Abstract
Life style modifications and optimization of the management of cardio-metabolic comorbidities are currently the mainstay of treatment for patients with non-alcoholic fatty liver disease (NAFLD). Pharmacotherapy to halt or reverse hepatic histological injury and prevent the development of end stage liver disease is specifically offered to patients with non-alcoholic steatohepatitis (NASH) and those with advanced fibrosis. In this review, we will discuss state of the art of various pharmacological agents for NASH. The efficacy of vitamin E and pioglitazone is reasonably well established in a select group of patients with NASH. Current data do not offer convincing evidence for efficacy of pentoxifylline, long-chain polyunsaturated fatty acids, angiotensin receptor blockers, metformin or ursodeoxycholic acid. We also discuss the state of several emerging agents for treating NASH including the farsenoid X receptor (FXR) ligand, obeticholic acid.
Keywords: NAFLD, NASH, vitamin E, thiazolidinedione, Obeticholic acid
Introduction
End stage liver disease (ESLD) due to non-alcoholic steatohepatitis (NASH) is currently the second leading indication for liver transplantation in the U.S.(1). NASH is expected to replace hepatitis C as the leading indication for liver transplantation within the next decade (2) While the progression of non-alcoholic fatty liver (NAFL) to fibrosing NASH has been recently been described (3, 4), older literature based on long term follow up studies has shown that progression to ESLD or liver related death is rare in these patients (5–7). On the other hand, patients with NASH and particularly with underlying fibrosis are at increased risk for progression to cirrhosis, liver failure and hepatocellular carcinoma (HCC) (5, 8–10). Fibrosis, typically seen as part of NASH, has emerged in several studies as the strongest predictor of long term outcomes in patients with NASH (5, 8, 9, 11, 12). While life style modifications and optimization of coexisting cardio-metabolic comorbidities have been recommended to all patients with NAFLD, liver directed pharmacotherapy to stop or reverse histological injury and to prevent liver related events and death, has been specifically targeted toward patients with NASH (13, 14). In this paper, we review the current literature pertaining to the efficacy of various pharmacological agents in NASH. Some proof-of-principle studies that tested therapies in patient with NAFLD but did not include histological phenotyping, are also discussed. We also review emerging agents, including those with anti-fibrotic effects that are in various stages of development.
Drugs with established efficacy in NASH
Vitamin E
In animal models of NASH, vitamin E supplementation reduces hepatic inflammation and lipid peroxidation (15). In patients with NASH, vitamin E reduces circulating levels of malondialdehyde and transforming growth factor-β1 (16, 17). Further, down-regulation of the hedgehog pathway and loss of sonic hedgehog positive hepatocytes, which promote liver injury in NASH, have recently been described in patients demonstrating histological response to vitamin E (18).
Vitamin E been used alone or with other agents in multiple clinical trials to treat NASH or NAFLD, with reported in improvement in liver biochemistries and histology (16, 17, 19–26), These trials varied in duration (4 to 96 weeks) and dose (100–1200 IU/day) of vitamin E used (25, 27). Beneficial effects for vitamin E were demonstrated even in trials of short duration; improvement in ALT was reported after 4 weeks and in histology after 6 months of vitamin E therapy (17, 21, 25). The best evidence for vitamin E efficacy in NASH comes from the PIVENS trial (Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis) (23). In this study, 247 adults with biopsy-proven NASH were randomized to receive vitamin E (800 IU daily, 84 subjects), pioglitazone (30 mg daily,80 subjects),or placebo (83 subjects) for 96 weeks. The primary outcome was improvement in histology defined as improvement by ≥1 points in ballooning score, no increase in the fibrosis score, and either a decrease in the NAFLD activity score (NAS) to ≤3 points or a decrease in NAS of ≤2 points, with at least a 1 point decrease in either the lobular inflammation or steatosis score. A p < 0.025 indicated statistical significance. Vitamin E and pioglitazone significantly improved ALT, steatosis, and lobular inflammation but neither drug had a significant effect on hepatic fibrosis. Although significantly more patients achieved resolution of NASH only with pioglitazone but not vitamin E (47% vs 36% respectively vs 21% for placebo), significant improvement in hepatocyte ballooning and achievement of the study primary outcome were observed only with vitamin E (Figure 1). This trial did not include patients with diabetes or cirrhosis. Similar beneficial effects on NAFLD histology were observed for vitamin E children with histologically proven NAFLD included in the TONIC trial (Treatment of nonalcoholic fatty liver disease in children) (26). In that study, vitamin E but not metformin given for 96 weeks significantly reduced the ballooning grade and NAS. Significantly more children had histological resolution of NASH on vitamin E (58%) versus placebo (28%, p 0.006) but not with metformin (41%, p 0.2).
Figure. 1.
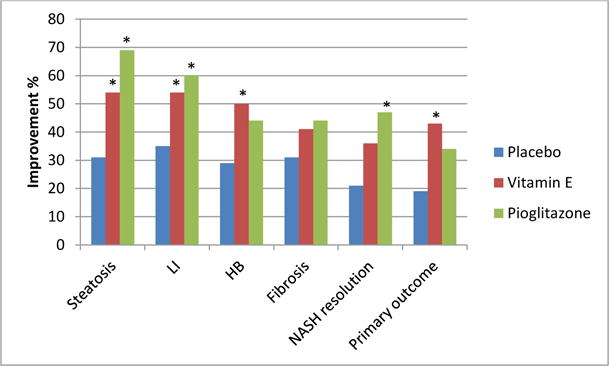
Effects of vitamin E and pioglitazone on liver histology in the PIVENS trial.
Footnote: * indicates statistical significance. LI: lobular inflammation, HB: hepatocellular ballooning. The primary outcome was improvement in histology defined as improvement by ≥1 points in ballooning score, no increase in the fibrosis score, and either a decrease in the NAS to ≤3 points or a decrease in NAS of ≤2 points, with at least a 1 point decrease in either the lobular inflammation or steatosis score.
There is continued debate about long term safety of vitamin E use and its possible association with increased mortality and prostate cancer (28–33). Despite these controversies, vitamin E at 800 IU daily is considered a first line therapy for patients with histologically confirmed NASH without cirrhosis or type 2 diabetes, according to the current multi-society practice guidelines (14).
Thiazolidinediones (TZDs)
Pioglitazone and rosiglitazone improve insulin sensitivity and adiponectin levels but reduce the levels of circulating resistin, tumor necrosis factor-α and free fatty acids (34–37). Both drugs exhibit a peroxisome proliferator-activated receptor-γ (PPAR-γ) agonistic effect but pioglitazone has also a PPAR-α agonistic effect (38).
Several studies have shown favorable effect for both drugs on NAFLD (17, 23, 39–47). As detailed above, the PIVENS trial established the efficacy of pioglitazone in improving NASH histology (Figure 1) (23). A recent meta-analysis of data from this study in addition to two prior trials of pioglitazone in NASH confirmed the improvement in steatosis, lobular inflammation, and hepatocyte ballooning (46–48). The pooled data also showed a significant improvement in fibrosis with pioglitazone (p 0.04). Prolonged use of pioglitazone may be necessary to maintain these effects. In one study, deterioration in ALT, HOMA, adiponectin, hepatic steatosis and inflammation were noted 48 weeks following pioglitazone discontinuation (49).
Another important issue is whether all or some metabolic and histological benefits are sustainable with continued TZDs use. Following an initial 1 year rosiglitazone placebo controlled trial, 2 year extension of rosiglitazone therapy in subjects who received it for a year prior did not result in further improvement in histology despite continued improvement in insulin sensitivity and liver transaminases (42). Eagerly awaited are the final results from a study conducted by Dr. Ken Cusi’s group which administered pioglitazone 45 mg/day for 18 months which presumably had a significant impact on steatosis, necroinflammation, and fibrosis.
Weight gain is the most common adverse event experienced by subjects with NASH during the TZD trials. Long term use of TZDs in patients with diabetes as raised safety concerns regarding the possible increase risk of congestive heart failure, bladder cancer, and bone fractures (50, 51). It has been suggested that pioglitazone may have a better cardiovascular profile than rosiglitazone presumably due to different effects on circulating lipids (52). Cautious use of pioglitazone is recommended by the current multi-society practice in non-diabetic NASH patients (14).
C. Drugs with equivocal efficacy in NASH
Metformin
Metformin exhibits several effects that result in increased insulin sensitivity: it inhibits hepatic gluconeogenesis, enhances peripheral tissue utilization of glucose, reduces circulating free fatty acids, and decreases food intake and body weight (53–55).
Despite early reports of favorable effect on liver transaminases and histology, other studies failed to confirm metformin effects on liver histology (26, 56–58). The best and largest study to examine the effect of metformin on NAFLD was the TONIC clinical trial (26). In this trial, 173 children (aged 8–17 years) with histologically proven NAFLD were randomized to receive metformin (1000 mg/day, n=57), vitamin E (800 IU/day, n=58), or placebo (n=58) for 96 weeks. The trial’s primary outcome, sustainable decrease in ALT, was not achieved in any group. In relation to liver histology, there was improvement in hepatocyte ballooning and NAS, and higher frequency of NASH resolution with both metformin and vitamin E, but these changes reached statistical significance only with vitamin E but not metformin therapy. Similarly, no effect for metformin on liver histology was noted in patients with NAFLD in a meta-analysis that pooled results from 4 studies (59).
Ursodeoxycholic acid (UDCA)
UDCA proposed mechanisms of action include altering bile acid pool, modulation of immune response, cell signaling and mitochondrial integrity in addition to potential anti-inflammatory and anti-apoptotic effects (60).
Contrary to the early reports of favorable effects of low (12–15mg/kg/day) and high (28–35 mg/kg/day) UDCA on liver biochemistries, steatosis and histology in NAFLD (22, 24, 61, 62), larger studies failed to validate these findings. Two large randomized studies in patients with biopsy-confirmed NASH showed no significant effects for UDCA on NASH histology with low (13–15 mg/kg/day for 2 years, n=166) or high dose (23–28 mg/kg/day for 1.5 years, n=185) UDCA (63, 64). Currently, there is no role for UDCA in patients with NASH.
Statins
Several small reports suggest that the HMG CoA reductase inhibitors, also known as statins, are safe when used in patients with NAFLD and have favorable effects on liver transaminases and hepatic steatosis on imaging (65–69). Improvement in NAFLD histology could not be consistently demonstrated in the small reported studies (70–72).
On the other hand, there is no evidence for increased hepatotoxicity when statins are used to treat dyslipidemia in patients with NAFLD or other chronic liver diseases (73–75). In a large study of dyslipidemic patients with elevated baseline liver transaminases who did (n=1342) or did not (n=2245) received statins, there was no difference in the incidence of hepatotoxicity over a 6 months follow up(74). The post-hoc analysis of the GREACE study evaluated 437 patients with increased liver biochemistry tests at baseline presumably due to NAFLD. The 227 who were treated with a statin (88% received atorvastatin) had substantial improvement in liver tests (p<0·0001) compared to the remaining 210 subjects whose liver tests did not improve. (73). The incidence of cardiovascular events was significantly lower compared to those with elevated liver tests who did not receive a statin (10% vs 30%, p<0.0001). The discontinuation of the statin due to rise in the transaminases to more than three-times the upper limit of normal per study protocol was reported in less than 1% of study subjects.
Fibrates
Fibrates activation of PPAR-α increases hepatic fatty acid oxidation and reduce hepatic triglyceride synthesis and VLDL production and export (76). The results of small studies yielded inconsistent effects of fibrates on NAFLD on ALT (61, 77, 78). Despite decreasing plasma triglycerides and VLDL, there was no effect for fenofibrate on hepatic fat content in a short 8 weeks study (79). Data on fibrates effect on liver histology in NAFLD are also conflicting, with one study showing improvement only in ballooning while another study showed no effect on histology (61, 78).
Long-chain polyunsaturated fatty acids (LC-PUFA)
LC-PUFA in the n−3 (ω-3) series including docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), are abundant in fish and fish oil supplements. LC-PUFA reduce triglycerides, adipose tissue inflammation, endothelial dysfunction, and increase HDL, adiponectin and insulin sensitivity (80–82). LC-PUFA desaturation is altered in rodent and human NASH with an increase in the pro-inflammatory (ω-6 pathway) and decrease in the anti-inflammatory (ω-3) pathways (83).
Several studies reported improvement of ALT and steatosis with LC-PUFA (84–89). The effect on hepatic steatosis but not ALT was corroborated in a meta-analysis of data pooled from these studies (90). In the WELCOME study 103 subjects with NAFLD were randomized to receive 15–18 months treatment with DHA+EPA (4 gm/day) (91). No significant decrease in hepatic fat content or serum markers of fibrosis was noted. Erythrocytes enrichment with DHA correlated with decreased hepatic fat content. No effects on ALT, liver histology or serum levels of Keratin-18, hyaluronic acid, C-reactive protein or insulin resistance were observed in a recent large multicenter trial that randomized 243 subjects with biopsy proven NASH to placebo, EPA 1800 mg/day, or EPA 2700 mg/day for 12 months (92). In another study from the United States which consisted of 41 non-cirrhotic NASH patients, n-3 fish oil administered for one year offered no histological benefit (93). Currently, there are no data supporting the use of fish oil to improve liver histology in patients with NASH.
Angiotensin antagonists
The renin-angiotensin system modulates hepatic stellate cells activation and fibrogenesis (94–96). There is emerging data to suggest that blocking this system may have an effect on NAFLD histology and particularly fibrosis. For example, the use of angiotensin-converting-enzyme inhibitors (ACEI) or angiotensin-receptor blockers (ARB) for treatment of hypertension in patients with NAFLD was associated decreased hepatic fibrosis and ballooning in a recent small retrospective study (97). In another study, 150 patients with NAFLD were randomized to receive either losartan or amlodipine for 6 months followed by simvastatin (98). Significant reduction in ultrasound measured hepatic steatosis, visceral adipose tissue, and insulin resistance were observed with Losartan compared to amlodipine. Addition of simvastatin enhanced these effects. Treatment of 54 patients with NASH and hypertension for 20 months with valsartan or telmisartan improved ALT, HOMA and steatosis. Significant improvement in lobular inflammation, ballooning, NAS and fibrosis and was only seen with telmisartan (99). Although these results are encouraging, to date there have not been rigorously conducted randomized controlled trials of sufficiently length and histological end points to definitely define the role of ACEI and ARBs in NASH.
Pentoxifylline
Based on its possible favorable effects on tumor necrosis factor-α, hepatic glutathione and hepatic inflammation (100–102), several studies have tested pentoxifylline as a potential therapy for NASH. Initial studies reported improvement in ALT as a treatment for NASH in a few small studies. Earlier reports suggested reduction of ALT and TNF-α (103, 104). Two small studies subsequently reported improvement in NASH histology with pentoxifylline (102, 105). However, these effects could not be demonstrated in another study in which there was no show significant improvement in NASH histology with pentoxifylline compared to placebo (106).
E. Emerging pharmacologic agents for NASH
Obeticholic acid (OCA)
OCA is a selective agonist of the bile acid nuclear receptor farnesoid X receptor (FXR). FXR biological effects include regulation of bile acids synthesis and transport, lipid and glucose homeostasis and hepatic inflammation (107, 108). In the first pilot human trial, 64 patients with NAFLD and type 2 diabetes mellitus were randomized to receive placebo, OCA at 25 mg, or OCA at 50 mg orally once daily for 6 weeks (109). OCA therapy resulted in improved insulin sensitivity, ALT and serum markers of fibrosis in addition to resulting in weight loss. Subsequently, OCA was studied in a large randomized clinical trial (FLINT) that recruited 283 subjects with biopsy proven non-cirrhotic NASH (110). Subjects were randomized to receive OCA 25 mg orally daily or matching placebo for 72 weeks. Type 2 diabetes was present in almost half of the study population. The primary outcome of this study was improvement in liver histology defined as a decrease in NAS by at least 2 points without worsening of fibrosis from baseline to the end of treatment. The trial’s design was modified midway after a planned interim analysis showed significant improvement in liver histology in subjects receiving OCA. As a result, it was deemed unnecessary to perform end of therapy liver biopsy on the last 64 subjects. Improvement in steatosis, lobular inflammation, ballooning and fibrosis was observed significantly more with OCA versus placebo (Figure 2). A significantly higher number of subjects achieved the primary study outcome on OCA as compared to placebo (45% versus 21%, relative risk 1.9, 95% CI 1.3 to 2.8; p=0·0002). Resolution of NASH was observed more frequently with OCA than placebo (22 versus 13%), but this did not reach statistical significance (p=0.08) (Figure 2).
Figure 2.
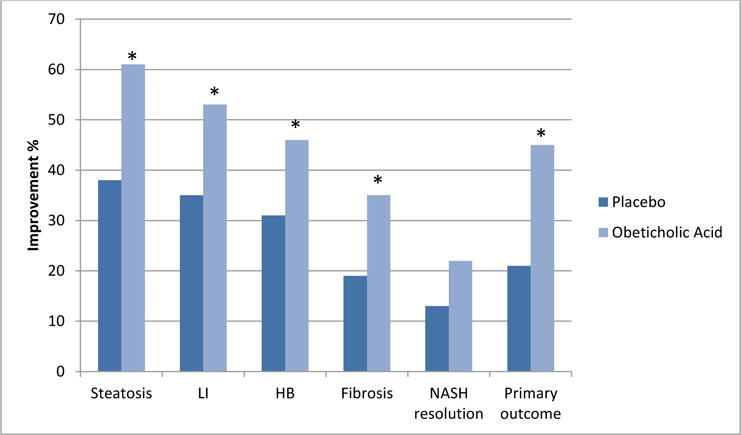
Effects of obeticholic acid on liver histology in the FLINT trial.
Footnote: * indicates statistical significance. LI: lobular inflammation, HB: hepatocellular ballooning. The primary outcome was an improvement in histology defined as a decrease of ≥2 points in NAS and no worsening in the fibrosis score;
The most common adverse event with OCA therapy was pruritus, which was reported in 23% on OCA versus 6% on placebo. In addition, a decrease in HDL and increase in total and LDL cholesterol were observed with OCA at 12 weeks of therapy. But these changes improved with therapy and resolved after stopping OCA. Patients receiving OCA also had a mild increase in alkaline phosphatase, similar to the prior pilot trial. Five severe or life threatening adverse events that were deemed related to OCA including possible cerebral ischemia (n=1), severe pruritus (n=3), and hyperglycemia (n=1). Two deaths in subjects receiving OCA were deemed not related to OCA; one from myocardial ischemia and another from sepsis and heart failure. OCA therapy was associated with a mean weight loss of 2.3 kg compared to no weight loss with placebo. Studies confirming the efficacy and demonstrating long term safety of OCA in NASH are being planned.
Fibroblast growth factor 21
Fibroblast growth factor 21 (FGF21) is regulator of metabolism and energy homeostasis (111, 112). FGF21 administration improves hepatic steatosis, inflammation and fibrosis in murine models of NAFLD and NASH (113–115)., FGF21 administration resulted in improvements in hepatic necroinflammation and fibrosis, insulin sensitivity, and post-prandial lipidemia n Ossabaw miniature swine with diet induced NASH (116). Authors are aware of two early phase clinical trials planned by Pharma in the United States in 2015–2016. One difficulty with these agents is their parenteral route of administration.
Exenatide and liraglutide
Exenatide is a synthetic glucagon-like peptide-1 (GLP-1) agonist with regulatory effects on post-prandial insulin secretion and glucose metabolism (117). It reduces free fatty acid induced endoplasmic reticulum stress and apoptosis (118), and improves hepatic steatosis in patients with type 2 diabetes by improving sensitivity to fibroblast growth factor 21 (119–121). An open label study of 8 patients with diabetes and biopsy proven NASH evaluated the effects of 28 weeks of exenatide on liver histology (122). There was improvement in NASH histological lesions and NAS redyction in 5 subjects. Fibrosis improved in 4 subjects (by 1 stage in 3 subjects and by 2 stages in one subject). There is currently one registered trial (NCT02303730) that aims to test the effects of exenatide on hepatic fat content in Chinese patients with type 2 diabetes in comparison to insulin glargine.
Recent data offer encouraging results for liraglutide, another GLP-1 agonist. A small single arm, open-label Japanese study (LEAN-J) showed some histological benefit with 96 weeks of liraglutide treatment in 10 patients with NASH who had paired liver biopsies (X). At the most recent International Liver Congress (Vienna 2015), Dr. Phil Newsome’s group presented the final results of their LEAN trial which consisted of 52 patients with biopsy-proven NASH and randomized participants to receive either 1.8 mg/day of liraglutide subcutaneously or placebo. The primary end of this trial, resolution of NASH with no worsening of fibrosis, was observed in 39% of patients receiving liraglutide as compared to 9% in the placebo group (x). Full results of this study as a peer reviewed manuscript are awaited.
Cysteamine Bitartrate
Cysteamine is a glutathione precursor that is more effective at crossing cellular membranes than glutathione. It has a protective effect against acetaminophen-induced liver injury in humans (123, 124). Enteric coated cysteamine given for 24 weeks to 13 children with biopsy proven NAFLD and elevated ALT (125), resulted in normalization or significant > 50% reduction in ALT in 7 children associated with an increase in mean serum adiponectin level and a decrease in keratin-18 levels. There is currently an ongoing clinical trial of in children with biopsy proven NAFLD (NCT01529268) to evaluate the effects of 3 doses of cysteamine given for 52 weeks on NAFLD histology.
Simtuzumab
Lysyl oxidases (LOX) are a family of extracellular matrix crosslinking enzymes involved in crosslinking collagen and elastin. Simtuzumab is a humanized monoclonal antibody to LOX like (LOXL) 2 (126). There are currently two clinical trials in patients with NASH and bridging fibrosis (NCT01672866) or cirrhosis (NCT01672879) evaluating simtuzumab’s safety and effects on hepatic venous pressure gradient, hepatic fibrosis, and overall and hepatic events free survival.
GR-MD-02
Galactin 3 protein is important in hepatic fibrogenesis. GR-MD-02 is a galactin 3 inhibitor that improved fibrosis and portal hypertension in toxin-induced cirrhosis, and resulted in regression of fibrosis in a murine model of NASH with fibrosis (127, 128). In a phase 1 trial in patients with NASH and bridging fibrosis,GR-MD-02 improved serum markers of fibrosis (FibroTest® and Keratin-18) and inflammatory markers (tumor necrosis factor-alpha and interleukin-6 and 8) in studied subjects (129). There no safety issues reported. A multicenter, phase 2 study of GR-MD-02 in patients with NASH cirrhosis is underway in the United States.
Cenicriviroc
Cenicriviroc (CVC) is an oral inhibitor of the C-C chemokine receptors (CCR) 2 and 5, which are involved in macrophage recruitment to the liver. It improves hepatic fibrosis and necroinflammation in a murine model of diet and streptozotocin induced NASHt (130). A phase 2 clinical trial (NCT02217475) that is currently evaluating the safety and efficacy of this agent in improving histology in patients with NASH and fibrosis but without cirrhosis.
Aramchol
Aramchol is a synthetic lipid molecule resulting from the conjugation of arachidic acid (saturated fatty acid) and cholic acid (bile acid). In rodent models of diet induced NAFLD, it suppresses stearoyl coenzyme Adesaturase 1 (SCD1) activity and improves hepatic steatosis(131, 132). In a recent controlled trial (133), 60 subjects with biopsy proven NAFLD were randomized to receive aramchol 100 mg or 300 mg daily versus placebo for 3 months. Significant reduction in hepatic fat content as measured by magnetic resonance spectroscopy was noted in the 300 mg group compared to placebo. No significant improvement in ALT, insulin sensitivity or endothelial function was observed. There is currently an ongoing clinical trial (NCT02279524) in pre-diabetic or diabetic patients with biopsy proven NASH to investigate the effect of 52 weeks of Aramchol therapy (400 mg or 600 mg once daily) on liver fat content and histology.
GFT505
GFT505 is a dual PPAR α and δ agonist that improves insulin sensitivity and exerts favorable effects on circulating lipids. Animal studies showed favorable effects for GFT505 on NASH histology (134, 135). It improved hepatic steatosis, fibrosis, and inflammation in different models of NASH and hepatic fibrosis in rodents (136). GFT505 has also been shown to result in improvement in liver enzymes in subjects with the metabolic syndrome in phase II trial (136). A phase2 multicenter randomized clinical trial (GOLDEN-505, NCT01694849) was recently completed and its promising preliminary results became available through the company’s press release (x) but full results are not yet available to the scientific community.
E. Conclusions
NAFLD importance as a clinical entity is continuing to increase and is resulting in increased utilization of liver transplantation. In addition to exercise and weight loss, vitamin E and pioglitazone have demonstrated efficacy in improving NASH histology in non-diabetic, non-cirrhotic patients. This is an exciting time indeed in the field with multiple emerging promising pharmacologic therapies for NASH that are aimed not only at improving fibrosis but also underlying disturbed metabolic pathways. The results of these clinical trials are anxiously awaited to fill in the many unmet needs that exist in NASH therapy such as treatment of the patients with diabetes or those with cirrhosis.
Table 1.
Emerging therapeutic agents for NASH
| Agent | Putative mechanism/s | Effects on NAFLD | Studies in human NAFLD |
|---|---|---|---|
| Obeticholic acid | FXR ligand Improves glucose and lipid metabolism | Improves histology including fibrosis | Yes |
| Fibroblast growth factor 21 | Regulates glucose and lipid metabolism | Improves histology in animal models including fibrosis | Not known |
| Exenatide and Liraglutide | GLP-1 agonist Regulates glucose metabolism and post-prandial insulin sectretion | Improves histology including fibrosis | Yes. Recently presented liraglutide data offer exciting results. |
| Cysteamine Bitartrate | glutathione precursor Antioxidant | Improves ALT and keratin-18 | Yes, Ongoing clinical trial |
| Simtuzumab | LOXL2 antagonist Antifibrotic | Unknown | Yes, Ongoing clinical trial |
| GR-MD-02 | galactin 3 inhibitor | Improves fibrosis in murine models | Yes, Ongoing clinical trial |
| Cenicriviroc | inhibitor of CCR 2 and 5 | Improves hepatic inflammation and fibrosis on murine models | Yes, Ongoing clinical trial |
| Aramchol | fatty acid–bile acid conjugate Inhibits stearoyl coenzyme A desaturase 1 | Decreased hepatic fat content | Yes, Ongoing clinical trial |
| GFT505 | PPAR α and δ agonist Imporves insulin sensitivity and lipid metabolism | Improves hepatic steatosis, inflammation and fibrosis in murine models | Yes, the scientific results of the GOLDEN 505 trial are awaited |
Acknowledgments
Authors acknowledge that there may be inevitable similarities between this review article and other review articles/text book chapters written by them on the same topic.
Drs. Chalasani and Gawrieh participate in multicenter clinical trials sponsored by various pharma in NASH. These clinical trials provide research grant support of their institution with no personal income.
Footnotes
Disclosures
Dr. Chalasani serves as a consultant to many pharmaceutical companies for both NASH and drug hepatotoxicity but none of them represent significant and direct conflict with this review article. His potential conflicts of interests are duly declared to his institution and there is a plan in place to manage his outside consulting interests vis a vis his research activities.
References
- 1.Wong RJ, Aguilar M, Cheung R, Perumpail RB, Harrison SA, Younossi ZM, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2015;148(3):547–55. doi: 10.1053/j.gastro.2014.11.039. [DOI] [PubMed] [Google Scholar]
- 2.Charlton MR, Burns JM, Pedersen RA, Watt KD, Heimbach JK, Dierkhising RA. Frequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United States. Gastroenterology. 2011;141(4):1249–53. doi: 10.1053/j.gastro.2011.06.061. [DOI] [PubMed] [Google Scholar]
- 3.Pais R, Charlotte F, Fedchuk L, Bedossa P, Lebray P, Poynard T, et al. A systematic review of follow-up biopsies reveals disease progression in patients with non-alcoholic fatty liver. J Hepatol. 2013;59(3):550–6. doi: 10.1016/j.jhep.2013.04.027. [DOI] [PubMed] [Google Scholar]
- 4.McPherson S, Hardy T, Henderson E, Burt AD, Day CP, Anstee QM. Evidence of NAFLD progression from steatosis to fibrosing-steatohepatitis using paired biopsies: Implications for prognosis and clinical management. J Hepatol. 2015;62(5):1148–55. doi: 10.1016/j.jhep.2014.11.034. [DOI] [PubMed] [Google Scholar]
- 5.Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116(6):1413–9. doi: 10.1016/s0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]
- 6.Teli MR, James OF, Burt AD, Bennett MK, Day CP. The natural history of nonalcoholic fatty liver: a follow-up study. Hepatology. 1995;22(6):1714–9. [PubMed] [Google Scholar]
- 7.Dam-Larsen S, Becker U, Franzmann MB, Larsen K, Christoffersen P, Bendtsen F. Final results of a long-term, clinical follow-up in fatty liver patients. Scand J Gastroenterol. 2009;44(10):1236–43. doi: 10.1080/00365520903171284. [DOI] [PubMed] [Google Scholar]
- 8.Ekstedt M, Hagstrom H, Nasr P, Fredrikson M, Stal P, Kechagias S, et al. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology. 2015;61(5):1547–54. doi: 10.1002/hep.27368. [DOI] [PubMed] [Google Scholar]
- 9.Ekstedt M, Franzen LE, Mathiesen UL, Thorelius L, Holmqvist M, Bodemar G, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44(4):865–73. doi: 10.1002/hep.21327. [DOI] [PubMed] [Google Scholar]
- 10.Angulo P, Machado MV, Diehl AM. Fibrosis in nonalcoholic Fatty liver disease: mechanisms and clinical implications. Semin Liver Dis. 2015;35(2):132–45. doi: 10.1055/s-0035-1550065. [DOI] [PubMed] [Google Scholar]
- 11.Younossi ZM, Stepanova M, Rafiq N, Makhlouf H, Younoszai Z, Agrawal R, et al. Pathologic criteria for nonalcoholic steatohepatitis: interprotocol agreement and ability to predict liver-related mortality. Hepatology (Baltimore, Md) 2011;53(6):1874–82. doi: 10.1002/hep.24268. [DOI] [PubMed] [Google Scholar]
- 12.Angulo P, Kleiner DE, Dam-Larsen S, Adams LA, Bjornsson ES, Charatcharoenwitthaya P, et al. Liver Fibrosis, but no Other Histologic Features, Associates with Long-term Outcomes of Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology. 2015 doi: 10.1053/j.gastro.2015.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sanyal AJ, Brunt EM, Kleiner DE, Kowdley KV, Chalasani N, Lavine JE, et al. Endpoints and clinical trial design for nonalcoholic steatohepatitis. Hepatology. 2011;54(1):344–53. doi: 10.1002/hep.24376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology. 2012;142(7):1592–609. doi: 10.1053/j.gastro.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 15.Chung MY, Yeung SF, Park HJ, Volek JS, Bruno RS. Dietary alpha- and gamma-tocopherol supplementation attenuates lipopolysaccharide-induced oxidative stress and inflammatory-related responses in an obese mouse model of nonalcoholic steatohepatitis. The Journal of nutritional biochemistry. 2010;21(12):1200–6. doi: 10.1016/j.jnutbio.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 16.Hasegawa T, Yoneda M, Nakamura K, Makino I, Terano A. Plasma transforming growth factor-beta1 level and efficacy of alpha-tocopherol in patients with non-alcoholic steatohepatitis: a pilot study. Aliment Pharmacol Ther. 2001;15(10):1667–72. doi: 10.1046/j.1365-2036.2001.01083.x. [DOI] [PubMed] [Google Scholar]
- 17.Sanyal AJ, Mofrad PS, Contos MJ, Sargeant C, Luketic VA, Sterling RK, et al. A pilot study of vitamin E versus vitamin E and pioglitazone for the treatment of nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol. 2004;2(12):1107–15. doi: 10.1016/s1542-3565(04)00457-4. [DOI] [PubMed] [Google Scholar]
- 18.Guy CD, Suzuki A, Abdelmalek MF, Burchette JL, Diehl AM. Treatment response in the PIVENS trial is associated with decreased Hedgehog pathway activity. Hepatology. 2015;61(1):98–107. doi: 10.1002/hep.27235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harrison SA, Torgerson S, Hayashi P, Ward J, Schenker S. Vitamin E and vitamin C treatment improves fibrosis in patients with nonalcoholic steatohepatitis. Am J Gastroenterol. 2003;98(11):2485–90. doi: 10.1111/j.1572-0241.2003.08699.x. [DOI] [PubMed] [Google Scholar]
- 20.Kugelmas M, Hill DB, Vivian B, Marsano L, McClain CJ. Cytokines and NASH: a pilot study of the effects of lifestyle modification and vitamin E. Hepatology. 2003;38(2):413–9. doi: 10.1053/jhep.2003.50316. [DOI] [PubMed] [Google Scholar]
- 21.Yakaryilmaz F, Guliter S, Ozenirler S, Erdem O, Akyol G. Vitamin E treatment in patients with nonalcoholic steatohepatitis: A six-month, open-label study of sixteen patients. Current therapeutic research, clinical and experimental. 2004;65(3):266–77. doi: 10.1016/S0011-393X(04)80077-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dufour JF, Oneta CM, Gonvers JJ, Bihl F, Cerny A, Cereda JM, et al. Randomized placebo-controlled trial of ursodeoxycholic Acid with vitamin e in nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol. 2006;4(12):1537–43. doi: 10.1016/j.cgh.2006.09.025. [DOI] [PubMed] [Google Scholar]
- 23.Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, et al. Pioglitazone, Vitamin E, or Placebo for Nonalcoholic Steatohepatitis. The New England journal of medicine. 2010 doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pietu F, Guillaud O, Walter T, Vallin M, Hervieu V, Scoazec JY, et al. Ursodeoxycholic acid with vitamin E in patients with nonalcoholic steatohepatitis: long-term results. Clinics and research in hepatology and gastroenterology. 2012;36(2):146–55. doi: 10.1016/j.clinre.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 25.Wang CL, Liang L, Fu JF, Zou CC, Hong F, Xue JZ, et al. Effect of lifestyle intervention on non-alcoholic fatty liver disease in Chinese obese children. World J Gastroenterol. 2008;14(10):1598–602. doi: 10.3748/wjg.14.1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lavine JE, Schwimmer JB, Van Natta ML, Molleston JP, Murray KF, Rosenthal P, et al. Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents: the TONIC randomized controlled trial. JAMA. 2011;305(16):1659–68. doi: 10.1001/jama.2011.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pacana T, Sanyal AJ. Vitamin E and nonalcoholic fatty liver disease. Current opinion in clinical nutrition and metabolic care. 2012;15(6):641–8. doi: 10.1097/MCO.0b013e328357f747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berry D, Wathen JK, Newell M. Bayesian model averaging in meta-analysis: vitamin E supplementation and mortality. Clinical trials. 2009;6(1):28–41. doi: 10.1177/1740774508101279. [DOI] [PubMed] [Google Scholar]
- 29.Dietrich M, Jacques PF, Pencina MJ, Lanier K, Keyes MJ, Kaur G, et al. Vitamin E supplement use and the incidence of cardiovascular disease and all-cause mortality in the Framingham Heart Study: Does the underlying health status play a role? Atherosclerosis. 2009;205(2):549–53. doi: 10.1016/j.atherosclerosis.2008.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller ER, 3rd, Pastor-Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2005;142(1):37–46. doi: 10.7326/0003-4819-142-1-200501040-00110. [DOI] [PubMed] [Google Scholar]
- 31.Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA. 2007;297(8):842–57. doi: 10.1001/jama.297.8.842. [DOI] [PubMed] [Google Scholar]
- 32.Gerss J, Kopcke W. The questionable association of vitamin E supplementation and mortality–inconsistent results of different meta-analytic approaches. Cellular and molecular biology. 2009;55(Suppl):OL1111–20. [PubMed] [Google Scholar]
- 33.Klein EA, Thompson IM, Jr, Tangen CM, Crowley JJ, Lucia MS, Goodman PJ, et al. Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2011;306(14):1549–56. doi: 10.1001/jama.2011.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yki-Jarvinen H. Thiazolidinediones. N Engl J Med. 2004;351(11):1106–18. doi: 10.1056/NEJMra041001. [DOI] [PubMed] [Google Scholar]
- 35.Sharma AM, Staels B. Review: Peroxisome proliferator-activated receptor gamma and adipose tissue–understanding obesity-related changes in regulation of lipid and glucose metabolism. The Journal of clinical endocrinology and metabolism. 2007;92(2):386–95. doi: 10.1210/jc.2006-1268. [DOI] [PubMed] [Google Scholar]
- 36.Libby P, Plutzky J. Inflammation in diabetes mellitus: role of peroxisome proliferator-activated receptor-alpha and peroxisome proliferator-activated receptor-gamma agonists. Am J Cardiol. 2007;99(4a):27b–40b. doi: 10.1016/j.amjcard.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 37.Lee CH, Olson P, Evans RM. Minireview: lipid metabolism, metabolic diseases, and peroxisome proliferator-activated receptors. Endocrinology. 2003;144(6):2201–7. doi: 10.1210/en.2003-0288. [DOI] [PubMed] [Google Scholar]
- 38.Orasanu G, Ziouzenkova O, Devchand PR, Nehra V, Hamdy O, Horton ES, et al. The peroxisome proliferator-activated receptor-gamma agonist pioglitazone represses inflammation in a peroxisome proliferator-activated receptor-alpha-dependent manner in vitro and in vivo in mice. Journal of the American College of Cardiology. 2008;52(10):869–81. doi: 10.1016/j.jacc.2008.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Neuschwander-Tetri BA, Brunt EM, Wehmeier KR, Oliver D, Bacon BR. Improved nonalcoholic steatohepatitis after 48 weeks of treatment with the PPAR-gamma ligand rosiglitazone. Hepatology. 2003;38(4):1008–17. doi: 10.1053/jhep.2003.50420. [DOI] [PubMed] [Google Scholar]
- 40.Tiikkainen M, Hakkinen AM, Korsheninnikova E, Nyman T, Makimattila S, Yki-Jarvinen H. Effects of rosiglitazone and metformin on liver fat content, hepatic insulin resistance, insulin clearance, and gene expression in adipose tissue in patients with type 2 diabetes. Diabetes. 2004;53(8):2169–76. doi: 10.2337/diabetes.53.8.2169. [DOI] [PubMed] [Google Scholar]
- 41.Ratziu V, Giral P, Jacqueminet S, Charlotte F, Hartemann-Heurtier A, Serfaty L, et al. Rosiglitazone for nonalcoholic steatohepatitis: one-year results of the randomized placebo-controlled Fatty Liver Improvement with Rosiglitazone Therapy (FLIRT) Trial. Gastroenterology. 2008;135(1):100–10. doi: 10.1053/j.gastro.2008.03.078. [DOI] [PubMed] [Google Scholar]
- 42.Ratziu V, Charlotte F, Bernhardt C, Giral P, Halbron M, Lenaour G, et al. Long-term efficacy of rosiglitazone in nonalcoholic steatohepatitis: results of the fatty liver improvement by rosiglitazone therapy (FLIRT 2) extension trial. Hepatology. 2010;51(2):445–53. doi: 10.1002/hep.23270. [DOI] [PubMed] [Google Scholar]
- 43.Omer Z, Cetinkalp S, Akyildiz M, Yilmaz F, Batur Y, Yilmaz C, et al. Efficacy of insulin-sensitizing agents in nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol. 2010;22(1):18–23. doi: 10.1097/MEG.0b013e32832e2baf. [DOI] [PubMed] [Google Scholar]
- 44.Torres DM, Jones FJ, Shaw JC, Williams CD, Ward JA, Harrison SA. Rosiglitazone versus rosiglitazone and metformin versus rosiglitazone and losartan in the treatment of nonalcoholic steatohepatitis in humans: a 12-month randomized, prospective, open-label trial. Hepatology. 2011;54(5):1631–9. doi: 10.1002/hep.24558. [DOI] [PubMed] [Google Scholar]
- 45.Promrat K, Lutchman G, Uwaifo GI, Freedman RJ, Soza A, Heller T, et al. A pilot study of pioglitazone treatment for nonalcoholic steatohepatitis. Hepatology. 2004;39(1):188–96. doi: 10.1002/hep.20012. [DOI] [PubMed] [Google Scholar]
- 46.Belfort R, Harrison SA, Brown K, Darland C, Finch J, Hardies J, et al. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med. 2006;355(22):2297–307. doi: 10.1056/NEJMoa060326. [DOI] [PubMed] [Google Scholar]
- 47.Aithal GP, Thomas JA, Kaye PV, Lawson A, Ryder SD, Spendlove I, et al. Randomized, placebo-controlled trial of pioglitazone in nondiabetic subjects with nonalcoholic steatohepatitis. Gastroenterology. 2008;135(4):1176–84. doi: 10.1053/j.gastro.2008.06.047. [DOI] [PubMed] [Google Scholar]
- 48.Boettcher E, Csako G, Pucino F, Wesley R, Loomba R. Meta-analysis: pioglitazone improves liver histology and fibrosis in patients with non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2012;35(1):66–75. doi: 10.1111/j.1365-2036.2011.04912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lutchman G, Modi A, Kleiner DE, Promrat K, Heller T, Ghany M, et al. The effects of discontinuing pioglitazone in patients with nonalcoholic steatohepatitis. Hepatology. 2007;46(2):424–9. doi: 10.1002/hep.21661. [DOI] [PubMed] [Google Scholar]
- 50.Azoulay L, Yin H, Filion KB, Assayag J, Majdan A, Pollak MN, et al. The use of pioglitazone and the risk of bladder cancer in people with type 2 diabetes: nested case-control study. BMJ (Clinical research ed) 2012;344:e3645. doi: 10.1136/bmj.e3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bennett WL, Maruthur NM, Singh S, Segal JB, Wilson LM, Chatterjee R, et al. Comparative effectiveness and safety of medications for type 2 diabetes: an update including new drugs and 2-drug combinations. Ann Intern Med. 2011;154(9):602–13. doi: 10.7326/0003-4819-154-9-201105030-00336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lincoff AM, Wolski K, Nicholls SJ, Nissen SE. Pioglitazone and risk of cardiovascular events in patients with type 2 diabetes mellitus: a meta-analysis of randomized trials. Jama. 2007;298(10):1180–8. doi: 10.1001/jama.298.10.1180. [DOI] [PubMed] [Google Scholar]
- 53.DeFronzo RA, Barzilai N, Simonson DC. Mechanism of metformin action in obese and lean noninsulin-dependent diabetic subjects. J Clin Endocrinol Metab. 1991;73(6):1294–301. doi: 10.1210/jcem-73-6-1294. [DOI] [PubMed] [Google Scholar]
- 54.Glueck CJ, Fontaine RN, Wang P, Subbiah MT, Weber K, Illig E, et al. Metformin reduces weight, centripetal obesity, insulin, leptin, and low-density lipoprotein cholesterol in nondiabetic, morbidly obese subjects with body mass index greater than 30. Metabolism. 2001;50(7):856–61. doi: 10.1053/meta.2001.24192. [DOI] [PubMed] [Google Scholar]
- 55.Pernicova I, Korbonits M. Metformin–mode of action and clinical implications for diabetes and cancer. Nature reviews Endocrinology. 2014;10(3):143–56. doi: 10.1038/nrendo.2013.256. [DOI] [PubMed] [Google Scholar]
- 56.Haukeland JW, Konopski Z, Eggesbo HB, von Volkmann HL, Raschpichler G, Bjoro K, et al. Metformin in patients with non-alcoholic fatty liver disease: a randomized, controlled trial. Scand J Gastroenterol. 2009;44(7):853–60. doi: 10.1080/00365520902845268. [DOI] [PubMed] [Google Scholar]
- 57.Nair S, Diehl AM, Wiseman M, H FG, Jr, Perrillo RP. Metformin in the treatment of non-alcoholic steatohepatitis: a pilot open label trial. Aliment Pharmacol Ther. 2004;20(1):23–8. doi: 10.1111/j.1365-2036.2004.02025.x. [DOI] [PubMed] [Google Scholar]
- 58.Shields WW, Thompson KE, Grice GA, Harrison SA, Coyle WJ. The Effect of Metformin and Standard Therapy versus Standard Therapy alone in Nondiabetic Patients with Insulin Resistance and Nonalcoholic Steatohepatitis (NASH): A Pilot Trial. Therapeutic advances in gastroenterology. 2009;2(3):157–63. doi: 10.1177/1756283X09105462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rakoski MO, Singal AG, Rogers MA, Conjeevaram H. Meta-analysis: insulin sensitizers for the treatment of non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2010;32(10):1211–21. doi: 10.1111/j.1365-2036.2010.04467.x. [DOI] [PubMed] [Google Scholar]
- 60.Lazaridis KN, Gores GJ, Lindor KD. Ursodeoxycholic acid ‘mechanisms of action and clinical use in hepatobiliary disorders’. J Hepatol. 2001;35(1):134–46. doi: 10.1016/s0168-8278(01)00092-7. [DOI] [PubMed] [Google Scholar]
- 61.Laurin J, Lindor KD, Crippin JS, Gossard A, Gores GJ, Ludwig J, et al. Ursodeoxycholic acid or clofibrate in the treatment of non-alcohol-induced steatohepatitis: a pilot study. Hepatology. 1996;23(6):1464–7. doi: 10.1002/hep.510230624. [DOI] [PubMed] [Google Scholar]
- 62.Ratziu V, de Ledinghen V, Oberti F, Mathurin P, Wartelle-Bladou C, Renou C, et al. A randomized controlled trial of high-dose ursodesoxycholic acid for nonalcoholic steatohepatitis. J Hepatol. 2011;54(5):1011–9. doi: 10.1016/j.jhep.2010.08.030. [DOI] [PubMed] [Google Scholar]
- 63.Leuschner UF, Lindenthal B, Herrmann G, Arnold JC, Rossle M, Cordes HJ, et al. High-dose ursodeoxycholic acid therapy for nonalcoholic steatohepatitis: a double-blind, randomized, placebo-controlled trial. Hepatology. 2010;52(2):472–9. doi: 10.1002/hep.23727. [DOI] [PubMed] [Google Scholar]
- 64.Lindor KD, Kowdley KV, Heathcote EJ, Harrison ME, Jorgensen R, Angulo P, et al. Ursodeoxycholic acid for treatment of nonalcoholic steatohepatitis: results of a randomized trial. Hepatology. 2004;39(3):770–8. doi: 10.1002/hep.20092. [DOI] [PubMed] [Google Scholar]
- 65.Athyros VG, Mikhailidis DP, Didangelos TP, Giouleme OI, Liberopoulos EN, Karagiannis A, et al. Effect of multifactorial treatment on non-alcoholic fatty liver disease in metabolic syndrome: a randomised study. Current medical research and opinion. 2006;22(5):873–83. doi: 10.1185/030079906X104696. [DOI] [PubMed] [Google Scholar]
- 66.Han KH, Rha SW, Kang HJ, Bae JW, Choi BJ, Choi SY, et al. Evaluation of short-term safety and efficacy of HMG-CoA reductase inhibitors in hypercholesterolemic patients with elevated serum alanine transaminase concentrations: PITCH study (PITavastatin versus atorvastatin to evaluate the effect on patients with hypercholesterolemia and mild to moderate hepatic damage) Journal of clinical lipidology. 2012;6(4):340–51. doi: 10.1016/j.jacl.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 67.Hatzitolios A, Savopoulos C, Lazaraki G, Sidiropoulos I, Haritanti P, Lefkopoulos A, et al. Efficacy of omega-3 fatty acids, atorvastatin and orlistat in non-alcoholic fatty liver disease with dyslipidemia. Indian journal of gastroenterology : official journal of the Indian Society of Gastroenterology. 2004;23(4):131–4. [PubMed] [Google Scholar]
- 68.Kiyici M, Gulten M, Gurel S, Nak SG, Dolar E, Savci G, et al. Ursodeoxycholic acid and atorvastatin in the treatment of nonalcoholic steatohepatitis. Can J Gastroenterol. 2003;17(12):713–8. doi: 10.1155/2003/857869. [DOI] [PubMed] [Google Scholar]
- 69.Foster T, Budoff MJ, Saab S, Ahmadi N, Gordon C, Guerci AD. Atorvastatin and antioxidants for the treatment of nonalcoholic fatty liver disease: the St Francis Heart Study randomized clinical trial. Am J Gastroenterol. 2011;106(1):71–7. doi: 10.1038/ajg.2010.299. [DOI] [PubMed] [Google Scholar]
- 70.Ekstedt M, Franzen LE, Mathiesen UL, Holmqvist M, Bodemar G, Kechagias S. Statins in non-alcoholic fatty liver disease and chronically elevated liver enzymes: a histopathological follow-up study. Journal of hepatology. 2007;47(1):135–41. doi: 10.1016/j.jhep.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 71.Hyogo H, Tazuma S, Arihiro K, Iwamoto K, Nabeshima Y, Inoue M, et al. Efficacy of atorvastatin for the treatment of nonalcoholic steatohepatitis with dyslipidemia. Metabolism. 2008;57(12):1711–8. doi: 10.1016/j.metabol.2008.07.030. [DOI] [PubMed] [Google Scholar]
- 72.Nelson A, Torres DM, Morgan AE, Fincke C, Harrison SA. A pilot study using simvastatin in the treatment of nonalcoholic steatohepatitis: A randomized placebo-controlled trial. J Clin Gastroenterol. 2009;43(10):990–4. doi: 10.1097/MCG.0b013e31819c392e. [DOI] [PubMed] [Google Scholar]
- 73.Athyros VG, Tziomalos K, Gossios TD, Griva T, Anagnostis P, Kargiotis K, et al. Safety and efficacy of long-term statin treatment for cardiovascular events in patients with coronary heart disease and abnormal liver tests in the Greek Atorvastatin and Coronary Heart Disease Evaluation (GREACE) Study: a post-hoc analysis. Lancet. 2010;376(9756):1916–22. doi: 10.1016/S0140-6736(10)61272-X. [DOI] [PubMed] [Google Scholar]
- 74.Chalasani N, Aljadhey H, Kesterson J, Murray MD, Hall SD. Patients with elevated liver enzymes are not at higher risk for statin hepatotoxicity. Gastroenterology. 2004;126(5):1287–92. doi: 10.1053/j.gastro.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 75.Lewis JH, Mortensen ME, Zweig S, Fusco MJ, Medoff JR, Belder R. Efficacy and safety of high-dose pravastatin in hypercholesterolemic patients with well-compensated chronic liver disease: Results of a prospective, randomized, double-blind, placebo-controlled, multicenter trial. Hepatology. 2007;46(5):1453–63. doi: 10.1002/hep.21848. [DOI] [PubMed] [Google Scholar]
- 76.Staels B, Dallongeville J, Auwerx J, Schoonjans K, Leitersdorf E, Fruchart JC. Mechanism of action of fibrates on lipid and lipoprotein metabolism. Circulation. 1998;98(19):2088–93. doi: 10.1161/01.cir.98.19.2088. [DOI] [PubMed] [Google Scholar]
- 77.Basaranoglu M, Acbay O, Sonsuz A. A controlled trial of gemfibrozil in the treatment of patients with nonalcoholic steatohepatitis. J Hepatol. 1999;31(2):384. doi: 10.1016/s0168-8278(99)80243-8. [DOI] [PubMed] [Google Scholar]
- 78.Fernandez-Miranda C, Perez-Carreras M, Colina F, Lopez-Alonso G, Vargas C, Solis-Herruzo JA. A pilot trial of fenofibrate for the treatment of non-alcoholic fatty liver disease. Dig Liver Dis. 2008;40(3):200–5. doi: 10.1016/j.dld.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 79.Fabbrini E, Mohammed BS, Korenblat KM, Magkos F, McCrea J, Patterson BW, et al. Effect of fenofibrate and niacin on intrahepatic triglyceride content, very low-density lipoprotein kinetics, and insulin action in obese subjects with nonalcoholic fatty liver disease. J Clin Endocrinol Metab. 2010;95(6):2727–35. doi: 10.1210/jc.2009-2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Flachs P, Rossmeisl M, Bryhn M, Kopecky J. Cellular and molecular effects of n-3 polyunsaturated fatty acids on adipose tissue biology and metabolism. Clin Sci (Lond) 2009;116(1):1–16. doi: 10.1042/CS20070456. [DOI] [PubMed] [Google Scholar]
- 81.Flachs P, Rossmeisl M, Kopecky J. The effect of n-3 fatty acids on glucose homeostasis and insulin sensitivity. Physiological research/Academia Scientiarum Bohemoslovaca. 2014;63(Suppl 1):S93–118. doi: 10.33549/physiolres.932715. [DOI] [PubMed] [Google Scholar]
- 82.Wu JH, Cahill LE, Mozaffarian D. Effect of fish oil on circulating adiponectin: a systematic review and meta-analysis of randomized controlled trials. J Clin Endocrinol Metab. 2013;98(6):2451–9. doi: 10.1210/jc.2012-3899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lopez-Vicario C, Gonzalez-Periz A, Rius B, Moran-Salvador E, Garcia-Alonso V, Lozano JJ, et al. Molecular interplay between Delta5/Delta6 desaturases and long-chain fatty acids in the pathogenesis of non-alcoholic steatohepatitis. Gut. 2014;63(2):344–55. doi: 10.1136/gutjnl-2012-303179. [DOI] [PubMed] [Google Scholar]
- 84.Capanni M, Calella F, Biagini MR, Genise S, Raimondi L, Bedogni G, et al. Prolonged n-3 polyunsaturated fatty acid supplementation ameliorates hepatic steatosis in patients with non-alcoholic fatty liver disease: a pilot study. Aliment Pharmacol Ther. 2006;23(8):1143–51. doi: 10.1111/j.1365-2036.2006.02885.x. [DOI] [PubMed] [Google Scholar]
- 85.Spadaro L, Magliocco O, Spampinato D, Piro S, Oliveri C, Alagona C, et al. Effects of n-3 polyunsaturated fatty acids in subjects with nonalcoholic fatty liver disease. Dig Liver Dis. 2008;40(3):194–9. doi: 10.1016/j.dld.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 86.Tanaka N, Sano K, Horiuchi A, Tanaka E, Kiyosawa K, Aoyama T. Highly purified eicosapentaenoic acid treatment improves nonalcoholic steatohepatitis. J Clin Gastroenterol. 2008;42(4):413–8. doi: 10.1097/MCG.0b013e31815591aa. [DOI] [PubMed] [Google Scholar]
- 87.Zhu FS, Liu S, Chen XM, Huang ZG, Zhang DW. Effects of n-3 polyunsaturated fatty acids from seal oils on nonalcoholic fatty liver disease associated with hyperlipidemia. World J Gastroenterol. 2008;14(41):6395–400. doi: 10.3748/wjg.14.6395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sofi F, Giangrandi I, Cesari F, Corsani I, Abbate R, Gensini GF, et al. Effects of a 1-year dietary intervention with n-3 polyunsaturated fatty acid-enriched olive oil on non-alcoholic fatty liver disease patients: a preliminary study. International journal of food sciences and nutrition. 2010;61(8):792–802. doi: 10.3109/09637486.2010.487480. [DOI] [PubMed] [Google Scholar]
- 89.Nobili V, Alisi A, Della Corte C, Rise P, Galli C, Agostoni C, et al. Docosahexaenoic acid for the treatment of fatty liver: randomised controlled trial in children. Nutrition, metabolism, and cardiovascular diseases : NMCD. 2013;23(11):1066–70. doi: 10.1016/j.numecd.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 90.Parker HM, Johnson NA, Burdon CA, Cohn JS, O’Connor HT, George J. Omega-3 supplementation and non-alcoholic fatty liver disease: a systematic review and meta-analysis. J Hepatol. 2012;56(4):944–51. doi: 10.1016/j.jhep.2011.08.018. [DOI] [PubMed] [Google Scholar]
- 91.Scorletti E, Bhatia L, McCormick KG, Clough GF, Nash K, Hodson L, et al. Effects of purified eicosapentaenoic and docosahexaenoic acids in non-alcoholic fatty liver disease: Results from the *WELCOME study. Hepatology. 2014 doi: 10.1002/hep.27289. [DOI] [PubMed] [Google Scholar]
- 92.Sanyal AJ, Abdelmalek MF, Suzuki A, Cummings OW, Chojkier M. No significant effects of ethyl-eicosapentanoic acid on histologic features of nonalcoholic steatohepatitis in a phase 2 trial. Gastroenterology. 2014;147(2):377–84.e1. doi: 10.1053/j.gastro.2014.04.046. [DOI] [PubMed] [Google Scholar]
- 93.Argo CK, Patrie JT, Lackner C, Henry TD, de Lange EE, Weltman AL, et al. Effects of n-3 fish oil on metabolic and histological parameters in NASH: a double-blind, randomized, placebo-controlled trial. J Hepatol. 2015;62(1):190–7. doi: 10.1016/j.jhep.2014.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Oakley F, Teoh V, Ching ASG, Bataller R, Colmenero J, Jonsson JR, et al. Angiotensin II activates I kappaB kinase phosphorylation of RelA at Ser 536 to promote myofibroblast survival and liver fibrosis. Gastroenterology. 2009;136(7):2334–44.e1. doi: 10.1053/j.gastro.2009.02.081. [DOI] [PubMed] [Google Scholar]
- 95.Moreno M, Gonzalo T, Kok RJ, Sancho-Bru P, van Beuge M, Swart J, et al. Reduction of advanced liver fibrosis by short-term targeted delivery of an angiotensin receptor blocker to hepatic stellate cells in rats. Hepatology. 2010;51(3):942–52. doi: 10.1002/hep.23419. [DOI] [PubMed] [Google Scholar]
- 96.Macedo SM, Antunes Guimaraes T, Feltenberger JD, Santos SH. The role of renin-angiotensin system modulation on treatment and prevention of liver diseases. Peptides. 2014;62c:189–96. doi: 10.1016/j.peptides.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 97.Goh GB, Pagadala MR, Dasarathy J, Unalp-Arida A, Sargent R, Hawkins C, et al. Renin-angiotensin system and fibrosis in non-alcoholic fatty liver disease. Liver Int. 2014 doi: 10.1111/liv.12611. [DOI] [PubMed] [Google Scholar]
- 98.Fogari R, Maffioli P, Mugellini A, Zoppi A, Lazzari P, Derosa G. Effects of losartan and amlodipine alone or combined with simvastatin in hypertensive patients with nonalcoholic hepatic steatosis. Eur J Gastroenterol Hepatol. 2012;24(2):164–71. doi: 10.1097/MEG.0b013e32834ba188. [DOI] [PubMed] [Google Scholar]
- 99.Georgescu EF, Ionescu R, Niculescu M, Mogoanta L, Vancica L. Angiotensin-receptor blockers as therapy for mild-to-moderate hypertension-associated non-alcoholic steatohepatitis. World J Gastroenterol. 2009;15(8):942–54. doi: 10.3748/wjg.15.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Koppe SW, Sahai A, Malladi P, Whitington PF, Green RM. Pentoxifylline attenuates steatohepatitis induced by the methionine choline deficient diet. J Hepatol. 2004;41(4):592–8. doi: 10.1016/j.jhep.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 101.Strieter RM, Remick DG, Ward PA, Spengler RN, Lynch JP, 3rd, Larrick J, et al. Cellular and molecular regulation of tumor necrosis factor-alpha production by pentoxifylline. Biochem Biophys Res Commun. 1988;155(3):1230–6. doi: 10.1016/s0006-291x(88)81271-3. [DOI] [PubMed] [Google Scholar]
- 102.Zein CO, Lopez R, Fu X, Kirwan JP, Yerian LM, McCullough AJ, et al. Pentoxifylline decreases oxidized lipid products in nonalcoholic steatohepatitis: new evidence on the potential therapeutic mechanism. Hepatology. 2012;56(4):1291–9. doi: 10.1002/hep.25778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Satapathy SK, Garg S, Chauhan R, Sakhuja P, Malhotra V, Sharma BC, et al. Beneficial effects of tumor necrosis factor-alpha inhibition by pentoxifylline on clinical, biochemical, and metabolic parameters of patients with nonalcoholic steatohepatitis. Am J Gastroenterol. 2004;99(10):1946–52. doi: 10.1111/j.1572-0241.2004.40220.x. [DOI] [PubMed] [Google Scholar]
- 104.Adams LA, Zein CO, Angulo P, Lindor KD. A pilot trial of pentoxifylline in nonalcoholic steatohepatitis. Am J Gastroenterol. 2004;99(12):2365–8. doi: 10.1111/j.1572-0241.2004.40064.x. [DOI] [PubMed] [Google Scholar]
- 105.Zein CO, Yerian LM, Gogate P, Lopez R, Kirwan JP, Feldstein AE, et al. Pentoxifylline improves nonalcoholic steatohepatitis: a randomized placebo-controlled trial. Hepatology. 2011;54(5):1610–9. doi: 10.1002/hep.24544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Van Wagner LB, Koppe SW, Brunt EM, Gottstein J, Gardikiotes K, Green RM, et al. Pentoxifylline for the treatment of non-alcoholic steatohepatitis: a randomized controlled trial. Annals of hepatology. 2011;10(3):277–86. [PubMed] [Google Scholar]
- 107.Adorini L, Pruzanski M, Shapiro D. Farnesoid X receptor targeting to treat nonalcoholic steatohepatitis. Drug discovery today. 2012;17(17–18):988–97. doi: 10.1016/j.drudis.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 108.Mazuy C, Helleboid A, Staels B, Lefebvre P. Nuclear bile acid signaling through the farnesoid X receptor. Cellular and molecular life sciences: CMLS. 2014 doi: 10.1007/s00018-014-1805-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mudaliar S, Henry RR, Sanyal AJ, Morrow L, Marschall HU, Kipnes M, et al. Efficacy and safety of the farnesoid X receptor agonist obeticholic acid in patients with type 2 diabetes and nonalcoholic fatty liver disease. Gastroenterology. 2013;145(3):574–82.e1. doi: 10.1053/j.gastro.2013.05.042. [DOI] [PubMed] [Google Scholar]
- 110.Neuschwander-Tetri BA, Loomba R, Sanyal AJ, Lavine JE, Van Natta ML, Abdelmalek MF, et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2014 doi: 10.1016/S0140-6736(14)61933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kharitonenkov A, Shanafelt AB. FGF21: a novel prospect for the treatment of metabolic diseases. Current opinion in investigational drugs. 2009;10(4):359–64. [PubMed] [Google Scholar]
- 112.Kharitonenkov A, Shiyanova TL, Koester A, Ford AM, Micanovic R, Galbreath EJ, et al. FGF-21 as a novel metabolic regulator. J Clin Invest. 2005;115(6):1627–35. doi: 10.1172/JCI23606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Coskun T, Bina HA, Schneider MA, Dunbar JD, Hu CC, Chen Y, et al. Fibroblast growth factor 21 corrects obesity in mice. Endocrinology. 2008;149(12):6018–27. doi: 10.1210/en.2008-0816. [DOI] [PubMed] [Google Scholar]
- 114.Xu J, Lloyd DJ, Hale C, Stanislaus S, Chen M, Sivits G, et al. Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet-induced obese mice. Diabetes. 2009;58(1):250–9. doi: 10.2337/db08-0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Fisher FM, Chui PC, Nasser IA, Popov Y, Cunniff JC, Lundasen T, et al. Fibroblast Growth Factor 21 Limits Lipotoxicity by Promoting Hepatic Fatty Acid Activation in Mice on Methionine and Choline-Deficient Diets. Gastroenterology. 2014 doi: 10.1053/j.gastro.2014.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gawrieh S, Alloosh M, Sheridan R, Liang R, Masuoka H, Chalasani N, et al. Fibroblast growth factor 21 treatment improves atherogenic diet-induced liver injury and metabolic syndrome in Ossabaw miniature swine. Hepatology. 2014;60:247A–9A. [Google Scholar]
- 117.Kolterman OG, Buse JB, Fineman MS, Gaines E, Heintz S, Bicsak TA, et al. Synthetic exendin-4 (exenatide) significantly reduces postprandial and fasting plasma glucose in subjects with type 2 diabetes. J Clin Endocrinol Metab. 2003;88(7):3082–9. doi: 10.1210/jc.2002-021545. [DOI] [PubMed] [Google Scholar]
- 118.Sharma S, Mells JE, Fu PP, Saxena NK, Anania FA. GLP-1 analogs reduce hepatocyte steatosis and improve survival by enhancing the unfolded protein response and promoting macroautophagy. PloS one. 2011;6(9):e25269. doi: 10.1371/journal.pone.0025269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sathyanarayana P, Jogi M, Muthupillai R, Krishnamurthy R, Samson SL, Bajaj M. Effects of combined exenatide and pioglitazone therapy on hepatic fat content in type 2 diabetes. Obesity. 2011;19(12):2310–5. doi: 10.1038/oby.2011.152. [DOI] [PubMed] [Google Scholar]
- 120.Samson SL, Sathyanarayana P, Jogi M, Gonzalez EV, Gutierrez A, Krishnamurthy R, et al. Exenatide decreases hepatic fibroblast growth factor 21 resistance in non-alcoholic fatty liver disease in a mouse model of obesity and in a randomised controlled trial. Diabetologia. 2011;54(12):3093–100. doi: 10.1007/s00125-011-2317-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Li Y, Wong K, Giles A, Jiang J, Lee JW, Adams AC, et al. Hepatic SIRT1 attenuates hepatic steatosis and controls energy balance in mice by inducing fibroblast growth factor 21. Gastroenterology. 2014;146(2):539–49.e7. doi: 10.1053/j.gastro.2013.10.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kenny PR, Brady DE, Torres DM, Ragozzino L, Chalasani N, Harrison SA. Exenatide in the treatment of diabetic patients with non-alcoholic steatohepatitis: a case series. Am J Gastroenterol. 2010;105(12):2707–9. doi: 10.1038/ajg.2010.363. [DOI] [PubMed] [Google Scholar]
- 123.Prescott LF, Newton RW, Swainson CP, Wright N, Forrest AR, Matthew H. Successful treatment of severe paracetamol overdosage with cysteamine. Lancet. 1974;1(7858):588–92. doi: 10.1016/s0140-6736(74)92649-x. [DOI] [PubMed] [Google Scholar]
- 124.Prescott LF, Sutherland GR, Park J, Smith IJ, Proudfoot AT. Cysteamine, methionine, and penicillamine in the treatment of paracetamol poisoning. Lancet. 1976;2(7977):109–13. doi: 10.1016/s0140-6736(76)92842-7. [DOI] [PubMed] [Google Scholar]
- 125.Dohil R, Schmeltzer S, Cabrera BL, Wang T, Durelle J, Duke KB, et al. Enteric-coated cysteamine for the treatment of paediatric non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2011;33(9):1036–44. doi: 10.1111/j.1365-2036.2011.04626.x. [DOI] [PubMed] [Google Scholar]
- 126.Van Bergen T, Marshall D, Van de Veire S, Vandewalle E, Moons L, Herman J, et al. The role of LOX and LOXL2 in scar formation after glaucoma surgery. Investigative ophthalmology & visual science. 2013;54(8):5788–96. doi: 10.1167/iovs.13-11696. [DOI] [PubMed] [Google Scholar]
- 127.Traber PG, Zomer E. Therapy of experimental NASH and fibrosis with galectin inhibitors. PloS one. 2013;8(12):e83481. doi: 10.1371/journal.pone.0083481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Traber PG, Chou H, Zomer E, Hong F, Klyosov A, Fiel MI, et al. Regression of fibrosis and reversal of cirrhosis in rats by galectin inhibitors in thioacetamide-induced liver disease. PloS one. 2013;8(10):e75361. doi: 10.1371/journal.pone.0075361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Harrison S, Chalasani N, Lawitz E, Marri S, Noureddin M, Sanyal A, et al. Early phase 1 clinical trial results of GR-MD-02, a galec-tin-3 inhibitor, in patients having non-alcoholic steato-hepatitis (NASH) with advanced fibrosis. Hepatology. 2014;60:224A–7A. [Google Scholar]
- 130.Lefebvre E, Hashiguchi T, Jenkins H, Nabhan A, Yoneyama H, Friedman S, et al. Anti-fibrotic and anti-inflammatory activity of the dual CCR2 and CCR5 antagonist cenicriviroc in a mouse model of NASH. Hepatology. 2013;58(S1):219A–22A. [Google Scholar]
- 131.Gilat T, Leikin-Frenkel A, Goldiner I, Juhel C, Lafont H, Gobbi D, et al. Prevention of diet-induced fatty liver in experimental animals by the oral administration of a fatty acid bile acid conjugate (FABAC) Hepatology. 2003;38(2):436–42. doi: 10.1053/jhep.2003.50348. [DOI] [PubMed] [Google Scholar]
- 132.Leikin-Frenkel A, Goldiner I, Leikin-Gobbi D, Rosenberg R, Bonen H, Litvak A, et al. Treatment of preestablished diet-induced fatty liver by oral fatty acid-bile acid conjugates in rodents. Eur J Gastroenterol Hepatol. 2008;20(12):1205–13. doi: 10.1097/MEG.0b013e3282fc9743. [DOI] [PubMed] [Google Scholar]
- 133.Safadi R, Konikoff FM, Mahamid M, Zelber-Sagi S, Halpern M, Gilat T, et al. The fatty acid-bile acid conjugate Aramchol reduces liver fat content in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2014;12(12):2085–91.e1. doi: 10.1016/j.cgh.2014.04.038. [DOI] [PubMed] [Google Scholar]
- 134.Cariou B, Staels B. GFT505 for the treatment of nonalcoholic steatohepatitis and type 2 diabetes. Expert opinion on investigational drugs. 2014;23(10):1441–8. doi: 10.1517/13543784.2014.954034. [DOI] [PubMed] [Google Scholar]
- 135.Quintero P, Arrese M. Nuclear control of inflammation and fibrosis in nonalcoholic steatohepatitis: therapeutic potential of dual peroxisome proliferator-activated receptor alpha/delta agonism. Hepatology. 2013;58(6):1881–4. doi: 10.1002/hep.26582. [DOI] [PubMed] [Google Scholar]
- 136.Staels B, Rubenstrunk A, Noel B, Rigou G, Delataille P, Millatt LJ, et al. Hepatoprotective effects of the dual peroxisome proliferator-activated receptor alpha/delta agonist, GFT505, in rodent models of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Hepatology. 2013;58(6):1941–52. doi: 10.1002/hep.26461. [DOI] [PubMed] [Google Scholar]


