Abstract
Background
Peri-coronary epicardial adipose tissue (cEAT) serves as a metabolic and paracrine organ that contributes to inflammation and is associated with macrovascular coronary artery disease (CAD) development. While there is a strong correlation in humans between cEAT volume and CAD severity, there remains a paucity of experimental data demonstrating a causal link of cEAT to CAD. The current study tested the hypothesis that surgical resection of cEAT attenuates inflammation and CAD progression.
Methods
Female Ossabaw miniature swine (n=12) were fed an atherogenic diet for 8 months and randomized into sham (n=5) or adipectomy (n=7) groups. Both groups underwent a thoracotomy, opening of the pericardial sac, and placement of radio-opaque clips to mark the proximal left anterior descending artery. Adipectomy swine underwent removal of 1–1.5 cm2 of cEAT from the proximal artery. Following sham or adipectomy, CAD severity was assessed with intravascular ultrasound. Swine recovered for an additional 3 months on atherogenic diet and CAD was assessed immediately prior to euthanasia. Artery sections were processed for histological and immunohistochemical analysis.
Results
CAD severity, as assessed by percent stenosis, was reduced in the adipectomy cohort compared to shams; however, plaque size remained unaltered, while sham-operated swine developed greater plaque sizes. Adipectomy resulted in an expanded arterial diameter, similar to the Glagov phenomenon of positive outward remodeling. No differences in inflammatory marker expression were observed.
Conclusions
These data indicate that cEAT resection did not alter inflammatory marker expression, but arrested CAD progression through increased positive outward remodeling and arrest of atherogenesis.
Classifications: Atherosclerosis, Coronary Artery Disease, Coronary Artery Imaging, Inflammatory Mediators, Obesity
Coronary epicardial adipose tissue (cEAT) is an adipose tissue depot located on the surface of the heart, encasing and interacting with the epicardial coronary arteries. Mounting evidence that cEAT serves as a metabolic and paracrine organ (1) provides an impetus for growing interest in cEAT as a causal factor in coronary artery disease (CAD) etiology. cEAT thickness and volume directly correlate with CAD severity in humans (1–10).
Metabolic syndrome (MetS), defined as the clustering of three of the following five risk factors: central obesity, hypertension, dyslipidemia, glucose intolerance, and insulin resistance, doubles the risk of developing CAD (11, 12). cEAT volume is increased in MetS (13–16), and the inflammatory adipokine profile is altered in cEAT during MetS and CAD (16–18). Thus, one of the mechanisms whereby MetS exacerbates CAD may be through effects of cEAT on the coronary vasculature.
Many human studies conducted on the link between cEAT and CAD development have been observational, showing a positive correlation between cEAT volume and CAD severity (2–7). There remains a need for experimental animal studies to determine a causal role of cEAT in CAD progression (16). To this end, we have developed an animal model, the Ossabaw miniature swine, which naturally recapitulates human components of MetS and CAD (19, 20). We previously examined the effect of cEAT removal from the middle segment of the left anterior descending coronary artery (LAD) in Ossabaw swine using an intra-artery control, demonstrating that CAD progression was attenuated in the surgical region, compared to adjacent regions (21). However, in the absence of sham-operated controls, questions remained regarding the control of surgery-induced inflammation, the robustness of the observations, and the nature of CAD attenuation. Reductions in coronary luminal encroachment may be explained by one of two vascular remodeling scenarios: 1) reductions in or curtailment of plaque size, or 2) enhancement of compensatory expansion of the coronary arterial wall, such as that classically described by Glagov, et al (22). Accordingly, we performed a sham-controlled study to test the hypothesis that cEAT removal attenuates CAD and is associated with outward vascular remodeling.
Material and Methods
Animal care and use
This protocol was approved by the Indiana University School of Medicine Animal Care and Use Committee and conducted in accordance with the Guide for the Care and Use of Laboratory Animals (23). Female Ossabaw miniature swine (n=12) were fed an excess-calorie, atherogenic diet for a total of 11 months (KT-324, Purina Test Diet, Richmond, IN; 16.3% kcal from protein, 40.8% kcal from complex carbohydrates, 19% kcal from fructose, and 42.9% kcal from fat; 1 kg once daily) supplemented with cholesterol (2.0%), hydrogenated coconut oil (4.70%), hydrogenated soybean oil (8.40%), cholate (0.70%), and high fructose corn syrup (5.0%) by weight, and consequently developed MetS and CAD, as previously described (20, 24, 25). All pigs were individually housed with a 12 hour light/dark cycle and free access to drinking water. Metabolic data were collected to confirm MetS and to identify any potential variables between the experimental and control groups (Table 1). After 8 months on diet, pigs were randomized to either an adipectomy (cEATx; n=7) or sham (n=5) group.
Table 1.
Metabolic Characteristics of Ossabaw Swine
| Sham (n=5) | cEATx (n=7) | P-value | ||
|---|---|---|---|---|
| Body Weight (kg) | Survival Surgery | 93.12 ± 1.36 | 88.09 ± 2.12 | 0.10 |
| Sacrifice | 110.14 ± 1.94 | 105.07 ± 3.30 | 0.26 | |
| Progression | 17.02* | 16.99* | 0.99 | |
| Total Cholesterol (mg/dL) | Survival | 471.80 ± 65.97 | 526.00 ± 56.71 | 0.55 |
| Sacrifice | 371.80 ± 79.41 | 533.00 ± 94.78 | 0.25 | |
| Progression | −100.00* | 7.00 | 0.10 | |
| Triglycerides (mg/dL) | Survival | 36.20 ± 7.21 | 38.57 ± 6.83 | 0.82 |
| Sacrifice | 55.20 ± 5.52 | 58.14 ± 7.35 | 0.77 | |
| Progression | 19.00 | 19.57 | 0.96 | |
| Fasting Glucose (mg/dL) | Survival | 79.74 ± 2.28 | 82.82 ± 2.66 | 0.42 |
| Systolic Blood Pressure (mmHg) | Survival | 155.94 ± 5.18 | 157.70 ± 3.49 | 0.77 |
| Diastolic Blood Pressure (mmHg) | Survival | 86.89 ± 4.27 | 82.74 ± 2.62 | 0.40 |
Indicates significant progression from survival to sacrifice, p < 0.05.
Anesthesia
After an overnight fast, anesthesia was induced with intramuscular administration of telazol (4.5–5.5 mg/kg) and xylazine (2.2 mg/kg). Following intubation, anesthesia was maintained with 2–4% isoflurane and 100% O2. Isoflurane levels were adjusted to maintain appropriate heart rate, blood pressure, respiratory rate, and cardiac electrical activity throughout the procedure.
Adipectomy
Following local anesthesia with lidocaine, a left-sided thoracotomy was performed and the lung was retracted to expose the heart. An incision was made in the pericardial sac above the region of the proximal LAD. Approximately 1–1.5 cm2 of cEAT was excised from the proximal LAD and radio-opaque ligation clips were placed around the surgical site. These clips confirmed the interrogated tissue upon euthanasia and tissue collection (Figure 1). Sham pigs also underwent a thoracotomy, opening of the pericardial sac, and placement of the ligation clips in efforts to control variables between groups.
Figure 1. Protocol for removal of coronary epicardial adipose tissue.
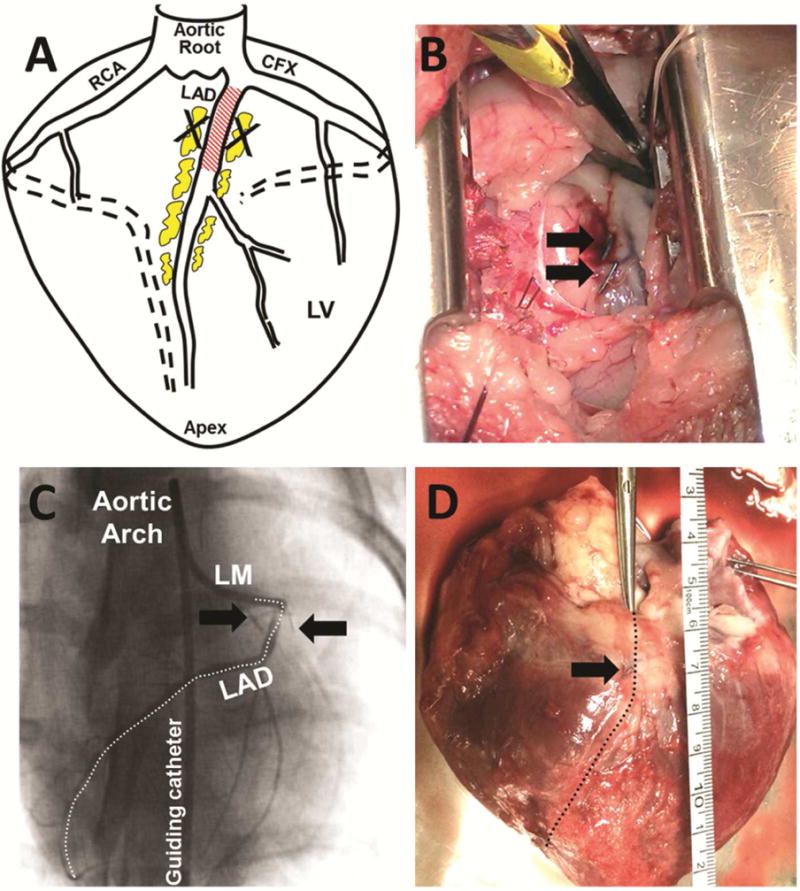
A) Diagram of coronary arteries depicting region of cEAT removal. B) Following removal of cEAT, radio-opaque ligation clips (black arrows) were placed near the surgery site. C) Representative coronary angiogram obtained following the adipectomy procedure. Radio-opaque ligation clips are visible markers of the adipectomy / sham site (black arrows). D) Following euthanasia, adipectomy site was identified by location of ligation clips (black arrow). CFX = Circumflex artery; LAD = Left anterior descending coronary artery; RCA = Right coronary artery; LM = left main coronary artery; LV = Left ventricle.
Intravascular Ultrasound imaging
Following the adipectomy or sham procedure, intravascular ultrasound (IVUS) imaging was conducted in the left anterior descending (LAD) as previously described (20, 21, 25), using a 45 MHz IVUS catheter (Volcano Corp., Rancho Cordova, CA). IVUS was repeated 3 months post-adipectomy prior to euthanasia.
IVUS analysis was performed by a single observer blinded to experimental group and time point. Location within each artery was determined by identification of the left main coronary artery bifurcation and IVUS still-frames were analyzed in the proximal 15 mm of the LAD utilizing ImageJ (NIH) software as previously described (19, 26, 27). External elastic lamina (EEL) cross-sectional area is indicated with a red dashed line in Figure 2A. Lumen cross-sectional area is indicated with the yellow dotted line in Figure 2A. Percent stenosis quantifies the percentage of the area within the EEL that is occupied by atherosclerotic plaque, using the formula (EEL area – lumen area)/EEL area*100%. We assessed percent stenosis progression with the equation Percent StenosisSacrifice – Percent StenosisSurvivaL Outward remodeling was assessed by examining the change in EEL area from survival to sacrifice. Unadjusted plaque area at survival surgery and at sacrifice was assessed with the following formula: EEL area – lumen area.
Figure 2. Removal of coronary epicardial adipose tissue attenuates coronary artery disease progression.
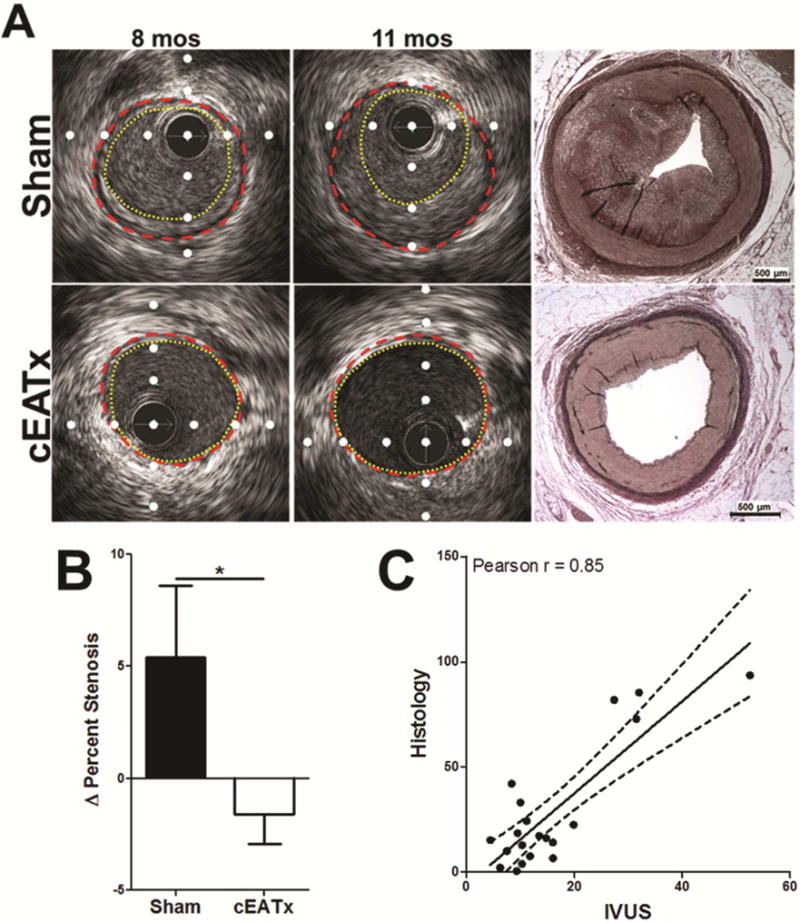
A) Representative IVUS still frames at survival surgery (8 mos) and euthanasia (11 mos). IVUS was verified with Verhoeff-Van Gieson histology. Red dashed line = external elastic lamina; Yellow dotted line = lumen; white dots are placed 1 mm apart. B) Removal of cEAT significantly attenuates CAD progression, as assessed by percent stenosis (p < 0.05). C) IVUS measurement of percent stenosis correlates with histological measurement of percent stenosis, verifying IVUS assessment of CAD severity.
Histology and immunohistochemistry
Specimens were collected from the healed surgical site of adipectomy pigs and from the corresponding region of the LAD of sham pigs, identified with clips placed in cEAT during survival surgery. The surrounding myocardium, adventitia, and adipose tissue were left intact, the specimen was fixed in 10% phosphate-buffered formalin for 24–48 hours, transferred to 70% ethanol, and embedded in paraffin. The Verhoeff-Van Gieson stain positively identified the elastic laminae (Figure 2A: far right images), allowing us to quantify percent stenosis. The results were compared between experimental and sham groups as well as with the IVUS percent stenosis data to confirm our in vivo technique. Immunohistochemistry was performed by the pathology core at Indiana University School of Medicine. Cell proliferation was quantified by positive Ki-67 staining of cells within the intima and expressed as a percentage of total cell count. Inflammation was assessed with macrophage scavenger receptor-A (1:100 dilution, Cosmo Bio USA, Inc. #KAL-KT022) and T-cadherin (1:1000 dilution, Abgent #CDH13, AP14346) as previously described (21) and expressed as a percentage of total area.
Statistics
Data are described as mean ± standard error. Statistical significance was set at p<0.05. Paired, one-tailed Student’s t-tests and unpaired one-tailed Student’s t-tests were conducted where appropriate using PRISM software (GraphPad Software Inc., La Jolla, CA).
Results
Metabolic data
All pigs continuously gained weight throughout the study and upon sacrifice there was no difference in body weights across groups. Assessment of total cholesterol, triglycerides, fasting blood glucose, and blood pressures revealed no metabolic differences between sham and cEATx pigs at euthanasia (Table 1).
Percent stenosis
Change in percent stenosis over the 3 month recovery period was significantly higher in the proximal LAD of sham pigs compared to cEATx (Figure 2B). Sham pigs displayed a positive change in percent stenosis, while a negative change in percent stenosis was seen in cEATx pigs.
The validity of our in vivo IVUS assessment of percent stenosis was confirmed by its significant correlation (r = 0.85) with histological measure of percent stenosis (Figure 2C).
Cell proliferation and plaque area
To investigate the apparent plaque regression of cEATx pigs, intimai Ki-67 positive cells were quantified. LAD segments from sham pigs demonstrated significantly more proliferating cells compared to cEATx pigs; however, both groups revealed evidence of cell proliferation (Figure 3 A–C).
Figure 3. Coronary epicardial adipose tissue removal does not alter plaque size, but attenuates intimal cell proliferation.
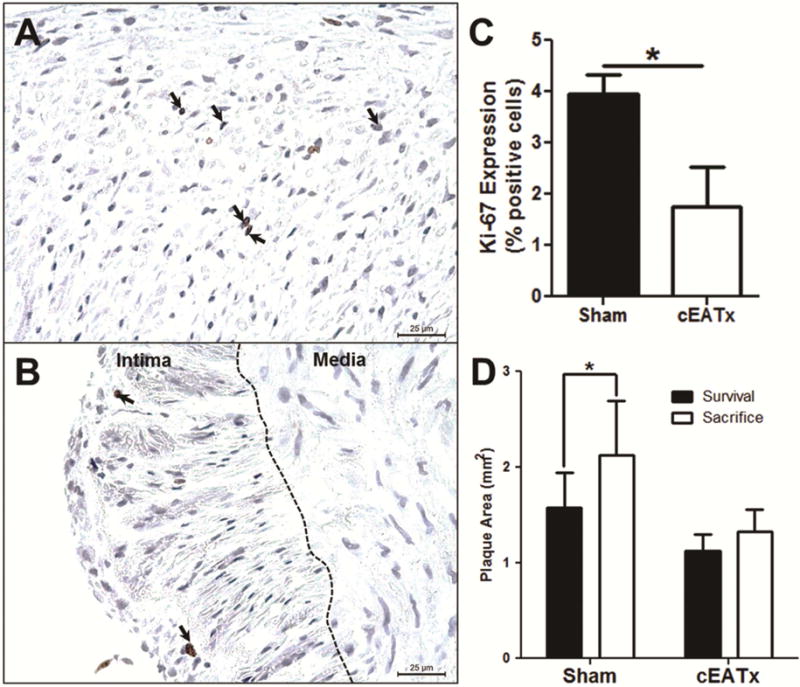
A) Representative sham-operated intimal section stained with Ki-67. Black arrows indicate positive staining. B) Representative cEATx intimal section stained with Ki-67. Black arrows indicate positive staining. C) Intimal cell proliferation reduced in cEATx arteries, compared to shams (p < 0.05). D) Plaque area significantly increased following survival procedure in sham-operated animals (p < 0.05), but was unchanged in cEATx swine (p = 0.2).
To directly assess changes in plaque size independent of vessel diameter, the unadjusted area of the plaque was examined. Plaque area in sham pigs significantly increased in size over the 3 month recovery period, while size of plaque in the analogous LAD segment of cEATx pigs was unchanged (Figure 3D).
Inflammatory marker expression
Expression of macrophage scavenger receptor A and T-cadherin were examined within atherosclerotic plaques and in surrounding adipose tissue. Removal of cEAT did not alter inflammatory marker expression in either region (Figure 4).
Figure 4. Expression of inflammatory markers is unaltered by removal of epicardial adipose tissue.
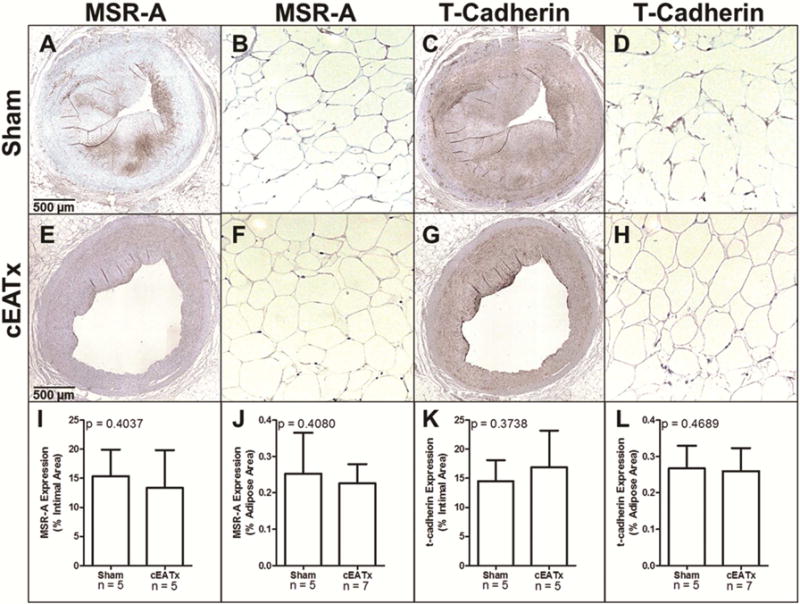
A–H) Representative images of macrophage scavenger receptor-A (MSR-A) and t-cadherin stained arterial (A,C,E,G) and adipose (B,D,F,H) sections from sham-operated (A–D) and cEATx-operated (E–H) swine. I) MSR-A expression within atherosclerotic plaques is unchanged by cEATx operation J) MSR-A expression within adipose tissue is unchanged by cEATx operation. K) T-cadherin expression within atherosclerotic plaques is unchanged by cEATx operation. L) T-cadherin expression within adipose tissue is unchanged by cEATx operation.
Outward remodeling
At euthanasia, cEATx pigs demonstrated a greater EEL area as assessed with IVUS, compared to shams (Figure 5A). To determine whether this increase in EEL area was compensatory outward remodeling, we examined the correlation between EEL and plaque size at both survival and sacrifice. At survival, there was no difference between this correlation across groups (Figure 5B). However, at the time of sacrifice (3 months after the procedure), cEATx pigs demonstrated a significantly more positive correlation compared to sham pigs (Figure 5C). The slopes of the regression lines are graphically represented in Figure 5D. In sham-operated controls, correlation of arterial expansion (change in EEL area) with plaque growth is reduced at sacrifice, compared to survival surgery. However, in cEATx-operated swine, arterial expansion during plaque growth was maintained from survival to sacrifice.
Figure 5. The Glagov phenomenon is potentiated with coronary epicardial adipose tissue resection.
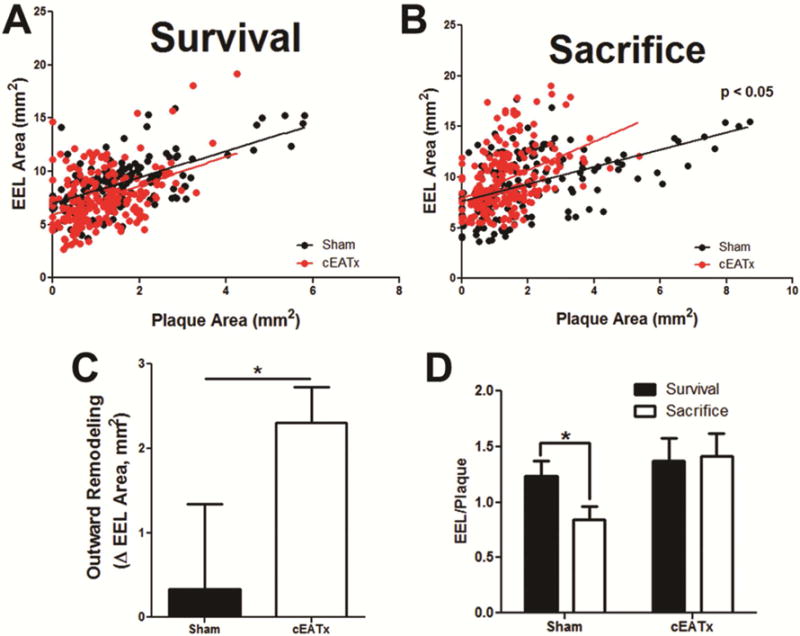
A – B) Removal of cEAT shifts the relationship between external elastic lamina (EEL) area and plaque area, thus potentiating outward remodeling during plaque growth. C) Outward remodeling, as assessed by the change in EEL area from survival to sacrifice, was increased in cEATx swine, compared to shams (p < 0.05). D) Graphical representation of the EEL/Plaque area slope. Outward remodeling is reduced with CAD progression in sham-operated swine (p < 0.05), but is preserved following cEATx (p = 0.4).
As a final measure of the effect of compensatory outward remodeling, the lumen area was compared across groups and time points. At the site of surgical intervention, the lumen area in cEATx pigs significantly increased from survival to sacrifice, but was unaltered in sham pigs (Figure 6).
Figure 6. Removal of coronary epicardial adipose tissue results in significant outward remodeling and increases lumen diameter.
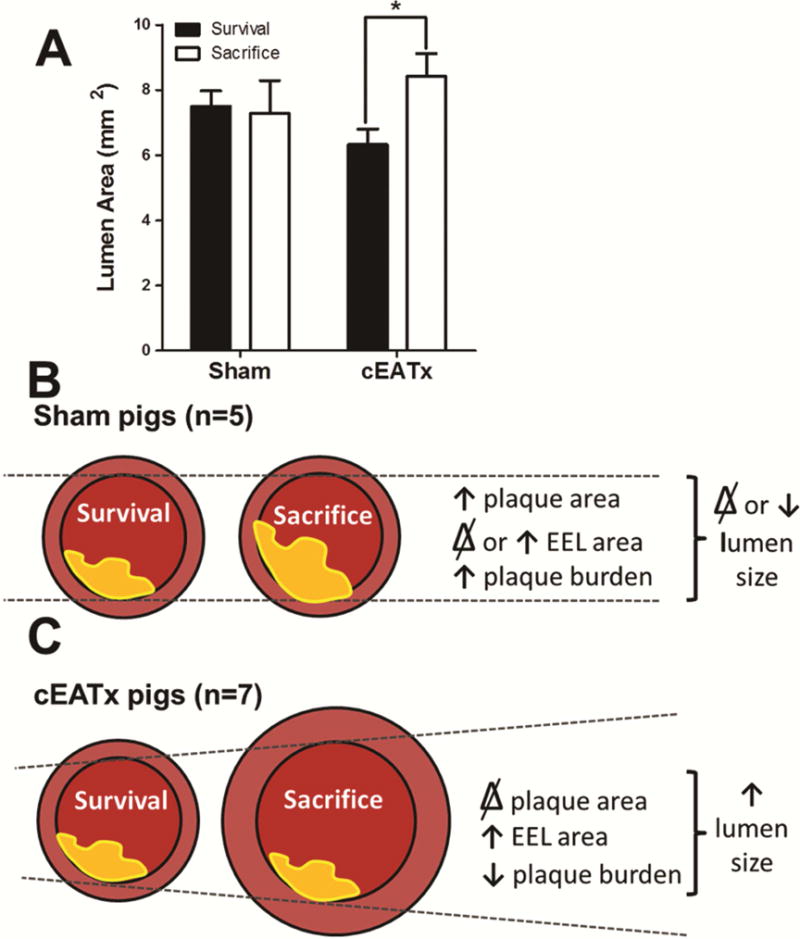
A) cEATx animals experienced an expansion in lumen size following surgery (p < 0.05). This expansion was not observed in sham-operated animals (p = 0.4). B) During normal (sham) CAD progression, plaque area increased with little change in EEL area, resulting in increased coronary stenosis. C) Following cEAT removal, significant outward remodeling is observed as an increase in EEL area. This, combined with inhibition of increases in plaque size, results in an increased lumen area, thus reducing percent stenosis.
Comment
We examined the effect of surgical removal of cEAT on CAD progression in vivo. We confirm that selective segmental cEAT resection in Ossabaw swine with MetS arrests atherogenesis, measured by percent stenosis (Fig 2). The measure of percent stenosis assesses plaque size as a function of external elastic lamina area. Thus, to determine whether the decreased percent stenosis associated with the cEATx cohort was due to reduction in plaque size or to other factors, we examined plaque cell proliferation and plaque size. Plaques in the cEATx group displayed less plaque cell proliferation, compared to sham-operated controls (Fig 3C). Plaque size increased in sham animals, but remained unchanged in cEATx animals (Fig 3D). Thus, we concluded that the reduced percent stenosis observed in the cEATx cohort was not due to plaque regression.
The arrest of atherogenesis is associated with expansion of the coronary segment lacking overlying cEAT (Fig 4C). This enlargement of coronary arterial lumen is reminiscent of the Glagov phenomenon (22). Fig 6B–C summarizes our findings, demonstrating that a Glagov-like phenomenon without plaque regression, is responsible for the luminal expansion observed in the cEATx cohort.
Hypothetically, cEAT might act as a scaffold, tethering the coronary artery to the epicardial surface and limiting movement during the cardiac cycle. The contiguity of cEAT with coronary arterial adventitia without an intervening fibrous layer (2, 8, 17, 28) might also restrict outward dilation of the proximal conduit coronary artery during diastole. Alternatively, cEAT from obese Ossabaw pigs expresses and secretes vasoconstrictors (29–31) which when removed might result in tonic vasodilation. It cannot be determined from our work whether the expansion of the EEL is due to outward medial enlargement or to enhanced vasodilation.
Molecular mechanisms for the arrest of atherogenesis have not been elucidated in our study. It is established that cEAT overlying atheromatous plaques contains dense numbers of inflammatory macrophages and lymphocytes and secretes pro-atherogenic cytokines (18, 32–37). Adipectomy might remove inflammatory cells and cytokines (21), thereby halting migration from outside the adventitia into the intima-media and reducing plaque expansion. However, we did not find any difference in some markers of atherogenesis in both the intima and cEAT layers of the adipectomised versus sham LAD segment. This suggests the removal of outside-to-inside paracrine factors less likely accounted for the beneficial anti-atherosclerotic effect; however, because of the limited number of inflammatory factors interrogated, it does not rule out a paracrine/vasocrine effect of cEAT on atherogenesis. Future studies should undertake a comprehensive examination of inflammatory gene expression to further elucidate an underlying mechanism for the role of cEAT in promoting atherogenesis. These paracrine/vasocrine factors should be distinguished from the role of cEAT vs. myocardium in mechanically compressing the coronary artery and restricting outward remodeling (38). Wall shear stress is important in enhancing endothelial function and halting atherogenesis (39, 40). Although we have no direct evidence, there is the possibility that in the cEATx vessel, shear stress was modified by luminal expansion. In our previous study (21), the manipulated LAD segment showed immunohistochemical evidence of less inflammation compared to the distal and proximal sections of the same LAD. The reasons for this lack of concordance between our prior and this current study are unclear, but a likely explanation is that the level of overall inflammatory marker expression was higher in the current study because the surgical sham group was included. Another possibility is a different effect of gender on the inflammatory response, since our previous study was on castrated males vs. females in this study.
Our current findings address the consideration raised in our earlier study (21) that cEAT adipectomy at the time of coronary artery bypass grafting might be used as adjunctive treatment to reduce the progression of atheroma post-grafting. Clearly, our results only apply to an early phase in the natural history of CAD without clinically significant luminal stenoses. In contrast, patients undergoing bypass grafting present with late stages of CAD who have substantial, flow-limiting stenoses (41). It will be of interest to investigate whether lifestyle modifications such as weight loss will result in similar cEAT mass/volume reduction, as this would be a safer and more appropriate strategy than invasive adipectomy.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Salazar J, Luzardo E, Mejias JC, et al. Epicardial fat: Physiological, pathological, and therapeutic implications. Cardiol Res Pract. 2016;2016:1291537. doi: 10.1155/2016/1291537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Erkan AF, Tanindi A, Kocaman SA, Ugurlu M, Tore HF. Epicardial adipose tissue thickness is an independent predictor of critical and complex coronary artery disease by gensini and syntax scores. Tex Heart Inst J. 2016;43(1):29–37. doi: 10.14503/THIJ-14-4850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang TD, Lee WJ, Shih FY, et al. Association of epicardial adipose tissue with coronary atherosclerosis is region-specific and independent of conventional risk factors and intra-abdominal adiposity. Atherosclerosis. 2010;213(1):279–287. doi: 10.1016/j.atherosclerosis.2010.07.055. [DOI] [PubMed] [Google Scholar]
- 4.Alexopoulos N, McLean DS, Janik M, Arepalli CD, Stillman AE, Raggi P. Epicardial adipose tissue and coronary artery plaque characteristics. Atherosclerosis. 2010;210(1):150–154. doi: 10.1016/j.atherosclerosis.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 5.Lu MT, Park J, Ghemigian K, et al. Epicardial and paracardial adipose tissue volume and attenuation - association with high-risk coronary plaque on computed tomographic angiography in the romicat ii trial. Atherosclerosis. 2016;251:47–54. doi: 10.1016/j.atherosclerosis.2016.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mahabadi AA, Berg MH, Lehmann N, et al. Association of epicardial fat with cardiovascular risk factors and incident myocardial infarction in the general population: The heinz nixdorf recall study. J Am Coll Cardiol. 2013;61(13):1388–1395. doi: 10.1016/j.jacc.2012.11.062. [DOI] [PubMed] [Google Scholar]
- 7.Okada K, Ohshima S, Isobe S, et al. Epicardial fat volume correlates with severity of coronary artery disease in nonobese patients. J Cardiovasc Med (Hagerstown) 2014;15(5):384–390. doi: 10.2459/JCM.0b013e32836094da. [DOI] [PubMed] [Google Scholar]
- 8.Ouwens DM, Sell H, Greulich S, Eckel J. The role of epicardial and perivascular adipose tissue in the pathophysiology of cardiovascular disease. J Cell Mol Med. 2010;14(9):2223–2234. doi: 10.1111/j.1582-4934.2010.01141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Verhagen SN, Visseren FL. Perivascular adipose tissue as a cause of atherosclerosis. Atherosclerosis. 2011;214(1):3–10. doi: 10.1016/j.atherosclerosis.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 10.Iozzo P. Myocardial, perivascular, and epicardial fat. Diabetes Care. 2011;34(Suppl 2):S371–379. doi: 10.2337/dc11-s250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: A joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood institute; american heart association; world heart federation; international atherosclerosis society; and international association for the study of obesity. Circulation. 2009;120(16):1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 12.Grundy SM. Pre-diabetes, metabolic syndrome, and cardiovascular risk. J Am Coll Cardiol. 2012;59(7):635–643. doi: 10.1016/j.jacc.2011.08.080. [DOI] [PubMed] [Google Scholar]
- 13.Gorter PM, van Lindert AS, de Vos AM, et al. Quantification of epicardial and peri-coronary fat using cardiac computed tomography; reproducibility and relation with obesity and metabolic syndrome in patients suspected of coronary artery disease. Atherosclerosis. 2008;197(2):896–903. doi: 10.1016/j.atherosclerosis.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 14.Yorgun H, Canpolat U, Hazirolan T, et al. Increased epicardial fat tissue is a marker of metabolic syndrome in adult patients. Int J Cardiol. 2013;165(2):308–313. doi: 10.1016/j.ijcard.2011.08.067. [DOI] [PubMed] [Google Scholar]
- 15.Okyay K, Balcioglu AS, Tavil Y, Tacoy G, Turkoglu S, Abaci A. A relationship between echocardiographic subepicardial adipose tissue and metabolic syndrome. Int J Cardiovasc Imaging. 2008;24(6):577–583. doi: 10.1007/s10554-008-9295-3. [DOI] [PubMed] [Google Scholar]
- 16.Sacks HS, Fain JN. Human epicardial fat: What is new and what is missing? Clin Exp Pharmacol Physiol. 2011;38(12):879–887. doi: 10.1111/j.1440-1681.2011.05601.x. [DOI] [PubMed] [Google Scholar]
- 17.Payne GA, Kohr MC, Tune JD. Epicardial perivascular adipose tissue as a therapeutic target in obesity-related coronary artery disease. BrJPharmacol. 2012;165(3):659–669. doi: 10.1111/j.1476-5381.2011.01370.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lau DC, Dhillon B, Yan H, Szmitko PE, Verma S. Adipokines: Molecular links between obesity and atheroslcerosis. Am J Physiol Heart Circ Physiol. 2005;288(5):H2031–H2041. doi: 10.1152/ajpheart.01058.2004. [DOI] [PubMed] [Google Scholar]
- 19.McKenney-Drake ML, Rodenbeck SD, Owen MK, et al. Biphasic alterations in coronary smooth muscle ca2+ regulation in a repeat cross-sectional study of coronary artery disease severity in metabolic syndrome. Atherosclerosis. 2016;249:1–9. doi: 10.1016/j.atherosclerosis.2016.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neeb ZP, Edwards JM, Alloosh M, Long X, Mokelke EA, Sturek M. Metabolic syndrome and coronary artery disease in ossabaw compared with yucatan swine. Comp Med. 2010;60(4):300–315. [PMC free article] [PubMed] [Google Scholar]
- 21.McKenney ML, Schultz KA, Boyd JH, et al. Epicardial adipose excision slows the progression of porcine coronary atherosclerosis. J Cardiothorac Surg. 2014;9:2–12. doi: 10.1186/1749-8090-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glagov S, Weisenberg E, Zarins CK, Stankunavicius R, Kolettis GJ. Compensatory enlargement of human atherosclerotic coronary arteries. N Engl J Med. 1987;316(22):1371–1375. doi: 10.1056/NEJM198705283162204. [DOI] [PubMed] [Google Scholar]
- 23.Institute for Laboratory. Animal Research Guide for the care and use of laboratory animals. Washington, D.C: National Academy Press; 2010. [Google Scholar]
- 24.Dyson MC, Alloosh M, Vuchetich JP, Mokelke EA, Sturek M. Components of metabolic syndrome and coronary artery disease in female ossabaw swine fed excess atherogenic diet. Comp Med. 2006;56(1):35–45. [PubMed] [Google Scholar]
- 25.Sturek M, Tune JD, Alloosh M. Ossabaw island miniature swine: Metabolic syndrome and cardiovascular assessment. In: Swindle MM, editor. Swine in the laboratory: Surgery, anesthesia, imaging, and experimental techniques. 3rd. Boca Raton: CRC Press; 2015. pp. 451–465. [Google Scholar]
- 26.Phillips-Eakley AK, McKenney-Drake ML, Bahls M, et al. Effect of high-calcium diet on coronary artery disease in ossabaw miniature swine with metabolic syndrome. JAHA. 2015;4 doi: 10.1161/JAHA.114.001620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mintz GS, Nissen SE, Anderson WD, et al. American college of cardiology clinical expert consensus document on standards for acquisition, measurement and reporting of intravascular ultrasound studies (ivus). A report of the american college of cardiology task force on clinical expert consensus documents. J Am Coll Cardiol. 2001;37(5):1478–1492. doi: 10.1016/s0735-1097(01)01175-5. [DOI] [PubMed] [Google Scholar]
- 28.Iacobellis G, Corradi D, Sharma AM. Epicardial adipose tissue: Anatomic, biomolecular and clinical relationships with the heart. Nat Clin Pract Cardiovasc Med. 2005;2(10):536–543. doi: 10.1038/ncpcardio0319. [DOI] [PubMed] [Google Scholar]
- 29.Owen MK, Witzmann FA, McKenney ML, et al. Perivascular adipose tissue potentiates contraction of coronary vascular smooth muscle: Influence of obesity. Circulation. 2013;128:9–18. doi: 10.1161/CIRCULATIONAHA.112.001238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Noblet JN, Owen MK, Goodwill AG, Sassoon DJ, Tune JD. Lean and obese coronary perivascular adipose tissue impairs vasodilation via differential inhibition of vascular smooth muscle k+ channels. Arterioscler Thromb Vasc Biol. 2015;35(6):1393–1400. doi: 10.1161/ATVBAHA.115.305500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Noblet JN, Goodwill AG, Sassoon DJ, Kiel AM, Tune JD. Leptin augments coronary vasoconstriction and smooth muscle proliferation via a rho-kinase-dependent pathway. Basic Res Cardiol. 2016;111(3):25. doi: 10.1007/s00395-016-0545-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sacks HS, Fain JN, Cheema P, et al. Depot-specific overexpression of proinflammatory, redox, endothelial cell, and angiogenic genes in epicardial fat adjacent to severe stable coronary atherosclerosis. Metab Syndr Relat Disord. 2011;(6):433–439. doi: 10.1089/met.2011.0024. (In press) [DOI] [PubMed] [Google Scholar]
- 33.Karastergiou K, Evans I, Ogston N, et al. Epicardial adipokines in obesity and coronary artery disease induce atherogenic changes in monocytes and endothelial cells. Arterioscler Thromb Vasc Biol. 2010;30(7):1340–1346. doi: 10.1161/ATVBAHA.110.204719. [DOI] [PubMed] [Google Scholar]
- 34.Cheng KH, Chu CS, Lee KT, et al. Adipocytokines and proinflammatory mediators from abdominal and epicardial adipose tissue in patients with coronary artery disease. Int J Obes (Lond) 2008;32(2):268–274. doi: 10.1038/sj.ijo.0803726. [DOI] [PubMed] [Google Scholar]
- 35.Dutour A, Achard V, Sell H, et al. Secretory type ii phospholipase a2 is produced and secreted by epicardial adipose tissue and overexpressed in patients with coronary artery disease. J Clin Endocrinol Metab. 2010;95(2):963–967. doi: 10.1210/jc.2009-1222. [DOI] [PubMed] [Google Scholar]
- 36.Chatterjee TK, Stoll LL, Denning GM, et al. Proinflammatory phenotype of perivascular adipocytes: Influence of high-fat feeding. Circ Res. 2009;104(4):541–549. doi: 10.1161/CIRCRESAHA.108.182998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chatterjee TK, Aronow BJ, Tong WS, et al. Human coronary artery perivascular adipocytes overexpress genes responsible for regulating vascular morphology, inflammation, and hemostasis. Physiol Genomics. 2013;45(16):697–709. doi: 10.1152/physiolgenomics.00042.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prati F, Arbustini E, Labellarte A, Sommariva L, Pawlowski T, Manzoli A, Pagano A, Motolese M, Boccanelli A. Eccentric atherosclerotic plaques with positive remodelling have a pericardial distribution: a permissive role of epicardial fat? A three-dimensional intravascular ultrasound study of left anterior descending artery lesions. Eur Heart J. 2003;24(4):329–36. doi: 10.1016/s0195-668x(02)00426-8. [DOI] [PubMed] [Google Scholar]
- 39.Heusch G, Libby P, Gersh B, et al. Cardiovascular remodelling in coronary artery disease and heart failure. Lancet. 2014;383(9932):1933–1943. doi: 10.1016/S0140-6736(14)60107-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pasterkamp G, de Kleijn DP, Borst C. Arterial remodeling in atherosclerosis, restenosis and after alteration of blood flow: Potential mechanisms and clinical implications. Cardiovasc Res. 2000;45(4):843–852. doi: 10.1016/s0008-6363(99)00377-6. [DOI] [PubMed] [Google Scholar]
- 41.Cornwell LD, Omer S, Rosengart T, Holman WL, Bakaeen FG. Changes over time in risk profiles of patients who undergo coronary artery bypass graft surgery: The veterans affairs surgical quality improvement program (vasqip) Jama surgery. 2015;150(4):308–315. doi: 10.1001/jamasurg.2014.1700. [DOI] [PubMed] [Google Scholar]


