Abstract
Background and Aims
Liver disease in Alagille syndrome is highly variable. Many of these patients presenting with severe cholestasis early in life improve spontaneously; 10–20% however, have progressive disease. It is currently not possible to predict long-term hepatic outcomes in Alagille syndrome. This international, multicenter study was aimed at identifying early life predictors of liver disease outcome.
Methods
Retrospective clinical, laboratory and radiographic data from a cohort of 144 Alagille syndrome patients, whose long-term hepatic outcomes had been determined a priori based on previously published criteria, were collected.
Results
Sixty-seven had mild and 77 severe hepatic outcome. Univariate analysis demonstrated that cholestasis and fibrosis on biopsy, as well as the presence of xanthomata were significantly different between the groups (p<0.05 for all). Mixed model analysis revealed that total serum bilirubin and serum cholesterol were also associated with outcome (p=0.001 and p=0.002, respectively). Graphical representation of the data revealed a change in total bilirubin levels between 12 and 24 months of age in the mild group. Recursive partitioning identified a threshold for total bilirubin of 3.8 mg/dL (65mmol/L) in that age-frame that differentiated between outcomes. A multivariable logistic regression model was developed using fibrosis, xanthomata and the total bilirubin cutoff of 3.8 mg/dL (65mmol/L), which generated an area under the ROC of 0.792.
Conclusions
The long-term hepatic outcomes of patients with Alagille syndrome can be predicted based on serum total bilirubin between the ages of 12–24 months combined with fibrosis on liver biopsy and presence of xanthomata on physical examination.
Keywords: Alagille syndrome, cholestasis, pediatric, outcome
INTRODUCTION
Alagille syndrome (ALGS) is the most common cause of familial intrahepatic cholestasis, affecting as many as 1:30,000 individuals [1, 2]. The diagnosis of ALGS is typically established on the basis of clinical criteria, which include paucity of intrahepatic bile ducts on liver histology in addition to 3 or more of the following: chronic cholestasis, structural heart disease, ocular abnormalities, skeletal anomalies, characteristic facies, renal anomalies and vascular involvement [3–5]. The vast majority of patients (~95%) carry an autosomal dominant mutation in JAGGED1 (JAG1) [6] and a minority have mutations in NOTCH2 [7]. However, there are no direct genotype-phenotype correlations and, hence, the presence of a disease-causing mutation does not determine the type or severity of clinical manifestations, nor does it predict long-term outcomes.
ALGS-related liver disease is highly variable and ranges from subtle biochemical abnormalities to profound cholestasis, portal hypertension and end-stage liver disease requiring liver transplantation [8]. Up to 50% of patients presenting with severe cholestasis in infancy or early childhood may require transplantation before they reach adulthood, indicating that in the other half, significant liver disease improves or stabilizes with time [9, 10]. This improvement in severity of cholestasis that is seen in a substantial number of children with ALGS typically occurs by age 5 years. To date it has not been possible to predict which children with ALGS will have this spontaneous improvement, which poses a unique management challenge and may result in some young patients undergoing surgical procedures to relieve pruritus [11] or unnecessary liver transplantation.
We previously performed a retrospective review of 33 patients to identify early predictors of long-term liver disease outcomes in ALGS [12]. The patients included in that study were older than 10 years of age and were stratified as having a mild or severe hepatic phenotype. Mean serial biochemical data from the first 5 years of life were compared between the mild and severe groups. A serum total bilirubin ≥ 6.5mg/dL (111 mmol/L), a serum conjugated bilirubin ≥ 4.5mg/dL (77 mmol/L), and a serum cholesterol ≥ 520mg/dL (13.5 mmol/L) served as thresholds that could reliably differentiate between the mild and severe hepatic outcome groups. Limitations of that study included a small sample size, and the use of mean values to determine the cutoffs.
In this study, we sought to expand the pilot study to a larger cohort and to include clinical, histological, and laboratory data in order to identify early predictors of long-term hepatic outcomes in ALGS.
MATERIALS AND METHODS
This was an international, multicenter, retrospective study. Eleven large pediatric liver and liver transplant centers from Europe and North America participated. Ethics Committee and Institutional Review Board (IRB) approvals were obtained at The Hospital for Sick Children, Ann & Robert H. Lurie Children’s Hospital of Chicago, University of Colorado School of Medicine and Children’s Hospital Colorado, Children’s Hospital of Philadelphia, Cincinnati Children’s Hospital, Baylor College of Medicine and Texas Children’s Hospital, Beatrix Children’s Hospital, Nemours/AI. duPont Hospital for Children, Children’s Hospital of Pittsburgh, UCSF Benioff Children’s Hospital and McMaster University to ensure that the study protocol conformed to the guidelines set by the 1975 Declaration of Helsinki. Parental/guardian consent was waivered by each site’s IRB since it was a retrospective study.
Inclusion criteria were a clinically or genetically confirmed diagnosis of ALGS [13] and age 10 years or older. This age was chosen based on literature suggesting that the severity of the hepatic phenotype of ALGS is evident after early childhood [9, 10]. Exclusion criteria were history of Kasai portoenterostomy, liver transplantation or death during the first 5 years of life. These children were excluded as these events interrupt the early natural history of Alagille liver disease. In addition, subjects with severe cardiac involvement (e.g. single ventricle physiology) were excluded to eliminate the potential confounding effect of significant heart disease on hepatic outcomes. Subjects were allocated to their hepatic outcome groups (mild or severe) based on data available for subjects at age 10 years or older. All available clinical, histological, and laboratory data from the first 5 years of life were retrospectively collected on standardized forms from each center (Table 1). Liver biopsy data were only used if they were performed during the first 5 years of life. If multiple biopsies were available during this period then the first one was used for consistency. The clinical and histological data were represented as binary outcomes, i.e. present or absent. For histology, the data were collected from biopsy reports.
Table 1.
Clinical, histologic, radiographic, laboratory and data collected for the study
| Histology | Liver biopsy evidence of bile duct paucity or proliferation, cholestasis, and hepatic fibrosis |
| Clinical and radiographic data | Hepatic: Pruritus, xanthomata |
| Cardiovascular: Heart murmur, PPS, VSD, ASD, TOF, valve stenosis or atresia, aortic coarctation, peripheral vascular anomalies | |
| Renal: RTA, cystic renal disease, abnormalities in renal u/s, Wilms tumor, ESRD and/or renal transplantation | |
| CNS: stroke, Moya-Moya | |
| Other: long bone fractures, butterfly vertebrae, posterior embryotoxon, ALGS facies | |
| Laboratory results | Hb, WBC, PLT, ALT, AST, GGT, ALP, TB, DB, IB, bile acids, cholesterol, triglycerides, creatinine, albumin, protein, INR, PT |
ALGS: Alagille syndrome; ALP: Alkaline phosphatase; ALT: Alanine transaminase; ASD: Atrial Septal Defect; AST: Aspartate aminotransferase; CNS: Central Nervous System; DB: Direct Bilirubin; ESRD: End-Stage Renal Disease; GGT: Gamma-Glutamyl Transferase; Hb: Hemoglobin; IB: Indirect Bilirubin; INR: International Normalized Ratio; PLT: platelets; PPS: Peripheral Pulmonic Stenosis; PT: Prothrombin Time; RTA: Renal Tubular Acidosis; TB: Total Bilirubin, TOF: Tetralogy of Fallot; WBC: White Blood cell Count; VSD: Ventricular Septal Defect
The patients were allocated to ‘mild’ or ‘severe’ hepatic outcome groups according to the criteria that had been used in the initial study (described in Table 2) and using the most recent clinical data available, i.e. at the oldest age possible over the age of 10 years in order to capture the most accurate, long-term hepatic outcome. This systematic stratification process distinguished between subjects whose liver disease was mild enough to be managed with medical interventions alone or severe enough to either require surgical interventions (e.g. partial external biliary diversion or liver transplantation) or be associated with severe comorbidities, such as pathologic long-bone fractures or presence of portal hypertension.
Table 2.
Criteria used to classify ALGS subjects aged 10 years or older as having a mild or severe hepatic outcome
| MILD | SEVERE |
|---|---|
| No overt clinical or biochemical evidence of liver disease | Death from complications of end-stage liver disease after age 5 years |
| Biochemical abnormalities without symptoms of cholestasis | Listed for or received a liver transplant after the age of 5 years |
| Symptoms of cholestasis (e.g. pruritus) adequately managed with medications | Cholestasis with significant complications (e.g. pathological bone fractures, pruritus requiring partial biliary diversion, etc.) |
| Portal Hypertension (splenomegaly, esophageal or gastric varices, or ascites) |
Statistical analyses
Clinical and histological data obtained during the first five years of life were expressed as binary variables, whereas the laboratory data were expressed as continuous variables. Univariate analyses were performed using a chi-square test to identify statistically significant differences in binary outcomes between the two hepatic outcome groups. Multivariable logistic regression models were developed and Receiver Operating Characteristic (ROC) curves were subsequently used to identify the combination of these significant variables with the best predictive ability for severe or mild long-term hepatic outcome. Continuous data were entered into a mixed model analysis to identify statistically significant variables. These were then represented graphically for each individual to visually detect the time (age) window that best differentiated the 2 groups. Recursive partitioning was subsequently used to determine cut-offs for these variables. All statistical analyses were performed using SAS version 9.2 software (SAS Institute, Cary, NC, USA). All statistical tests were two-sided, and p <0.05 was considered statistically significant.
RESULTS
The study cohort consisted of 144 patients (55% male) with clinically and/or genetically confirmed ALGS. Of those, 67 were classified as having mild and 77 as severe hepatic long-term outcome. Their mean age at last follow-up used in data collection was 13.2 years. One hundred patients had had a liver biopsy performed within the first year of life (39 with a mild and 61 with a severe phenotype). The age at liver biopsy did not differ between those with mild and severe phenotypes. The median age at liver biopsy was 3 months (range 1–60).
A univariate analysis was performed to determine differences in clinical and histological parameters between subjects with mild and severe hepatic outcomes (Table 3). Of all the variables assessed, the presence of any degree of cholestasis (p=0.041) and fibrosis (p=0.006) on liver biopsy (as mentioned in the histopathology report), as well as the presence of any xanthomata (p=0.003) were significantly different between the 2 groups. The risk of a severe long-term hepatic phenotype increased more than 3-fold in patients found to have fibrosis on a liver biopsy done during the first 5 years of life (OR: 3.3, 95% CI 1.4–7.9). Cholestasis on biopsy early in life was associated with an odds ratio of 2.8 for the eventual outcome of a severe phenotype (95% CI 1.0–7.6)
Table 3.
Results of the univariate analyses
| HISTOLOGY & CLINICAL DATA | MILD PHENOTYPE | SEVERE PHENOTYPE | p value |
|---|---|---|---|
| Bile duct paucity (n=86) | (33/86) 39% | (53/86) 61% | 0.31 |
| Bile duct proliferation (n=15) | (7/15) 44% | (8/15) 56% | 1.00 |
| Cholestasis (on liver biopsy; n=80) | (28/80) 38% | (52/80) 62% | 0.04 |
| Fibrosis (on liver biopsy; n=61) | (20/61) 32% | (41/61) 68% | <0.01 |
| Pruritus (n=82) | (36/82) 43% | (46/82) 57% | 0.10 |
| Xanthomata (n=32) | (8/32) 25% | (24/32) 75% | <0.01 |
Only a subset of the variables assessed is shown in this table.
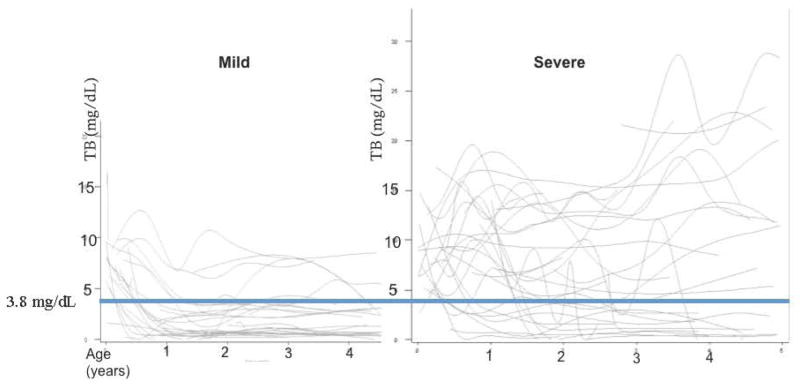
Mixed model analyses of the laboratory data revealed that only serum total bilirubin (p=0.001) and cholesterol (p=0.002) early in life were significantly different between the mild and severe hepatic outcome groups. All serial serum total bilirubin and cholesterol values obtained during the first 5 years of life were represented graphically for each hepatic outcome group (Figure 1). In the mild outcome group the total bilirubin levels fell rapidly between 12 and 24 months of age. This phenomenon was not observed in patients who had a severe hepatic outcome (Figure 1). [Mean TB +/− SD between 12–24 months was 2.1mg/dL +/− 2.3 for the mild group and 7.4mg/dL +/− 5.4 for the severe group; p<0.001]. No clear trend was apparent in the plots of serial cholesterol values (data not shown). [Mean cholesterol +/− SD between 12–24 months was 456mg/dL +/− 439 for the mild group and 515mg/dL +/− 356, for the severe group; p=0.6]. Given the change in the total bilirubin values observed in the mild group during the 12–24 month age period, recursive partitioning was applied to the mean serum total bilirubin values from that period of time, and a threshold of 3.8mg/dL (65mmol/L) was identified as the optimal cutoff value to differentiate between the 2 groups. Of those who had a serum total bilirubin greater than 3.8 mg/dL (65mmol/L) between 12 and 24 months of age, 87% ultimately developed a severe phenotype (p<0.001). A serum total bilirubin higher greater than 3.8 mg/dL (65mmol/L) between 12 and 24 months of age was associated with an almost 10 fold increased risk of developing a severe hepatic phenotype (OR: 9.9, 95% CI: 3.5–28). Only 9% of children with a mild phenotype had a total bilirubin above this level during this age interval.
Figure 1. Graphical Plot of all available serial total bilirubin values for each patient in the mild and severe hepatic outcome groups.
In the graphs, each line represents a single patient. The lines between the values have been smoothed to visually aid in separating trends between the groups. After the first year of life, most patients who are classified in the mild hepatic phenotype group, have a total bilirubin that falls below the threshold of 3.8 mg/dL (65mmol/L); such a pattern is not observed in the severe outcome group. (TB: total bilirubin)
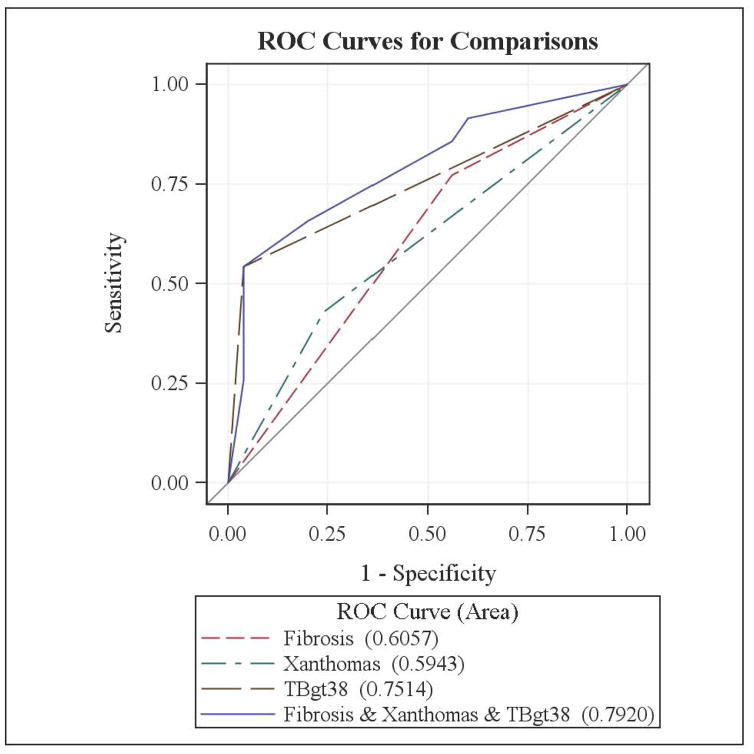
Utilizing the clinical variables that were shown to be significantly different between the two hepatic outcome groups on the univariate analysis (fibrosis on liver biopsy and the presence of xanthomata) and the total bilirubin cut-off of above ) mg/dL, as identified by recursive partitioning, a multivariable logistic regression model was developed. Serum cholesterol could not be used in this model as a cut-off value could not be identified on the serial plot and therefore it could not be represented as a binary variable. Cholestasis on liver biopsy was also not used in the model because its inclusion led to multicollinearity (meaning that cholestasis on liver biopsy could be predicted by other outcome variables already included in the model). Figure 2 represents the logistic regression model for each variable (fibrosis on liver biopsy, presence of xanthomata and serum total bilirubin above 3.8 mg/dL) and combining these three variables, the latter generating an area under the ROC curve of 0.792. Although serum total bilirubin alone yielded an ROC of 0.751, adding the other variables improved the predictive value in this model.
Figure 2. Receiver Operating Characteristic curves demonstrating the predictive ability of statistically significant histological and clinical variables for severe hepatic outcome.
Values in parentheses represent the area under the curve.
TBgt38: Total Bilirubin above 3.8 mg/dL (65mmol/L) during the second year of life (12–24 months)
DISCUSSION
Utilizing a large multi-center cohort of children with ALGS, we present a simple and clinically applicable model to predict long-term outcomes of liver disease based on data available in the first 2 years of life. We determined that a combination of xanthomata on physical examination, fibrosis on liver biopsy obtained in the first five years of life and a serum total bilirubin cut-off of 3.8mg/dL (65mmol/L) between the first and second years of life, reliably predict the eventual hepatic outcome of patients with ALGS.
This study confirmed and expanded on the results of our pilot study, which identified serum total bilirubin and cholesterol as early makers of liver disease outcome [12]. In the current study, serum total bilirubin, cholesterol and the clinical correlate of hypercholesterolemia, i.e., xanthomata, also reached statistical significance as predictive variables. The cutoff point for a differentiating total bilirubin value in the pilot study, however, was much higher than that of the current study (6.5mg/dl [111 mmol/L] vs. 3.8 mg/dL [65 mmol/L]). This difference is likely due to the fact that in the pilot study all the available total bilirubin levels obtained during the first five years of life were averaged to determine the cutoff, whereas in the current study we carefully examined each patient’s bilirubin levels serially over time and identified the time frame (between ages 12–24 months) in which the levels became different between the groups. It was only during this interval that serum total bilirubin values were averaged to determine the cut-off.
The current study also expanded beyond the pilot study by also examining clinical variables. The univariate analysis identified histological evidence of cholestasis and fibrosis on liver biopsy as having predictive value. It is difficult to ascertain the predictive value of liver biopsies in ALGS from these data as the information was compiled from histopathology reports only, rather than having slides read by a single pathologist, though it is appealing to consider the role of liver biopsy in ALGS for this purpose in future studies with central histology reading. The most promising clinical variable identified in this study as an early predictive marker of long-term outcome was the presence of xanthomata, which likely functioned as a surrogate marker for the severity of cholestasis. In this study we were only able to score this characteristic as present or absent, however it would be useful to consider a method of scoring severity of xanthomata to explore this marker in future studies.
Predictive tools frequently guide clinical decisions in patients with liver disease, as with the Model for End-stage Liver Disease (MELD) and Pediatric End-Stage Liver Disease (PELD) scoring systems for allocation of donor liver organs [14]. Although there are known limitations to tools such as these, there is a clear need to predict liver disease outcomes in ALGS as the hepatic phenotype evolves during the early years of life in a unique fashion. Children with ALGS-related liver disease that is mild in infancy rarely, if ever, have progression of liver disease later in childhood. However infants with ALGS and substantial cholestasis may have either spontaneous improvement, progression to end-stage liver disease or persistent symptomatic cholestasis [15]. The potential to have a mild hepatic outcome in later life despite cholestasis during the first few years of life should be recognized and considered in decision-making regarding evaluation for liver transplantation or surgery for treatment of pruritus. This study identified easily measurable markers of liver disease outcome in ALGS that may assist in decision making about the optimal clinical care for an individual child.
Limitations of this study include its retrospective nature, however children with ALGS generally have frequent blood tests drawn and clinical visits during the first 5 years of life. It would be of interest to confirm the utility of this predictive model (including the value of serum total bilirubin between 12 and 24 months of age) as a predictive tool in a replication cohort or a prospective cohort. As all participating centers in the current study are tertiary/quaternary level hospitals where liver transplantation is performed, there was likely a referral bias in this study that could have affected the relative frequency of patients with mild versus severe liver disease. However, this should not bias the results of the study, as allocation to the mild and severe outcomes groups was determined a priori. As mentioned above, the lack of centralized reading of the hepatic histopathology is also a limiting factor but could be evaluated in future prospective studies. However, experienced pediatric pathologists performed all the histological interpretations in a real-world setting in this study, which has merit for generalizing the findings of this study to the general population of ALGS patients.
In conclusion, this study has shown that it is possible to predict long-term hepatic outcomes in ALGS based on serum total bilirubin levels in the first two years of life, combined with the identification of fibrosis on liver biopsy performed in the first 5 years of life and the presence of xanthomata by age 5 years. In addition, in a cholestatic child with ALGS, an average total bilirubin of above 3.8mg/dL between the ages of 12 and 24 months represents a cut-off that by itself has significant predictive value for severe or mild hepatic outcomes in later life. This predictive model may assist clinicians in the complex decision-making regarding liver transplantation in a patient with ALGS, and potentially avoiding unnecessary liver transplantation in young children destined to have spontaneous improvement in their cholestasis. Conversely, earlier interventions may be implemented in preparation for transplant in patients who are predicted to have poor outcome. This model should of course be placed in the wider context of other important factors such as quality of life and nutrition that impact this complicated population, however we propose that these data add objective elements to the discussion surrounding hepatic prognostication and liver transplantation in ALGS.
Key Points.
This multi-centre analysis has generated the first clinically applicable model to predict long-term outcomes of liver disease in Alagille syndrome. The model includes:
Xanthomata on physical examination,
Fibrosis on liver biopsy obtained in the first five years of life, and
A serum total bilirubin cut-off of 3.8mg/dL (65mmol/L) between the first and second years of life, that in combination, reliably predict the eventual hepatic outcome of patients with Alagille syndrome.
Acknowledgments
Financial support: This study was supported by the American Liver Foundation Blowitz Ridgeway Foundation Seed Grant, the National Institute of Health (NIH) Clinical and Translational Science Awards (CTSA) grant UL1 TR001082, U01 DK062453, NIH CTSA grant UL1 TR000077, U01 DK62497, R01DK081702, U01 DK62500.
Abbreviations
- ALGS
Alagille syndrome
- IRB
Institutional Review Board
- ROC
Receiver Operating Characteristic curves
- SAS
Statistical Analysis System
- MELD
Model for End-stage Liver Disease
- PELD
Pediatric End-Stage Liver Disease
Footnotes
Conflicts: The authors have no conflicts to declare.
Author contributions:
Marialena Mouzaki: study design, acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content,
Lee M. Bass: acquisition of data; analysis and interpretation of data; critical revision of the manuscript for important intellectual content,
Ronald J. Sokol: acquisition of data; analysis and interpretation of data; critical revision of the manuscript for important intellectual content,
David A. Piccoli: acquisition of data; analysis and interpretation of data; critical revision of the manuscript for important intellectual content,
Claudia Quammie: acquisition of data; analysis and interpretation of data; critical revision of the manuscript for important intellectual content,
Kathleen M. Loomes: acquisition of data; analysis and interpretation of data; critical revision of the manuscript for important intellectual content,
James E. Heubi: acquisition of data; analysis and interpretation of data; critical revision of the manuscript for important intellectual content,
Paula M. Hertel: acquisition of data; analysis and interpretation of data; critical revision of the manuscript for important intellectual content,
Rene Scheenstra: acquisition of data; analysis and interpretation of data; critical revision of the manuscript for important intellectual content,
Katryn Furuya: acquisition of data; analysis and interpretation of data; critical revision of the manuscript for important intellectual content,
Erica Kutsch: acquisition of data; analysis and interpretation of data,
Nancy B. Spinner: acquisition of data; analysis and interpretation of data; critical revision of the manuscript for important intellectual content,
Kristen N. Robbins: acquisition of data; analysis and interpretation of data,
Veena Venkat: acquisition of data; analysis and interpretation of data; critical revision of the manuscript for important intellectual content,
Philip Rosenthal: acquisition of data; analysis and interpretation of data; critical revision of the manuscript for important intellectual content,
Joseph Beyene: analysis and interpretation of data; critical revision of the manuscript for important intellectual content; statistical analysis
Alastair Baker: acquisition of data; analysis and interpretation of data; critical revision of the manuscript for important intellectual content,
Binita M. Kamath: study concept and design; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; obtained funding
References
- 1.Kamath BM, et al. Consequences of JAG1 mutations. J Med Genet. 2003;40(12):891–5. doi: 10.1136/jmg.40.12.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Danks DM, et al. Studies of the aetiology of neonatal hepatitis and biliary atresia. Arch Dis Child. 1977;52(5):360–7. doi: 10.1136/adc.52.5.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alagille D, et al. Syndromic paucity of interlobular bile ducts (Alagille syndrome or arteriohepatic dysplasia): review of 80 cases. J Pediatr. 1987;110(2):195–200. doi: 10.1016/s0022-3476(87)80153-1. [DOI] [PubMed] [Google Scholar]
- 4.Kamath BM, Spinner NB, Rosenblum ND. Renal involvement and the role of Notch signalling in Alagille syndrome. Nat Rev Nephrol. 2013;9(7):409–18. doi: 10.1038/nrneph.2013.102. [DOI] [PubMed] [Google Scholar]
- 5.Kamath BM, et al. Vascular anomalies in Alagille syndrome: a significant cause of morbidity and mortality. Circulation. 2004;109(11):1354–8. doi: 10.1161/01.CIR.0000121361.01862.A4. [DOI] [PubMed] [Google Scholar]
- 6.Li L, et al. Alagille syndrome is caused by mutations in human Jagged1, which encodes a ligand for Notch1. Nat Genet. 1997;16(3):243–51. doi: 10.1038/ng0797-243. [DOI] [PubMed] [Google Scholar]
- 7.McDaniell R, et al. NOTCH2 mutations cause Alagille syndrome, a heterogeneous disorder of the notch signaling pathway. Am J Hum Genet. 2006;79(1):169–73. doi: 10.1086/505332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kamath BM, et al. Outcomes of liver transplantation for patients with Alagille syndrome: the studies of pediatric liver transplantation experience. Liver Transpl. 2012;18(8):940–8. doi: 10.1002/lt.23437. [DOI] [PubMed] [Google Scholar]
- 9.Emerick KM, et al. Features of Alagille syndrome in 92 patients: frequency and relation to prognosis. Hepatology. 1999;29(3):822–9. doi: 10.1002/hep.510290331. [DOI] [PubMed] [Google Scholar]
- 10.Hoffenberg EJ, et al. Outcome of syndromic paucity of interlobular bile ducts (Alagille syndrome) with onset of cholestasis in infancy. J Pediatr. 1995;127(2):220–4. doi: 10.1016/s0022-3476(95)70298-9. [DOI] [PubMed] [Google Scholar]
- 11.Emerick KM, Whitington PF. Partial external biliary diversion for intractable pruritus and xanthomas in Alagille syndrome. Hepatology. 2002;35(6):1501–6. doi: 10.1053/jhep.2002.33332. [DOI] [PubMed] [Google Scholar]
- 12.Kamath BM, et al. A longitudinal study to identify laboratory predictors of liver disease outcome in Alagille syndrome. J Pediatr Gastroenterol Nutr. 2010;50(5):526–30. doi: 10.1097/MPG.0b013e3181cea48d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kamath BM. Alagille syndrome. In: Suchy SRFJ, Balistreri WF, editors. Liver Disease in Children. Cambridge University Press; United Kingdom: 2014. p. 216. [Google Scholar]
- 14.Malinchoc M, et al. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 2000;31(4):864–71. doi: 10.1053/he.2000.5852. [DOI] [PubMed] [Google Scholar]
- 15.Lykavieris P, et al. Outcome of liver disease in children with Alagille syndrome: a study of 163 patients. Gut. 2001;49(3):431–5. doi: 10.1136/gut.49.3.431. [DOI] [PMC free article] [PubMed] [Google Scholar]