Abstract
Purpose
Male infertility is a multifactorial disorder with impressively genetic basis; besides, sperm abnormalities are the cause of numerous cases of male infertility. In this study, we evaluated the genetic variants in exons 4 and 5 and their intron-exon boundaries in RABL2B gene in infertile men with oligoasthenoteratozoospermia (OAT) and immotile short tail sperm (ISTS) defects to define if there is any association between these variants and human male infertility.
Methods
To this purpose, DNA was extracted from peripheral blood and after PCR reaction and sequencing, the results of sequenced segments were analyzed. In the present study, 30 infertile men with ISTS defect and 30 oligoasthenoteratozoospermic infertile men were recruited. All men were of Iranian origin and it took 3 years to collect patient’s samples with ISTS defect.
Results
As a result, the 50776482 delC intronic variant (rs144944885) was identified in five patients with oligoasthenoteratozoospermia defect and one patient with ISTS defect in heterozygote form. This variant was not identified in controls. The allelic frequency of the 50776482 delC variant was significantly statistically higher in oligoasthenoteratozoospermic infertile men (p < 0.05). Bioinformatics studies suggested that the 50776482 delC allele would modify the splicing of RABL2B pre-mRNA. In addition, we identified a new genetic variant in RABL2B gene.
Conclusions
According to the present study, 50776482 delC allele in the RABL2B gene could be a risk factor in Iranian infertile men with oligoasthenoteratozoospermia defect, but more genetic studies are required to understand the accurate role of this variant in pathogenesis of human male infertility.
Keywords: Immotile short tail sperm, Oligoasthenoteratozoospermia, RABL2B gene, Male infertility
Introduction
According to World Health Organization (WHO) definition, human infertility is the 1-year-long failure of pregnancy in sexually active couples without using any contraceptives. Based on this definition, about 15% of couples do not achieve pregnancy within 1 year and look for medical treatment of infertility [1]. Male factors are implicated in approximately 50% of all infertility cases [2]. Male infertility is a multifactorial disorder and the main cause of this defect is not clear in many patients [3]. Considerably, many of the infertile couples suffered from idiopathic infertility; however, recent studies have revealed that genetic factors play a significant role in male infertility [4]. Abnormal sperm development is the cause of numerous cases of male infertility [2]; however, in spite of significant efforts to identify genes involved in male infertility, up to now, just a few genes have been definitely correlated with human sperm defects [5]. In mammalian, spermatogenesis is defined as the development of specialized spermatozoa, during an intricate process from diploid spermatogonia. This process includes considerably morphological and biochemical changes which are controlled by specifically organized genes expression. Germ cells are supported at all spermatogenetic stages by somatic sertoli cells [6].
Immotile short tail sperm (ISTS) defect mostly is associated with fertility disorders due to inefficiency of sperm cells to oocyte-capacitation. In human, ISTS defect is described by Baccetti et al. in two unrelated individuals [9].
ISTS defect is characterized by (1) lowered sperm counts, (2) highly reduced length of the sperm tails in the total sperm population, and (3) entirely immotile spermatozoa (sometimes poor vibration could be detected in the low number of spermatozoa; but sperm head, nuclei, and acrosome are generally normal [7, 8]. The other common manifestations of this defect are the total absence of axoneme or the severely disorganized axoneme, when axoneme is present, generally devoid of the couple of central tubules and is made up of doublets where the arms are absent or poorly assembled with undetectable dynein and disassembled mitochondria [8, 9, 18]. Although these are the usual manifestations of ISTS defect, in some patients, only some of them could be observed. Actually, this defect gives a great opportunity to make an analysis of the function of genes and proteins which are affecting sperm tail and cilia development [7].
Oligoasthenoteratozoospermia (OAT) defect is a common phenotype which leads to male infertility and refers to a combination of abnormalities in three semen parameters (sperm count, motility, and morphology). OAT defect includes oligozoospermia (low sperm count), asthenozoospermia (inadequate sperm motility), and teratozoospermia (abnormal sperm morphology) [10]. In other words, it is defined by a combination of qualitative and quantitative sperm defects [19].
Recently, RABL2 (RAB, member of the Ras oncogene family-like 2) was found as a substantial regulator for male fertility in mouse. RABL2 is nearly related to the RAB family and is a GTPase member of the Ras superfamily [2]. It is lately demonstrated that RABL2 is a sperm intra-flagellar transporter and has an essential role in the delivery of proteins into the growing sperm tail and male fertility. According to the recent study performed by Lo JCY et al., male mice with homozygous point mutation (a single A to G substitution in the first codon of exon 5 of RABL2 gene) were infertile due to a short-tailed sperm with poor motility and decreased sperm output [11].
In human genome, the RABL2 gene is located in the subtelomeric region of chromosome 22 (22q13.33) called RABL2B (88.7% protein identity); it should be noted that subtelomeric regions are common locations for members of many gene families in human [11, 12].
RT-PCR for RABL2B using whole human testis messenger RNA (mRNA) revealed that RABL2B is expressed in human testis; besides expressed sequence tag (EST) expression, data for human suggests that RABL2B is mainly expressed in the brain, testis, and uterus with lesser amounts in a range of other tissues [11]. Just recently, it was demonstrated that RABL2 has existed within the mid piece and to a lesser amount to the principle piece of the human sperm flagella [2].
In the present study, a sequencing approach was used to determine a potential correlation between genetic variants of the human RABL2B gene and male infertility as a consequence of oligoasthenoteratozoospermia and immotile short tail sperm defects.
Materials and methods
Participants
This study included semen samples of 90 participants who referred to the Department of Andrology at the Reproductive Biomedicine Research Center at Royan Institute (Tehran, Iran) from 2011 to 2014. After semen analysis and providing spermogram for each participant, Papanicolaou (PAP) staining was used to identify sperm morphology. PAP staining is one of the most important and established staining technique that is routinely utilized in andrology laboratories and fertility clinics which provides a valid staining of spermatozoa [20]. Accordingly, we divided the participants to three groups: 30 infertile men with immotile short tail sperm defect (P1–P30) (more than 80% of spermatozoa were immotile short-tail in total sperm population), 30 infertile men with oligoasthenoteratozoospermia defect (O1–O30); and 30 normozoospermic men as controls (C1–C30). None of the subjects had familiar relationship to each other. The selection criteria for the two patient groups were (a) normal 46,XY karyotype, (b) no history of varicocele and congenital absence of vas deferens, (c) none of the patients manifested any of the other symptoms encountered in primary ciliary dyskinesia (PCD) such as bronchiectasis and chronic sinusitis, and (d) inclusion criteria for OAT defect was based on standard values for semen analysis according to 2010 World Health Organization (WHO) criteria. All men were of Iranian origin. This study was approved by the Reproductive Biomedicine Research Center and Ethics Committee at Royan Institute (Tehran, Iran), and all participants gave their informed consent.
Genomic DNA samples were obtained from the DNA Bank Center of Royan Institute (Tehran, Iran) from 2011 to 2014. DNA was extracted from peripheral blood leucocytes by salting out method [13]. It should be noted that because of the rarity of immotile short tail sperm defect (80% ≤ short-tail sperm) in Iranian infertile men, collecting the DNA samples lasted about 3 years (from 2011 to 2014). During these years, 154 infertile men with ISTS defect (80% ≤ short-tail sperm) referred to the Reproductive Biomedicine Research Center at Royan Institute (Tehran, Iran) and 30 of them accepted to donate their blood samples for research use.
RABL2B genotype analysis
Based on a related article [11], in which a point mutation (a single A to G substitution in the first codon of exon 5) has been observed in the RABL2 gene in mice, and due to the fact that all male mice carrying the mentioned point mutation in homozygous form were sterile, according to Ensembl database, primers were designed for exons 4 and 5 of RABL2B gene (ENSG00000079974; Chromosome 22: 50,767,501–50,783,663, Ensembl release 83, December 2015) and intron-exon boundaries of these two exons. It should be noted that there is a gene in human genome with a high degree of identity to RABL2B; it is considered in primer designing and primers were designed with high precision so the primers are 100% identical to the RABL2B gene, ENSG00000079974, Ensembl release 83 (Table 1).
Table 1.
Primers used for PCR and sequencing
| Primer name | Sequence | Product size (bp) | TM (°C) |
|---|---|---|---|
| RABL2B.ex4.F | 5′-CACCCCTCACCCTTCCATGGAGT-3′ | 592 | 67 |
| RABL2B.ex4.R | 5′-TGCCTCGTCGAAGGCCTCTTAC-3′ | 592 | 67 |
| RABL2B.ex5.F | 5′-CTCTCACATCACACTCAGGTA-3′ | 656 | 62 |
| RABL2B.ex5.R | 5′-ACTCAGTCCCAGCGTGC-3′ | 656 | 62 |
Exon and intron numbers are according to transcript ENST00000395598, Ensembl release 83.
Polymerase chain reaction and sequencing
PCR amplification was performed in a volume of 50 μl reaction mixture containing 1.5 μl MgCl2, 1 μl dNTP, 5 μl PCR buffer (1X), 100 ng of the genomic DNA, 0.9 μl for each 10 pmol/ul primer, and 1 U/μl Taq DNA polymerase enzyme (CinnaGen, Iran). The PCR cycle included an initial DNA denaturation at 94 °C for 4 min, followed by 30 cycles of DNA denaturation at 94 °C for 45 s, annealing at melting temperature (TM) set for 45 s (Table 1), extension at 72 °C for 45 s, and a final extension at 72 °C for 7 min.
The PCR products were sequenced using an ABI automated DNA sequencer (Macro gen, Korea). The results were analyzed by Finch TV software version 1.4.0 and Sequencher software version 5.3 and they were compared with data obtained from the Ensembl database.
Statistical analysis
The difference of genotype and the variation of allele frequencies in control and patient groups were evaluated using Chi-square test. All statistical analyses were conducted using SPSS version 20.0 (SPSS Inc., Chicago, IL, USA) and a p value of less than 0.05 (p < 0.05) was considered statistically significant.
Results
According to the phenotype of the RABL2 mutant male mice and based on the fact that RABL2 protein is evolutionary conserved and has about 88.7% identity between mouse and human [11], DNA sequencing approach was used to screen for genetic variants in RABL2B gene in two groups of patients: (a) infertile men with oligoasthenoteratozoospermia defect and (b) infertile men with immotile short tail sperm defect. Identified genetic variants were compared with normozoospermic men as controls.
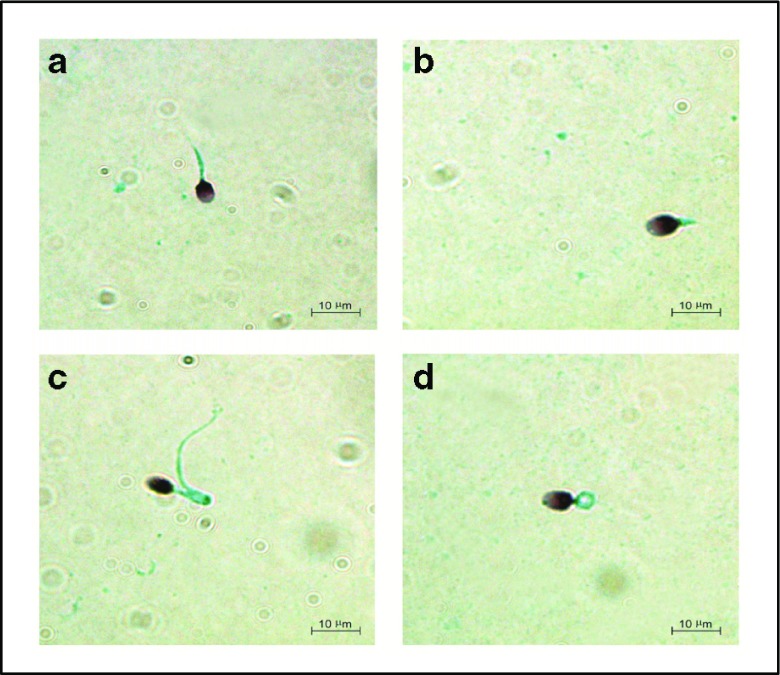
PAP staining clearly identified morphologic sperm defects in the patients with OAT and ISTS defects (Fig. 1).
Fig. 1.
Papanicolaou staining showing morphologic defects of spermatozoa. a Short tail sperm in patient (P11). b Short tail sperm in patient (P23). c Bent tail sperm in patient (O2). d Coiled tail sperm in patient (O14)
The primers covered exons 4 and 5 of the RABL2B gene and about 150–400 bp of exon-intron boundaries of these two exons. So in addition to these two coding exons, some regulatory parts in untranslated regions (UTRs) of the RABL2B gene were sequenced.
The sequencing results revealed two genetic variants:
-
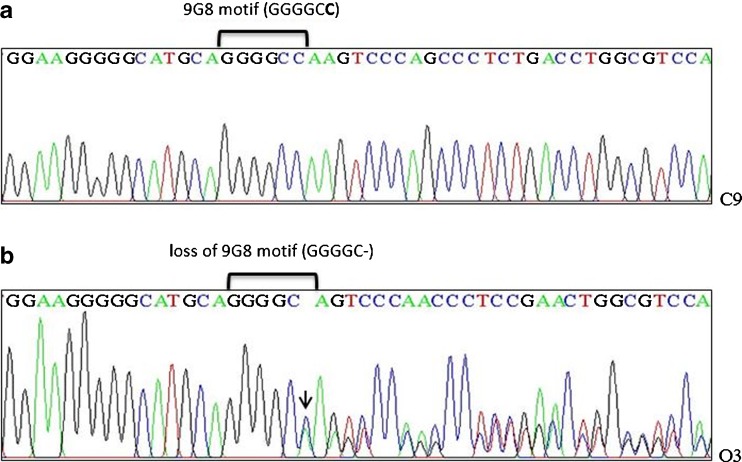
The 50776482 delC (rs144944885) variant was identified in six patients in heterozygous form (Fig. 2b). Five of them were infertile men with oligoasthenoteratozoospermia defect and one of them was an infertile man with immotile short tail sperm defect. This variant was not identified in controls (Fig. 2a). The allelic frequency of the 50776482 delC variant was significantly increased in oligoasthenoteratozoospermic infertile men (p < 0.05).
The 50776482 C nucleotide is located inside 9G8 motif (ggggcc) (Fig. 2a) into intron 4 of RABL2B gene. Bioinformatics analysis suggested that splicing factor 9G8 is a binding protein for 9G8 motif and 50776482 delC variant would modify consensus-binding sites for the splicing factor 9G8 (Fig. 2b). This splicing factor also has been known as serine/arginine-rich splicing factor 7 (SASF7) [2]. In the present study, bioinformatics studies were performed using Internet-based splicing analysis tools such as Human Splicing Finder Version 2.4.1.
The other variant which has not been previously reported was also identified in only one patient with immotile short tail sperm defect (P26). It was an intronic single heterozygous G to A substitution, in RABL2B gene, at a position of 201 nucleotides downstream of exon 5 (ENSG00000079974; chromosome 22: 50,775,571 G>A) (Fig. 3). We submitted this variant in the National Center for Biotechnology Information (NCBI) and received a GenBank accession number (the GenBank accession number: KT757686).
Fig. 2.
Sequencing analysis. a Sequencing results showing part of RABL2B intron 4 of a control subject (C9), 9G8 motif is revealed in intron 4 of RABL2B; the 50776482 C is located in intron 4 and at a position of 188 nucleotides downstream of exon 4 (shown in bold). b Sequencing results showing the 50776482 delC of a heterozygous individual (O3); loss of 9G8 motif is revealed in a mutant subject with OAT defect (the heterozygous 50776482 delC was shown with arrow and from this point, the arrangement of the peaks are obviously disrupted)
Fig. 3.
The 50775571 G>A variant (shown in red) is located in intron 5 (5755 nucleotides in length). This variant is at a position of 201 nucleotides downstream of exon 5 (the GenBank accession number: KT757686)
Discussion
Male infertility is becoming an increasing disorder. The male factor infertility is often defined by abnormal semen parameters which mainly include abnormality in sperm concentration, motility, and morphology; besides, considerably, many problems with sperm defects arise from genetic factors [8, 21]. Despite many efforts to identify genes involved in spermatogenesis, little is known about genetic basis of human male infertility [5].
The ultrastructure of mammalian sperm cell is evolutionary very conserved, so knowledge of genes, proteins, and molecular mechanisms involved in spermatozoa development in different mammals can be used across species [8]. According to the previous study on mutant mice model which was conducted by Lo JCY et al. [11], in the present study, RABL2B gene was selected as a candidate gene related to human sperm abnormalities. Further study performed by Jamsia D et al. indicated that “the human RABL2 localization pattern was analogous to the mouse ortholog: in the midpiece and to a lesser extent in the principal piece of the sperm tails” [2]. These findings suggest that RABL2B could play a significant role in sperm tail assembly, sperm motility, and male fertility in human.
Based on previous study on male mutant mice [11], we selected two groups of patients (infertile men with ISTS and OAT defects) and normozoospermic men as controls for our study. Abnormalities of sperm tail always induce asthenozoospermia [19]. In our study, patients with ISTS defect were infertile due entirely to sperm immotility; in these patients, more than 80% of spermatozoa revealed thick, short, and irregular flagellum in total sperm population. In previous studies, electronic microscopy disclosed common features of sever asthenozoospermia defect resulted from short tail spermatozoa, involving the lack of axoneme or the severely disorganized axoneme and the absence or intensive disruption of central complex, doublets, peripheral junctions, dynein arms, and mitochondrial sheath [22–24]. The fertilizing capacity of spermatozoa requires progressive sperm motility, so it is clear that this defect has highly destructive effects on sperm fertilizing capacity and male fertility.
On the other hand, OAT is one of the most common phenotype of male infertility that shows high phenotypic variability. OAT involves a reduction in motility and number of spermatozoa with abnormal sperm morphology. Several causes for OAT have been stated, such as varicocele, infections, hormone preparations, and genetic factors [10].
In our study, two intronic variants were identified which one of them had not been previously reported and it was recognized in one patient (P26) with ISTS defect. The other intronic variant 50776482 delC was found in heterozygous form in both groups of our patients and it was not identified in controls. Allelic frequency of 50776482 delC variant was statistically increased in oligoasthenoteratozoospermic infertile men (p < 0.05). The result of bioinformatics investigations suggested that the 50776482 delC in homozygous form would result in modifying binding site for the splicing factor 9G8 [2]. Importantly, it is demonstrated that 9G8 is expressed in male germ cells in combination with RBMY (RNA-binding protein) and Tra2-β and has a crucial role in the regulation of specific splicing events in male germline [2, 15]. As it was mentioned, only the heterozygous form of this variant was identified in the present study; accordingly, the physiological consequences and the degree of splicing modification caused by this heterozygous variant need further analysis.
It should be considered that single-nucleotide polymorphisms (SNPs) and mutations in introns can have a significant effect on altering pre-mRNA splicing [2]. It is also demonstrated that genetic variants which affect splicing efficiency cause human illnesses and are remarkably correlated with disease severity [14].
Nearly all transcripts of protein-coding genes undergo splicing which is regulated by specific factors that recognize splicing sites on pre-mRNA. Splicing increases the coding capacity of eukaryotic genome and plays a critical role in human gene expression. Notably, considerable numbers of human diseases arise from splicing defects [16]. Up to 50% of mutations which cause diseases in human might affect splicing [15]; therefore, a deep study about the role of various splicing factors and their targets will help us to comprehend the pathogenicity of human diseases and will assist the development of new treatments that are focused on well-known splicing events and their related biological pathways [17].
In conclusion, 50776482 delC in intron 4 of human RABL2B was described as a splicing alteration which could be a risk factor in Iranian infertile men with oligoasthenoteratozoospermia defect; however, further studies using larger populations are recommended to proceed with these preliminary data.
Acknowledgements
We thank Amir Amiri-Yekta and Sajjad Sarikhan for kindly helping during the study. Also, we appreciate all patients who cooperated in this research.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Funding
This work was supported by Royan Institute funds.
Footnotes
Capsule This is the first report concerning the role of RABL2B gene in human male infertility. Nucleotide deletion (rs144944885) in RABL2B could alter a splicing factor target site and would be a risk factor in oligoasthenoteratozoospermic infertile men. Also a new genetic variant of RABL2B was identified in this study.
Anahita Mohseni Meybodi and Marjan Sabbaghian contributed equally to this work.
References
- 1.Rowe P, Comhaire F, Hargreave T, Mellows H. WHO manual for the standardized investigation and diagnosis of the infertile couple. Cambridge: Press Syndicate of the University of Cambridge; 1993. [Google Scholar]
- 2.Jamsai D, Lo JC, et al. Genetic variants in the RABL2A gene in fertile and oligoasthenospermic infertile men. Fertil Steril. 2014;102(1):223–9. doi: 10.1016/j.fertnstert.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 3.O’Brien KLF, Varghese AC, Agarwal A. The genetic causes of male factor infertility: a review. Fertil Steril. 2010;93(1):1–12. doi: 10.1016/j.fertnstert.2009.10.045. [DOI] [PubMed] [Google Scholar]
- 4.Chemes HE. Phenotypes of sperm pathology: genetic and acquired forms in infertile men. J Androl. 2000;21(6):799–808. [PubMed] [Google Scholar]
- 5.Ben Khelifa M, Coutton C, Zouari R, Karaouzene T, Rendu J, Bidart M, et al. Mutations in DNAH1, which encodes an inner arm heavy chain dynein, lead to male infertility from multiple morphological abnormalities of the sperm flagella. Am J Hum Genet. 2014;94(1):95–104. doi: 10.1016/j.ajhg.2013.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sironen A, Hansen J, Thomsen B, Andersson M, Vilkki J, Toppari J, et al. Expression of SPEF2 during mouse spermatogenesis and identification of IFT20 as an interacting protein. Biol Reprod. 2010;82(3):580–90. doi: 10.1095/biolreprod.108.074971. [DOI] [PubMed] [Google Scholar]
- 7.Sironen A, Thomsen B, Andersson M, Ahola V, Vilkki J. An intronic insertion in KPL2 results in aberrant splicing and causes the immotile short-tail sperm defect in the pig. Proc Natl Acad Sci U S A. 2006;103(13):5006–11. doi: 10.1073/pnas.0506318103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sironen A. Molecular genetics of the immotile short tail sperm defect. 2009.
- 9.Baccetti B, Burrini AG, Capitani S, Collodel G, Moretti E, Piomboni P, et al. Notulae seminologicae. 2. The ‘short tail’ and ‘stump’ defect in human spermatozoa. Andrologia. 1993;25(6):331–5. doi: 10.1111/j.1439-0272.1993.tb02736.x. [DOI] [PubMed] [Google Scholar]
- 10.Wei TC, Huang WJ, Lin AT, Chen KK. The role of hormones on semen parameters in patients with idiopathic or varicocele-related oligoasthenoteratozoospermia (OAT) syndrome. J Chin Med Assoc: JCMA. 2013;76(11):624–8. doi: 10.1016/j.jcma.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 11.Lo JC, Jamsai D, O’Connor AE, Borg C, Clark BJ, Whisstock JC, et al. RAB-like 2 has an essential role in male fertility, sperm intra-flagellar transport, and tail assembly. PLoS Genet. 2012;8(10):e1002969. [DOI] [PMC free article] [PubMed]
- 12.Wong AC, Shkolny D, Dorman A, Willingham D, Roe BA, McDermid HE. Two novel human RAB genes with near identical sequence each map to a telomere-associated region: the subtelomeric region of 22q13.3 and the ancestral telomere band 2q13. Genomics. 1999;59(3):326–34. doi: 10.1006/geno.1999.5889. [DOI] [PubMed] [Google Scholar]
- 13.Chacon-Cortes D, Griffiths LR. Methods for extracting genomic DNA from whole blood samples: current perspectives. J Biorepos Sci Appl Med. 2014;2014(2):1–9. [Google Scholar]
- 14.Orengo JP, Cooper TA. Alternative splicing in disease. Adv Exp Med Biol. 2007;623:212–23. doi: 10.1007/978-0-387-77374-2_13. [DOI] [PubMed] [Google Scholar]
- 15.Dreumont N, Bourgeois CF, Lejeune F, Liu Y, Ehrmann IE, Elliott DJ, et al. Human RBMY regulates germline-specific splicing events by modulating the function of the serine/arginine-rich proteins 9G8 and Tra2-{beta} J Cell Sci. 2010;123(Pt 1):40–50. doi: 10.1242/jcs.055889. [DOI] [PubMed] [Google Scholar]
- 16.Tazi J, Bakkour N, Stamm S. Alternative splicing and disease. Biochim Biophys Acta (BBA)-Molecul Basis Dis. 2009;1792(1):14–26. doi: 10.1016/j.bbadis.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cieply B, Carstens RP. Functional roles of alternative splicing factors in human disease. Wiley Interdisciplin Rev: RNA. 2015;6(3):311–26. doi: 10.1002/wrna.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moretti E, Collodel G. Electron microscopy in the study of human sperm pathologies. Translocat. 2012;33:34. [Google Scholar]
- 19.Coutton C, et al. Teratozoospermia: spotlight on the main genetic actors in the human. Hum Reprod Update. 2015;21(4):455–85. doi: 10.1093/humupd/dmv020. [DOI] [PubMed] [Google Scholar]
- 20.McAlister DA. A comparison of motility and head morphology of sperm using different semen processing methods and three different staining techniques. Stellenbosch: University of Stellenbosch; 2010. [Google Scholar]
- 21.Medicine, P.C.o.t.A.S.f.R Diagnostic evaluation of the infertile male: a committee opinion. Fertil Steril. 2012;98(2):294–301. doi: 10.1016/j.fertnstert.2012.05.033. [DOI] [PubMed] [Google Scholar]
- 22.Chemes H, et al. Ultrastructural pathology of the sperm flagellum: association between flagellar pathology and fertility prognosis in severely asthenozoospermic men. Hum Reprod. 1998;13(9):2521–6. doi: 10.1093/humrep/13.9.2521. [DOI] [PubMed] [Google Scholar]
- 23.Chemes HE, et al. Dysplasia of the fibrous sheath: an ultrastructural defect of human spermatozoa associated with sperm immotility and primary sterility. Fertil Steril. 1987;48(4):664–9. doi: 10.1016/S0015-0282(16)59482-5. [DOI] [PubMed] [Google Scholar]
- 24.Escalier D, David G. Pathology of the cytoskeleton of the human sperm flagellum: axonemal and peri‐axonemal anomalies. Biol Cell. 1984;50(1):37–52. doi: 10.1111/j.1768-322X.1984.tb00253.x. [DOI] [PubMed] [Google Scholar]