Abstract
Background
The National Comprehensive Cancer Network (NCCN) surgical resection guidelines for non-small-cell lung cancer (NSCLC) recommend anatomic resection, negative margins, examination of hilar/intrapulmonary lymph nodes, and examination of 3 or more mediastinal nodal stations. We examined the survival impact of these guidelines.
Methods
Population-based observational study using patient-level data from all curative-intent NSCLC resections from 2004–2013 at 11 institutions in 4 contiguous Dartmouth Hospital Referral Regions in 3 US states. We used an adjusted Cox proportional hazards model to assess the overall survival impact of attaining NCCN guidelines.
Results
Of 2,429 eligible resections,91% were anatomic, 94% had negative margins, 51% sampled hilar nodes, and 26% examined three or more mediastinal nodal stations. Only 17% of resections met all four criteria, however there was a significant increasing trend from 2% in 2004 to 39% in 2013 (p<0.001). Compared to patients whose surgery missed one or more parameters, the hazard ratio for patients whose surgery met all four criteria was 0.71 (95% confidence interval: 0.59–0.86, p<0.001). Margin status and the nodal staging parameters were most strongly linked with survival.
Conclusions
Attainment of NCCN surgical quality guidelines was low, but improving, over the past decade in this cohort from a high lung cancer mortality region of the US. The NCCN quality criteria, especially the nodal examination criteria, were strongly associated with survival. The quality of nodal examination should be a focus of quality improvement in NSCLC care.
Provider- and institutional-level disparities in patient survival after curative-intent lung cancer surgery suggest the existence of potentially correctable gaps in the quality of surgical care [1–6]. Such gaps affect short-term outcomes, such as postoperative mortality and hospital readmission rates [7,8]. Gaps in the oncologic quality of resection may be more difficult to measure because of their delayed manifestation [9]. Such gaps exist in the quality of pathologic nodal staging and rates of resection with positive margins [10–13].
Quality improvement requires validated, survival-impactful benchmarks. The National Comprehensive Cancer Network (NCCN) has established principles of surgical therapy which can be condensed into a composite benchmark consisting of a recommendation for anatomic resection, negative margins, hilar and intrapulmonary lymph node examination, and examination of 3 or more mediastinal lymph node stations [14].
We examined the rate of attainment, and the survival impact, of these quality parameters in a diverse population-based cohort.
PATIENTS AND METHODS
Study design and participants
The Mid-South Quality of Surgical Resection (MS-QSR) database
With the approval of the Institutional Review Boards of all participating hospitals, we conducted a population-based observational study of all curative-intent non-small cell lung cancer (NSCLC) resections in 11 hospitals within 4 contiguous Dartmouth Hospital Referral Regions in North Mississippi, East Arkansas, and West Tennessee. Eligible hospitals had 5 or more annual lung cancer resections. We identified patients who had undergone NSCLC resection from institutional records. Trained data abstractors conducted a structured retrieval of demographic and clinical information from clinical records of eligible patients.
Current study cohort
The current report includes data on resections in 7 metropolitan Memphis hospitals from 2004 to 2008 (the early era), and resections in 11 hospitals in the tristate region (including Metropolitan Memphis) from 2009 to 2013 (the recent era). We hierarchically excluded patients with small cell lung cancer, previous lung cancer, neoadjuvant therapy, and no information on the extent of resection.
Survival outcomes
Patients’ vital status and date of death were obtained from hospital and state tumor registries. Vital statistics were updated up to April 1, 2015, on which date vital status was censored for patients alive or with no death information. The cause of death was not available, precluding cause-specific survival analysis.
NCCN parameters and assumptions
We distilled the NCCN surgical resection principles into 4 parameters: anatomic resection (segmentectomy, or greater); negative margins; examination of the hilar lymph node station; and resection of 3 or more mediastinal lymph node stations. We examined the rate of attainment of each of these preferred quality parameters individually, and in combination, and also examined their relationship to survival. Lymph nodes retrieved during pre-operative invasive staging tests, such as mediastinoscopy, were also recorded and included in the analysis of lymph node stations retrieved during the curative resection.
Covariables
Analysis variables included demographic information such as age, race, sex, and insurance status, and clinical information including comorbid conditions used in the Charlson score. A surgical quality improvement intervention with a lymph node specimen collection kit was introduced in some institutions during the recent era [15]. The kit is described in the Supplemental Material. All information entered into the MS-QSR database is systematically cross-audited.
Statistical analysis plan
We used descriptive analysis to summarize patient characteristics, analyzed NCCN criteria attainment rates according to patient characteristics, and tested for differences with the chi-squared test. We used Kaplan-Meier survival curves to visually display the survival patterns associated with the four criteria individually and cumulatively. Statistical tests were based on the multivariable Cox proportional hazards model. All criteria were entered individually and together to assess the relative impact on survival. Postoperative chemotherapy use was also examined in the Cox model, as chemotherapy may be appropriate for some patients after surgery. The proportional hazards assumption for NCCN criteria was assessed visually through log-log survival curves and statistically through the interaction with time in the Cox model.
We analyzed data in the whole population, and in subsets restricted to stage I and II patients, non-kit cases, and patients with surgery in the more recent era (as some database information was more complete during the recent era). We performed additional analyses stratifying pN0 non-kit cases by T-category, and the whole cohort by surgical technique. Finally, we repeated the whole analysis after excluding patients who died within 30 days of surgery. Results were similar. All statistical analyses were performed in SAS 9.4 (2013, SAS Institute Inc., Cary NC).
RESULTS
Cohort characteristics
The analytic cohort of 2429 patients consists of 37% from the early era, 2004 – 2008, and 63% from the recent era, 2009 – 2013 (Table 1). These operations were performed by 43 board-certified cardiothoracic surgeons and 4 board-certified general surgeons. The mean cohort age was 67 years, 79% were white and 21% black. From 2009 on, most patients (79%) had one or more major comorbidity, had a preoperative PET/CT scan (80%), and no preoperative invasive staging procedure (85%). Data on preoperative staging procedures was not systematically collected in the early era and therefore not reported.
Table 1.
patient characteristics
| Characteristics | No. (%) |
|---|---|
| Total | 2.429 (100) |
| Period | |
| 2004–2008 | 892 (36.7) |
| 2009–2013 | 1537 (63.3) |
| Age (mean/SD) | 66.9 (9.7) |
| Age group | |
| <65 | 881 (36.3) |
| 65 – 74 | 1000 (41.2) |
| 75 – 84 | 516 (21.2) |
| >=85 | 32 (1.3) |
| Sex | |
| Male | 1266 (52.1) |
| Female | 1163 (47.9) |
| Race | |
| White | 1912 (78.7) |
| Black | 497 (20.5) |
| Other | 20 (0.8) |
| Insurance | |
| Medicare only | 1207 (49.7) |
| Medicaid | 293 (12.1) |
| Commercial insurance/supplement | 835 (34.4) |
| Self-pay/no insurance | 94 (3.9) |
| Chest CT * | |
| Yes | 1,419 (92.3) |
| No/missing | 118 (7.7) |
| PET-CT* | |
| Yes | 1,223 (79.6) |
| No | 314 (20.4) |
| Invasive staging exam* | |
| Yes | 231 (15.0) |
| No | 1306 (85.0) |
| Pathologic T classification | |
| T1 | 1122 (46.2) |
| T2 | 949 (39.1) |
| T3 | 256 (10.5) |
| T4 | 88 (3.6) |
| Tx | 14 (0.6) |
| Pathologic N classification | |
| N0 | 1687 (69.5) |
| N1 | 316 (13.0) |
| N2 | 199 (8.2) |
| NX | 227 (9.3) |
| Pathologic stage | |
| I | 1581 (65.1) |
| II | 480 (19.8) |
| III | 316 (13) |
| IV | 39 (1.6) |
| Unknown | 13 (0.5) |
| Total Lymph nodes examined pre- and post-operative: median (IQR) | 6 (3, 11) |
| Number of mediastinal lymph nodes examined: median (IQR) | 2 (0, 5) |
| Number of mediastinal lymph node stations sampled: median (IQR) | 1 (0, 2) |
| Histology | |
| Adenocarcinoma | 1285 (52.9) |
| Squamous cell | 842 (34.7) |
| Adenosquamous | 68 (2.8) |
| Large cell | 111 (4.6) |
| Other | 123 (5.1) |
| Grade | |
| Well differentiated | 259 (10.7) |
| Moderately differentiated | 1037 (42.7) |
| Poorly differentiated | 732 (30.1) |
| Undifferentiated | 52 (2.1) |
| Not reported | 349 (14.4) |
| Extent of Resection | |
| Pneumonectomy | 192 (7.9) |
| Bilobectomy | 152 (6.3) |
| Lobectomy | 1782 (73.4) |
| Segmentectomy | 72 (3.0) |
| Wedge | 231 (9.5) |
| Surgical Technique | |
| Open | 1861 (76.7) |
| Robotically-assisted | 226 (9.3) |
| Video-assisted | 340 (14) |
| Surgical kit use (2011 – 2013) | |
| Yes | 233 (25.4) |
| No | 684 (74.6) |
| Postoperative chemotherapy* | |
| Yes | 235 (15.3) |
| No | 1297 (84.7) |
| Number of comorbidities* | |
| 0 | 319 (20.8) |
| 1 | 603 (39.2) |
| 2 | 376 (24.5) |
| 3 | 155 (10.1) |
| 4 + | 84 (5.5) |
| Mortality Rates | |
| 30 days | 109 (4.5) |
| 60 days | 163 (6.7) |
| 90 days | 203 (8.4) |
recent era (2009–2013); IQR=interquartile range
The surgical resection technique was minimally invasive in 23% of cases. A lymph node specimen collection kit, introduced in 2011 for intraoperative collection of hilar and mediastinal lymph nodes, was used in 25% of cases performed from 2011 onward. Most patients had early-stage disease: pathologic (p) T1 or T2 (85%), pN0 (70%), and stage I or II (85%). However, 9% of patients had resection without nodal examination (pNX). A median of 6 lymph nodes were examined (interquartile range [IQR], 3–11), including a median of 2 mediastinal nodes (IQR, 0–5).
Attainment of surgical resection quality parameters
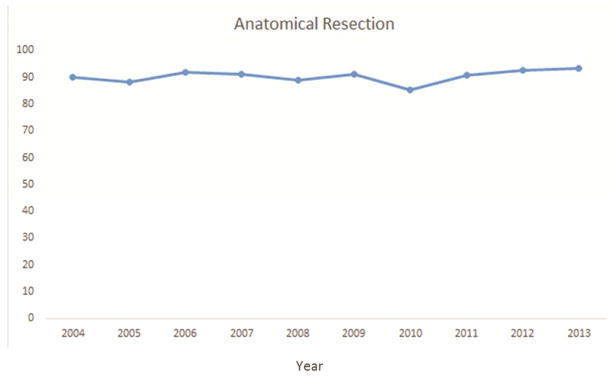
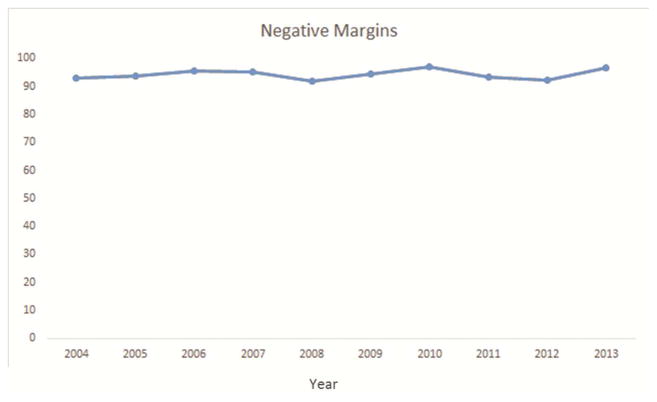
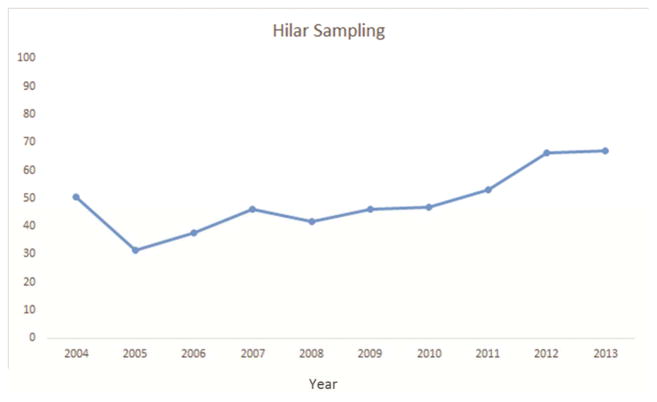
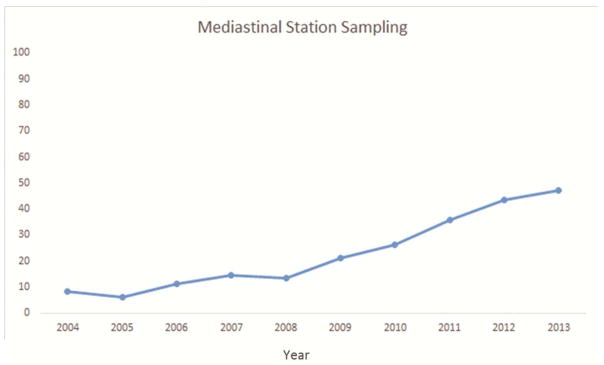
Approximately 91% of resections were anatomic, 94% had negative margins, 51% had at least one hilar lymph node, and 26% had three or more mediastinal nodal stations examined. Although all but 4 (<1%) resections met at least one NCCN criterion, only 17% met all four criteria (Table 2). Pathologic nodal staging was the major quality deficit, especially mediastinal staging.
Table 2.
Patients’ clinical and demographic factors and rates of attainment of quality parameters.
| Characteristics | Anatomic resection | Negative margin | Hilar station sampled | >=3 Mediastinal stations sampled | Met all four criteria |
|---|---|---|---|---|---|
| Total | 2198 (90.5) | 2294 (94.4) | 1226 (50.5) | 629 (25.9) | 423 (17.4) |
| Period | |||||
| 2004–2008 | 804 (90.1) | 838 (93.9) | 371 (41.6) | 97 (10.9) | 40 (4.5) |
| 2009–2013 | 1394 (90.7) | 1456 (94.7) | 855 (55.6) | 532 (34.6) | 383 (24.9) |
| Age Group | |||||
| <65 | 823 (93.4) | 825 (93.6) | 451 (51.2) | 234 (26.6) | 162 (18.4) |
| 65 – 74 | 895 (89.5) | 952 (95.2) | 528 (52.8) | 272 (27.2) | 181 (18.1) |
| 75 – 84 | 454 (88) | 488 (94.6) | 233 (45.2) | 116 (22.5) | 76 (14.7) |
| >=85 | 26 (81.3) | 29 (90.6) | 14 (43.8) | 7 (21.9) | 4 (12.5) |
| Sex | |||||
| Male | 1161 (91.7) | 1183 (93.4) | 612 (48.3) | 313 (24.7) | 203 (16) |
| Female | 1037 (89.2) | 1111 (95.5) | 614 (52.8) | 316 (27.2) | 220 (18.9) |
| Insurance | |||||
| Medicare only | 1064 (88.2) | 1138 (94.3) | 593 (49.1) | 288 (23.9) | 189 (15.7) |
| Medicaid | 269 (91.8) | 280 (95.6) | 150 (51.2) | 82 (28) | 63 (21.5) |
| Commercial insurance/supplement | 780 (93.4) | 785 (94) | 432 (51.7) | 234 (28) | 155 (18.6) |
| Self-pay/no insurance | 85 (90.4) | 91 (96.8) | 51 (54.3) | 25 (26.6) | 16 (17) |
| Pet CT | |||||
| Yes | 1403 (90.6) | 1463 (94.5) | 822 (53.1) | 459 (29.7) | 314 (20.3) |
| No | 795 (90.2) | 831 (94.3) | 404 (45.9) | 170 (19.3) | 109 (12.4) |
| Invasive preoperative nodal staging procedure | |||||
| Yes | 219 (94.8) | 214 (92.6) | 136 (58.9) | 83 (35.9) | 57 (24.7) |
| No | 1175 (90) | 1242 (95.1) | 719 (55.1) | 449 (34.4) | 326 (25) |
| Pathologic T classification | |||||
| T1 | 981 (87.4) | 1095 (97.6) | 549 (48.9) | 255 (22.7) | 182 (16.2) |
| T2 | 896 (94.4) | 896 (94.4) | 509 (53.6) | 270 (28.5) | 184 (19.4) |
| T3 | 235 (91.8) | 215 (84) | 134 (52.3) | 89 (34.8) | 49 (19.1) |
| T4 | 77 (87.5) | 75 (85.2) | 31 (35.2) | 10 (11.4) | 5 (5.7) |
| TX | 9 (64.3) | 13 (92.9) | 3 (21.4) | 5 (35.7) | 3 (21.4) |
| Pathologic N classification | |||||
| N0 | 1604 (95.1) | 1613 (95.6) | 918 (54.4) | 460 (27.3) | 310 (18.4) |
| N1 | 311 (98.4) | 283 (89.6) | 188 (59.5) | 83 (26.3) | 56 (17.7) |
| N2 | 188 (94.5) | 176 (88.4) | 120 (60.3) | 86 (43.2) | 57 (28.6) |
| NX | 95 (41.9) | 222 (97.8) | 0 (0) | 0 (0) | 0 (0) |
| Pathologic stage | |||||
| I | 1406 (88.9) | 1539 (97.3) | 755 (47.8) | 360 (22.8) | 249 (15.7) |
| II | 457 (95.2) | 433 (90.2) | 281 (58.5) | 143 (29.8) | 96 (20) |
| III | 298 (94.3) | 274 (86.7) | 169 (53.5) | 110 (34.8) | 67 (21.2) |
| IV | 28 (71.8) | 36 (92.3) | 18 (46.2) | 11 (28.2) | 8 (20.5) |
| Unknown | 9 (69.2) | 12 (92.3) | 3 (23.1) | 5 (38.5) | 3 (23.1) |
| Extent of Resection | |||||
| Pneumonectomy | 192 (100) | 169 (88) | 90 (46.9) | 60 (31.3) | 25 (13) |
| Bilobectomy | 152 (100) | 132 (86.8) | 82 (53.9) | 35 (23) | 22 (14.5) |
| Lobectomy | 1782 (100) | 1702 (95.5) | 998 (56) | 494 (27.7) | 361 (20.3) |
| Segmentectomy | 72 (100) | 67 (93.1) | 28 (38.9) | 19 (26.4) | 15 (20.8) |
| Wedge | 0 (0) | 224 (97) | 28 (12.1) | 21 (9.1) | 0 (0) |
| Surgical Technique | |||||
| Open | 1705 (91.6) | 1744 (93.7) | 911 (49) | 462 (24.8) | 286 (15.4) |
| Robotic | 219 (96.9) | 219 (96.9) | 155 (68.6) | 102 (45.1) | 90 (39.8) |
| Video | 273 (80.3) | 330 (97.1) | 159 (46.8) | 64 (18.8) | 47 (13.8) |
| Post-operative Chemotherapy | |||||
| Yes | 218 (92.8) | 214 (91.1) | 150 (63.8) | 90 (38.3) | 66 (28.1) |
| No | 1171 (90.3) | 1237 (95.4) | 700 (54) | 441 (34) | 316 (24.4) |
| Number of Comorbidities | |||||
| 0 | 304 (95.3) | 301 (94.4) | 183 (57.4) | 114 (35.7) | 80 (25.1) |
| 1 | 555 (92) | 569 (94.4) | 341 (56.6) | 232 (38.5) | 165 (27.4) |
| 2 | 326 (86.7) | 359 (95.5) | 198 (52.7) | 109 (29) | 82 (21.8) |
| 3 | 135 (87.1) | 148 (95.5) | 87 (56.1) | 50 (32.3) | 33 (21.3) |
| 4 + | 74 (88.1) | 79 (94) | 46 (54.8) | 27 (32.1) | 23 (27.4) |
| Surgical Kit Use | |||||
| Yes | 225 (96.6) | 219 (94) | 206 (88.4) | 216 (92.7) | 176 (75.5) |
| No | 621 (90.8) | 644 (94.2) | 362 (52.9) | 170 (24.9) | 112 (16.4) |
Note: All factors listed in this table are factors that had at least one significant difference in the rates of attainment for quality measures.
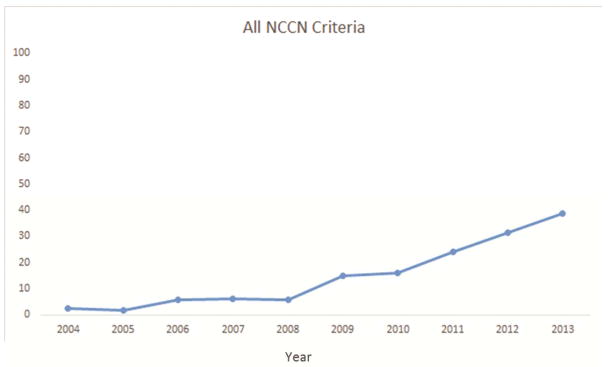
Several factors were significantly associated with the likelihood of attaining all four criteria. Resections performed in the recent era (2009–2013) were more likely to attain the four criteria (Figure 1a–e). The rate of attainment of all four criteria increased from 2% in 2004 to 15% in 2009 to 39% in 2013 (p<0.001). Additionally, patients who had a PET/CT scan, pT1, T2, or T3 tumors (compared to T4) and pN2 were more likely to have surgery meeting all four criteria. Finally, robotically-assisted resections and those using a surgical specimen collection kit were significantly more likely to attain all four NCCN criteria (Table 2).
Figure 1.
National Comprehensive Cancer Network lung cancer resection quality criteria attainment by year: a) anatomic resection; b) negative margins; c) hilar lymph node examination; d) examination of 3 or more mediastinal lymph node stations; e) all four criteria.
Survival impact of NCCN quality criteria
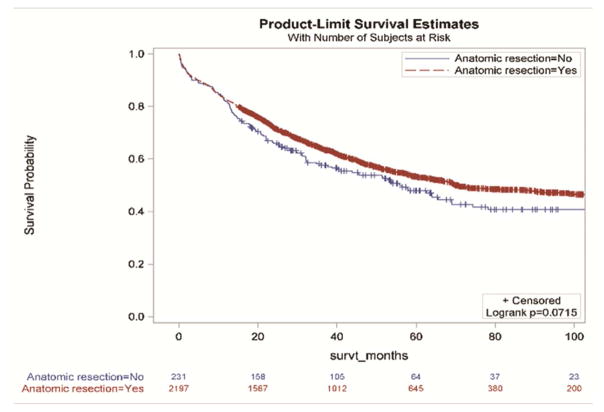
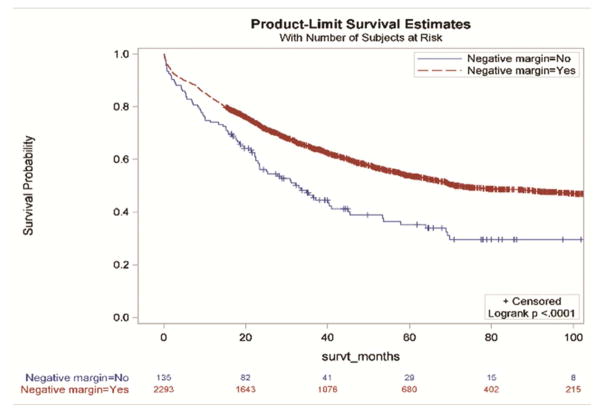
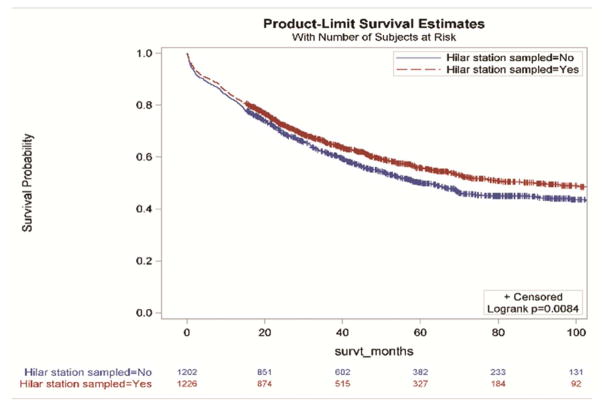
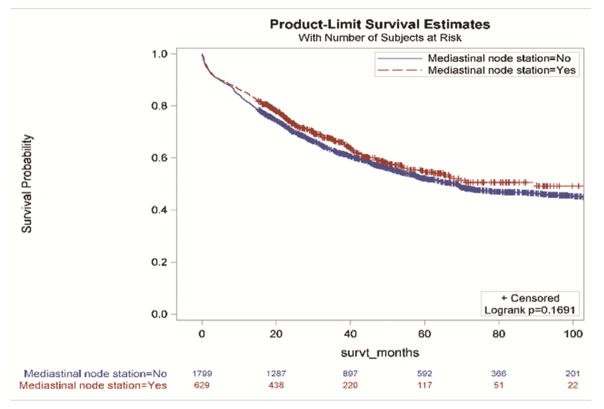
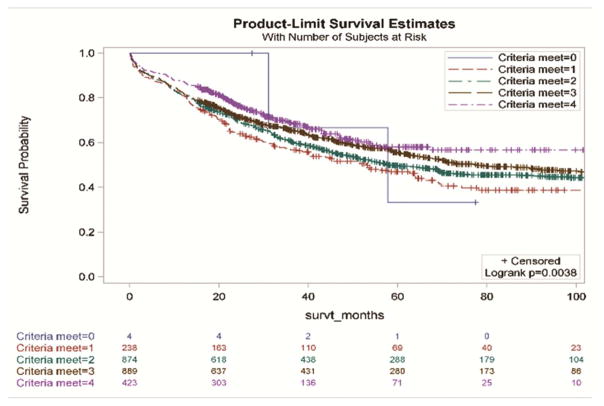
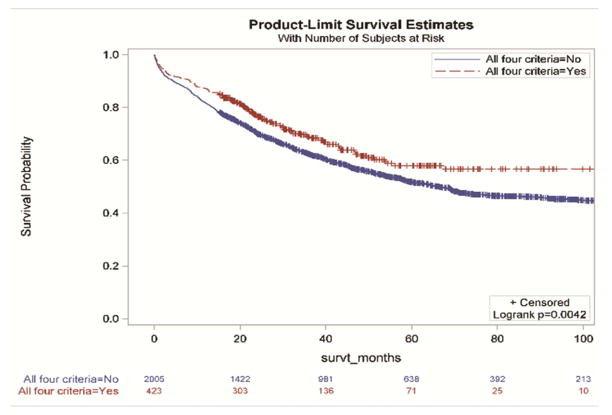
Without accounting for demographic and clinical characteristics, the extent of resection and the examination of three or more mediastinal stations were not associated with improved survival (Figure 2a and 2d,). However, resections with negative margins and examination of hilar lymph nodes were each individually associated with significantly better survival (Figure 2b and 2c). Resections in which all four criteria were attained had significantly better survival than those in which one or more of the individual criteria were not achieved (Figures 3a and 3b).
Figure 2.
Survival impact of attaining National Comprehensive Cancer Network resection quality criteria: a) anatomic resection; b) negative margins; c) hilar node examination; d) three or more mediastinal stations.
Figure 3.
Survival impact of attaining National Comprehensive Cancer Network lung cancer resection quality criteria stratified by: a) the number of criteria met; b) whether, or not, all four criteria were met.
In the multivariable analysis (Table 3) for the entire population, adjusting for period, age, sex, race, insurance, pathologic stage, histology, grade, and surgical technique, anatomic resections were associated with a hazard ratio (HR) of 0.81 (95% confidence interval [CI]: 0.67–0.99, p=0.035) and attainment of all four criteria was associated with an HR of 0.71 (CI: 0.59–0.86, p=0.0004). With further adjustment for extent of resection, resection with negative margins was associated with an HR of 0.74 (CI:0.59–0.93, p=0.01), hilar lymph node examination was associated with an HR of 0.84 (CI:0.74–0.96, p=0.008), and examination of three or more mediastinal stations was associated with an HR of 0.83 (CI:0.72–0.97, p=0.018). In the presence of all criteria in the model, resections with negative margins and hilar stations sampled were associated with lower hazard ratios (HR: 0.74, CI: 0.59–0.94, p=0.012 and HR: 0.86, CI: 0.76–0.98, p=0.019, respectively).
Table 3.
Survival impact of attaining NCCN lung cancer surgical resection quality parameters
| All patientsa | Stage I and IIb | p value | ||||
|---|---|---|---|---|---|---|
| N (%) | Hazard Ratio | p value | N (%) | Hazard Ratio | ||
| Separate modelsc | ||||||
| Anatomic resection | 2198 (90.5) | 0.81 (0.67, 0.99) | 0.0352 | 2198 (90.5) | 0.91 (0.73, 1.13) | 0.3732 |
| Negative margins | 2294 (94.4) | 0.74 (0.59, 0.93) | 0.0103 | 2294 (94.4) | 0.76 (0.57, 1.03) | 0.0761 |
| Hilar station sampled | 1226 (50.5) | 0.84 (0.74, 0.96) | 0.0077 | 1226 (50.5) | 0.82 (0.71, 0.95) | 0.0064 |
| ≥mediastinal stations sampled | 629 (25.9) | 0.83 (0.72, 0.97) | 0.0183 | 629 (25.9) | 0.81 (0.68, 0.97) | 0.0231 |
| All four criteria met | 423 (17.4) | 0.71 (0.59, 0.86) | 0.0004 | 423 (17.4) | 0.71 (0.57, 0.88) | 0.0023 |
| Cumulative number of criteria met | ||||||
| 0 | 4 (0.2) | 0.52 (0.13, 2.11) | 0.3587 | 4 (0.2) | 0.71 (0.18, 2.91) | 0.6397 |
| 1 | 238 (9.8) | Reference | 238 (9.8) | Reference | ||
| 2 | 875 (36) | 0.91 (0.67, 1.24) | 0.5543 | 875 (36) | 0.8 (0.55, 1.18) | 0.2607 |
| 3 | 889 (36.6) | 0.79 (0.57, 1.08) | 0.1409 | 889 (36.6) | 0.69 (0.47, 1.02) | 0.0628 |
| 4 | 423 (17.4) | 0.62 (0.43, 0.88) | 0.0073 | 423 (17.4) | 0.54 (0.35, 0.83) | 0.0051 |
| All criteria in one model | ||||||
| Anatomic resection | 2198 (90.5) | 0.88 (0.72, 1.08) | 0.2136 | 2198 (90.5) | 0.99 (0.79, 1.24) | 0.9219 |
| Negative margins | 2294 (94.4) | 0.74 (0.59, 0.94) | 0.0117 | 2294 (94.4) | 0.71 (0.53, 0.95) | 0.0208 |
| Hilar station sampled | 1226 (50.5) | 0.86 (0.76, 0.98) | 0.0194 | 1226 (50.5) | 0.85 (0.74, 0.98) | 0.0247 |
| Three or more mediastinal stations sampled | 629 (25.9) | 0.88 (0.75, 1.03) | 0.1059 | 629 (25.9) | 0.86 (0.72, 1.03) | 0.0983 |
Models are adjusted for period, age, sex, race, insurance, pathological stage, histology, grade, extent of resection, and surgical technique.
Pathological staging covariate was not included for adjustment.
Extent of resection covariate was not included for adjustment when modeling anatomical resection, all four criteria met, and all criteria in one model.
The pattern of low hazard ratios associated with criteria attainment was similar for resections with hilar stations sampled, three or more mediastinal stations sampled, and when all four criteria were met in analysis restricted to stage I and II (Table 3). We found similar patterns when we restricted the analysis to non-kit cases (Supplemental Table 1), the 2009–2013 era (Supplemental Table 2), pN0 non-kit cases stratified for pathologic T-category (Supplemental Table 3a,b), the whole cohort excluding patients who died within 30 days (Supplemental Table 4), a cohort from the largest healthcare system only (Supplemental Table 5) and the whole cohort stratified by surgical technique (data not shown).
COMMENT
Multiple reports indicate the existence of major lung cancer care and outcome disparities [1–13]. In Donabedian’s construct of 3 quality improvement domains - structures, processes, and outcomes - process measures are the most readily susceptible to intervention [16]. However, process measures must be linked to meaningful outcomes, such as survival [17]. Such linkages raise the political will for disseminating improved processes. Multiple recommendations defining good-quality surgical resection have been proposed [14,18–20]. The NCCN guidelines are influential to multiple oncology disciplines [14]. Their survival impact needs validation.
Only 17% of resections in this regional cohort met all four components of the composite NCCN surgical guidelines. Lymph node examination was the most frequent quality defect, with 49% of resections failing to examine the hilar station and 74% failing to examine three or more mediastinal nodal stations. The use of anatomic resection had the least, and resection with negative margins had the greatest, individual survival impact. The nodal staging parameters were intermediate, especially in patients with stage I and II (Table 3). The risk of death was reduced by 29% in the cohort of patients whose resection achieved all four parameters.
The evidence for lobectomy as the preferred extent of resection has been questioned, ever since the Lung Cancer Study Group’s lobectomy vs sub-lobar resection trial report in 1995 [21]. The soundness of non-anatomic resection for patients with relatively small tumors, vulnerable patients such as the elderly and those with limited lung function, and patients with certain low-risk histologic variants, although still disputed, is supported by observational data [22–28].
The immensely negative survival impact of incomplete resection is clearly established [29]. The clinical importance of examining the hilar lymph node station is indicated by its inclusion in definitions of optimal staging [18,30]. However, some guidelines, such as those of the American Joint Committee on Cancer, and the Commission on Cancer of the American Cancer Society, have not specifically emphasized the need to examine the hilar nodal station [20,31]. Nevertheless, hilar nodal metastasis connotes a worse prognosis than involvement of more peripheral N1 nodal stations only [32]. We emphasized this particular station because its retrieval completely depends on surgical processes.
Multiple reports have shown that the quality of mediastinal nodal staging is generally poor, with major negative implications for patients [10–12]. The definition of the minimum required quality of mediastinal nodal staging remains open to debate [30]. Recommendations include lobe-specific directives, systematic sampling or mediastinal lymph node dissection, and examination of a certain minimum number of nodal stations or lymph nodes [14,18–20,31]. We have not compared the various existing recommendations for mediastinal nodal examination.
Despite our use of statistical methods to account for them, this study has all the limitations of retrospective analyses, including potential confounding by missing data, misclassification bias, unrelated secular changes in postoperative management and survival, the inclusion of different institutions with plausibly different practice patterns and different points of data entry, and the lack of causal inference.
Our findings provide justification for using the NCCN criteria for benchmarking quality. Future work should examine if these criteria can distinguish between high- and low-achieving surgeons and institutions, and how low-achieving surgeons and institutions can use this feedback to improve their performance.
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bach PB, Cramer LD, Schrag D, Downey RJ, Gelfand SE, Begg CB. The influence of hospital volume on survival after resection for lung cancer. N Engl J Med. 2001;345:181–8. doi: 10.1056/NEJM200107193450306. [DOI] [PubMed] [Google Scholar]
- 2.Birkmeyer JD, Siewers AE, Finlayson EVA, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;846:1128–37. doi: 10.1056/NEJMsa012337. [DOI] [PubMed] [Google Scholar]
- 3.Meguid RA, Brooke BS, Chang DC, Sherwood JT, Brock MV, Yang SC. Are surgical outcomes for lung cancer resections improved at teaching hopsitals? Ann Thorac Surg. 2008;85:1015–25. doi: 10.1016/j.athoracsur.2007.09.046. [DOI] [PubMed] [Google Scholar]
- 4.Farjah F, Glum DR, Varghese TK, Symons RG, Wood DE. Surgeon specialty and long-term survival after pulmonary resection for lung cancer. Ann Thorac Surg. 2009;87:995–1006. doi: 10.1016/j.athoracsur.2008.12.030. [DOI] [PubMed] [Google Scholar]
- 5.Schipper PH, Diggs BS, Ungerleider RM, Welke KF. The influence of surgeon specialty on outcomes in general thoracic surgery: a national sample 1996 to 2005. Ann Thorac Surg. 2009;88:1566–73. doi: 10.1016/j.athoracsur.2009.08.055. [DOI] [PubMed] [Google Scholar]
- 6.Ferraris VA, Saha SP, Davenport DL, Zwischenberger JB. Thoracic surgery in the real world: does surgical specialty affect outcomes in patients having general thoracic operations? Ann Thorac Surg. 2012;93:1041–8. doi: 10.1016/j.athoracsur.2011.12.061. [DOI] [PubMed] [Google Scholar]
- 7.Farjah F, Backhus L, Cheng A, et al. Failure to rescue and pulmonary resection for lung cancer. J Thorac Cardiovasc Surg. 2015;149(5):1365–71. doi: 10.1016/j.jtcvs.2015.01.063. [DOI] [PubMed] [Google Scholar]
- 8.Grenda TR, Revels SL, Yin H, Birkmeyer JD, Wong SL. Lung Cancer Resection at Hospitals With High vs Low Mortality Rates. JAMA Surg. 2015;150:1034–40. doi: 10.1001/jamasurg.2015.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bilimoria KY, Bentrem DJ, Feinglass JM, et al. Directing surgical quality improvement initiatives: comparison of perioperative mortality and long-term survival for cancer surgery. J Clin Oncol. 2008;26:4626–33. doi: 10.1200/JCO.2007.15.6356. [DOI] [PubMed] [Google Scholar]
- 10.Little AG, Rusch VW, Bonner JA, et al. Patterns of surgical care of lung cancer patients. Ann Thorac Surg. 2005;80:2051–6. doi: 10.1016/j.athoracsur.2005.06.071. [DOI] [PubMed] [Google Scholar]
- 11.Osarogiagbon RU, Yu X. Mediastinal lymph node examination and survival in resected early-stage non-small-cell lung cancer in the surveillance, epidemiology, and end results database. J Thorac Oncol. 2012;7(12):1798–806. doi: 10.1097/JTO.0b013e31827457db. [DOI] [PubMed] [Google Scholar]
- 12.Osarogiagbon RU, Yu X. Nonexamination of lymph nodes and survival after resection of non-small cell lung cancer. Ann Thorac Surg. 2013;96(4):1178–89. doi: 10.1016/j.athoracsur.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 13.Osarogiagbon RU, Ogbata O, Yu X. Number of lymph nodes associated with maximal reduction of long-term mortality risk in pathologic node-negative non-small cell lung cancer. Ann Thorac Surg. 2014;97(2):385–93. doi: 10.1016/j.athoracsur.2013.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.NCCN Clinical Practice Guildelines in Oncology. [Accessed on 02.08.16];Non-Small Cell Lung Cancer. at http://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf.
- 15.Osarogiagbon RU, Miller LE, Ramirez RA, et al. Use of a surgical specimen-collection kit to improve mediastinal lymph-node examination of resectable lung cancer. J Thorac Oncol. 2012;7(8):1276–82. doi: 10.1097/JTO.0b013e318257fbe5. [DOI] [PubMed] [Google Scholar]
- 16.Donabedian A. The quality of care. How can it be assessed? JAMA. 1988;260(12):1743–8. doi: 10.1001/jama.260.12.1743. [DOI] [PubMed] [Google Scholar]
- 17.Birkmeyer JD, Dimick JB, Birkmeyer NJ. Measuring the quality of surgical care: structure, process, or outcomes? J Am Coll Surg. 2004;198:626–32. doi: 10.1016/j.jamcollsurg.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 18.Lardinois D, De Leyn P, Van Schil P, et al. ESTS guidelines for intraoperative lymph node staging in non-small cell lung cancer. Eur J Cardiothorac Surg. 2006;30(5):787–92. doi: 10.1016/j.ejcts.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 19.Howington JA, Blum MG, Chang AC, Balekian AA, Murthy SC. Treatment of stage I and II non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 Suppl):e278S–313S. doi: 10.1378/chest.12-2359. [DOI] [PubMed] [Google Scholar]
- 20.Commission on Cancer. Cancer Programs Practice Profile Reports (CP3R) [Accessed on 02.08.16];Lung measure specifications. at https://www.facs.org/~/media/files/quality%20programs/cancer/lungmeasuredocumentation_05272015.ashx.
- 21.Ginsberg RJ, Rubinstein LV Lung Cancer Study Group. Randomized trial of lobectomy versus limited resection for T1N0 non-small cell lung cancer. Ann Thorac Surg. 1995;60:615–23. doi: 10.1016/0003-4975(95)00537-u. [DOI] [PubMed] [Google Scholar]
- 22.Mery CM, Pappas AN, Bueno R, et al. Similar long-term survival of elderly patients with non-small cell lung cancer treated with lobectomy or wedge resection within the Surveillance, Epidemiology, and End Results Database. CHEST. 2005;128:237–45. doi: 10.1378/chest.128.1.237. [DOI] [PubMed] [Google Scholar]
- 23.Little AG. The “Goldilocks” principle. CHEST. 2005;128:13–14. doi: 10.1378/chest.128.1.13. [DOI] [PubMed] [Google Scholar]
- 24.Schuchert MJ, Pettiford BL, Leeley S, et al. Anatomic segmentectomy in the treatment of stage I non-small cell lung cancer. Ann Thorac Surg. 2007;84:926–33. doi: 10.1016/j.athoracsur.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 25.Veluswamy RR, Ezer N, Mhango G, et al. Limited Resection Versus Lobectomy for Older Patients With Early-Stage Lung Cancer: Impact of Histology. J Clin Oncol. 2015;33:3447–53. doi: 10.1200/JCO.2014.60.6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rami-Porta R, Tsuboi M. Sublobar resection for lung cancer. Eur Respir J. 2009;33:426–35. doi: 10.1183/09031936.00099808. [DOI] [PubMed] [Google Scholar]
- 27.Khullar OV, Liu Y, Gillespie T, et al. Survival After Sublobar Resection versus Lobectomy for Clinical Stage IA Lung Cancer: An Analysis from the National Cancer Data Base. J Thorac Oncol. 2015;10(11):1625–33. doi: 10.1097/JTO.0000000000000664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Donington JS. Survival After Sublobar Resection Versus Lobectomy for Clinical Stage IA Lung Cancer: Analysis From the National Cancer Database. J Thorac Oncol. 2015;10:1513–4. doi: 10.1097/JTO.0000000000000674. [DOI] [PubMed] [Google Scholar]
- 29.Osarogiagbon RU, Lin CC, Smeltzer MP, Jemal A. Prevalence, Prognostic Implications, and Survival Modulators of Incompletely Resected Non-Small Cell Lung Cancer in the U.S. National Cancer Data Base. J Thorac Oncol. 2016;11(1):e5–e16. doi: 10.1016/j.jtho.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Darling GE, Allen MS, Decker PA, et al. Randomized trial of mediastinal lymph node sampling versus complete lymphadenectomy during pulmonary resection in the patient with N0 or N1 (less than hilar) non-small cell carcinoma: results of the American College of Surgery Oncology Group Z0030 Trial. J Thorac Cardiovasc Surg. 2011;141(3):662–70. doi: 10.1016/j.jtcvs.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. Lung. AJCC Cancer Staging Handbook. 7. Springer; NY: 2009. pp. 299–323. [Google Scholar]
- 32.Rena O, Boldorini R, Papalia E, et al. Metastasis to subsegmental and segmental lymph nodes in patients resected for non-small cell lung cancer: Prognostic impact. Ann Thorac Surg. 2014;97(3):987–992. doi: 10.1016/j.athoracsur.2013.11.051. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.