Abstract
Clostridium botulinum type B was detected by multiplex PCR in the intestinal contents of a suddenly deceased 11-week-old infant and in vacuum cleaner dust from the patient's household. C. botulinum was also isolated from the deceased infant's intestinal contents and from the household dust. The genetic similarity of the two isolates was demonstrated by pulsed-field gel electrophoresis and randomly amplified polymorphic DNA analysis, thereby confirming that dust may act as a vehicle for infant botulism that results in sudden death.
Clostridium botulinum is an anaerobic spore-forming bacterium that produces botulinum neurotoxin, the causative agent of botulism. The spores of C. botulinum, considered harmless when ingested by healthy adults, may cause a toxigenic disease when ingested by infants less than 1 year of age, in whom the ingested spores may germinate, multiply, and produce toxin in the intestinal lumen (12, 15). The severity of the resultant illness may vary from an outpatient illness simulating failure to thrive to death without preceding signs, thus resembling sudden infant death syndrome (SIDS) (1, 2). Honey is the only spore-containing food item causally associated with infant botulism, but for the majority of cases the source of the causative spores remains unclear (11). Identical toxin types of C. botulinum isolated from the patient's surroundings, as well as from the patient, have been identified (1). However, C. botulinum organisms have also been detected in the environment of control infants who remained healthy (1). There are no previous reports on the genotyping of such isolates that could aid in ascertaining their epidemiologic association with the clinical illness.
An 11-week-old boy died unexpectedly in January 2002 a few hours after being fed, the final feeding being slower than normal. The baby was healthy and developing normally until his sudden and unexpected death. The death of the infant was classified by the pathologist as SIDS on the basis of the autopsy findings.
The autopsy specimens of the child were investigated as a part of a survey on the association of C. botulinum with SIDS in Finland. Serum, feces, and autopsy sections from the intestine and spleen were examined, as was the opened package of the infant's last meal of corn gruel.
The tissue samples and the corn gruel were inoculated in several parallel 10-ml Trypticase-peptone-glucose-yeast extract (TPGY) broths (8) and incubated under anaerobic conditions at 37 or 30°C. Cells from the enrichment culture were harvested and washed with Tris-EDTA buffer in accordance with a previously described method (6) in order to prepare a template for PCR. The samples were studied with a multiplex PCR assay targeted to the neurotoxin genes of C. botulinum types A, B, E, and F (9). Toxin detection was performed on serum with a mouse bioassay (13). Animal testing was performed in accordance with the national guidelines and with the permission of the committee for animal experiments, University of Helsinki. The amount of feces and intestinal contents was inadequate for the detection of toxin.
The C. botulinum type B toxin gene was identified by PCR in two parallel samples of the infant's intestinal contents, both incubated at 37°C. C. botulinum was isolated from the PCR-positive samples by plating 0.1 ml of TPGY broth on egg yolk agar plates and incubating them under anaerobic conditions at 37°C for 2 days (19). C. botulinum type B was successfully isolated from one of the intestinal specimens. All other autopsy specimens were negative for C. botulinum, and the serum did not contain detectable botulinum toxin.
Family members of the deceased child were asked about the previous health of the infant, his feeding and nursing habits, the nearby environment, possible pets, and travel. Samples collected from the household included soil from flower pots and a vacuum cleaner dust bag. The child had been healthy since birth except for a 2-day period of constipation at the age of 4 weeks, but no signs of constipation preceded his death. He had been breast fed until 6 weeks of age, after which the breast milk had been gradually replaced with commercial formula milk and corn gruel. Honey was not included at any point in the infant's diet. No environmental factors predisposing to infant botulism, e.g., nearby construction (10), were noted.
The household samples were studied by multiplex PCR in a total of 240 parallel assays to estimate the quantity of possible C. botulinum spores in the patient's environment by the most-probable-number technique. The sample sizes were 40 g of soil from the flower pots and 100 g of vacuum cleaner dust. Each soil or dust subsample inoculated in TPGY broth weighed 1.0 or 0.5 g, respectively. The results obtained with these one-dilution most-probable-number series were used to calculate an estimated spore count with Thomas's approximation (18).
C. botulinum type B was detected by PCR in, and also isolated from, the vacuum cleaner dust. The quantity of spores in the dust was estimated to be 10/kg of examined material. The other household samples were negative for C. botulinum by TPGY culture and PCR analysis.
The C. botulinum isolates from the patient's intestinal contents and from the vacuum cleaner dust were confirmed to produce botulinum toxin with a mouse bioassay. The proteolytic activity of the isolates was demonstrated by plating the strains on reinforced clostridium medium containing 5% (vol/wt) skim milk (14). The two C. botulinum type B isolates were genotyped by pulsed-field gel electrophoresis (PFGE) typing (5) and randomly amplified polymorphic DNA (RAPD) analysis (7).
Isolation of DNA for PFGE was performed as previously described (5). Samples were electrophoresed at 8°C through a 1% (wt/vol) agarose gel (SeaKem Gold agarose; BMA, Rockland, Maine) in 0.5× TBE buffer (Amresco, Solon, Ohio) at 200 V with a Gene Navigator system (Pharmacia, Uppsala, Sweden) with a hexagonal electrode. With SmaI the running time was 22 h with a pulse time ramp of 1 to 26 s, and with XhoI the running time was 18 h with a pulse time ramp of 1 to 15 s. Low Range PFG marker (New England Biolabs, Beverly, Mass.) was used for fragment size evaluation. RAPD analysis was performed as previously described (7) with Ready-To-Go RAPD analysis beads (Amersham Pharmacia Biotech Inc., Piscataway, N.J.). The thermal profile consisted of denaturation at 95°C for 5 min, following 45 cycles of denaturation at 95°C for 1 min, annealing at 36°C for 1 min, and extension at 72°C for 2 min.
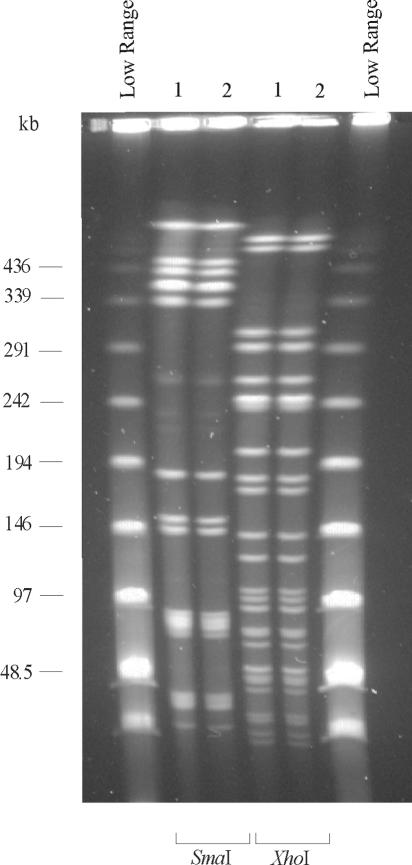
Both molecular typing methods indicated that the patient intestinal isolate and the dust isolate of C. botulinum type B were clonal (Fig. 1 and 2).
FIG. 1.
PFGE patterns of the C. botulinum strains isolated from the infant (lane 1) and from vacuum cleaner dust (lane 2). Digestion was performed with restriction enzymes SmaI and XhoI. The outermost lanes contain Low Range PFG marker.
FIG. 2.
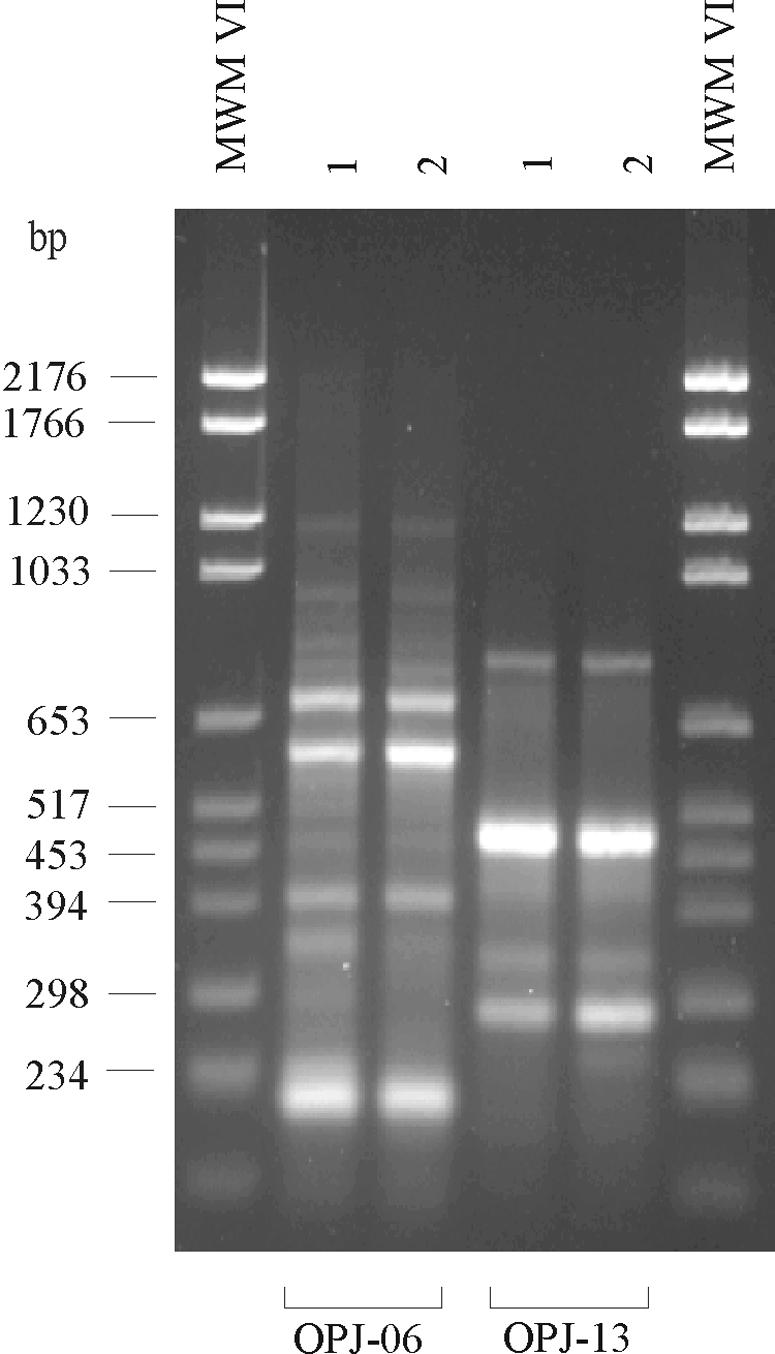
RAPD patterns of the C. botulinum strains isolated from the infant (lane 1) and from vacuum cleaner dust (lane 2). The patterns were generated with universal primers OPJ-6 and OPJ-13. The outermost lanes contain molecular weight marker (MWM) VI.
This study was the first instance in which PCR methodology was successfully used to detect the botulinum toxin gene in human intestinal contents. In addition, for the first time the C. botulinum isolates from an intestinally colonized infant and from household dust from the infant's home were demonstrated to be genetically similar. This genetic similarity suggests that airborne spores of C. botulinum in an infant's surroundings may cause infant botulism and, as in this case, also result in the fulminant form of the illness that resembles SIDS. Previous studies have shown that C. botulinum is not a part of the normal human infant intestinal microflora (4, 16, 17). Also, because C. botulinum ordinarily competes poorly with other intestinal bacteria, isolation of C. botulinum from the intestinal contents suggests that a relatively large number of spores was initially ingested, the organism multiplied substantially in the intestinal lumen, or both. According to the case definition of infant botulism used in the United States (3), infant botulism is an illness consistent with the known paralyzing action of botulinum toxin in which C. botulinum toxin and/or organisms are identified in serum or fecal specimens. However, in practice botulinum toxin is rarely found in the serum of infant botulism patients with fecally diagnosed illness (4). In accordance with the U.S. definition, the case reported here may be considered to be infant botulism and is the first case of infant botulism to be recognized in Finland. Our experience suggests that the possibility of this diagnosis should not be overlooked in sudden infant death cases in countries with a low reported incidence of infant botulism.
REFERENCES
- 1.Arnon, S. S., K. Damus, and J. Chin. 1981. Infant botulism: epidemiology and relation to sudden infant death syndrome. Epidemiol. Rev. 3:45-66. [DOI] [PubMed] [Google Scholar]
- 2.Arnon, S. S., T. F. Midura, K. Damus, R. M. Wood, and J. Chin. 1978. Intestinal infection and toxin production by Clostridium botulinum as one cause of sudden infant death syndrome. Lancet i:1273-1277. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. 1997. Case definitions for infectious conditions under public health surveillance. Morb. Mortal. Wkly. Rep. 46(RR-10):1-55. [PubMed] [Google Scholar]
- 4.Hatheway, C. L., and L. M. McCroskey. 1987. Examination of feces and serum for diagnosis of infant botulism in 336 patients. J. Clin. Microbiol. 25:2334-2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hielm, S., J. Björkroth, E. Hyytiä, and H. Korkeala. 1998. Genomic analysis of Clostridium botulinum group II by pulsed-field gel electrophoresis. Appl. Environ. Microbiol. 64:703-708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hielm, S., E. Hyytiä, J. Ridell, and H. Korkeala. 1996. Detection of Clostridium botulinum in fish and environmental samples using polymerase chain reaction. Int. J. Food Microbiol. 31:357-365. [DOI] [PubMed] [Google Scholar]
- 7.Hyytiä, E., S. Hielm, J. Björkroth, and H. Korkeala. 1999. Biodiversity of Clostridium botulinum type E strains isolated from fish and fishery products. Appl. Environ. Microbiol. 65:2057-2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lilly, T., S. M. Harmon, D. A. Kautter, H. M. Solomon, and R. K. Lynt. 1971. An improved medium for detection of Clostridium botulinum type E. J. Milk Food Technol. 34:492-497. [Google Scholar]
- 9.Lindström, M., R. Keto, A. Markkula, M. Nevas, S. Hielm, and H. Korkeala. 2001. Multiplex PCR assay for detection and identification of Clostridium botulinum types A, B, E, and F in food and fecal material. Appl. Environ. Microbiol. 67:5694-5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Long, S. S., J. L. Gajewski, L. W. Brown, and P. H. Gilligan. 1985. Clinical, laboratory, and environmental features of infant botulism in southeastern Pennsylvania. Pediatrics 75:935-941. [PubMed] [Google Scholar]
- 11.Midura, T. F. 1996. Update: infant botulism. Clin. Microbiol. Rev. 9:119-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Midura, T. F., and S. S. Arnon. 1976. Infant botulism: identification of Clostridium botulinum and its toxin in faeces. Lancet ii:934-936. [DOI] [PubMed] [Google Scholar]
- 13.Nordic Committee on Food Analysis. 1991. Botulinum toxin. Detection in foods, blood and other test materials. NCFA method no. 79, 2nd ed. Nordic Committee on Food Analysis, Espoo, Finland.
- 14.Peck, M. W., D. A. Fairbarn, and B. M. Lund. 1992. The effect of recovery medium on the estimated heat-inactivation of spores of non-proteolytic Clostridium botulinum. Lett. Appl. Microbiol. 15:146-151. [DOI] [PubMed] [Google Scholar]
- 15.Pickett, J., B. Berg, E. Chaplin, and M.-A. Brunstetter-Schafer. 1976. Syndrome of botulism in infancy: clinical and electrophysiologic study. N. Engl. J. Med. 295:770-772. [DOI] [PubMed] [Google Scholar]
- 16.Stark, P. L., and A. Lee. 1982. Clostridia isolated from the feces of infants during the first year of life. J. Pediatr. 100:362-365. [DOI] [PubMed] [Google Scholar]
- 17.Stark, P. L., and A. Lee. 1982. The microbial ecology of the large bowel of breast-fed and formula-fed infants during the first year of life. J. Med. Microbiol. 15:189-203. [DOI] [PubMed] [Google Scholar]
- 18.Thomas, H. A. 1942. Bacterial densities from fermentation tube tests. J. Am. Water Works Assoc. 34:572-576. [Google Scholar]
- 19.U.S. Food and Drug Administration. 2001. Bacteriological analytical manual online. [Online.] http://www.cfsan.fda.gov/∼ebam/bam-toc.html. Accessed 15 August 2004.