Abstract
Background
Reproductive maturation is initiated with the onset of puberty, which activates the hypothalamic-pituitary-gonadal axis and coincidences with increased expression of the hormone kisspeptin within the hypothalamus. Maturational events are sensitive to environmental factors, including alcohol, which is known to delay reproductive development. We hypothesized that, similar to alcohol's adverse effects during reproductive maturation, prenatal alcohol exposure (PAE) would alter pubertal markers, sex hormone profiles, and kisspeptin expression in the hypothalamus.
Methods
Female offspring from control (C), pair-fed (PF), and PAE groups were sacrificed prior to puberty onset (postnatal day [PND] 30), during puberty (PND 35), or in adulthood (PND 65). Estradiol (E2), progesterone (P4), prolactin (PRL) and luteinizing hormone (LH) levels, and Kiss1 mRNA expression were measured in the arcuate (ARC) and anteroventral periventricular (AVPV) nuclei of the hypothalamus. Pubertal markers (vaginal opening, uterus/body weight ratio) were assessed.
Results
Our findings indicate that: 1) PAE inhibits the expected increases in E2 levels with age and delays maturational increases of P4 levels; 2) PAE and pair-feeding have similar adverse effects on: i) vaginal opening and uterus/body weight ratio; 3) Differential relationships between PRL and P4 suggest that different mechanisms may underlie delayed maturation in PAE and PF; that is, PF females have low PRL levels and no increase in P4 with age, whereas PAE animals, despite low PRL, show the expected age-related increase in P4. 4) There is higher mean density of Kiss1 mRNA in the ARC of adult PAE females and altered Kiss1 expression in the AVPV of both PAE and PF females.
Conclusions
PAE and pair-feeding have some overlapping but important differential effects on hormonal profiles and Kiss1 mRNA expression during reproductive development. Pre-adolescent alterations in Kiss1 expression in the AVPV and ARC, which may change the balance of function in these two nuclei, may differentially contribute to delayed reproductive maturation in PAE and PF compared to C females.
Keywords: prenatal alcohol (ethanol), pair-feeding, kisspeptin, puberty, sex hormones
INTRODUCTION
Acquisition of reproductive maturity involves one of the most important series of developmental events in an organism's life. In female mammals, reproductive maturation is initiated with the onset of puberty, which activates the hypothalamic-pituitary-gonadal (HPG) axis, leading to gonadotropin-releasing hormone (GnRH) release. This in turn activates luteinizing hormone (LH) release and the occurrence of the first pre-ovulatory surge of gonadotropins [LH and follicle-stimulating hormone (FSH)] from the pituitary (Ojeda et al., 2006). This process can be affected by adverse environmental factors such as stress, undernutrition, and alcohol. To date neither the specific processes necessary for reproductive maturation, nor their sensitivity to environmental factors, are fully understood (Soliman et al., 2014, Dees et al., 2015).
Similar to alcohol's adverse effects during reproductive maturation, prenatal alcohol exposure (PAE) may also adversely impact the reproductive system. While there is a paucity of clinical research, the few studies that have examined individuals with fetal alcohol spectrum disorder (FASD) have revealed adverse effects of PAE on the reproductive system (Robe et al., 1979, Carter et al., 2014). Daughters of women who drank heavily during pregnancy showed a trend to late menarche (Robe et al., 1979). Similarly, PAE was related to elevated testosterone concentrations and decreased tissue responsiveness to testosterone for both males and females but not to changes in Tanner stages or age at menarche (Carter et al., 2014).
Studies using animal models of PAE show clear evidence of altered HPG development and function in offspring. PAE delays the onset of puberty, reduces LH responses to estradiol (E2) and progesterone (P4) priming (Handa et al., 1985), delays FSH secretion, reduces perinatal and postnatal surges of testosterone (Wilson and Handa, 1997, Emanuele et al., 2002a, Dees et al., 2015), delays onset of spermatogenesis (Lan et al., 2013), and results in the early onset of acyclicity (McGivern et al., 1995). Thus, the effects of PAE on the reproductive system are manifest as alterations in reproductive function and a shortening of the reproductive window. However, the mechanism(s) of these reproductive deficits are not fully understood.
Previously, we reported that a potential mechanism of PAE effects on adult HPG functions involves the actions of alcohol on the kisspeptin system (Sliwowska et al., 2014). Kisspeptins, products of the Kiss1 gene, play a crucial role in regulation of the HPG axis. Female and male rats show an increase in Kiss1 in the hypothalamus that coincides with the onset of puberty (Navarro et al., 2004a). Chronic central administration of kisspeptin-10 (KP-10, a potent endogenous ligand of the kisspeptin receptor [KISS1R, known also as GPR54]) to immature female rats induces precocious activation of the HPG axis manifested by advanced vaginal opening (VO - complete canalization of vagina, an external sign of puberty), elevated uterus weight and increased LH and E2 levels (Navarro et al., 2004b). Conversely, mutations of the kisspeptin receptor have been associated with a failure to progress through puberty, resulting in hypogonadotropic hypogonadism and infertility both in mice and humans (de Roux et al., 2003, Seminara et al., 2003). In light of our findings in adults (Sliwowska et al., 2014) and the role of kisspeptin in the onset of puberty, we undertook the present study to investigate potential mechanisms of delayed puberty in PAE females in our rat model of moderate PAE.
Female offspring from control (C), pair-fed (PF), and PAE dams were sacrificed prior to puberty onset (postnatal day 30 - PND 30), around the time of puberty (PND 35), or in young adulthood (PND 65). Body and uterus weights, plasma levels of E2, P4, prolactin (PRL), and LH, and expression of Kiss 1 mRNA in the anteroventral periventricular (AVPV) and arcuate (ARC) nuclei of the hypothalamus were assessed. VO, as an index of sexual maturation, was also noted. We hypothesized that, compared to C and PF controls, PAE would result in both short-term and long-lasting consequences for HPG function that would manifest as a delay in the onset of puberty (as indicated by VO and uterus/body wt ratios), as well as altered reproductive hormone profiles, including deficits or delays in attainment of adult levels of E2, P4, PRL, and LH. Further, we hypothesized that alterations in Kiss1 mRNA expression in the AVPV and ARC nuclei may contribute to the observed changes in reproductive development in PAE compared to C and PF females.
MATERIALS AND METHODS
Breeding and feeding
Sprague-Dawley rats (Charles River Laboratories, St Constant, PQ, Canada) were group-housed by sex for a period of 1-2 weeks, with ad libitum access to laboratory chow (Jamieson's Pet Food Distributors Ltd, Delta, BC, Canada) and water, with temperature (21-22°C) and lighting (on from 0600 h to 1800 h). Next males were individually housed with females in suspended stainless steel cages with wire mesh front and floor, and checked daily for the presence of vaginal plugs, which indicated gestation day 1 (GD 1). On GD 1, females were singly housed and assigned to groups: (1) Alcohol (ethanol) (PAE), n = 9; ad libitum access to a liquid ethanol diet (36% ethanol-derived calories); (2) pair-fed (PF), n = 11; liquid control diet with maltosedextrin isocalorically substituted for ethanol and intake matched by gestation day to the amount consumed by a yoked alcohol-consuming dam (g/kg body weight); and (3) control (C), n = 11; ad libitum access to a pelleted version of the liquid control diet. Dams all had ad libitum access to water. Liquid diets were formulated to provide adequate nutrition to pregnant rats regardless of ethanol intake, and prepared by Dyets Inc., Bethlehem, PA (Weinberg/Keiver High Protein Ethanol [#710324] and Control [#710109] Diets; Weinberg/Keiver High Protein Pelleted Control Diet #710109) (Sliwowska et al., 2008, Uban et al., 2013, Sliwowska et al., 2014). For all diets, 25% kcal was derived from protein and 16.4% from fat. Both liquid and pelleted Control diets had 58.6% kcal derived from carbohydrate, whereas the liquid ethanol diet had 23% kcal derived from carbohydrate and 35.5% kcal from ethanol. Over many years of using this model, we have shown that consumption of this liquid ethanol diet results in blood alcohol levels of ~120-150 mg/dl (Sliwowska et al., 2008, Uban et al., 2013).
Females were handled only during cage changing and weighing. On GD 22, experimental diets were replaced with ad libitum access to laboratory chow and water. On postnatal day (PND) 1, dams and pups were weighed and litters randomly culled to 10 (5 females, 5 males when possible). Pups were weighed and cages changed weekly. On PND 22, pups were weaned and group-housed by litter and sex. Only female offspring were used in this study.
Brain, Blood and Organ Collection
Animals were weighed prior to brain and blood collection, between 0900 h and 1200 h. To control for litter effects, no more than 1 or in a few instances 2 female offspring per litter from each of C, PF, and PAE dams were sacrificed on PND 30, 35, or 65, n = 8-14 females/prenatal group/age. Ages were chosen based on the mean timing of VO and hormonal changes (Navarro et al., 2004a, Navarro et al., 2004b, Lan et al., 2009). Animals were decapitated and trunk blood collected into tubes containing 100μL 0.5M EDTA per 2 ml blood collected (to prevent coagulation), centrifuged (1,880xg, 10 min at 4°C), and plasma stored at −80°C. Brains were flash-frozen and stored at −80°C until sectioning. Uteri were collected and weighed. VO was assessed at the time of sacrifice in PND 30 and PND 35 females.
Coronal sections (20μm) were collected through the AVPV (from Bregma 0.48 mm to at Bregma −1.08 mm), and the ARC, (from Bregma 11.32 mm to Bregma −2.92 mm), using a microtome cryostat (HM 505E; MICROM International GmbH, Germany). Sections were placed on slides (twelve slides with four sections each for each nucleus) and stored at −80°C. Sections from the AVPV (n=8/animal/prenatal group/age) and ARC (middle ARC, n=4/animal/prenatal group/age) were chosen for in situ hybridization.
In situ hybridization for Kiss1 mRNA
KISS-1 (409bp) inserted into pAMP1 was generously provided by Dr. RA Steiner (University of Washington, Seattle, WA). Plasmid stocks were prepared by transformation and GenElute HP plasmid midi-preps (Sigma Aldrich, Oakville ON). Plasmids were linearized by restriction enzymes, HIND111 and EcoR1 for an anti-sense strand and sense strand respectively. Pilot in situs assessed probe specificity. The riboprobe was transcribed using 33-P UTP (Perkin-Elmer, Waltham, MA) and polymerases T7 (antisense) and SP6 (sense), purified using Roche RNA G50 Sephadex Quick Spin columns (Sigma-Aldrich, Oakville, ON) then stored at −20°C. Slides were thawed at RT, fixed in 10% buffered formalin (30min); rinsed twice in 1xPBS (10min), then Proteinase K (0.1ug/ml, 37°C, 9min), NF water (5min), 0.1M TEA/0.09% NaCl + 0.25% Acetic Acid (10min), 2xSSC (5min), dehydrated 50,75,95,100% ethanol (1min), chloroform (5min) then 100% EtOH (1min). Slides were dried (30min) prior to probe application (1×106cpm/slide) in hyb buffer (75% formamide, 3xSSC, 1x Denhardt's solution, 0.2mg/ml tRNA, 0.5% 1M NaPO4 pH7.4, and 10% Dextran Sulphate), Hybrislips (Sigma-Aldrich, Oakville,ON) applied and slides incubated in trays humidified with 75% formamide overnight at 55°C. The next day, hybrislips were removed and slides washed as follows; 2xSSC (30min twice), RNase A (100mg/L, 37°C, 1hr), 2xSSC (10min), 1xSSC (10min), 0.5xSSC (10min), 0.1xSSC at 62°C (1hr) and 0.1xSSC RT (5min). Slides were dehydrated through 50,75,95,100% ethanol (1min) and allowed to dry for at least 24 hours before being dipped in NTB emulsion (Eastman Kodak Co., Rochester, NY) diluted 1:1 with distilled water. Slides were exposed for 15 days, developed using Kodak D-19 developer and Kodak Fixer at 14 °C, and counterstained with Cresyl Violet prior to image analysis.
Analysis of Kiss1 mRNA signal
Slides were analyzed by investigators double-blind to prenatal group and age. Kiss1 mRNA positive cells were visualized with a Q-imaging monochrome 12-bit camera attached to an Axioskop 2 microscope (Zeiss, Jena, Germany). Images were captured using Northern Elite 6.0v (Empix Imaging Inc., Mississauga, ON, Canada) and semi-quantitative densitometric analyses were performed using Image J 1.33v software (National Institutes of Health, Bethesda, MD, USA). The mean optical density (OD) of hybridization signal was measured under dark-field illumination. The AVPV and ARC were traced by outlining under the bright field. Illumination was then switched to dark-field, the tracing was restored over the image, and OD measurements were taken. Background signal was measured over a region adjacent to the AVPV and ARC, and corrected grey levels were averaged from 4 measurements.
Radioimmunoassays (RIA)
Estradiol
Plasma E2 levels were measured using the Double Antibody Estradiol RIA kit (Siemens, Oakville, ON, Canada), using [125I] estradiol as the tracer. The minimum detectable E2 concentration was 1.4 pg/mL, and the intra- and inter-assay coefficients of variation were 4.8% and 5.5%, respectively.
Progesterone
Plasma P4 levels were measured by RIA using the ImmuChem Double Antibody Progesterone Kit (MP Biomedical, Costa Mesa, CA, USA) using [125I] as the tracer. The minimum detectable P4 concentration was 0.2ng/ml and the intra- and inter-assay coefficients of variation were 3.6% and 6.7% respectively.
Prolactin
Plasma PRL levels were measured using the NIDDK rat kit, which was supplied by Dr. A.F. Parlow at the National Hormone Pituitary Program. This kit included reference preparation NIDDK-rPRL-RP-3 and anti-rat prolactin S-9. Assays sensitivity averaged at 0.5 ng/ml, inter-assay and intra-assay coefficients of variation were 9% and 5% respectively.
Luteinizing Hormone
The rLH assays were performed by the Endocrine Laboratory, ARBL, Colorado State University, Fort Collins, CO. The kit utilized includes reference preparation NIDDK-rLH-RP-3 from Dr. A.F. Parlow at NIDDK's National Hormone and Peptide Program.
Statistical Analyses
Statistical analyses were performed with Statistica 12.0 software (StatSoft, Inc.). Developmental data were analyzed using repeated-measures analyses of variance (RM-ANOVA) with prenatal group (C, PF, PAE) as the between-subjects factor; within-subjects factors were day of gestation or lactation for the dams, or postnatal day for the offspring. Female offspring from this experiment were part of larger study and developmental data were reported previously (Uban et al., 2013). Uterus/body weight ratios, hormonal, and kisspeptin data were analyzed using ANOVAs for the factors of prenatal group (C, PF, PAE) and age (PND 30, PND 35, PND 65). Significant main and interaction effects were further analyzed by Fisher least significant differences (LSD) post-hoc tests. In addition, a priori tests were used in some analyses, as indicated, according to the stated hypotheses. Statistical significance was set at p < 0.05, and a trend was noted for p values between 0.05 and 1.0.
RESULTS
Developmental data
Body weights of dams and pups during gestation and lactation were reported in detail previously (Uban et al., 2013), as littermates of animals in the present study were utilized in this previous study. Briefly, weight gain was reduced overall in PAE and PF dams during gestation and in PAE and PF pups at birth. However, catch up growth occurred such that there were no differences in pup body weight among groups by weaning or thereafter.
There were no significant main or interaction effects for uterus/body wt ratios (p > 0.10). However, in agreement with our a priori hypothesis, C females showed an increased uterus/body weight ratio over days, with a lower ratio at PND 30 (0.13 ± 0.01) than PND 35 (0.17 ± 0.01) and 65 (0.18 ± 0.009) (PND 30 < PND 35 = PND 65, p < 0.05 and p > 0.561, respectively), whereas PAE (p > 0.53) and PF (p > 0.38) females showed no significant change in uterus/body weight ratio with age (for PAE, PND 30 = 0.17 ± 0.02, PND 35 = 0.16 ± 0.01, PND 65 = 0.16 ± 0.008); for PF, PND 30 = 0.17 ± 0.01, PND 35 = 0.19 ± 0.01, PND 65 = 0.18 ± 0.01). VO was also delayed in PAE and PF females. At PND 30, 9% of C showed VO but VO was not observed in any PAE or PF females. By PND 35, 50% of C, 25% of PAE, and 0% of PF females displayed VO.
Hormonal data
Estradiol
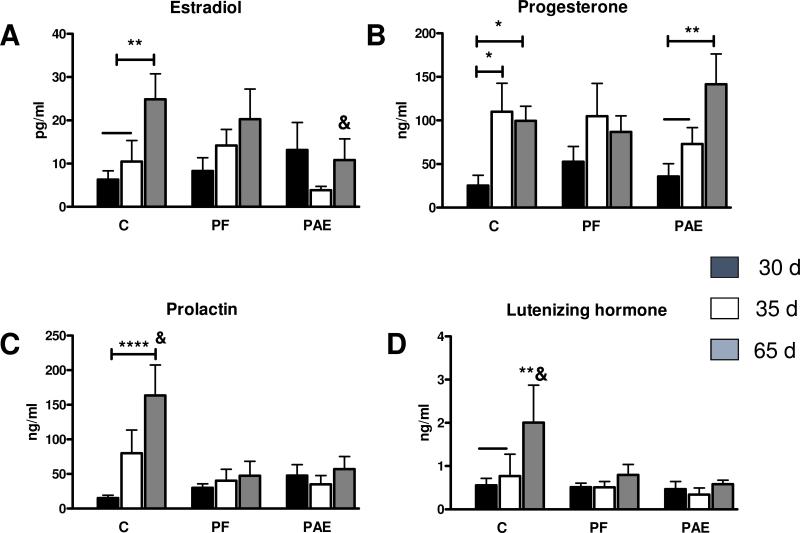
ANOVA revealed a main effect of age [F(2,90) = 3.66, p = 0.03]; overall, PND 65 females had higher levels of E2 compared to PND 30 and 35 females (Fig. 1A). However, in line with our hypothesis, a priori tests revealed that the increase in estradiol was driven primarily by an increase with age in C females (PND 30 = PND 35 < PND 65, p < 0.01) and that there were no significant changes with age for PAE (p > 0.25) or PF (p > 0.36) females. A priori comparisons further indicated that at PND 65, PAE females had lower levels of E2 compared to PND 65 C and PF females (PAE < C = PF, p < 0.05 and p = 0.47, respectively).
Fig. 1.
Hormone profile for estradiol (A), progesterone (B), prolactin (C) and luteinizing hormone (D) for PND 30, 35 and 65, in control (C), pair-fed (PF), and prenatally alcohol exposed (PAE) females. (A) For C: PND 30 = PND 35 < PND 65, p = 0.56, **p < 0.01; for PND 65: PAE < C = PF, &p < 0.05, p = 0.47; (B) For C: PND 30 < PND 35 = PND 65, *p< 0.02 and p = 0.75, respectively; for PAE: PND 30 = PND 35 < PND 65, p = 0.37; **p<0.01; (C) For C: PND 30 < PND 65, ****p < 0.0002; for PND 65; C > PAE = PF, &p < 0.003, p = 0.79; (D) For C: PND 30 = PND 35 < PND 65, p = 0.74 and **p < 0.01); for C P65 only: C > PAE = PF, &p < 0.04, p = 0.73, respectively.
Progesterone
A main effect of age [F (2,89) = 6.68, p = 0.002] indicated that overall, P4 levels increased from PND 30 to PND 65 (Fig. 1B). Further a priori tests showed that for PAE females, P4 levels did not increase over PND 30 levels until PND 65 (PND 30 = PND 35 < PND 65, p = 0.37 and p < 0.01, respectively). By contrast, for C females, P4 levels reached adult values by PND 35 (PND 30 < PND 35 = PND 65, p < 0.02 and p = 0.75, respectively), whereas PF females showed no significant change in P4 levels as a function of age, p > 0.11. Thus, compared to C females, PAE females showed a delayed increase of P4, while PF animals showed no significant increase over time.
Prolactin
For PRL levels, analysis revealed a main effect of age [F(2, 87)=3.922, p = 0.023], and trends for an effect of group [F(2, 87)=2.876, p = 0.062], and a group × age interaction [F(4, 87)=2.271, p = 0.068]. Post hoc analysis revealed that overall, prolactin levels were higher at PND 65 compared to PND 30 (p < 0.01) and 35, (p < 0.04); Fig. 1 C). However, as noted for estradiol, further a priori analysis indicted that the overall PRL increase was driven primarily by the increase in C females (PND 30 = 35 < 65, p=0.11, p < 0.0002, p < 0.02, respectively). By contrast, neither PAE nor PF females showed significant differences in PRL levels as a function of age (p > 0.760 and p > 0.604, respectively). Moreover, at PND 65, C females had higher PRL levels than both PAE and PF females (C > PAE = PF, p < 0.003 and p = 0.79, respectively).
LH
There were no significant effects of group or age for LH levels, all p > 0.15. However, a priori analyses, consistent with our hypothesis, again revealed effects driven primary by C females. C females showed a significant increase in LH levels on PND 65 (PND 30 = PND 35 < PND 65, p = 0.74 and p < 0.01), whereas LH levels in PAE and PF females showed no change across age (p > 0.73). Additionally, at PND 65, C females had higher LH levels than both PAE and PF females (PAE = PF < C, p < 0.04, respectively; Fig. 1 D).
Kiss1 mRNA data
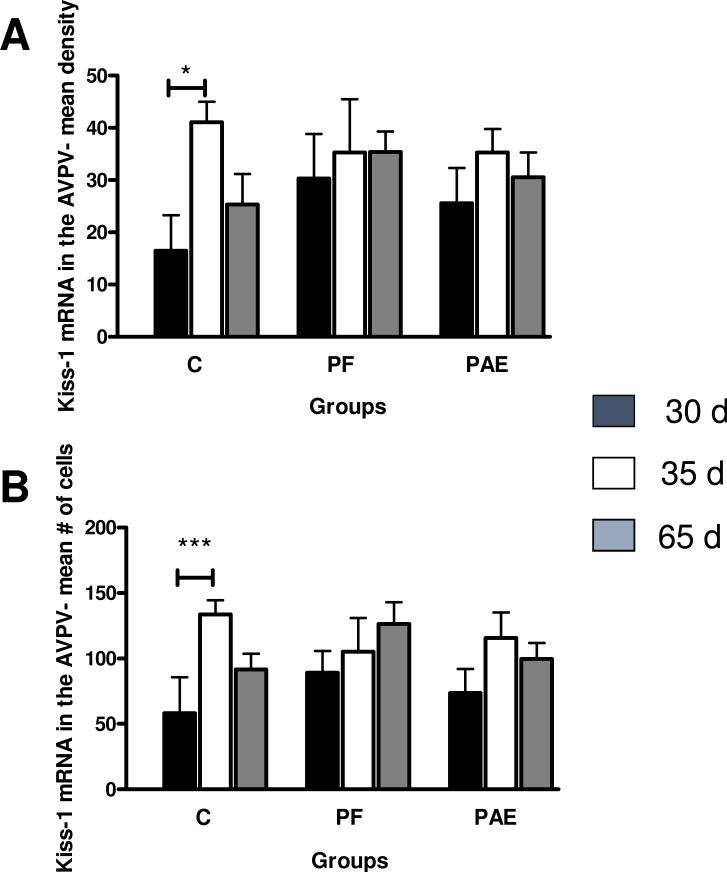
Kiss1 mRNA was detected in the AVPV (Fig, 2 A-C). There were no effects of prenatal group on Kiss1 mRNA density (p > 0.504. However, a priori analysis indicated an age-dependent change in Kiss1 mRNA density in C but not PAE or PF females. C females showed an increase in Kiss1 mRNA density from PND 30 to PND 35 (PND 35 > PND 30, p < 0.05; Figs. 2 A-C and 3 A), whereas PAE (p > 0.32) and PF (p > 0.55) females showed no changes over time (Fig 3 A). There was an age effect for mean Kiss1 mRNA cell numbers in the AVPV [F(2,39)=4.7344, p = 0.014], with an overall increase in the number of Kiss1 mRNA cells on PND 35 (p < 0.005) and PND 65 (p < 0.05) compared to PND 30. However, further a priori analysis indicated once again that changes in cell numbers with age were evident only in C females (PND 35 > PND 30, p < 0.01), and not in PAE (p > 0.12) or PF (p > 0.11) females (Fig 3 B).
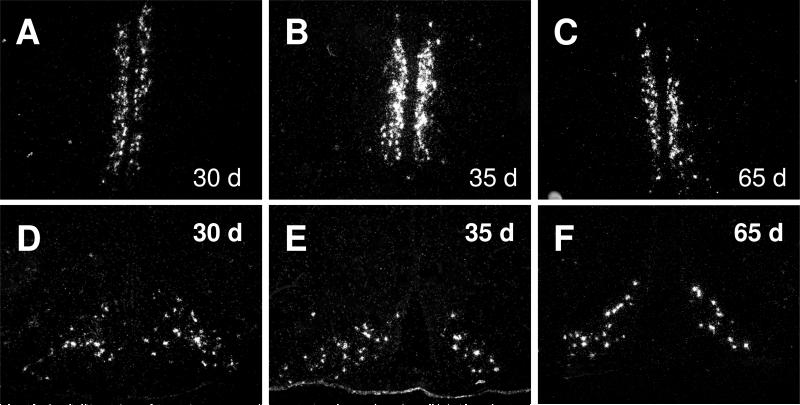
Fig. 2.
Dark-field photomicrographs show representative sections of the anteroventral periventricular nucleus (AVPV) - (A-C) and arcuate nucleus (ARC) - (D-F) of 30, 35 and 65 days old control female rats with Kiss1 mRNA-expressing neurons (as indicated by the presence of silver grain clusters).
Fig. 3.
Mean density of Kiss-1 mRNA (A) and mean number of Kiss-1 mRNA cells (B) in the anteroventral periventricular nucleus (AVPV) of control (C), pair-fed (PF) and prenatally alcohol exposed (PAE) females at PND 30, 35 and 65. (A) For C only: PND 30 < PND 35, *p < 0.05; (B) For C only: PND 30 < PND 35, ***p < 0.005. Bars represent mean of density and mean number of Kiss-1 mRNA ±SEM.
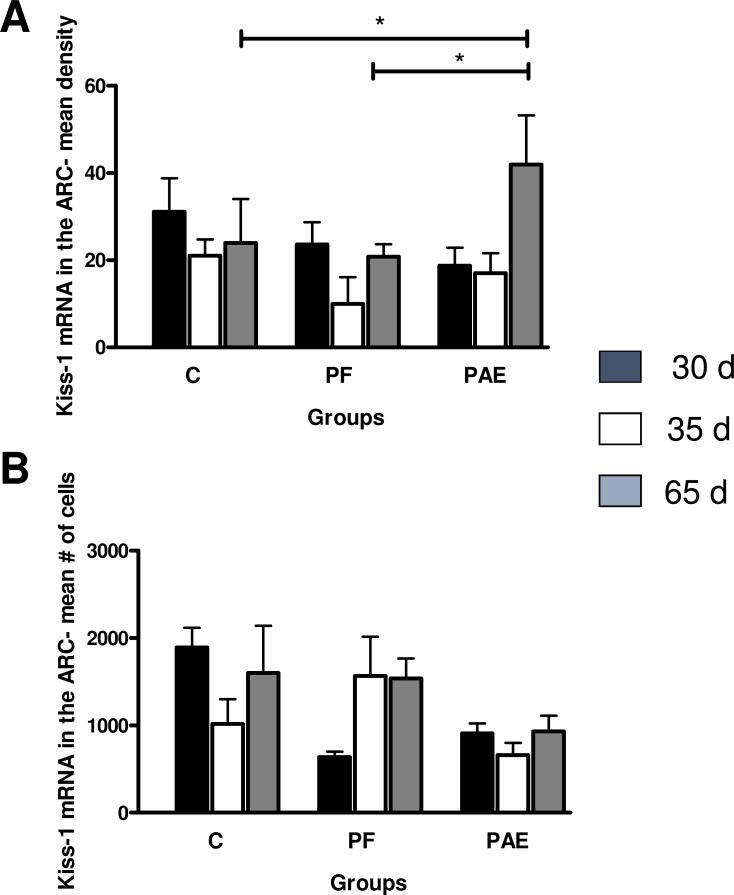
Kiss1 mRNA was also detected in the ARC. While there was no main effect of prenatal group on Kiss1 mRNA cell density, p > 0.13 (Figs. 2 D-F, 4 A), a priori analyses revealed that on PND 65, Kiss1 mRNA density was higher in PAE females compared to their C and PF counterparts, p < 0.05. There were no significant effects of prenatal group or age and no group × age interaction for Kiss1 cell numbers in the ARC, all p > 0.28 (Fig. 4 B).
Fig. 4.
Mean density of Kiss1 mRNA (A) and mean number of Kiss-1 mRNA cells (B) in the arcuate nucleus (ARC) of control (C), pair-fed (PF) and prenatally alcohol exposed (PAE) females at PND 30, 35 and 65. (A) For PND 65: PAE > C and PAE > PF, *p < 0.05 (B) For C: no statistically significant differences p > 0.95. Bars represent mean of density and mean number of Kiss-1 mRNA ± SEM.
DISCUSSION
We show differential PAE- and PF-related changes to the reproductive system associated not only with a delay in sexual maturation, but also with alterations in the sex hormone profile and hypothalamic expression of Kiss1 mRNA: 1) PAE inhibits the expected increases in E2 levels as a function of age and delays maturational increases of P4; 2) PAE and pair-feeding have similar adverse effects on vaginal opening and uterus/body weight ratio; 3) While neither PAE nor PF females show age-related increases in levels of PRL and LH, differential relationships between PRL and P4 help to dissociate PAE and PF effects: PF females have low PRL levels and no increase in P4 with age, whereas despite low PRL, PAE animals show an age-related increase in P4, suggesting that delays in maturation in the two groups may occur via different mechanisms; 4) PAE results in higher mean density of Kiss1 mRNA in the ARC, while both PAE and pair-feeding blunt the normal changes in Kiss1 cell density and cell numbers in the AVPV. These findings suggest that while PAE and pair-feeding have some overlapping effects, there are important differential effects on hormonal markers of reproductive development as well as Kiss1 mRNA expression in the hypothalamus. We suggest that a possible mechanism leading to delayed reproductive maturation may be related to pre-adolescent alterations in Kiss1 expression, possibly resulting in a change in the balance of function between the ARC and AVPV, with differential consequences for PAE and PF females.
Absence of an age-dependent increase in E2 levels is an important finding specific to PAE females. These data support and extend our previous studies in adult PAE females, where we uncovered alterations in E2 levels (Sliwowska et al., 2008; Lan et al., 2009; Sliwowska et al., 2014b), corticosteroid and serotonin Type 1A (5-HT1A) receptors, and HPA-HPG interactions that were estrous stage-dependent (Sliwowska et al., 2008, Sliwowska et al., 2014b), and found differential sensitivity of PAE females to the modulatory effects of sex steroids on the serotonergic system (Sliwowska et al., 2014b).
Our present finding of increased uterus/body weight ratios in adult compared to prepubertal control females is consistent with previous data (Romeo et al., 2004), and confirms the normal maturational trajectory in controls. By contrast, the absence of a developmental increase in uterus weight in PAE females likely reflects alterations in secretion of E2, and suggests that uterus weight might be used as an index of estrogen secretion. Together with our previous findings in adult females (Sliwowska et al., 2014a), the present data strongly support a role for ovarian sex steroids in the adverse effects of alcohol on a number of outcome measures.
The present study provides the first investigation of both pre- and post-pubertal changes in P4 levels of PAE females, and supports our previous results showing no significant differences in adult P4 levels among groups (Sliwowska et al., 2008, Hellemans et al., 2010, Sliwowska et al., 2013). Importantly, we now show that the normal increase in P4 levels is delayed during pubertal development in PAE females. Although the majority of P4 originates from the ovaries, the adrenal glands provide a secondary source of this hormone, as shown by previous data demonstrating that an acute alcohol injection into adult ovariectomized rats increases P4 levels (Bude et al., 2002 ). We have previously reported an increase in P4 levels in adult PAE females following restraint (Lan et al., 2009) and chronic mild stress (Hellemans et al., 2008). Thus, it is possible that in our PAE model, the altered P4 secretion seen during pubertal development reflects the broad programming effects by alcohol exposure in utero on adrenal sensitivity or responsiveness to challenge.
In addition to PAE-specific effects on E2 and P4 levels, we show that neither PAE nor PF females display age-related increases in PRL levels, consistent with previous reports (Becu-Villalobos et al., 1992). Our finding of reduced PRL levels in adult PAE females is in agreement with previous data (Hannigan et al., 1997). PRL is known to be involved in the onset and regulation of puberty (Dohler and Wuttke, 1975). PRL implantation into the median eminence (Advis et al., 1982), or PRL injections (Wuttke et al., 1976) of immature female rats markedly advance the onset of puberty, as measured by the age at VO and at first ovulation, and initiate regular estrous cycles. It has been demonstrated that PRL, like stress, stimulates P4 secretion from the adrenal glands (Advis and Ojeda, 1978). Indeed, in the current experiment, high PRL levels in control rats were associated with high P4 levels, whereas low levels of PRL in PF females were associated with the lack of an increase in P4 levels with age. On the other hand, despite their relatively low levels of PRL, PAE animals showed an age-related increase in P4 levels. Thus, while both PAE and pair feeding lead to delayed puberty, differential relationships between PRL and P4 suggest that delays in maturation in the two groups may occur via different mechanisms. Further research is needed to elucidate these mechanisms, as in contrast to previous studies (Advis and Ojeda, 1978), females in the current study were intact, which did not allow us to determine the origin (ovarian, adrenal or both) of P4 secretion.
Finally, we revealed an absence of the normal age-related increases in LH levels in our intact adult PAE females. These results are in agreement with findings by Handa et al. (1985), showing that ovariectomized adult PAE females had lower levels of LH and a reduced LH response to E2 and P4 compared to controls (Handa et al., 1985), and with those of Esquifino et al (1986) and Morris et al (1989), who found lower LH levels in PAE females, particularly at the time of VO (Esquifino et al., 1986, Morris et al., 1989). Lower LH levels in adult PAE females could contribute to the reproductive abnormalities and earlier acyclicity reported in these animals (McGivern et al., 1995, Wilson and Handa, 1997). The finding that PF females also showed a lack of LH increase with age is discussed further below. Of note, the altered hormonal profile in PAE and PF females in the current study is in agreement with the delayed maturation (assessed by VO) observed here and in previous studies in rats and mice (Boggan et al., 1979, Esquifino et al., 1986, McGivern et al., 1992, McGivern and Yellon, 1992, Emanuele et al., 2002b, Lan et al., 2009). Importantly, in spite delayed VO, we have previously reported that both PAE and PF females display normal estrous cycles (Lan et al., 2009).
We previously demonstrated marked alterations in the kisspeptin system in PAE adult females that were ovariectomized and replaced with E2 and P4 (Sliwowska et al., 2014a), suggesting one potential mechanism responsible for the alterations in reproductive function in PAE offspring. Here we provide novel evidence that PAE leads to alterations in Kiss1 mRNA in the hypothalamus during sexual maturation, as well as producing differential effects in the AVPV and ARC. Recent studies indicate that while kisspeptin neurons in the AVPV play a role in the control of pubertal timing, both AVPV and ARC populations are essential for normal LH surges and estrous cyclicity (Hu et al., 2015).
We found that while PAE and PF females both fail to show an increase in Kiss1 mRNA in the AVPV on PND 35 (peripubertal period), which may contribute to delayed sexual maturation, a PAE-specific increase in adult (PND 65) Kiss1 mRNA density in the ARC, together with low E2 levels, may contribute to decreased estradiol feedback and result in alterations in LH levels. Previously, it was reported that short-term (6 days) alcohol exposure in juvenile females suppressed Kiss1 mRNA in both the AVPV and ARC (Srivastava et al., 2009). Moreover, acute alcohol exposure blocked insulin-like growth factor-1 (IGF-1)-induced Kiss1 gene expression in the AVPV but not in the ARC of juvenile animals (Hiney et al., 2010). Of note, both kisspeptin and IGF-1 act centrally to stimulate GnRH secretion at puberty in female rats (Hiney et al., 1996) and IGF-1 injection has been shown to advance first ovulation in rhesus monkeys (Wilson, 1998), and advance puberty in growth hormone-receptor knockout mice expressing low levels of the peptide (Danilovich et al., 1999). Thus, both acute and chronic exposure to alcohol affect IGF-1 signaling, suppress GnRH/LH secretion and alter pubertal development in rats and monkeys (Hiney et al., 1998, Wilson, 1998, Srivastava et al., 2009). Hiney et al. (Hiney et al., 1998) have shown that IGF-1-induced Kiss1 gene expression in the AVPV depends on adequate levels of E2. In support of an E2-dependent change in Kiss1 expression, we found no increase of Kiss1 mRNA levels in the AVPV together with low levels of E2 in 35 day old PAE females. This is in agreement with studies showing that changes in serum levels of E2 can differentially influence Kiss1 expression in both the AVPV and ARC in control animals (Smith et al., 2005).
In the current study, a number of pair-feeding effects were also observed, including delays in VO [similar to (Lan et al., 2009)], lack of the normal change in uterus/body weight ratio and LH levels with age, and altered Kiss1 mRNA levels in the AVPV. We suggest that these findings may be, at least in part, linked to the known complexity and confounds of the pair-feeding procedure. While PF is the accepted and indeed standard procedure utilized to control for the reduced food intake that is typical of ethanol-consuming animals, pair-feeding is in many ways a treatment in itself. Pair-feeding can never control for the significant nutritional effects (e.g., on absorption and utilization of nutrients) of ethanol. Moreover, the pair-feeding procedure may in itself result in mild prenatal stress. While PAE dams consume their diet ad libitum throughout the 24-h period, PF dams get a restricted food ration, equivalent in amount to that consumed by a PAE partner, and less than they would consume if given the same control liquid diet ad libitum. Thus they are hungry and typically consume their entire daily ration within a few hours of presentation This is not only an abnormal feeding pattern, but also introduces a mild prenatal stress component to the pair-feeding paradigm beyond the nutritional aspect of receiving a reduced food ration, which itself can program offspring behavioral and physiological (including HPA) function. Indeed previous experiments have shown that CRH and AVP regulation differ between PAE and PF animals under both basal and stress conditions (Glavas et al., 2007; Lan et al., 2016). Similarly, it was shown previously that both prenatal food restriction and stress can decrease LH levels (Gawalek and Sliwowska, 2015, Iwasa et al., 2015). Moreover, in response to food deprivation, prepubertal rats were shown to have decreased hypothalamic Kiss1 mRNA and increased GPR54 (Castellano et al., 2005). Studies using much more severe food restriction (50%) similarly reveal delays in the onset of puberty (Leonhardt et al., 2003) and disturbed development of reproductive function. Interestingly, delayed VO was overcome by chronic administration of kisspeptin, which also elicited gonadotropin and estrogen responses in a majority of the animals (Castellano et al., 2005). While the PF condition in our experiments represents mild rather than severe food restriction/undernutrition, nevertheless our findings are in agreement with these previous data, and support the suggestion that pair-feeding is a treatment in itself with unique adverse effects.
To summarize, our findings extend the literature showing that prenatal alcohol and dietary restriction (pair feeding) can have both short- and long-term effects on development of the reproductive system. While PAE and pair-feeding had some overlapping effects, there were important differential effects of these prenatal treatments on hormonal markers of reproductive development and Kiss1 mRNA expression in the hypothalamus. We postulate that the effects of in utero alcohol exposure and pair feeding on kisspeptin expression during the periadolescent period could possibly change the balance of activity in the AVPV and ARC, and thus play a role in altered reproductive maturation and function. Indeed, given the different roles of the AVPV and ARC, alterations in Kiss1 mRNA expression could differentially influence the timing of puberty, the relationship between PRL and P4 during development, pulsatile LH secretion, the LH surge, and ultimately estrous cyclicity and reproductive function in PAE and PF females.
ACKNOWLEDGEMENTS
The authors are grateful to Wayne Yu for expertise in the radioimmunoassays.
Sources of support:
This work was supported by National Institutes of Health/National Institute on Alcohol Abuse and Alcoholism Grants R37 AA007789 and RO1 AA022460, and NeuroDevNet (Canadian Networks of Centres of Excellence) to J.W.; Natural Sciences and Engineering Research Council of Canada CGS-D to T.S.B.; and a Fellowship from IMPART (CIHR Strategic Training Initiative in Health Research) to JHS.
REFERENCES
- Advis JP, Andrews WW, Ojeda SR. Studies on the central effect of prolactin in inducing precocious puberty in the female rat. Brain Res Bull. 1982;8:449–458. doi: 10.1016/0361-9230(82)90002-8. [DOI] [PubMed] [Google Scholar]
- Advis JP, Ojeda SR. Hyperprolactinemia-induced precocious puberty in the female rat: ovarian site of action. Endocrinology. 1978;103:924–935. doi: 10.1210/endo-103-3-924. [DOI] [PubMed] [Google Scholar]
- Becu-Villalobos D, Lacau-Mengido IM, Diaz-Torga GS, Libertun C. Ontogenic studies of the neural control of adenohypophyseal hormones in the rat. II. Prolactin. Cell Mol Neurobiol. 1992;12:1–19. doi: 10.1007/BF00711635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggan WO, Randall CL, Dodds HM. Delayed sexual maturation in female C57BL/6J mice prenatally exposed to alcohol. Res Commun Chem Pathol Pharmacol. 1979;23:117–125. [PubMed] [Google Scholar]
- Bude M, Koko V, Milovanovi T, Balint-Peri L, Petkovi A. Acute ethanol treatment increases level of progesterone in ovariectomized rats. Alcohol. 2002;26:173–178. doi: 10.1016/s0741-8329(02)00197-0. [DOI] [PubMed] [Google Scholar]
- Carter RC, Jacobson JL, Dodge NC, Granger DA, Jacobson SW. Effects of prenatal alcohol exposure on testosterone and pubertal development. Alcohol Clin Exp Res. 2014;38:1671–1679. doi: 10.1111/acer.12395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellano JM, Navarro VM, Fernandez-Fernandez R, Nogueiras R, Tovar S, Roa J, Vazquez MJ, Vigo E, Casanueva FF, Aguilar E, Pinilla L, Dieguez C, Tena-Sempere M. Changes in hypothalamic KiSS-1 system and restoration of pubertal activation of the reproductive axis by kisspeptin in undernutrition. Endocrinology. 2005;146:3917–3925. doi: 10.1210/en.2005-0337. [DOI] [PubMed] [Google Scholar]
- Danilovich N, Wernsing D, Coschigano KT, Kopchick JJ, Bartke A. Deficits in female reproductive function in GH-R-KO mice; role of IGF-I. Endocrinology. 1999;140:2637–2640. doi: 10.1210/endo.140.6.6992. [DOI] [PubMed] [Google Scholar]
- de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci U S A. 2003;100:10972–10976. doi: 10.1073/pnas.1834399100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dees WL, Hiney JK, Srivastava VK. Alcohol alters hypothalamic glial-neuronal communications involved in the neuroendocrine control of puberty: In vivo and in vitro assessments. Alcohol. 2015;49:631–637. doi: 10.1016/j.alcohol.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohler KD, Wuttke W. Changes with age in levels of serum gonadotropins, prolactin and gonadal steroids in prepubertal male and female rats. Endocrinology. 1975;97:898–907. doi: 10.1210/endo-97-4-898. [DOI] [PubMed] [Google Scholar]
- Emanuele MA, Wezeman F, Emanuele NV. Alcohol's effects on female reproductive function. Alcohol Res Health. 2002a;26:274–281. [PMC free article] [PubMed] [Google Scholar]
- Emanuele N, Ren J, LaPaglia N, Steiner J, Emanuele MA. EtOH disrupts female mammalian puberty: age and opiate dependence. Endocrine. 2002b;18:247–254. doi: 10.1385/ENDO:18:3:247. [DOI] [PubMed] [Google Scholar]
- Esquifino AI, Sanchis R, Guerri C. Effect of prenatal alcohol exposure on sexual maturation of female rat offspring. Neuroendocrinology. 1986;44:483–487. doi: 10.1159/000124690. [DOI] [PubMed] [Google Scholar]
- Gawalek M, Sliwowska JH. Neuronal basis of reproductive dysfunctions associated with diet and alcohol: From the womb to adulthood. Reprod Biol. 2015;15:69–78. doi: 10.1016/j.repbio.2015.04.001. [DOI] [PubMed] [Google Scholar]
- Glavas MM, Ellis L, Yu WK, Weinberg J. Effects of prenatal ethanol exposure on basal limbic-hypothalamic-pituitary-adrenal regulation: role of corticosterone. Alcohol Clin Exp Res. 2007;31:1598–1610. doi: 10.1111/j.1530-0277.2007.00460.x. [DOI] [PubMed] [Google Scholar]
- Handa RJ, McGivern RF, Noble ES, Gorski RA. Exposure to alcohol in utero alters the adult patterns of luteinizing hormone secretion in male and female rats. Life Sci. 1985;37:1683–1690. doi: 10.1016/0024-3205(85)90295-4. [DOI] [PubMed] [Google Scholar]
- Hannigan JH, Hackett JA, Tilak J, Subramanian MG. Sulpiride-induced increases in serum prolactin levels in female rats exposed prenatally to alcohol. Alcohol. 1997;14:585–592. doi: 10.1016/s0741-8329(97)00053-0. [DOI] [PubMed] [Google Scholar]
- Hellemans KG, Verma P, Yoon E, Yu W, Weinberg J. Prenatal alcohol exposure increases vulnerability to stress and anxiety-like disorders in adulthood. Ann N Y Acad Sci. 2008;1144:154–175. doi: 10.1196/annals.1418.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellemans KG, Verma P, Yoon E, Yu WK, Young AH, Weinberg J. Prenatal alcohol exposure and chronic mild stress differentially alter depressive- and anxiety-like behaviors in male and female offspring. Alcohol Clin Exp Res. 2010;34:633–645. doi: 10.1111/j.1530-0277.2009.01132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiney JK, Srivastava V, Lara T, Dees WL. Ethanol blocks the central action of IGF-1 to induce luteinizing hormone secretion in the prepubertal female rat. Life Sci. 1998;62:301–308. doi: 10.1016/s0024-3205(97)01111-9. [DOI] [PubMed] [Google Scholar]
- Hiney JK, Srivastava VK, Les Dees W. Insulin-like growth factor-1 stimulation of hypothalamic KiSS-1 gene expression is mediated by Akt: effect of alcohol. Neuroscience. 2010;166:625–632. doi: 10.1016/j.neuroscience.2009.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiney JK, Srivastava VK, Nyberg CL, Ojeda SR, Dees WL. Insulin-like growth factor-1 (IGF-1) of peripheral origin acts centrally to accelerate the initiation of female puberty. Endocrinology. 1996;137:3717–3727. doi: 10.1210/endo.137.9.8756538. [DOI] [PubMed] [Google Scholar]
- Hu MH, Li XF, McCausland B, Li SY, Gresham R, Kinsey-Jones JS, Gardiner JV, Sam AH, Bloom SR, Poston L, Lightman SL, Murphy KG, O'Byrne KT. Relative Importance of the Arcuate and Anteroventral Periventricular Kisspeptin Neurons in Control of Puberty and Reproductive Function in Female Rats. Endocrinology. 2015;156:2619–2631. doi: 10.1210/en.2014-1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasa T, Matsuzaki T, Tungalagsuvd A, Munkhzaya M, Kuwahara A, Yasui T, Irahara M. LH and testosterone production are more sensitive to the suppressive effects of food deprivation in prenatally undernourished male rats. Int J Dev Neurosci. 2015;43:66–69. doi: 10.1016/j.ijdevneu.2015.04.001. [DOI] [PubMed] [Google Scholar]
- Lan N, Vogl AW, Weinberg J. Prenatal ethanol exposure delays the onset of spermatogenesis in the rat. Alcohol Clin Exp Res. 2013;37:1074–1081. doi: 10.1111/acer.12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan N, Yamashita F, Halpert AG, Sliwowska JH, Viau V, Weinberg J. Effects of prenatal ethanol exposure on hypothalamic-pituitary-adrenal function across the estrous cycle. Alcohol Clin Exp Res. 2009;33:1075–1088. doi: 10.1111/j.1530-0277.2009.00929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan N, Hellemans KG, Ellis L, Weinberg J. Exposure to chronic mild stress differentially alters corticotropin-releasing hormone and arginine vasopressin mRNA expression in the stress-responsive neurocircuitry of male and female rats prenatally exposed to alcohol. Alcohol Clin Exp Res. 2015;39:2414–2421. doi: 10.1111/acer.12916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonhardt M, Lesage J, Croix D, Dutriez-Casteloot I, Beauvillain JC, Dupouy JP. Effects of perinatal maternal food restriction on pituitary-gonadal axis and plasma leptin level in rat pup at birth and weaning and on timing of puberty. Biol Reprod. 2003;68:390–400. doi: 10.1095/biolreprod.102.003269. [DOI] [PubMed] [Google Scholar]
- McGivern RF, McGeary J, Robeck S, Cohen S, Handa RJ. Loss of reproductive competence at an earlier age in female rats exposed prenatally to ethanol. Alcohol Clin Exp Res. 1995;19:427–433. doi: 10.1111/j.1530-0277.1995.tb01526.x. [DOI] [PubMed] [Google Scholar]
- McGivern RF, Raum WJ, Handa RJ, Sokol RZ. Comparison of two weeks versus one week of prenatal ethanol exposure in the rat on gonadal organ weights, sperm count, and onset of puberty. Neurotoxicol Teratol. 1992;14:351–358. doi: 10.1016/0892-0362(92)90042-9. [DOI] [PubMed] [Google Scholar]
- McGivern RF, Yellon SM. Delayed onset of puberty and subtle alterations in GnRH neuronal morphology in female rats exposed prenatally to ethanol. Alcohol. 1992;9:335–340. doi: 10.1016/0741-8329(92)90077-n. [DOI] [PubMed] [Google Scholar]
- Morris DL, Harms PG, Petersen HD, McArthur NH. LHRH and LH in peripubertal female rats following prenatal and/or postnatal ethanol exposure. Life Sci. 1989;44:1165–1171. doi: 10.1016/0024-3205(89)90311-1. [DOI] [PubMed] [Google Scholar]
- Navarro VM, Castellano JM, Fernandez-Fernandez R, Barreiro ML, Roa J, Sanchez-Criado JE, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M. Developmental and hormonally regulated messenger ribonucleic acid expression of KiSS-1 and its putative receptor, GPR54, in rat hypothalamus and potent luteinizing hormone-releasing activity of KiSS-1 peptide. Endocrinology. 2004a;145:4565–4574. doi: 10.1210/en.2004-0413. [DOI] [PubMed] [Google Scholar]
- Navarro VM, Fernandez-Fernandez R, Castellano JM, Roa J, Mayen A, Barreiro ML, Gaytan F, Aguilar E, Pinilla L, Dieguez C, Tena-Sempere M. Advanced vaginal opening and precocious activation of the reproductive axis by KiSS-1 peptide, the endogenous ligand of GPR54. J Physiol. 2004b;561:379–386. doi: 10.1113/jphysiol.2004.072298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojeda SR, Roth C, Mungenast A, Heger S, Mastronardi C, Parent AS, Lomniczi A, Jung H. Neuroendocrine mechanisms controlling female puberty: new approaches, new concepts. Int J Androl. 2006;29:256–263. doi: 10.1111/j.1365-2605.2005.00619.x. discussion 286-290. [DOI] [PubMed] [Google Scholar]
- Robe LB, Robe RS, Wilson PA. Maternal heavy drinking related to delayed onset of daughters menstruation. Curr Alcohol. 1979;7:515–520. [PubMed] [Google Scholar]
- Romeo RD, Lee SJ, McEwen BS. Differential stress reactivity in intact and ovariectomized prepubertal and adult female rats. Neuroendocrinology. 2004;80:387–393. doi: 10.1159/000084203. [DOI] [PubMed] [Google Scholar]
- Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr., Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O'Rahilly S, Carlton MB, Crowley WF, Jr., Aparicio SA, Colledge WH. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349:1614–1627. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- Sliwowska JH, Bodnar TS, Weinberg J. Prenatal alcohol exposure alters response of kisspeptin-ir neurons to estradiol and progesterone in adult female rats. Alcohol Clin Exp Res. 2014a;38:2780–2789. doi: 10.1111/acer.12561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sliwowska JH, Lan N, Yamashita F, Halpert AG, Viau V, Weinberg J. Effects of prenatal ethanol exposure on regulation of basal hypothalamic-pituitary-adrenal activity and hippocampal 5-HT1A receptor mRNA levels in female rats across the estrous cycle. Psychoneuroendocrinology. 2008;33:1111–1123. doi: 10.1016/j.psyneuen.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sliwowska JH, Song HJ, Bodnar T, Weinberg J. Prenatal Alcohol Exposure Results in Long-Term Serotonin Neuron Deficits in Female Rats: Modulatory Role of Ovarian Steroids. Alcohol Clin Exp Res. 2014b doi: 10.1111/acer.12224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology. 2005;146:3686–3692. doi: 10.1210/en.2005-0488. [DOI] [PubMed] [Google Scholar]
- Soliman A, De Sanctis V, Elalaily R. Nutrition and pubertal development. Indian J Endocrinol Metab. 2014;18:S39–47. doi: 10.4103/2230-8210.145073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava VK, Hiney JK, Dees WL. Short-term alcohol administration alters KiSS-1 gene expression in the reproductive hypothalamus of prepubertal female rats. Alcohol Clin Exp Res. 2009;33:1605–1614. doi: 10.1111/j.1530-0277.2009.00992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uban KA, Comeau WL, Ellis LA, Galea LA, Weinberg J. Basal regulation of HPA and dopamine systems is altered differentially in males and females by prenatal alcohol exposure and chronic variable stress. Psychoneuroendocrinology. 2013;38:1953–1966. doi: 10.1016/j.psyneuen.2013.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson ME. Premature elevation in serum insulin-like growth factor-I advances first ovulation in rhesus monkeys. J Endocrinol. 1998;158:247–257. doi: 10.1677/joe.0.1580247. [DOI] [PubMed] [Google Scholar]
- Wilson ME, Handa RJ. Gonadotropin secretion in infantile rats exposed to ethanol in utero. Alcohol. 1997;14:497–501. doi: 10.1016/s0741-8329(97)00037-2. [DOI] [PubMed] [Google Scholar]
- Wuttke W, Dohler KD, Gelato M. Oestrogens and prolactin as possible regulators of puberty. J Endocrinol. 1976;68:391–396. doi: 10.1677/joe.0.0680391. [DOI] [PubMed] [Google Scholar]