Abstract
Psychosis—an impaired contact with reality—is a hallmark of schizophrenia. Many psychotic symptoms are associated with disruptions in agency—the sense that I cause my actions. A failure to predict sensory consequences of one’s own actions may underlie agency disturbances. Such predictions rely on corollary discharge (CD) signals, “copies” of movement commands sent to sensory regions prior to action execution. Here, we make a case that the oculomotor system is a promising model for understanding CD in psychosis, building on advances in our understanding of the behavioral and neurophysiological correlates of CD associated with eye movements. We provide an overview of recent evidence for disturbed oculomotor CD in schizophrenia, potentially linking bizarre and disturbing psychotic experiences with basic physiological processes.
Keywords: psychosis, schizophrenia, eye movements, corollary discharge, efference copy, prediction
Oculomotor probes of agency disturbances in psychosis
Our sense of reality relies on the rapid perception and interpretation of our world. The brain’s ability to make predictions based on current context form an integral part of shaping and adapting to reality: When we push a swing, we expect it to swing back, and—as adaptive systems—we are able to effectively adjust our responses accordingly [1]: we stop the swing or get out of the way. This simple example is illustrative of how biological systems exist in dynamic and symbiotic relationships with their environment. When a system is unable to exist in such a relationship, it is inherently impaired. It is notable that this relatively simple framework, termed predictive coding (see Glossary), is now being applied to the understanding of psychiatric illnesses in general [2], and psychosis in particular.
Psychosis evokes separation from reality and includes experiences like hallucinations (false perceptions) and delusions (irrational beliefs). An individual may hear a voice criticizing their appearance or be convinced that the government controls their mind through an implanted device. These are not illusions or vague notions—they are experienced as reality and are notoriously immune to evidence of the contrary. Acute psychosis occurs in many medical and psychiatric conditions and is sometimes considered “the fever” of mental illness. In no disorder, however, is psychosis as enduring and profound as in schizophrenia. Schizophrenia is an epigenetic puzzle, a complex polygenic disorder whose etiological bases remains poorly understood. The world appears radically different to the schizophrenia patient, and this difference is the basis for the illness’ core characteristics.
Unsurprisingly, the notion of disordered prediction has featured prominently in mechanistic theories of psychosis [3–5]. These theories effectively argue that psychosis arises due to an abnormality in forming or using stored regularities that structure and help us understand the environment. This abnormality leads to misinterpretations of sensory information and over-interpretation of random associations, thus potentially explaining both hallucinations and delusions [3]. More generally, it has been argued that these prediction disruptions cause a fragmented interaction with the external world and a disjointed self. Within this framework, psychosis can be explained by a failure both in predicting how external stimuli co-occur as well as predicting the sensory consequences of actions. In this opinion piece, we argue that the oculomotor system provides an ideal framework for investigating the latter: Understanding how the brain anticipates the sensory consequences of movement provides a powerful model for understanding how these processes could go awry in psychosis.
The brain’s mechanism for anticipating the sensory consequences of movement is remarkably elegant: “copies” of motor commands—so-called corollary discharge signals (CD)—are used to form a prediction of the sensation expected from the self-generated movement (sometimes referred to as the forward model), which is then sent to sensory areas prior to or during movement execution (Figure 1a, Key Figure). When the actual self-generated sensation is consistent with the prediction, it can be largely ignored, sparing resources for information that does violate our predictions. Further, these predictions allow self-generated actions to be recognized as such: when the predicted sensation matches the actual sensation we have a subjective experience of agency. Many psychotic experiences carry traces of an external misattribution of self-generated action, and a disturbance in CD has offered a plausible explanation for these agency-related phenomena across a range of sensorimotor domains (Box 1). Although CD disturbances provide the most compelling biological explanation for agency disturbances in psychosis, the evidence remains largely indirect.
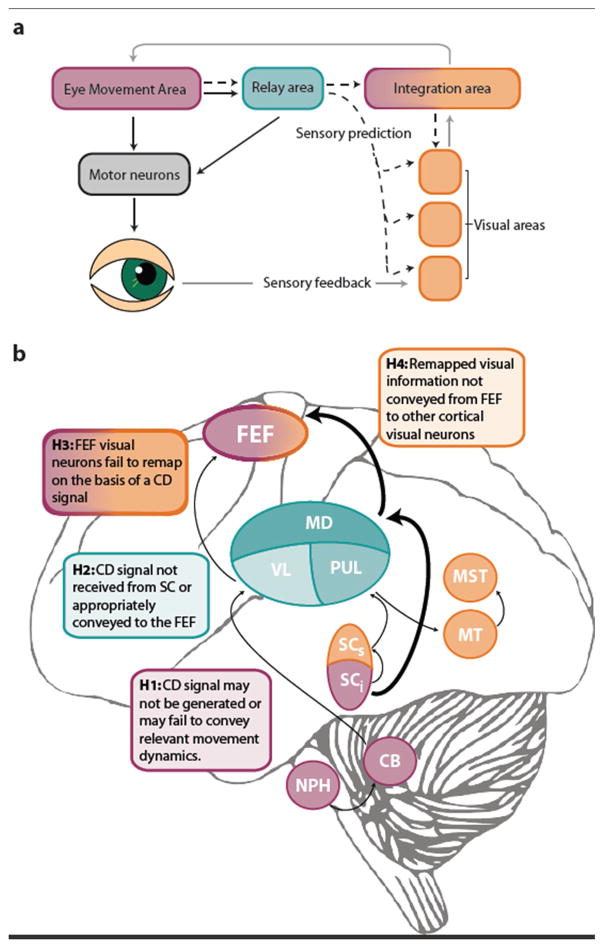
Figure 1. Circuits conveying a corollary discharge (CD) signal in the oculomotor system.
a Functional architecture. A motor command (solid black line) issued from an area generating eye movements is sent to motor neurons controlling the eye muscles, either directly or via a relay node. An accompanying CD signal (dotted black line) conveys information about the spatial and temporal dynamics of this movement to visual areas, either directly, or via higher-order integration areas. Along with CD signals, visual and integration areas also receive sensory feedback regarding the retinal stimulation following the eye movement (grey line) and can compare the CD-based predictions with the actual sensory consequences (i.e. reafferent information). Although a relay node is not strictly necessary, it may facilitate the dissemination of the CD signals across multiple functional nodes and ensure that the timing of these signals is commensurate with the purpose it fulfills in the target sensory and higher processing areas. b Anatomical circuitry. Thicker arrows indicate pathways that have received more empirical support. CB: cerebellum; FEF: frontal eye fields MD: mediodorsal nucleus of the thalamus; MST: medial superior temporal area; MT: middle temporal area; NPH: nucleus prepositus hypoglossi; PUL: pulvinar; SCi: intermediate layers of the superior colliculus; SCs: superficial layers of the superior colliculus; VL: ventrolateral nucleus of the thalamus. Colors indicate mapping onto functions in panel a.
Box 1. Disturbed corollary discharge as a mechanism of psychosis.
An altered sense of agency is at the heart of many psychotic symptoms—both hallucinations and delusions [90–92]. Passivity delusions, where the individual experiences thoughts or actions as under external control, provide a clear example. Similarly, auditory hallucinations are construed as subvocal or inner speech that is attributed externally. Models of sensorimotor control, which posit a system that predicts the sensory consequences of a movement command based on an internal signal (corollary discharge; CD) [93], have been co-opted to explain these symptoms. CD is theorized to serve a function outside of the sensorimotor domain by supporting a subjective sense of agency over action. Sensory input that is consistent with prediction is tagged as self-generated. A breakdown in these CD signals may underlie the disturbed sense of agency in psychosis [91]: sensory events resulting from voluntary actions are unexpected and thus attributed externally. This notion conceptualizes thinking as an active motor process [94] to explain the external attribution of not only willful action but also of imagery and thoughts. Within this framework, the integrity of sensorimotor processing is central to a forward fed normative construction of the self.
Indirect evidence for abnormal CD signals in psychosis exists in multiple sensory systems [95]:
Audition. Electrophysiological responses in auditory cortex to self-generated speech are attenuated in healthy individuals [96]. Individuals with schizophrenia show reduced modulation of auditory cortex during speech [96–100]. Furthermore, schizophrenia patients appear to correct speech errors based on auditory feedback rather than a CD signal from the phonetic plan [101].
Somatosensation. CD diminishes tactile responses to self-generated movement (indeed, we cannot tickle ourselves [102]); schizophrenia patients with passivity symptoms and auditory hallucinations show reduced attenuation of tactile responsivity to self-produced stimuli [103].
Proprioception. When healthy individuals are instructed to match the force they feel pressed on a lever, they consistently apply too much pressure, arguably because CD attenuates the sensation on the lever when they are actively producing the movement [104]. Individuals with schizophrenia are more accurate, consistent with altered CD [105]. In addition, they correct fewer erroneous joystick movements in the absence of external feedback [106, 107].
Notably, other explanations for the experience of agency have been proposed. Cognitive theories posit that we experience agency when an action’s outcome is consistent with our expectation [108]. Accordingly, goals, biases, and beliefs influence perceived agency. Inappropriate weighting of these different sources of information may contribute to altered agency experiences [109].
Because the most robust evidence for CD in non-human primates and healthy human populations stems from studies of eye movements, we argue that the oculomotor system is an ideally suited model for testing hypotheses of abnormal CD in psychosis [6]. Indeed, eye movement impairments have been recognized in schizophrenia patients since the early 1900’s [7], and CD-based predictions appear to underlie several sensorimotor functions including: (1) the continuity of perception despite eye movements constantly displacing the retinal image [8]; (2) the rapid production of accurate successive eye movements [9, 10]; (3) the precise tracking of moving stimuli [11], and, (4), the adaptation of saccade commands in the face of consistent visual errors [12]. Objective and well-established paradigms that produce data with high signal-to-noise ratios have been developed to study each of these phenomena, and they allow clear and grounded predictions about the precise way in which CD should be evidenced behaviorally in clinical populations (Box 2).
Box 2. Tapping CD signals in the oculomotor system at the behavioral level.
CD signals convey information about an impending movement—its onset, the overall vector (direction and amplitude), or even the entire trajectory. In principle, therefore, any task in which performance is correlated to an eye movement in space and/or time is potentially informed by CD signals. A number of tasks aim at isolating CD-based predictions in perceptual and motor processes (in some cases, visual and proprioceptive signals are also potential sources of information).
In motor tasks, CD may influence movement execution or subsequent behavior. Predictive pursuit, for instance, occurs when the motion of a predictably moving target is interrupted (blanked or altered) for a brief moment [110] or stabilized on the fovea [111], eliminating the retinal error signal that would normally drive the movement. Thus, smooth tracking must rely on an internal prediction of motion and a corollary of the eye movement. In the saccadic system, the double-step task [9, 10], in which two flashed stimuli cue a rapid sequence of two saccades, has become the litmus test of CD. To accurately land the second saccade, the oculomotor system must discount the vector of the first saccade from the encoded retinal vector of the second target. Similarly, CD signals are thought to enable fast corrections of erroneous saccades in paradigms requiring higher-level control (e.g. inhibition) of eye movements [112]. Finally, CD-based predictions drive saccadic adaptation: prediction errors experienced after previous saccades alter the metrics of future movements, and deviations from the intended trajectory may change saccades in midflight [113, 114].
In perceptual tasks, correlates of a CD signal may be observed as soon as it is available—even before movement onset. Pre-saccadic shifts of attention are a case in point: these predictive boosts in visual performance are spatially-selective to the saccade target and occur time-locked to movement onset [115, 116] The most direct perceptual test of the precision of saccadic CD is the trans-saccadic localization task [37], in which the saccade target disappears with movement onset and reappears with a delay after saccade landing. This manipulation results in conspicuous spatiotopic apparent motion: the target is seen to jump from its origin to its new post-saccadic location with little (but systematic) error. Given the vastly different retinal locations at which the pre- and post-saccadic target are seen (in the periphery vs. near the fovea), judging the direction of motion in this task must rely on a very precise CD signal [38].
In addition, an active research program investigating oculomotor prediction at the neurophysiological level has significantly advanced our understanding of CD associated with eye movements. We now know that certain types of visual neurons exhibit predictive remapping: they show vigorous visual responses even before the impending saccade brings a stimulus into their receptive field [13]. To do so, they must receive information about the time and target of the next saccade. A pathway between subcortical saccade neurons and cortical visual neurons appears to convey the CD signals necessary for these predictions via the mediodorsal thalamus [14–16]. The recent surge in publications investigating remapping and CD in the oculomotor system of individuals with schizophrenia underscores the utility of understanding abnormal prediction of self-generated actions and agency in psychosis through eye movement physiology [6]. This surge coincides with an expanding interest in understanding the role of thalamo-cortical interactions in psychotic disorders.
Evidence for disturbed oculomotor CD in psychosis
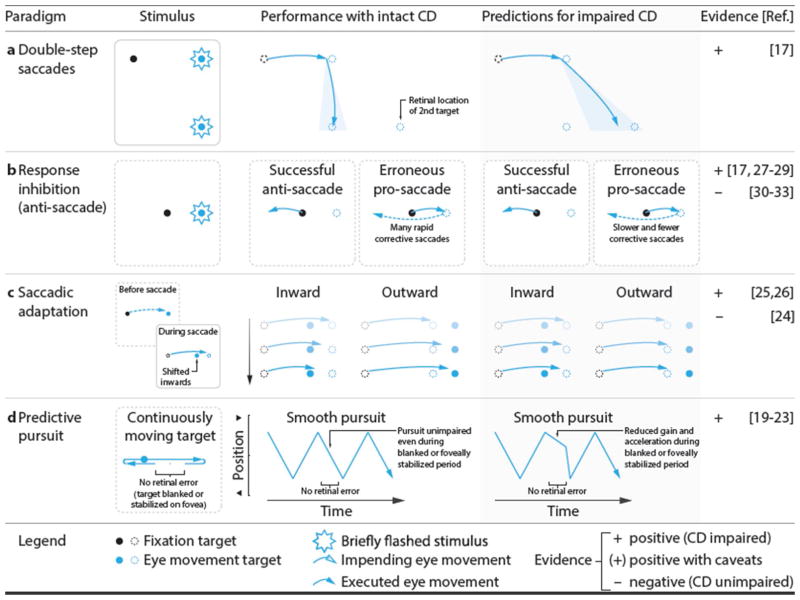
A number of behavioral paradigms have been developed to assess CD in the oculomotor system. Successful performance on these tasks relies on prediction of the (future) location of gaze, as reafferent processing is relatively slow. A putative CD signal sub serves this prediction and informs both action plans and visual perception (Box 2). Patients with schizophrenia often show specific deficits on those tasks for both saccadic and smooth pursuit eye movements, suggesting an impaired or non-veridical CD signal that affects action plans (Figure 2) and visual perception (Figure 3). At the level of action control, individuals with schizophrenia show performance deficits consistent with altered CD on the double-step [17], smooth pursuit [18], predictive pursuit [19–23], and saccade adaptation [24–26] tasks. One exception here is in the rapid correction of saccades that are inconsistent with higher-level goals: for instance, fast corrections after errors in the anti-saccade test are purported to rely on CD. Both equal [27–29] and impaired [30–33] antisaccade error correction latencies and rates have been reported. A notable aspect of these studies, however, is that visual feedback indicating an error (i.e., the visual target) was available to subjects. It is possible that error-correction impairments are only present when no external information is available to inform these corrections—that is, when they rely most heavily on CD. Indeed, a recent study using a modified double-step paradigm, where the second target was the cue to inhibit the response to the first target and saccade immediately to the second target [17], was conducted in complete darkness, and visual stimuli were flashed only briefly. Thus, no visual information could indicate that an error had been committed. Despite equal error rates and latencies in this task, individuals with schizophrenia made fewer and slower error corrections. In sum, evidence from a range of experimental paradigms indicates that schizophrenia patients show impairments in using CD for action.
Figure 2. Oculomotor paradigms used to study the role of CD signals in action.
a Double-step task. Two targets flash to cue a sequence of two saccades. An accurate second saccade requires accounting for the metrics of the first saccade; patients show incomplete compensation. b Anti-saccade task. After erroneous pro-saccades, CD of the first saccade might play a role in rapidly executing a corrective saccade. c Saccade adaptation. The saccade target is displaced during the saccade. Provided an intact CD signal, consistent landing errors across trials result in a reduction of the saccade amplitude for inward adaptation, and an increase for outward adaptation. In patients, adaptation can be weakened or slowed. d Predictive pursuit. While tracking a predictably moving target, intact CD in the pursuit system facilitates smooth tracking during periods of zero visual error (e.g., due to blanking or image stabilization).
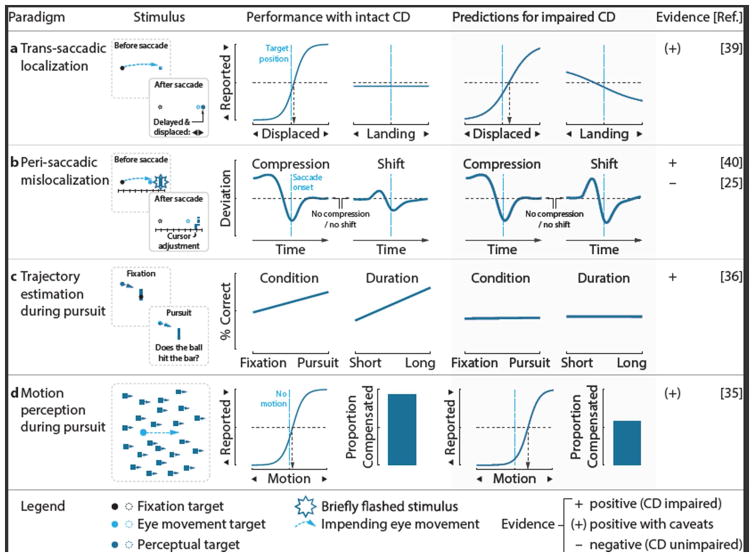
Figure 3. Oculomotor paradigms used to study the role of CD signals in perception.
a Trans-saccadic localization. Observers judge the displacement of the saccade target, blanked upon saccade landing before reappearing displaced in one of two directions. In healthy observers, displacement reports do not depend on saccade landing site, as CD signals inform this judgment (predicted target location is the difference between the initial target distance and the amplitude of the executed saccade). If CD is disturbed, the saccadic landing site can be used as a proxy (e.g., the target is to the right of where I am looking); the displacement report thus depends on landing site. b Peri-saccadic mislocalization. Observers perceive stimuli flashed briefly before saccades to be shifted in the direction of the saccades and closer to the saccade target. Impairment of CD is theorized to cause increased shifts. c Trajectory estimation. Pursuit of the ball facilitates performance in the collision judgment task suggesting a role of CD in the task. d Motion perception. During pursuit, retinal motion of the background should be cancelled by CD signals.
CD is also argued to support perceptual stability [34] in the face of disruptions in visual input generated by the moving eye. Accordingly, CD abnormalities in schizophrenia may also reveal themselves through altered perception during and immediately following eye movements. During smooth pursuit eye movements, CD fails to ensure visual stability of a static background image in those patients with the most severe psychotic symptoms [35]. Moreover, whereas in healthy controls estimation of the future location of a smoothly moving target is enhanced by smooth pursuit, schizophrenia patients fail to show that benefit, putatively due to abnormal CD [36].
For perceptual judgments around the time of a saccadic eye movement, the evidence is mixed. Schizophrenia patients show altered performance on the trans-saccadic localization task, in which the target is blanked during the saccade and reappears at a new location only after saccade landing [37, 38]. This task relies on CD to inform the visual system of the vector of the executed saccade. Altered CD, therefore, may cause impaired post-saccadic localization of visual targets in schizophrenia patients [39]. Two further studies investigated mislocalization of visual targets flashed around the time of a saccade, but their implications are unclear. One study found that the flashed target showed greater perisaccadic mislocalization in the direction of the saccade target in schizophrenia patients, consistent with a disturbed CD signal associated with a continuous readout of (the predicted) eye position during the saccade [40]. Using a model of perisaccadic mislocalization, the authors argued that the effect was consistent with a noisy, rather than delayed or absent, CD signal. A second study did not observe differences in perisaccadic mislocalization between schizophrenia patients and controls [25]; however the localization target was flashed at only one delay following the saccade target, when large mislocalizations would not be expected [41]. Despite group differences in the shift towards the saccade target, no group differences in compression toward the saccade target were observed [40]. Traditionally, perisaccadic compression has been thought to arise due to the perceptual effects of a transient CD signal associated with the saccade vector [42]. More recent work, however, has revealed that compression can be observed in the absence of a saccade, arguing instead that it is due to reduced perisaccadic visual sensitivity [43, 44]. Thus, these findings of unaltered perisaccadic compression in schizophrenia do not necessarily challenge other evidence for a disturbed oculomotor CD signal.
Theories positing disturbed CD as a mechanism of psychosis predict that performance on the aforementioned oculomotor tasks should be related specifically to the psychotic symptoms of schizophrenia, the key features being hallucinations and delusions. Moreover, CD abnormalities should be associated with other disorders with prominent psychotic symptoms. Consistent with this notion, CD abnormalities have been observed in individuals with bipolar disorder who have a history of psychotic symptoms [45, 46] and in healthy individuals with schizophrenia-like traits [47, 48]. Although there is some evidence for a relationship between psychotic symptom severity and CD alterations in schizophrenia [29, 35, 39], these relationships are inconsistent across studies. Indeed, many studies of CD find no such relationship, leaving open the possibility that altered CD is related to other clinical factors in schizophrenia and bipolar disorder. The lack of such symptom correlations does not necessarily preclude a relationship between CD and psychosis, however. Clinical interviews may fail to enquire about psychotic experiences most closely aligned to CD abnormalities. They are further limited by what the patient is willing to report. Indeed, implicit neurobiological measures, like oculomotor indices of CD, might be a better (or at least more reliable) index of psychopathology than interview measures. Moreover, CD disturbances could reflect symptom vulnerability, rather being a proximal mechanism of psychosis. Consistent with that notion, abnormal predictive pursuit [23, 49, 50] (and smooth pursuit more generally [18]), as well as abnormal antisaccade error corrections [28] have also been observed in asymptomatic relatives of schizophrenia patients, suggesting a genetically mediated trait. Retrospective data showing CD abnormalities in high-risk individuals who eventually develop psychosis versus those who do not [51] would be particularly illuminating in this regard, but to our knowledge, these studies have not been performed.
Although we interpret the studies presented above as supporting a disturbance in CD-based oculomotor prediction that is related to schizophrenia, and potentially psychosis broadly, we would like to highlight two potential caveats. First, the possibility of medication-related effects is relevant, given that schizophrenia is typically treated with dopamine antagonists. Yet, there are two main reasons to discount medication effects as driving impaired oculomotor CD. First, impairments have been observed in unmedicated, healthy relatives of schizophrenia patients and unmedicated individuals high in schizophrenia-like traits [23, 49, 50]. Second, in the aforementioned studies, antipsychotic dose is not predictive of task performance.
Potential group differences in the integrity and utilization of sensory signals used for oculomotor processing is another consideration. Sensory signals are crucial for oculomotor behavior—at least for the initiation of eye movements. There is abundant evidence for low-level sensory abnormalities in schizophrenia [52], including in the visual domain [53]. Certainly, a failure in sensory processing of visual targets would lead to disrupted oculomotor behavior. The kind of visual processing necessary for generation of saccadic eye movements seems to be spared in schizophrenia patients as the dynamics of visually-guided saccades are generally intact [54]. Schizophrenia patients have robust deficits in motion perception [55], however, which is crucial for accurate smooth pursuit eye movements. Indeed, previous studies have observed that altered pursuit initiation in schizophrenia patients was related to impaired velocity discrimination [56]. In our opinion, it is unlikely that altered visual processing can fully explain performance on the oculomotor tasks tapping CD presented above, though it is possible that a mismatch between the actual and predicted sensory signals in schizophrenia could arise due to a disrupted or absent prediction or due to noise in the sensory system.
Furthermore, the balance of using retinal versus CD-based extraretinal information to guide oculomotor behavior could be altered in schizophrenia. In the presence of external visual signals to guide eye movements, extraretinal signals play less of a role [57, 58]. Much of the work investigating CD and predictive remapping in human and non-human primates has been performed in impoverished visual environments that would either force, or at least bias, participants to use extraretinal information. If predictive mechanisms are disrupted in schizophrenia due to abnormal CD, they might show a stronger bias towards using retinal, versus extraretinal, information to perform these tasks (see Outstanding Questions).
Outstanding Questions.
How are CD signals disturbed? We need to develop new paradigms or utilize existing ones to identify the nature of CD disturbances in psychosis: is CD slower, less accurate, or not generated?
What is the neural basis of abnormal CD in psychosis? If further work were to establish causal relationships between activity in the FEF, SC, and MD, we could probe whether behavioral abnormalities have their basis in reduced connectivity within this network.
What are the phenomenological consequences of failing to perceive ourselves as the agent of an eye movement? Abnormal oculomotor CD should have consequences associated with the clinical picture of psychosis. The unpredicted changes in visual input caused by a failure in saccade-related CD may cause inappropriate assignment of salience, and thereby meaning, to irrelevant aspects of the environment
Does the thalamus convey CD in other sensory domains? The clinical relevance of disturbed oculomotor CD rides on the assumption of common networks for transmitting CD across sensorimotor domains. Given its role as a versatile multimodal relay station, the thalamus is a promising candidate that should be targeted in future studies.
Do schizophrenia patients use alternate sources of information to infer causality? Unreliable CD signals may lead patients to weigh other sources of information more strongly to establish the causes of events, potentially rendering them more prone to arrive at the wrong conclusion. In the oculomotor system, for instance, individuals with schizophrenia may rely more on visual or proprioceptive signals than healthy controls to estimate the location of gaze.
Is there a link between CD abnormalities and cognitive dysfunction in schizophrenia? Recent studies suggest a possible link between CD and working memory ability, thereby creating avenues for investigating the relationship between CD and cognitive function in schizophrenia.
Can the oculomotor system shed light on CD beyond the sensorimotor domain? Despite prevalent motor abnormalities, schizophrenia is fundamentally a disorder of thought. Thus, the clinical relevance of this proposal relies on the notion of thought as a motor process that is associated with CD signals functioning analogously to those in the oculomotor system.
Finally, we would like to speculate on the relationship between altered oculomotor CD and cognitive deficits. Although not part of the formal diagnostic criteria, individuals with schizophrenia show cognitive deficits across a range of domains [59]. Impairments in visuospatial working memory are particularly robust [60]. As far as we are aware, the potential contributions of CD abnormalities to cognitive dysfunction have not received any attention. However, a recent study in healthy individuals observed enhanced maintenance of information at a future saccade target in working memory, arguably via CD signals [61]. These findings suggest a possible link between CD abnormalities and working memory impairments in schizophrenia and create an avenue for investigating the relationship between CD and cognitive function more broadly.
Neural pathways carrying CD signals
In their elegant neurophysiological work aimed at identifying pathways conveying CD in the monkey brain, Sommer and Wurtz [15] required a set of clear-cut criteria: 1) CD must originate from neurons involved in movement generation, occur prior to movement onset, and represent the movement’s spatial and temporal dynamics; 2) Rather than travelling towards the muscles, CD must travel away from lower movement areas and thus not influence simple movement production, and 3) disrupting a pathway involved in transmitting CD should disrupt performance on tasks requiring CD. A rich body of neurophysiology and human lesion work suggests that a pathway involving the mediodorsal thalamus (MD) satisfies these criteria and that the MD plays a crucial role in the transmission of CD associated with saccadic eye movements (Figure 1b). Specifically, inactivating MD neurons that relay signals from the superior colliculus (SC) to the frontal eye fields (FEF) disrupts performance on the double-step task in non-human primates, causing saccade targeting errors of the second, but not first, movement [14, 15]. Similar double-step-task impairments in humans with MD lesions further bolster the notion that this pathway transmits CD signals [62–64].
In addition, the CD signal transmitted from the MD may be the basis of the predictive remapping properties of FEF neurons [16] and aid in postsaccadic perceptual processing [65–67]. Neurons that show predictive remapping anticipate the consequence of the eye movement and update the landscape of neural activity in an otherwise retinotopic map. Such neurons are common in many oculomotor brain regions (i.e. FEF, SC, lateral intraparietal sulcus) as well as in visual cortex [reviewed in 68]. Recently, however, the story of predictive updating of neural maps has become more nuanced. By sampling a large number of locations in the visual field, it was found that receptive fields in FEF converge onto the saccade target, rather than shift in the direction of the impeding saccade [69]. Follow-up recordings of pre-saccadic changes in the receptive field of V4 neurons indicated that predictive remapping as well as convergence onto the saccade target can occur in the same neuron, albeit at different timescales and dependent on saccade direction [70]. In sum, there is considerable evidence that predictive remapping does occur on the basis of a CD signal, presumably transmitted via the MD. There is indirect evidence that remapping may begin in FEF, from where it is disseminated across the brain [71].
In the smooth pursuit eye movement system, CD signals must convey information about eye velocity. Although CD pathways associated with pursuit have not been studied with the same rigor as in the saccade system, several potential thalamo-cortical pathways emerge. Neurons in the ventrolateral thalamus (VL), which project to frontal oculomotor regions, contain eye velocity information consistent with a CD signal [72]. Neurons in the cerebellar flocculus, which projects to VL, also convey a putative CD signal that it likely receives from brainstem nuclei [73]. Thus, one potential pathway for CD associated with smooth pursuit, projects from brainstem oculomotor nuclei to cortical oculomotor pursuit regions (e.g., FEF) via the cerebellum and VL. Another potential pathway involves the medial superior temporal area (MST), a region that processes visual motion and projects to cortical oculomotor regions. During smooth pursuit eye movements, a class of MST neurons continue to fire in the absence of retinal motion (i.e. when the target is momentarily blanked or stabilized on the retina), consistent with the notion that MST neurons also carry a putative CD signal [74, 75]. Moreover, MST receives projections from motion-sensitive neurons in the middle temporal area (MT), which in turn receive the effect of a CD signal conveyed from visual neurons in the superior colliculus via the pulvinar nuclei in the thalamus [76, 77]. Thus, a pathway from SC to frontal oculomotor regions via pulvinar, MT, and MST, is another candidate pathway for pursuit-related CD signals. Finally, given overlap between the saccade and pursuit systems [78], the SC-MD-FEF pathway could also transmit CD signals necessary for pursuit. This remains to be investigated.
Possible network mechanisms of disturbed oculomotor CD in psychosis
The relevant questions in psychosis are: 1) in which sub-networks is functioning altered and 2) how do abnormalities in functional brain networks give rise to performance deficits requiring a CD signal. The precise neurophysiological characterization of a CD pathway for saccadic eye movements and the way in which it influences sensory neurons allows formulation of several predictions regarding macroscopic brain network function (and dysfunction) (Figure 1b):
Movement neurons in the SC could fail to generate a CD signal or the relevant movement dynamics might not be conveyed in this signal.
Dysfunction in MD neurons could prevent an accurate CD signal from being received from SC or appropriately conveyed to the FEF.
FEF visual neurons could fail to remap visual responses on the basis of a CD signal.
Remapped visual information could fail to be conveyed from FEF to other visual areas.
Although these hypotheses remain to be tested, we argue that the second one (involving the thalamus) is particularly plausible. The active motor processes putatively implicated in the sense of agency depend heavily on the cortical-subcortical interface. In primates, a dense network architecture underpins this interface, with the thalamus poised in a principal role [79]. The cortex functions under a high input regime from the thalamus [80–82], and cortical-thalamic functional transactions are highly modifiable in ascending and descending directions [83]. Thus, the thalamus may be a central neuronal unit linking psychosis with neurobiology. Indeed, thalamus dysfunction and dysconnectivity is considered fundamental for explaining typical symptoms such as thought disorder; these inevitably reflect a lack of appropriate integration of functional signals across cortical-thalamic networks [84]. Widespread empirical support corroborates this link, including reliable evidence of thalamic structural deficits [85], dysfunctional activation profiles during saccade tasks [86], and—of particular relevance—dysfunctional thalamo-cortical networks. Especially salient is evidence of dysfunctional effective connectivity of cortical-thalamic network interactions, specifically involving the MD [87], and generally reduced cortical-thalamic functional connectivity at rest [88, 89].
We realize that the hypotheses outlined above and in Figure 1b are not comprehensive. We have outlined other thalamo-cortical pathways that can convey CD in the oculomotor system and additional, redundant pathways almost certainly exist. However, the SC-MD-FEF pathway has received the most empirical support and thus forms the most solid base on which to make predictions about dysfunction in clinical populations.
Concluding remarks and future perspectives
Investigation of CD in the oculomotor system capitalizes on the translational advantages that oculomotor paradigms afford, allowing novel and exciting interpretations of patient findings in the context of rich non-human primate neurophysiology data: using this system as a framework for understanding agency dysfunction in schizophrenia can lead to great strides in our understanding of the specific mechanisms of those symptoms of the illness that have been the most challenging to explain at the level of physiology. That is, oculomotor disturbances could share a common mechanism with, and potentially contribute to, the bizarre subjective experiences in psychosis. The behavioral findings in patient populations reviewed here, along with an emerging psychophysical and physiological understanding of CD signals in the primate brain, create avenues for further investigation (see Outstanding Questions). Future research should explore the full translational possibilities of this work by adapting the experimental paradigms reviewed here for use as simple tests that can be performed in clinical contexts to aid in the prediction of psychosis in at-risk individuals, assist in diagnostic decisions, and track treatment-related changes over time.
Trends Box.
Psychosis, a defining feature of schizophrenia, is associated with profound disruptions in the sense of agency. A failure to predict the sensory consequences of actions may underlie these agency disturbances.
Predicting consequences of self-generated action relies on corollary discharge (CD) signals, “copies” of movement commands that are sent to sensory regions prior to action execution.
The oculomotor system is a promising model for understanding abnormal CD in psychosis, building on significant advances in our understanding of the behavioral and neurophysiological correlates of CD associated with eye movements in humans and non-human primates.
A surge of recent evidence suggests disturbed CD associated with eye movements in schizophrenia, shedding light on the mechanisms of the symptoms that have been the most challenging to explain at the level of physiology.
Acknowledgments
K.N.T. is supported by a NARSAD Young Investigator award from the Brain and Behavior Research Foundation. V.A.D. is supported by a Charles H. Gershenson Distinguished Faculty Fellowship, the National Institute of Mental Health (MH111177; MH059299), the Children’s Hospital of Michigan Foundation, the Children’s Research Center of Michigan, The Prechter World Bipolar Foundation, the Cohen Neuroscience Endowment, and the Lyckaki-Young Fund from the State of Michigan. M.R. is supported by the Deutsche Forschungsgemeinschaft (grants RO 3579/2-1 and RO 3579/3-1). The authors would like to thank Miriam Spering, Florian Ostendorf, and Sohee Park for reading a draft of our manuscript and providing constructive feedback.
Glossary
- Agency
The subjective experience that one controls the volitional action one produces
- Bipolar disorder
a disorder characterized by bouts of depression and elevated mood (mania). Psychosis is common during mood episodes.
- Corollary discharge
“Copies” of motor commands that, rather than being sent to lower movement areas, are sent to sensory and higher processing areas.
- Perceptual stability
The perception of the world as stationary despite the fact that eye movements incessantly displace the visual input on the retina.
- Perisaccadic compression
a type of perisaccadic mislocalization in which visual stimuli flashed around the time of a saccade are perceived as closer to the saccade target than they actually were.
- Perisaccadic mislocalization
The phenomenon that visual stimuli around the time of a saccade (before, after, or during) are perceived at a different location than the one in which they were presented.
- Predictive coding
A framework for brain function, positing that the brain continually generates a model of the world based on current context and past experience and updates this model when the predicted and actual input do not match.
- Predictive remapping
The finding that neurons in retinotopic brain areas start responding to a visual stimulus before an impending eye movement brings it into their receptive field (or, earlier than expected from reafferent processing).
- Psychosis
A clinical phenomenon characterized by a loss of contact with reality.
- Reafferent processing
Processing of sensory input produced by the execution of one’s own movement. In the case of eye movements, this input is primarily visual in nature.
- Receptive field
the part of space in which stimuli alter a neuron’s activity.
- Retinal error
distance between a visual target and the location of gaze.
- Retinotopic
organization of visual brain areas such that visual neurons in the brain have receptive fields encoding specific locations on the retina. Eye movements therefore redirect the cells’ receptive fields to new locations in the world.
- Saccade
A rapid gaze shift redirecting the fovea (and thus, high-acuity vision) to a new location in space.
- Saccade neuron
Neuron that is silent during fixation and fires in relation to saccades to a particular location.
- Schizophrenia
A neurodevelopmental disorder with genetic and environmental causes whose typical onset is in early adulthood. Diagnostic symptoms include psychosis, blunted affect and motivation, and disorganized thought and language. Cognitive and sensorimotor dysfunction is also common.
- Smooth pursuit
Eye movements that keep a moving stimulus in the foveal region.
- Spatiotopic
organization of visual brain areas such that visual neurons in the brain have receptive fields encoding specific locations in external space, independent of gaze location.
- Thought disorder
symptom of psychosis in which disorganized thought becomes evident through disorganized language.
- Visual neurons
Neuron that fires in response to visual information in its receptive field.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Friston K. The free-energy principle: a rough guide to the brain? Trends Cogn Sci. 2009;13:293–301. doi: 10.1016/j.tics.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 2.Friston KJ, et al. Computational psychiatry: the brain as a phantastic organ. Lancet Psychiatry. 2014;1:148–158. doi: 10.1016/S2215-0366(14)70275-5. [DOI] [PubMed] [Google Scholar]
- 3.Corlett PR, et al. Prediction error, ketamine and psychosis: An updated model. J Psychopharmacol. 2016 doi: 10.1177/0269881116650087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fletcher PC, Frith CD. Perceiving is believing: a Bayesian approach to explaining the positive symptoms of schizophrenia. Nat Rev Neurosci. 2009;10:48–58. doi: 10.1038/nrn2536. [DOI] [PubMed] [Google Scholar]
- 5.Hemsley DR. An experimental psychological model for schizophrenia. In: Hafner H, et al., editors. Search for the causes of schizophrenia. Springer; 1987. pp. 179–188. [Google Scholar]
- 6.Pack CC. Eye movements as a probe of corollary discharge function in schizophrenia. ACS Chem Neurosci. 2014;5:326–328. doi: 10.1021/cn5000869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diefendorf AR, Dodge R. An experimental study of the ocular reactions of the insane from photographic records. Brain. 1908;31:451–489. [Google Scholar]
- 8.Cavanagh P, et al. Visual stability based on remapping of attention pointers. Trends Cogn Sci. 2010;14:147–153. doi: 10.1016/j.tics.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hallett PE, Lightstone AD. Saccadic eye movements towards stimuli triggered by prior saccades. Vision Res. 1976;16:99–106. doi: 10.1016/0042-6989(76)90083-3. [DOI] [PubMed] [Google Scholar]
- 10.Hallett PE, Lightstone AD. Saccadic eye movements to flashed targets. Vision Res. 1976;16:107–114. doi: 10.1016/0042-6989(76)90084-5. [DOI] [PubMed] [Google Scholar]
- 11.Lisberger SG, et al. Visual motion processing and sensory-motor integration for smooth pursuit eye movements. Annu Rev Neurosci. 1987;10:97–129. doi: 10.1146/annurev.ne.10.030187.000525. [DOI] [PubMed] [Google Scholar]
- 12.Bahcall DO, Kowler E. The control of saccadic adaptation: implications for the scanning of natural visual scenes. Vision Res. 2000;40:2779–2796. doi: 10.1016/s0042-6989(00)00117-6. [DOI] [PubMed] [Google Scholar]
- 13.Duhamel JR, et al. The updating of the representation of visual space in parietal cortex by intended eye movements. Science. 1992;255:90–92. doi: 10.1126/science.1553535. [DOI] [PubMed] [Google Scholar]
- 14.Sommer MA, Wurtz RH. A pathway in primate brain for internal monitoring of movements. Science. 2002;296:1480–1482. doi: 10.1126/science.1069590. [DOI] [PubMed] [Google Scholar]
- 15.Sommer MA, Wurtz RH. What the brain stem tells the frontal cortex. II. Role of the SC-MD-FEF pathway in corollary discharge. J Neurophysiol. 2004;91:1403–1423. doi: 10.1152/jn.00740.2003. [DOI] [PubMed] [Google Scholar]
- 16.Sommer MA, Wurtz RH. Influence of the thalamus on spatial visual processing in frontal cortex. Nature. 2006;444:374–377. doi: 10.1038/nature05279. [DOI] [PubMed] [Google Scholar]
- 17.Thakkar KN, et al. Disrupted Saccadic Corollary Discharge in Schizophrenia. J Neurosci. 2015;35:9935–9945. doi: 10.1523/JNEUROSCI.0473-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levy DL, et al. Eye tracking dysfunction in schizophrenia: characterization and pathophysiology. Curr Top Behav Neurosci. 2010;4:311–347. doi: 10.1007/7854_2010_60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hong LE, et al. Components of the smooth pursuit function in deficit and nondeficit schizophrenia. Schizophr Res. 2003;63:39–48. doi: 10.1016/s0920-9964(02)00388-2. [DOI] [PubMed] [Google Scholar]
- 20.Hong LE, et al. Response to unexpected target changes during sustained visual tracking in schizophrenic patients. Exp Brain Res. 2005;165:125–131. doi: 10.1007/s00221-005-2276-z. [DOI] [PubMed] [Google Scholar]
- 21.Thaker GK, et al. Smooth pursuit eye movements to extra-retinal motion signals: deficits in patients with schizophrenia. Psychiatry Res. 1999;88:209–219. doi: 10.1016/s0165-1781(99)00084-0. [DOI] [PubMed] [Google Scholar]
- 22.Hong LE, et al. Specific motion processing pathway deficit during eye tracking in schizophrenia: a performance-matched functional magnetic resonance imaging study. Biol Psychiatry. 2005;57:726–732. doi: 10.1016/j.biopsych.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 23.Hong LE, et al. Refining the predictive pursuit endophenotype in schizophrenia. Biol Psychiatry. 2008;63:458–464. doi: 10.1016/j.biopsych.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Picard HJ, et al. Correlates between neurological soft signs and saccadic parameters in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:676–681. doi: 10.1016/j.pnpbp.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 25.Lencer R, et al. Instability of visual error processing for sensorimotor adaptation in schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2016 doi: 10.1007/s00406-016-0716-3. [DOI] [PubMed] [Google Scholar]
- 26.Coesmans M, et al. Cerebellar motor learning deficits in medicated and medication-free men with recent-onset schizophrenia. J Psychiatry Neurosci. 2014;39:E3–11. doi: 10.1503/jpn.120205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McDowell JE, Clementz BA. The effect of fixation condition manipulations on antisaccade performance in schizophrenia: studies of diagnostic specificity. Exp Brain Res. 1997;115:333–344. doi: 10.1007/pl00005702. [DOI] [PubMed] [Google Scholar]
- 28.Mazhari S, et al. Revisiting the suitability of antisaccade performance as an endophenotype in schizophrenia. Brain Cogn. 2011;77:223–230. doi: 10.1016/j.bandc.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 29.Waters F, et al. Electrophysiological brain activity and antisaccade performance in schizophrenia patients with first-rank (passivity) symptoms. Psychiatry Res. 2009;170:140–149. doi: 10.1016/j.psychres.2008.10.033. [DOI] [PubMed] [Google Scholar]
- 30.Brownstein J, et al. Antisaccade performance is abnormal in schizophrenia patients but not in their biological relatives. Schizophr Res. 2003;63:13–25. doi: 10.1016/s0920-9964(02)00438-3. [DOI] [PubMed] [Google Scholar]
- 31.Polli FE, et al. Reduced error-related activation in two anterior cingulate circuits is related to impaired performance in schizophrenia. Brain. 2008;131:971–986. doi: 10.1093/brain/awm307. [DOI] [PubMed] [Google Scholar]
- 32.Polli FE, et al. Schizophrenia patients show intact immediate error-related performance adjustments on an antisaccade task. Schizophr Res. 2006;82:191–201. doi: 10.1016/j.schres.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 33.Reuter B, et al. Antisaccade performance of schizophrenia patients: evidence of reduced task-set activation and impaired error detection. J Psychiatr Res. 2006;40:122–130. doi: 10.1016/j.jpsychires.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 34.Wurtz RH. Neuronal mechanisms of visual stability. Vision Res. 2008;48:2070–2089. doi: 10.1016/j.visres.2008.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lindner A, et al. Disorders of agency in schizophrenia correlate with an inability to compensate for the sensory consequences of actions. Curr Biol. 2005;15:1119–1124. doi: 10.1016/j.cub.2005.05.049. [DOI] [PubMed] [Google Scholar]
- 36.Spering M, et al. Efference copy failure during smooth pursuit eye movements in schizophrenia. J Neurosci. 2013;33:11779–11787. doi: 10.1523/JNEUROSCI.0578-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deubel H, et al. Postsaccadic target blanking prevents saccadic suppression of image displacement. Vision Res. 1996;36:985–996. doi: 10.1016/0042-6989(95)00203-0. [DOI] [PubMed] [Google Scholar]
- 38.Collins T, et al. Post-saccadic location judgments reveal remapping of saccade targets to non-foveal locations. J Vis. 2009;9:29, 21–29. doi: 10.1167/9.5.29. [DOI] [PubMed] [Google Scholar]
- 39.Rosler L, et al. Failure to use corollary discharge to remap visual target locations is associated with psychotic symptom severity in schizophrenia. J Neurophysiol. 2015;114:1129–1136. doi: 10.1152/jn.00155.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Richard A, et al. Perisaccadic perception of visual space in people with schizophrenia. J Neurosci. 2014;34:4760–4765. doi: 10.1523/JNEUROSCI.4744-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ross J, et al. Compression of visual space before saccades. Nature. 1997;386:598–601. doi: 10.1038/386598a0. [DOI] [PubMed] [Google Scholar]
- 42.Pola J. An explanation of perisaccadic compression of visual space. Vision Res. 2011;51:424–434. doi: 10.1016/j.visres.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 43.Zimmermann E, et al. Perifoveal spatial compression. J Vis. 2013;13:21. doi: 10.1167/13.5.21. [DOI] [PubMed] [Google Scholar]
- 44.Ostendorf F, et al. Perisaccadic mislocalization without saccadic eye movements. Neuroscience. 2006;137:737–745. doi: 10.1016/j.neuroscience.2005.09.032. [DOI] [PubMed] [Google Scholar]
- 45.Ivleva EI, et al. Smooth pursuit eye movement, prepulse inhibition, and auditory paired stimuli processing endophenotypes across the schizophrenia-bipolar disorder psychosis dimension. Schizophr Bull. 2014;40:642–652. doi: 10.1093/schbul/sbt047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moates AF, et al. Predictive pursuit association with deficits in working memory in psychosis. Biol Psychiatry. 2012;72:752–757. doi: 10.1016/j.biopsych.2012.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kattoulas E, et al. Predictive smooth eye pursuit in a population of young men: II. Effects of schizotypy, anxiety and depression. Exp Brain Res. 2011;215:219–226. doi: 10.1007/s00221-011-2888-4. [DOI] [PubMed] [Google Scholar]
- 48.Malassis R, et al. Corollary Discharge Failure in an Oculomotor Task Is Related to Delusional Ideation in Healthy Individuals. PLoS One. 2015;10:e0134483. doi: 10.1371/journal.pone.0134483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thaker GK, et al. A model of smooth pursuit eye movement deficit associated with the schizophrenia phenotype. Psychophysiology. 2003;40:277–284. doi: 10.1111/1469-8986.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thaker GK, et al. Smooth pursuit eye movements to extraretinal motion signals: deficits in relatives of patients with schizophrenia. Arch Gen Psychiatry. 1998;55:830–836. doi: 10.1001/archpsyc.55.9.830. [DOI] [PubMed] [Google Scholar]
- 51.Cannon TD, et al. Prediction of psychosis in youth at high clinical risk: a multisite longitudinal study in North America. Arch Gen Psychiatry. 2008;65:28–37. doi: 10.1001/archgenpsychiatry.2007.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Javitt DC. Sensory processing in schizophrenia: neither simple nor intact. Schizophr Bull. 2009;35:1059–1064. doi: 10.1093/schbul/sbp110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Butler PD, et al. Visual perception and its impairment in schizophrenia. Biol Psychiatry. 2008;64:40–47. doi: 10.1016/j.biopsych.2008.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nuechterlein KH. Reaction time and attention in schizophrenia: a critical evaluation of the data and theories. Schizophr Bull. 1977;3:373–428. doi: 10.1093/schbul/3.3.373. [DOI] [PubMed] [Google Scholar]
- 55.Chen Y, et al. Motion perception in schizophrenia. Arch Gen Psychiatry. 1999;56:149–154. doi: 10.1001/archpsyc.56.2.149. [DOI] [PubMed] [Google Scholar]
- 56.Chen Y, et al. Psychophysical isolation of a motion-processing deficit in schizophrenics and their relatives and its association with impaired smooth pursuit. Proc Natl Acad Sci U S A. 1999;96:4724–4729. doi: 10.1073/pnas.96.8.4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Deubel H, et al. Landmarks facilitate visual space constancy across saccades and during fixation. Vision Res. 2010;50:249–259. doi: 10.1016/j.visres.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 58.Deubel H. Localization of targets across saccades: Role of landmark objects. Visual Cognition. 2004;11:173–202. [Google Scholar]
- 59.Barch DM, Ceaser A. Cognition in schizophrenia: core psychological and neural mechanisms. Trends in cognitive sciences. 2012;16:27–34. doi: 10.1016/j.tics.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee J, Park S. Working memory impairments in schizophrenia: a meta-analysis. J Abnorm Psychol. 2005;114:599–611. doi: 10.1037/0021-843X.114.4.599. [DOI] [PubMed] [Google Scholar]
- 61.Ohl S, Rolfs M. Saccadic Eye Movements Impose a Natural Bottleneck on Visual Short-Term Memory. J Exp Psychol Learn Mem Cogn. 2016 doi: 10.1037/xlm0000338. [DOI] [PubMed] [Google Scholar]
- 62.Bellebaum C, et al. The role of the human thalamus in processing corollary discharge. Brain. 2005;128:1139–1154. doi: 10.1093/brain/awh474. [DOI] [PubMed] [Google Scholar]
- 63.Gaymard B, et al. Impairment of extraretinal eye position signals after central thalamic lesions in humans. Exp Brain Res. 1994;102:1–9. doi: 10.1007/BF00232433. [DOI] [PubMed] [Google Scholar]
- 64.Ostendorf F, et al. Human thalamus contributes to perceptual stability across eye movements. Proc Natl Acad Sci U S A. 2010;107:1229–1234. doi: 10.1073/pnas.0910742107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cavanaugh J, et al. Saccadic Corollary Discharge Underlies Stable Visual Perception. J Neurosci. 2016;36:31–42. doi: 10.1523/JNEUROSCI.2054-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rolfs M, et al. Predictive remapping of attention across eye movements. Nat Neurosci. 2011;14:252–256. doi: 10.1038/nn.2711. [DOI] [PubMed] [Google Scholar]
- 67.Rolfs M, Szinte M. Remapping Attention Pointers: Linking Physiology and Behavior. Trends Cogn Sci. 2016;20:399–401. doi: 10.1016/j.tics.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 68.Hall NJ, Colby CL. Remapping for visual stability. Phil Trans R Soc B. 2011;366:528–539. doi: 10.1098/rstb.2010.0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zirnsak M, et al. Visual space is compressed in prefrontal cortex before eye movements. Nature. 2014;507:504–507. doi: 10.1038/nature13149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Neupane S, et al. Two distinct types of remapping in primate cortical area V4. Nat Commun. 2016;7:10402. doi: 10.1038/ncomms10402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rao HM, et al. Circuits for Presaccadic Visual Remapping. J Neurophysiol. 2016 doi: 10.1152/jn.00182.2016. jn 00182 02016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tanaka M. Involvement of the central thalamus in the control of smooth pursuit eye movements. J Neurosci. 2005;25:5866–5876. doi: 10.1523/JNEUROSCI.0676-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stone LS, Lisberger SG. Visual responses of Purkinje cells in the cerebellar flocculus during smooth-pursuit eye movements in monkeys. I. Simple spikes. J Neurophysiol. 1990;63:1241–1261. doi: 10.1152/jn.1990.63.5.1241. [DOI] [PubMed] [Google Scholar]
- 74.Newsome WT, et al. Relation of cortical areas MT and MST to pursuit eye movements. II. Differentiation of retinal from extraretinal inputs. J Neurophysiol. 1988;60:604–620. doi: 10.1152/jn.1988.60.2.604. [DOI] [PubMed] [Google Scholar]
- 75.Mustari MJ, et al. Signal processing and distribution in cortical-brainstem pathways for smooth pursuit eye movements. Ann N Y Acad Sci. 2009;1164:147–154. doi: 10.1111/j.1749-6632.2009.03859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Berman RA, Wurtz RH. Functional identification of a pulvinar path from superior colliculus to cortical area MT. J Neurosci. 2010;30:6342–6354. doi: 10.1523/JNEUROSCI.6176-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Berman RA, Wurtz RH. Signals conveyed in the pulvinar pathway from superior colliculus to cortical area MT. J Neurosci. 2011;31:373–384. doi: 10.1523/JNEUROSCI.4738-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Krauzlis RJ. Recasting the smooth pursuit eye movement system. J Neurophysiol. 2004;91:591–603. doi: 10.1152/jn.00801.2003. [DOI] [PubMed] [Google Scholar]
- 79.Modha DS, Singh R. Network architecture of the long-distance pathways in the macaque brain. Proc Natl Acad Sci U S A. 2010;107:13485–13490. doi: 10.1073/pnas.1008054107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Douglas RJ, Martin KA. Mapping the matrix: the ways of neocortex. Neuron. 2007;56:226–238. doi: 10.1016/j.neuron.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 81.Logothetis NK, et al. How not to study spontaneous activity. Neuroimage. 2009;45:1080–1089. doi: 10.1016/j.neuroimage.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 82.Sherman SM, Guillery RW. The role of the thalamus in the flow of information to the cortex. Philos Trans R Soc Lond B Biol Sci. 2002;357:1695–1708. doi: 10.1098/rstb.2002.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sherman SM. The thalamus is more than just a relay. Curr Opin Neurobiol. 2007;17:417–422. doi: 10.1016/j.conb.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Andreasen NC. A unitary model of schizophrenia: Bleuler’s “fragmented phrene” as schizencephaly. Arch Gen Psychiatry. 1999;56:781–787. doi: 10.1001/archpsyc.56.9.781. [DOI] [PubMed] [Google Scholar]
- 85.Glahn DC, et al. Meta-analysis of gray matter anomalies in schizophrenia: application of anatomic likelihood estimation and network analysis. Biol Psychiatry. 2008;64:774–781. doi: 10.1016/j.biopsych.2008.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tu PC, et al. Neural correlates of antisaccade deficits in schizophrenia, an fMRI study. J Psychiatr Res. 2006;40:606–612. doi: 10.1016/j.jpsychires.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 87.Dauvermann MR, et al. The application of nonlinear Dynamic Causal Modelling for fMRI in subjects at high genetic risk of schizophrenia. Neuroimage. 2013;73:16–29. doi: 10.1016/j.neuroimage.2013.01.063. [DOI] [PubMed] [Google Scholar]
- 88.Li T, et al. Brain-Wide Analysis of Functional Connectivity in First-Episode and Chronic Stages of Schizophrenia. Schizophr Bull. 2016 doi: 10.1093/schbul/sbw099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Woodward ND, et al. Thalamocortical dysconnectivity in schizophrenia. The American journal of psychiatry. 2012;169:1092–1099. doi: 10.1176/appi.ajp.2012.12010056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Frith CD. The positive and negative symptoms of schizophrenia reflect impairments in the perception and initiation of action. Psychol Med. 1987;17:631–648. doi: 10.1017/s0033291700025873. [DOI] [PubMed] [Google Scholar]
- 91.Feinberg I. Efference copy and corollary discharge: implications for thinking and its disorders. Schizophr Bull. 1978;4:636–640. doi: 10.1093/schbul/4.4.636. [DOI] [PubMed] [Google Scholar]
- 92.Feinberg I, Guazzelli M. Schizophrenia--a disorder of the corollary discharge systems that integrate the motor systems of thought with the sensory systems of consciousness. Br J Psychiatry. 1999;174:196–204. doi: 10.1192/bjp.174.3.196. [DOI] [PubMed] [Google Scholar]
- 93.Wolpert DM, Ghahramani Z. Computational principles of movement neuroscience. Nat Neurosci. 2000;3(Suppl):1212–1217. doi: 10.1038/81497. [DOI] [PubMed] [Google Scholar]
- 94.Graybiel AM. The basal ganglia and cognitive pattern generators. Schizophr Bull. 1997;23:459–469. doi: 10.1093/schbul/23.3.459. [DOI] [PubMed] [Google Scholar]
- 95.Pynn LK, DeSouza JF. The function of efference copy signals: implications for symptoms of schizophrenia. Vision Res. 2013;76:124–133. doi: 10.1016/j.visres.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 96.Ford JM, Mathalon DH. Electrophysiological evidence of corollary discharge dysfunction in schizophrenia during talking and thinking. J Psychiatr Res. 2004;38:37–46. doi: 10.1016/s0022-3956(03)00095-5. [DOI] [PubMed] [Google Scholar]
- 97.Ford JM, et al. Acquiring and inhibiting prepotent responses in schizophrenia: event-related brain potentials and functional magnetic resonance imaging. Arch Gen Psychiatry. 2004;61:119–129. doi: 10.1001/archpsyc.61.2.119. [DOI] [PubMed] [Google Scholar]
- 98.Ford JM, et al. Cortical responsiveness during inner speech in schizophrenia: an event-related potential study. Am J Psychiatry. 2001;158:1914–1916. doi: 10.1176/appi.ajp.158.11.1914. [DOI] [PubMed] [Google Scholar]
- 99.Ford JM, et al. Cortical responsiveness during talking and listening in schizophrenia: an event-related brain potential study. Biol Psychiatry. 2001;50:540–549. doi: 10.1016/s0006-3223(01)01166-0. [DOI] [PubMed] [Google Scholar]
- 100.Ford JM, et al. Neurophysiological evidence of corollary discharge dysfunction in schizophrenia. Am J Psychiatry. 2001;158:2069–2071. doi: 10.1176/appi.ajp.158.12.2069. [DOI] [PubMed] [Google Scholar]
- 101.Leudar I, et al. Self-monitoring in speech production: effects of verbal hallucinations and negative symptoms. Psychol Med. 1994;24:749–761. doi: 10.1017/s0033291700027902. [DOI] [PubMed] [Google Scholar]
- 102.Blakemore SJ, et al. Why can’t you tickle yourself? Neuroreport. 2000;11:R11–16. doi: 10.1097/00001756-200008030-00002. [DOI] [PubMed] [Google Scholar]
- 103.Blakemore SJ, et al. The perception of self-produced sensory stimuli in patients with auditory hallucinations and passivity experiences: evidence for a breakdown in self-monitoring. Psychol Med. 2000;30:1131–1139. doi: 10.1017/s0033291799002676. [DOI] [PubMed] [Google Scholar]
- 104.Shergill SS, et al. Two eyes for an eye: the neuroscience of force escalation. Science. 2003;301:187. doi: 10.1126/science.1085327. [DOI] [PubMed] [Google Scholar]
- 105.Shergill SS, et al. Evidence for sensory prediction deficits in schizophrenia. Am J Psychiatry. 2005;162:2384–2386. doi: 10.1176/appi.ajp.162.12.2384. [DOI] [PubMed] [Google Scholar]
- 106.Malenka RC, et al. Impaired central error-correcting behavior in schizophrenia. Arch Gen Psychiatry. 1982;39:101–107. doi: 10.1001/archpsyc.1982.04290010073013. [DOI] [PubMed] [Google Scholar]
- 107.Malenka RC, et al. Central error-correcting behavior in schizophrenia and depression. Biol Psychiatry. 1986;21:263–273. doi: 10.1016/0006-3223(86)90047-8. [DOI] [PubMed] [Google Scholar]
- 108.Wegner DM. Who is the controller of controlled processes? In: Hassin R, et al., editors. The New Unconscious. Oxford University Press; 2005. pp. 19–36. [Google Scholar]
- 109.van der Weiden A, et al. Self-other integration and distinction in schizophrenia: A theoretical analysis and a review of the evidence. Neurosci Biobehav Rev. 2015;57:220–237. doi: 10.1016/j.neubiorev.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 110.Becker W, Fuchs AF. Prediction in the oculomotor system: smooth pursuit during transient disappearance of a visual target. Exp Brain Res. 1985;57:562–575. doi: 10.1007/BF00237843. [DOI] [PubMed] [Google Scholar]
- 111.Newsome WT, et al. Relation of cortical areas MT and MST to pursuit eye movements. II. Differentiation of retinal from extraretinal inputs. J Neurophysiol. 1988;60:604–620. doi: 10.1152/jn.1988.60.2.604. [DOI] [PubMed] [Google Scholar]
- 112.Murthy A, et al. Frontal eye field contributions to rapid corrective saccades. J Neurophysiol. 2007;97:1457–1469. doi: 10.1152/jn.00433.2006. [DOI] [PubMed] [Google Scholar]
- 113.Ethier V, et al. Changes in control of saccades during gain adaptation. J Neurosci. 2008;28:13929–13937. doi: 10.1523/JNEUROSCI.3470-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chen-Harris H, et al. Adaptive control of saccades via internal feedback. J Neurosci. 2008;28:2804–2813. doi: 10.1523/JNEUROSCI.5300-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rolfs M, Carrasco M. Rapid simultaneous enhancement of visual sensitivity and perceived contrast during saccade preparation. J Neurosci. 2012;32:13744–13752a. doi: 10.1523/JNEUROSCI.2676-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Deubel H, Schneider WX. Saccade target selection and object recognition: evidence for a common attentional mechanism. Vision Res. 1996;36:1827–1837. doi: 10.1016/0042-6989(95)00294-4. [DOI] [PubMed] [Google Scholar]