Abstract
Traumatic brain injury (TBI) causes sustained disability due to compromised neurorepair mechanisms. Crucial to neurorepair and functional recovery following both TBI and stroke is hypoxia-inducible factor-1 alpha (HIF-1α). Based on reports that HIF-1α could be stabilized via S-nitrosylation, we tested the hypothesis that the S-nitrosylating agent S-nitrosoglutathione (GSNO) would stabilize HIF-1α, thereby stimulating neurorepair mechanisms and aiding in functional recovery. TBI was induced by controlled cortical impact (CCI) in adult rats. GSNO (0.05 mg/kg) was administered at two hours after CCI. The treatment was repeated daily until the 14th day after CCI. Functional recovery was assessed by motor and cognitive functions, and the recovery was compared with the expression of HIF-1α. The mechanisms of GSNO-mediated S-nitrosylation of HIF-1α were determined using brain endothelial cells. While non-treated TBI animals showed sustained neurobehavioral deficits, GSNO treatment of TBI improved neurobehavioral functions. GSNO also increased the expression of HIF-1α and VEGF. The beneficial effects of GSNO on neurobehavioral functions in TBI animals were blocked by treatment with the HIF-1α inhibitor 2-methoxyestradiol (2-ME). The stimulatory effect of GSNO on VEGF was reversed not only by 2-ME but also by the denitrosylating agent dithiothreitol, confirming our hypothesis that GSNO's benefits are mediated by the stabilization of HIF-1α via S-nitrosylation. GSNO's S-nitrosylation of HIF-1α was further confirmed using a biotin switch assay in endothelial cells. The data provide evidence that GSNO treatment of TBI aids functional recovery through stabilizing HIF-1α via S-nitrosylation. GSNO is a natural component of the human brain/body, and its exogenous administration has not shown adverse effects in humans. Therefore, the translational potential of GSNO therapy in TBI is high.
Keywords: TBI, GSNO, HIF-1α, VEGF, neurobehavioral function, neurorepair, S-nitrosylation
1. Introduction
Traumatic brain injury (TBI) is the leading cause of long-term disability worldwide [1; 2]. About 3 million patients with TBI receive medical care annually, and more than 5.3 million Americans live with neurological deficits caused by TBI, a heavy burden for individuals and societies [3]. In spite of TBI's disabling consequences and significant financial burdens, no currently approved therapy exists due to limited understanding of TBI's neuroprotection/neurorepair mechanisms and functional recovery processes [4]. For functional recovery, clinical trials in human TBI show that neuroprotective drugs fail due to a lack of efficacy in the chronic phase. Therefore, an ideal therapy must ameliorate acute as well as chronic phases of the injury by well-understood mechanisms [4]. Previously, we documented that an S-nitrosylating agent S-nitrosoglutathione (GSNO) confers neuroprotection and protects against endothelial dysfunction in TBI's acute phase in animal models [5; 6]. Using an animal model of controlled cortical impact (CCI) to assess the chronic phase of TBI, we focused on delineating the mechanisms of GSNO-mediated neurorepair leading to improvements in neurobehavioral functions.
GSNO, a biologically active member of the nitric oxide (NO) metabolome and a mediator of S-nitrosylation, is present in the brain and other organs [7]. GSNO is formed by a reaction between glutathione and NO in the presence of oxygen [8]; however, its synthesis is also influenced by redox [9]. Pharmacokinetic studies indicate that the half-life of GSNO varies in human [10] and rodent [11]. It is anticipated that GSNO/S-nitrosothiols levels are decreased in brain trauma due to an instantaneous reaction between nitric oxide synthase (NOS)-derived NO and superoxide, forming peroxynitrite. This pathological peroxynitrite formation hampers the biosynthesis of GSNO/nitrosothiols, a reaction product of NO and glutathione (GSH)/protein thiols (PSH). GSNO, via S-nitrosylation, regulates the activities of key enzymes involved in TBI such as NF-κB, STAT3, COX-2, caspase-3, nitric oxide synthases (NOS) [12; 13; 14; 15; 16]. An exogenous administration of GSNO protects against cardiac ischemic injury via the mechanism of S-nitrosylation [17], supporting the notion that, when channeled adequately into S-nitrosylation, GSNO shows therapeutic potential [18]. A recent stroke study showed that S-nitrosylation of PTEN inhibits its activity, leading to the activation of Akt [19]. Akt activation results in hypoxia-inducible factor-1 alpha (HIF-1α) stabilization, which, in turn, induces vascular endothelial growth factor (VEGF) and angiogenesis/neurogenesis and thus stimulates the neurorepair process [20].
HIF-1, a nuclear transcription factor, is characterized as the master regulator of cellular oxygen homeostasis. HIF-1 is a combination of the HIF-1α (120 kDa) and HIF-1β (91-94 kDa) subunits. There are three HIF-α isoforms (HIF-1α, HIF-2α and HIF-3α). The beta class includes HIF-1β. The HIF-1β subunit is a constitutively expressed protein, but the expression of the HIF-1α subunit (a cytosolic protein) is largely dependent on oxygen levels. HIF-1α is rapidly up regulated in response to hypoxia and is rapidly degraded upon reoxygenation/reperfusion. Admittedly, it is directly involved in both pathological (hypoxia) and neurorepair (normoxia) pathways following brain trauma [21]. The HIF-1α stabilizers/inducers, such as desferrioxamine (an iron chelator approved for haemochromatosis treatment), promote a number of survival pathways, including neuroprotection, angiogenesis and neurotrophins. They also reduce brain infarctions when administered pre- or post-stroke [21]. HIF-1α hydroxylating enzyme (prolyl-4-hydroxylase domain proteins; PHDs) inhibitors, such as FG-4539, are presently in a phase II anemia trial because of their activity to stabilize HIF-1α by preventing degradation with the ubiquitin proteasome system [22]. However, early inhibition of HIF-1α in the acute phase of stroke and TBI has also been reported to be neuroprotective [22; 23; 24]. Under normoxic conditions, studies are lacking on direct stabilization of HIF-1α by secondary modification and the induction of consequent protective genes. An S-nitrosylation reaction has been shown to stabilize HIF-1 protein expression and thus enhance its activity in endothelial cells [25]. Later, it was found that, while GSNO stabilizes HIF-1α by S-nitrosylation, reactive oxygen species (peroxynitrite, superoxide) destabilize HIF-1α, likely via thiol oxidation [26]. Furthermore, GSNO attenuates PHD activity during normoxia, thus inhibiting proteasomal degradation of HIF-1α [27]. S-nitrosylation-mediated stabilization of HIF-1α has been shown to protect against myocardial injury via the VEGF/angiogenesis pathway in GSNO reductase (GSNOR) knockout mice [28], indicating that HIF-1 is one of the key players in tissue repair processes. Using a rat model of ischemia reperfusion (IR), we observed that stabilization of HIF-1α by GSNO results in enhanced angiogenesis and the stimulation of neurorepair processes, leading to functional recovery [29]. The data presented in this study support that the GSNO-mediated neurorepair process and functional recovery in TBI are invoked by the stabilization of HIF-1α via S-nitrosylation.
2. Methods
2.1. Reagents
GSNO was obtained from World Precision Instruments (Sarasota, FL) and recrystallized before use. 2-Methoxyestradiol (2-ME), dithiothreitol (DDT), and all other chemicals and reagents were from Sigma-Aldrich (St. Louis, MO), unless stated otherwise.
2.2. Animals
Sprague-Dawley (SD) male rats approximately three months (250-300 grams) old were obtained from Harlan Laboratory (Wilmington, MA). TBI (controlled cortical impact; CCI) surgery was performed in animals weighing 260-300 grams. All animals received humane care in compliance with the Medical University of South Carolina's (MUSC) guidance and the National Research Council's criteria for humane care. Animal procedures were approved by the institutional animal care and use committee of MUSC. The animals were allowed to acclimatize for at least 3-5 days before the experiments. They were randomly divided into 4 groups: 1) sham-operated control without treatment (Sham) for 14 days, 2) TBI for 14 days (TBI), 3) TBI+GSNO for 14 days (GSNO), 4) TBI+GSNO+2-ME (GSNO+2-ME) for 14 days. At least 7 animals per group were used, and the number of animals used in each experiment is indicated in the figure legends.
2.3 Controlled cortical impact (CCI) rat model of focal TBI
Ketamine (90 mg/kg body weight) and xylazine (10 mg/kg body weight) as surgical anesthesia were administered intraperitoneally (ip) [30]. Analgesic buprenorphine was administered preemptively to alleviate pain following surgery. Utilizing aseptic techniques, a midline scalp incision was made, and the skin and fascia were reflected to expose the skull. A bone flap (5 mm, diameter) was removed from the right side. A 3-mm diameter craniotomy (centered at the +3.0 mm posterior and 2.7 mm lateral to the bregma) was made with a handheld Michele trephine [31]. The craniotomy was enlarged further with cranial rongeurs. This process does not cause rupture or significant bleeding acutely. CCI injury was produced as previously described in the extensive literature [32; 33; 34]. A cortical contusion was produced on the exposed cortex using a controlled impactor device reported by Bilgen [35] and described in our TBI studies [5; 6]. Briefly, the impacting shaft was extended, and the impact tip was centered and lowered over the craniotomy site until it touched the dura mater. Then, the rod was retracted and the impact tip was advanced to produce a brain injury of moderate severity (tip diameter, 4 mm; cortical contusion depth, 3 mm; impact velocity, 1.5 m/sec) [6]. The impact tip was wiped clean with sterile alcohol after each impact and cleaned/disinfected further with cidex after surgery. Core temperature was maintained at 37 ± 0.5°C with a heating pad during surgery as recorded with a rectal probe. Immediately after injury, the skin incision was closed with nylon sutures. Lidocaine jelly (2%) was applied to the lesion site to minimize any possible infection/discomfort. Sham animals had no cortical impact but underwent the same procedure otherwise.
2.4. Drug Treatment
In the GSNO-treated groups, GSNO (0.05 mg/kg body weight) in saline (~250μl) was slowly infused by iv at 2 h after CCI. From one day after CCI, the same dose of GSNO was gavage fed daily until 14 days following CCI. Physiological parameters did not alter after GSNO treatment. Details of the study and physiologic parameters in TBI and GSNO-treated rats have been reported earlier [30]. In GSNO+2-ME groups, GSNO was administered for 14 days as described above. 2-ME (5 mg/kg, ip) administration to the GSNO group was initiated 24 h after the first dose of GSNO treatment and continued until the 14th day following CCI. The selected dose of 2-ME was based on studies from our [29] and another laboratory [24].
2.5. Evaluation of motor behavior and cognitive function
Animals were pre-trained with a rotarod task and novel object recognition (NOR) test, then randomized. After CCI, animals in each group were then evaluated for functional recovery for 14 days using the rotarod task and NOR test. All tasks described below were performed by trained personnel blinded to the study groups and experimental conditions.
In the motor function test, latency on the rotarod was measured using the Rotarod system (Rotomex 5) from Columbus Instruments, Columbus, OH as described [36; 37; 38; 39]. Animals were pre-trained on an automated 4-lane rotarod unit, and the task was performed for two weeks after CCI. The animal was placed on the rod and its latency period was assessed. The starting speed was set to 0, and the speed was increased by 2 rpm every 5 seconds up to 30 rpm. The total time in seconds that the animal could stay on the drum was recorded. Each animal was given 3 trials, and the mean latency of three trials was calculated for each animal.
Non-spatial memory was determined by the novel object recognition (NOR) test as described [5; 40]. The NOR test evaluates the ability of the animal to recognize a novel object in the environment. The NOR test wooden box (36cm × 22cm × 18 cm) was located in an isolated and illuminated animal testing room. The animals were allowed to explore the test box for 15 min per day before the actual experiments. The NOR test box was devoid of any object during the habituation trial. During session I, two objects, A1 and A2, with identical texture, color, and size were presented to the test animal for 10 min. After a 24 h delay in the home cage, the rats were again exposed to the same area with one novel object of different texture, color, and size included (A1 and B) for 4 min. Both the objects and the box were cleaned with alcohol and dried after each trial to remove olfactory cues. Latency (% preference for a novel object) was recorded using a video camera. The advantage of this test for assessing cognition is that, unlike the Morris water-maze task, its score is independent of motor function [41].
2.6. Western blot studies
In the traumatic penumbra area from the ipsilateral injured brain tissue, western blot was performed using antibodies against HIF-1α (Abcam, Cambridge, MA); VEGF, brain-derived neurotrophic factor (BDNF; Santa Cruz Biotechnology, Santa Cruz, CA); and β-actin, as described previously [29]. Protein concentrations were determined using protein assay dye from Bio-Rad Laboratories (Hercules, CA). Densitometry of protein expression was performed using a GS800 calibrated densitometer from Bio-Rad laboratories (Hercules, CA).
2.7. mRNA expression of VEGF, BDNF and PECAM-1 in a mouse brain capillary endothelial cell line (bEnd3)
bEnd3, an immortalized mouse brain endothelial cell line [42], used in our studies [14], is commercially available at American Type Culture Collection, Manassas, VA (CRL-2299). It is a well characterized endothelial cell line that shows the expression of ICAM-1 and VCAM-1[14]. Cells were grown according to the supplier's instructions and were allowed to grow to ~70%-80% confluence. Cells were treated with freshly prepared GSNO (100 μM) in the presence or absence of DDT (200 μM) or 2-ME (5 μM) for 24 h. Both DDT and 2-ME were treated 2 h prior to GSNO treatment. Effective doses of GSNO, DDT, and 2-ME were determined by LDH cell death assay.
mRNA expression levels were determined using RT-PCR as described previously [43]. Briefly, after extraction of RNA using TRIzol (Invitrogen, USA), single-stranded cDNA was synthesized from total RNA using an ‘iscript cDNA synthesis’ kit. (BIO-RAD). Real time PCR was performed using Bio-Rad iCycler (iCycler iQ Multi-Color Real Time PCR Detection System; Bio-Rad) analysis, performed as described previously [44]. The primer sets for VEGF, forward: 5′-ctcaccaaagccagcacata-3′, reverse: 5′-aaatgcttcctccgctctga-3′ and BDNF, forward: 5′-cctgcatctgttggggag-3′, reverse: 5′-gccttgtccgtggacgtt-3′; were received from IDT, Coralville, IA, USA. The primer set for platelet endothelial cell adhesion molecule (PECAM-1) was obtained from Qiagen, Valencia, CA (Catalogue # PPM03802C). Thermal cycling conditions were as follows: activation of DNA polymerase at 95 °C for 10 min, followed by 40 cycles of amplification at 95 °C for 30 s and 60 °C for 30 s. The expression of the target genes was normalized with β-actin, forward 5’–ccatcatgaagtgtgacgtgg-3’, reverse 5’-gtccgcctagaagcatttgcg-3’.
2.8. Biotin switch assay for detection of S-nitrosylated HIF-1α protein
Protein S-nitrosylation was detected using the biotin switch method as described previously [14; 16]. Briefly, 70-80% confluent bEnd3 cells treated with different concentrations of GSNO for 2 h were lysed in 250mM HEPES, pH 7.7, 1mM EDTA, 0.1mM neocuproine, 1% Nonidet P-40, 150mM NaCl, 1mM PMSF, 20 lM methyl methanethiosulfonate (MMTS), 80 lM carmustine, protease inhibitor mixture (Sigma), and mixed with an equal volume of 25mM HEPES, pH 7.7, 0.1mM EDTA, 10 lM neocuproine, 5% SDS, 20 lM MMTS and incubated at 50°C for 20 min. After acetone precipitation, the precipitates were resuspended in 25mM HEPES, pH 7.7, 0.1mM DTA, 10 lM neocuproine, 1% SDS and mixed with two volumes of 20mM HEPES, pH 7.7, 1mMEDTA, 100mM NaCl, and 0.5% Triton X-100. The S-nitrosylated proteins were then modified with biotin in 25mM HEPES, pH 7.7, 0.1mMEDTA, 1% SDS, 10 lMneocuproine, 10mMascorbate sodium salt, and 0.2mM N-[6-(biotinamido)hexyl]-30-(20- pyridyldithio) propionamide (biotin-HPDP; Pierce). After acetone precipitation, biotinylated proteins were assessed with western immunoblotting, and another set was subjected to pull down with neutravidin-agarose and separated by SDS-PAGE followed by immunoblotting with anti-HIF-1α antibody.
2.9. Statistical evaluation
Statistical analysis was performed as described [29] using software Graph pad Prism 5.01. Unless otherwise stated, all values are expressed as mean ± SD of n determinations. The results were examined by unpaired Student t-test. Multiple comparisons were performed by one-way ANOVA followed by the post-hoc Tukey test. A p value less than 0.05 was considered significant.
3. Results
3.1. HIF-1α inhibition by 2-ME blocked the beneficial effects of GSNO on functional recovery
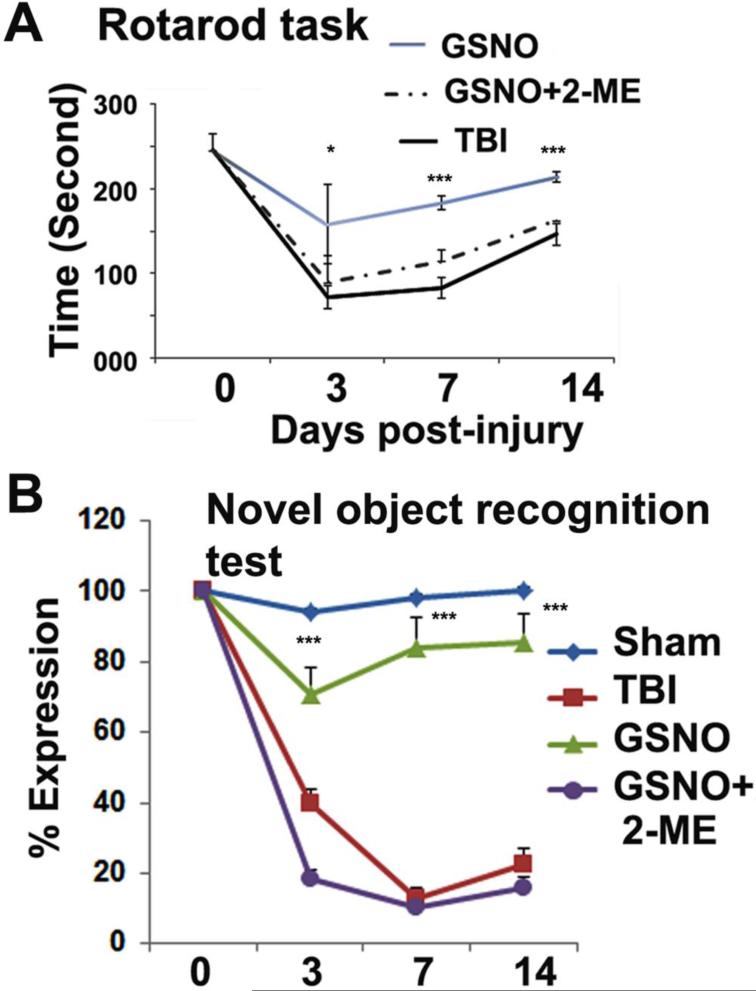
Both motor and cognitive functions contribute significantly to disability following TBI. Using the HIF-1α inhibitor 2-ME, we tested the hypothesis that GSNO's effect on motor and cognitive function recovery is mediated by the stabilization of HIF-1α. 2-ME, a metabolite of estradiol/estrogen, inhibits HIF-1α stabilization and thus its transcriptional activity [45]. We observed that GSNO treatment of TBI significantly improved motor function (rotarod task; Fig 1A) and increased NOR performance (Fig 1B) from day 3 onward. However, 2-ME treatment of GSNO group animals significantly reversed the improvement of both rotarod and NOR functions (Fig 1A, B). 2-ME was first administered 24 h after TBI to investigate the role of HIF-1α for neurorepair and functional recovery in the chronic phase.
Figure 1.
Effect of inhibition of HIF-1α by 2-ME on GSNO-induced improvement of neurobehavioral functions [rotarod task and novel object recognition (NOR) test] for 14 days. GSNO treatment of TBI animals improved motor function (rotarod task, A) and cognitive behavior (NOR, B) with increasing time whereas treatment with 2-ME of GSNO-treated TBI animals reversed the effects of GSNO. Sham animals showed deficits neither in rotarod task (base line latency 260±20 seconds) nor in NOR. Statistically significant differences were not observed between TBI and GSNO+2-ME group. Data are presented as mean±SE (n=7). *p<0.05 and ***p<0.001 vs. TBI and GSNO+2-ME.
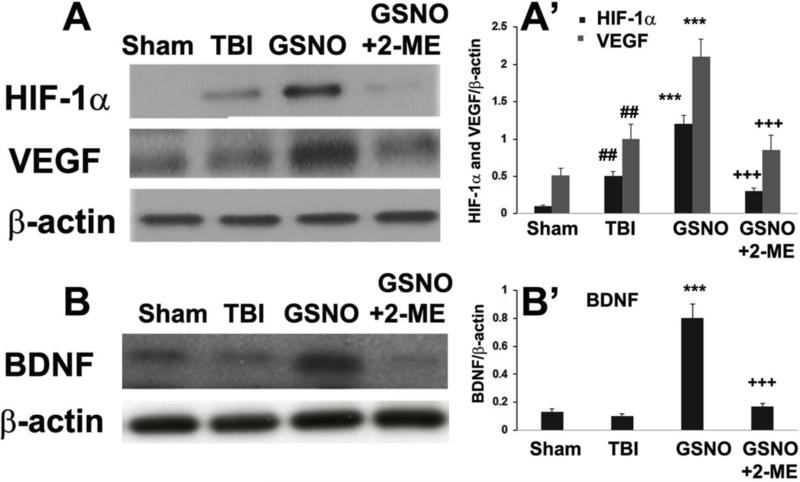
3.2. GSNO-mediated stabilization of HIF-1α and the consequent stimulation of VEGF and BDNF were reversed by HIF-1α inhibitor 2-ME
Administration of 2-ME to GSNO-treated TBI animals for 13 days consecutively (first dose of 2-ME was administered 24 h after TBI to investigate its effect after the acute phase) remarkably inhibited HIF-1α protein expression determined at the 14th day of GSNO treatment (Fig 2A, A’). Similarly, treatment with 2-ME also significantly blocked GSNO-mediated enhanced expression VEGF (Fig 2A, A’) and BDNF (Fig 2B, B’). The regulatory activity of GSNO and 2-ME on neurorepair mediators HIF-1α, VEGF, and BDNF correlated well with the recovery of function as depicted in Fig 1.
Figure 2.
Effect of inhibition of HIF-1α by 2-ME on GSNO-mediated stabilization of HIF-1α and stimulation of VEGF and BDNF at 14th day after TBI. TBI animals showed higher protein levels of both HIF-1α and VEGF; however, treatment of TBI animals with GSNO further increased the levels as shown by western blot studies (A) and densitometry (A’). 2-ME treatment of GSNO group animals reversed the effect of GSNO (A, A’). Similar effects of GSNO and 2-ME were observed on the levels of BDNF, as shown by western blot studies (B) and densitometry (B’). Data are presented as mean±SD (n=7). ##p<.01 vs. Sham, ***p<0.001 vs. TBI, +++p<0.001 vs. GSNO.
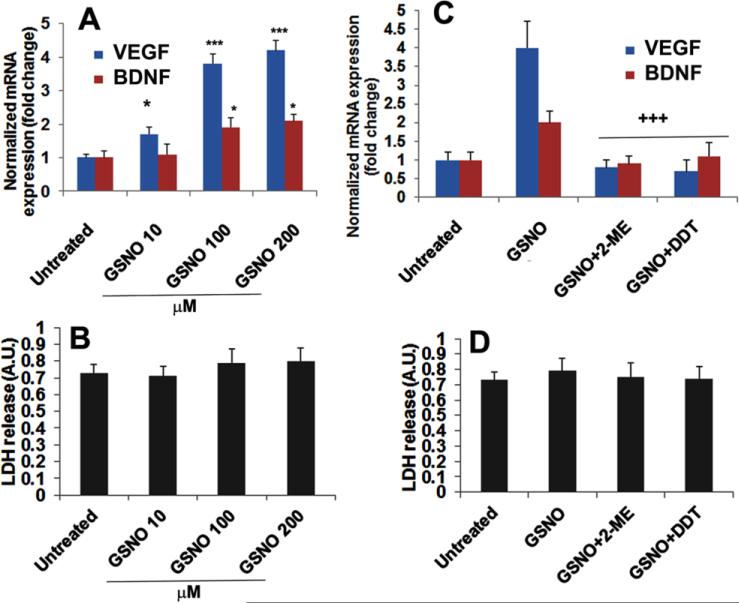
3.3. GSNO-mediated enhanced mRNA expression of VEGF and BDNF was blocked by treatment with HIF-1α inhibitor 2-ME as well as denitrosylating agent DDT in endothelial cells
To determine the effect of GSNO on HIF-1α stabilization and the consequent induction of the expression of the angiogenic and neurorepair gene VEGF, we measured the mRNA expression of VEGF in the presence and absence of 2-ME and DDT in mouse brain capillary endothelial (bEnd3) cells (Fig 3). The induction of VEGF stimulates increased expression of BDNF, a neurotrophic molecule that promotes neurorepair process. Therefore, we also measured the mRNA levels of BDNF. GSNO treatment for 24 h significantly increased the expression of VEGF and BDNF (Fig 3A) in a dose dependent manner, indicating that GSNO-mediated neuroprotection and neurorepair may be modulated via the VEGF/BDNF pathway. The expression of both VEGF and BDNF was reduced after treatment with the HIF-1α inhibitor 2-ME (Fig 3C), indicating that HIF-1α is necessary for GSNO-mediated induction of VEGF/BDNF. Furthermore, DTT (a reducing agent, known to denitrosylate) also reduced the expression VEGF/BDNF (Fig 3C), documenting that the activity of GSNO was mediated via the mechanisms of S-nitrosylation. Figs 3B and 3D of LDH release show no significant cell death under these experimental conditions.
Figure 3.
Effect of GSNO, HIF-1α inhibitor 2-ME, and denitrosylating agent DDT on the expression of VEGF and BDNF in endothelial (bEnd3) cells. GSNO treatment increased the mRNA expression levels of VEGF and BDNF in a dose dependent manner (A). Pretreatment (2 h) with 2-ME (5 μM) or DDT (200 μM) reversed the effect of GSNO on the expression levels of VEGF and BDNF (C). The expression of VEGF and BDNF was determined at 24 h using RT-PCR and normalized against β-actin. Under the described experimental conditions, cell viability was assayed using LDH release assay (B, D), showing no significant cell death. Data are presented as mean +SD. n=5. ***p<0.001, *p<0.05 vs. Untreated, +++p<0.001 vs. GSNO.
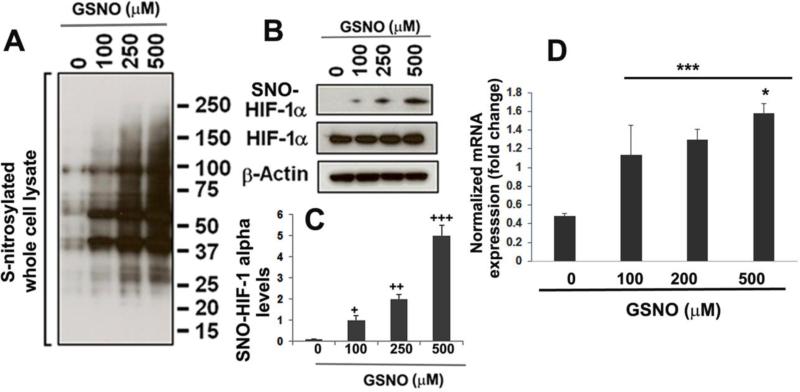
3.4. GSNO treatment of bEnd3 endothelial cells increased the levels of total S-nitrosylated proteins and the expression of S-nitrosylated HIF-1α protein determined by the biotin-switch assay and enhanced mRNA expression of PECAM-1
To determine that GSNO the levels of S-nitrosylated proteins (PSNO) and directly S-nitrosylates HIF-1α leading to its stabilization, we treated 70-80% confluent endothelial cells with different concentrations of GSNO for 2 h and determined the levels of PSNO (Fig 4A) and S-nitrosylated HIF-1α proteins (Fig 4B, C) by the biotin switch assay followed by immunoblotting analysis with an anti-HIF-1α antibody. GSNO-treated cells had dose dependently increased levels of total PSNO (Fig 4A). Parallel to increased levels of PSNO, GSNO treatment also enhanced S-nitrosylated HIF-1α levels (Fig 4B, and its densitometry Fig 4C) and mRNA expression of PECAM-1 (Fig 4D) in a dose dependent manner.
Figure 4.
Effect of GSNO (2 h treatment) on the total S-nitrosylated proteins, S-nitrosylation of HIF-1α using biotin switch assay and mRNA expression of PECAM-1 in endothelial (bEnd3) cells. Total S-nitrosylated proteins were determined by western blot analysis for biotin labeled proteins (A). A dose-wise effect of GSNO (100, 250, 500 μM) for 2 h treatment on increased S-nitrosylation of HIF-1α was examined by immunoblotting (B) and densitometry (C) and normalized with β-actin. mRNA expression of PECAM-1 was determined at 24 h using RT-PCR and normalized against β-actin (D). Data are presented as mean±SD. n=3. +p<0.05 vs. untreated (0 μM), ++p<0.01 vs. GSNO (100 μM), +++p<0.001 vs. GSNO (200 μM), ***p<0.001 vs. untreated (0 μM), *p<0.05 vs. GSNO (100, and200 μM).
4. Discussion
GSNO promotes improvement of neurobehavioral functions, and GSNO-aided functional recovery is blocked by the inhibition of HIF-1α in a rat CCI model of TBI. Therefore, GSNO confers its beneficial effects by stabilizing HIF-1α. Enhancing the S-nitrosylation of HIF-1α with exogenous GSNO has been shown to stabilize HIF-1α, leading to induction and increased expression of VEGF, angiogenesis, and neurorepair [20; 29]. S-nitrosylation-mediated stabilization of HIF-1α is also reported to provide myocardial protection via angiogenesis [28], indicating a protective role of HIF-1α. HIF-1α-induced neuroprotection and neurorepair are also supported by a recent report showing that deletion/inhibition of PHD1, a dioxygenase hydroxylating HIF-1α, protects against ischemic stroke injury [46]. A recent study in human brain infarctions showed that HIF-1α upregulation and stabilization attenuated vascular cognitive impairment and provided neuroprotection [47]. This finding supports our results of HIF-1α's neurorestorative activity [29]. However, inhibiting HIF-1α was shown to be neuroprotective early after injury in TBI animals [23]. Interestingly, this study shows that animals with TBI (comparatively, a less severe injury) had increased HIF-1α levels. In another TBI study, stabilizing HIF-1α was also found to be neuroprotective in another study [48]. In this study, TBI animals with ischemia (comparatively, more severe) had significantly reduced HIF-1α protein levels, suggesting that HIF-1α is expressed by comparatively healthy/surviving cells. Recently, a HIF-1α activator has also been reported to provide neuroprotection following TBI [49]. To better understand the role of HIF-1α for the stimulation of neurorepair mechanisms and functional recovery following TBI, the focus of this study was to investigate whether stabilizing HIF-1α would stimulate neurorepair mechanisms that aid in functional recovery following TBI.
In TBI, a disturbed NO metabolome results in decreased NO bioavailability and increased peroxynitrite levels [5; 30] due to an instantaneous reaction between NO and superoxide. Reduced NO levels result in decreased biosynthesis and, thus, reduced levels of GSNO because GSNO is formed by a reaction of NO with GSH in the presence of oxygen [7]. GSNO is the most prominent small S-nitrosothiol cellular molecule in mammals, and it regulates cellular functions related with cell survival, anti-inflammatory activities, and tissue repair [50]. Both S-nitrosylated proteins (PSNO) and GSNO are in dynamic equilibrium in the human body, and their dysregulation hampers cellular functions. Restoration of GSNO homeostasis by supplementation of exogenous GSNO improves neurological function following hypoxia-induced neurotrauma via the stabilization of HIF-1α [29]. Under normoxic conditions, GSNO-mediated S-nitrosylation has been shown to stabilize HIF-1α (a transcription factor of VEGF) [51], inducing VEGF activity [28; 52]. VEGF stimulates angiogenesis and neurogenesis following experimental stroke. Our results showed that HIF-1α stabilization-based functional recovery was reversed by 2-ME (Fig 1), an inhibitor of HIF-1α [53; 54].
2-ME, a natural metabolite of estrogen/estradiol, is a potent HIF-1α inhibitor, and its activity is associated with antiproliferative and antiangiogenic effects [45]. Mechanistically, it induces microtubule depolymerization and reactive oxygen species production and they, in turn, inhibit HIF-1α by down regulating HIF-1α protein expression [55]. Inhibition of HIF-1α reduces its transcription activity and thus decreases expression of target genes, including VEGF [52]. To delineate a clear role of HIF-1α in TBI's chronic phase for the stimulation of neurorepair mechanisms and functional recovery, we treated TBI+GSNO animals with 2-ME after 24 h of TBI (Figs 1, 2). The 2-ME treatment for 2 weeks decreased GSNO-mediated increased protein expression of HIF-1α, which correlated well with decreased VEGF protein levels (Fig 2A) and functional recovery (Fig 1). Because the activity of HIF-1/VEGF is linked with angiogenesis and angiogenesis is intrinsically associated with neurogenesis, growth factors, and functional recovery [56], inhibition of GSNO-mediated HIF-1α stabilization reverses GSNO-induced functional recovery. GSNO also stimulated the expression of BDNF (Fig 2B), as previously reported in TBI [30]. BDNF is known to stabilize HIF-1α [57], and we observed that inhibition of HIF-1α by 2-ME down regulates BDNF (Fig 2B), indicating that BDNF expression may be synergistically regulated via feedback with HIF-1α.
To examine the direct effect of GSNO on HIF-1α, its neurorepair target gene VEGF, and the mediator BDNF, we used endothelial cells treated with GSNO in the presence and absence of 2-ME (Fig 3). DDT was used to provide evidence that GSNO's effects are mediated via the mechanism of S-nitrosylation (Fig 3). GSNO increased VEGF mRNA levels while 2-ME and DDT decreased these levels. Therefore, we conclude that GSNO treatment stabilized HIF-1α, likely via its S-nitrosylation, resulting in the transcriptional induction of VEGF and the consequent increased expression of BDNF (Fig 3A, C). These data indicate that HIF-1α and VEGF are targeted by GSNO-mediated mechanisms, supporting the notion that GSNO stimulates the neurorepair process and aids in functional recovery via stimulation of the HIF-1α/VEGF pathway by S-nitrosylation of HIF-1α. S-nitrosylation of HIF-1α by GSNO was confirmed by an S-nitrosylation study of HIF-1α in endothelial cells (Fig 4). GSNO treatment for 2 h increased total S-nitrosylated proteins (Fig 4A) and enhanced the degree of S-nitrosylation of HIF-1α protein in a dose dependent manner. Furthermore, S-nitrosylation of HIF-1α by GSNO paralleled (Fig 4B-C) the enhanced expression of PECAM-1 (Fig 4D), an adhesion molecule highly expressed at endothelial cells. PECAM-1 maintains endothelial barrier function and plays a significant role in migration and functional organization of angiogenesis [58; 59], indicating GSNO's activity in stimulating neurorepair via angiogenesis, as previously reported [29]. Our observations of GSNO-mediated S-nitrosylation are supported by another study showing S-nitrosylation and thus stabilization of HIF-1α [60]. Based on these findings and premises, S-nitrosylation of HIF-1α is anticipated to protects HIF-1α from proteasomal degradation, resulting in its stability and transcriptional activity for VEGF. VEGF, in turn, stimulates angiogenesis/neurogenesis and the expression of neurotrophic factors[56], ultimately leading to functional recovery.
Limitations
In previous studies, we have reported dose and time windows of GSNO treatment in a rat model of TBI [6; 30]. Therefore, we used only one effective dose and a single treatment time in this study. In support of reversing of functional recovery by 2-ME, we selected only the rotarod task and NOR test. However, we have previously reported GSNO-induced improvement of several other neurological functions in TBI [5; 6]. Furthermore, we used only male animals in this study because our previous TBI studies were performed using male animals [5; 6; 30]. In view of the efficacy of GSNO in our spinal cord compression/injury model studies using female animals [61; 62], we anticipate that GSNO will be effective in female rat models of TBI. Another weakness is that our studies are limited to only a CCI model of TBI. The efficacy of GSNO in other models remains to be evaluated. Clearly, more pharmacokinetic studies are also required to advance understanding of GSNO's neuroprotective mechanisms in neurodegenerative diseases, including TBI.
Conclusions
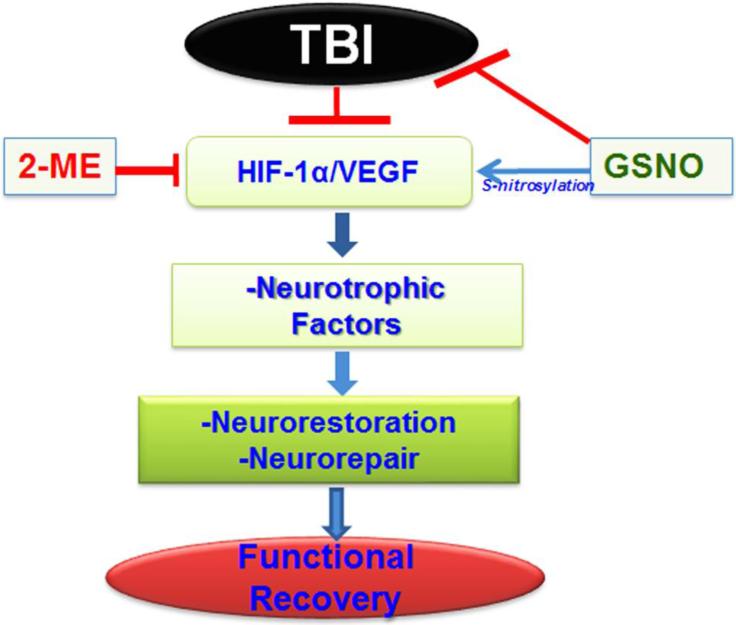
HIF-1α/VEGF is a significant pathway that aids and accelerates functional recovery in TBI, and this pathway is mechanistically stimulated by GSNO via S-nitrosylation of HIF-1α (Fig 5). HIF-1α stabilization for functional recovery in TBI may be achieved by regulation of several indirect pathways, such as inhibition of PHDs or activation of Akt by chemical compounds. However, such inhibitors/activators also show non-target effects. In contrast, S-nitrosylation-mediated HIF-1α stabilization has an advantage in that it targets and S-nitrosylates HIF-1α directly, conferring robust stimulation of neurorepair processes and inducing functional recovery. Furthermore, GSNO is a natural component of the human body, and its exogenous administration to humans has not been associated with toxicity [63; 64]. Additionally, GSNO is inexpensive, readily available, and easily administered in clinic. In view of the safety profile of GSNO in humans and animals and its observed mechanism-based stimulation of neurorepair, GSNO is a drug candidate to be investigated in human TBI.
Figure 5.
Schematic showing the hypothesized deleterious events in a CCI rat model of TBI, GSNO-mediated stimulation of neurorepair mechanisms, and induction of functional recovery via stabilization of HIF-1α/VEGF following TBI. HIF-1α inhibitor 2-ME treatment of GSNO-treated TBI animals reverses the effect of GSNO.
HIGHLIGHTS.
GSNO aids functional recovery in TBI animals by stimulating neurorepair
GSNO invokes neurorepair through S-nitrosylation of HIF-1α
S-nitrosylation stabilizes HIF-α and thus increases its activity
Inhibiting HIF-1α blocks GSNO-mediated neurorepair mechanisms and functional recovery
Acknowledgements
This work was supported by grants from NIH (NS-72511) and VA merit award (RX2090 and BX3401). This work was also supported by the NIH, Grants C06 RR018823 and No C06 RR015455 from the Extramural Research Facilities Program of the National Center for Research Resources. We thank Ms. Joyce Bryan for her technical help. We also acknowledge Dr. Tom Smith from the MUSC Writing Center for his valuable suggestions and editing of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors’ Contributions
This study is based on an original idea of MK, AKS and IS. MK wrote the manuscript and all authors reviewed the manuscript. TS D, MKP, JK and MB carried out animal and biochemical studies. MK, AKS, MB, and IS critically examined biochemical studies. TSD and MB performed immunoblotting studies. All authors have approved the manuscript.
Conflicts of interest
The author(s) declare that they have no competing interests.
References
- 1.Blennow K, Hardy J, Zetterberg H. The neuropathology and neurobiology of traumatic brain injury. Neuron. 2012;76:886–99. doi: 10.1016/j.neuron.2012.11.021. [DOI] [PubMed] [Google Scholar]
- 2.Chauhan NB. Chronic neurodegenerative consequences of traumatic brain injury. Restor Neurol Neurosci. 2014;32:337–65. doi: 10.3233/RNN-130354. [DOI] [PubMed] [Google Scholar]
- 3.Levin HS, Diaz-Arrastia RR. Diagnosis, prognosis, and clinical management of mild traumatic brain injury. Lancet Neurol. 2015;14:506–17. doi: 10.1016/S1474-4422(15)00002-2. [DOI] [PubMed] [Google Scholar]
- 4.Haddad SH, Arabi YM. Critical care management of severe traumatic brain injury in adults. Scand J Trauma Resusc Emerg Med. 2012;20:12. doi: 10.1186/1757-7241-20-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khan M, Dhammu TS, Matsuda F, Annamalai B, Dhindsa TS, Singh I, Singh AK. Targeting the nNOS/peroxynitrite/calpain system to confer neuroprotection and aid functional recovery in a mouse model of TBI. Brain Res. 2016;1630:159–70. doi: 10.1016/j.brainres.2015.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khan M, Im YB, Shunmugavel A, Gilg AG, Dhindsa RK, Singh AK, Singh I. Administration of S-nitrosoglutathione after traumatic brain injury protects the neurovascular unit and reduces secondary injury in a rat model of controlled cortical impact. J Neuroinflammation. 2009;6:32. doi: 10.1186/1742-2094-6-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kluge I, Gutteck-Amsler U, Zollinger M, Do KQ. S-Nitrosoglutathione in Rat Cerebellum: Identification and Quantification by Liquid Chromatography-Mass Spectrometry. J Neurochem. 1997;69:2599–2607. doi: 10.1046/j.1471-4159.1997.69062599.x. [DOI] [PubMed] [Google Scholar]
- 8.Broniowska KA, Diers AR, Hogg N. S-nitrosoglutathione. Biochim Biophys Acta. 2013;1830:3173–81. doi: 10.1016/j.bbagen.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jourd'heuil D, Jourd'heuil FL, Feelisch M. Oxidation and nitrosation of thiols at low micromolar exposure to nitric oxide. Evidence for a free radical mechanism. J Biol Chem. 2003;278:15720–6. doi: 10.1074/jbc.M300203200. [DOI] [PubMed] [Google Scholar]
- 10.Jourd'heuil D, Hallen K, Feelisch M, Grisham MB. Dynamic state of S-nitrosothiols in human plasma and whole blood. Free Radic Biol Med. 2000;28:409–17. doi: 10.1016/s0891-5849(99)00257-9. [DOI] [PubMed] [Google Scholar]
- 11.Bryan NS, Rassaf T, Maloney RE, Rodriguez CM, Saijo F, Rodriguez JR, Feelisch M. Cellular targets and mechanisms of nitros(yl)ation: an insight into their nature and kinetics in vivo. Proc Natl Acad Sci U S A. 2004;101:4308–13. doi: 10.1073/pnas.0306706101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chiueh CC, Rauhala P. The redox pathway of S-nitrosoglutathione, glutathione and nitric oxide in cell to neuron communications. Free Radic Res. 1999;31:641–50. doi: 10.1080/10715769900301211. [DOI] [PubMed] [Google Scholar]
- 13.Marshall HE, Stamler JS. Inhibition of NF-kappa B by S-nitrosylation. Biochemistry. 2001;40:1688–93. doi: 10.1021/bi002239y. [DOI] [PubMed] [Google Scholar]
- 14.Prasad R, Giri S, Nath N, Singh I, Singh AK. GSNO attenuates EAE disease by S-nitrosylation-mediated modulation of endothelial-monocyte interactions. Glia. 2007;55:65–77. doi: 10.1002/glia.20436. [DOI] [PubMed] [Google Scholar]
- 15.Khan M, Sekhon B, Giri S, Jatana M, Gilg AG, Ayasolla K, Elango C, Singh AK, Singh I. S-Nitrosoglutathione reduces inflammation and protects brain against focal cerebral ischemia in a rat model of experimental stroke. J Cereb Blood Flow Metab. 2005;25:177–92. doi: 10.1038/sj.jcbfm.9600012. [DOI] [PubMed] [Google Scholar]
- 16.Kim J, Won JS, Singh AK, Sharma AK, Singh I. STAT3 regulation by S-nitrosylation: implication for inflammatory disease. Antioxid Redox Signal. 2014;20:2514–27. doi: 10.1089/ars.2013.5223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Konorev EA, Tarpey MM, Joseph J, Baker JE, Kalyanaraman B. S-nitrosoglutathione improves functional recovery in the isolated rat heart after cardioplegic ischemic arrest-evidence for a cardioprotective effect of nitric oxide. J Pharmacol Exp Ther. 1995;274:200–6. [PubMed] [Google Scholar]
- 18.Que LG, Liu L, Yan Y, Whitehead GS, Gavett SH, Schwartz DA, Stamler JS. Protection from experimental asthma by an endogenous bronchodilator. Science. 2005;308:1618–21. doi: 10.1126/science.1108228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Numajiri N, Takasawa K, Nishiya T, Tanaka H, Ohno K, Hayakawa W, Asada M, Matsuda H, Azumi K, Kamata H, Nakamura T, Hara H, Minami M, Lipton SA, Uehara T. On-off system for PI3-kinase-Akt signaling through S-nitrosylation of phosphatase with sequence homology to tensin (PTEN) Proc Natl Acad Sci U S A. 2011;108:10349–54. doi: 10.1073/pnas.1103503108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng XW, Kuzuya M, Kim W, Song H, Hu L, Inoue A, Nakamura K, Di Q, Sasaki T, Tsuzuki M, Shi GP, Okumura K, Murohara T. Exercise training stimulates ischemia-induced neovascularization via phosphatidylinositol 3-kinase/Akt-dependent hypoxia-induced factor-1 alpha reactivation in mice of advanced age. Circulation. 2010;122:707–16. doi: 10.1161/CIRCULATIONAHA.109.909218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kasivisvanathan V, Shalhoub J, Lim CS, Shepherd AC, Thapar A, Davies AH. Hypoxia-inducible factor-1 in arterial disease: a putative therapeutic target. Curr Vasc Pharmacol. 2011;9:333–49. doi: 10.2174/157016111795495602. [DOI] [PubMed] [Google Scholar]
- 22.Harten SK, Ashcroft M, Maxwell PH. Prolyl hydroxylase domain inhibitors: a route to HIF activation and neuroprotection. Antioxid Redox Signal. 2010;12:459–80. doi: 10.1089/ars.2009.2870. [DOI] [PubMed] [Google Scholar]
- 23.Schaible EV, Windschugl J, Bobkiewicz W, Kaburov Y, Dangel L, Kramer T, Huang C, Sebastiani A, Luh C, Werner C, Engelhard K, Thal SC, Schafer MK. 2-Methoxyestradiol confers neuroprotection and inhibits a maladaptive HIF-1alpha response after traumatic brain injury in mice. J Neurochem. 2014;129:940–54. doi: 10.1111/jnc.12708. [DOI] [PubMed] [Google Scholar]
- 24.Chen C, Hu Q, Yan J, Lei J, Qin L, Shi X, Luan L, Yang L, Wang K, Han J, Nanda A, Zhou C. Multiple effects of 2ME2 and D609 on the cortical expression of HIF-1 alpha and apoptotic genes in a middle cerebral artery occlusion-induced focal ischemia rat model. J Neurochem. 2007;102:1831–41. doi: 10.1111/j.1471-4159.2007.04652.x. [DOI] [PubMed] [Google Scholar]
- 25.Palmer LA, Gaston B, Johns RA. Normoxic stabilization of hypoxia-inducible factor-1 expression and activity: redox-dependent effect of nitrogen oxides. Mol Pharmacol. 2000;58:1197–203. doi: 10.1124/mol.58.6.1197. [DOI] [PubMed] [Google Scholar]
- 26.Wellman TL, Jenkins J, Penar PL, Tranmer B, Zahr R, Lounsbury KM. Nitric oxide and reactive oxygen species exert opposing effects on the stability of hypoxia-inducible factor-1alpha (HIF-1alpha) in explants of human pial arteries. Faseb J. 2004;18:379–81. doi: 10.1096/fj.03-0143fje. [DOI] [PubMed] [Google Scholar]
- 27.Metzen E, Zhou J, Jelkmann W, Fandrey J, Brune B. Nitric oxide impairs normoxic degradation of HIF-1alpha by inhibition of prolyl hydroxylases. Mol Biol Cell. 2003;14:3470–81. doi: 10.1091/mbc.E02-12-0791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lima B, Lam GK, Xie L, Diesen DL, Villamizar N, Nienaber J, Messina E, Bowles D, Kontos CD, Hare JM, Stamler JS, Rockman HA. Endogenous S-nitrosothiols protect against myocardial injury. Proc Natl Acad Sci U S A. 2009;106:6297–302. doi: 10.1073/pnas.0901043106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khan M, Dhammu TS, Matsuda F, Baarine M, Dhindsa TS, Singh I, Singh AK. Promoting endothelial function by S-nitrosoglutathione through the HIF-1alpha/VEGF pathway stimulates neurorepair and functional recovery following experimental stroke in rats. Drug Des Devel Ther. 2015;9:2233–47. doi: 10.2147/DDDT.S77115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khan M, Sakakima H, Dhammu TS, Shunmugavel A, Im YB, Gilg AG, Singh AK, Singh I. S-Nitrosoglutathione reduces oxidative injury and promotes mechanisms of neurorepair following traumatic brain injury in rats. J Neuroinflammation. 2011;8:78. doi: 10.1186/1742-2094-8-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cole JT, Yarnell A, Kean WS, Gold E, Lewis B, Ren M, McMullen DC, Jacobowitz DM, Pollard HB, O'Neill JT, Grunberg NE, Dalgard CL, Frank JA, Watson WD. Craniotomy: true sham for traumatic brain injury, or a sham of a sham? J Neurotrauma. 2011;28:359–69. doi: 10.1089/neu.2010.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheng JP, Hoffman AN, Zafonte RD, Kline AE. A delayed and chronic treatment regimen with the 5-HT1A receptor agonist 8-OH-DPAT after cortical impact injury facilitates motor recovery and acquisition of spatial learning. Behav Brain Res. 2008;194:79–85. doi: 10.1016/j.bbr.2008.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoffman AN, Cheng JP, Zafonte RD, Kline AE. Administration of haloperidol and risperidone after neurobehavioral testing hinders the recovery of traumatic brain injury-induced deficits. Life Sci. 2008;83:602–7. doi: 10.1016/j.lfs.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kline AE, Wagner AK, Westergom BP, Malena RR, Zafonte RD, Olsen AS, Sozda CN, Luthra P, Panda M, Cheng JP, Aslam HA. Acute treatment with the 5-HT(1A) receptor agonist 8-OH-DPAT and chronic environmental enrichment confer neurobehavioral benefit after experimental brain trauma. Behav Brain Res. 2007;177:186–94. doi: 10.1016/j.bbr.2006.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bilgen M. A new device for experimental modeling of central nervous system injuries. Neurorehabil Neural Repair. 2005;19:219–26. doi: 10.1177/1545968305278635. [DOI] [PubMed] [Google Scholar]
- 36.Fox GB, Fan L, Levasseur RA, Faden AI. Sustained sensory/motor and cognitive deficits with neuronal apoptosis following controlled cortical impact brain injury in the mouse. J Neurotrauma. 1998;15:599–614. doi: 10.1089/neu.1998.15.599. [DOI] [PubMed] [Google Scholar]
- 37.Monville C, Torres EM, Dunnett SB. Comparison of incremental and accelerating protocols of the rotarod test for the assessment of motor deficits in the 6-OHDA model. J Neurosci Methods. 2006;158:219–23. doi: 10.1016/j.jneumeth.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 38.Wang H, Lynch JR, Song P, Yang HJ, Yates RB, Mace B, Warner DS, Guyton JR, Laskowitz DT. Simvastatin and atorvastatin improve behavioral outcome, reduce hippocampal degeneration, and improve cerebral blood flow after experimental traumatic brain injury. Exp Neurol. 2007;206:59–69. doi: 10.1016/j.expneurol.2007.03.031. [DOI] [PubMed] [Google Scholar]
- 39.Chen SF, Hung TH, Chen CC, Lin KH, Huang YN, Tsai HC, Wang JY. Lovastatin improves histological and functional outcomes and reduces inflammation after experimental traumatic brain injury. Life Sci. 2007;81:288–98. doi: 10.1016/j.lfs.2007.05.023. [DOI] [PubMed] [Google Scholar]
- 40.Huang TN, Chuang HC, Chou WH, Chen CY, Wang HF, Chou SJ, Hsueh YP. Tbr1 haploinsufficiency impairs amygdalar axonal projections and results in cognitive abnormality. Nat Neurosci. 2014;17:240–7. doi: 10.1038/nn.3626. [DOI] [PubMed] [Google Scholar]
- 41.Dhawan J, Benveniste H, Luo Z, Nawrocky M, Smith SD, Biegon A. A new look at glutamate and ischemia: NMDA agonist improves long-term functional outcome in a rat model of stroke. Future Neurol. 2011;6:823–834. doi: 10.2217/fnl.11.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Montesano R, Pepper MS, Mohle-Steinlein U, Risau W, Wagner EF, Orci L. Increased proteolytic activity is responsible for the aberrant morphogenetic behavior of endothelial cells expressing the middle T oncogene. Cell. 1990;62:435–45. doi: 10.1016/0092-8674(90)90009-4. [DOI] [PubMed] [Google Scholar]
- 43.Baarine M, Khan M, Singh A, Singh I. Functional Characterization of IPSC-Derived Brain Cells as a Model for X-Linked Adrenoleukodystrophy. PLoS One. 2015;10:e0143238. doi: 10.1371/journal.pone.0143238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baarine M, Beeson C, Singh A, Singh I. ABCD1 deletion-induced mitochondrial dysfunction is corrected by SAHA: implication for adrenoleukodystrophy. J Neurochem. 2015;133:380–96. doi: 10.1111/jnc.12992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mooberry SL. Mechanism of action of 2-methoxyestradiol: new developments. Drug Resist Updat. 2003;6:355–61. doi: 10.1016/j.drup.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 46.Quaegebeur A, Segura I, Schmieder R, Verdegem D, Decimo I, Bifari F, Dresselaers T, Eelen G, Ghosh D, Davidson SM, Schoors S, Broekaert D, Cruys B, Govaerts K, De Legher C, Bouche A, Schoonjans L, Ramer MS, Hung G, Bossaert G, Cleveland DW, Himmelreich U, Voets T, Lemmens R, Bennett CF, Robberecht W, De Bock K, Dewerchin M, Ghesquiere B, Fendt SM, Carmeliet P. Deletion or Inhibition of the Oxygen Sensor PHD1 Protects against Ischemic Stroke via Reprogramming of Neuronal Metabolism. Cell Metab. 2016;23:280–91. doi: 10.1016/j.cmet.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ke XJ, Zhang JJ. Changes in HIF-1alpha, VEGF, NGF and BDNF levels in cerebrospinal fluid and their relationship with cognitive impairment in patients with cerebral infarction. J Huazhong Univ Sci Technolog Med Sci. 2013;33:433–7. doi: 10.1007/s11596-013-1137-4. [DOI] [PubMed] [Google Scholar]
- 48.Thelin EP, Frostell A, Mulder J, Mitsios N, Damberg P, Aski SN, Risling M, Svensson M, Morganti-Kossmann MC, Bellander BM. Lesion Size Is Exacerbated in Hypoxic Rats Whereas Hypoxia-Inducible Factor-1 Alpha and Vascular Endothelial Growth Factor Increase in Injured Normoxic Rats: A Prospective Cohort Study of Secondary Hypoxia in Focal Traumatic Brain Injury. Front Neurol. 2016;7:23. doi: 10.3389/fneur.2016.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sen T, Sen N. Treatment with an activator of hypoxia-inducible factor 1, DMOG provides neuroprotection after traumatic brain injury. Neuropharmacology. 2016;107:79–88. doi: 10.1016/j.neuropharm.2016.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hess DT, Stamler JS. Regulation by S-nitrosylation of protein post-translational modification. J Biol Chem. 2012;287:4411–8. doi: 10.1074/jbc.R111.285742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li F, Sonveaux P, Rabbani ZN, Liu S, Yan B, Huang Q, Vujaskovic Z, Dewhirst MW, Li CY. Regulation of HIF-1alpha stability through S-nitrosylation. Mol Cell. 2007;26:63–74. doi: 10.1016/j.molcel.2007.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kimura H, Ogura T, Kurashima Y, Weisz A, Esumi H. Effects of nitric oxide donors on vascular endothelial growth factor gene induction. Biochem Biophys Res Commun. 2002;296:976–82. doi: 10.1016/s0006-291x(02)02029-6. [DOI] [PubMed] [Google Scholar]
- 53.Zhou D, Matchett GA, Jadhav V, Dach N, Zhang JH. The effect of 2-methoxyestradiol, a HIF-1 alpha inhibitor, in global cerebral ischemia in rats. Neurol Res. 2008;30:268–71. doi: 10.1179/016164107X229920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang Z, Tsai LK, Munasinghe J, Leng Y, Fessler EB, Chibane F, Leeds P, Chuang DM. Chronic valproate treatment enhances postischemic angiogenesis and promotes functional recovery in a rat model of ischemic stroke. Stroke. 2012;43:2430–6. doi: 10.1161/STROKEAHA.112.652545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Carbonaro M, Escuin D, O'Brate A, Thadani-Mulero M, Giannakakou P. Microtubules regulate hypoxia-inducible factor-1alpha protein trafficking and activity: implications for taxane therapy. J Biol Chem. 2012;287:11859–69. doi: 10.1074/jbc.M112.345587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sun Y, Jin K, Xie L, Childs J, Mao XO, Logvinova A, Greenberg DA. VEGF-induced neuroprotection, neurogenesis, and angiogenesis after focal cerebral ischemia. J Clin Invest. 2003;111:1843–51. doi: 10.1172/JCI17977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gao Y, Jing M, Ge R, Lang L. Induction of hypoxia-inducible factor-1alpha by BDNF protects retinoblastoma cells against chemotherapy-induced apoptosis. Mol Cell Biochem. 2016;414:77–84. doi: 10.1007/s11010-016-2660-y. [DOI] [PubMed] [Google Scholar]
- 58.Privratsky JR, Newman PJ. PECAM-1: regulator of endothelial junctional integrity. Cell Tissue Res. 2014;355:607–19. doi: 10.1007/s00441-013-1779-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chistiakov DA, Orekhov AN, Bobryshev YV. Endothelial PECAM-1 and its function in vascular physiology and atherogenic pathology. Exp Mol Pathol. 2016;100:409–15. doi: 10.1016/j.yexmp.2016.03.012. [DOI] [PubMed] [Google Scholar]
- 60.Yasinska IM, Sumbayev VV. S-nitrosation of Cys-800 of HIF-1alpha protein activates its interaction with p300 and stimulates its transcriptional activity. FEBS Lett. 2003;549:105–9. doi: 10.1016/s0014-5793(03)00807-x. [DOI] [PubMed] [Google Scholar]
- 61.Shunmugavel A, Khan M, Hughes FM, Jr., Purves JT, Singh A, Singh I. S Nitrosoglutathione protects the spinal bladder: novel therapeutic approach to post-spinal cord injury bladder remodeling. Neurourol Urodyn. 2015;34:519–26. doi: 10.1002/nau.22619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shunmugavel A, Khan M, Martin MM, Copay AG, Subach BR, Schuler TC, Singh I. S-Nitrosoglutathione administration ameliorates cauda equina compression injury in rats. Neurosci Med. 2012;3:294–305. doi: 10.4236/nm.2012.33034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Radomski MW, Rees DD, Dutra A, Moncada S. S-nitroso-glutathione inhibits platelet activation in vitro and in vivo. Br J Pharmacol. 1992;107:745–9. doi: 10.1111/j.1476-5381.1992.tb14517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hornyak I, Pankotai E, Kiss L, Lacza Z. Current developments in the therapeutic potential of S-nitrosoglutathione, an endogenous NO-donor molecule. Curr Pharm Biotechnol. 2011;12:1368–74. doi: 10.2174/138920111798280983. [DOI] [PubMed] [Google Scholar]