Abstract
Historically, most clinical microbiology laboratories report that 80 to 90% of enterococci are Enterococcus faecalis, whereas E. faecium accounts for 5 to 10% of isolates. At our medical center from 1993 to 2002, we evaluated the percentages of E. faecium among all enterococcal isolates and the percentages of E. faecium isolates that were vancomycin resistant. Over this 10-year period, the percentage of enterococci that were identified as E. faecium increased from 12.7 to 22.2% (P < 0.001) and the proportion of E. faecium that was vancomycin resistant increased from 28.9 to 72.4% (P < 0.001). Both the percentage of E. faecium among the enterococci and the proportion of vancomycin-resistant E. faecium increased significantly over this 10-year period.
Enterococci are normal inhabitants of the gastrointestinal tract, but they may be associated with invasive disease (7). Common sites of clinical infection include the urinary tract, the bloodstream, and intra-abdominal or pelvic wounds. Previously, due to the low pathogenicity of enterococci, some investigators advocated not treating this organism, but targeted antimicrobial therapy is now common (3). However, with the emergence of antimicrobial resistance among enterococci, treatment options present ongoing challenges.
Enterococcus faecalis and Enterococcus faecium are the predominant enterococcal species identified in clinical microbiology laboratories. Historically, these laboratories report that 80 to 90% of enterococci are E. faecalis, whereas E. faecium accounts for 5 to 10% of enterococci (2, 6). The enterococci E. durans, E. avium, E. raffinosus, E. gallinarum, and E. casseliflavus are reported much less frequently than E. faecalis and E. faecium (6). More recently, the SENTRY Antimicrobial Resistance Surveillance Program reported that in 2001, E. faecium accounted for 20% of clinical enterococcal infections in the United States (5). This finding is of potential concern, as E. faecium is more commonly associated with vancomycin resistance than are the other enterococci (14).
E. faecium strains expressing high levels of ampicillin resistance (MIC > 128 μg/ml) emerged in medical centers in the United States in the late 1980s (14). Subsequently, vancomycin-resistant enterococci (VRE) were reported in France in 1986 and in the United States in 1989 (6). Since that time, the incidence of VRE has continued to increase, and VRE make up in excess of 25% of enterococci in some intensive care units (11). Although any of the enterococcal species may be associated with ampicillin or glycopeptide resistance, E. faecium more commonly acquires resistance relative to the other enterococcal species (14).
Clinical microbiology laboratories often identify enterococci to the species level for clinical as well as surveillance isolates. Over the past decade, we have observed a significant increase in the percentage of E. faecium isolates, a result which suggests a changing epidemiology of this organism at our medical center. Despite our adherence to standard infection control practices, the percentage of E. faecium isolates identified and the concomitant vancomycin resistance continued to increase. Therefore, we reviewed the changing incidence of enterococci at our hospital.
(This work was presented in part at the 41st Annual Meeting of the Infectious Diseases Society of America, San Diego, Calif., October 2003.)
Data from the clinical microbiology laboratory at Northwestern Memorial Hospital (Chicago, Ill.) were extracted to include all enterococcal isolates found from 1993 to 2002. These data were evaluated by a search of the Sunquest Information Systems (Tucson, Ariz.) database for the following bacterial organisms: E. faecalis, E. faecium, E. avium, E. durans, E. casseliflavus, and E. gallinarum. All enterococci were identified to the species level according to standard methods. Each positive culture result was reviewed to determine whether the isolate was a clinical or surveillance (rectal swab) specimen and was categorized by the year of identification. The data are for one isolate per patient per month (duplicates and surveillance cultures were removed). Vancomycin resistance was determined according to NCCLS standards (10), recorded for each isolate, and categorized by the year of identification. The isolates were categorized and graphed by using Microsoft Excel (Seattle, Wash.). Only clinical isolates were included in the data analysis. The percentage of E. faecium among total enterococci and the rate of VRE over time were analyzed by linear regression analysis. A P value of ≤0.05 was considered statistically significant.
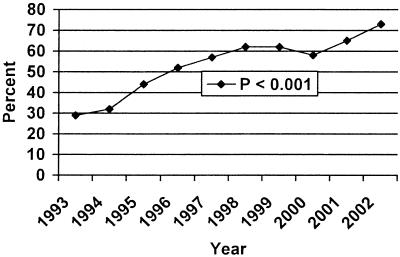
From 1993 to 2002, 18,856 enterococci were identified in our clinical microbiology laboratory. The percentages of enterococci that were identified as E. faecium increased from 12.7% in 1993 to 22.2% in 2002 (P < 0.001) (Fig. 1). The percentages of E. faecium that were vancomycin resistant increased from 28.9% in 1993 to 72.4% in 2002 (P < 0.001). The percentages of E. faecalis that were vancomycin resistant increased from 0.2% in 1993 to 0.5% in 2002, but this increase was not statistically significant. Figure 1 illustrates the percentages of E. faecium found over time (from 1993 to 2002), and Fig. 2 depicts the rates of vancomycin resistance during this 10-year time period.
FIG. 1.
Percentage of E. faecium among clinical enterococcal isolates.
FIG. 2.
Percentage of E. faecium isolates that are vancomycin resistant.
Since the total numbers of E. faecalis remained stable during the 10-year period, the increasing numbers of E. faecium were responsible for the increase in total enterococci. We observed significant increases in the rates of incidence of E. faecium and VRE during the study period. Both of these trends are potentially problematic, as increasing rates of these organisms limit treatment options. While new antimicrobials, such as linezolid, daptomycin, and quinupristin-dalfopristin, have recently been developed to treat serious enterococcal infections, resistance to these agents has already emerged (4, 13, 16).
Addressing this trend for enterococci requires a combined infection control and antimicrobial utilization approach (12). An active surveillance program to detect colonization with VRE has been in place at our medical center for 8 years and is associated with a high rate of compliance. Despite utilization of the Centers for Disease Control Hospital Infection Control Practice Advisory Committee (HICPAC) (1) and Society for Healthcare Epidemiology of America (SHEA) (9) guidelines regarding control of this organism, we continue to observe increasing rates of VRE. Antibiotic prescribing practices also contribute to increasing rates of VRE (8, 15). While there is some controversy regarding which antimicrobial agents are most likely responsible, cephalosporins and vancomycin are consistent risk factors (8, 15).
Our study has several limitations. Since we reviewed only microbiological records to determine the numbers of enterococci, we were not able to determine the role infection control practices or antimicrobial utilization played in this changing epidemiology. We also did not determine whether these organisms represented community pathogens or were health-care associated. Finally, since this was a clinical microbiology laboratory-based project, we were unable to distinguish colonization from infection.
In summary, there has been a significant increase in the numbers of enterococci that are E. faecium and the proportion that are vancomycin resistant over a 10-year period at our medical center. Nearly three-quarters of our E. faecium are vancomycin resistant, and this changing epidemiology has important clinical and infection control implications.
REFERENCES
- 1.Centers for Disease Control and Prevention. 1995. Recommendations for preventing the spread of vancomycin resistance: recommendations of the Hospital Infection Control Practices Advisory Committee (HICPAC). Morb. Mortal. Wkly. Rep. Recomm. Rep. 44(RR-12):1-13. [PubMed] [Google Scholar]
- 2.Facklam, R. R., D. F. Sahm, and L. M. Teixeira. 1999. Enterococcus, p. 297-305. In P. R. Murray, E. J. Baron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken (ed.), Manual of clinical microbiology, 7th ed. American Society for Microbiology, Washington, D.C.
- 3.Hoge, C. W., J. Adam, B. Buchanan, and S. Sears. 1991. Enterococcal bacteremia: to treat or not to treat, a reappraisal. Rev. Infect. Dis. 13:600-605. [DOI] [PubMed] [Google Scholar]
- 4.Gonzales, R. D., P. C. Schreckenberger, M. B. Graham, S. Kelkar, K. Denbesten, and J. P. Quinn. 2001. Infections due to vancomycin-resistant Enterococcus faecium resistant to linezolid. Lancet 357:1179. [DOI] [PubMed] [Google Scholar]
- 5.Low, D. L., N. Keller, A. Barth, and R. Jones. 2001. Clinical prevalence, antimicrobial susceptibility, and geographic resistance patterns of enterococci: results from the SENTRY antimicrobial surveillance program, 1997-1999. Clin. Infect. Dis. 32(Suppl. 2):S133-S145. [DOI] [PubMed] [Google Scholar]
- 6.Moellering, R. C. 2000. Enterococcus species, Streptococcus bovis, and Leuconostoc species, p. 2147-2152. In G. L. Mandell, J. E. Bennett, and R. Dolin (ed.), Principles and practice of infectious diseases, 5th ed. Churchill Livingstone, New York, N.Y.
- 7.Moellering, R. C. 1992. Emergence of enterococcus as a significant pathogen. Clin. Infect. Dis. 14:1173-1176. [DOI] [PubMed] [Google Scholar]
- 8.Moellering, R. C. 1998. Vancomycin-resistant enterococci. Clin. Infect. Dis. 26:1196-1199. [DOI] [PubMed] [Google Scholar]
- 9.Muto, C. A., J. A. Jernigan, B. E. Ostrowsky, N. M. Richet, W. R. Jarvis, J. M. Boyce, and B. M. Farr. 2003. SHEA guideline for preventing nosocomial transmission of multidrug-resistant strains of Staphylococcus aureus and Enterococcus. Infect. Control Hosp. Epidemiol. 24:362-386. [DOI] [PubMed] [Google Scholar]
- 10.National Committee for Clinical Laboratory Standards. 2000. Methods for dilution antimicrobial susceptibility testing for bacteria that grow aerobically, 5th ed. Approved standard M7-A5. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 11.National Nosocomial Infections Surveillance (NNIS) System. 2003. National Nosocomial Infections Surveillance (NNIS) System report, data summary from January 1992 through June 2003, issued August 2003. Am. J. Infect. Control 31:481-498. [DOI] [PubMed] [Google Scholar]
- 12.Perencevich, E. N., D. N. Fisman, M. Lipsitch, A. D. Harris, J. G. Morris, and D. L. Smith. 2004. Projected benefits of active surveillance for vancomycin-resistant enterococci in intensive care units. Clin. Infect. Dis. 38:1108-1115. [DOI] [PubMed] [Google Scholar]
- 13.Prystowsky, J., F. Siddiqui, J. Chosay, D. L. Shinabarger, J. Millichap, L. R. Peterson, and G. A. Noskin. 2001. Resistance to linezolid: characterization of mutations in rRNA and comparison of their occurrences in vancomycin-resistant enterococci. Antimicrob. Agents Chemother. 45:2154-2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rice, L. B. 2001. Emergence of vancomycin-resistant enterococci. Emerg. Infect. Dis. 7:183-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stosor, V., L. R. Peterson, M. Postelnick, and G. A. Noskin. 1998. Enterococcus faecium bacteremia: does vancomycin resistance make a difference? Arch. Intern. Med. 158:522-527. [DOI] [PubMed] [Google Scholar]
- 16.Tsiodras, S., H. S. Gold, G. Sakoulas, G. M. Eliopoulos, C. Wennersten, L. Venkataraman, R. C. Moellering, and M. J. Ferraro. 2001. Linezolid resistance in a clinical isolate of Staphylococcus aureus. Lancet 358:207-208. [DOI] [PubMed] [Google Scholar]