Abstract
Paraquat can lead to injury of multiple organs. As there is no specific treatment for paraquat poisoning, it represents a serious clinical problem. Antioxidants are required to treat oxidative stress in paraquat poisoning and paraquat-induced injury. In this study, we report on successful treatment of a rare case of epilepsy and acute pancreatitis caused by paraquat poisoning. A 29-year-old woman at our hospital presented with a 13-day history of nausea and vomiting after ingesting 20 mL of paraquat (20% W/V). After therapies, the symptoms were controlled. The patient remained asymptomatic during 1 year of follow-up.
Keywords: Epilepsy, acute pancreatitis, severe paraquat poisoning
Introduction
Paraquat, a quaternary nitrogen herbicide, has been widely used agriculturally in many countries since the 1960s. It is highly toxic to both humans and animals. Paraquat poisoning can lead to injury of multiple organs, including the kidneys, lungs, and gastrointestinal tract. When severe, ingestion of paraquat in humans and animals can be fatal. As there is no specific treatment for paraquat poisoning, it represents a serious clinical problem. The major cause of death in paraquat poisoning is respiratory failure caused by pulmonary fibrosis. Here, we report on successful treatment of a rare case of epilepsy and acute pancreatitis caused by paraquat poisoning.
Case presentation
A 29-year-old woman at our hospital presented with a 13-day history of nausea and vomiting after ingesting 20 mL of paraquat (20% W/V). After being transported to a local hospital, her vital signs were nearly stable. The concentrations of albumin and creatinine in blood were 28 g/L and 717 µmol/L, respectively. The diagnosis was severe paraquat poisoning. Initial interventions included admission for supportive care, hemoperfusion, and hemodialysis. She was concurrently treated with dexamethasone, tiopronin and antioxidants. Because of chest oppression and suffocation, she was transferred to our hospital for further therapy.
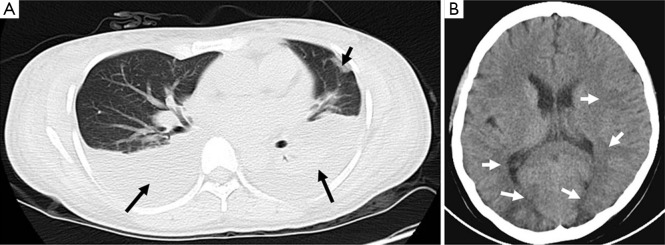
Upon admission, the patient was conscious, and her vital signs were normal. A chest spiral computed tomography (CT) showed pleural effusion and pulmonary exudate (Figure 1A). Basic blood laboratory testing showed a white blood cell count of 11.71×109/L, neutrophil 84.4%, hemoglobin 115 g/L, albumin 25 g/L, creatinine 371 µmol/L, K+ 3.4 mmol/L, and Na+ 132 mmol/L. The patient was treated with methylprednisolone sodium succinate (injection), N-acetyl cysteine (NAC) to scavenge free radicals, and piperacillin and sulbactam to prevent infection; other therapies were applied to protect the vital organs. On post-ingestion day #16 the patient lost consciousness due to new-onset seizures. She was treated with somatostatin and antiepileptic drugs until the epilepsy assuaged. The symptoms reappeared 1 h later, and diazepam and torsemide were administered for symptom relief. A cranial spiral CT showed low-density lesions on the parietal lobe, occipital lobe, and left basal ganglia (Figure 1B). These lesions were symptomatic of epilepsy. One day following the new-onset seizure activity, the patient developed increasing abdominal pain and distension; during evaluation it was noted that serum amylase and lipase levels were 435 and 1,298 U/L, respectively. These results are indicative of acute pancreatitis. The patient was then treated with octreotide acetate and ornidazole sodium chloride. After these therapies, the symptoms were controlled and further reduced. The patient was discharged from our hospital on the 31st day following paraquat poisoning. An electroencephalogram taken approximately 2 weeks after discharge appeared normal (Figure 2), and the patient remained asymptomatic during one year of follow-up.
Figure 1.
The chest and cranial spiral CT. (A) There were pleural effusion and pulmonary exudation in chest spiral CT; (B) there were low-density lesions on the parietal lobe, occipital lobe, and left basal ganglia in cranial spiral CT.
Figure 2.
An electroencephalogram appeared normal approximately 2 weeks after discharge.
Discussion
Pulmonary exudation, which can cause pulmonary fibrosis, was an important indicator of paraquat poisoning. The diagnosis depends on the patient’s description and toxicological test. The lung injury in our patient was serious after paraquat poisoning, and some complications were appeared. It can cause a large number of free radicals in vivo by paraquat. Free radicals can cause epileptic seizures (1). Elevated pancreatic enzymes have been found in patients with severe paraquat poisoning, and pancreatitis has been observed in the autopsies of paraquat poisoning patients (2). The usage of octreotide acetate and ornidazole sodium chloride in this patient is general usage when the patient appears to be intoxicated with paraquat and the amylase and lipase levels changes. Antioxidants are required to treat oxidative stress in paraquat poisoning (3-5) and paraquat-induced acute pancreatitis (6). Our patient, who had epilepsy and acute pancreatitis, was cured after receiving timely treatment. The symptoms were alleviated with hormones, antioxidants, and antibiotics. Therefore, a timely diagnosis and prompt treatment are beneficial for curing complications from severe paraquat poisoning.
Acknowledgements
None.
Informed Consent: Patient consent about data treatment was obtained upon patient admission as for routine practice at Beijing 307 Hospital.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Willmore LJ, Ueda Y. Posttraumatic epilepsy: hemorrhage, free radicals and the molecular regulation of glutamate. Neurochem Res 2009;34:688-97. 10.1007/s11064-008-9841-3 [DOI] [PubMed] [Google Scholar]
- 2.Gil HW, Yang JO, Lee EY, et al. The level and clinical significance of pancreatic enzymes in survivors of acute paraquat poisoning. Clin Toxicol (Phila) 2009;47:308-11. 10.1080/15563650902834497 [DOI] [PubMed] [Google Scholar]
- 3.Blanco-Ayala T, Andérica-Romero AC, Pedraza-Chaverri J. New insights into antioxidant strategies against paraquat toxicity. Free Radic Res 2014;48:623-40. 10.3109/10715762.2014.899694 [DOI] [PubMed] [Google Scholar]
- 4.Dinis-Oliveira RJ, Duarte JA, Sánchez-Navarro A, et al. Paraquat poisonings: mechanisms of lung toxicity, clinical features, and treatment. Crit Rev Toxicol 2008;38:13-71. 10.1080/10408440701669959 [DOI] [PubMed] [Google Scholar]
- 5.Díaz S, Martín-González A, Cubas L, et al. High resistance of Tetrahymena thermophila to paraquat: Mitochondrial alterations, oxidative stress and antioxidant genes expression. Chemosphere 2016;144:909-17. 10.1016/j.chemosphere.2015.09.010 [DOI] [PubMed] [Google Scholar]
- 6.Booth DM, Murphy JA, Mukherjee R, et al. Reactive oxygen species induced by bile acid induce apoptosis and protect against necrosis in pancreatic acinar cells. Gastroenterology 2011;140:2116-25. 10.1053/j.gastro.2011.02.054 [DOI] [PubMed] [Google Scholar]