Analyses of the last two data sets gave trees congruent with the tree constructed from the D1 and D2 sequences, supporting the concept that C. pintolopesii, C. bovina, and C. slooffiae are distinct species. The analyses also detected an additional, previously unknown species. Microscopic examination of the cultures revealed that one or more strains from each of these four clades formed ascospores. Consequently, we propose placement of the ascosporic states of C. pintolopesii, C. bovina, C. slooffiae, and the newly detected species in the teleomorphic genus Kazachstania.
MATERIALS AND METHODS
Initial identification of yeasts from laboratory animals.
The yeast-like organisms were first found during necropsy and by histopathology in sentinel and research mice of various strains and stocks from a facility where caging, food, and bedding were not autoclaved. These organisms were not observed in an adjacent barrier facility where caging, food, and bedding were autoclaved. Similar organisms were found frequently in hamsters and sporadically in rat sentinels from the same facility and in nude and heterozygous nude mice in a facility at an unrelated institution.
Histology.
Multiple tissues from laboratory animals were fixed in 10% neutral buffered formalin and embedded in paraffin blocks. The specimens were sectioned and stained with hematoxylin-eosin and periodic acid-Schiff.
Organisms and culture media.
Organisms from the infected mice at the National Institutes of Health were cultured from stool specimens, gastric contents, and homogenized tissues onto Sabouraud glucose agar supplemented with gentamicin and chloramphenicol. All strains examined in this study, their sources of isolation, and observations on ascospore formation are provided in Table 1. The composition of the culture media and the procedures for morphological examination and characterization from fermentation and assimilation tests were given previously (25). Representative strains of each species group were tested with the seven fermentable sugars and by the 44 growth tests often used in yeast taxonomy (7). The small group of compounds likely to give positive responses was then tested with all strains in this study. Because growth responses were sometimes weak, the yeast nitrogen base basal medium was supplemented with 0.1% yeast extract, which resulted in stronger positive reactions but which gave no false-positive results. Assimilation reactions were determined in liquid medium with manual shaking of the tubes every 2 to 3 days. Incubation was at 37°C. Yeast extract-malt extract-peptone-glucose (YM) agar slants were used for growth tests at 40 and 42°C.
TABLE 1.
Sources of strains and their taxonomic designations as proposed in this study
| Species | Strain designation
|
Source (description of strain)a | Ascospore formationb | |
|---|---|---|---|---|
| NRRL | CBSc | |||
| Kazachstania bovina | Y-7283T | 9732 | Nasal passage, pigeon, USA | + |
| Y-27501 | 5812 | Human, France | + | |
| Y-27502 | 6264 | Nasal passage, pigeon, USA | + | |
| Y-27511 | 2760 | Cecum, cow, Portugal (strain of Candida bovina) | −? | |
| Y-27536 | 2678 | Crop, turkey, UK | − | |
| Kazachstania heterogenica | Y-27499T | 2675 | Feces, rodent, New Zealand | + |
| Y-2306 | Lungs, spleen, mouse, Portugal | −? | ||
| Y-27537 | 2778 | Cecum, rat, Portugal | − | |
| Y-27541 | 3046 | Peritoneal cavity, mouse, USA | −? | |
| Kazachstania pintolopesii | Y-27500T | 2985 | Peritoneal fluid, dead guinea pig, Indonesia | ++ |
| Y-2251 | Mouse, Paraguay? | − | ||
| Y-2260 | 1787 | Liver, mouse, Portugal (type strain of Candida pintolopesii) | −? | |
| Y-7492 | Stomach, mouse, USA | − | ||
| Y-27461 | Stomach, mouse, NIH, USA | − | ||
| Y-27462 | Stomach, mouse, NIH, USA | −? | ||
| Y-27463 | Stomach, mouse, NIH, USA | + | ||
| Y-27464 | Stomach, mouse, NIH, USA | −? | ||
| Y-27465 | Feces, mouse, NIH, USA | − | ||
| Y-27466 | Feces, mouse, NIH, USA | + | ||
| Y-27467 | Feces, mouse, NIH, USA | −? | ||
| Y-27510 | 2676 | Udder, mouse, The Netherlands | −? | |
| Y-27539 | 3011 | Feces, rat, UK | − | |
| Y-27540 | 3045 | Mouse, Uruguay | − | |
| Y-27542 | 3047 | Gut, pigeon, USA | − | |
| Y-27543 | 3084 | Gut, chinchilla, Canada | − | |
| Y-27544 | 6261 | Unknown | − | |
| YB-1785 | Mouse, USA | + | ||
| YB-2574 | Rodent?, USA | + | ||
| YB-2576 | Rodent?, USA | −? | ||
| YB-2577 | Rodent?, USA | − | ||
| YB-2578 | Rodent?, USA | −? | ||
| YB-2579 | Rodent?, USA | − | ||
| YB-2580 | Rodent?, USA | − | ||
| YB-2581 | Rodent?, USA | − | ||
| YB-2582 | Rodent?, USA | −? | ||
| YB-3291 | Lungs, spleen, mouse, USA | − | ||
| YB-3357 | Intestines, rat, USA | − | ||
| YB-3360 | Intestines, rat, USA | −? | ||
| YB-3359 | Intestines, rat, USA | − | ||
| Kazachstania slooffiae | YB-4349T | 9733 | Cecum, horse, Portugal | + |
| Y-27498 | 2419 | Cecum, horse, Portugal (type strain of Candida slooffiae) | − | |
| Y-27538 | 2783 | Cecum, horse, Portugal | − | |
| Y-27546 | 4068 | Cecum, pig, Portugal | − | |
| YB-4351 | Cecum, horse, Portugal | − | ||
| Kazachstania (Arxiozyma) telluris | YB-4302T | 2685 | Soil, South Africa | +++ |
| Y-7284 | 6258 | Nasal passage, pigeon, USA | +++ | |
| Y-7285 | Nasal passage, pigeon, USA | ++ | ||
| Y-27545 | 6265 | Nasal passage, pigeon, USA | + | |
Abbreviations: NIH, National Institutes of Health; UK, United Kingdom; USA, United States.
−, no ascospores; −?, possible poorly formed ascospores; +, infrequent ascospores; ++, occasional ascospores; +++, ascospores relatively common.
CBS, Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands.
Ascospore formation.
Strains were examined for ascospore formation on YM and McClary's acetate agars. The cultures were initially incubated for 2 days at 37°C to facilitate growth and were then further incubated at 25°C to induce ascospore formation. The cultures were examined for ascospores at approximately weekly intervals for 4 weeks.
Gene sequencing and phylogenetic analysis.
Three gene regions were sequenced: D1 and D2 (nucleotides 63 to 642) of the large-subunit (26S) rRNA gene, the mitochondrial small-subunit rRNA gene bounded by primers MS-1 and MS-2, and the RNA polymerase II gene (nucleotides 2447 to 3127). Primers and details of the sequencing reactions were given by Kurtzman and Robnett (11). Sequences were determined on an Applied Biosystems model 3730 capillary DNA sequencer.
Estimates of phylogenetic relatedness among species were determined by using the maximum parsimony (MP) and neighbor-joining programs of the PAUP*4.063a software package (16). The Kimura two-parameter distance correction was used for neighbor-joining analyses. Bootstrap support for the phylogenetic trees was determined from 1,000 replications.
Nucleotide sequence accession numbers.
The GenBank accession numbers of the sequences determined from the analysis of each gene, except for those of the RNA polymerase II gene (GenBank accession numbers AY552467 to AY552472) and the mitochondrial small-subunit rRNA gene (GenBank accession numbers AF442281 to AF442286), which were used for comparison with species in the Saccharomyces cerevisiae clade, are given on the phylogenetic trees.
RESULTS
Initial identification of yeasts from laboratory animals.
In addition to the mice at the National Cancer Institute that were observed to have yeasts within the gastrointestinal tract, a line of knockout mice with a B6:129 background from that facility were shipped to another large research facility in another state for rederivation and eventual research. The mice were routinely maintained in a receiving and quarantine room. The gastrointestinal infection had not been seen at that research facility for many years. The transported infected mice were found to have gastric infection with chronic gastritis. DBA/2NCr mice placed in the same cage as infected null mice for 4 months also developed gastric infection.
Histopathology.
The histopathological gastric changes consisted of numerous but variable numbers of yeast-like organisms in gastric epithelial surface debris and along the surfaces of epithelial cells throughout all regions of the glandular stomach. Hematoxylin-eosin staining revealed that the organisms were small, approximately 3 μm in diameter, spherical to ovoid yeast-like cells with a dark blue outer wall and some internal dot-like structures. These organisms were densely positive by periodic acid-Schiff staining. Chronic inflammation with lymphocytes or myeloid cells was observed in a minority of animals. However, no inflammation was observed in most animals.
Gene sequence comparisons.
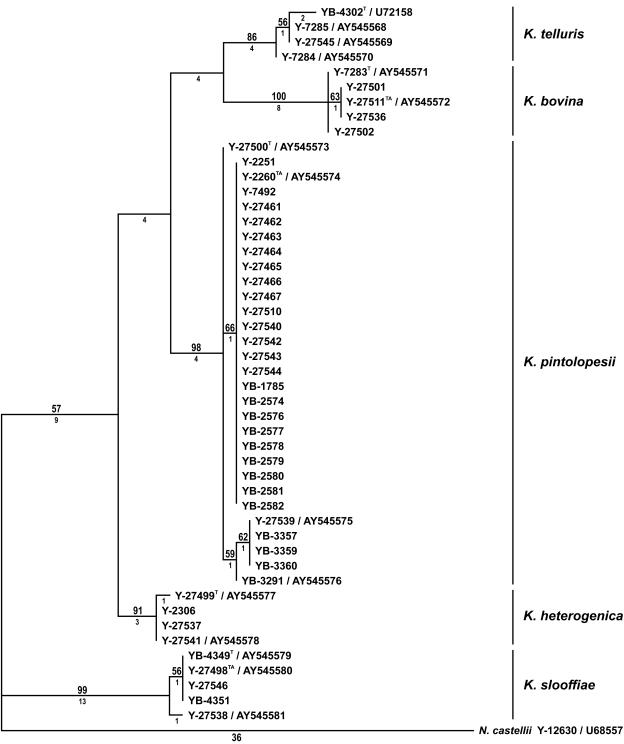
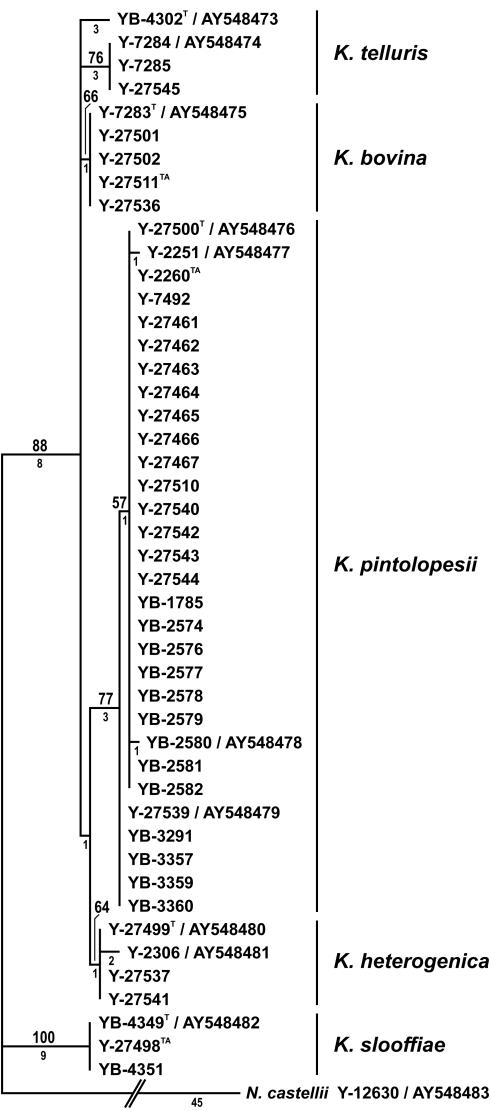
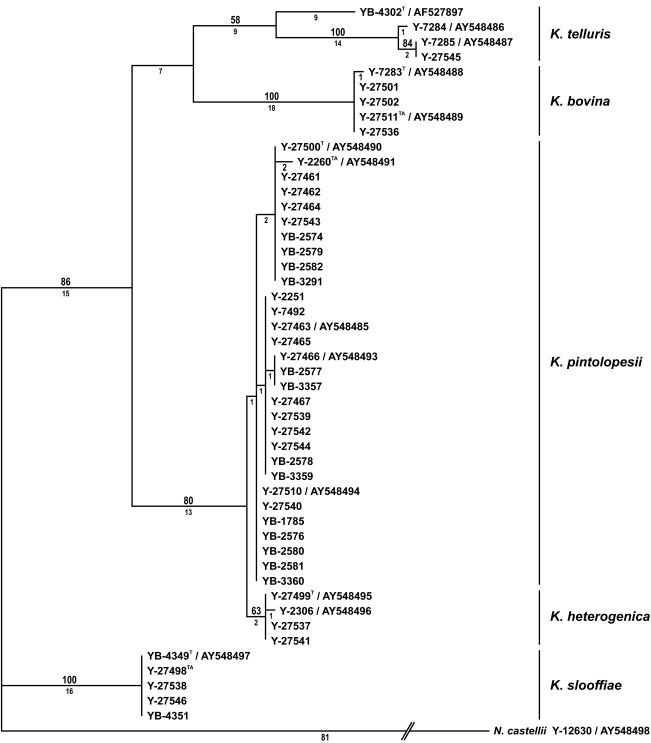
Phylogenetic analysis of the D1 and D2 26S rRNA gene nucleotide sequences resolved strains of the K. telluris complex into five clades, with the level of intraclade divergence ranging from 1 to 3 nucleotides (Fig. 1). The clades correspond to K. telluris, C. bovina, C. pintolopesii, C. slooffiae, and a previously unrecognized species. Analysis of mitochondrial small-subunit rRNA gene sequences also gave five clades (Fig. 2) which were congruent with those in the tree derived from the D1 and D2 sequences. However, analysis of RNA polymerase II gene nucleotide sequences resolved just four clades (Fig. 3). Strains of C. pintolopesii and the previously undetected species formed a single clade, which suggests that the new species is a hybrid of C. pintolopesii and another species that is not among those sequenced in this study. In the work of James et al. (3), K. telluris, C. bovina, C. pintolopesii, and C. slooffiae were resolved into separate clades from analysis of the 18S rRNA gene and D1 and D2 of the 26S rRNA gene, as shown here. Additionally, James et al. (3) sequenced the mitochondrially encoded COX II gene; and the analysis resolved C. pintolopesii and C. slooffiae into distinct populations, but C. bovina and K. telluris were found to be members of the same clade. These results suggest that either K. telluris or C. bovina represents a hybrid species, which further suggests that additional species of the K. telluris complex are to be discovered and that interspecific hybridization in the complex may be common.
FIG. 1.
One of four most parsimonious trees from MP analysis of the D1 and D2 26S rRNA gene sequences from species of the K. telluris clade. Tree length = 95, consistency index = 0.853, retention index = 0.956, rescaled consistency index = 0.815, homoplasy index = 0.147. Bootstrap values ≥50% are given above the branches; and branch lengths, indicated as the number of steps, are given below the branches. Naumovia castellii was the designated outgroup species. Strains are designated by NRRL accession numbers followed by GenBank accession numbers for strains with unique DNA sequences. T, type strain of the designated Kazachstania species; TA, type strain of the previously described Candida anamorph.
FIG. 2.
One of 100 most parsimonious trees from MP analysis of mitochondrial small-subunit rRNA gene sequences from species of the K. telluris clade. Tree length = 79, consistency index = 0.924, retention index = 0.947, rescaled consistency index = 0.875, homoplasy index = 0.076. Other designations are as defined in the legend to Fig. 1. Because of sequencing difficulties, NRRL Y-27538 and NRRL Y-27546 were excluded from the analysis.
FIG. 3.
One of 100 most parsimonious trees from MP analysis of RNA polymerase II nucleotide sequences from species of the K. telluris clade. Tree length = 198, consistency index = 0.798, retention index = 0.926, rescaled consistency index = 0.739, homoplasy index = 0.202. Other designations are as defined in the legend to Fig. 1.
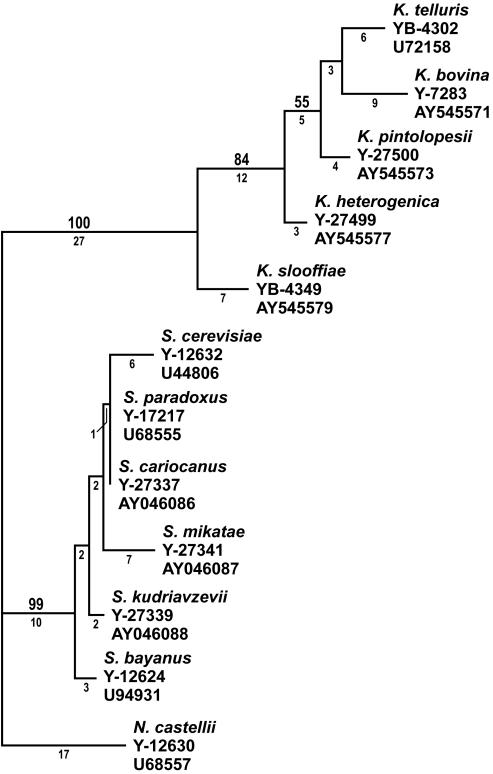
Do the clades seen in this study and that of James et al. (3) represent individual species or genetically unique populations within a single species? On the basis of earlier comparisons, strains that differ from one another by 1% or greater nucleotide substitutions in the D1 and D2 domains of the 26S rRNA gene have proven to be separate species on the basis of the results from genetic crosses and from the extent of nuclear DNA complementarity, as measured from DNA reassociation analysis (10). The most closely related species pair in this study, K. telluris and C. bovina, differed from one another by ca. 2% substitutions (13 of 575 positions). The divergence in the mitochondrial small-subunit rRNA gene and the RNA polymerase II gene data sets is also consistent with the concept of separate species. For comparison, there was greater divergence among populations of the K. telluris clade than among species of the S. cerevisiae clade in the D1 and D2 26S rRNA gene sequences (Fig. 4), as well as for divergence in the mitochondrial small-subunit rRNA gene and the RNA polymerase II gene sequences (data not shown). Members of the S. cerevisiae clade have been recognized as separate species from nuclear DNA reassociation analyses (21, 22), gene sequence comparisons (10, 11, 14), and genetic crosses (13). By comparison, the five population groups in the K. telluris clade appear to represent separate species.
FIG. 4.
Comparison of divergence among type strains of species of Saccharomyces and Kazachstania from MP analysis of the D1and D2 domains of the 26S rRNA gene sequences. One of three most parsimonious trees is shown. Tree length = 126, consistency index = 0.794, retention index = 0.867, rescaled consistency index = 0.688, homoplasy index = 0.206. Other designations are as defined in the legend to Fig. 1.
Habitat and host specificity.
Members of the K. telluris species complex are mainly known from infections in rodents and birds and to a lesser extent in humans, horses, pigs, and cows (Table 1). The clades determined from sequence analysis allow analysis of whether there is host specificity among these species. As shown in Table 1, strains of C. slooffiae were from horses and a pig, C. pintolopesii and the new species were almost exclusively from rodents, and K. telluris was isolated from soil and the nasal passages of pigeons. The least host specificity was shown by C. bovina, which has been isolated from a cow, birds, and a human. On the basis of the hybrid nature of the NRRL Y-27499 clade and the apparent hybrid detected by James et al. (3), it appears that there are additional closely related species yet to be discovered within the K. telluris complex, some of which may be specific to other animal hosts.
Description of new ascosporic species.
On the basis of the molecular analyses described above, five distinct species have been resolved in the K. telluris complex: K. telluris, C. bovina, C. pintolopesii, C. slooffiae, and a previously unrecognized species. The last four species were found to form ascospores, and on the basis of multigene phylogenetic analyses, their placement in the teleomorphic genus Kazachstania is proposed.
Latin diagnosis of Kazachstania bovina Kurtzman & Robnett sp. nov.
In agaro YM post dies 2 ad 37°C, cellulae vegetativae globosae (2.8 to 6.0 μm) aut ellipsoideae (2.8-7.1 × 3.8-11.0 μm), singulae, binae et catenas brevis. In agaro morphologico post dies 7 ad 37°C, incrementum fuscum pallidum, nitens aut hebes et butyrosum; centrum coloniae deprimere; margo glabro vel lobate. Pseudohyphae et hyphae verae non fiunt. Species homothallica. Asci liberi, ascospora globosa singulara, non liberi. Glucosum fermentatur. Galactosum, sucrosum, maltosum, lactosum, raffinosum et trehalosum non fermentantur. Assimilantur glucosum, ethanolum, dl-acidum lacticum (variabile) et acidum succinicum (variabile). Non assimilantur galactosum, l-sorbosum, maltosum, sucrosum, cellobiosum, trehalosum, lactosum, melibiosum, raffinosum, melezitosum, inulinum, amylum solubile, d-xylosum, l-arabinosum, d-arabinosum, d-ribosum, l-rhamnosum, d-glucosaminum, N-acetyl-d-glucosaminum, glycerolum, methanolum, erythritolum, ribitolum, galactitolum, mannitolum, glucitolum, methyl α-d-glucosidum, salicinum, d-gluconas, 2-keto-d-gluconas, 5-keto-d-gluconas, saccharatas, acidum citricum, inositolum, hexadecanum et potassii nitras. Augmentum non fiunt in 10% sal-5% glucosum. Amylum non formatur. Vitaminae externae ad crescentiam necessaria sunt. Augmentum fiunt in temperatura 40 et 42°C (variabile). Species nova a speciebus aliis sequentibus nucleotiditis D1 et D2 26S rRNA gene, mitochondrial submonas parvus rRNA gene et RNA polymerase II distinguenda. Typus: NRRL Y-7283 (CBS 9732) designat stirpem typicam. Isolata naris Columba livida, USA, depositata in Collectione Culturarum ARS (NRRL), Peoria, Ill.
Description of Kazachstania bovina Kurtzman & Robnett, sp. nov.
Vegetative reproduction. After growth for 2 days on YM agar at 37°C, the cells are spherical (2.8 to 6.0 μm) to ellipsoidal (2.8 to 7.1 × 3.8 to 11.0 μm) and occur singly, in pairs, or in short chains (Fig. 5A). Budding is multilateral. Growth is white to tannish white, semiglistening, and butyrous. Aerobic growth after 7 days at 37°C in Dalmau plate culture on yeast morphology agar is tannish white, dull to semiglistening, and butyrous with a depressed center. Margins are entire to finely lobed. Neither hyphae nor pseudohyphae are formed under the cover glass.
FIG. 5.
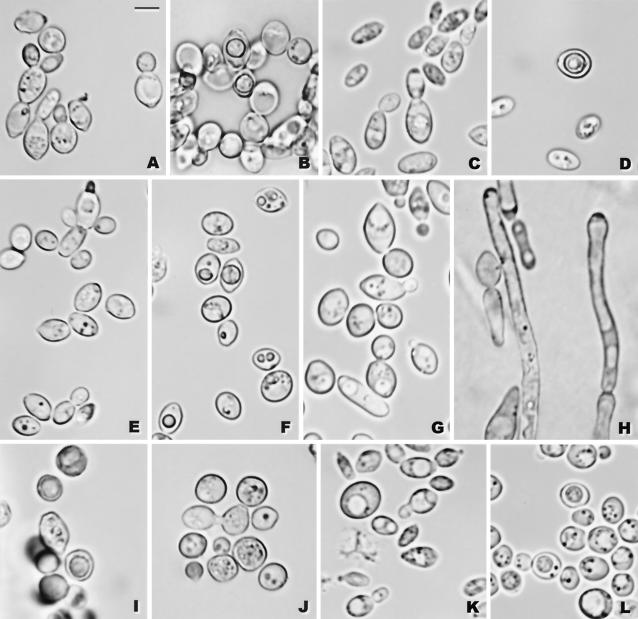
(A and B) K. bovina NRRL Y-7283: (A) budding cells; (B) asci with a single ascospore, YM agar, 15 days. (C and D) K. heterogenica NRRL Y-27499: (C) budding cells; (D) ascus with an ascospore, YM agar, 25 days. (E and F) K. pintolopesii NRRL Y-27500: (E) budding cells; (F) ascus with an ascospore, YM agar, 23 days. (G to J) K. slooffiae NRRL YB-4349: (G) budding cells; (H) pseudohyphae, Dalmau plate with yeast morphology agar, 7 days, 37°C (NRRL Y-27498); (I) asci with a single ascospore, acetate agar, 22 days; (J) conjugation between two cells, acetate agar, 22 days. (K and L) K. telluris NRRL YB-4302: (K) budding cells; (L) asci with ascospores, acetate agar, 8 days. For all panels, budding cells, YM agar, 2 days, 37°C and ascospore formation, 25°C. Bar, 5 μm.
Ascospore formation.
Three of the five available strains formed ascospores, albeit at a low frequency. Ascospores formed on YM and McClary's acetate agars after 10 to 20 days at 25°C; asci are persistent and unconjugated and contain a single, finely roughened ascospore (Fig. 5B). Although asci are unconjugated, the occasional conjugations observed between independent cells, as well as apparent conjugations between buds and their parent cells, suggest that the species is homothallic.
Fermentation, assimilation, and other growth reactions.
Glucose is the only sugar fermented in standard tests; and only glucose, ethanol, lactic acid (variable), and succinic acid (variable) are assimilated (Table 2). All strains grow at 40°C, but only three of the five strains grow at 42°C (Table 2).
TABLE 2.
Physiological and morphological characteristics of Kazachstania species in the K. telluris clade
| Species | Glucose fermentation | Growtha
|
Presence of pseudohyphae | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Glucose | Ethanol | dl-Lactic acid | Succinic acid | 10% NaCl-5% glucose | 40°C | 42°C | |||
| K. bovina | 5/5b | 5/5 | 5/5 | 4/5 | 2/5 | 0/5 | 5/5 | 3/5 | 0/5 |
| K. heterogenica | 4/4 | 4/4 | 3/4 | 3/4 | 0/4 | 0/4 | 3/4 | 0/4 | 0/4 |
| K. pintolopesii | 29/29 | 29/29 | 4/29 | 13/29 | 0/29 | 0/29 | 29/29 | 1/29 | 0/29 |
| K. slooffiae | 5/5 | 5/5 | 0/5 | 0/5 | 0/5 | 0/5 | 5/5 | 2/5 | 5/5 |
| K. telluris | 4/4 | 4/4 | 4/4 | 4/4 | 0/4 | 2/4 | 4/4 | 4/4 | 0/4 |
The full set of seven sugars and 44 growth tests typically used for strain characterization are given by Kurtzman (7) and are also listed in the Latin description of each new species.
Data represent number of strains positive/total number of strains tested.
Type strain.
NRRL Y-7283 (CBS 9732) is preserved in the lyophilized state, as well as in liquid nitrogen storage, in the Agricultural Research Service Culture Collection, NRRL, Peoria, Ill. The strain was received in 1971 from H. F. Hasenclever, National Institutes of Health, Bethesda, Md., as strain B-3197 and had been isolated from the nasal passage of a pigeon. This isolate was selected as the type strain because it produced the greatest number of ascospores of the three ascosporogenous strains examined.
Etymology.
The species name bovina comes from that of the anamorphic state, Candida bovina van Uden & do Carmo-Sousa.
Latin diagnosis of Kazachstania heterogenica Kurtzman & Robnett sp. nov.
In agaro YM post dies 2 ad 37°C, cellulae vegetativae globosae (4.0 to 8.0 μm) aut ellipsoideae (2.1-7.0 × 4.0-12.0 μm), singulae, binae et catenas brevis. In agaro morphologico post dies 7 ad 37°C, incrementum fuscum pallidum, nitens aut hebes et butyrosum; centrum coloniae planae; margo glabro vel lobate. Pseudohyphae et hyphae verae non fiunt. Species homothallica. Asci liberi, ascospora globosa singulara, non liberi. Glucosum fermentatur. Galactosum, sucrosum, maltosum, lactosum, raffinosum et trehalosum non fermentantur. Assimilantur glucosum, ethanolum (variabile) et dl-acidum lacticum (variabile). Non assimilantur galactosum, l-sorbosum, maltosum, sucrosum, cellobiosum, trehalosum, lactosum, melibiosum, raffinosum, melezitosum, inulinum, amylum solubile, d-xylosum, l-arabinosum, d-arabinosum, d-ribosum, l-rhamnosum, d-glucosaminum, N-acetyl-d-glucosaminum, glycerolum, methanolum, erythritolum, ribitolum, galactitolum, mannitolum, glucitolum, methyl α-d-glucosidum, salicinum, d-gluconas, 2-keto-d-gluconas, 5-keto-d-gluconas, saccharatas, acidum succinicum, acidum citricum, inositolum, hexadecanum et potassii nitras. Augmentum non fiunt in 10% sal-5% glucosum. Amylum non formatur. Vitaminae externae ad crescentiam necessaria sunt. Augmentum fiunt in temperatura 40°C (variabile), non 42°C. Species nova a speciebus aliis sequentibus nucleotiditis D1 et D2 26S rRNA gene et mitochondrial submonas parvus rRNA gene distinguenda. Typus: NRRL Y-27499 (CBS 2675) designat stirpem typicam. Isolata ex feces, rodentia incognita, Nova Zealandia, depositata in NRRL.
Description of Kazachstania heterogenica Kurtzman & Robnett, sp. nov.
Reproduction is vegetative. After growth for 2 days on YM agar at 37°C, the cells are spherical (4.0 to 8.0 μm) to ellipsoidal (2.1 to 7.0 × 4.0 to 12.0 μm) and occur singly in pairs or in short chains (Fig. 5C). Budding is multilateral. Growth is tannish white, semiglistening, and butyrous. Aerobic growth after 7 days at 37°C in Dalmau plate culture on yeast morphology agar is tan in color, dull to semiglistening, and butyrous in texture. Colonies are low and flat and have finely lobed margins. Neither hyphae nor pseudohyphae are formed under the cover glass.
Ascospore formation.
Of the four strains examined, only NRRL Y-27499 formed ascospores. Ascosporulation is quite sparse and occurs on YM and McClary's acetate agars after 10 to 15 days at 25°C. Asci are persistent and unconjugated and contain a single, finely roughened ascospore (Fig. 5D). Occasionally, conjugation between independent cells as well as conjugation between a bud and its parent cell is observed, which suggests that the species is homothallic.
Fermentation, assimilation, and other growth reactions.
Glucose is the only sugar fermented. Growth occurs on glucose, ethanol (variable), and dl-lactic acid (variable). Three of the four known strains grow at 40°C, but none grow at 42°C (Table 2).
Type strain.
NRRL Y-27499 (CBS 2675) is preserved in the lyophilized state, as well as in liquid nitrogen storage, at NRRL. The strain was isolated from the feces of an unidentified rodent in New Zealand.
Etymology.
The species name heterogenica refers to the possibility that this species is a hybrid between C. pintolopesii and a presently unknown species.
Latin diagnosis of Kazachstania pintolopesii Kurtzman, Robnett, J. N. Ward & T. J. Walsh, sp. nov.
In agaro YM post dies 2 ad 37°C, cellulae vegetativae globosae (2.1 to 6.0 μm) aut ellipsoideae (2.1-8.0 × 2.5-11.0 μm), singulae et binae. In agaro morphologico post dies 7 ad 37°C, incrementum fuscum pallidum, nitens aut hebes, butyrosum; centrum coloniae deprimere aut planae, margo lobate. Pseudohyphae et hyphae verae non fiunt. Species homothallica. Asci liberi, ascospora globosa singulara, non liberi. Glucosum fermentatur. Galactosum, sucrosum, maltosum, lactosum, raffinosum et trehalosum non fermentantur. Assimilantur glucosum, ethanolum (variabile), et dl-acidum lacticum (variabile). Non assimilantur galactosum, l-sorbosum, maltosum, sucrosum, cellobiosum, trehalosum, lactosum, melibiosum, raffinosum, melezitosum, inulinum, amylum solubile, d-xylosum, l-arabinosum, d-arabinosum, d-ribosum, l-rhamnosum, d-glucosaminum, N-acetyl-d-glucosaminum, glycerolum, methanolum, erythritolum, ribitolum, galactitolum, mannitolum, glucitolum, methyl α-d-glucosidum, salicinum, d-gluconas, 2-keto-d-gluconas, 5-keto-d-gluconas, saccharatas, acidum succinicum, acidum citricum, inositolum, hexadecanum et potassii nitras. Augmentum non fiunt in 10% sal-5% glucosum. Amylum non formatur. Vitaminae externae ad crescentiam necessaria sunt. Augmentum fiunt in temperatura 40 et 42°C (variabile). Species nova a speciebus aliis sequentibus nucleotiditis D1 et D2 26S rRNA gene, mitochondrial submonas parvus rRNA gene et RNA polymerase II distinguenda. Typus: NRRL Y-27500 (CBS 2985) designat stirpem typicam. Isolata humor peritonealis ex Cavia porcellus, Iabada, depositata in NRRL.
Description of Kazachstania pintolopesii Kurtzman, Robnett, J. N. Ward & T. J. Walsh, sp. nov.
Vegetative reproduction. After growth for 2 days on YM agar at 37°C, the cells are spherical (2.1 to 6.0 μm) to ellipsoidal (2.1 to 8.0 × 2.5 to 11.0 μm) and occur singly or in pairs (Fig. 5E). Budding is multilateral. Growth is tannish white, semiglistening, and butyrous. Aerobic growth after 7 days at 37°C in Dalmau plate culture on yeast morphology agar is tannish white, semiglistening, and butyrous. Colonies may be flat or have a depressed center and have a finely lobed margin. Neither hyphae nor pseudohyphae are formed under the cover glass.
Ascospore formation.
Five of the 30 available strains formed ascospores. Ascosporulation occurs on YM and McClary's acetate agars after 10 to 15 days at 25°C. Ascospore formation is generally sparse with one finely roughened ascospore in each persistent ascus (Fig. 5F). Asci are unconjugated, but the presence of apparent conjugations between individual cells and between cells and their buds suggests that the species is homothallic.
Fermentation, assimilation, and other growth tests.
Glucose is the only sugar fermented; and only glucose, ethanol (variable), and dl-lactic acid (variable) are assimilated. All strains grow at 40°C, but just one strain grows at 42°C (Table 2).
Type strain.
NRRL Y-27500 (CBS 2985) is preserved in the lyophilized state, as well as in liquid nitrogen storage, at NRRL. The strain was isolated from the peritoneal fluid of a dead guinea pig in Indonesia. This isolate was selected as the type strain because it produced the greatest number of ascospores of the five ascosporogenous strains examined.
Etymology.
The species name pintolopesii comes from that of the anamorphic state, Candida pintolopesii (van Uden) S. A. Meyer & Yarrow.
Latin diagnosis of Kazachstania slooffiae Kurtzman & Robnett, sp. nov.
In agaro YM post dies 2 ad 37°C, cellulae vegetativae globosae (4.0 to 11.0 μm) aut ellipsoideae (3.0-11.5 × 4.8-14.0 μm), singulae, binae et catenas brevis. In agaro morphologico post dies 7 ad 37°C, incrementum fuscum pallidum, nitens aut hebes et butyrosum. Centrum coloniae planae, margo lobate. Pseudohyphae fiunt, hyphae verae non fiunt. Species homothallica. Asci liberi, ascospora globosa singulara, non liberi. Glucosum fermentatur. Galactosum, sucrosum, maltosum, lactosum, raffinosum et trehalosum non fermentantur. Assimilantur glucosum. Non assimilantur galactosum, l-sorbosum, maltosum, sucrosum, cellobiosum, trehalosum, lactosum, melibiosum, raffinosum, melezitosum, inulinum, amylum solubile, d-xylosum, l-arabinosum, d-arabinosum, d-ribosum, l-rhamnosum, d-glucosaminum, N-acetyl-d-glucosaminum, ethanolum, glycerolum, methanolum, erythritolum, ribitolum, galactitolum, mannitolum, glucitolum, methyl α-d-glucosidum, salicinum, d-gluconas, 2-keto-d-gluconas, 5-keto-d-gluconas, saccharatas, dl-acidum lacticum, acidum succinicum, acidum citricum, inositolum, hexadecanum et potassii nitras. Augmentum non fiunt in 10% sal-5% glucosum. Amylum non formatur. Vitaminae externae ad crescentiam necessaria sunt. Augmentum fiunt in temperatura 40 et 42°C (variabile). Species nova a speciebus aliis sequentibus nucleotiditis D1 et D2 26S rRNA gene, mitochondrial submonas parvus rRNA gene et RNA polymerase II distinguenda. Typus: NRRL YB-4349 (CBS 9733) designat stirpem typicam. Isolata ex caecum, Equus caballus, Portugallia, depositata in NRRL.
Description of Kazachstania slooffiae Kurtzman & Robnett sp. nov.
Vegetative reproduction. After growth for 2 days on YM agar at 37°C, the cells are spherical (4.0 to 11.0 μm) to ellipsoidal (3.0 to 11.5 × 4.8 to 14.0 μm) and occur singly in pairs or infrequently in short chains (Fig. 5G). Budding is multilateral. Growth is tannish white, semiglistening, and butyrous. Aerobic growth after 7 days at 37°C in Dalmau plate culture on yeast morphology agar is white to tannish white, semiglistening, and butyrous in texture. Colonies are low and flat and have finely lobed margins. Sparingly differentiated pseudohyphae are formed under the cover glass (Fig. 5H), but true hyphae are not formed.
Ascospore formation.
Of the five strains examined, only NRRL YB-4349 formed ascospores. Ascosporulation is infrequent and occurs on YM agar after 15 to 20 days at 25°C. Asci are unconjugated and persistent and form a single roughened ascospore (Fig. 5I). Although asci are unconjugated, apparent conjugations between individual cells (Fig. 5J), as well as between cells and their buds, are observed and are interpreted as evidence that the species is homothallic.
Fermentation, assimilation, and other growth tests.
Glucose is the only sugar fermented and assimilated. All strains grow at 40°C, but three of the five strains available fail to grow at 42°C (Table 2).
Type strain.
NRRL YB-4349 (CBS 9733) is preserved in the lyophilized state, as well as in liquid nitrogen storage, at NRRL. The strain was received from N. van Uden and had been isolated from the cecum of a horse in Portugal.
Etymology.
The species name slooffiae comes from that of the anamorphic state, Candida slooffiae van Uden & do Carmo-Sousa.
Comparisons with K. telluris.
The vegetative morphology of K. telluris is indistinguishable from that of the four new species described here (Fig. 5K). The one exception is that strains of the C. slooffiae clade produce relatively undifferentiated pseudohyphae (Fig. 5H), whereas strains of the other species do not. Ascosporulation by strains of K. telluris appears to be identical to that of the new species (Fig. 5L), although asci from K. telluris may form two ascospores rather than one. As with the other species, K. telluris ferments only glucose. All four strains of K. telluris tested assimilate glucose, ethanol, and dl-lactic acid; and two of the strains grow in a medium with 10% NaCl and 5% glucose. The four strains grow at both 40 and 42°C (Table 2).
DISCUSSION
For more than 20 years and from reviews of several thousand sentinel animals, gastric infections in mice have seldom been observed (15); but within the past 2 years numerous animals with gastric yeast infections have been found. Whether this increase in frequency of observed infections reflects the emergence of a more virulent yeast, decreased resistance to infection among present-day mouse breeds, or pathogen introduction through poor sanitation is uncertain. However, mouse colonies that received sterilized food, bedding, and cages did stay free of infection. Consequently, the microbiological identity and mode of transmission reported here for these yeasts may alert others to a serious source of infection in laboratory mice.
Separation of yeast species by phenotype has been a long-standing problem, and the application of molecular comparisons to yeast systematics has further emphasized that it is often impossible to separate species by using cellular morphology and physiological responses (9, 23). Watson et al. (24) reported some differences in temperature optima among species of the K. telluris complex, but as with other physiological criteria, strain variation precluded use of this criterion for the reliable recognition of species. Consequently, with the possible exception of K. slooffiae, the only known means for the reliable separation of species in the K. telluris complex is from gene sequence analysis. The sequences of domains D1 and D2 of the 26S rRNA gene have been quite helpful for rapid species identification (10), but as we have demonstrated here and earlier (8, 14), single gene sequences will not detect hybrids nor provide a full understanding of strain diversity within species. In addition to this study, interspecific hybrids have been detected in Williopsis (6) and Saccharomyces (1), suggesting that hybrids are widespread among species and common to the speciation process.
Acknowledgments
Amy Morgan is gratefully acknowledged for skillful operation of the nucleic acid sequencer and oligonucleotide synthesizer, Eleanor Basehoar-Powers is acknowledged for conducting fermentation and assimilation tests, Grace Lidl is acknowledged for assistance in identifying infected animals, and Don Fraser is acknowledged for preparation of the final figures.
The mention of firm names or trade products does not imply that they are endorsed or recommended by the U.S. Department of Agriculture over other firms or similar products not mentioned.
This study was supported, in part, with federal funds from the NCI under contract N01-CO-56000 to SA1C Frederick.
REFERENCES
- 1.Groth, C., J. Hansen, and J. Piskur. 1999. A natural chimeric yeast containing genetic material from three species. Int. J. Syst. Bacteriol. 49:1933-1938. [DOI] [PubMed] [Google Scholar]
- 2.Hurt, R. A. 1997. A molecular analysis of the relatedness of anamorphic yeasts currently classified as Candida pintolopesii. Ph.D. thesis. University of Tennessee, Knoxville.
- 3.James, S. A., M. D. Collins, and I. N. Roberts. 2001. Phylogenetic analysis of the psychrophobic yeast Arxiozyma telluris and the reinstatement of Candida pintolopesii (van Uden) Meyer et Yarrow and Candida slooffii van Uden et do Carmo-Sousa. Int. J. Syst. E vol. Microbiol. 51:1917-1925. [DOI] [PubMed] [Google Scholar]
- 4.Kreger-van Rij, N. J. W. 1958. The relationship between Saccharomyces tellustris and Candida bovina. Antonie Leeuwenhoek 24:137-144. [DOI] [PubMed] [Google Scholar]
- 5.Kreger-van Rij, N. J. W. 1966. Taxonomy of the genus Pichia, p. 51-58. In A. Kocková-Kratochvilová (ed.), Proc. 2nd Int. Symp. Yeasts. Slovak Academy of Sciences, Bratislava, Czecholslovakia.
- 6.Kurtzman, C. P. 1987. Prediction of biological relatedness among yeasts from comparisons of nuclear DNA complementarity, p. 459-468. In G. S. de Hoog, M. T. Smith, and A. C. M. Wiejman (ed.), The expanding realm of yeast-like fungi. Elsevier, Amsterdam, The Netherlands.
- 7.Kurtzman, C. P. 1998. Arxiozyma van der Walt & Yarrow, p. 134-135. In C. P. Kurtzman and J. W. Fell (ed.), The yeasts, a taxonomic study, 4th ed. Elsevier Science BV, Amsterdam, The Netherlands.
- 8.Kurtzman, C. P. 2003. Phylogenetic circumscription of Saccharomyces, Kluyveromyces and other members of the Saccharomycetaceae, and the proposal of the new genera Lachancea, Nakaseomyces, Naumovia, Vanderwaltozyma and Zygotorulaspora. FEMS Yeast Res. 4:233-245. [DOI] [PubMed] [Google Scholar]
- 9.Kurtzman, C. P., and H. J. Phaff. 1987. Molecular taxonomy, p. 63-94. In A. H. Rose and J. S. Harrison (ed.), The yeasts, vol. 1, 2nd ed. Academic Press, London, United Kingdom.
- 10.Kurtzman, C. P., and C. J. Robnett. 1998. Identification and phylogeny of ascomycetous yeasts from analysis of nuclear large subunit (26S) ribosomal DNA partial sequences. Antonie Leeuwenhoek 73:331-371. [DOI] [PubMed] [Google Scholar]
- 11.Kurtzman, C. P., and C. J. Robnett. 2003. Phylogenetic relationships among yeasts of the ′Saccharomyces complex' determined from multigene sequence analyses. FEMS Yeast Res. 3:417-432. [DOI] [PubMed] [Google Scholar]
- 12.Mendonça-Hagler, L. C., and H. J. Phaff. 1975. Deoxyribonucleic acid base composition and deoxyribonucleic acid/deoxyribonucleic acid hybrid formation in psychrophobic and related yeasts. Int. J. Syst. Bacteriol. 25:222-229. [Google Scholar]
- 13.Naumov, G. I., S. A. James, E. S. Naumova, E. J. Louis, and I. N. Roberts. 2000. Three new species in the Saccharomyces sensu stricto complex: Saccharomyces cariocanus, Saccharomyces kudriavzevii and Saccharomyces mikatae. Int. J. Syst. E vol. Microbiol. 50:1931-1942. [DOI] [PubMed] [Google Scholar]
- 14.Peterson, S. W., and C. P. Kurtzman. 1991. Ribosomal RNA sequence divergence among sibling species of yeasts. Syst. Appl. Microbiol. 14:124-129. [Google Scholar]
- 15.Savage, D. C., and R. J. Dubos. 1967. Localization of indigenous yeast in the murine stomach. J. Bacteriol. 91:1811-1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Swofford, D. L. 1998. PAUP* 4.0: phylogenetic analysis using parsimony. Sinauer Associates, Sunderland, Mass.
- 17.van der Walt, J. P. 1957. Three new sporogenous yeasts from soil. Antonie Leeuwenhoek 23:23-29. [DOI] [PubMed] [Google Scholar]
- 18.van der Walt, J. P., and D. Yarrow. 1984. The genus Arxiozyma gen. nov. (Saccharomycetaceae). S. Afr. J. Bot. 3:340-342. [Google Scholar]
- 19.van Uden, N., and H. R. Buckley. 1970. Candida Berkhout, p. 893-1087. In J. Lodder (ed.), The yeasts, a taxonomic study, 2nd ed. North-Holland, Amsterdam, The Netherlands.
- 20.van Uden, N., and M. Vidal-Leiria. 1970. Torulopsis Berlese, p. 1235-1308. In J. Lodder (ed.), The yeasts, a taxonomic study, 2nd ed. North-Holland, Amsterdam, The Netherlands.
- 21.Vaughan-Martini, A., and C. P. Kurtzman. 1985. Deoxyribonucleic acid relatedness among species of the genus Saccharomyces sensu stricto. Int. J. Syst. Bacteriol. 35:508-511. [Google Scholar]
- 22.Vaughan-Martini, A., and A. Martini. 1987. Three newly delimited species of Saccharomyces sensu stricto. Antonie Leeuwenhoek 53:77-84. [DOI] [PubMed] [Google Scholar]
- 23.Vaughan-Martini, A., and A. Martini. 1998. Saccharomyces Meyen ex Reess, p. 358-371. In C. P. Kurtzman and J. W. Fell (ed.), The yeasts, a taxonomic study, 4th ed. Elsevier Science BV, Amsterdam, The Netherlands.
- 24.Watson, K., H. Arthur, and M. Blakey. 1981. Biochemical and morphological correlations in the thermophilic enteric yeasts, Saccharomyces telluris, Candida slooffii, Torulopsis bovina and T. pintolopesii, p. 449-503. In G. G. Stewart and I. Russell (ed.), Current developments in yeast research. Pergamon Press, Toronto, Ontario, Canada.
- 25.Yarrow, D. 1998. Methods for the isolation, maintenance and identification of yeasts, p. 77-100. In C. P. Kurtzman and J. W. Fell (ed.), The yeasts, a taxonomic study, 4th ed. Elsevier Science BV, Amsterdam, The Netherlands.