Abstract
Purpose
The aim of this study was to evaluate a new predisposition factor, M2/ANXA5 (RPRGL3), in recurrent pregnancy loss (RPL) patients of Malay origin, since it was previously known that the prevalence of this condition is relatively high among the Malay population of Malaysia, where conventional hereditary thrombophilia factors have been generally ruled out.
Methods
A total of 232 women who had experienced ≥2 unexplained RPL and 141 available male partners were recruited, with 360 healthy Malay and 166 parous female controls. Prevalence of M2 carriage and RPL odds ratios were calculated in (a) control and patient groups; (b) clinically defined subgroups in categories of pregnancy loss, primary, secondary, and tertiary; and (c) timing of pregnancy loss in early, ≤15th gestation week and “late” fetal losses, and >15th gestation week subgroups.
Results
Both male and female subjects had similar M2/ANXA5 allele frequencies. The carrier rate of M2/ANXA5 for the general Malay population was 42.2 and 34.9% for parous controls. These carrier rates compared to Malay RPL subjects (52% M2 carriers) resulted in elevated odds ratios (95% confidence interval) of 1.53 (1.1 to 2.1) and 1.97 (1.3 to 3.1) accordingly for early fetal losses. Moreover, exceeding copy numbers of M2/ANXA5 alleles seemed to afflict a greater chance of RPL in couples, especially when both partners were M2 carriers.
Conclusion
This study confirmed the proposed role of M2/ANXA5 as embryonic, genetically associated thrombophilia predisposition factor for early RPL among ethnic Malay of Malaysia.
Electronic supplementary material
The online version of this article (doi:10.1007/s10815-017-0871-0) contains supplementary material, which is available to authorized users.
Keywords: Annexin A5, M2/ANXA5, Recurrent pregnancy loss (RPL), Miscarriage
Introduction
“Recurrent pregnancy loss” or “repeated pregnancy loss” (RPL), also named “recurrent spontaneous abortions” (RSA), has an adverse psychosocial impact in women and their families, resulting in depression or anxiety for the next pregnancy [1]. RPL is a complex and multifactorial obstetric problem with polygenic background and it involves a variety of genetic, physiological, and environment factors [2]. About 30–40% of RPL cases remain unexplained, categorized as “idiopathic.”
In the last decade, evidence has linked hereditary thrombophilia to the etiology of RPL by a mechanism of impeded placental perfusion, ultimately resulting in adverse pregnancy outcome [3–7]. Although factor V Leiden (FVL) and prothrombin G20210A (PTm) variants are accounted for 70% of inherited thrombophilia patients [7], both of these factors are rare in Asian population [8, 9].
Malaysia, located in South East Asia, has a multi-ethnic society. Due to different cultural and socioeconomic backgrounds, the Malay ethnic group (55% of the population) has a higher prevalence of spontaneous abortion and RPL compared to other ethnics [10]. A low incidence of FVL and PTm (1 and 0.3%) in Malay RPL women has been previously reported [11, 12]. There are no official data regarding RPL incidence among Malay to date. Based on the statistics available from the Malaysian Population and Family Survey (MPSF), 14% of early pregnancy losses are recorded from total pregnancies [13]. However, this reported abortion rate could be an underestimate. Notably, a high proportion of pregnancy losses at the Sultan Abdul Halim Hospital from 2013 to 2015, 2296/2575 (89.2%), occurred in Malay women. The spontaneous abortion rate in Malay women at this hospital was 20.3% from a total of 11,306 admissions, although no records were available on RPL. Higher spontaneous abortion rates were recorded among Malay with advancing age, growing number, and shorter intervals of subsequent pregnancies [10]. A conservative average estimate would be between 20 and 25% considering maternal age group 30–40 with multiple consecutive pregnancies. Assuming an equal to the worldwide RPL proportion of 20 to 30%, these would result in a deduced recurrent miscarriage rate of 5 to 8% judging from correlative distribution.
In 2007, a new hereditary predisposition factor associated with thrombophilia-related RPL, termed M2, was reported, a haplotype in the proximal core promoter region of the annexin A5 (ANXA5) gene, defined as a constellation of four single nucleotide substitutions (SNPs), rs112782763, rs28717001, rs28651243, and rs113588187, respectively [14]. This genetic variant has been acknowledged as a third gene for RPL predisposition, RPRGL3, OMIM entry 614391.
Annexin A5 is a placental anticoagulant protein, which binds to the apical surface of placental syncytiotrophoblasts as a putative protective shield [15]. Available evidence demonstrated that M2 haplotype significantly lowers the expression of ANXA5 in a reporter gene assay [14, 16]. The reduction of M2/ANXA5 mRNA [17, 18] and its protein levels were further confirmed in thrombophilia-associated placental complication cases [19].
The M2 haplotype has been reported in the German and Bulgarian populations with odds ratios for M2 carriers ranging between 1.3 and 2, as compared to random population controls. The estimated odds ratios in M2 carriers were somewhat elevated, 1.8 to 3.0, when compared to healthy controls with at least one live birth and with negative history of pregnancy losses in German [5, 14], Italian [4], Bulgarian [5], and Japanese [20] cohorts. However, the criteria for RPL subjects varied slightly between the studies such as the definition of RPL and categories of embryonic development. In addition to available evidence supporting reduced expression of ANXA5 in chorionic placenta of RPL women, who were M2/ANXA5 carriers [18, 19], the male M2/ANXA5 carriers in RPL couples also showed a rather similar risk that would corroborate impeded embryonic anticoagulant function [5, 21–23].
The assessment of thrombophilia-associated obstetric complications should not be a trivial consideration among the Malay ethnic group based solely on the rare incidence of FVL and PTm factors reported previously. Therefore, the aim of the present study was to obtain more information on the prevalence of M2/ANXA5 as RPL predisposition factor, noted in a preceding pilot investigation, and answer the relevance of the haplotype concerning timing of miscarriage and allelic dependence.
Patients and methods
Study populations
The present genetic association study was approved by the Human Ethics Research Committee of the Universiti Sains Malaysia (USMKK/PPP/JEPeM [245.3.(2)]) and from the National Institutes of Health, Ministry of Health, Malaysia (NMRR-11-1044-9519). The study was carried out in accordance with The Code of Ethics of the World Health Organization (Declaration of Helsinki), and the criteria of strengthening the reporting of genetic association studies were observed as far as applicable. The volunteer subjects who agreed to participate have signed an informed consent before collection of peripheral blood samples.
All cases and controls were of Malay origin verified across three generations that did not have intermarriage. Subjects who had experienced RPL (n = 237) were recruited from the Department of Obstetrics and Gynaecology at Hospital Sultan Abdul Halim, Sungai Petani; Hospital Tuanku Jaafar, Seremban; Hospital Tengku Ampuan Afzan, Kuantan; and Hospital Sultanah Bahiyah, Alor Setar, between January 2013 and May 2015. Male partners of 146 of these women agreed to participate in the study. RPL subjects were pre-screened for potential causes of their repeated pregnancy loss as described previously [21, 24]. Uterine anomalies and endocrine dysfunctions (polycystic ovary syndrome according to the Rotterdam criteria [25] and thyroidal dysfunctions, if anamnestic) were not included. Four abortions in cases of fetal chromosomal abnormalities (mostly numerical aberrations) were excluded from this study through morphological high-resolution ultrasonic examination [26]. Inherited thrombophilia (FVL, PTm) and deficiencies in anti-thrombotic factors (protein C, protein S, factor XII, antithrombin III) were ruled out. Stillbirths were not included in this study. After completion of this diagnostic protocol for RPL patients, 232 subjects with unexplained fetal losses remained; of these, 141 had presented with male partners.
RPL was defined as the occurrence of ≥2 pregnancy loss before the 20th gestational week (GW). Primary RPL comprises ≥2 consecutive RPL before the 20th GW, with no history of live birth; secondary RPL have ≥2 consecutive abortions before the 20th GW, after a live birth; and tertiary RPL represent ≥2 nonconsecutive miscarriages that occurred before the 20th GW but are interspersed with live births [27, 28]. RPL was further subdivided into two subgroups based on timing of pregnancy losses: subgroup 1, “early” fetal losses, GW ≤15, and subgroup 2, “late” fetal losses, GW >15 [4].
Random Malay population subjects were recruited at the Universiti Sains Malaysia, Penang campus from January 2011 to May 2013 with appropriate informed consent. The control group consisted of 360 participants, with 188 (52.2%) men and 172 (47.8%) women. All control participants had healthy status according to the medical register of the university’s campus occupational safety and health administration. A history of previous pregnancies and pregnancy losses was not recorded. Another 166 anonymized female control samples with at least one successful pregnancy and no previous gestational pathology (parous controls) were recruited from the Department of Obstetrics and Gynaecology at Hospital Sultan Abdul Halim, Sungai Petani, and Hospital Tuanku Jaafar, Seremban. Clinically relevant features of patients and control groups are summarized in Table 1.
Table 1.
Clinical features of cases and control groups
| RPL (n = 232) | Parous controls (n = 166) | Population controls (n = 360) | |
|---|---|---|---|
| Age, median (range) | 32 (21–45) | 30 (20–47) | 32 (18–55) |
| Gravidity, median (range) | 5 (2–11) | 2 (1–9) | / |
| Parity, median (range) | 7 (0–7) | 2 (1–9) | / |
| No. of fetal losses, median (range) | 5 (2–7) | / | / |
| Weeks of early fetal losses, median (range) | 8 (6–14) | / | / |
| Weeks of late fetal losses, median (range) | 16 (16–20) | / | / |
| GDM | 20 | 6 | / |
| GHT | 5 | 0 | / |
RPL recurrent pregnancy loss, GDM gestational diabetes mellitus, GHT gestational hypertension
Genotyping and statistical analysis
Genotyping of DNA extracted from peripheral blood was performed for the M2 haplotype of the ANXA5 promoter region (RPRGL3) for all patient and control subjects of this study. The 360 Malay controls, 77 RPL patients, and 41 male partners thereof were genotyped by amplicon sequencing as previously described [23]. Subsequently, a direct genotyping protocol was developed that utilized allele-specific PCR (AS-PCR) reactions for the M2 and “normal” haplotypes. This protocol was verified blinded on 100 sequenced samples and the rest of 166 parous controls, 155 RPL women, and 100 available male partners were genotyped with AS-PCR.
AS-PCR for the M2/ANXA5 haplotype was designed as two nested PCR reactions, the first using common primers for the ANXA5 5′ UTR and the second with allele-specific primers sets. The first PCR reactions contained 1× PCR reaction buffer (Biotools, Spain), 1 mM MgCl2 (Biotools, Spain), 5% dimethyl sulfoxide (DMSO), 0.2 μM of each (forward and reverse) common primer, 200 μM dNTPs, approximately 100 ng genomic DNA, and 1 U Taq polymerase (Biotools, Spain). The common primers sequences were as reported previously [14]. Cycling conditions were as follows: initial denaturation at 95°C for 5 min, followed by 25 cycles of amplification, 95°C for 30 s, 65°C for 30 s, and 72°C for 30 s. The final extension was performed at 72°C for 5 min.
Next, two parallel allele-specific reactions (“normal” and M2/ANXA5 haplotype) were carried out by using “normal” primers (F: 5′-TGGCGCGGCCGGCCTGCGGTTGG-3′; R: 5′-GAGATGCAGACGCTGAAGGATC-3′) and M2/ANXA5 primers (F: 5′-TGGCGCGGCCGGCCTGCGGTTGA-3′; R: 5′- GAGATGCAGACGCTGAAGGATCT -3′) in separate PCR mixes. The template was 1 μl of 5× diluted first PCR reaction product. The cycling conditions and PCR mixture were similar to the first PCR reaction, but the next amplification round was performed with 0.5 mM MgCl2, 0.2 μM of each primer, and 1.25 U Taq polymerase. The PCR product thereof with amplicon size of 139 bp was analyzed on 1.5% ethidium bromide-stained agarose gels.
Odds ratios (OR), 95% confidence intervals (CI), and adjusted OR and 95% CI were estimated with a minimal power of 70% in comparisons with population controls and reached 80% when comparing with parous controls by using multiple logistic regression models that controlled for potential confounding variables, such as age and gravidity (SPSS version 22.0, Chicago, USA). Differences in (a) clinical subgroups, (b) pregnancy loss subgroups, (c) number of alleles, and (d) number of carriers were evaluated using the Pearson chi-square test (n > 30) or the two-tailed Fisher’s exact test (n ≤ 30). Deviations from the Hardy-Weinberg equilibrium (HWE) were calculated using a Monte Carlo Markov chain (MCMC) implementation of an exact test, part of the Genepop package (http://genepop.curtin.edu.au/) [29]. Mean and standard deviations of age and number of miscarriages were evaluated with Microsoft Excel 2013 (Microsoft Redmond Campus, Redmond, WA, USA). Statistical significance was defined as p ≤ 0.05.
Results
Genotyping by AS-PCR
The genotyping method developed accurately discriminated the M2 and “normal” haplotypes as a sum of two independent reactions (Supplementary Fig. S1) with 100% reliability as verified blinded on 100 previously sequenced samples. As already documented in numerous studies and lately confirmed in the pilot investigation [23], the M1 haplotype was not a predisposing factor for RPL. Therefore, the screening of M1 was not included in further analysis.
Overall RPL predisposition
The RPL dependence of M2 carrier status was first assessed by comparison to the random Malay population (Table 2). As previously shown [23], the control group of random Malay subjects (n = 360) fulfilled HWE for ANXA5 promoter haplotypes with P = 0.6622. The M2 carrier rate of the random control group was 42.2%. The second control group comprised of parous female controls (n = 166) without gestational pathology had an M2 carrier rate of 34.9% (Table 2) and was not in HWE for M2/ANXA5 (P = 0.036), due to a lack of M2 heterozygotes (respective excess of M2 homozygotes). This control group was specifically used to assess M2 predisposition in RPL patients and not in their male partners, because the post hoc statistical analyses involved the confounders age and gravidity/parity specifically applying to the repeated miscarriages phenotype in women.
Table 2.
Genotype distributions of female and male partners in the RPL cohort, according to clinically relevant pregnancy loss categories
| Index | Population controls | Parous controls | All RPL | Primary and secondary RPL | Tertiary RPL | |||
|---|---|---|---|---|---|---|---|---|
| n (%) | n (%) | Women n (%) |
Men n (%) |
Women n (%) |
Men n (%) |
Women n (%) |
Men n (%) |
|
| Genotypes | n = 360 | n = 166 | n = 232 | n = 141 | n = 179 | n = 107 | n = 53 | n = 34 |
| N/N | 208 (57.8) | 108 (65.1) | 119 (51.3) | 72 (51.1) | 88 (49.2) | 49 (45.8) | 31 (58.5) | 23 (67.6) |
| N/M2 | 134 (37.2) | 46 (27.7) | 105 (45.3) | 65 (46.1) | 83 (46.4) | 55 (51.4) | 22 (41.5) | 10 (29.4) |
| M2/M2 | 18 (5.0) | 12 (7.2) | 8 (3.4) | 4 (2.8) | 8 (4.5) | 3 (2.8) | 0 (0.0) | 1 (2.9) |
| M2 AF | 0.236 | 0.211 | 0.261 | 0.259 | 0.277 | 0.285 | 0.208 | 0.176 |
| M2 carriage | 152 (42.2) | 58 (34.9) | 113 (48.7) | 69 (48.9) | 91 (50.9) | 58 (54.2) | 22 (41.5) | 11 (32.3) |
RPL recurrent pregnancy loss, N “normal” allele including M1 heterozygous and homozygous combinations, AF allele frequency
A total of 182 out of 373 subjects (RPL women and partners thereof, median age of 33, range 21 to 64) experiencing RPL (in ≤20th GW) were M2/ANXA5 carriers (48.8%), which resulted in adjusted odds ratio of 1.32 (95% CI 0.99 to 1.78, p = 0.062) compared to random population controls.
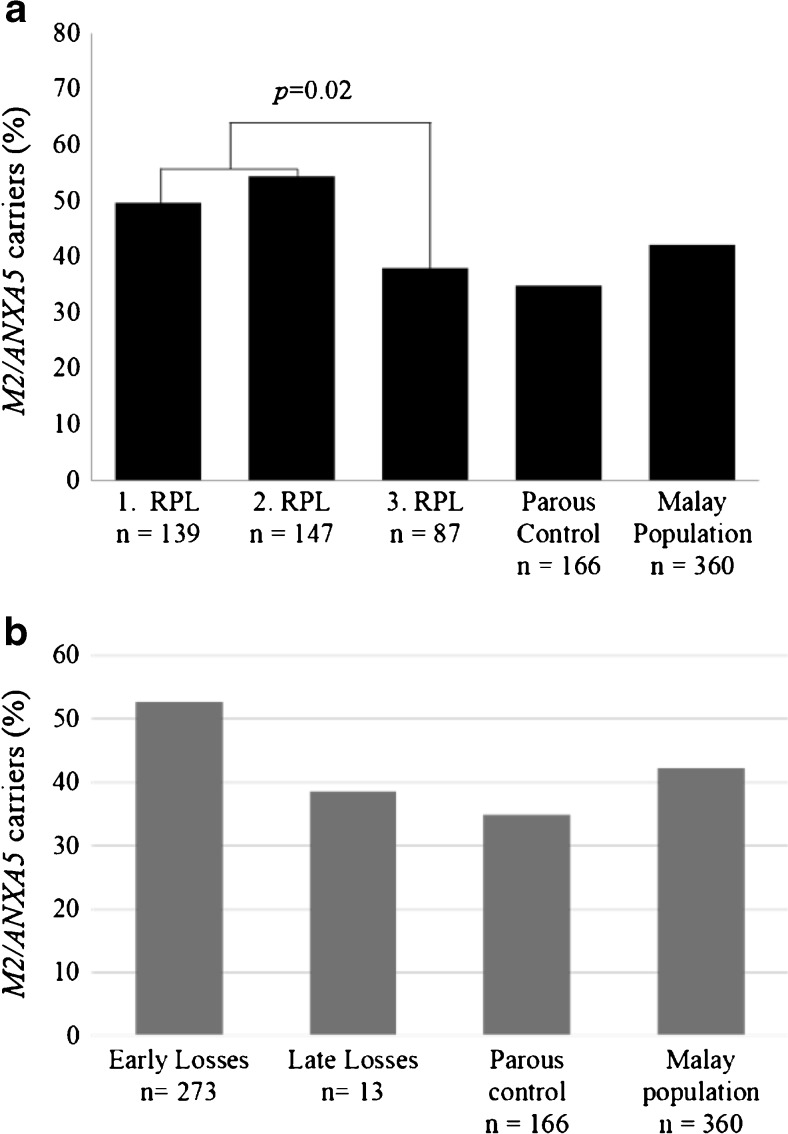
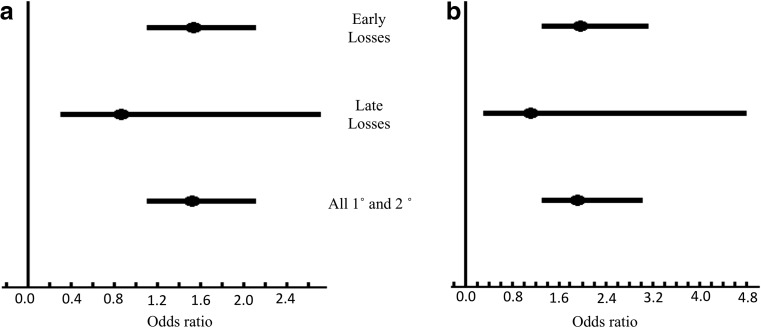
RPL subjects were further divided into three clinically relevant subgroups and carrier rates of the M2 haplotype were reassessed. As shown in Fig. 1a, the primary (1°) RPL, n = 139, and secondary (2°) RPL, n = 147, subgroups had M2 carrier rates of 49.6 and 54.4% accordingly, with an overall combined prevalence of 52.0% (Table 2). The estimated M2 carrier rate for the tertiary (3°) RPL subgroup, n = 87, was 37.9%, somewhat lower than the random population prevalence of 42.2%, and it showed a statistically significant difference when compared to the first two subgroups (p = 0.02). Therefore, the consequence of M2 carriage was reassessed for the 1° and 2° RPL subgroups (n = 286) and further compared to the random Malay population. The comparison yielded an adjusted overall odds ratio of 1.52 (95% CI 1.1 to 2.1, p = 0.01) for M2 carriers of the first two RPL subgroups (Fig. 2a). For subsequent analyses, the focus was on the clinically relevant 1° and 2° RPL subjects, where simple logistic regression was used for further assessment in subgroups.
Fig. 1.
Distribution of M2 carriers (%) in relevant clinical subgroups of Malay patients and male partners who had experienced RPL according to repeated pregnancy loss categories (a). Embryonic categories, gestational weeks 6 to 15 and >15 (b)
Fig. 2.
Forrest plot showing odds ratios for M2 carriers in RPL subgroups compared to random Malay population (a) and to parous controls (b)
RPL predispositions of women and their male partners as carriers of the M2 haplotype
To determine whether the M2/ANXA5 haplotype would be an RPL predisposing factor of importance in both sexes, 1° and 2° RPL subjects were subdivided into female patients and their male partners to further assess the independent contributions to estimated M2 carrier rates. The 1° and 2° RPL subgroups comprised of 179 women and 107 male partners accordingly. When compared to random Malay population, the subgroup of RPL women yielded an odds ratio of 1.42 (95% CI 1.0 to 2.0, p = 0.05) for M2 carriers. The comparison to parous controls without gestational complications resulted in an odds ratio of 1.93 (95% CI 1.25 to 2.97, p < 0.01). When male partners carrying M2 were compared to the population controls, the odds ratio was 1.62 (95% CI 1.1 to 2.5, p = 0.03) that is comparable to the general population predisposition in RPL females. Male partners with 54.2% M2 carrier rate (Table 2) had a carrier status only three percentage points higher (95% CI 0.7 to 1.9, p = 0.74) than RPL women from this study.
RPL predispositions of M2 carriers in patient subgroups according to timing of miscarriage
The miscarriage proportions in the early (n = 273) and late (n = 13) fetal losses subgroups were 95.5 and 4.5% accordingly. Table 3 shows genotype distributions in each subgroup of fetal losses related to the predisposition haplotype M2. M2 carrier rate in RPL subjects was higher in early, compared to late fetal losses, with 52.7 vs. 38.5% (Fig. 1b).
Table 3.
Genotype distributions of female and male partners in the RPL cohort according to fetal losses categories of miscarriage
| Genotype | All 1° and 2° | RPL | Subgroup 1: early fetal losses, ≤15th GW | Subgroup 2: late fetal losses, >15 GW | Population controls | Parous controls | ||
|---|---|---|---|---|---|---|---|---|
| Women n (%) |
Men n (%) |
Women n (%) |
Men n (%) |
Women n (%) |
Men n (%) |
n (%) | n (%) | |
| Genotypes | n = 179 | n = 107 | n = 171 | n = 102 | n = 8 | n = 5 | n = 360 | n = 166 |
| N/N | 88 (49.2) | 49 (45.8) | 83 (48.5) | 46 (45.1) | 5 (62.5) | 3 (0.6) | 208 | 108 (65.1) |
| N/M2 | 83 (46.4) | 55 (51.4) | 80 (46.8) | 53 (51.9) | 3 (37.5) | 2 (0.4) | 134 | 46 (27.7) |
| M2/M2 | 8 (4.5) | 3 (2.8) | 8 (4.7) | 3 (3.0) | 0 (0.0) | 0 (0.0) | 18 | 12 (7.2) |
| M2 AF | 0.277 | 0.285 | 0.281 | 0.289 | 0.188 | 0.200 | 0.236 | 0.211 |
| M2 carriage | 91 (50.9) | 58 (54.2) | 88 (51.5) | 56 (54.9) | 3 (37.5) | 2 (33.3) | 134 (42.2) | 58 (34.9) |
RPL recurrent pregnancy loss, N “normal” allele including M1 heterozygous and homozygous combinations, AF allele frequency, GW gestational week
Next, the relative prevalence of M2 carriers in fetal loss categories was estimated by comparing patients and their male partners (combined) of these subgroups against the population controls. Because of the relatively small number of individuals in the late fetal loss subgroup, the comparison performed used Fisher’s exact test. Obtained odds ratios were 1.53 (95% CI 1.1 to 2.1, p = 0.01) for early fetal losses and 0.86 (95% CI 0.3 to 2.7, p = 1.0) for late fetal losses (Fig. 2a). The comparison of M2-carrying patients of these subgroups to parous controls yielded odds ratios of 1.97 (95% CI 1.3 to 3.1, p < 0.01) and 1.12 (95% CI 0.3 to 4.8, p = 1.0) accordingly (Fig. 2b).
Assessment of RPL predisposition in M2-carrying couples
In order to better assess the M2 carrier predisposition for RPL, the comparisons were drawn to couples’ (n = 141) rather than to individuals’ status. M2/ANXA5 carrier rates among patients were 47.5 and 48.9% for their male partners accordingly (Supplementary Table S1). Statistical comparisons on the relative distribution of M2 carriers and RPL associated attributable population predisposition per couple in the pregnancy loss categories (1°, 2°, and 3°) yielded odds ratios that were similar to those obtained for individuals with appropriately higher significance in individuals, due to the increased sample size. Estimated odds ratios of M2 carriers in early (≤15th GW) and late (>15th GW) couples were 1.53 (95% CI, 1.1 to 2.1, p < 0.01) and 0.86 (95% CI, 0.3 to 2.7, p = 1.0).
M2/ANXA5 allelic and carrier status dependence of RPL predisposition in couples
To elucidate the effect of increasing M2 allele numbers in couples, Pearson’s chi squared tests were used to estimate the difference in relative abundances. M2 alleles number ranged from zero (in cases in which both partners were wild type for ANXA5) to three copies (in cases in which one partner was a M2 homozygote and the other was M2 heterozygous) per couple. There were no homozygous M2 couples detected in this study. The fractions of 1° and 2° RPL couples with one or more copies of the M2 allele in relation to non-carriers were compared in a 2 × 2 table with couples of the tertiary RPL subgroup and among the fetal losses categories (Supplementary Tables S1 and S2). Couples who had ≥2 alleles (32/102 compared to 5/34 of 3° couples) were significantly more frequent among 1° and 2° RPL subjects (p = 0.01), with a greater share in the early fetal losses subgroup (Supplementary Table S2).
In order to estimate the odds of increasing carrier numbers per couple, RPL couples were categorized into non-M2 carrier (both partners are wild type for ANXA5), one M2 carrier (one of the partners is heterozygous or M2 homozygous), and two M2 carriers (in cases that two partners are heterozygous or in cases that one partner is heterozygous and the other is M2 homozygous). Thus, the substantial difference between the M2 copy number and carrier status analyses is that heterozygous and homozygous individuals were both counted as carriers. Relative abundances of two carriers per couple were compared to the occurrences of non-carrier couples among RPL categories. There was higher prevalence of two-carrier M2 couples (26/102 vs. 5/34) among 1° and 2° RPL subjects (p = 0.03), further corroborated with their relative share in the early fetal losses subgroup, ≤15th GW.
Discussion
The M2 haplotype of the ANXA5 gene is significantly associated with RPL. As reported in previous studies, M2 prevalence in population and healthy subject controls of German, Italian, Bulgarian, and UK white European origin are about 15–17% [4, 5, 14]; whereas in Asian populations, the reported carrier rate is 11% with 5.4% M2 allelic frequency for the Japanese [20]. A recent study finding no association of M2/ANXA5 with RPL in 86 Estonian and 227 Danish subjects reported 27.3% prevalence with 15.2% allelic frequency in Estonian and 23.5% prevalence with 12.6% allelic frequency in Danish parous women [30]. Surprisingly, the M2 carrier rate estimated for the Malay population of Malaysia is relative high with 42.2%. Although the Japanese and Malay share a common ancestral haplogroup origin in the Austronesian population [31], they show markedly different M2 prevalences that could be explained with diverse phylogenetics. It is thus tempting to speculate that the relatively high spontaneous abortion and according RPL rates recorded for the Malay [10, 32] are at least in part due to the abundance of the M2 haplotype, as previously suggested in a pilot study [23].
The diagnostic protocol for RPL workup in Malaysia still involves the “classic” genetic thrombophilia screening, i.e., factor V Leiden (FVL) and prothrombin (PTm) variants. However, these two genetic thrombophilia lesions have very low prevalence in Malay RPL women [11, 12]. The second study reported that despite the extensive screening, 38% of their recurrent miscarriage cases remained idiopathic, which could at least partly involve the risk haplotype M2 [12].
The current extended study involving a total of 232 Malay RPL women and 141 male partners thereof corroborated the risk estimates for M2 carriers from the initial pilot study [23]. This study also confirmed the increased odds ratios for RPL patients from the primary and secondary pregnancy loss clinical categories as opposed to the subgroup of tertiary pregnancy losses, where a genetic factor would be of lesser importance, due to the relatively increased sporadic share of miscarriages. The clinical consideration of the first and second subgroups is rather important for the diagnostic workup of ethnic Malay, as it is common for a Malay family to have more than three children.
The parous controls had a M2 carrier rate of 34.9%, comparable to the tertiary RPL couples group with 37.9%, where RPL per definition appears as a condition much more dependent on environmental and physiological than on genetic factors. The observed HWE deviation in M2 distribution for parous controls is analogous to other selected fertile control groups from previous studies [14, 22]. A possible explanation is the positive ascertainment bias resulting from selection of the Malay parous control group comprised only of women with at least one successful pregnancy and without miscarriages or other gestational pathology, whereas spontaneous abortion generally occurs in about 10% of women worldwide [33]. The observed ascertainment bias is thus indirectly indicative for the proposed role of M2 as RPL factor, apparently the consequence of adjustment for phenotype.
The odds ratios of M2-carrying RPL patients from this study, 1.4 with population controls and 1.9 with parous women, are similar to these of European RPL cohorts, 1.5 to 2.5 with population controls and 3 to 5 with parous controls [5, 21, 22], as well as to the reported odds ratio of 2.4 with parous women from the Japanese study [20]. Attributable predisposition of male M2 carriers in Malay RPL couples is thus in general agreement with these previous studies, supporting the proposed pathophysiological expression of M2/ANXA5 in impediment of embryonic anticoagulation.
Therefore, stratifying RPL according to timing of miscarriage (categories of fetal loss) was necessary, in order to specifically determine the role of annexin A5 in pregnancy-relevant pathology. Because of the relatively high conception rate in Malay women, and the systemic influence of M2/ANXA5 as hereditary factor, the last recorded pregnancy loss was considered as reference index loss. The highest M2 carrier rates were observed in the early fetal losses subgroup, less than 15 gestational weeks, in primary and secondary RPL patients. This is in agreement with the proposed role of M2/ANXA5 as “early” RPL factor [4, 5], in contrast to the “classic” thrombophilia factors FVL and PTm, of greater importance for late miscarriages, >20th week [2, 34]. It should also be noted that for this study, uncertainties in the exact timing of conception should allow for ±1 to 2 weeks difference, when recording the timing of pregnancy loss similar to previous studies. Furthermore, even if some more patients with fetal aneuploidies undetected by morphological ultrasound in addition to the four that were already excluded, would have participated in this study, the average age of the RPL patients included was below 35 years, which should considerably reduce the chance of fetal chromosomal aberrations.
Raising the number of predisposing alleles in couples should generally increase the chance of their pathologic expression in the progeny. A higher count of M2/ANXA5 alleles would elevate the miscarriage risk of RPL couples accordingly. Without exception, the analysis showed that 2 and 3 M2/ANXA5 alleles are significantly associated with “early” RPL (p = 0.01). Likewise, if both partners are M2/ANXA5 carriers, they would have higher chances to experience RPL (p = 0.03), specifically enriched in the subgroup of “early” fetal losses between the 6th and 15th gestational weeks, similar to the UK cohort study [22].
In conclusion, M2/ANXA5 seems to be a predisposing factor for primary and secondary RPL in Malays with unusually high abundance in the general population, even more so on the background of insignificant occurrences of “classic”’ inherited thrombophilia reported [11, 12]. Male and female M2 carriers are subject to similar RPL predisposition with the highest exposure for early pregnancies, less than 15 weeks of gestation. Considering the relatively high pregnancy loss rates of Malay women and the estimated high general prevalence of the M2 haplotype, couples who have experienced primary and secondary RPL might be screened for M2 carrier status as a biomarker of possible successful anticoagulant treatment, as suggested by recent genetic evidence from the EThIGII and the CARE Fertility Group clinical trials [35, 36]. Related to this, Malay RPL couples appear as a very suitable population model for a properly powered therapeutic randomized clinical trial.
Electronic supplementary material
Below is the link to the electronic supplementary material.
(DOCX 54 kb)
(DOCX 55 kb)
AS-PCR- lane 1: negative control (1st PCR); lanes 2 and 3: negative control (2nd PCR) for ‘normal’ and M2/ANXA5 specific primers; lanes 4: M2 heterozygous DNA template; lanes 5: DNA sample of a ‘normal’ genotype; lanes 6: M2 homozygous DNA template. Double lanes 4, 5 and 6: 1st track is specific for the ‘normal’ allele, 2nd track discriminates the M2 haplotype. (GIF 91 kb)
Acknowledgements
The authors thank the women and their partners who participated in this study.
Compliance with ethical standards
The present genetic association study was approved by the Human Ethics Research Committee of the Universiti Sains Malaysia (USMKK/PPP/JEPeM [245.3.(2)]) and from the National Institutes of Health, Ministry of Health, Malaysia (NMRR-11-1044-9519). The study was carried out in accordance with The Code of Ethics of the World Health Organization (Declaration of Helsinki), and the criteria of strengthening the reporting of genetic association studies were observed as far as applicable. The volunteer subjects who agreed to participate have signed an informed consent before collection of peripheral blood samples. Random Malay population subjects were recruited at the Universiti Sains Malaysia, Penang campus from January 2011 to May 2013 with appropriate informed consent.
Funding
This work was supported by Universiti Sains Malaysia Research Universiti Grant (USM RU grant no: 1001/CIPPT/812100) awarded to TTH. AM was funded by a PI grant of the German Research Community, DFG, MA-6288/1-1. AKC was supported by MyBrain15 Program (KPM (b) 850304015158) under the Malaysian Ministry of Education.
Conflict of Interest
The authors declare that they have no conflict of interest.
Contributor Information
Arseni Markoff, Phone: ++492518355403, Email: markoff@uni-muenster.de.
Thean-Hock Tang, Phone: ++6045622302, Email: tangth@usm.my.
References
- 1.Sutan R, Miskam HM. Psychosocial impact of perinatal loss among Muslim women. BMC Womens Health. 2012;12:1–9. doi: 10.1186/1472-6874-12-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kutteh WH, Park VM, Deitcher SR. Hypercoagulable state mutation analysis in white patients with early first-trimester recurrent pregnancy loss. Fertil Steril. 1999;71:1048–53. doi: 10.1016/S0015-0282(99)00133-8. [DOI] [PubMed] [Google Scholar]
- 3.Grandone E, Margaglione M. Inherited thrombophilia and gestational vascular complications. Best Pract Res Clin Haematol. 2003;16:321–32. doi: 10.1016/S1521-6926(03)00017-3. [DOI] [PubMed] [Google Scholar]
- 4.Tiscia G, Colaizzo D, Chinni E, Pisanelli D, Sciannamè N, Favuzzi G, et al. Haplotype M2 in the annexin A5 (ANXA5) gene and the occurrence of obstetric complications. Thromb Haemost. 2009;102:309–13. doi: 10.1160/TH09-02-0123. [DOI] [PubMed] [Google Scholar]
- 5.Tüttelmann F, Ivanov P, Dietzel C, Sofroniou A, Tsvyatkovska TM, Komsa-Penkova RS, et al. Further insights into the role of the annexin A5 M2 haplotype as recurrent pregnancy loss factor, assessing timing of miscarriage and partner risk. Fertil Steril. 2013;100:1321–5. doi: 10.1016/j.fertnstert.2013.06.046. [DOI] [PubMed] [Google Scholar]
- 6.Younis JS, Samueloff A. Gestational vascular complications. Best Pract Res Clin Haematol. 2003;16:135–51. doi: 10.1016/S1521-6926(02)00099-3. [DOI] [PubMed] [Google Scholar]
- 7.Bogdanova N, Markoff A. Hereditary thrombophilic risk factors for recurrent pregnancy loss. J Community Genet. 2010;1:47–53. doi: 10.1007/s12687-010-0011-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosendaal FR, Doggen CJ, Zivelin A, Arruda VR, Aiach M, Siscovick DS, et al. Geographic distribution of the 20210 G to A prothrombin variant. Thromb Haemost. 1998;79:706–8. [PubMed] [Google Scholar]
- 9.De Stefano V, Chiusolo P, Paciaroni K, Leone G. Epidemiology of factor V Leiden: clinical implications. Semin Thromb Hemost. 1998;24:367–79. doi: 10.1055/s-2007-996025. [DOI] [PubMed] [Google Scholar]
- 10.Arshat H, Tan Boon A, Tey Nai P. The effects of life cycle and family formation variables on pregnancy outcome. Malaysian journal of reproductive health: a publication of the Reproductive Research Centre of the National Population and Family Development Board, Malaysia. 1985;3:115–25 [PubMed]
- 11.Ayadurai T, Muniandy S, Omar SZ. Thrombophilia investigation in Malaysian women with recurrent pregnancy loss. J Obstet Gynaecol Res. 2009;35:1061–8. doi: 10.1111/j.1447-0756.2009.01067.x. [DOI] [PubMed] [Google Scholar]
- 12.Yusoff NM, Abdullah WZ, Ghazali S, Othman MS, Baba AA, Abdullah N, et al. The absence of factor V Leiden mutation in Malays with recurrent spontaneous abortions. Aust N Z J Obstet Gynaecol. 2002;42:164–6. doi: 10.1111/j.0004-8666.2002.00164.x. [DOI] [PubMed] [Google Scholar]
- 13.Tey NP, Ng ST, Yew SY. Proximate determinants of fertility in Peninsular Malaysia. Asia Pac J Public Health. 2012;24:495–505. doi: 10.1177/1010539511401374. [DOI] [PubMed] [Google Scholar]
- 14.Bogdanova N, Horst J, Chlystun M, Croucher PJ, Nebel A, Bohring A, et al. A common haplotype of the annexin A5 (ANXA5) gene promoter is associated with recurrent pregnancy loss. Hum Mol Genet. 2007;16:573–8. doi: 10.1093/hmg/ddm017. [DOI] [PubMed] [Google Scholar]
- 15.Rand JH, Wu X-X, Quinn AS, Chen PP, McCrae KR, Bovill EG, et al. Human monoclonal antiphospholipid antibodies disrupt the annexin A5 anticoagulant crystal shield on phospholipid bilayers: evidence from atomic force microscopy and functional assay. Am J Pathol. 2003;163:1193–200. doi: 10.1016/S0002-9440(10)63479-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tiscia GL, Dørum E, Myklebust CF, Grandone E, Sandset PM, Skretting G. Functional characterization of annexin A5 gene promoter allelic variants. Thromb Res. 2016;144:93–9. doi: 10.1016/j.thromres.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 17.Chinni E, Tiscia GL, Colaizzo D, Vergura P, Margaglione M, Grandone E. Annexin V expression in human placenta is influenced by the carriership of the common haplotype M2. Fertil Steril. 2009;91:940–2. doi: 10.1016/j.fertnstert.2007.12.056. [DOI] [PubMed] [Google Scholar]
- 18.Markoff A, Gerdes S, Feldner S, Bogdanova N, Gerke V, Grandone E. Reduced allele specific annexin A5 mRNA levels in placentas carrying the M2/ANXA5 allele. Placenta. 2010;31:937–40. doi: 10.1016/j.placenta.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 19.Ota S, Miyamura H, Nishizawa H, Inagaki H, Inagaki A, Inuzuka H, et al. Contribution of fetal ANXA5 gene promoter polymorphisms to the onset of pre-eclampsia. Placenta. 2013;34:1202–10. doi: 10.1016/j.placenta.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 20.Miyamura H, Nishizawa H, Ota S, Suzuki M, Inagaki A, Egusa H, et al. Polymorphisms in the annexin A5 gene promoter in Japanese women with recurrent pregnancy loss. Mol Hum Reprod. 2011;17:447–52. doi: 10.1093/molehr/gar008. [DOI] [PubMed] [Google Scholar]
- 21.Rogenhofer N, Engels L, Bogdanova N, Tüttelmann F, Markoff A, Thaler C. Paternal and maternal carriage of the annexin A5 M2 haplotype are equal risk factors for recurrent pregnancy loss: a pilot study. Fertil Steril. 2012;98:383–8. doi: 10.1016/j.fertnstert.2012.04.026. [DOI] [PubMed] [Google Scholar]
- 22.Demetriou C, Abu-Amero S, White S, Peskett E, Markoff A, Stanier P, et al. Investigation of the annexin A5 M2 haplotype in 500 white European couples who have experienced recurrent spontaneous abortion. Reprod BioMed Online. 2015;31:681–8. doi: 10.1016/j.rbmo.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 23.Thean Hock T, Bogdanova N, Kai Cheen A, Kathirgamanathan S, Bin Abdullah R, Mohd Yusoff N, et al. M2/ANXA5 haplotype as a predisposition factor in Malay women and couples experiencing recurrent spontaneous abortion: a pilot study. Reprod BioMed Online. 2015;30:434–9. doi: 10.1016/j.rbmo.2014.12.014. [DOI] [PubMed] [Google Scholar]
- 24.Li TC, Makris M, Tomsu M, Tuckerman E, Laird S. Recurrent miscarriage: aetiology, management and prognosis. Hum Reprod Update. 2002;8:463–81. doi: 10.1093/humupd/8.5.463. [DOI] [PubMed] [Google Scholar]
- 25.The Rotterdam Eshre/Asrm-Sponsored PCOS Consensus Workshop Group Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19–25. doi: 10.1016/j.fertnstert.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 26.Kagan KO, Sonek J, Berg X, Berg C, Mallmann M, Abele H, et al. Facial markers in second- and third-trimester fetuses with trisomy 18 or 13, triploidy or Turner syndrome. Ultrasound Obstet Gynecol. 2015;46:60–5. doi: 10.1002/uog.14655. [DOI] [PubMed] [Google Scholar]
- 27.Carp H, editor. Recurrent pregnancy loss: causes, controversies and treatment. London: CRC Press; 2007. [Google Scholar]
- 28.Practice Committee of the American Society for Reproductive Medicine Definitions of infertility and recurrent pregnancy loss. Fertil Steril. 2008;89:1603. doi: 10.1016/j.fertnstert.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 29.Rousset F. genepop’007: a complete re-implementation of the genepop software for Windows and Linux. Mol Ecol Resour. 2008;8:103–6. doi: 10.1111/j.1471-8286.2007.01931.x. [DOI] [PubMed] [Google Scholar]
- 30.Nagirnaja L, Nõmmemees D, Rull K, Christiansen OB, Nielsen HS, Laan M. Annexin A5 promoter haplotype M2 is not a risk factor for recurrent pregnancy loss in Northern Europe. PLoS One. 2015;10:e0131606. doi: 10.1371/journal.pone.0131606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soares PA, Trejaut JA, Rito T, Cavadas B, Hill C, Eng KK, et al. Resolving the ancestry of Austronesian-speaking populations. Hum Genet. 2016;135:309–26. doi: 10.1007/s00439-015-1620-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Irwan NM. Prevalence and risk factors of pregnancy loss in Malaysia. 2nd International Conference on Demography and Population Studies, 2015; Athens, Greece.
- 33.Everett C. Incidence and outcome of bleeding before the 20th week of pregnancy: prospective study from general practice. BMJ. 1997;315:32–4. doi: 10.1136/bmj.315.7099.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roqué H, Paidas MJ, Funai EF, Kuczynski E, Lockwood CJ. Maternal thrombophilias are not associated with early pregnancy loss. Thromb Haemost. 2004;91:290–5. doi: 10.1160/TH03-09-0596. [DOI] [PubMed] [Google Scholar]
- 35.Rogenhofer N, Markoff A, Wagner A, Klein HG, Petroff D, Schleussner E, et al. Lessons from the EThIGII trial: proper putative benefit assessment of low-molecular-weight heparin treatment in M2/ANXA5 haplotype carriers. Clinical and Applied Thrombosis/Hemostasis. 2016. [DOI] [PubMed]
- 36.Fishel S, Baker D, Elson J, Ragunath M, Atkinson G, Shaker A, et al. Precision medicine in assisted conception: a multicenter observational treatment cohort study of the annexin A5 M2 haplotype as a biomarker for antithrombotic treatment to improve pregnancy outcome. EBioMedicine. 2016;10:298–304. doi: 10.1016/j.ebiom.2016.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 54 kb)
(DOCX 55 kb)
AS-PCR- lane 1: negative control (1st PCR); lanes 2 and 3: negative control (2nd PCR) for ‘normal’ and M2/ANXA5 specific primers; lanes 4: M2 heterozygous DNA template; lanes 5: DNA sample of a ‘normal’ genotype; lanes 6: M2 homozygous DNA template. Double lanes 4, 5 and 6: 1st track is specific for the ‘normal’ allele, 2nd track discriminates the M2 haplotype. (GIF 91 kb)