The considerable progress achieved in the past 10 years in the field of tumor biology and therapeutics has strengthened the idea that cancer is not only a cellular but also a tissue disease. This concept is likely to apply to nasopharyngeal carcinoma (NPC), characterized by the consistent expression of oncogenic viral proteins in a context of inflammation and immune escape (1). In its typical undifferentiated form, NPC is constantly associated with the Epstein-Barr virus, whose genome is contained in the nuclei of all malignant cells, but not in the surrounding tissue. Latency is the predominant mode of virus-cell interactions, meaning that most viral genes are silent in the vast majority of malignant cells. However, a small fraction of them are consistently expressed coding a handful of viral products, both viral proteins and untranslated RNAs, most of them with proven oncogenic properties. The inflammatory context of NPC is obvious for pathologists: almost all NPC primary tumors are heavily infiltrated by non-malignant leucocytes, mainly T-lymphocytes but also B lymphocytes, macrophages, dendritic cells and neutrophils. This inflammatory infiltration often disappears in metastatic lesions. The immune escape is also obvious because of the rapid proliferation of malignant cells despite the consistent expression of EBNA1, LMP1 and LMP2 which are known to be the targets of CD4+ and CD8+ cytotoxic T-cells in EBV-carriers. One interpretation of the paradox of tumor inflammation combined with tumor immune escape is that malignant cells in the primary tumor benefit to some extent from the proximity of leucocytes while developing mechanisms of immune escape.
High-scale genomic studies have brought evidence that the immune escape mechanisms in NPC can be cellular intrinsic alterations, for example defects in the expression of HLA class I molecules (Lo KW, 17th International Symposium on Epstein-Barr virus and associated diseases, Zurich, August 2016, abstract EBV2016-1040). These alterations are probably the most difficult to deal with for the oncologist. However, there is also evidence of a major contribution of extra-cellular “micro-environmental” immunosuppressive factors. Data from previous studies support the role of immunosuppressive proteins either secreted in a soluble form or carried by tumor exosomes, for example CCL20, galectin-9 or IDO (indoleamine 2, 3-dioxygenase) (2-4). One recent elegant publication from Jiang Li’s group in Guangzhou provides new insight on the role of tumor exosomes carrying immunosuppressive microRNAs (5) (Figure 1). For the sake of brevity, one can distinguish two types of results in this study. Most data are based on in vitro experiments. They demonstrate that malignant cells mixed with T-cells from healthy donors can deliver miR-24-3p to these T-cells using exosomes as intercellular carriers. Then it is shown that miR-24-3p decreases the proliferation of target T-cells by down-regulation of FGF11 and subsequent modifications of ERK and STAT protein phosphorylation. Simultaneously, there is a decrease in the expression of interferon-γ and IL-17 expression in CD4 T-cells suggesting the impairment of Th1 and Th17 differentiation. From these data, the authors infer that similar interactions are likely to occur in the tumor microenvironment where malignant cells are in close contact with tumor infiltrating lymphocytes (TILs). Other data, based on investigations of serum samples or tumor tissue sections, support this inference. First, the authors show that the abundance of FGF11 in TILs is inversely correlated with the serum concentration of miR-24-3p. Later, they show on tumor sections that a low abundance of CD4+ and CD8+ TILs correlates with a low abundance of FGF11 in TILS (and in malignant cells as well). Moreover, a high concentration of exosomal miR-24-3p in the serum and a low amount of FGF11 in TILs and malignant cells were associated with a shorter disease-free survival. A few more experiments demonstrated that, at least in vitro, hypoxia enhances the concentration of miR-24-3p in tumor exosomes. The data are less consistent with regard to regulatory T-cells (T-regs). Indeed, in vitro T-regs’ expansion and Fox-P3 expression were enhanced by the uptake of miR-24-3p and FGF11 down-regulation. However, in vivo, there was no significant correlation between the abundance of Fox-P3-positive cells among stromal cells and the depletion of FGF11 in TILs and malignant cells. This reminds us that the role of T-regs in NPC remains controversial (3,6).
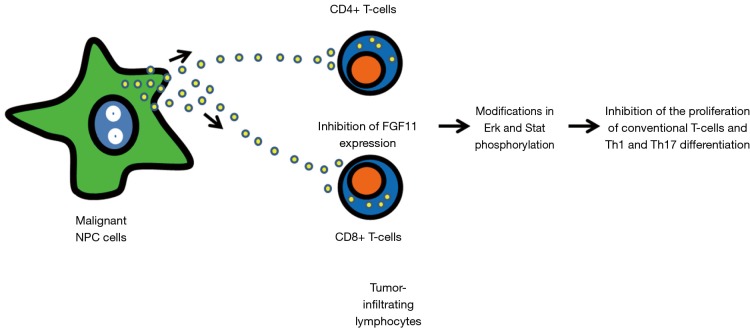
Figure 1.
Main steps of the scenario linking the inhibition of the proliferation and differentiation of tumor-infiltrating T-lymphocytes to the release of tumor exosomes carrying miR-24-3p in the microenvironment of nasopharyngeal carcinomas. Exosomes carrying miR-24-3p are released by malignant NPC cells and up-taken by tumor-infiltrating lymphocytes. The internalization of miR-24-3p in target cells results in the downregulation of FGF11 with subsequent modifications in the phosphorylation of Erk and Stat proteins. These signaling events result in the inhibition of the proliferation of conventional T-cells (CD4+ and CD8+) and probably impair Th1 and Th17 differentiation. This scenario is based on in vitro and in vivo investigations reported by Ye et al. (5).
In terms of methodology, it is important to note that almost all in vitro investigations were done using an EBV-negative malignant epithelial cell line, TW03. This approach is likely to have both positive and negative consequences. TW03 cells are easier to handle in vitro that genuine NPC cells carrying an endogenous EBV genome. The authors have largely taken advantage of the ease of DNA transfection into these cells to make an intense use of microRNA mimics, microRNA sponges and reporter assays. However, in many respects, TW03 cells lack several major characteristics of NPC cells. For example, NPC cells carry on their surface an array of inflammatory molecules like HLA class II molecules, CD54 and CD70 which are not found on TW03 (1). Moreover, a huge fraction of the total microRNAs produced by NPC cells—often as much as one sixth or even one third of them—are EBV—encoded microRNAs of which some might have an impact on T-cell functions (7). On the other hand, to a large extent, TW03 cells have a phenotype which is reminiscent of the phenotype of malignant cells from squamous cell carcinomas of the upper aero-digestive tract. Therefore, the findings reported by Ye et al. may have applications for non-NPC epithelial malignancies, for example squamous carcinomas of the upper aero-digestive tract where hypoxia is often highly prevalent.
Tumor immunosuppression is usually a multifactorial process. As mentioned previously, other immuno-suppressive factors, especially proteins are known to be released by NPC cells either in a soluble form or conveyed by exosomes. Therefore an integrated approach will be required to assess the respective contributions to the immune evasion of NPCs of the various immuno-suppressive agents, regardless of their chemical nature, proteins, nucleic acids and probably lipids like prostaglandins. It is noteworthy that in virtually all experiments reported by Ye et al., the reduction of the T-cell proliferation induced by exosomes carrying the miR-24-3p did not exceed 20%. Thus, there is ample room for potential synchronic or non-synchronic cooperation of miR-24-3p with other immunosuppressive factors. In future research, one major challenge will be to identify the predominant mechanisms of immune suppression for each clinical and molecular subtype of NPC or even for a given patient, at each stage of his treatment and surveillance. NPC clearly is a heterogeneous disease, in terms of growth pattern (with either early metastases or predominantly local growth), in terms of malignant cell phenotypes (with more or less epithelio-mesenchymal transition) or in terms of immune “contexture” (variable relative abundance of various types of T-lymphocytes, macrophages, NK cells and dendritic cells) (1,6,8). Although currently there is no consensus on molecular subcategories, it is obvious that the NPC malignant phenotypes can be supported by different genetic and epigenetic alterations as well as different modes of virus-cell interactions, for example a high, low or very low level of LMP1 expression (Lo KW, 17th International Symposium on Epstein-Barr virus and associated diseases, Zurich, august 2016, abstract EBV2016-1040). The work published by Ye et al. is also quite exemplary insofar as it shows the importance of combining the investigations on the tumor tissue with investigations on serum or plasma samples (5). One can presume that, in the future, the diagnosis of the immune “contexture” and immune suppressive mechanisms will rely on tumor tissue analysis combined with assays performed on serum or plasma factors including microRNAs and proteins like CCL20 and galectin-9 (8).
What are the consequences of the findings reported by Ye et al., in terms of therapeutics? One option seems to be the use of miR antagonists (antagomiRs or anti-miRs) to neutralize plasma miR-24-3p. Vectorization of these antagonists to the malignant cells or the tumor-infiltrating leucocytes remains a major challenge. Another option is to attempt a capture and depletion of tumor exosomes using systemic injections of therapeutic antibodies. Because exosomes released by endothelial cells or leukocytes are very abundant in plasma and many interstitial fluids, it will be necessary to use antibodies reacting with molecules expressed selectively on the surface of NPC exosomes. Some years ago, we made the empirical and surprising observation that selective capture of tumor exosomes from plasma samples from NPC patients was facilitated by the use of anti-HLA class II antibodies (2). Galectin-9 is another protein present on the surface of NPC tumor exosomes (ibid.). Thus, the use of anti-galectin-9 or anti-HLA II antibodies might play a role in a future strategy of NPC exosome capture and depletion.
Acknowledgements
The authors would like to thank Bristol-Myers Squibb Foundation (2016-2017) for research funding.
Provenance: This is a Guest Editorial commissioned by Section Editor Mingzhu Gao (Department of Laboratory Medicine, Wuxi Second Hospital, Nanjing Medical University, Wuxi, China).
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Gourzones C, Barjon C, Busson P. Host-tumor interactions in nasopharyngeal carcinomas. Semin Cancer Biol 2012;22:127-36. 10.1016/j.semcancer.2012.01.002 [DOI] [PubMed] [Google Scholar]
- 2.Klibi J, Niki T, Riedel A, et al. Blood diffusion and Th1-suppressive effects of galectin-9-containing exosomes released by Epstein-Barr virus-infected nasopharyngeal carcinoma cells. Blood 2009;113:1957-66. 10.1182/blood-2008-02-142596 [DOI] [PubMed] [Google Scholar]
- 3.Mrizak D, Martin N, Barjon C, et al. Effect of nasopharyngeal carcinoma-derived exosomes on human regulatory T cells. J Natl Cancer Inst 2014;107:363. 10.1093/jnci/dju363 [DOI] [PubMed] [Google Scholar]
- 4.Ben-Haj-Ayed A, Moussa A, Ghedira R, et al. Prognostic value of indoleamine 2,3-dioxygenase activity and expression in nasopharyngeal carcinoma. Immunol Lett 2016;169:23-32. 10.1016/j.imlet.2015.11.012 [DOI] [PubMed] [Google Scholar]
- 5.Ye SB, Zhang H, Cai TT, et al. Exosomal miR-24-3p impedes T-cell function by targeting FGF11 and serves as a potential prognostic biomarker for nasopharyngeal carcinoma. J Pathol 2016;240:329-40. 10.1002/path.4781 [DOI] [PubMed] [Google Scholar]
- 6.Zhang YL, Li J, Mo HY, et al. Different subsets of tumor infiltrating lymphocytes correlate with NPC progression in different ways. Mol Cancer 2010;9:4. 10.1186/1476-4598-9-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee KT, Tan JK, Lam AK, et al. MicroRNAs serving as potential biomarkers and therapeutic targets in nasopharyngeal carcinoma: A critical review. Crit Rev Oncol Hematol 2016;103:1-9. 10.1016/j.critrevonc.2016.04.006 [DOI] [PubMed] [Google Scholar]
- 8.Becht E, Giraldo NA, Germain C, et al. Immune Contexture, Immunoscore, and Malignant Cell Molecular Subgroups for Prognostic and Theranostic Classifications of Cancers. Adv Immunol 2016;130:95-190. 10.1016/bs.ai.2015.12.002 [DOI] [PubMed] [Google Scholar]