Abstract
Two noninvasive diagnostic tests, (1→3)-β-d-glucan (BG) (Glucatell) and galactomannan (GM) (Platelia Aspergillus), were used retrospectively in a twice-weekly screening for the diagnosis of invasive aspergillosis (IA) in 40 treatment episodes (one hospital visit per patient) in 40 neutropenic adult patients at high risk for IA. Five proven IA cases, three probable IA cases, and three possible IA cases were diagnosed. Diagnostic levels of both BG and GM were detected in 100% of patients with proven IA cases and in 66% of patients with probable IA cases. The kinetics of both markers in patients with IA were similar. The sensitivity, specificity, and positive and negative predictive values for GM and BG were identical, namely, 87.5, 89.6, 70, and 96.3%, respectively. False-positive reactions occurred at a rate of 10.3% in both tests, but the patients showing false-positive results were different in each test. Both tests anticipated the clinical diagnosis, computed tomography abnormalities, and the initiation of antifungal therapy in most patients, but BG tended to become positive earlier than GM. A combination of the two tests improved the specificity (to 100%) and positive predictive value (to 100%) of each individual test without affecting the sensitivity and negative predictive values. In conclusion, BG and GM detection are useful tests for the diagnosis of IA in high-risk hematological patients, but a combination of the two tests was very useful to identify false-positive reactions by each test.
Invasive aspergillosis (IA) is an increasingly common infection among hematological cancer patients receiving cytotoxic chemotherapy (7, 34). Steroid-treated allogenic bone marrow transplant recipients are particularly at risk (10, 19). The crude mortality rate of IA is very high despite appropriate antifungal treatment, since the difficulty in obtaining an early diagnosis results in a delay in establishing treatment (15). The diagnosis of IA is frequently established postmortem. Prompt initiation of antifungal therapy in patients with IA is critical in improving the outcome of this disease (37). Conventional diagnostic methods are insensitive, and the “gold standard” diagnostic procedures (histological examination and cultures of deep tissues) require an aggressive approach which often precludes their use due to profound thrombocytopenia, hypoxemia, and the critical condition of these patients (1).
Clinically, IA is nonspecific, and clinical and radiological signs appear late in the course of the infection (11). In recent years a number of rapid diagnostic techniques have become available: high-resolution computed tomography (HRCT) and non-culture-based methods such as detection of circulating fungal antigens and nucleic acids. These techniques, when combined with risk stratification as described by Prentice et al. (27), permit the early diagnosis of IA and the implementation of preemptive therapeutic strategies. The rapid serological diagnostic methods appear to be most useful when used prospectively to screen high-risk patients (11). Several prospective clinical trials with neutropenic patients have shown the utility of Aspergillus galactomannan (GM) detection by enzyme immunosorbent assay (ELISA) (Platelia Aspergillus; Bio-Rad, Marnes-La-Coquette, France) for the early diagnosis of IA (13, 16-18, 25, 31, 35, 36).
(1→3)-β-d-Glucan (BG) is a cell wall polysaccharide component specific for fungi except for zygomycetes and, to a lesser extent, cryptococci (21). Prokaryotes and viruses, as well as human cells, lack BG. Its presence in blood and normally sterile body fluids may be a marker of invasive fungal infection (IFI) including infection with the most common pathogens such as Aspergillus and Candida. Although there are a number of commercially available methods to detect BG (FungiTec G, Seikagaku Kogyo Corp., Tokyo, Japan; β-d-glucan Test Wako, Wako Pure Chemical Industries, Tokyo, Japan; B-G Star, Maruha Corp., Tokyo, Japan), there is little experience in the use of this marker outside Japan. A new chromogenic test to detect BG (Glucatell; Associates of Cape Cod, Falmouth, Mass.) has been recently commercialized, and a preliminary study has documented its potential for the diagnosis of IFI in humans (24).
The aim of this study was to assess the usefulness of BG detection in sera by the Glucatell test for the diagnosis and therapeutic monitoring of IA in neutropenic adult patients at increased risk for IA. BG detection was compared with the widely used GM detection in an attempt to study the kinetics of both markers and to assess whether a combination of the tests may result in an early and specific diagnosis of IA.
MATERIALS AND METHODS
Patient selection.
From April 2001 to June 2002, all adult hematological cancer patients (n = 154) treated at the Hospital 12 de Octubre, Madrid, Spain, and stratified as high-risk individuals as defined by Prentice et al. (27), were prospectively analyzed twice weekly for quantitative values of GM by using the commercially available sandwich ELISA (Platelia Aspergillus) until the high-risk condition for developing IFI had subsided. The prospective study aiming to evaluate the value of GM in the diagnosis of IA has been published elsewhere (25). The availability of serial serum samples together with complete clinical records gave us the opportunity to assess retrospectively the usefulness of the Glucatell test for the diagnosis of IA in a selection of 40 patients, including 5 with proven IA, 3 with probable IA, 3 with possible IA, and 29 without IA. The patient characteristics and sample distributions are summarized in Tables 1 and 2.
TABLE 1.
Characteristics of patients with proven, probable, and possible IA
| Patient no. | Gender/age (yr)a | Underlying diseaseb | Type of IA | Steroidsc | Duration of neutropenia (days) | No. of positive samples/total no. of samples | Highest level of glucan (pg/ml) | Organ involvement of IA | Site(s) of isolation/Aspergillus speciesb | HRCT scan | Death in relation to IA |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M/70 | MDS | Proven | − | 26 | 9/13 | >523 | Lungs | TBB/A. fumigatus | Nodules in lungs | No |
| 2 | F/65 | NHL | Proven | + | 10 | 3/6 | >523 | Lungs | TBB/A. fumigatus | Nodules in lungs | Yes |
| 3 | F/29 | AML | Proven | + | 34 | 9/6 | >523 | Lungs, brain | Sputum/A. fumigatus | Nodules in lung | No |
| Abcess/A. fumigatus | Pleural effusion | ||||||||||
| 4 | F/30 | AML | Proven | − | 66 | 10/24 | >523 | Lungs, subcutaneous tissue | Subcutaneous nodule/A. flavus | Nodules in lungs | No |
| 5 | F/44 | CLL | Proven | + | 32 | 4/12 | >523 | Lungs | TBB/A. fumigatus | Bilateral infiltrate in lungs | No |
| 6 | M/45 | AML | Probable | + | 30 | 0/9 | Lungs | BAL/A. fumigatus | Nodules in lungs | No | |
| 7 | M/70 | MDS | Probable | + | 23 | 8/18 | >523 | Lungs | Sputum/A. fumigatus | Nodules in lungs | No |
| 8 | F/32 | AML | Probable | + | 26 | 9/9 | >523 | Lungs | Sputum/A. fumigatus | Nodules in lungs | Yes |
| Pleural effusion | |||||||||||
| 9 | M/20 | ALL | Possible | − | 28 | 0/8 | Lungs | Nodule in lungs | No | ||
| 10 | M/25 | ALL | Possible | + | 31 | 0/14 | Lungs | Nodules in lungs | No | ||
| 11 | F/54 | AML | Possible | − | 60 | 12/16 | >523 | Lungs | Pleural effusion | Yes |
M, male; F, female.
MDS, myelodysplastic syndrome; NHL, non-Hodgkin's lymphoma; AML, acute myelogenous leukemia; CLL, chronic lymphocytic leukemia; ALL, acute lymphocytic leukemia; TBB, transbronchial biopsy.
+, administered; −, not administered.
TABLE 2.
Characteristics of patients at risk for IA in whom serum BG and GM was evaluated
| Characteristic | Proven IA | Probable IA | Possible IA | No IA | Total |
|---|---|---|---|---|---|
| No. of patients | 5 | 3 | 3 | 29 | 40 |
| Age (yr)a | 48 (29-70) | 49 (32-70) | 33 (20-54) | 45 (18-70) | 44 (18-70) |
| Gender (M/F)b | 2/3 | 2/1 | 2/1 | 17/12 | 23/17 |
| No. (%) with underlying diseasec | |||||
| ALL | 2 (66.7) | 2 (5) | |||
| AML | 2 (40) | 2 (66.7) | 1 (33.3) | 4 (13.8) | 9 (22.5) |
| CLL | 1 (20) | 8 (27.5) | 9 (22.5) | ||
| MM | 3 (10.34) | 3 (7.5) | |||
| MDS | 1 (20) | 1 (33.3) | 1 (3.4) | 3 (7.5) | |
| NHL | 1 (20) | 8 (27.6) | 9 (22.5) | ||
| HD | 4 (13.8) | 4 (10) | |||
| SAA | 1 (3.4) | 1 (2.5) | |||
| No. (%) receiving steroids | 3 (60) | 3 (100) | 1 (33.3) | 6 (20.7) | 13 (32.5) |
| Mean duration of neutropenia (days) | 42.8 | 37.33 | 43.6 | 22.44 | 27.7 |
| Range of duration of neutropenia (days) | 10-70 | 27-56 | 26-56 | 6-70 | 6-70 |
| No. of episodes of antifungal therapy | 5 (100) | 3 (100) | 3 (100) | 12 (30) | 23 (57.5) |
| No. of samples (total) | 69 | 36 | 39 | 181 | 325 |
| No. of samples/episode | 13.8 | 12 | 12.66 | 6.24 | 8.12 |
| No.d (%) of positive episodes | 5 (100) | 2 (66) | 1 (33) | 3 (10.34) | 11 (27.5) |
| No. (%) of positive samples for BG | 35 (50.7) | 17 (47.2) | 12 (30.7) | 6 (3.3) | 64 (19.7) |
| No. (%) of positive samples for GM | 28 (40.6) | 13 (36.1) | 11 (28.2) | 6 (3.3) | 58 (17.8) |
Values in parentheses are ranges.
M/F, male/female.
ALL, acute lymphocytic leukemia; AML, acute myeloid leukemia; CLL, Chronic lymphocytic leukemia; MM, Multiple myeloma; MDS, Myelodisplastic syndrome; NHL, Non-Hodgkin's lymphoma; HD, Hodgkin disease; SAA, Severe aplastic anemia.
Values in parentheses are percentages of patients who received antifungal therapy.
Definition of invasive aspergillosis.
IA episodes were classified on the basis of the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC-IFICG and NIAID-MSG) case definitions (1). In the prospective study (25), GM results were excluded as microbiological criteria.
Diagnostic work-up of IFI.
In cases of clinical suspicion of IFI, or when the GM index was above 1.5, a diagnostic work-up was started; this included a pulmonary HRCT scan followed, when possible, by bronchoalveolar lavage and/or biopsy for bacterial, mycobacterial, fungal, and viral cultures. Direct examination for bacteria and fungi (including Pneumocystis jiroveci) was performed for all patients. The presence of Legionella antigen in urine was tested.
Management of patients.
All patients were nursed in rooms with HEPA filtration. Antifungal prophylaxis with fluconazole (200 mg once daily) was given to 9 (22.5%) of 40 patients. One patient (2.5%) with possible IA (patient 11, Table 1) received prophylactic liposomal amphotericin B because she had had a previous episode of possible IA, and another patient (2.5%) with proven IA (patient 2, Table 1) received itraconazole due to a previous episode of Aspergillus tracheobronchitis. Initial antibiotics for febrile neutropenia included a β-lactam and aminoglycoside; vancomycin was added 48 h later if fever persisted. Antimicrobial therapy could be modified on the basis of microbiological findings. Criteria for initiating antifungal therapy with liposomal amphotericin B included (i) persistent fever after 5 days of intravenous antibiotic treatment, (ii) development of pulmonary infiltrates while receiving antibacterial therapy, (iii) isolation of mycelial fungi from the respiratory tract, and (iv) recurrence of fever after an afebrile interval of at least 48 h in neutropenic patients still receiving broad-spectrum antibiotics.
Collection and storage of serum samples.
Blood samples (5 ml of whole blood) were collected by venipuncture twice weekly until the risk for IFI had ended. Serum was separated from the blood and tested prospectively twice weekly for GM, and serum samples were stored frozen at −70°C until tested for BG.
BG detection.
BG was detected with the Glucatell test kit essentially as recommended by the manufacturer. Briefly, serum samples (5 μl) were pretreated for 10 min at 37°C with 20 μl of a solution containing 0.6 M KCl and 0.125 M KOH and assayed with the Glucatell reagent in a kinetic, chromogenic format for 25 to 40 minutes at 37°C. Optical densities at 405 nm (OD405) were read. The concentration of BG in each sample was calculated by using a calibration curve with standard solutions of 6.25 to 100 pg/ml. Patients were judged positive if the level of BG was ≥120 pg/ml in at least one serum sample.
GM detection.
The ELISA was performed as recommended by the manufacturer in Europe (32). Results were expressed as the ratio of the OD obtained from the patient serum sample and the control (index = OD of the sample/OD of the control). A result was considered a true positive when two consecutive samples for a patient tested positive, including the retesting of the first sample (an index of 1.5 or greater was considered positive). Results between 1.0 and 1.5 (gray zone) were considered undetermined. An index below 1.0 was negative.
Surveillance cultures.
Semiquantitative surveillance cultures for yeasts were performed weekly. Oropharyngeal, nasal, perineal skin, vulvovaginal or balanoprepucial, rectal, and pericatheter skin specimens were planted onto CHROMagar and Sabouraud chloramphenicol (0.4 g/liter), and the plates were incubated at 37°C for 2 weeks. Cultures were evaluated using the following score: negative (0 colonies), light (<10 colonies), moderate (11 to 20 colonies), and heavy (>20 colonies). The yeast isolates were identified by the API 32 system (Bio-Mérieux, Marcy L'Etoile, France).
Mycological studies.
When judged necessary, specimens from clinically infected foci were collected and processed as described by Denning et al. (6). Blood samples for culture were inoculated in a BACTEC Plus aerobic/F bottle and incubated for up to 15 days with the BACTEC 9240 blood culture system (Becton Dickinson Franklin Lakes, N.J.). Aspergillus species were identified by their macroscopic and microscopic culture characteristics.
Statistical analysis.
Sensitivity, specificity, and positive and negative predictive values were calculated as described by Kozinn et al. (14). According to Mennink-Kersten et al. (20), only proven and probable IA were considered truly positive and only no IA cases were considered truly negative.
RESULTS
BG detection.
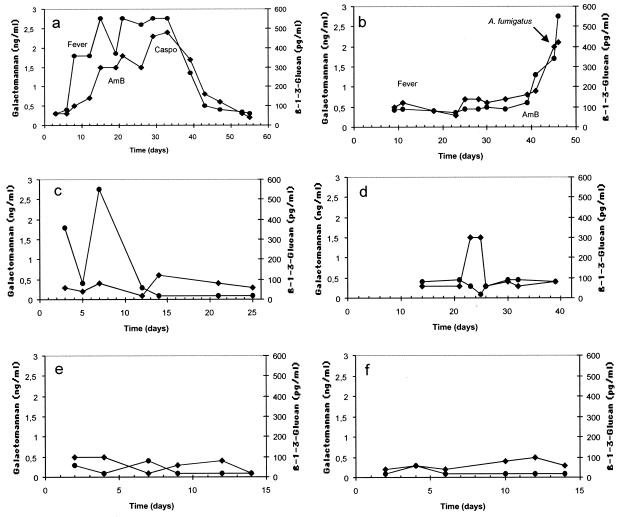
Of 40 patients, 11 (27.5%) had BG levels of ≥120 pg/ml in at least one serum sample. In most patients, BG positivity was detected in two or more samples (median, 6.4 positive serum samples per patient). All five patients with proven IA (100%), two (66%) of three patients with probable IA, and one (33%) of three patients with possible IA tested positive for BG (Tables 1 and 2). In patients with proven IA, BG levels showed a constant rise before clinical and microbiological evidence of IA existed and then decreased and eventually became negative if the patient responded to antifungal therapy (Fig. 1a). However, patients not responding to antifungal treatment did not show a decrease in the levels of BG (Fig. 1b).
FIG. 1.
Representative kinetics of BG (•) and GM (⧫) in different patients. (a) Proven IA in a patient with acute myeloid leukemia who responded to treatment with amphotericin B and caspofungin. (b) Proven IA in a patient with chronic lymphocytic leukemia who did not respond to treatment with amphotericin B. (c) False-positive BG results in a patient with multiple myeloma and no IA. (d) False-positive GM results in a patient with non-Hodgkin's lymphoma and no IA. (e) Negative BG and GM results in a patient with acute myeloid leukemia and no IA who was colonized by C. albicans and C. glabrata. (f) Negative BG and GM results in a patient with chronic lymphocytic leukemia and no IA who was colonized by C. albicans. Abbreviations: AmB, amphotericin B; Caspo, caspofungin.
Of 29 patients with no IA, 3 (10.3%) were positive for BG detection. In these three patients, IFI was excluded after the careful assessment of clinical, microbiological, and radiological records and outcome of the patients without antifungal treatment. None of these patients had mucositis. The first patient was not colonized by yeasts but had Escherichia coli bacteremia. The second patient had a single site colonized with Candida albicans (low count), and the third individual had high counts of both C. albicans and Candida glabrata in four different sites. Although the theoretical occurrence of invasive candidiasis in the last two patients cannot be ruled out, the likelihood of invasive candidiasis was very low because neither patient had antibodies to Candida albicans germ tubes (CAGT) (reference 9 and data not shown). Analysis of the kinetics of BG levels helped in the identification of false-positive results since in these patients BG levels showed abrupt rises and falls. An example of this type of kinetics is shown in Fig. 1c, where high levels of BG were detected in a patient with multiple myeloma at the time E. coli was isolated in a blood culture. BG levels became negative during the following days in the absence of any antifungal treatment. Most patients with no IA showed very low levels of BG during the period studied (Fig. 1e and f).
The temporal relationship between positive BG values in serum and other findings in patients with proven and probable IA is shown in Table 3. BG preceded (n = 2) the development of fever by 4 and 6 days. One febrile patient with probable IA had negative BG antigenemia. This patient had acute myeloid leukemia and E. coli bacteremia. After observation of pulmonary nodules in the HRCT scan and the growth of A. fumigatus in bronchoalveolar lavage fluid, the patient was treated with amphotericin B. BG preceded (n = 4) the development of clinical signs (cough and/or dyspnea and/or hemoptysis and/or thoracic pain) by 4, 10, 8, and 21 days. A positive BG result preceded the demonstration of abnormalities on HRCT scan in all seven patients (100%) by a median of 9.3 days (range, 1 to 21 days). Positive BG results preceded the initiation of antifungal therapy in five patients by a median of 14 days (range, 4 to 25 days).
TABLE 3.
Temporal onset of BG antigenemia in patients with proven and probable IA
| Time pointa | No. of evaluable patients | No (%) of patients with BG antigen | Days between BG detection and time point median (range) |
|---|---|---|---|
| First day of fever | 7 | 2 (28.2) | 5 (4-6) |
| First day of clinical signsb | 7 | 4 (57.1) | 10.7 (4-21) |
| Pulmonary HRCT scan | 7 | 7 (100) | 9.3 (1-21) |
| Initation of antifungal therapy | 7 | 5 (71.4) | 14 (4-25) |
At or before time point.
Cough and/or dyspnea and/or hemoptysis and/or thoracic pain.
Considering true positives as only the results obtained for patients with proven and probable IA cases and true negatives as the results in the no-IA group of patients, the sensitivity, specificity, and positive and negative predictive values of BG monitoring were 87.5, 89.6, 70, and 96.3%, respectively.
GM detection.
Of 40 patients, 11 (27.5%) repeatedly tested positive for GM detection. This group of GM-positive patients included 100% of patients with IA (five of five), 66% of patients with probable IA (two of three), and 33% of patients with possible IA (one of three) (Table 2). Of 29 patients with no IA, 3 (10.3%) were positive for GM detection. One of these patients with false-positive results had a relapse of acute myelogenous leukemia and severe mucositis, cytomegalovirus viremia, and graft-versus-host disease. The second patient had acute myelogenous leukemia, severe mucositis, and Pseudomonas aeruginosa bacteriemia. The third patient had Hodgkin's lymphoma and Staphylococcus aureus and Staphylococcus epidermidis bacteremia and had been treated with cyclophosphamide (Fig. 1d).
Considering true positives as only the results obtained for patients with proven and probable IA cases and true negatives as the results in the no-IA group of patients, the sensitivity, specificity, and positive and negative predictive values of GM monitoring were 87.5, 89.6, 70, and 96.3%, respectively.
The temporal relationship between GM antigenemia and other diagnostic markers in patients with proven and probable IA is shown in Table 4. Antigen GM detection preceded the development of fever by 4 days in one patient. GM antigenemia preceded the development of clinical signs (n = 3) by 4, 8, and 15 days. Antigen GM detection preceded the demonstration of abnormalities in HRCT scans in six patients by a median of 7.2 days (range, 1 to 15 days). Positive GM antigenemia preceded the initiation of antifungal therapy in four patients by a median of 12.5 days (range, 1 to 23 days).
TABLE 4.
Temporal onset of GM antigenemia in patients with proven and probable IA
| Time pointa | No. of evaluable patients | No (%) of patients with GM antigen | Days between GM antigen detection and time point median (range) |
|---|---|---|---|
| First day of fever | 7 | 1 (14.3) | 4 (4) |
| First day of clinical signsb | 7 | 3 (42.8) | 9 (4-15) |
| Pulmonary HRCT scan | 7 | 6 (85.7) | 7.2 (1-15) |
| Initation of antifungal therapy | 7 | 4 (57.1) | 12.5 (1-23) |
At or before time point.
Cough and/or dyspnea and/or hemoptysis and/or thoracic pain.
Combined analysis of both markers.
The results obtained by BG and GM detection in each patient were combined in an attempt to assess whether a combination of the two markers resulted in an early and specific diagnosis of IA. Interestingly, both tests were positive in the same patients with IA and the kinetics of both markers were very similar in most patients. BG tended to become positive earlier than GM. Discrepancies were observed in patients with false-positive results, since patients without IA but positive for BG detection were negative by GM detection and patients with false-positive results for GM were negative by BG detection. Interestingly, these discrepancies were important to identify the patients with false-positive results since it was only when both markers were positive that the patients had IA.
Consideration of the results obtained for both markers in combination showed in an improvement of the diagnostic efficacy of each individual test to predict IA. The sensitivity, specificity, and positive and negative predictive values were 87.5, 100, 100, and 96.3%, respectively.
DISCUSSION
IA is one of the most frequent fungal infections in neutropenic patients, in whom it is a major cause of morbidity and mortality, in part due to the inability to identify infected patients at an early stage of the disease (4, 5). The diagnosis of IA is a challenge for the clinician, as are the poor prognosis and the limited efficacy of current available antifungal drugs (7). Traditional microbiological studies (direct microscopy and culture of respiratory specimens) have low sensitivity and appear positive only in the late stage of IA. Furthermore, positive cultures do not discriminate between colonization, contamination, and IA (26). In recent years, the detection of different circulating surrogate markers such as fungal cell wall components (BG and GM) and genomic fungal DNA have emerged and improved the diagnosis of IA (5, 13, 20, 26, 28). The Platelia Aspergillus kit for the detection of GM has been widely used in Europe for several years, and the Food and Drug Administration has recently approved its clinical use in the United States (5, 11, 13, 16-18, 25, 28, 31, 32, 35, 36). While the prospective detection of GM in patients at high risk for IA shows that the Platelia Aspergillus test is highly specific (above 85%), the reported sensitivity varies widely, between 30 and 100%, due to several factors discussed by Mennink-Kersten et al. (20). One of these factors is the cutoff value of a positive GM result. In Europe the manufacturer recommends a cutoff of 1.5 ng/ml, while in the United States 0.5 ng/ml is the recommended value.
A strategy to overcome the deficiencies of the Platelia Aspergillus kit could be to combine it with another surrogate marker of IA in an attempt to complement the diagnostic usefulness of GM detection. The results presented in this paper appear to suggest that BG may be such a complementary marker since the combination of BG and GM detection was more effective than either test alone in diagnosing IA.
The information about the diagnostic potential of BG for the diagnosis of IA is currently scarce. In most studies, BG detection has been used as a tool for screening IFI. An early study by Obayashi et al. (23), published a decade ago, showed that cases of deep mycoses were associated with high concentrations of BG in plasma as measured by the FungiTec G test. In their study of over 200 febrile episodes in patients with hematological malignancy, they were able to detect 37 of 41 cases of IFI by using a cutoff of 20 pg/ml. An interesting feature of their study is that fungal superficial colonization (including oral, urinary, and bronchial colonization) did not raise the concentration of BG above 20 pg/ml. Four patients with Aspergillus pneumonia verified at autopsy had high concentrations of BG in plasma.
Several studies have reported that the sensitivity of BG detection for diagnosing IFI ranges between 50 and 63% (12, 13, 29). This is in contrast to data presented in this study, which show that detection of BG by the Glucatell test is a quite sensitive tool for the diagnosis of IA (87.5%). The high sensitivity observed in our study could be inherent to the Glucatell test, since commercialized tests for BG detection differ widely in sensitivity (13, 38). A preliminary evaluation of the Glucatell test has shown sensitivities between 60 and 100% for the diagnosis of IFI in patients with acute myeloid leukemia or myelodysplastic syndrome who were receiving antifungal prophylaxis (24). Although the existence of differences in the kinetics of BG release during invasive growth by Aspergillus and other fungi causing IFI cannot be ruled out at present, it is possible that the frequency of sampling in our study (twice weekly) may have increased the sensitivity of the test, since some studies have detected BG on a once-a-week basis or once per episode (13, 23). Odabasi et al. (24) used a cutoff of 60 pg/ml with the aim of detecting patients with IFI. The use of that cutoff for our patients would had decreased the specificity and positive predictive value in comparison with values obtained when using 120 pg/ml. Currently, the kinetics of BG release from the infected sites and its circulation in blood and clearance are poorly understood. The kinetics shown in this study suggest that an increase in the concentration of BG from 60 to greater than 360 pg/ml (Fig. 1a) and from 90 to 120 pg/ml (Fig. 2b) can occur in the course of 5 days. Therefore, a twice-weekly sampling seems to be a reasonable frequency for a BG screening strategy.
BG was consistently negative in one patient with probable IA (who had received prophylaxis with fluconazole) and two patients with possible IA (who had received no prophylaxis). Although the IA episodes were classified in this study on the basis of the EORTC-IFICG/NIAID-MSG case definitions (1), it cannot be excluded that the clinicoradiological pictures of the patients were due to a variety of nonfungal diseases. In fact, the clinical applicability of EORTC-IFICG/NIAID-MSG case definitions is controversial, as has been recently shown (30). Interestingly, these three patients were also negative by GM detection.
One of the problems observed in the detection of BG is the existence of false-positive results that decrease the specificity of the test (13). The reason for the BG positivity in the three patients with false-positive results remains obscure. While none of them was undergoing hemodialysis with cellulose membranes, a well-known cause of false-positive results in BG detection tests (22, 23), one of the patients had E. coli bacteremia and the other two were colonized by Candida species. However, Candida colonization is unlikely to be the cause of the false-positive results, since other patients with intense colonization by Candida species had negative BG levels (Fig. 1e and f), and it has been reported that isolation of a number of bacteria in blood, as well as colonization by yeasts, did not produce BG positive results by the FungiTec G test (23). In addition, neither Candida-colonized patient had antibodies to CAGT, which is a marker of invasive candidiasis (9). Since BG is a panfungal marker that could detect undiagnosed fungal infections, the possibility of an infection caused by a number of unusual fungal species such as Trichosporon spp., Saccharomyces spp., Acremonium spp., and P. jiroveci cannot be totally ruled out. An interesting feature of the kinetics of BG levels in patients with false-positive results is the sudden rise and fall in BG levels in serum in the absence of antifungal treatment (Fig. 1c). This type of kinetics is also shared by false-positive GM results (33), while a more protracted rise in BG levels in serum suggests the presence of Aspergillus infection, as shown in Fig. 1a and b.
Detection of circulating surrogate markers of IA may be useful not only for the diagnosing the mycosis but also for assessing the effectiveness of therapy. This has been demonstrated for GM antigenemia, since declining levels of GM have been found in patients responding to treatment while rising GM antigenemia is associated with treatment failure (2, 3, 17, 25). Results presented in this study show, for the first time, that monitoring BG antigenemia is useful in predicting the therapeutic outcome of patients with IA. Decreasing levels of BG were observed in patients who recovered from IA, while patients not responding to antifungal treatment showed a continuous rise in serum BG levels.
Although it did not improve the sensitivity of each test, the combination of BG and GM detection was very useful in confirming the existence of IA, since both markers were positive in patients with IA and, as expected for two cell wall molecules, their kinetics of release to the blood was very similar. Given the concordance of the two markers in patients with IA, discrepancies in positivity by each test were very useful in identifiying false-positive results by each test. If this concordance in positivity is confirmed in other studies, combined detection of BG and GM may be an important tool to solve the problem raised by the false-positive results detected by each test (8, 13, 20).
Although the sensitivities were equal for both markers and both tests are usually positive before the diagnosis of IA could be suspected or demonstrated by other means, results presented in this study suggest that BG tended to become positive earlier than GM (Tables 3 and 4). This precocity, and the fact that BG can also be a marker of invasive infections caused by a number of different fungi, should lead to initiation of the diagnostic workup of IFI when a positive BG result is detected and should enable prompt provision of preemptive antifungal treatment with a broad-spectrum antifungal drug. BG positivity should be confirmed by GM detection or by detection of antibodies to CAGT (9) and, when available, by PCR to identify the type of IFI and to exclude the possibility of a false-positive result.
In conclusion, BG and GM detection are useful tests for the diagnosis of IA in high-risk patients with hematological malignancies. However, a combination of the two tests was very useful to identify false-positive reactions by each test. The results presented in this study encourage a prospective evaluation of the Glucatell test to assess its role in the diagnosis of IA and follow-up of patients at high risk for developing this disease.
Acknowledgments
This investigation was supported by grants v-iig-27 from Fundación Fundesa (to A.D.P.) and 9/UPV 0093.327-13550/2001 from the Universidad del País Vasco (to J.P.).
We thank Pelayo Fontsaré and Marta Pérez from Fontlab 2000 Santa Eulalia de la Ronçana, Barcelona, Spain, for their technical help with the detection of BG.
REFERENCES
- 1.Ascioglu, S., J. H. Rex, B. de Pauw, J. E. Bennett, J. Bille, F. Crokaert, D. W. Denning, J. P. Donnelly, J. E. Edwards, Z. Erjavec, D. Fiere, O. Lortholary, J. Maertens, J. F. Meis, T. F. Patterson, J. Ritter, D. Selleslag, P. M. Shah, D. A. Stevens, and T. J. Walsh. 2002. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin. Infect. Dis. 34:7-14. [DOI] [PubMed] [Google Scholar]
- 2.Boutboul, F., C. Alberti, T, Leblanc, A. Sulahian, E. Gluckman, F. Derovin, and P. Ribaud. 2002. Invasive aspergillosis in allogenic stem cell transplant recipients: increasing antigenemia is associated with progressive disease. Clin. Infect. Dis. 43:939-943. [DOI] [PubMed] [Google Scholar]
- 3.Bretagne, S., A. Marmorat-Khvong, M. Kventz, J. P. Latgé, E. Bart-Delabasse, and C. Cordonnier. 1997. Serum Aspergillus galactomannan antigen testing by sandwich ELISA: a practical use in neutropenic patients. J. Infect. Dis. 35:7-15. [DOI] [PubMed] [Google Scholar]
- 4.Del Palacio, A., M. S. Cuétara, and J. Pontón. 2003. La aspergilosis invasora. Rev. Iberoam. Micol. 20:77-78. [PubMed] [Google Scholar]
- 5.Del Palacio, A., M. S. Cuétara, and J. Pontón. 2003. El diagnóstico de laboratorio de la aspergilosis invasora. Rev. Iberoam. Micol. 20:90-98. [PubMed] [Google Scholar]
- 6.Denning, D. W., E. G. Evans, C. C. Kibbler, M. D. Richardson, M. M. Roberts, T. R. Rogers, D. W. Warnock, and R. E. Warren. 1997. Guidelines for the investigation of invasive fungal infections in haematological malignancy and solid organ transplantation. Eur. J. Clin. Microbiol. Infect. Dis. 16:424-436. [DOI] [PubMed] [Google Scholar]
- 7.Denning, D. W., A. Marinus, J. Cohen, D. Spence, R. Herbrecht, L. Pagano, C. Kibbler, V. Kermery, F. Offner, C. Cordonnier, U. Jehn, M. Ellis, L. Collette, and R. Sylvester. 1998. An EORTC multicentre prospective survey of invasive aspergillosis in haematological patients: diagnosis and therapeutic outcome. J. Infect. 37:173-180. [DOI] [PubMed] [Google Scholar]
- 8.Digby, J., J. Kalbfleisch, A. Glenn, A. Larsen, W. Browder, and D. Williams. 2003. Serum glucan levels are not specific for presence of fungal infections in intensive care unit patients. Clin. Diagn. Lab. Immunol. 10:882-885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.García-Ruiz, J. C., M. C. Arilla, P. Regúlez, G. Quindós, A. Alvarez, and J. Pontón. 1997. Detection of antibodies to Candida albicans germ tubes for the diagnosis and therapeutic monitoring of invasive candidiasis in patients with hematologic malignancies. J. Clin. Microbiol. 35:3284-3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jantunen, E., P. Ruutu, L. Niskanen, L. Volin, T. Parkkali, P. Koukila-Kahkola, and T. Ruutu. 1997. Incidence and risk factors for invasive fungal infections in allogeneic BMT recipients. Bone Marrow Transplant. 19:801-808. [DOI] [PubMed] [Google Scholar]
- 11.Jones, B. L., and L. A. McLintock. 2003. Impact of diagnostic markers on early antifungal therapy. Curr. Opin. Infect. Dis. 16:521-526. [DOI] [PubMed] [Google Scholar]
- 12.Kami, M., Y. Tanaka, Y. Kanda, S. Ogawa, T. Masumoto, K. Ohtomo, T. Matsumura, T. Saito, U. Machida, T. Kashima, and H. Hirai. 2000. Computer tomographic scan of the chest, latex agglutination test and plasma (1→3)-β-d-glucan assay in early diagnosis of invasive pulmonary aspergillosis: a prospective study of 215 patients. Haematologica 85:745-752. [PubMed] [Google Scholar]
- 13.Kawazu, M., Y. Kanda, Y. Nannya, K. Aoki, M. Kurokawa, S. Chiba, T. Motokura, H. Hirai, and S. Ogawa. 2004. Prospective comparison of the diagnostic potential of real-time PCR, double-sandwich enzyme-linked immnosorbent assay for galactomannan, and (1→3)-β-d-glucan test in weekly screening for invasive aspergillosis in patients with hematological disorders. J. Clin. Microbiol. 42:2733-2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kozinn, P. J., C. L. Taschdjian, P. K. Goldberg, W. P. Protzmann, D. W. R. Mackenzie, J. S. Remington, S. Anderson, and M. S. Seelig. 1978. Efficiency of serologic tests in the diagnosis of systemic candidiasis. Am. J. Clin. Pathol. 70:893-898. [DOI] [PubMed] [Google Scholar]
- 15.Lin, S. J., J. Schranz, and S. M. Teutsch. 2001. Aspergillosis case-fatality rate: systematic review of the literature. Clin. Infect. Dis. 32:358-366. [DOI] [PubMed] [Google Scholar]
- 16.Maertens, J., J. Van Eldere, J. Verhaegen, E. Verbeken, J. Verschakelen, and M. Boogaerts. 2002. Use of circulating galactomannan screening for early diagnosis of invasive aspergillosis in allogeneic stem cell transplant recipients. J. Infect. Dis. 186:1297-1306. [DOI] [PubMed] [Google Scholar]
- 17.Maertens, J., J. Verhaegen, H. Demuynck, P. Brock, G. Verhoef, P. Vandenberghe, J. Van Eldere, L. Verbist, and M. Boogaerts. 1999. Autopsy-controlled prospective evaluation of serial screening for circulating galactomannan by a sandwich enzyme-linked immunosorbent assay for hematological patients at risk for invasive aspergillosis. J. Clin. Microbiol. 37:3223-3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maertens, J., J. Verhaegen, K. Lagrou, J. Van Eldere, and M. Boogaerts. 2001. Screening for circulating galactomannan as a noninvasive diagnostic tool for invasive aspergillosis in prolonged neutropenic patients and stem cell transplantation recipients: a prospective validation. Blood 97:1604-1610. [DOI] [PubMed] [Google Scholar]
- 19.Marr, K. A., R. A. Carter, M. Boeckh, P. Martin, and L. Corey. 2002. Invasive aspergillosis in allogeneic stem cell transplant recipients: changes in epidemiology and risk factors. Blood 100:4358-4366. [DOI] [PubMed] [Google Scholar]
- 20.Mennink-Kersten, M. A. S. H., J. P. Donnelly, and P. E. Verweij. 2004. Detection of circulating galactomannan for the diagnosis and management of invasive aspergillosis. Lancet Infect. Dis. 4:349-357. [DOI] [PubMed] [Google Scholar]
- 21.Miyazaki, T., S. Kohno, K. Mitsutake, S. Maesaki, K. Tanaka, N. Ishikawa, and K. Hara. 1995. Plasma (1→3)-beta-d-glucan and fungal antigenemia in patients with candidemia, aspergillosis, and cryptococcosis. J. Clin. Microbiol. 33:3115-3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Obayashi, T., T. Tamura, S. Tamaka, M. Onki, S. Takahashi, and T. Kawai. 1986. Endotoxin-inactivating activity in normal and pathological human blood samples. Infect. Immun. 53:294-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Obayashi, T., M. Yoshida, T. Mori, H. Goto, A. Yasuoka, H. Iwasaki, H. Teshima, S. Kohno, A. Horiuchi, A. Ito, H. Yamaguchi, K. Shimada, and T. Kawai. 1995. Plasma (1→3)-beta-d-glucan measurement in diagnosis of invasive deep mycosis and fungal febrile episodes. Lancet 345:17-20. [DOI] [PubMed] [Google Scholar]
- 24.Odabasi, Z., G. Mattiuzzi, E. Estey, H. Kantarjian, F. Saeki, R. J. Ridge, P. A. Ketchum, M. A. Finkelman, J. H. Rex, and L. Ostrosky-Zeichner. 2004. β-d-Glucan as a diagnostic adjunct for invasive fungal infections: validation, cutoff development, and performance in patients with acute myelogenous leukemia and myelodysplastic syndrome. Clin. Infect. Dis. 39:199-205. [DOI] [PubMed] [Google Scholar]
- 25.Pazos, C., and A. del Palacio. 2003. Diagnóstico precoz de la aspergilosis invasora en enfermos neutropénicos mediante la detección bisemanal de galactomanano en suero con Platelia Aspergillus. Rev. Iberoam. Micol. 20:99-102. [PubMed] [Google Scholar]
- 26.Perfect, J. R., G. M. Cox, J. Y. Lee, C. A. Kauffman, L. de Repentigny, S. W. Chapman, V. A. Morrison, P. Pappas, J. W. Hiemenz, and D. A. Stevens. 2001. The impact of culture isolation of Aspergillus species: a hospital-based survey of aspergillosis. Clin. Infect. Dis. 33:1824-1833. [DOI] [PubMed] [Google Scholar]
- 27.Prentice, H. G., C. C. Kibbler, and A. G. Prentice. 2000. Towards a targeted, risk-based, antifungal strategy in neutropenic patients. Br. J. Haematol. 110:273-284. [DOI] [PubMed] [Google Scholar]
- 28.Ruhnke, M., and G. Maschmetes. 2002. Management of mycoses in patients with hematologic disease and cancer-review of the literature. Eur. J. Med. Res. 7:227-235. [PubMed] [Google Scholar]
- 29.Sakai, T., K. Ikegami, E. Yoshinaga, R. Uesugi-Hayakawa, and A. Wakizaka. 2000. Rapid, sensitive and simple detection of candida deep mycosis by amplification of 18S ribosomal RNA gene; comparison with assay of serum beta-d-glucan level in clinical samples. Tohoku J. Exp. Med. 190:119-128. [DOI] [PubMed] [Google Scholar]
- 30.Subirá, M., R. Martino, M. Rovira, L. Vázquez, D. Serrano, and R. de la Cámara. 2003. Clinical applicatibility of the new EORTC/MSG classification for invasive pulmonary aspergillosis in patients with hematological malignancies and autopsy-confirmed invasive aspergillosis. Ann. Hematol. 82:80-82. [DOI] [PubMed] [Google Scholar]
- 31.Sulahian, A., F. Boutboul, P. Ribaud, T. Leblanc, C. Lacroix, and F. Derouin. 2001. Value of antigen detection using an enzyme immunoassay in the diagnosis and prediction of invasive aspergillosis in two adult and pediatric hematology units during a 4-year prospective study. Cancer 91:311-318. [DOI] [PubMed] [Google Scholar]
- 32.Sulahian, A., M. Tabouret, P. Ribaud, J. Sarfati, E. Gluckman, J. P. Latgé, and F. Derouin. 1996. Comparison of an enzyme immunoassay and latex agglutination test for detection of galactomannan in the diagnosis of invasive aspergillosis. Eur. J. Clin. Microbiol. Infect. Dis. 15:139-145. [DOI] [PubMed] [Google Scholar]
- 33.Swanink, C. M. A., J. F. G. Meis, A. J. M. M. Rijs, J. P. Donnelly, and P. E. Verweij. 1997. Specificity of a sandwich enzyme-linked immunosorbent assay for detecting Aspergillus galactomannan. J. Clin. Microbiol. 35:257-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Verweij, P. E., and D. W. Denning. 1997. The challenge of invasive aspergillosis: increasing numbers in diverse patient groups. Int. J. Infect. Dis. 2:61-63. [Google Scholar]
- 35.Verweij, P. E., E. C. Dompeling, J. P. Donnelly, A. V. Schattenberg, and J. F. Meis. 1997. Serial monitoring of Aspergillus antigen in the early diagnosis of invasive aspergillosis. Preliminary investigations with two examples. Infection 25:86-89. [DOI] [PubMed] [Google Scholar]
- 36.Verweij, P. E., J. P. Latgé, A. J. Rijs, W. J. Melchers, B. E. De Pauw, J. A. Hoogkamp-Korstanje, and J. F. Meis. 1995. Comparison of antigen detection and PCR assay using bronchoalveolar lavage fluid for diagnosing invasive pulmonary aspergillosis in patients receiving treatment for hematological malignancies. J. Clin. Microbiol. 33:3150-3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.von Eiff, M., N. Roos, R. Schulten, M. Hesse, M. Zuhlsdorf, and J. van de Loo. 1995. Pulmonary aspergillosis: early diagnosis improves survival. Respiration 62:341-347. [DOI] [PubMed] [Google Scholar]
- 38.Yoshida, K., Y. Niki, H. Mitekura, M. Nakajima, H. Kawane, and T. Matsushima. 2001. A discrepancy in the values of serum (1-3)-beta-d-glucan measured by two kits using different methods. Nippon Ishinkin Gakkai Zasshi 42:237-242. (In Japanese.) [DOI] [PubMed] [Google Scholar]