Abstract
To validate the identification of Pasteurella multocida-like bacteria negative for acid formation from sucrose, including isolates from bite wounds caused by large cats, 17 strains were phenotypically and genotypically characterized. Phylogenetic analysis of partially sequenced rpoB and infB genes showed the monophyly of the strains characterized and the reference strains of P. multocida. The sucrose-negative strains formed two groups, one related to reference strains of P. multocida and the other related to a separate species-like group (taxon 45 of Bisgaard). DNA-DNA hybridization further documented the species-like nature of this group. Ribotyping showed the heterogeneity of all strains except four strains that shared the same ribotype and that were isolated from bovine lungs. Phylogenetic analysis by 16S rRNA sequence comparison showed the monophyly of the strains characterized and the reference strains of P. multocida. Two strains isolated from leopard bite wounds were related to the type strain of P. dagmatis; however, they represented a new taxon (taxon 46 of Bisgaard), in accordance with their distinct phenotypic and genotypic identifications. The present study documents that sucrose-negative strains of P. multocida-like bacteria belong to two genotypically distinct groups. The study further confirms the phenotypic heterogeneity of P. multocida strains and documents two new species-like taxa of Pasteurella related to P. multocida. Until diagnostic tools have been further elaborated, special care should be taken in the identification of Pasteurella-like bacteria isolated from bite wounds caused by large cats. The evidence of phenotypic and genotypic divergence calls for the further development of PCR tests and DNA sequencing to document doubtful isolates.
Pasteurella multocida has been isolated from a multitude of hosts (26), and different lineages of P. multocida may be responsible for various diseases in both birds and mammals (9, 15). Human infections with P. multocida are in most cases of animal origin and are most often related to the bites of carnivores. However, other types of infections are also occasionally reported (25, 28, 34).
Significant variations in the phenotypic properties of P. multocida have been reported (27), leading to confusion in the definition and identification of this organism. Mutters et al. (37) reclassified the genus Pasteurella on the basis of DNA-DNA hybridization studies. Three clusters of P. multocida showing 84 to 100, 91 to 100, and 89 to 100% DNA reassociation between strains subsequently described as P. multocida subsp. multocida, P. multocida subsp. gallicida, and P. multocida subsp. septica, respectively, were identified. Representatives of the existing capsular types were found to be closely related on the basis of DNA-DNA hybridization (44), despite the diversity of disease manifestations and hosts. This is contradictory to the diversity shown by outer membrane protein profiling (17, 18, 19), multilocus enzyme electrophoresis (5), and ribotyping (43). The study of Petersen et al. (43) showed a great diversity of ribotypes among strains classified as P. multocida subsp. multocida, P. multocida subsp. gallicida, and P. multocida subsp. septica. However, comparisons of the sequences of the 16S rRNA gene and the atpD gene (which encodes the β subunit of ATP synthase) confirmed the overall homogeneity of P. multocida (43). The study of Kuhnert et al. (33) also showed that variant phenotypes of P. multocida shared at least 98.5% 16S rRNA sequence similarity with the recognized subspecies of this species.
P. avium and P. canis were reported as new species by Mutters et al. (36, 37). Both species were separated into two biovars. Strains of P. canis biovar 2 and P. avium biovar 2 and strains of P. multocida deviating in key phenotypic characters were subsequently genotyped to examine their relationship with P. multocida (13). Surprisingly, these investigations allowed the reclassification of P. multocida to include biovars 2 of both P. avium and P. canis, and on the basis of this background, the existence of biovars 2 of P. avium and P. canis was questioned. The redefined species P. multocida is genotypically homogeneous, although phenotypically diverse lineages exist with respect to the key characteristics ornithine decarboxylase, indole, and mannitol fermentation, which have been regarded as essential for identification of P. multocida to the species level (13).
P. multocida and P. multocida-like bacteria have occasionally been isolated from the wounds of humans bitten by large cats, like lions and tigers (6, 25, 29, 48, 50). Similar organisms have also been isolated from the dental-gingival junction of several species of large cats (50). The majority of these isolates do not ferment sucrose (25, 50) and, consequently, differ from Pasteurella sensu stricto, which is defined as sucrose positive (9). Strain SSI P 876, proposed as “P. leonis” (Table 1), was isolated from a man bitten by a lion, and this strain was found to be genotypically related to P. multocida (30); however, the polyamine pattern of the strain differed from that of the type strain (7). Strain ATCC BAA 600 (Table 1) was isolated from a girl bitten by a tiger. The name “Pasteurella multocida subsp. tigris” was proposed for this strain on the basis of phenotypic characterization and 16S rRNA gene sequence comparisons (8). The phenotypic characteristics reported were in accordance with those of P. multocida, except for the lack of fermentation of sucrose and mannitol, the fermentation of which being which specific characteristics of P. multocida (39). Disregarding the results for sucrose and mannitol fermentation, the combination of sorbitol fermentation and a lack of dulcitol, xylose, and trehalose fermentation would have identified the isolate as P. multocida subsp. multocida (39).
TABLE 1.
Strains used for investigation of P. multocida, including isolates from large cats and sucrose-negative variants
| Species and identification | Straina | Country, yr of isolation | Source (animal, disease, or organ) | Ribotype | PCR result | Capsular type | GenBank accession no.
|
||
|---|---|---|---|---|---|---|---|---|---|
| 16S rRNA | rpoB | infB | |||||||
| Reference strains | |||||||||
| P. multocida subsp. multocida | NCTC 10322T (ATCC 43137T) | 5 | +b | A | AF294410c | AY170216c | AJ289690c | ||
| P. multocida subsp. gallicida | NCTC 10204T (ATCC 51689T) | 6 | + | A | AF326323c | AY362969c | AY683511 | ||
| P. multocida subsp. septica | CIP A125T (NCTC 11995T, ATCC 51687T) | 7 | + | A | AF326325c | AY362970c | AY683512 | ||
| P. canis | NCTC 11621T | NTd | AY362919c | AY314038c | |||||
| P. multocida subsp. septica | 5e | Germany | Calf pneumonia | NDf | + | A | AY316314c | AY683493 | AY683513 |
| P. multocida subsp. septica | 25e | Germany | Calf pneumonia | ND | + | A | Identical to AY316314c | AY683494 | AY683514 |
| P. multocida subsp. septica | A 285/86e | Germany, 1986 | Calf pneumonia | ND | + | A | Identical to AY316314c | AY683495 | AY683515 |
| P. multocida subsp. septica | W 208e | Germany | Bovine organs | ND | + | A | AY316317c | AY683496 | AY683516 |
| Strains investigated | |||||||||
| P. multocida subsp. multocida, ornithine, indole, and sucrose negative | B80/20g | United Kingdom | Bovine pneumonia | 4 | + | A | Identical to AY316316c | AY683497 | AY683517 |
| P. multocida subsp. multocida, ornithine, indole, and sucrose negative | B80/26g | United Kingdom | Bovine pneumonia | 4 | + | A | Identical to AY316316c | AY683498 | AY683518 |
| P. multocida subsp. multocida, sucrose negative | W 819 | United Kingdom | Bovine pneumonia | 4 | + | A | AY683485 | AY683499 | AY683519 |
| P. multocida subsp. multocida, mannitol and sucrose negative | RA 12/2e | United Kingdom | Bovine pneumonia | 10 | + | A | AY316316c | AY683500 | AY683520 |
| P. multocida subsp. septica, mannitol, indole, and sucrose negative | K 323e | Denmark | Bovine pneumonia | 1 | + | A | Identical to AY316316c | AY683501 | AY683521 |
| P. multocida subsp. septica, indole and sucrose negative | Schmid W 87-227-9 (HIM 1057-3) | Germany, 1987 | Sheep | 3 | + | A | Identical to AY316317c | AY683502 | AY683522 |
| P. multocida subsp. septica, sucrose and mannitol negative | Younan S2 (HIM 996-8, MCCM 00657) | Syria, 1986 | Sheep, nose | 2 | + | A | Identical to AF224298c | AY683503 | AY683523 |
| P. multocida subsp. septica, Ornithine, sucrose and mannitol negative | 288g | Germany | Calf pneumonia | 4 | + | A | Identical to AY316315c | AY683504 | AY683524 |
| Bisgaard taxon 45 | 14589/75g | United Kingdom | Source unknown | 11 | + | A | AY683486 | AY683505 | AY683525 |
| Bisgaard taxon 45 | CDC F 4484 | United States | Man, tiger bite wound | 9 | + | NT | AY683487 | AY683506 | AY683526 |
| Bisgaard taxon 45 | CDC G 9955 | United States | Man, tiger bite wound | ND | + | ND | AY683488 | ND | ND |
| Bisgaard taxon 45 “P. multocida subsp. tigris” | ATCC BAA-600 | United States | Man, tiger bite | 12 | + | NT | AY057994c | AY683507 | AY683527 |
| Bisgaard taxon 45 “P. leonis” | SSI P 876 (HIM 969-4, FsK11447, MCCM 00659) | Kenya, 1984 | Man, bite from lion | 8 | − | NT | AY683489 | AY683508 | AY683528 |
| Bisgaard taxon 45 | 47182 | United Kingdom, 1975 | Man, lion bite wound | ND | ND | ND | ND | ND | ND |
| Bisgaard taxon 45 | HIM 1004-6 (Pasteurella sp. strain Schmid 351) | Germany, 1967 | Chipmunk | 13 | + | A | AY683490 | AY683509 | AY683529 |
| Bisgaard taxon 46 | CDC A996 | United States | Man, leopard bite wound | 14 | − | NT | AY683491 | AY683510 | AY683530 |
| Bisgaard taxon 46 | CDC F 4646 | United States | Man, leopard bite wound | ND | − | ND | AY683492 | ND | ND |
ATCC, American Type Culture Collection; NCTC, National Collection of Type Cultures; CIP, Collection de I'Institut Pasteur; HIM, Hygienischer Institut Marburg; MCCM, Microbiology Culture Collection Marburg; CDC, Centers for Disease Control and Prevention; SSI, Statens Serum Institut.
+, positive reaction by the P. multocida-specific PCR of Miflin and Blackall (35).
Accession number of a sequence determined in a previous report.
NT, no type obtained by the capsular PCR test.
From an earlier investigation by Christensen et al. (13).
ND, not determined.
From an earlier investigation by Bisgaard et al. (4).
Among 1,268 cultures of P. multocida characterized by Heddleston (27), none were reported to be sucrose negative. Among some 1,500 strains characterized in the laboratory of one of the authors (M. Bisgaard, unpublished data), only 17 were sucrose negative.
The objective of the present study was to characterize sucrose-negative variant strains of P. multocida-like bacteria, including isolates obtained from large cats, in order to investigate their taxonomic position and host relationship and to identify methods for the identification of these bacteria.
MATERIALS AND METHODS
Selection of bacterial strains and phenotypic characterization.
A total of 25 bacterial strains were investigated, including reference strains and strains that have been described previously (4, 8, 13) (Table 1). Phenotypic characterization of 15 strains was performed on the basis of 79 characteristics, as reported previously (4).
Ribotyping.
Ribotyping of 17 strains selected to represent phenotypic variants, including type strains of the three subspecies of P. multocida, was done as described by Christensen et al. (16). Briefly, 6 μg of DNA was digested with the HpaII enzyme (New England Biolabs, Beverly, Mass.) at 37°C for 2 h. The digests were separated on a 0.8% agarose gel for 16 h at 35 V. The gel was stained in ethidium bromide, and the DNA was vacuum blotted onto a nitrocellulose filter. The filter was reacted with a 16S-23S rRNA-specific probe overnight at 56°C. Ribotype patterns were rendered visible with a digoxigenin wash and block buffer kit (Roche A/S, Hvidovre, Denmark), according to the instructions of the manufacturer. Bacteriophage λ DNA digested with HindIII (New England Biolabs) was included as a size marker. Ribotype patterns were analyzed with Gelcompar software (version 4.0; Applied Maths, Kortrijk, Belgium), and Dice coefficients were used to construct a neighbor-joining dendrogram with the PHYLIP software package (24).
Sequencing of 16S rRNA, rpoB, and infB genes.
The 16S rRNA gene sequences of 18 strains were determined, and the rpoB and infB sequences of 21 strains were compared (Table 1). The bacteria were cultured overnight in brain heart infusion broth (Difco, Detroit, Mich.) at 37°C. The fragments amplified by PCR were purified and cycle sequenced on an automatic sequencer (ABI 377; Chemistry Guide; Applied Biosystems, Foster City, Calif.), as described recently (14, 31, 40). DNA sequencing resulted in at least 1,278, 470, and 446 bp of the 16S rRNA, rpoB, and infB genes, respectively.
Analysis of sequence data.
Searches for homologous DNA sequences in the GenBank database were performed with the BLAST program (1). Pairwise similarities were calculated with the BESTFIT program (Wisconsin Sequence Analysis Package; Genetics Computer Group, Madison, Wis.). Phylogenetic analysis by parsimony analysis was performed with the PROTPARS and CONSENSE programs with the default settings included with the PHYLIP package (24). Maximum-likelihood analysis, including bootstrap analysis, was performed with fastDNAml software (41) run on a Linux (version 7.2)-compatible server.
DNA-DNA hybridization.
DNA-DNA hybridization was determined for 11 pairs of strains by the microwell method (11) with the modifications reported previously (13), including growth with EDDA (ethylenediamine-N,N′-diacetic acid) and hyaluronidase to limit capsule formation of isolates with capsular type A.
P. multocida-specific PCR and capsular typing by PCR.
The PCR procedure of Miflin and Blackall (35) was followed, as reported recently (13). Briefly, a loopful of an overnight culture was taken from the surface of a blood agar plate and suspended in sterile water. The suspension was boiled, the cells were spun down, and the supernatant was used as the template for the PCR. PCR was performed with primers PM23F1 and PM23R2 (35). After electrophoresis and ethidium bromide staining, an amplicon of 1,432 bp could be visualized under UV light. Capsular typing by PCR was performed as described by Townsend et al. (49).
ITS analysis.
PCR typing by the 16S-23S rRNA internal transcribed spacer (ITS) analysis approach was performed for five strains, as reported by Christensen et al. (13).
Nucleotide sequence accession numbers.
The nucleotide sequences described in this report have been deposited in GenBank under accession numbers AY683485 to AY683530 and are indicated in Table 1 and Fig. 2 to 4.
FIG. 2.
Phylogenetic relationships between strains of Pasteurella, including the type strain of P. multocida subsp. septica only (left panel) and 11 additional strains of this taxon (right panel), based on maximum-likelihood analysis of 16S rRNA gene sequences. The support for monophyletic groups by bootstrap analysis is indicated as percentages. Nodes recognized by maximum-parsimony analysis are marked with an asterisk. Strains sequenced in the present study are in boldface type.
FIG. 4.
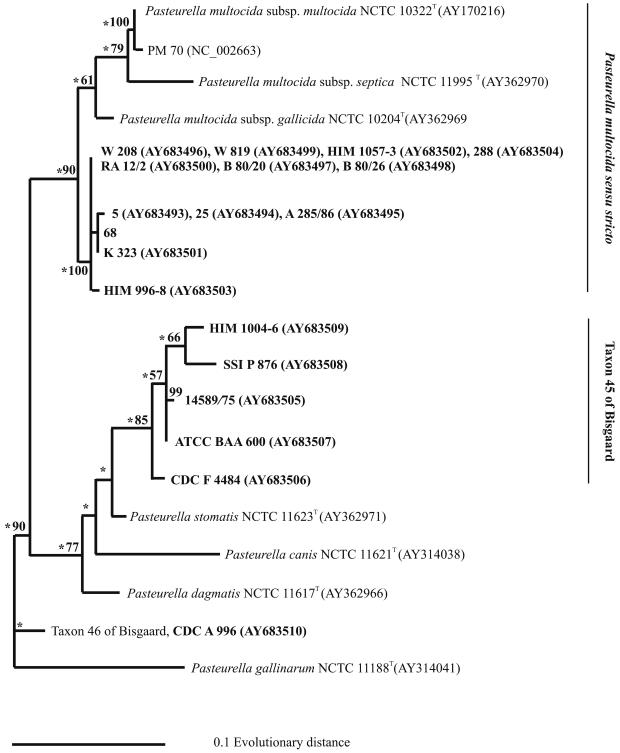
Phylogenetic relationships between strains of Pasteurella based on maximum-likelihood analysis of the infB gene DNA sequences. The support for monophyletic groups by bootstrap analysis is indicated as percentages. Nodes recognized in maximum parsimony analysis are labeled with an asterisk. Strains sequenced in the present study are in bold face type.
RESULTS
Phenotypic characterization.
Seventeen of the 79 characteristics investigated varied between the 22 strains analyzed (Table 2), all of which were in accordance with the species description of P. multocida described by Mutters et al. (39) and Christensen and Bisgaard (9) (Table 2). Eight and four strains were classified as P. multocida subsp. septica and P. multocida subsp. multocida, respectively, while nine strains remained unclassified. Seven of these were phenotypically related to P. multocida subsp. gallicida but could be separated from this subspecies by sucrose and glycerol utilization, and some could be separated by the formation of gas from (+)-d-glucose (Table 2). These organisms, tentatively named Bisgaard taxon 45, were obtained from tiger (n = 3) and lion (n = 2) bite wounds in humans, a chipmunk, and an unknown source. Two strains, both of which were obtained from leopard bite wounds in humans, also remained unclassified. Both of these isolates differed from the type strain of P. dagmatis in (−)-d-sorbitol, sucrose, and trehalose fermentation, key characteristics for the separation of members of the family Pasteurellaceae. For the same reasons mentioned above, these organisms might represent a new taxon and are consequently named Bisgaard taxon 46.
TABLE 2.
Variable phenotypic characteristics of P. multocida and taxon 45 of Bisgaard
| Characteristic | Result fora:
|
|||||
|---|---|---|---|---|---|---|
| P. multocida subsp. multocida NCTC10322T | P. multocida subsp. gallicida NCTC10204T | P. multocida subsp. septica NCTC11995T | P. multocida subsp. septica (eight atypical strainsb) | P. multocida subsp. multocida (four sucrose-negative strains) | Taxon 45 of Bisgaard (seven strains) | |
| Oxidase | + | + | + | d (4)c | d (1) | + |
| Ornithine decarboxylase | + | + | + | d (4) | d (2) | + |
| Indole | + | + | + | d (2) | d (2) | + |
| Glycerol | − | − | + | − | − | d (6) |
| (+)-l-Arabinose | − | + | − | − | − | − |
| (−)-d-Arabinose | − | − | (+) | − | − | − |
| (+)-d-Xylose | (+) | − | + | d (3) | − | d (5) |
| Dulcitol | −d | + | − | − | − | d (3) |
| (−)-d-Mannitol | (+) | + | + | d (2) | d (2) | − |
| (−)-d-Sorbitol | (+) | + | − | − | +/(+) | + |
| (−)-l-Fucose | − | − | (+) | − | d (1) | − |
| Gas from (+)-d-glucose | − | − | − | − | − | d (3) |
| Lactose | − | − | − | d (1) | − | − |
| ONPGe | − | − | − | d (1) | − | − |
| Sucrose | + | + | + | d (4) | − | − |
| Trehalose | + | − | + | + | + | − |
| PNPGf | + | − | + | + | + | d (1) |
+, positive within 1 to 2 days; (+), positive within 3 to 14 days; −, negative after 14 days; d, variable.
Including four reference strains (Table 1).
The number of positive strains is given in parentheses.
Key characteristics used for separation of taxa are in boldface.
ONPG, β-galactosidase test performed with o-nitro-phenyl-d-galactopyranoside.
PNPG, α-glucosidase test determined with 4-nitrophenyl-α-d-glucopyranoside.
Ribotyping.
Seventeen strains were compared by ribotyping, including the three type strains of P. multocida. Between three and nine bands were registered at 13 positions in 14 ribotypes (Fig. 1). One band of approximately 5 kb was common to all strains. One group included the two type strains of P. multocida subsp. multocida and P. multocida subsp. gallicida and seven other strains, all of which were sucrose negative. These nine strains were closely related and shared at least four bands. Four strains even shared the same ribotype. The remaining strains were less uniform with respect to ribotype. This group included taxa 45 and 46; however, strain RA 12/2 of P. multocida subsp. multocida was also located with these strains by ribotyping.
FIG. 1.
Genotypic relationships between P. multocida and related atypical strains of Pasteurella investigated by ribotyping. Strains with identical profiles for specific ribotypes are indicated at the branch tips. The dendrogram was based on neighbor-joining cluster analysis of the Dice coefficients formed by comparison of the fragment profiles.
16S rRNA sequence comparison.
Identical 16S rRNA gene sequences were found between sucrose-negative strains HIM 996-8 and 288 and sucrose-positive strain 214 (GenBank accession no. AF224298) (13). Strains B 80/20, B 80/26, and K 323 had the same sequence as previously sequenced strain RA12/2 (GenBank accession no. AY316316) (13), and strain HIM 1057-3 showed a sequence identical to the previously published sequence of sucrose-positive strain W208 (GenBank accession no. AY316317) (13). This shows that sucrose-positive and -negative isolates can have identical 16S rRNA sequences.
The phylogenetic analysis based on 16S rRNA gene sequence comparison showed the monophyly of all strains of P. multocida, including the sucrose-negative variants of both P. multocida and taxon 45 (Fig. 2, left panel). The level of 16S rRNA gene sequence similarity was 98.4% or higher within this group, and the level of similarity was at least 98.6% between all the strains and the type strain of P. multocida. If only the type strain of P. multocida subsp. septica was included in the phylogenetic analysis, a tendency for two subgroups to appear within the major P. multocida group was observed (Fig. 2, left panel). Strains classified as taxon 45 (strains ATCC BAA 600, 14589/75, SSI P 876, HIM 1004-6, and CDC F 4484) and the type strain of P. multocida subsp. septica showed at least 99.4% gene sequence similarity. At least 98.4% similarity was found between this group and the other group, including the type strains of the other two subspecies. Low bootstrap values were found for this tree, including the node supporting the two groups of P. multocida-like strains. The reason for these low bootstrap values was probably the low levels of sequence variation. For this reason the position of P. multocida subsp. septica should also be taken with caution. Repeat analysis after the inclusion of 11 additional P. multocida subsp. septica sequences published previously by Kuhnert et al. (33) changed the positions of the type strain and related strains such that they formed a new group with strain CDC A 996 and CDC F 4646 (taxon 46) and the type strain of P. dagmatis (Fig. 2, right panel). In this new version of the tree, the remaining strains of P. multocida could no longer be separated from taxon 45.
The two strains of taxon 46 formed a monophyletic group with the type strain of P. dagmatis if only the type strain of P. multocida subsp. septica was included (Fig. 2, left panel). The highest degree of similarity found for strain CDC A 996 was to strain F 4646 (99.6%) and the type strain of P. dagmatis (98.4%). At most, 97.4% similarity to the sucrose-negative and -positive strains of P. multocida investigated was found.
The reason for the instability of the phylogenetic trees based on 16S rRNA sequences when a few more related strains are included is probably caused by a low degree of sequence variation. For this reason, sequencing of the housekeeping genes infB and rpoB was initiated.
infB and rpoB sequence comparison.
The two groups of sucrose-negative strains found by ribotyping (except for RA 12/2) were also recognized by phylogenetic analysis of partially sequenced infB and rpoB genes (Fig. 3, and 4). The four strains of P. multocida isolated from wounds caused by large-cat bites and the strain isolated from a chipmunk formed their own monophyletic group. This group was provisionally named taxon 45 of Bisgaard by phenotypic classification (see above). Strains classified as Bisgaard taxon 46 and represented by strain CDC A 996 were place into a group unrelated to P. multocida and taxon 45, and comparison of the rpoB and infB sequences did not allow classification of Bisgaard taxon 46 with P. dagmatis (Fig. 3 and 4).
FIG. 3.
Phylogenetic relationships between strains of Pasteurella based on maximum-likelihood analysis of the rpoB gene DNA sequences. The support for monophyletic groups by bootstrap analysis is indicated as percentages. Nodes recognized by maximum-parsimony analysis are labeled with an asterisk. Strains sequenced in the present study are in boldface type.
DNA-DNA hybridization.
On basis of the present results and results published by Eckert et al. (22), Stenzel (47), and Christensen et al. (13), an overall high degree of DNA reassociation of at least 78% seems to exist for strains classified in the single subspecies of P. multocida (Table 3), in accordance with the general levels of DNA reassociation of the three subspecies (37). Slightly lower DNA reassociation values were observed between subspecies of P. multocida, which represent phenotypically deviating strains. For these strains the rates of DNA reassociation varied between 53 and 91% (Table 3). Strains classified as taxon 45 all showed DNA binding values below the species level (31 to 51%), with the strains representing the three subspecies of P. multocida; however, 90% DNA reassociation was found between the two strains of taxon 45 (Table 3). When the rpoB and infB sequence analysis results are compared with the ribotyping results, taxon 45 seems to differ from P. multocida and probably represents a new species-like taxon of Pasteurella.
TABLE 3.
DNA-DNA hybridization between strains of P. multocida and taxon 45 of Bisgaard in relation to that for sucrose-negative variants
| Taxon | Strain(s) | Taxon | Strain(s) | % DNA reassociation |
|---|---|---|---|---|
| P. multocida subsp. multocida | NCTC 10322T | P. multocida subsp. multocida | B 80/20 | 78 (9)a |
| P. multocida subsp. septica | 5 | P. multocida subsp. septica | A 285/86 | 96 (9)b |
| HIM 996-8 | HIM1057-3 | 95 (7)c | ||
| W 208 | A 285/86 | 94 (19)b | ||
| 25 | A 285/86 | 81 (13)b | ||
| P. multocida subsp. multocida | RA 12/2 | P. multocida subsp. septica | A 285/86 | 91 (18)b |
| NCTC 10322T | HIM 996-8 | 66 (7)c | ||
| NCTC 10322T | A 285/86 | 63 (17)b | ||
| P. multocida subsp. multocida | B 80/20 | P. multocida subsp. gallicida | NCTC10204T | 70 (9) |
| P. multocida subsp. gallicida | NCTC 10204T | P. multocida subsp. septica | A 285/86 | 53 (12)b |
| P. multocida subsp. multocida | NCTC10322T | Taxon 45 of Bisgaard | HIM 1004-6 | 48 (12) |
| NCTC10322T | SSI P 876 | 41 (4)c | ||
| NCTC10322T | 14589/75 | 40 (4) | ||
| RA 12/2 | HIM 1004-6 | 34 (8) | ||
| P. multocida subsp. gallicida | NCTC10204T | Taxon 45 of Bisgaard | 14589/75 | 42 (8) |
| NCTC10204T | HIM 1004-6 | 36 (15) | ||
| P. multocida subsp. septica | HIM 1057-3 | Taxon 45 of Bisgaard | SSI P 876 | 51 (3)c |
| HIM 1057-3 | HIM 1004-6 | 50 (2)c | ||
| HIM 996-8 | HIM 1004-6 | 47 (6)c | ||
| 5 | HIM 1004-6 | 43 (6) | ||
| NCTC 11995T | SSI P 876 | 40 (8)c | ||
| HIM 996-8 | SSI P 876 | 38 (5)c | ||
| 25 | HIM 1004-6 | 38 (4) | ||
| A 285/86 | HIM 1004-6 | 32 (5) | ||
| W 208 | HIM 1004-6 | 31 (10) | ||
| Taxon 45 of Bisgaard | SSI P 876 | Taxon 45 of Bisgaard | HIM 1004-6 | 90 (6)c |
PCR.
With the exception of a single strain, all strains of taxon 45 surprisingly tested positive by the PCR test of Miflin and Blackall (35) for P. multocida. Both strains of taxon 46 tested negative by the PCR test. In addition, the results of capsular typing remained negative for this taxon; however, negative capsular typing results were also observed for strains of taxon 45. The typeable strains were all of type A.
ITS analysis.
ITS analysis showed that strains 288, B 80/20, and B 80/26 had the same profile as the type strain of P. multocida; however, another profile was found for strains 14589/75 and HIM 1004-6 (data not shown). These results confirm the existence of two groups of sucrose-negative strains that are recognized by ribotyping, rpoB and infB sequence comparison, and DNA-DNA hybridization.
DISCUSSION
The present study has documented that sucrose-negative strains of P. multocida-like bacteria belong to two genotypically distinct groups. One group that is genotypically similar to P. multocida contained strains mainly isolated from cows with pneumonia, whereas the other group was isolated mainly from large-cat bite wounds and formed a new species-like taxon (taxon 45 of Bisgaard). The study supplements previous investigations of phenotypically divergent P. multocida strains (5, 13, 23, 42), including V factor-dependent strains of P. multocida subsp. multocida isolated from pigs with pneumonia (32). The documentation of phenotypic variations in key characteristics of P. multocida, including maltose (42), ornithine decarboxylase, indole, and mannitol (13), and sucrose (the present study) fermentation, makes routine diagnosis on the basis of phenotypic characteristics difficult and uncertain.
The present investigation confirms previous difficulties associated with the use of 16S rRNA gene sequence comparisons for separation of the subspecies of P. multocida, as well as with the use of phenotypic characteristics for the separation of P. gallinarum and related species (12, 43). This problem has also been reported for other taxa (46). On the basis of 16S rRNA gene sequence comparisons, the four strains of P. multocida subsp. septica were expected to show a tight relationship with the type strain of this taxon. Surprisingly, these strains were more closely related to P. multocida subsp. multocida (Fig. 2). The four strains probably represent sorbitol-negative variants of P. multocida subsp. multocida, and they will therefore be misidentified as P. multocida subsp. septica by phenotyping. Similar observations were made in a previous study (13).
The 16S rRNA phylogenetic tree that incorporated all strains of P. multocida and related taxa was rather unstable in relation to perturbations of input sequences (Fig. 2). For this reason, housekeeping gene sequences were compared. The present study is the first to use housekeeping gene sequence comparison for classification of a larger set of strains of P. multocida and related bacteria. Davies et al. (20) recently used housekeeping gene sequence comparison for the diagnostic comparison of P. multocida isolates. The results for the two genes rpoB and infB analyzed in the present study corresponded well, and the analysis seemed to provide a deeper resolution at the species level compared to that achieved by 16S rRNA gene sequence comparison. Recently, infB sequence comparison has successfully been used for phylogenetic analysis of Haemophilus and Actinobacillus sensu stricto and rpoB sequence comparison has been used for investigation of Histophilus somni and members of the family Pasteurellaceae (3, 14, 31, 40). Although comparison of housekeeping gene sequences has been recommended for classification to the species level (46), a consensus has not been reached.
The results of phenotypic and phylogenetic analyses of sequences of the housekeeping genes rpoB and infB point to the existence of a new sucrose-negative species of Pasteurella isolated mainly from the wounds of bites caused by large cats and tentatively named taxon 45 of Bisgaard. The resolution obtained by 16S rRNA sequence analysis did not enable a clear separation of the group from P. multocida. However, except for one strain, the members of taxon 45 were recognized by ribotyping. Two strains from the divergent group showed a DNA reassociation rate at the species level of 90% and were well separated from other taxa by less than 51% DNA reassociation. The limit for defining a species on the basis of DNA reassociation within the family Pasteurellaceae has varied between 80 and 85% (2, 10, 38).
Since “P. multocida subsp. tigris” strain ATCC BAA 600 (8) was found to be a member of taxon 45 and strain SSI P 876, tentatively named “P. leonis” (47), also belongs to this taxon, priority should be given to SSI P 876 in future studies on the final classification of this taxon. However, it is of vital importance that the present knowledge be used to preserve additional strains from large-cat bite wounds to allow final classification of organisms classified as taxon 45 of Bisgaard.
The polyamine pattern of SSI P 876 deviated significantly from those of the type strains of the subspecies of P. multocida (7). The members P. multocida, P. canis, P. dagmatis, P. stomatis, and Pasteurella species strain B of 16S rRNA cluster 3B (21) and strain SSI P 876 mentioned above could all be separated from most other members of the family Pasteurellaceae by the presence of the polyamine sym-nor-spermidine (7), suggesting that strain SSI P 876 is part of 16S rRNA cluster 3B.
Strains HIM 1004-6 and W 208 were included in the study of Schmid et al. (45). Despite their phenotypic variation from P. multocida, both strains were found to be closely related to the type strains of the P. multocida subspecies by crossed immunoelectrophoresis analysis, in accordance with the results obtained in the present study, including the fact that they belong to capsular type A.
Strains tentatively named taxon 46 seem to represent a new species-like group within the genus Pasteurella sensu stricto. These organisms are urease positive and might easily be misidentified as P. dagmatis since they are associated with cat-bite lesions in humans. However, this taxon differs from P. dagmatis in (−)-d-sorbitol, sucrose, and trehalose fermentation; and laboratories that come across similar organisms are encouraged to keep these isolates or submit them to reference laboratories to enable final studies on the classification of this taxon.
The G+C contents of strains SSI P 876 and HIM 1004-6 were found to be 37.2 and 41.8 mol%, respectively, and the genome masses were found to be 1.8 and 1.7 GDa, respectively (22, 47). These values are within the range of G+C contents of 37.7 to 45.9 mol% and genome masses of 1.4 to 1.9 GDa reported for Pasteurella sensu stricto, even though the G+C content of strain SSI P 876 is slightly below this limit.
Surprisingly, all strains of Bisgaard taxon 45 tested positive for the P. multocida species test of Miflin and Blackall (35), and the reasons for this remain to be investigated. The test is based on the 23S rRNA gene sequence as a target for the PCR, and sufficient variation might not be present within this gene for separation of P. multocida from taxon 45. Further elaboration of DNA sequence-based tests might contribute to a more accurate means of identification of P. multocida and related members of the genus Pasteurella.
In conclusion, the present study showed that sucrose-negative variant strains of P. multocida-like bacteria, including isolates obtained from large cats, belong to two taxa. One group of strains mainly isolated from cows with pneumonia belonged to P. multocida, whereas the other strains mainly isolated from human bite wounds caused by large cats belonged to a new taxon (taxon 45 of Bisgaard). Two other strains isolated from bite wounds caused by leopards formed a new taxon of Pasteurella sensu stricto (taxon 46 of Bisgaard) with similarity to P. dagmatis. The study showed the limitations of phenotype-based tests as well as those of genotype-based tests, such as 16S rRNA sequence comparison and PCR, for the identification of these bacteria. For the identification of isolates with doubtful identities, the use of a combination of phenotype- and genotype-based tests test is recommended. Only with the collection and deposition of more isolates like these will further characterization in reference laboratories be able to be performed and the taxonomy of these bacteria improved. Further elaboration of DNA sequence-based tests, including PCR, might then contribute to a safer means of identification of P. multocida and related members of the genus Pasteurella.
Acknowledgments
Katrine Madsen and Lisbeth Dam are thanked for excellent technical assistance. Special thanks is given to Paul N. Levett, Special Bacteriology Reference Laboratory, Centers for Disease Control and Prevention (CDC), Atlanta, Ga., for providing access to the CDC strains.
The study was financed by the Danish Agricultural and Veterinary Research Council (grant 9702797).
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angen, Ø., R. Mutters, D. A. Caugant, J. E. Olsen, and M. Bisgaard. 1999. Taxonomic relationships of the [Pasteurella] haemolytica complex as evaluated by DNA-DNA hybridizations and 16S rRNA sequencing with proposal of Mannheimia haemolytica gen. nov., comb. nov., Mannheimia granulomatis comb. nov., Mannheimia glucosida sp. nov., Mannheimia ruminalis sp. nov., and Mannheimia varigena sp. nov. Int. J. Syst. Bacteriol. 49:67-86. [DOI] [PubMed] [Google Scholar]
- 3.Angen, Ø., P. Ahrens, P. Kuhnert, H. Christensen, and R. Mutters. 2003. Proposal of Histophilus somni gen. nov., sp. nov. for the three species incertae sedis“Haemophilus somnus,” “Haemophilus agni” and “Histophilus ovis.” Int. J. Syst. Evol. Microbiol. 53:1449-1456. [DOI] [PubMed] [Google Scholar]
- 4.Bisgaard, M., S. B. Houghton, R. Mutters, and A. Stenzel. 1991. Reclassification of German, British and Dutch isolates of so-called Pasteurella multocida obtained from pneumonic calf lungs. Vet. Microbiol. 26:115-126. [DOI] [PubMed] [Google Scholar]
- 5.Blackall, P. J., N. Fegan, G. T. I. Chew, and D. J. Hampson. 1998. Population structure and diversity of avian isolates of Pasteurella multocida from Australia. Microbiology 144:279-289. [DOI] [PubMed] [Google Scholar]
- 6.Burdge, D. R., D. Scheifele, and D. P. Speert. 1985. Serious Pasteurella multocida infections from lion and tiger bites. JAMA 253:3296-3297. [PubMed] [Google Scholar]
- 7.Busse, H.-J., S. Bunka, A. Hensel, and W. Lubitz. 1997. Discrimination of members of the family Pasteurellaceae based on polyamine patterns. Int. J. Syst. Bacteriol. 47:698-708. [Google Scholar]
- 8.Capitini, C. M., I. A. Herrero, R. Patel, and M. B. Ishitani. 2002. Wound infection with Neisseria weaveri and a novel subspecies of Pasteurella multocida in a child who sustained a tiger bite. Clin. Infect. Dis. 34:E74-E76. [DOI] [PubMed] [Google Scholar]
- 9.Christensen, H., and M. Bisgaard. 2003. The genus Pasteurella. In M. Dworkin (ed.), The prokaryotes, release 3.14. Springer, New York, N.Y. http://141.150.157.117:8080/prokWIP/index.htm.
- 10.Christensen, H., and M. Bisgaard. 2004. Revised definition of Actinobacillus sensu stricto isolated from animals. A review with special emphasis on diagnosis. Vet. Microbiol. 99:13-30. [DOI] [PubMed] [Google Scholar]
- 11.Christensen, H., Ø. Angen, J. E. Olsen, and M. Bisgaard. 2000. DNA-DNA hybridization determined in micro-wells utilizing covalent attachment of DNA. Int. J. Syst. Evol. Microbiol. 50:1095-1102. [DOI] [PubMed] [Google Scholar]
- 12.Christensen, H., F. Dziva, J. E. Olsen, and M. Bisgaard. 2002. Genotypical heterogeneity of Pasteurella gallinarum as shown by ribotyping and 16S rRNA sequencing. Avian Pathol. 31:603-610. [DOI] [PubMed] [Google Scholar]
- 13.Christensen, H., Ø. Angen, J. E. Olsen, and M. Bisgaard. 2004. Revised description and classification of atypical isolates of Pasteurella multocida from bovine lungs based on genotypical characterization to include variants previously classified as biovar 2 of P. canis and P. avium. Microbiology 150:1757-1767. [DOI] [PubMed] [Google Scholar]
- 14.Christensen, H., P. Kuhnert, J. E. Olsen, and M. Bisgaard. 2004. Comparative phylogeny between the housekeeping genes atpD, infB, rpoB and 16S rDNA within Pasteurellaceae. Int. J. Syst. Evol. Microbiol. 54:1601-1609. [DOI] [PubMed] [Google Scholar]
- 15.Christensen, J. P., and M. Bisgaard. 1997. Avian pasteurellosis: taxonomy of the organisms involved and aspects of pathogenesis. Avian Pathol. 26:461-483. [DOI] [PubMed] [Google Scholar]
- 16.Christensen, J. P., J. E. Olsen, and M. Bisgaard. 1993. Ribotypes of Salmonella enterica serovar Gallinarum biovars gallinarum and pullorum. Avian Pathol. 22:725-738. [DOI] [PubMed] [Google Scholar]
- 17.Davies, R. L., R. MacCorquodale, S. Baillie, and B. Caffrey. 2003. Characterization and comparison of Pasteurella multocida strains associated with porcine pneumonia and atrophic rhinitis. J. Med. Microbiol. 52:59-67. [DOI] [PubMed] [Google Scholar]
- 18.Davies, R. L., P. J. Watson, and B. Caffrey. 2003. Comparative analyses of Pasteurella multocida strains associated with the ovine respiratory and vaginal tracts. Vet. Rec. 152:7-10. [DOI] [PubMed] [Google Scholar]
- 19.Davies, R. L., R. MacCorquodale, and B. Caffrey. 2003. Diversity of avian Pasteurella multocida strains based on capsular PCR typing and variation of the OmpA and OmpH outer membranes proteins. Vet. Microbiol. 91:169-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davies, R. L., R. MacCorquodale, and S. Reilly. 2004. Characterisation of bovine strains of Pasteurella multocida and comparison with isolates of avian, ovine and porcine origin. Vet. Microbiol. 99:145-158. [DOI] [PubMed] [Google Scholar]
- 21.Dewhirst, F. E., B. J. Paster, I. Olsen, and G. J. Fraser. 1993. Phylogeny of the Pasteurellaceae as determined by comparison of 16S ribosomal ribonucleic acid sequences. Zentbl. Bakteriol. Parasitenkd. Infektkrankh. Hyg. Abt. 1 Orig. 279:35-44. [DOI] [PubMed] [Google Scholar]
- 22.Eckert, F., A. Stenzel, R. Mutters, W. Frederiksen, and W. Mannheim. 1991. Some unusual members of the family Pasteurellaceae isolated from human sources—phenotypic features and genomic relationships. Zentbl. Bakteriol. Parasitenkd. Infektkrankh. Hyg. Abt. 1 Orig. 275:143-155. [DOI] [PubMed] [Google Scholar]
- 23.Fegan, N., P. J. Blackall, and J. L. Pahoff. 1995. Phenotypic characterization of Pasteurella multocida isolates from Australian poultry. Vet. Microbiol. 47:281-286. [DOI] [PubMed] [Google Scholar]
- 24.Felsenstein, J. 1995. PHYLIP (Phylogeny Inference Package), version 3.5c. Department of Genetics, University of Washington, Seattle.
- 25.Frederiksen, W. 1989. Pasteurellosis of man, p. 303-320. In C. Adlam and J. M. Ruter (ed.), Pasteurella and pasteurellosis. Academic Press, London, United Kingdom.
- 26.Frederiksen, W. 1993. Ecology and significance of Pasteurellaceae in man—an update. Zentbl. Bakteriol. Parasitenkd. Infektkrankh. Hyg. Abt. 1 Orig. 279:27-34. [DOI] [PubMed] [Google Scholar]
- 27.Heddleston, B. S. 1976. Physiologic characteristics of 1,268 cultures of Pasteurella multocida. Am. J. Vet. Res. 37:745-747. [PubMed] [Google Scholar]
- 28.Hubbert, W. T., N. Merton, and M. A. Rosen. 1970. Pasteurella multocida infection in man unrelated to animal bite. Am. J. Public Health 60:1109-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Isotalo, P. A., D. Edgar, and B. Toye. 2000. Polymicrobial tenosynovitis with Pasteurella multocida and other gram negative bacilli after a Siberian tiger bite. J. Clin. Pathol. 53:871-872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kainz, A., W. Lubitz, and H. J. Busse. 2000. Genomic fingerprints, ARDRA profiles and quinone systems for classification of Pasteurella sensu stricto. Syst. Appl. Microbiol. 23:494-503. [DOI] [PubMed] [Google Scholar]
- 31.Korczak, B., H. Christensen, S. Emler, J. Frey, and P. Kuhnert. 2004. Phylogeny of the family Pasteurellaceae based on rpoB sequences. Int. J. Syst. Evol. Microbiol. 54:1393-1399. [DOI] [PubMed] [Google Scholar]
- 32.Krause, T., H. U. Bertschinger, L. Corboz, and R. Mutters. 1987. V-factor dependent strains of Pasteurella multocida subsp. multocida. Zentbl. Bakteriol. Mikrobiol. Hyg. Reihe A 266:255-260. [DOI] [PubMed] [Google Scholar]
- 33.Kuhnert, P., P. Boerlin, S. Emler, M. Krawinkler, and J. Frey. 2000. Phylogenetic analysis of Pasteurella multocida subspecies and molecular identification of feline P. multocida subsp. septica by 16S rRNA gene sequencing. Int. J. Med. Microbiol. 290:599-604. [DOI] [PubMed] [Google Scholar]
- 34.Liu, W., R. F. Chemaly, M. J. Tuohy, M. M. LaSalvia, and G. W. Procop. 2003. Pasteurella multocida urinary tract infection with molecular evidence of zoonotic transmission. Clin. Infect. Dis. 36:E58-E60. [DOI] [PubMed] [Google Scholar]
- 35.Miflin, J. K., and P. J. Blackall. 2001. Development of a 23S rRNA based PCR assay for the identification of Pasteurella multocida. Lett. Appl. Microbiol. 33:216-221. [DOI] [PubMed] [Google Scholar]
- 36.Mutters, R., K. Piechulla, K.-H. Hinz, and W. Mannheim. 1985. Pasteurella avium (Hinz and Kunjara 1977) comb. nov. and Pasteurella volantium sp. nov. Int. J. Syst. Bacteriol. 35:5-9. [Google Scholar]
- 37.Mutters, R., P. Ihm, S. Pohl, W. Frederiksen, and W. Mannheim. 1985. Reclassification of the genus Pasteurella Trevisan 1887 on the basis of DNA homology with proposals for the new species Pasteurella dagmatis, Pasteurella canis, Pasteurella stomatis, Pasteurella anatis, and Pasteurella langaa. Int. J. Syst. Bacteriol. 35:309-322. [Google Scholar]
- 38.Mutters, R., W. Mannheim, and M. Bisgaard. 1989. Taxonomy of the group, p. 3-34. In C. Adlam and J. M. Rutter (ed.), Pasteurella and pasteurellosis. Academic Press, London, United Kingdom.
- 39.Mutters, R., H. Christensen, and M. Bisgaard. Genus Pasteurella Trevisan 1887, 94,AL Nom. cons. Opin. 13, Jud. Comm. 1954. In G. M. Garrity (ed.), Bergey's manual of systematic bacteriology, 2nd ed., vol. 2, in press. Springer, New York, N.Y.
- 40.Nørskov-Lauritsen, N., H. Christensen, H. Okkels, M. Kilian, and B. Bruun. 2004. Delineation of the genus Actinobacillus by comparison of partial infB sequences. Int. J. Syst. Evol. Microbiol. 54:635-644. [DOI] [PubMed] [Google Scholar]
- 41.Olsen, G. J., H. Matsuda, R. Hagstrom, and R. Overbeek. 1994. FastDNAml: a tool for construction of phylogenetic trees of DNA sequences using maximum likelihood. Comput. Appl. Biosci. 10:41-48. [DOI] [PubMed] [Google Scholar]
- 42.Petersen, K. D., J. P. Christensen, and M. Bisgaard. 1998. Phenotypic and genotypic diversity of organisms previously classified as maltose positive Pasteurella multocida. Zentbl. Bakteriol. Parasitenkd. Infektkrankh. Hyg. Abt. 1 Orig. 288:1-12. [DOI] [PubMed] [Google Scholar]
- 43.Petersen, K. D., H. Christensen, M. Bisgaard, and J. E. Olsen. 2001. Genetic diversity of Pasteurella multocida isolated from fowl cholera as demonstrated by ribotyping, 16S rRNA and partial atpD sequence comparisons. Microbiology 147:2739-2748. [DOI] [PubMed] [Google Scholar]
- 44.Pohl, S. 1981. DNA relatedness among members of Haemophilus, Pasteurella and Actinobacillus, p. 245-253 In M. Kilian, W. Frederiksen, and E. L. Biberstein (ed.), Haemophilus, Pasteurella and Actinobacillus. Academic Press, London, United Kingdom.
- 45.Schmid, H., M. Hartung, and E. Hellmann. 1991. Crossed immunoelectrophoresis applied to representative strains from 11 different Pasteurella species under taxonomic aspects. Zentbl. Bakteriol. Parasitenkd. Infektkrankh. Hyg. Abt. 1 Orig. 275:16-27. [DOI] [PubMed] [Google Scholar]
- 46.Stackebrandt, E., W. Frederiksen, G. M. Garrity, P. A. Grimont, P. Kampfer, M. C. Maiden, X. Nesme, R. Rossello-Mora, J. Swings, H. G. Truper, L. Vauterin, A. C. Ward, and W. B. Whitman. 2002. Report of the ad hoc committee for the re-evaluation of the species definition in bacteriology. Int. J. Syst. Evol. Microbiol. 52:1043-1047. [DOI] [PubMed] [Google Scholar]
- 47.Stenzel, A. 1992. Untersuchungen zur Taxononie atypischen Pasteurella und Pasteurella-ähnlichen Bakterien-Stamme: DNA:DNA hybridisierung und phenotüpische Characterisierung. Inaugural-dissertation der Philipps-Universität, Marburg, Germany.
- 48.Talan, D. A., D. M. Citron, F. M. Abrahamian, G. J. Moran, E. J. Goldstein, et al. 1999. Bacteriologic analysis of infected dog and cat bites. N. Engl. J. Med. 340:85-92. [DOI] [PubMed] [Google Scholar]
- 49.Townsend, K. M., J. D. Boyce, J. Y. Chung, A. J. Frost, and B. Adler. 2001. Genetic organization of Pasteurella multocida cap loci and development of a multiplex capsular PCR typing system. J. Clin. Microbiol. 39:924-929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Woolfrey, B. F., C. O. Quall, and R. T. Lally. 1985. Pasteurella multocida in an infected tiger bite. Arch. Pathol. Lab. Med. 109:744-746. [PubMed] [Google Scholar]