Abstract
HIV-1 infection results in blood-brain barrier (BBB) disruption, which acts as a rate-limiting step for HIV-1 entry into the CNS and for subsequent neuroinflammatory/neurotoxic actions. One mechanism by which HIV may destabilize the BBB involves actions of the HIV-1 regulatory protein, trans-activator of transcription (Tat). We utilized a conditional, Tat-expressing transgenic murine model to examine the influence of Tat1-86 expression on BBB integrity and to assess the relative numbers of phagocytic perivascular macrophages and microglia within the CNS in vivo. The effects of Tat exposure on sodium-fluorescein (Na-F; 0.376 kDa), horseradish peroxidase (HRP; 44 kDa), and Texas Red-labeled dextran (70 kDa) leakage into the brain were assessed in Tat-exposed (Tat+) and control (Tat−) mice. Exposure to HIV-1 Tat significantly increased both Na-F and HRP, but not the larger sized Texas Red-labeled dextran, confirming BBB breakdown and also suggesting the breach was limited to molecules <70 kDa. Additionally, at 5 d after Tat induction, Alexa Fluor® 488-labeled dextran was bilaterally infused into the lateral ventricles 5 d before the termination of the experiment. Within the caudate/putamen, Tat induction increased the proportion of dextran-labeled Iba-1+ phagocytic perivascular macrophages (~5-fold) and microglia (~3-fold) compared to Tat− mice. These data suggest that HIV-1 Tat exposure is sufficient to destabilize BBB integrity and to increase the presence of activated, phagocytic, perivascular macrophages and microglia in an in vivo model of neuroAIDS.
Keywords: Blood-brain barrier, caudate/putamen, human immunodeficiency virus, microglia, perivascular macrophages, trans-activator of transcription
Introduction
About 37 million people globally are living with human immunodeficiency virus-1 (HIV-1), with over 1.2 million HIV-infected individuals residing within the United States [1]. Despite the use of combination antiretroviral therapy (cART), approximately half of infected individuals experience HIV-associated neurocognitive disorders (HAND) and display deficits in memory and learning, an increased prevalence of neuropsychiatric disorders, and motor impairments [2,3]. Understanding the ability of HIV infection to disrupt the blood-brain barrier (BBB), and to alter the migration of inflammatory cells to the brain is critical to understanding the pathology of neuroAIDS.
The BBB is a major barrier against HIV-1 entry into the CNS. HIV is largely thought to enter the CNS through the trafficking of HIV-infected monocyte-derived macrophages (MDMs) (i.e., Trojan Horse model) [4,5]. It is also hypothesized that some virions may enter the CNS via endothelial transcytosis or cross the BBB paracellularly, and that viral entry would be aided by breaches in BBB integrity [6]. Indeed, early post-mortem analyses of HIV-infected brains reveal an accumulation of serum proteins, consistent with barrier breakdown [7,8]. Molecular alterations critical for leukocyte transmigration across the BBB are increased in the HIV-infected CNS [9] and expression of the tight junction protein, claudin-5, is reduced [10]. However, the mechanisms by which HIV disrupts BBB integrity are only partially understood.
One mechanism by which HIV may destabilize the BBB involves actions of the HIV-1 regulatory protein, trans-activator of transcription (Tat). Tat is an early-expressed gene product, secreted from infected cells [11], that induces the expression and release of cytokines, chemokines, and adhesion proteins in vitro and in vivo in mice receiving intracerebral Tat injections [12,13]. In vitro, Tat decreases expression of the tight junction proteins, claudin-1, claudin-5, and zonula occludens-1 (ZO-1) and/or zonula occludens-2 (ZO-2) in brain microvascular endothelial cells [14–16]. Tat-mediated effects on tight junction proteins may be dynamic given that Tat-promoted neuroinflammation may further contribute to BBB permeability, and inhibiting Tat-mediated translocation of NF-κB attenuates changes in claudin-5 [17]. However, the extent to which these in vitro findings reflect changes in BBB function and integrity in vivo is uncertain [18]. Accordingly, the present investigation assessed BBB integrity in Tat transgenic mice.
We hypothesized that expression of HIV-1 Tat protein in a transgenic murine model would disrupt the BBB, resulting in increased barrier permeability and in the increased recruitment/activation of macrophages/microglia within the dorsal striatum. The dorsal striatum is reported to be selectively vulnerable in HIV-infected individuals [19] and the acute and chronic effects of Tat-induced neuropathogenesis in this region are well-characterized in our transgenic mouse model [20–22]. To assess this, mice that conditionally expressed HIV-1 Tat (Tat+), or their control counterparts (Tat−), received transcardial injections of sodium fluorescein (Na-F; 0.376 kDa), horseradish peroxidase (HRP; 44 kDa), or Texas Red®-labeled dextran (70 kDa) to determine the nature and extent of BBB leakiness. To assess phagocytic activity, mice were administered bilateral i.c.v. infusions of Alexa Fluor® 488-labeled dextran and numbers of phagocytic perivascular macrophages and microglia were examined 5 d later by quantitative fluorescence microscopy.
Materials and Methods
The use of mice in these studies was approved by the Institutional Animal Care and Use Committee at Virginia Commonwealth University and the experiments were conducted in accordance with ethical guidelines defined by the National Institutes of Health (NIH Publication No. 85-23).
Subjects and housing
Adult, male mice (approximately 70 days of age) that expressed the HIV-1 tat transgene (Tat+; N = 10), and their control counterparts that lacked the transgene (Tat−; N = 12), were generated in the vivarium at Virginia Commonwealth University. Briefly, Tat+ mice conditionally-expressed the HIV-1 Tat1-86 protein in a CNS-targeted manner via a GFAP-driven Tet-on promoter (activated via consumption of chow containing doxycycline). Tat− controls expressed only the doxycycline-responsive rtTA transcription factor as previously described [20,23]. All mice were placed on doxycycline chow (Dox Diet #2018; 6 g/kg) obtained from Harlan Laboratories (Madison, WI) for the duration of the experiment (10 d). Mice were housed 4–5/cage and were maintained in a temperature- and humidity-controlled room on a 12:12 h light/dark cycle (lights off at 18:00 h) with ad libitum access to food and water.
Surgical manipulation
All mice underwent bilateral stereotaxic infusions as modified from prior reports [24,25]. Briefly, mice received bilateral i.c.v. infusions (4 μL) under isoflurane (4%) anesthesia (Bregma: AP: −0.5 mm, Lat: ±1.6 mm, DV: −2 mm; [26,27]). Following surgery, mice were monitored to ensure weight gain, muscle tone, and proper neurological response and general health [28].
Experiment 1: assessment of blood-brain barrier permeability
To assess the influence of HIV-1 Tat on BBB integrity, Tat− and Tat+ mice were transcardially infused with 50 μL of ~0.376 kDa Na-F (2%, w/v; 10 min prior to perfusion with 15 mL PBS), 10 μL ~44 kDa HRP (5 mg/mL; 5 min prior to perfusion with 15 mL PBS followed by 20 mL 4% paraformaldehyde), or 10 μL ~70 kDa dextran conjugated to Texas Red® (4 mg/mL; 10 min prior to perfusion with 15 mL PBS) per prior methods [29–31]. BBB permeability was assessed via multiple methods: HRP brain penetration was measured immunohistochemically in whole-brain sections, whereas Na-F and Texas Red®-labeled dextran were measured in brain homogenates. For HRP experiments, frozen coronal slices (40 μm; obtained 0.845–1.245 mm from Bregma) were labeled with primary anti-HRP and visualized via appropriate secondary antibody conjugated to Alexa Fluor® 647 (Alexa 647, Thermo Fisher, Rockford, IL; far-red fluorescence). Slices were counterstained with Hoechst 33342 nuclear stain (Thermo Fisher; blue fluorescence) and imaged as described [32]. HRP signal was normalized to background (signal intensity in the off-tissue area of the tiled image). HRP signal above background levels indicates BBB disruption and leakage of HRP into the brain [29]. Tiled images of HRP and Hoechst dual-labeled sections in the dorsal striatum were acquired from within a single z-plane (~0.50 μm-depth) within 5 μm from the surface of the section. HRP immunofluorescence was detected in the far-red range (Alexa 647) using diode laser excitation (637 nm) with a 640 nm long-pass filter. All images were acquired using a Zeiss LSM700 confocal microscope (Oberkochen, Germany) equipped with a 20× 1.0 NA objective. During image acquisition, the laser intensity, detector gain, and all other parameters were held constant within an identical volume of tissue across all treatment groups. Fluorescent densitometry was assessed in unaltered tagged image file (tif) format images using ImageJ software (National Institutes of Health).
For Na-F and 70 kDa dextran experiments, brains were homogenized and Na-F and fluorescein-labeled dextrans were measured via spectrophotometry (Na-F: 440/525 nm, ex/em; Texas Red®-dextrans: 575/620 nm, ex/em) using a PHERAstar FS Plus microplate reader (BMG Labtech). Na-F and 70 kDa dextran data are expressed as fold-change in fluorescent intensity/well (200 uL volume) compared to Tat− control mice [33].
Experiment 2: in vivo labeling of phagocytic macrophages/microglia in the CNS
To assess the effects of Tat on the number of phagocytic macrophages/microglia within the brain, Tat+ and Tat− mice received a bilateral i.c.v. infusion of ~10 kDa Alexa Fluor® 488-dextran (Alexa 488-dextran; 4 mg/kg; Thermo Fisher; cat. # D22910) on day 5 of Tat exposure (approximately half-way through the Tat induction period). On day 10 of Tat exposure, mice were transcardially perfused with PBS followed by 4% paraformaldehyde and were prepared for immunohistochemistry as previously described [32]. Coronal slices (40 μm; 0.845–1.245 mm from Bregma) were counterstained with Hoechst 33342 to detect cell nuclei. Alexa 488-dextran was infused at 5 days following induction when Tat causes significant pathology in the striatum; astrogliosis and microgliosis is evident at 48 h following Tat induction [20], and synaptodendritic injury occurs 7–10 days following Tat induction [21]. To further demonstrate that the Alexa 488-dextran-labeled phagocytes were macrophages/microglia, anti-Iba-1 primary antibodies (Wako Pure Chemical Industries, Richmond, VA) were visualized using Alexa 647-conjugated secondary antibodies (Thermo Fisher) and co-localized with Alexa 488-dextran. Alexa 488-dextran and Iba-1 co-localization was confirmed in the same cell by 3-dimensional reconstruction of multiple z-stack images using a Zeiss LSM 700 microscope (63× 1.4 NA objective) and presented as a single compressed image in the present paper. To determine the relative number of phagocytic macrophages/microglia in the caudate/putamen of Tat− and Tat+ mice, Hoechst+ cells were counted in sequential fields until a criterion of 200 cells/slice was met; the number of Alexa 488-dextran cells is reported as a proportion of the total number of Hoechst+ cells.
Statistical analyses
Dependent measures for BBB permeability and phagocytic activity in perivascular macrophages/microglia were assessed by one-way analysis of variance (ANOVA) in the HRP experiment and by Student’s one-tailed t-tests for remaining experiments. Group differences in main effects were determined using Fisher’s Protected Least Significant Difference post-hoc tests determined group. No interactions were detected. Analyses were considered significant if p < 0.05.
Results
Experiment 1: HIV-1 Tat disrupts the blood-brain barrier of Tat-transgenic mice
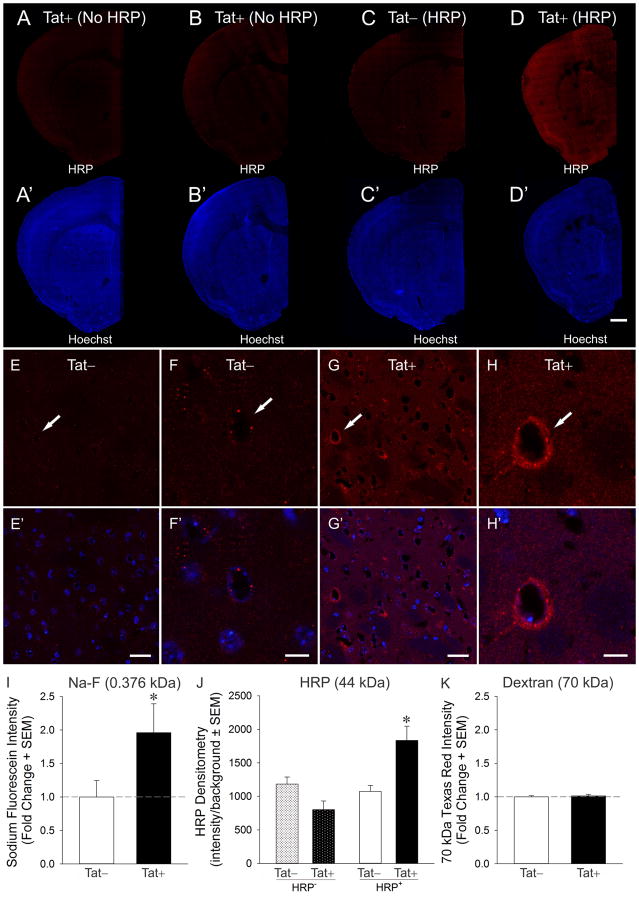
Inducing HIV-1 Tat in transgenic mice significantly altered BBB permeability as assessed by HRP accumulation in brain [F(3,9) = 8.20, p < 0.05] (Fig, 1A–H′). Tat+ mice receiving transcardial infusions of HRP demonstrated significantly greater HRP signal in the brain compared to HRP-infused, Tat− control mice (p = 0.008), or the negative controls [Tat+ (p = 0.001) or Tat− (p = 0.02) mice that were not infused with HRP] (Fig. 1J). Moreover, Tat exposure compromised barrier integrity as assessed by Na-F accumulation in brain [t(10) = 2.09, p < 0.05] (Fig. 1I). However, the accumulation of Texas Red+ 70 kDa dextran in the brain did not significantly differ between Tat− or Tat+ mice (Fig. 1K), suggesting that there is an upper limit to barrier disruption induced by Tat under these experimental conditions.
Fig. 1.
Effects of HIV-1 Tat expression on the penetration of horseradish peroxidase (HRP; red) from the vasculature into the forebrain of Tat transgenic mice (A–D; Scale bar = 1 mm). Tat expressing (Tat+) mice that were transcardially injected with HRP showed increased BBB permeability (D), while mice lacking the Tat transgene (Tat−) (C) or mice that were injected with saline instead of HRP (A–B) served as controls. The same sections as in A–D were counterstained with Hoechst 33342 (blue) to reveal the underlying cytoarchitecture (A′–D′). Higher magnification image showing a gradient of HRP penetration from some small blood vessels (capillaries and some venules, indicated by arrows) into the striatal parenchyma in Tat+ mice (G–H′) that was minimally evident in Tat− control mice (E–F′). Scale bar = 50 μm (E, E′, G, G′) or 10 μm (F, F′, H, H′). Effects of Tat expression on mice transcardially injected with 0.376 kDa sodium fluorescein (Na-F) (I), 44 kDa HRP (J), and 70 kDa Texas Red®-labeled dextran (K). Tat expression increased the permeability of the BBB to Na-F and HRP, but not to the higher molecular weight Texas Red®-labeled dextran. * indicates p < 0.05 compared to all other groups.
Experiment 2: Phagocytic macrophage/microglial-activity is greater following HIV-1 Tat exposure
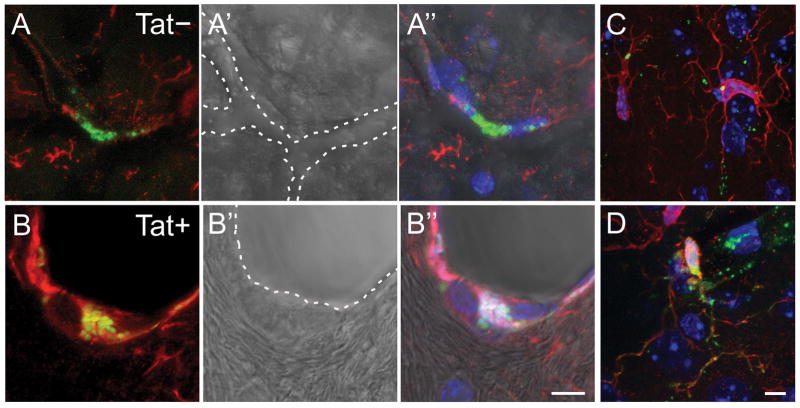
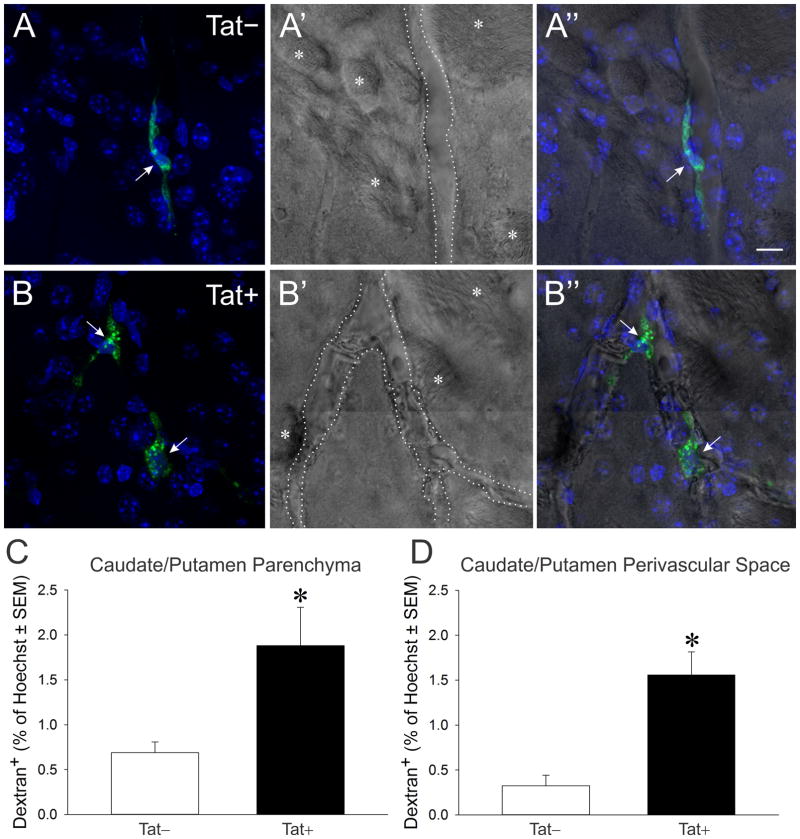
Alexa 488-dextran (i.c.v.) co-localized with perivascular Iba-1-labeled cells within the caudate/putamen of Tat− (Fig. 2A-A″) and Tat+ (Fig. 2B-B″) mice. Morphology and dextran accumulation were consistent with phagocytic perivascular macrophages (2A-2B″). Within the parenchyma of the caudate/putamen, Iba-1-labeled cells with microglial morphology co-localized with Alexa 488-dextran (Fig. 2C). Compared to Iba-1-labeled macrophages within the perivascular space, microglia within the parenchyma internalized notably less dextran; however, greater amounts of dextran were observed within some microglia that were proximally closer to vascular boundaries (Fig. 2D). The co-localization of Alexa 488-dextran in phagocytic cells associated with the perivascular space (Fig. 3A–B″) and within the parenchyma (not shown) was quantified. Compared to Tat− controls, HIV-1 Tat exposure significantly increased the proportion of dextran-labeled phagocytes both in the parenchyma [F(1,20) = 9.08, p < 0.05] (Fig. 3C) and in the perivascular space [F(1,20) = 23.58, p < 0.05] (Fig. 3D) of the caudate/putamen.
Fig. 2.
Photomicrographs of phagocytic perivascular macrophages within the caudate/putamen of Tat− (A-A″) and Tat+ (B-B″) transgenic mice (A–B). Perivascular macrophages labeled with Iba-1 (red) typically phagocytosed Alexa 488-conjugated dextran (infused i.c.v. at 5 d following continuous Tat induction; green). Dotted lines indicate the boundaries of small blood vessels. Alexa 488-dextran was also observed within Iba-1-labeled microglia residing in the caudate/putamen parenchyma of Tat− (C) and Tat+ (D) transgenic mice. Scale bar = 10 μm.
Fig. 3.
Photomicrographs of phagocytic perivascular macrophages within the caudate/putamen of Tat− (A-A) and Tat+ (B-B″) transgenic mice (A–B). Macrophages (arrows) were labeled with Alexa 488-dextran (infused i.c.v. at 5 d following continuous Tat induction; green). The dotted line indicates the boundaries of small blood vessel, while the asterisk (*) indicates white matter tracts indicative of the striatum. Scale bar = 10 μm. HIV-1 Tat exposure significantly increased the proportion of dextran-labeled phagocytes compared to Tat− control mice (C–D). Quantification of the proportion of phagocytic cells labeled with green within the parenchyma (C) or perivascular space (D). * indicates p < 0.05 compared to Tat− mice.
Discussion
Our findings support the hypothesis that Tat is a critical component mediating the known BBB disruptive effects of HIV-1. The results suggest that Tat-dependent disruptions to the BBB also contribute to the glial activation, inflammation, and neuronal injury seen in the dorsal striatum in the transgenic Tat mouse [20–22].
The leakage of Na-F and HRP tracers into the brains of mice was significantly increased in Tat+ compared with Tat− mice. These data are consistent with previous in vitro work demonstrating that Tat exposure increases brain vascular endothelial permeability to paracellular compounds such as Evans Blue or FITC-dextrans [16,34]. BBB disruption is not an “all-or-none” phenomenon and therefore varying the size of molecular tracers can infer the magnitude of BBB disruption [35]. In our studies, BBB breach occurred not only for a relatively small compound (Na-F; 0.376 kDa) but also for a compound ~100-fold larger (HRP; 44 kDa), while a 70 kDa dextran conjugate failed to cross, suggesting an intermediate level of disruption of the endothelium upon 10 d exposure to Tat. Interestingly, HRP immunofluorescence was not uniformly distributed within the Tat+ mouse brain (Fig. 1G–1H′). Although differences in HRP leakage within the brain may be a specific feature of the Tat model, it may also reflect regional differences in the response of the BBB to Tat exposure. Future investigations may aim to examine these endpoints using additional models of central Tat expression, perhaps in a between-subjects design in which dextrans of varying size are assessed against the same fluorophore to eliminate any variance caused by differences in fluorescent labeling and/or the differences in the tracers themselves (e.g., Na-F vs. HRP vs. dextran).
We also hypothesized that Tat exposure would increase the recruitment and/or activity of phagocytic macrophages and microglia within the brain, which was confirmed by the observed increases in Alexa 488-dextran labeled cells within the parenchyma and the perivascular space of the caudate/putamen. We observed strong co-localization of labeled dextran within perivascular macrophages (Fig. 2A-A″ and 2B-B″). Of interest, we also saw dextran-labeled Iba-1-immunoreactive microglia within the parenchyma (Fig. 2C). Dextran accumulation within microglia was notably reduced compared to that observed in perivascular macrophages; albeit, some microglia situated closer to the brain vasculature demonstrated increased amounts of dextran internalization (Fig. 2D) and dextran was not co-localized with some Iba-1+ microglia especially in the Tat− mice. Although astrogliosis and microgliosis have been observed in the Tat transgenic mouse [20], alterations in macrophage/microglial function, as assessed by phagocytic activity, have not been previously characterized. Similar increases in microglia and perivascular macrophages are seen following intrahippocampal injections of Tat [13], though in these studies the BBB was partially disrupted by the stereotaxic injection of Tat. Additionally, others have demonstrated using in vitro models that Tat exposure increases transmigration of peripheral monocytes across a BBB model via increased production of CCL2 (MCP-1) and upregulation of CCR5 on monocytes [36]. HIV infection increases expression of junctional adhesion molecule-A (JAM-A) and activated leukocyte cell adhesion molecule (ALCAM) on monocytes, both of which can mediate the enhanced transmigration of human CD14+, CD16+ monocytes (infected and uninfected) into the brain resulting in accumulation of these inflammatory leukocytes within the CNS. Despite the importance of JAM-A and ALCAM in monocyte trafficking, Tat’s specific role in this process is not yet well described [37]. It is also noteworthy that the rarefaction of brain capillaries and altered hemodynamics seen with chronic (6 months) Tat exposure in this Tat transgenic mouse model [38] are likely a direct result of sustained BBB disruption, macrophage activation, and inflammation that we observe after 10 d of Tat induction. Given Tat’s dynamic capacity to decrease tight junction expression in brain microvascular endothelial cells [14–16], and to facilitate NF-κB signaling which may inhibit occludin expression [39], future studies are warranted that examine the importance of timing of Tat expression on BBB disruption and neuroinflammation.
Perivascular macrophages are phenotypically distinct from resident brain macrophages, the microglia, and play a central role in HIV neuropathogenesis [40–42]. Perivascular macrophages can be productively infected with HIV and produce soluble inflammatory mediators and cytokines which contribute to breakdown of the BBB [43]. Additionally, the perivascular space is a major site of infiltration of blood-derived cells under normal and inflammatory conditions [44,45]. They are continuously repopulated from bone marrow [41,46] and the rate of this repopulation can be accelerated in inflammation and infection [47–49]. Accumulation of perivascular macrophages is a feature of HIV infection, including HIV encephalitis and HIV-associated dementia [41,44,50]. Our findings support the hypothesis that Tat has a critical role in mediating the increased numbers of phagocytic macrophages and microglia within the brain that are observed in HIV infection.
HIV-1-infected cells within the CNS may secrete viral proteins besides Tat (such as gp120), which activate surrounding macrophages, microglia and astrocytes to increase the release of inflammatory factors and escalate recruitment of monocytes into the CNS [42,51–54]. Gp120 can affect the BBB and may act in concert with Tat to further exacerbate BBB disruption and monocyte expansion and transmigration [55,56]. Exposure to gp120 in vitro results in the expansion of high CD16 expressing monocytes, which is similar to the expansion observed in vivo following HIV infection [57,58]. Unlike most other HIV proteins, Tat appears to continue to be expressed by infected cells despite the suppression of viral replication by cART [59], and our studies demonstrate that this sustained expression of Tat could be of clinical importance to BBB alterations in virally suppressed patients. Current antiretroviral therapy does not target the early phase of HIV-1 mRNA transcription when Tat is expressed [60].
Conclusions
The present findings demonstrate HIV-1 Tat to be a critical mediator of HIV-associated disruption of the intact BBB. Furthermore, in vivo Tat exposure resulted in increases in the proportion of dextran-labeled macrophages within the perivascular space and striatal tissue, which is a region of clinical significance in HAND. This Tat transgenic mouse model may be a useful tool in further examining BBB dynamics in monocyte trafficking within the context of HIV-1 Tat exposure.
Highlights.
HIV-1 Tat exposure disrupted the BBB, as evidenced by Na-F and HRP leakage into the brain
HIV-1 Tat exposure increased phagocytic macrophages/microglia within the caudate/putamen
HIV-1 Tat activated both perivascular and tissue-resident macrophages/microglia
Acknowledgments
This work was supported by funds from the National Institutes of Health: K99 DA039791 (JJP), R01 DA024461 (PEK), R01 DA034231 (PEK and KFH), K02 DA027374 (KFH), and R01 DA033200 (KFH).
Abbreviations
- ALCAM
activated leukocyte cell adhesion molecule
- ANOVA
analysis of variance
- BBB
blood-brain barrier
- cART
combination antiretroviral therapy
- CNS
central nervous system
- HAND
HIV associated neurocognitive disorders
- HIV-1
human immunodeficiency virus-1
- HRP
horseradish peroxidase
- Iba-1
ionized calcium-binding adaptor molecule 1
- ICV
intracerebroventricular
- JAM-A
junctional adhesion molecule-A
- Na-F
sodium fluorescein
- SIV
simian immunodeficiency virus
- Tat
trans-activator of transcription
- ZO
zonula occludens
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.UNAIDS. Global AIDS Update 2016. 2016 http://www.unaids.org/sites/default/files/media_asset/global-AIDS-update-2016_en.pdf.
- 2.Saylor D, Dickens AM, Sacktor N, Haughey N, Slusher B, Pletnikov M, Mankowski JL, Brown A, Volsky DJ, McArthur JC. HIV-associated neurocognitive disorder — pathogenesis and prospects for treatment. Nat Rev Neurol. 2016 doi: 10.1038/nrneurol.2016.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, Clifford DB, Cinque P, Epstein LG, Goodkin K, Gisslen M, Grant I, Heaton RK, Joseph J, Marder K, Marra CM, McArthur JC, Nunn M, Price RW, Pulliam L, Robertson KR, Sacktor N, Valcour V, Wojna VE. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=17914061&retmode=ref&cmd=prlinks. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meltzer MS, Skillman DR, Gomatos PJ, Kalter DC, Gendelman HE. Role of mononuclear phagocytes in the pathogenesis of human immunodeficiency virus infection. Annu Rev Immunol. 1990;8:169–94. doi: 10.1146/annurev.iy.08.040190.001125. [DOI] [PubMed] [Google Scholar]
- 5.Verani A, Gras G, Pancino G. Macrophages and HIV-1: dangerous liaisons. Mol Immunol. 2005;42:195–212. doi: 10.1016/j.molimm.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 6.Mattson MP, Haughey NJ, Nath A. Cell death in HIV dementia. Cell Death Differ. 2005;12(Suppl 1):893–904. doi: 10.1038/sj.cdd.4401577. [DOI] [PubMed] [Google Scholar]
- 7.Petito CK, Cash KS. Blood-brain barrier abnormalities in the acquired immunodeficiency syndrome: immunohistochemical localization of serum proteins in postmortem brain. Ann Neurol. 1992;32:658–66. doi: 10.1002/ana.410320509. [DOI] [PubMed] [Google Scholar]
- 8.Power C, Kong Pa, Crawford TO, Wesselingh S, Glass JD, McArthur JC, Trapp BD. Cerebral white matter changes in acquired immunodeficiency syndrome dementia: alterations of the blood-brain barrier. Ann Neurol. 1993;34:339–350. doi: 10.1002/ana.410340307. [DOI] [PubMed] [Google Scholar]
- 9.Eugenin EA. CCL2/Monocyte Chemoattractant Protein-1 Mediates Enhanced Transmigration of Human Immunodeficiency Virus (HIV)-Infected Leukocytes across the Blood-Brain Barrier: A Potential Mechanism of HIV-CNS Invasion and NeuroAIDS. J Neurosci. 2006;26:1098–1106. doi: 10.1523/JNEUROSCI.3863-05.2006. http://www.jneurosci.org/cgi/doi/10.1523/JNEUROSCI.3863-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chaudhuri A, Yang B, Gendelman HE, Persidsky Y, Georgette D, Kanmogne GD. STAT1 signaling modulates HIV-1 – induced inflammatory responses and leukocyte transmigration across the blood-brain barrier. Blood. 2008;111:2062–2072. doi: 10.1182/blood-2007-05-091207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ensoli B, Barillari G, Salahuddin SZ, Gallo RC, Wong-Staal F. Tat protein of HIV-1 stimulates growth of cells derived from Kaposi’s sarcoma lesions of AIDS patients. Nature. 1990;345:84–6. doi: 10.1038/345084a0. [DOI] [PubMed] [Google Scholar]
- 12.Conant K, Garzino-Demo A, Nath A, McArthur JC, Halliday W, Power C, Gallo RC, Major EO. Induction of monocyte chemoattractant protein-1 in HIV-1 Tat-stimulated astrocytes and elevation in AIDS dementia. Proc Natl Acad Sci U S A. 1998;95:3117–21. doi: 10.1073/pnas.95.6.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pu H, Tian J, Flora G, Woo Lee Y, Nath A, Hennig B, Toborek M. HIV-1 tat protein upregulates inflammatory mediators and induces monocyte invasion into the brain. Mol Cell Neurosci. 2003;24:224–237. doi: 10.1016/S1044-7431(03)00171-4. [DOI] [PubMed] [Google Scholar]
- 14.Andras IE, Pu H, Deli MA, Nath A, Hennig B, Toborek M. HIV-1 Tat Protein Alters Tight Junction Protein Expression and Distribution in Cultured Brain Endothelial Cells. J Neurosci Res. 2003;74:255–265. doi: 10.1002/jnr.10762. [DOI] [PubMed] [Google Scholar]
- 15.Mahajan SD, Aalinkeel R, Sykes DE, Reynolds JL, Bindukumar B, Fernandez SF, Chawda R, Shanahan TC, Schwartz SA. Tight junction regulation by morphine and HIV-1 tat modulates blood-brain barrier permeability. J Clin Immunol. 2008;28:528–541. doi: 10.1007/s10875-008-9208-1. http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=18574677&retmode=ref&cmd=prlinks. [DOI] [PubMed] [Google Scholar]
- 16.Pu H, Hayashi K, Andras IE, Eum S, Hennig B, Toborek M. Limited role of COX-2 in HIV Tat-induced alterations of tight junction protein expression and disruption of the blood-brain barrier. Brain Res. 2007;1184:333–344. doi: 10.1016/j.brainres.2007.09.063. [DOI] [PubMed] [Google Scholar]
- 17.András IE, Pu H, Tian J, Deli MA, Nath A, Hennig B, Toborek M, Andras IE, Pu H, Tian J, Deli MA, Nath A, Hennig B, Toborek M. Signaling mechanisms of HIV-1 Tat-induced alterations of claudin-5 expression in brain endothelial cells. J Cereb Blood Flow Metab. 2005;25:1159–1170. doi: 10.1038/sj.jcbfm.9600115. http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=15815581&retmode=ref&cmd=prlinks. [DOI] [PubMed] [Google Scholar]
- 18.Bakri Y, Amzazi S, Mannioui A, Benjouad A. The susceptibility of macrophages to human immunodeficiency virus type 1 X4 isolates depends on their activation state. Biomed Pharmacother. 2001;55:32–38. doi: 10.1016/S0753-3322(00)00015-9. [DOI] [PubMed] [Google Scholar]
- 19.Nath A. Eradication of human immunodeficiency virus from brain reservoirs. J Neurovirol. 2015;21:227–34. doi: 10.1007/s13365-014-0291-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bruce-Keller AJ, Turchan-Cholewo J, Smart EJ, Geurin T, Chauhan A, Reid R, Xu R, Nath A, Knapp PE, Hauser KF. Morphine causes rapid increases in glial activation and neuronal injury in the striatum of inducible HIV-1 tat transgenic mice. Glia. 2008;56:1414–1427. doi: 10.1002/glia.20708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fitting S, Xu R, Bull C, Buch SK, El-Hage N, Nath A, Knapp PE, Hauser KF. Interactive comorbidity between opioid drug abuse and HIV-1 Tat: chronic exposure augments spine loss and sublethal dendritic pathology in striatal neurons. Am J Pathol. 2010;177:1397–1410. doi: 10.2353/ajpath.2010.090945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paris JJ, Zou S, Hahn YK, Knapp PE, Hauser KF. 5α-reduced progestogens ameliorate mood-related behavioral pathology, neurotoxicity, and microgliosis associated with exposure to HIV-1 Tat. Brain Behav Immun. 2016;55:202–14. doi: 10.1016/j.bbi.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hauser KF, Hahn YK, Adjan VV, Zou S, Buch SK, Nath A, Bruce-Keller AJ, Knapp PE. HIV-1 Tat and morphine have interactive effects on oligodendrocyte survival and morphology. Glia. 2009;57:194–206. doi: 10.1002/glia.20746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.El-Hage N, Wu G, Wang J, Ambati J, Knapp PE, Reed JL, Bruce-Keller AJ, Hauser KF. HIV-1 Tat and opiate-induced changes in astrocytes promote chemotaxis of microglia through the expression of MCP-1 and alternative chemokines. Glia. 2006;53:132–146. doi: 10.1002/glia.20262. http://doi.wiley.com/10.1002/glia.20262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.El-Hage N, Wu G, Ambati J, Bruce-Keller AJ, Knapp PE, Hauser KF. CCR2 mediates increases in glial activation caused by exposure to HIV-1 Tat and opiates. J Neuroimmunol. 2006;178:9–16. doi: 10.1016/j.jneuroim.2006.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Naumenko VS, Kondaurova EM, Bazovkina DV, Tsybko AS, Tikhonova MA, Kulikov AV, Popova NK. Effect of brain-derived neurotrophic factor on behavior and key members of the brain serotonin system in genetically predisposed to behavioral disorders mouse strains. Neuroscience. 2012;214:59–67. doi: 10.1016/j.neuroscience.2012.04.031. [DOI] [PubMed] [Google Scholar]
- 27.Naumenko VS, Bazovkina DV, Morozova MV, Popova NK. Effects of brain-derived and glial cell line-derived neurotrophic factors on startle response and disrupted prepulse inhibition in mice of DBA/2J inbred strain. Neurosci Lett. 2013;550:115–118. doi: 10.1016/j.neulet.2013.06.056. [DOI] [PubMed] [Google Scholar]
- 28.Crawley JN, Paylor R. A proposed test battery and constellations of specific behavioral paradigms to investigate the behavioral phenotypes of transgenic and knockout mice. Horm Behav. 1997;31:197–211. doi: 10.1006/hbeh.1997.1382. [DOI] [PubMed] [Google Scholar]
- 29.Ben-Zvi A, Lacoste B, Kur E, Andreone BJ, Mayshar Y, Yan H, Gu C. Mfsd2a is critical for the formation and function of the blood-brain barrier. Nature. 2014;509:507–511. doi: 10.1038/nature13324. http://dx.doi.org/10.1038/nature13324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hawkins BT, Egleton RD. Fluorescence imaging of blood-brain barrier disruption. J Neurosci Methods. 2006;151:262–267. doi: 10.1016/j.jneumeth.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 31.Ramirez SH, Hasko J, Skuba A, Fan S, Dykstra H, McCormick R, Reichenbach N, Krizbai I, Mahadevan A, Zhang M, Tuma R, Son YJ, Persidsky Y. Activation of Cannabinoid Receptor 2 Attenuates Leukocyte-Endothelial Cell Interactions and Blood-Brain Barrier Dysfunction under Inflammatory Conditions. J Neurosci. 2012;32:4004–4016. doi: 10.1523/JNEUROSCI.4628-11.2012. http://www.jneurosci.org/cgi/doi/10.1523/JNEUROSCI.4628-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marks WD, Paris JJ, Schier CJ, Denton MD, Fitting S, McQuiston AR, Knapp PE, Hauser KF. HIV-1 Tat causes cognitive deficits and selective loss of parvalbumin, somatostatin, and neuronal nitric oxide synthase expressing hippocampal CA1 interneuron subpopulations. J Neurovirol. 2016:1–16. doi: 10.1007/s13365-016-0447-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ramirez SH, Skuba A, Fan S, Dykstra H, Mccormick R, Reichenbach N, Krizbai I, Mahadevan A, Zhang M, Tuma R, Son Y, Persidsky Y. Activation of Cannabinoid Receptor 2 Attenuates Leukocyte – Endothelial Cell Interactions and Blood – Brain Barrier Dysfunction under Inflammatory Conditions. J Neurosci. 2012;32:4004–4016. doi: 10.1523/JNEUROSCI.4628-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gandhi N, Saiyed ZM, Napuri J, Samikkannu T, Reddy PVB, Agudelo M, Khatavkar P, Saxena SK, Nair MPN. Interactive role of human immunodeficiency virus type 1 (HIV-1) clade-specific Tat protein and cocaine in blood-brain barrier dysfunction: implications for HIV-1-associated neurocognitive disorder. J Neurovirol. 2010;16:294–305. doi: 10.3109/13550284.2010.499891. http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=20624003&retmode=ref&cmd=prlinks. [DOI] [PubMed] [Google Scholar]
- 35.Hoffmann A, Bredno J, Wendland M, Derugin N, Ohara P, Wintermark M. High and Low Molecular Weight Fluorescein Isothiocyanate (FITC)–Dextrans to Assess Blood-Brain Barrier Disruption: Technical Considerations. Transl Stroke Res. 2010;2:106–111. doi: 10.1007/s12975-010-0049-x. http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=21423333&retmode=ref&cmd=prlinks. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weiss JM, Nath A, Major EO, Berman JW. HIV-1 Tat induces monocyte chemoattractant protein-1-mediated monocyte transmigration across a model of the human blood-brain barrier and up-regulates CCR5 expression on human monocytes. J Immunol. 1999;163:2953–2959. http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=10453044&retmode=ref&cmd=prlinks. [PubMed] [Google Scholar]
- 37.Williams DW, Anastos K, Morgello S, Berman JW. JAM-A and ALCAM are therapeutic targets to inhibit diapedesis across the BBB of CD14+CD16+ monocytes in HIV-infected individuals. J Leukoc Biol. 2015;97:401–412. doi: 10.1189/jlb.5A0714-347R. http://www.jleukbio.org/cgi/doi/10.1189/jlb.5A0714-347R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Silva JN, Polesskaya O, Wei HS, Rasheed IYD, Chamberlain JM, Nishimura C, Feng C, Dewhurst S. Chronic Central Nervous System Expression of HIV-1 Tat Leads to Accelerated Rarefaction of Neocortical Capillaries and Loss of Red Blood Cell Velocity Heterogeneity. Microcirculation. 2014;21:664–676. doi: 10.1111/micc.12145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wachtel M, Bolliger MF, Ishihara H, Frei K, Bluethmann H, Gloor SM. Down-regulation of occludin expression in astrocytes by tumour necrosis factor (TNF) is mediated via TNF type-1 receptor and nuclear factor-kB activation. J Neurochem. 2001;78:155–162. doi: 10.1046/j.1471-4159.2001.00399.x. [DOI] [PubMed] [Google Scholar]
- 40.Kim WK, Alvarez X, Fisher J, Bronfin B, Westmoreland S, McLaurin J, Williams KC. CD163 identifies perivascular macrophages in normal and viral encephalitic brains and potential precursors to perivascular macrophages in blood. Am J Pathol. 2006;168:822–834. doi: 10.2353/ajpath.2006.050215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fischer-Smith T, Croul S, Sverstiuk aE, Capini C, L’Heureux D, Régulier EG, Richardson MW, Amini S, Morgello S, Khalili K, Rappaport J. CNS invasion by CD14+/CD16+ peripheral blood-derived monocytes in HIV dementia: perivascular accumulation and reservoir of HIV infection. J Neurovirol. 2001;7:528–541. doi: 10.1080/135502801753248114. [DOI] [PubMed] [Google Scholar]
- 42.Buckner CM, Calderon TM, Williams DW, Belbin TJ, Berman JW. Characterization of monocyte maturation/differentiation that facilitates their transmigration across the blood-brain barrier and infection by HIV: Implications for NeuroAIDS. Cell Immunol. 2011;267:109–123. doi: 10.1016/j.cellimm.2010.12.004. http://dx.doi.org/10.1016/j.cellimm.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hong S, Banks WA. Role of the immune system in HIV-associated neuroinflammation and neurocognitive implications. Brain Behav Immun. 2015;45:1–12. doi: 10.1016/j.bbi.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim W-K, Avarez X, Williams KC. The role of monocytes and perivascular macrophages in HIV and SIV neuropathogenesis: information from non-human primate models. Neurotox Res. 2005;8:107–15. doi: 10.1007/BF03033823. http://www.ncbi.nlm.nih.gov/pubmed/16260389. [DOI] [PubMed] [Google Scholar]
- 45.Ransohoff RM, Kivisäkk P, Kidd G. Three or more routes for leukocyte migration into the central nervous system. Nat Rev Immunol. 2003;3:569–81. doi: 10.1038/nri1130. [DOI] [PubMed] [Google Scholar]
- 46.Hickey WF, Vass K, Lassmann H. Bone marrow-derived elements in the central nervous system: an immunohistochemical and ultrastructural survey of rat chimeras. J Neuropathol Exp Neurol. 1992;51:246–56. doi: 10.1097/00005072-199205000-00002. http://www.ncbi.nlm.nih.gov/pubmed/1583531. [DOI] [PubMed] [Google Scholar]
- 47.Burdo TH, Soulas C, Orzechowski K, Button J, Krishnan A, Sugimoto C, Alvarez X, Kuroda MJ, Williams KC. Increased monocyte turnover from bone marrow correlates with severity of SIV encephalitis and CD163 levels in plasma. PLoS Pathog. 2010;6:e1000842. doi: 10.1371/journal.ppat.1000842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hasegawa A, Liu H, Ling B, Borda JT, Alvarez X, Sugimoto C, Vinet-Oliphant H, Kim W-K, Williams KC, Ribeiro RM, Lackner Aa, Veazey RS, Kuroda MJ. The level of monocyte turnover predicts disease progression in the macaque model of AIDS. Blood. 2009;114:2917–25. doi: 10.1182/blood-2009-02-204263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim WK, Corey S, Alvarez X, Williams KC. Monocyte/Macrophage Traffic in HIV and SIV Encephalitis. J Leukoc Biol. 2003;74:650–656. doi: 10.1189/jlb.0503207.1. [DOI] [PubMed] [Google Scholar]
- 50.Nowlin BT, Burdo TH, Midkiff CC, Salemi M, Alvarez X, Williams KC. SIV Encephalitis Lesions Are Composed of CD163+ Macrophages Present in the Central Nervous System during Early SIV Infection and SIV-Positive Macrophages Recruited Terminally with AIDS. Am J Pathol. 2015;185:1649–1665. doi: 10.1016/j.ajpath.2015.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Williams DW, Eugenin EA, Calderon TM, Berman JW. Monocyte maturation, HIV susceptibility, and transmigration across the blood brain barrier are critical in HIV neuropathogenesis. J Leukoc Biol. 2012;91:401–415. doi: 10.1189/jlb.0811394. http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=22227964&retmode=ref&cmd=prlinks. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gendelman HE, Ding S, Gong N, Liu J, Ramirez SH, Persidsky Y, Mosley RL, Wang T, Volsky DJ, Xiong H. Monocyte chemotactic protein-1 regulates voltage-gated K+ channels and macrophage transmigration. J Neuroimmune Pharmacol. 2009;4:47–59. doi: 10.1007/s11481-008-9135-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Carvallo L, Lopez L, Che FY, Lim J, Eugenin EA, Williams DW, Nieves E, Calderon TM, Madrid-Aliste C, Fiser A, Weiss L, Angeletti RH, Berman JW. Buprenorphine Decreases the CCL2-Mediated Chemotactic Response of Monocytes. J Immunol. 2015;194:3246–3258. doi: 10.4049/jimmunol.1302647. http://www.jimmunol.org/cgi/doi/10.4049/jimmunol.1302647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Eugenin EA, Berman JW. Chemokine-dependent mechanisms of leukocyte trafficking across a model of the blood-brain barrier. Methods. 2003;29:351–361. doi: 10.1016/s1046-2023(02)00359-6. http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=12725802&retmode=ref&cmd=prlinks. [DOI] [PubMed] [Google Scholar]
- 55.Zembala M, Bach S, Szczepanek A, Mancino G, Colizzi V. Phenotypic changes of monocytes induced by HIV-1 gp120 molecule and its fragments. Immunobiology. 1997;197:110–21. doi: 10.1016/S0171-2985(97)80061-7. [DOI] [PubMed] [Google Scholar]
- 56.Nakamuta S, Endo H, Higashi Y, Kousaka A, Yamada H, Yano M, Kido H. Human immunodeficiency virus type 1 gp120-mediated disruption of tight junction proteins by induction of proteasome-mediated degradation of zonula occludens-1 and -2 in human brain microvascular endothelial cells. J Neurovirol. 2008;14:186–195. doi: 10.1080/13550280801993630. [DOI] [PubMed] [Google Scholar]
- 57.Thieblemont N, Weiss L, Sadeghi HM, Estcourt C, Haeffner-Cavaillon N. CD14lowCD16high: a cytokine-producing monocyte subset which expands during human immunodeficiency virus infection. Eur J Immunol. 1995;25:3418–3424. doi: 10.1002/eji.1830251232. [DOI] [PubMed] [Google Scholar]
- 58.Pulliam L, Gascon R, Stubblebine M, McGuire D, McGrath MS. Unique monocyte subset in patients with AIDS dementia. Lancet. 1997;349:692–5. doi: 10.1016/S0140-6736(96)10178-1. [DOI] [PubMed] [Google Scholar]
- 59.Johnson TP, Patel K, Johnson KR, Maric D, Calabresi PA, Hasbun R, Nath A. Induction of IL-17 and nonclassical T-cell activation by HIV-Tat protein. Proc Natl Acad Sci U S A. 2013;110:13588–13593. doi: 10.1073/pnas.1308673110. http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=23898208&retmode=ref&cmd=prlinks. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Karn J, Stoltzfus CM. Transcriptional and posttranscriptional regulation of HIV-1 gene expression. Cold Spring Harb Perspect Med. 2012;2:1–17. doi: 10.1101/cshperspect.a006916. [DOI] [PMC free article] [PubMed] [Google Scholar]