Abstract
Toxicological studies of defined chemical mixtures assist human health risk assessment by establishing how chemicals interact with one another to induce an effect. This paper reviews how antiandrogenic chemical mixtures can alter reproductive tract development in rats with a focus on the reproductive toxicant phthalates. The reviewed studies compare observed mixture data to mathematical mixture model predictions based on dose addition or response addition to determine how the individual chemicals in a mixture interact (e.g., additive, greater, or less than additive). Phthalate mixtures were observed to act in a dose additive manner based on the relative potency of the individual phthalates to suppress fetal testosterone production. Similar dose additive effects have been reported for mixtures of phthalates with antiandrogenic pesticides of differing mechanisms of action. Overall, data from these phthalate experiments in rats can be used in conjunction with human biomonitoring data to determine individual hazard indices, and recent cumulative risk assessments in humans indicate an excess risk to antiandrogenic chemical mixtures that include phthalates only or phthalates in combination with other antiandrogenic chemicals.
Keywords: cumulative risk assessment, phthalates, antiandrogens, pesticides, male reproductive tract development, mixtures
Introduction
Risk assessment has traditionally been conducted on an individual chemical basis. However, human biomonitoring and environmental monitoring studies have detected the presence of multiple chemical exposures, including many endocrine disrupting chemicals. Of special interest, multiple chemical exposures have been documented for sensitive periods of human development, including in pregnant women (Enke et al., 2013; Mitro et al., 2015; Woodruff et al., 2011; Ye et al., 2009), amniotic fluid samples (Silva et al., 2004), infants (Enke et al., 2013), and children (Becker et al., 2009; Blount et al., 2000; Eskenazi et al., 1999; Koch et al., 2011; Teitelbaum et al., 2008). Environmental monitoring studies have documented exposure to multiple chemicals in wildlife, including fish and birds (Ankley et al., 2007; Baxter et al., 2015; Jaspers et al., 2006; Jobling and Tyler, 2006; Kendall et al., 2010; Tyler et al., 1998). Furthermore, environmental monitoring efforts have detected multiple chemicals in freshwater samples, including: pesticides (Hela et al., 2005; Maruya et al., 2016), hormones (Kolok et al., 2007; Kolpin et al., 2002), pharmaceuticals and personal care products (Kolpin et al., 2002; Wu et al., 2014), and industrial chemicals (Durhan et al., 2006; Maruya et al., 2016).
Regulatory agencies have begun to consider how to conduct cumulative risk assessment for toxic chemicals. In 1996, the United States Congress implemented the Food Quality Protection Act (FQPA), which mandated that the United States Environmental Protection Agency (US EPA) consider the cumulative risk of pesticides found in food that operate via a common mechanism of toxicity (US Congress, 1996). For example, the US EPA has risk assessment programs in the area of mixtures toxicology in the Office of Water, the Office of Air and Radiation, Superfund, and the Office of Research and Development’s National Center for Environmental Assessment (Sexton, 2012; US EPA, 2015). In addition, many international health agencies have either recently established or are actively developing cumulative risk assessment guidelines (Boobis et al., 2008; Health Canada, 2016; RIVM, 2016; Solecki et al., 2014).
Many of the chemicals detected in human biomonitoring can disrupt normal hormone-signaling; for example, in utero exposure to several phthalates induces reproductive toxicity in male rats due to inhibition testosterone production during sexual differentiation. In 2006, the US EPA requested that the National Academy of Sciences (NAS) establish a panel to provide the US EPA with recommendations on whether to perform a cumulative risk assessment for the phthalates due to their ubiquitous presence in the environment and human urine samples. The NAS panel concluded that the US EPA should conduct cumulative risk assessments on phthalates that are known to inhibit fetal testosterone production (National Academies of Science, 2008). Furthermore, the NAS panel recommended that the US EPA also include other antiandrogenic environmental chemicals in their risk assessment of phthalates based on their common adverse outcome of impaired androgen-dependent male reproductive tract development, instead of cumulative risk assessment based on a narrowly-defined common mechanism of action (US EPA, 2002).
In this paper, we review published animal research from our laboratory on impaired male rat reproductive tract development following in utero exposure during the critical period of sexual differentiation. This research was intended to contribute to the development of a guidance framework for assessing the cumulative risk of mixtures of phthalates, or phthalates in combination with other antiandrogenic chemicals. Finally, we describe how our data have been applied to assess human hazard identification and assessment for phthalate and antiandrogenic chemical mixtures.
Adverse Outcome Pathways for Antiandrogenic Chemicals
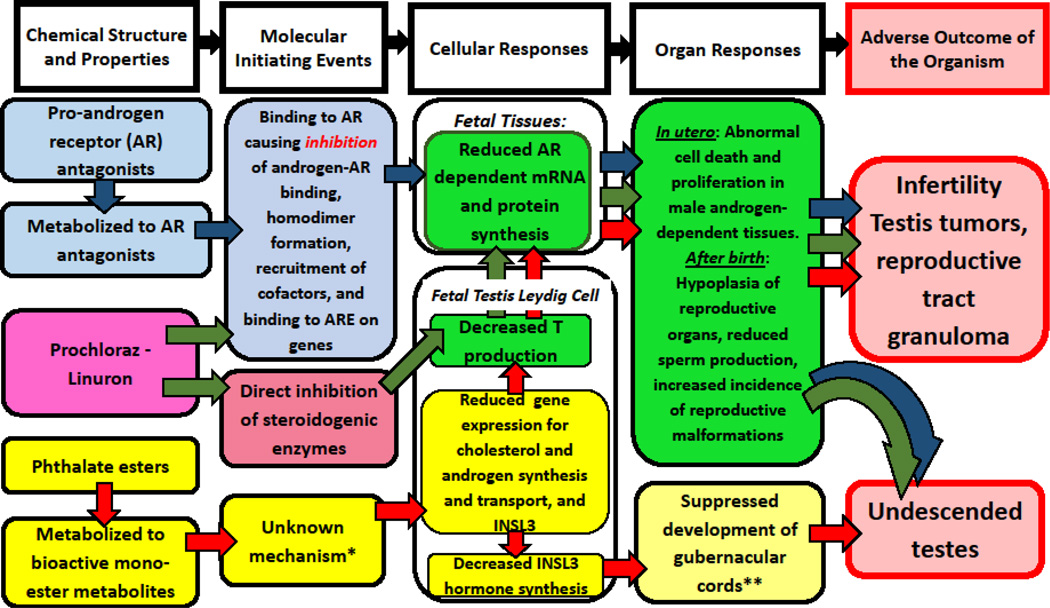
There are multiple mechanisms (i.e., molecular initiating events) by which chemicals can interfere with the androgen signaling pathway and, thereby, disrupt male reproductive development. In risk assessment, this series of events with multiple molecular initiating events leading to an adverse outcome can be outlined as an adverse outcome pathway (AOP) network (Edwards et al., 2016)(Figure 1). Among these are androgen receptor (AR) antagonism and inhibition of androgen synthesis enzymes, as well as the unknown mechanism by which phthalates reduce testosterone production. Depending on the timing of the exposure, these chemicals can disrupt development of androgen-sensitive reproductive organs (e.g., reducing organ weights or inducing malformations (e.g., hypospadias, undescended testes)), lead to reduced fertility, and, possibly, testicular cancer. Exposure to antiandrogenic chemicals in utero can cause many different alterations in the developing male rodent. Developmental effects of antiandrogenic chemicals include a shortening of the anogenital distance (AGD) at birth relative to control males, and the retention of areolae and/or nipples in juvenile and adult males (absent in control male rats). While these developmental effects (reduced AGD or retention of areolae and/or nipples) are not considered adverse, they are predictive of changes in adult reproductive tissues following in utero exposure to antiandrogenic chemicals during the period of sexual differentiation (Hotchkiss et al., 2004; McIntyre et al., 2001).
Figure 1.
Adverse outcome pathway network for disrupted androgen- and insulin-like hormone 3 (Insl3)-dependent reproductive development in male rats. The first column of the AOP network identifies three classes of chemicals known to disrupt the androgen-signaling pathways via three different mechanisms of action. Different colored arrows indicate the pathway through which each set of chemicals exerts its affects: blue arrows, androgen receptor (AR) antagonists; green arrows, dual mechanism of action chemicals (AR agonists antagonists and steroid enzyme inhibitors; and red arrows, phthalates (molecular initiating event unknown, but known to inhibit fetal testosterone (T) production. *Reproductive toxicant effects of phthalates are not mediated via the AR or the peroxisome proliferator activated receptor alpha (PPARα). **INSL3 hormone is required for maturation of the gubernacular cords, which leads to transabdominal descent of the testes (the first phase of testes descent).
The effects of antiandrogenic chemicals (e.g., phthalates) in the rat share a striking similarity to the human testicular dysgenesis syndrome (TDS) (Sharpe and Skakkebaek, 2008). Developmental exposure to phthalates has also been reported to negatively impact reproductive development in humans (Arbuckle et al., 2014)(reviewed in (Marie et al., 2015)) and in animal models other than the rat (e.g., rabbit, African clawed frog and mouse) (reviewed in (Howdeshell et al., 2008a)). Some studies have suggested possible species specificity in responsiveness to phthalates; however, the dose, route, and timing of exposure may have been significant contributing factors to the lack of inhibition of testosterone observed in these experiments (reviewed in (CHAP, 2014; Zarean et al., 2016)).
Mixture models
As interest in the study of chemical mixtures has grown over the past several decades, so too have efforts to accurately predict mixture responses based on individual chemical data. Predicted mixture responses can be compared to empirical mixture data to test hypotheses regarding the types of interactions occurring between multiple chemicals. These models are based on the concepts of dose addition, response addition (also called independent action or independent joint action), or integrated addition. Each of these models and their applications are discussed briefly below. In comparing empirical data to modeled predictions, the data can either fit one or more of the models, or diverge from modeled predictions to indicate potential greater than additive or less than additive interactions among mixture constituents.
Dose addition is commonly used for chemicals which share the same mechanism of action (Altenburger et al., 2003; Rider et al., 2008; Rider et al., 2010; Silva et al., 2002). The current US EPA guidance for cumulative risk assessment of chemical mixtures recommends that the chemicals share a common narrowly-defined mechanism of action (US EPA, 2002); for example, inhibition of acetylcholinesterase by phosphorylation and induction of cholinergic effects were identified as a common mechanism of action for organochlorine pesticides. There are many different approaches for calculating predicted mixture responses based on the concept of dose addition. However, all approaches involve adding together individual chemicals at the dose level. Often, this is accomplished by converting individual chemical doses to equivalent terms (Rider et al., 2008; Rider et al., 2010). Here, we accomplish this by dividing the dose of the individual chemical in the mixture by the effective dose 50% (ED50; i.e., the dose that results in a 50% response of a study population) of that chemical. The resulting adjusted ‘doses’ can then be added together to get on overall “total mixture dose” (Rider et al., 2008; Rider et al., 2010). This overall dose can then be used to predict the response expected from the mixture of interest. Below is a mathematical formula used for dose addition:
| (Equation 1) |
where R is the response to the mixture, Di is the concentration of chemical i in the mixture, ED50i is the concentration of chemical i that causes a 50% response, and ρ′ is the average Hill slope (i.e. slope associated with a logistic fit of the individual chemical dose-response curve) associated with the all chemicals (Equation 1).
The effects of a mixture comprised of chemicals acting via different mechanisms of action are traditionally thought to be most accurately predicted using the response addition model (Greco et al., 1992). This model uses probability theory to arrive at the predictions for mixtures.
| (Equation 2) |
where R represents the response to the mixture, and Ri is the response to individual chemical i in the mixture (Equation 2). The methods for predicting the dose addition and response addition effects of mixtures have been described in detail elsewhere (Howdeshell et al., 2007; Howdeshell et al., 2008a; Rider and LeBlanc, 2005).
More recently, a model has been suggested that incorporates aspects of both dose addition and response addition called integrated addition (Altenburger et al., 2003; Rider and LeBlanc, 2005; Teuschler et al., 2004). Integrated addition is a two-step process that first groups the chemicals that operate via the same mechanism of action and calculates a total dose based on dose addition. Mechanism-based groups are then combined using the response addition model to arrive at mixture response predictions. An equation for the integrated addition model is displayed below (Equation 3; abbreviations are defined in Equation 1):
| (Equation 3) |
Using these models, one can investigate whether chemicals conform to one of the additivity models, or differ from a specified model, indicating a potential greater than or less than additive interaction. These studies help us to understand how chemicals behave when they are present together, and they can inform decisions about which chemicals to include in cumulative risk assessments. Model selection has significant implications for risk assessment. To illustrate the importance of the difference between dose addition and response addition consider that with response addition, chemicals below their No Observed Adverse Effect Level (NOAEL) do not contribute to mixture toxicity; whereas with dose addition, incremental increases in total mixture dose can lead to significant mixture effects even when all chemicals are present below their NOAEL.
Mixture Study Design
Our mixture studies followed a similar basic study design. Pregnant Sprague-Dawley rats were given oral doses of individual chemicals or a combination of chemicals during a critical window of male reproductive tract development (Carruthers and Foster, 2005). Male offspring were assessed for fetal testicular testosterone production (referred to here as fetal testosterone production), fetal testicular gene expression, or postnatal reproductive parameters.
Our early mixture studies were simple two-by-two factorial designs with binary combinations of chemicals. During these studies, pregnant rats were dosed during fetal sexual differentiation with chemicals singly or in pairs at doses levels roughly equivalent to one half of the effective dose to induce a 50% incidence (ED50) of epididymal agenesis or hypospadias (Gray et al., 2001; Hotchkiss et al., 2004; Howdeshell et al., 2007; Howdeshell et al., 2008a). At the selected doses, each individual chemical produced no or low rates of malformations, but the combination of chemicals produced significant dose-additive effects on reproductive tissues. Subsequent mixture research studies combined the individual chemicals in equipotent doses based on the ED50 for a postnatal malformation or fetal testosterone production. Importantly, the mixture studies from our laboratory were designed to produce significant responses to test the model predictions of dose or response additivity. Our experiments were not designed to test chemical mixtures based on levels of the individual chemicals present in the environment.
Mixtures with Similar Mechanism of Action
Chemicals that share a similar mechanism of action are predicted to have cumulative effects that would most accurately be predicted by dose addition. Our laboratory has investigated several different classes of chemicals sharing a similar mechanism of action (e.g., androgen receptor antagonists) or a similar mode of action (e.g., phthalates, which inhibit fetal testosterone production) to alter male reproductive development (Table 1). First, a mixture of the dicarboximide fungicides vinclozolin and procymidone was investigated (Rider et al., 2009). Both vinclozolin and procymidone are known androgen receptor antagonists (Hosokawa et al., 1993; Kelce et al., 1994; Nellemann et al., 2003; Ostby et al., 1999; Vinggaard et al., 1999). Following in utero exposure to the chemicals during the critical period of sex differentiation, male offspring exposed to vinclozolin alone displayed a 10% incidence of hypospadias and 0% incidence of vaginal pouch development, while procymidone exposure resulted in 0% incidence of either malformation. Exposure to both chemicals together resulted in 96% incidence of hypospadias and 54% incidence of vaginal pouch in treated animals. These data suggest that these two chemicals with similar mechanisms can act in a dose additive fashion on androgen-sensitive reproductive endpoints.
Table 1.
List of chemical mixtures targeting male reproductive development in the Earl Gray, Jr. laboratory, USEPA (as of October, 2016)
| Type of mixture | Mixture study designa: Chemicals in mixture |
Mechanism of individual chemicals | Mixture model(s) testedb |
Reference |
|---|---|---|---|---|
| Similar mechanisms of action, same signaling pathwayc |
B, EQ: Vinclozolin (VIN) + Procymidon (PROCYM) |
VIN and PROCYM: Androgen receptor (AR) antagonists | RA | (Andrew Hotchkiss and Earl Gray, Jr., unpublished; Howdeshell et al., 2008) |
| B, EQ: Di(n)butyl phthalate (DBP) + Benzyl butyl phthalate (BBP) |
DBP and BBP: inhibitors of fetal testosterone (T) synthesis with a one common active metabolite (monobutyl phthalate (MBP) |
RA | (Andrew Hotchkiss and Earl Gray, Jr., unpublished; Howdeshell et al., 2008) | |
| B, EQ: DBP + Diethylhexyl phthlate (DEHP) |
DBP and DEHP: inhibitors of fetal T synthesis with different active metabolites (MBP and monoethylhexyl phthalate (MEHP) |
DA, RA | Howdeshell et al., 2007 | |
| FR-D, EQ: BBP + DBP + DEHP + Diisobutyl phthalate (DiBP) + Dipentyl phthalate (DPeP) |
BBP, DBP, DEHP, DiBP, and DPeP: inhibitors of fetal T synthesis |
DA, RA | Howdeshell et al, 2008; Howdeshell et al. 2015 | |
| FR-D, EQ: BBP + DBP + DEHP + DiBP + DPeP + Dihexyl phthlate (DHP) + Diheptyl phthalate (DHeP) + Diisoheptyl phthalate (DiHeP) + dicyclohexyl phthalate (DCHP) |
BBP, DBP, DEHP, DiBP, DHP, DHeP, DiHeP, DCHP, and DPeP: inhibitors of fetal T synthesis |
DA, RA | Hannas et al., 2012 | |
| B, EQ: BBP + Linuron (LIN) |
BBP: inhibitor of fetal T synthesis LIN: AR antagonist and direct inhibitor of T synthesis |
DA | Hotchkiss et al. 2004 | |
| FR-D, EQ: DBP + PROCYM |
DBP: inhibitor of fetal T synthesis PROCYM: AR antagonist |
DA, RA | Hotchkiss et al. 2010 | |
| FR-D, EQ: VIN + PROCYM + Prochloraz (PROCL) + LIN + BBP + DBP + DEHP |
VIN and PROCYM: AR antagonists LIN: AR antagonist and direct inhibitor of T synthesis PROCL: AR antagonist and direct inhibitor of steroid hormone synthesis BBP, DBP, and DEHP: inhibitors of fetal T synthesis |
DA, RA, IA, TEQ |
Rider et al., 2008 | |
| FR-D, EQ: VIN + PROCYM + PROCL + LIN + BBP + DBP + DEHP + DiBP + DiHeP + DPeP |
VIN and PROCYM: AR antagonists LIN: AR antagonist and direct inhibitor of T synthesis PROCL: AR antagonist and inhibitor of steroid hormone synthesis BBP, DBP, DEHP, DiBP, DiHeP, DPeP: inhibitors of fetal T synthesis |
DA, RA, IA | Rider et al., 2010 | |
| FR-D, EQ: DBP + Pyrifluquinazon (PFQ) |
DBP: inhibitor of fetal T synthesis PFQ: Possible AR antagonist |
DA, RA | Earl Gray, Jr., under preparation | |
| Different signaling pathways, same target tissue |
B, EQ DBP + 2,3,7,8- Tetrachlorodibenzo-p-dioxin (TCDD) |
DBP: inhibitor of fetal T synthesis TCDD: Aryl hydrocarbon receptor (AhR) agonist |
RA | Rider et al., 2010 |
| Converging AOPs, same target tissue |
B, EQ: DPeP + Simvastatin (SIM) |
DPeP: inhibitor of fetal T synthesis SIM: inhibitor of cholesterol synthesis (via inhibition of 3- hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase) resulting in reduced fetal T synthesis |
RA | Beverly et al., 2014 |
| Converging AOPs, same target tissue and different mechanisms of action, same signaling pathway |
18 chemical, FR-D, LOEL study: VIN + PROCYM + PROCL + PFQ + p,p’- dichlorodiphenyl dichloroethylene (pp’DDE) + LIN + PHTHALATES (BBP + DBP + DEHP + DiBP + DiHeP + DPeP+ DHeP+ DCHP+ DHP) + flutamide (FLUT) + finasteride (FIN) + SIM |
VIN, PFQ, FLUT, p,p’DDE and PROCYM: AR antagonists LIN: AR antagonist and direct inhibitor of T synthesis PROCL: AR antagonist and direct inhibitor of steroid hormone synthesis BBP + DBP + DEHP + DiBP + DiHeP + DPeP+ DHeP+ DCHP+ DHP: inhibitors of fetal T synthesis SIM: inhibitor of cholesterol synthesis (via inhibition of 3- hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase) resulting in reduced fetal T synthesis FIN: direct inhibitor of dihydrotestosterone (DHT) synthesis (via 5 alpha reductase) |
DA, RA, IA, | Justin Conley and Earl Gray, Jr., under preparation |
| 15 chemical FR-D, NOEL study: VIN + PROCYM + PROCL +PFQ+ pp’DDE+LIN + PHTHALATES (BBP + DBP + DEHP + DiBP + DiHeP + DPeP+ DHeP+ DCHP+ DHP) |
VIN, PFQ, p,p’DDE and PROCYM: AR antagonists LIN: AR antagonist and direct inhibitor of T synthesis PROCL: AR antagonist and direct inhibitor of steroid hormone synthesis BBP + DBP + DEHP + DiBP + DiHeP + DPeP+ DHeP+ DCHP+ DHP: inhibitors of fetal T synthesis |
DA, RA, IA, | Justin Conley and Earl Gray, Jr., ongoing |
Mixture study design: binary (B) or fixed ratio dilution (FR-D) design with equipotent doses (EQ), doses based on one-fifth the lowest observed effect level (LOEL), or doses based on twice the,no observed effect level (NOEL) for the individual chemicals for the top dose.
Mixture models tested include: dose addition (DA), integrated addition (IA), response addition (RA), and toxic equivalency factor (TEQ). Studies that did not specifically test a mixture model, but compared the data to the individual chemical responses were considered to have tested RA.
AOP: adverse outcome pathway.
Another set of studies was conducted with mixtures of phthalates, which are chemicals that inhibit fetal testosterone production and alter androgen-sensitive male reproductive development. Phthalates are commonly found in the environment and the potential for co-exposure to multiple phthalates was the impetus for the previously discussed NAS review (National Academies of Science, 2008). The specific mechanism of action for phthalates is unknown, but altered reproductive tract development by phthalates in the male rat involves altered Leydig cell migration and differentiation and abnormal gonocyte development (reviewed in (Howdeshell et al., 2008a)). These changes result in reductions in fetal testicular testosterone production and mRNA levels for proteins in the steroidogenic pathway and insulin-like hormone 3 (insl3), a hormone critical for gubernacular cord development and the early phase of testis descent (Hughes and Acerini, 2008; Kumagai et al., 2002). The cumulative effects of phthalates were investigated in two binary studies: one study of in utero exposure to two phthalates with shared active metabolites (di-n-butyl phthalate (DBP) and benzylbutyl phthalate (BBP) and another study of phthalates with different active metabolites (DBP and diethylhexyl phthalate (DEHP). Dosing was based on combining the two phthalates at the one-half of the ED50 of each chemical to induce epididymal agenesis (Howdeshell et al., 2007). The results from the binary studies were consistent with the predictions for dose addition. For example, exposure to the individual chemicals resulted in little or no malformations; whereas, the exposure to a combination of chemicals generally resulted in 50% or greater incidences of malformations (Howdeshell et al., 2007). Next, we designed a mixture study of five phthalates (DBP, DEHP, BBP, diisobutyl phthalate (DiBP), and dipentyl phthalate (DPeP)) with each phthalate represented in the mixture at an equipotent dose for inhibition of fetal testicular testosterone production (Howdeshell et al., 2008b). Dose addition accurately predicted the observed effects of the five phthalate mixture to reduce fetal testosterone production and induce fetal mortality (fetal resorptions or dead fetuses) when administered to the pregnant dam from gestation day 8 to 18.
Up to this point, mixture studies in our laboratory had been based on data for a reproductive endpoint (e.g., hypospadias or epididymal agenesis) for the individual chemical; however, as is often a challenge in cumulative risk assessments, there was insufficient data on each individual phthalate in the mixture for many of the postnatal reproductive endpoints evaluated. In a companion study, we evaluated whether dose addition based on the fetal testosterone production data of individual phthalates would predict the effects the same 5 phthalate mixture on androgen-sensitive postnatal male reproductive tract development (Howdeshell et al., 2015) In brief, the observed mixture responses were compared to predictions of dose addition based on the previously published potencies of the individual phthalates to reduce fetal testosterone production (Howdeshell et al., 2008b) relative to a reference chemical and published postnatal data for the reference chemical (e.g., DBP). As hypothesized, dose addition based on fetal testosterone production accurately predicted the observed mixture effect on 11 of 14 endpoints, including the induction of reproductive malformations, suppression of androgen-sensitive reproductive organ weights, and inhibition of gene expression of insl3, and genes involved in steroidogenesis (Howdeshell et al., 2015). Similar dose additive effects have been observed for a mixture of 9 phthalates (DBP, DEHP, BBP, DiBP, DPeP, dihexyl (DHP), diheptyl (DHeP), diisononyl (DiNP), or diisodecyl phthalate (DiDP) on inhibition of fetal testosterone and several genes involved in steroidogenesis and steroid hormone transport (Hannas et al., 2012).
Mixtures with Multiple Mechanisms of Action, Same Pathway
We have also investigated the potential for cumulative effects of mixtures of antiandrogenic chemicals operating via different molecular or cellular mechanisms of toxicity to alter male reproductive development (Table 1). This broader approach focuses on chemicals that either disrupt a common signaling pathway and/or a common target tissue, rather than a common mechanism of toxicity. Grouping chemicals that affect a common target tissue is in line with the NAS recommendations (National Academies of Science, 2008) and would represent a considerable broadening of the chemicals considered for cumulative risk.
In one of the first mixture studies combining antiandrogenic chemicals with different mechanisms of action, we investigated whether the phthalate BBP (a fetal testosterone inhibitor) would act in a cumulative fashion if combined with the pesticide linuron (an androgen receptor antagonist as well as an enzyme inhibitor). In this study, BBP, linuron, or a mixture of BBP and linuron were administered to pregnant rats from gestational day 14 to18. In utero exposure to BBP alone elicited a 0% incidence of hypospadias and vaginal pouch formation and 12% incidence of epididymal agenesis in male rats. In utero exposure to linuron alone resulted in 0% incidence of hypospadias and vaginal pouch development and 63% incidence of epididymal agenesis. However, the mixture of BBP and linuron resulted in hypospadias in 56% of males, vaginal pouch in 40% of males, and epididymal agenesis in 97% of the males (Hotchkiss et al., 2004). This study was one of the first to suggest that chemical mixtures with a variety of mechanisms of action can act in a cumulative fashion; this study did not evaluate dose addition.
Next, we conducted a binary study with another phthalate, DBP (a fetal testosterone production inhibitor), and procymidone (a pure androgen receptor antagonist) to evaluate whether chemicals that do not share any similarities in their mechanism of action are able to act in a cumulative fashion on a common adverse outcome; in this case, the adverse outcome was androgen-sensitive reproductive tract development (Hotchkiss et al., 2010). If these chemicals act in a cumulative fashion, then the dose addition model would most accurately predict the responses of the mixtures. If, as would be predicted by the response addition model, the chemicals with different mechanisms of action did not act in a cumulative fashion, then the response addition model would most accurately predict the empirical data. This study built on previously published experiments with the individual chemicals from our laboratory in which determined the shape of the dose response curves. We then conducted an experiment with a mixture of procymidone and DBP given at a dose that was expected to induce 100% incidence (ED100) of reproductive tract malformations in male rats. In addition to this high dose, we conducted a second experiment where we treated the pregnant rats with a mixture at 83, 67, 50, 33, 17, 8, 4, or 0% of the maximal dose. In the binary mixture, we observed that each chemical alone induced low incidences of hypospadias (DBP, 0% and procymidone, 1.5%) or vaginal pouch (DBP or procymidone, 0%). In contrast, the males exposed in utero to the mixture of DBP and procymidone displayed 49% incidence of hypospadias and a 27% incidence of vaginal pouch indicating that the interaction was at least dose additive. In the second experiment, interstitial cell hyperplasia and adenoma were observed in the testes of rats from the high dose groups of the mixture, but not in either the procymidone or DBP treatment groups (Hotchkiss et al., 2010).
Another set of experiments added further clarity to whether chemicals can act on different parts of a common signaling pathway and induce cumulative effects (Rider et al., 2008; Rider et al., 2010). In the first experiment, pregnant rats were dosed from gestation day 14 to 18 with a dose range of an antiandrogenic chemical mixture comprised of: two androgen receptor antagonists (procymidone and vinclozolin), three phthalates (e.g., testosterone synthesis inhibitors (BBP, DBP, DEHP; testosterone synthesis inhibitors), and two pesticides with dual mechanisms of androgen receptor antagonism and testosterone synthesis inhibition (prochloraz and linuron) (Rider et al., 2008). The second experiment involved the same antiandrogenic chemicals and added three more phthalates (DiBP, diisooheptyl phthalate (DiHeP), and DPeP; testosterone synthesis inhibitors) (Rider et al., 2010). The chemicals were combined such that the high dose of each chemical contributed equally to induce 100% malformations (ED100) at the highest mixture dose tested. We had multiple chemicals acting via the same mechanism as well as groups of chemicals acting on different parts of the androgen signaling pathway by different mechanisms, so we tested dose addition, response addition as well as integrated addition models. Thus, we also tested whether the integrated addition model would provide the most accurate prediction of the effects. The dose addition model provided the best fit to observed mixture effects to disrupt androgen-sensitive reproductive tract development and induce reproductive malformations in the male rat, even though not all chemicals acted via a common cellular mechanism of action (Rider et al., 2008; Rider et al., 2010). Given that dose addition most closely predicted the effects observed, consideration of the cumulative effects of chemicals affecting multiple aspects of the same signaling pathway is warranted.
Mixtures with Different Pathways, Same Target Tissue
From our previous work, we established that chemicals that act via the same or disparate mechanisms of action should be considered for their cumulative effects if they alter the AOP network for disrupted androgen- and insl3-dependent male reproductive development. One extension of this is to ask whether chemicals that affect the same target tissue via different signaling pathways would also act in a cumulative manner (Table 1). An initial study investigating this possibility used two chemicals that affect reproductive tissues via distinct signaling pathways - the androgen signaling pathway and the aryl hydrocarbon receptor (AhR) signaling pathway (Rider et al., 2010). The two chemicals used were DBP, and 2,3,7,8 tetrachlorodibenzo dioxin (TCDD). Dams were dosed with either DBP, TCDD, or one of two different doses of a mixture of DBP and TCDD. The doses of the mixture were selected to produce a significant incidence of malformations in the adult male rats. This mixture of DBP and TCDD reduced the weight of the epididymides, a common androgen-sensitive target tissue, which were greater than that which would have been predicted by response addition alone (independent action). In other tissues classically affected by DBP displayed alterations that were either greater than expected via response addition or anticipated by response addition. These initial data suggest that the universe of chemicals that should be considered for cumulative effects may, in fact, be much larger than simply chemicals which disrupt a common signaling pathway. Further studies are needed to determine whether the mixture of DBP and TCDD act in a dose-additive manner.
Mixture with Different Mechanism of Action, Converging AOPs and Same Pathway
Disruption of different key events along the steroidogenic pathway was investigated in a mixture study examining the potential cumulative effects of combining the fetal Leydig cell toxicant, dipentyl phthalate (DPeP) with the cholesterol lowering drug, Simvastatin (Beverly et al., 2014) (Table 1). Although both these chemicals potentially affect the steroid biosynthesis pathway, they do so via different modes of action. DPeP is a fetal androgen synthesis inhibitor (molecular initiating event unknown), while Simvastatin directly inhibits an enzyme, HMG-CoA reductase, in the cholesterol synthesis pathway (e.g., part of the cholesterol AOP). Because cholesterol is the precursor in steroid hormone production, we hypothesized that Simvastatin alone would reduce cholesterol and decrease the level of fetal testosterone production when administered during the critical period of sexual differentiation. Secondly, we hypothesized that a mixture of DPeP and Simvastatin would reduce fetal testosterone production in a cumulative fashion. In the mixture study, pregnant rats were dosed with either Simvastatin, DPeP, or a mixture of both chemicals. The doses were based on previous studies and were expected to partially but not completely reduce testosterone levels in fetal rats exposed to the individual chemicals. The results of the mixture could then be examined for cumulative or antagonistic effects. The results suggested that the mixture of DPeP and Simvastatin had a cumulative effect on fetal testosterone production despite operating via two different MOAs (Beverly et al., 2014).
Our laboratory is working on several studies which test the accuracy of the dose addition model to predict effects chemical mixtures with different mechanisms of action on the same AOP network and in combination with a chemical from a converging AOP (i.e., Simvastatin) on male reproductive development in rats (Table 1). The DBP and pyrifluquinazon (PFQ) mixture study combines two chemicals with different molecular initiating events in the same androgen AOP network to test for dose and response additive effects on F1 male rat reproductive development (Justin Conley and Earl Gray, Jr., under preparation). An 18 chemical study has been designed to test the accuracy of dose, response, or integrated addition models to predict the effects on F1 male reproductive development when the top dose of the mixture includes each chemical at one-fifth of the lowest observed effect level (LOEL) for inducing a developmental reproductive effect; the study tested 100, 50, 25, 12.5, 6.25 and 0% of the top dose of the mixture (Justin Conley and Earl Gray, Jr, under preparation). Finally, we have designed a 15 chemical mixture to determine if 15 chemicals, each chemical present in the top dose at twice its no observed effect level (NOEL) for inducing a developmental reproductive effect, would behave in a dose, response, or integrated addition manner (Justin Conley and Earl Gray, Jr, ongoing study); the dose range of the 15 chemical mixture included two very low dose groups (100, 50, 3, 0.5, 0.05, and 0% of the top dose).
Animal to human extrapolation
The metabolism of phthalates is similar between rats and humans (Kluwe, 1982), and the fetuses of both species are exposed to similar metabolites of these chemicals (Calafat et al., 2006; Silva et al., 2004). Silva et al. (2004) reported that MBP and MEHP, monoester metabolites of DBP and DEHP respectively, were detected in 93% (MBP) and 24% (MEHP) of 54 human amniotic fluid samples tested. Two percent of the human amniotic fluid samples had MBP and MEHP levels that differed by only five-fold (MBP) and 24-fold (MEHP) from the levels in rat amniotic fluid from dams treated with oral dosage levels near their lowest observed adverse effect levels (LOAEL) (Calafat et al., 2006; Gray et al., 2009; Mylchreest et al., 2000). This margin of exposure is within the default uncertainty factor of 100, which is normally applied to risk assessment when considering experimental animal data to human data. It is possible that the human fetus is exposed levels of phthalate metabolites greater than reported in Silva (Silva et al., 2004) because analytical methods for measuring phthalate metabolites have since improved and a number of oxidative metabolites of monoesters have been detected in greater quantity monoester metabolites in human urine samples (Barr et al., 2003; Koch et al., 2004; Silva et al., 2006). Research on phthalate metabolism in rats has also been useful in identifying potential human phthalate metabolites. Our laboratory has collaborated with the Centers for Disease Controls (Atlanta, GA) to analyze phthalate metabolites in the urine samples of adult Sprague-Dawley rats treated with di-isodecyl phthalate (Kato et al., 2007), di-n-octyl phthalate (Silva et al., 2005), and DPeP (Silva et al., 2011). Similar metabolism studies have also been conducted on the phthalate containing flame retardant tetrabromophthalate (BEH-TEBP) (Silva et al., 2016) and the phthalate alternative di(isononyl)cyclohexane-1,2-dicarboxylate (DINCH) (Silva et al., 2012).
Implications for cumulative risk assessment for human health
Developmental reproductive toxicity studies of phthalates in laboratory animals have important implications for human health risk assessments. The US Consumer Products Safety Commission convened a panel of experts to conduct a risk assessment on the phthalates used in consumer products (Lioy et al., 2015). The panel, called the Chronic Hazard Advisory Panel on Phthalates and Phthalate Alternatives (referred to here as CHAP) used animal data for the basis of the phthalate risk assessment due to a lack of human data to directly quantify risk. A hazard index (HI) approach was applied for the 5 reproductive toxicant phthalates known to be present in consumer products: DBP, DiBP, BBP, DEHP, and DiNP. The HI is the sum of hazard quotients (HQs), which are the ratio of exposure (e.g., estimate of daily intake) to intakes deemed acceptable for an individual chemical for the same period of time (e.g., usually daily). Acceptable daily intakes (also called tolerable daily intakes or TDIs) and reference doses (RfDs) are examples of values that have been used as the denominator of HQs for assessing cumulative risk (Lioy et al., 2015). Daily intake was based on human biomonitoring data of specific phthalates metabolites in pregnant women (15–44 years old; NHANES 2005–2006 (CDC, 2012)) and infants (ages 2–36 months old; (Sathyanarayana et al., 2008a; Sathyanarayana et al., 2008b)). The HI estimate was based on an individual’s urinary concentrations of mixtures of phthalates, instead of population percentiles, which allowed for an analysis of the distribution of HI among the individuals sampled. A HI of >1.0 indicates possible concern for adverse health effects in the exposed individual. The authors reported an elevated hazard for pregnant women and infants with approximately 10% of pregnant women and 4–5% of infants exceeding a HI >1.0. Furthermore, the CHAP also reported that the number of individuals with a HI>1.0 would increase if the cumulative risk assessment included these 5 phthalates in combination with 10 antiandrogenic chemicals with different modes of action; specifically, they estimated a HI of 0.88 in the 75th percentile and that 20% of pregnant women would have a HI>1.0 (CHAP, 2014; Lioy et al., 2015). The estimated HI for this complex mixture represents a worst case exposure scenario based on biomonitoring data for the five phthalates and three of the antiandrogenic chemicals (bisphenol A, butylparaben, and propylparaben) as well as the high intake of 7 additional antiandrogenic chemicals of different modes of action reported in Kortenkamp and Faust (2010) (vinclozolin, prochloraz, procymidone, linuron, fenitrothion, p,p’-DDE, and BDE99).
In addition to the CHAP report, many international agencies have conducted cumulative risk assessments on select phthalates detected in their biomonitoring programs. Many of the assessments reported that children were more likely to have a HI>1 than adults. Beko et al. (2013) reported that 30% of 439 Danish children exceeded the TDI levels of DBP, DiBP and DEHP when considering exposure from dust ingestion, inhalation and dermal absorption to assess the cumulative tolerable daily intake (TDIcum). In contrast when the tolerable daily intake was based on each phthalate individually, the recommended TDI was exceeded for only two phthalates: DBP (5.1% of the children) and DiBP (5.3% of the children tested) only (Beko et al., 2013). A cumulative risk assessment of phthalates in the Belgian population reported that 25% of children had a HI >1.0 based primarily on exposure to DEHP, DiBP and DBP (Dewalque et al., 2014). Hartmann et al. (2015) reported that 4% of Austrian children of age 7 years old and 13% of children of age 11 years old exceeded the HI of 1.0 when the assessment was based on TDI, but no children were in excess of the HI of 1.0 when the assessment was based on reference dose (RfD; the estimate of the daily oral dose) of the antiandrogenic phthalates.
Conclusions
In conclusion, chemicals that disrupt reproductive tract development can contribute to cumulative risk, even though the individual chemicals may act via different mechanisms of action to disrupt the androgen signaling pathway. As would be predicted, chemicals that share the same mechanism of action (e.g., androgen receptor antagonists) elicited a cumulative effect on the development of androgen-sensitive tissues. However, chemicals that operate via different mechanisms of action also can act in a dose additive fashion on the androgen signaling pathway. While there are different mechanisms at play (e.g., androgen receptor antagonism and inhibition of fetal testosterone synthesis), these alterations can act in a dose additive manner by reducing the quantity of activated androgen receptor and subsequent gene expression in the reproductive tissues resulting in adverse effects for the developing male rat. In addition to chemicals which operate via different mechanisms of action on a common pathway, recent research has suggested that alterations on entirely different pathways that converge on a single tissue can also have at least additive effects. This was demonstrated with the co-exposure of chemicals which affect the AR signaling pathway with a chemical (TCDD) that alters the AhR pathway. Both of these pathways converge on several common reproductive tissues and results from this initial study support the conclusions reached by the NAS panel in 2008 which recommended considering all chemicals that affect a common tissue in a cumulative assessment (National Academies of Science, 2008). Furthermore, the laboratories of Ulla Hass and Andreas Kortenkamp have corroborated our observations that dose addition accurately predicts the effects of mixtures of antiandrogenic chemicals on androgen-dependent reproductive development, regardless of whether the individual chemical share a mechanism of action or not (Christiansen et al., 2009; Hass et al., 2007; Metzdorff et al., 2007; Orton et al., 2014; Orton et al., 2012). They also report that mixtures of endocrine disrupting chemicals combined at environmentally-relevant dose levels induce effects greater than expected based on individual chemical doses levels (Axelstad et al., 2014; Isling et al., 2014). Research on mixtures of antiandrogens and other combinations of endocrine-disrupting chemicals is an important area of study that, in combination with human biomonitoring, will inform more accurate cumulative risk assessments to preserve human reproductive health.
Highlights.
Many chemicals disrupt androgen-sensitive male reproductive development in the rat.
Mixtures of antiandrogenic chemicals act via dose addition, regardless of mechanism.
Cumulative risk assessments report a HI>1 for phthalates with other antiandrogens.
Acknowledgments
We thank Drs. Justin Conley and Cynthia V. Rider for their comments on earlier drafts of this manuscript. Disclaimer: The research described in this article has been reviewed by the National Health and Environmental Effects Research Laboratory at the U.S. Environmental Protection Agency and approved for publication. Approval does not signify that the contents necessarily reflect the views and policies of the Agency nor does mention of trade names of commercial products constitute endorsement or recommendation for use. This research was supported in part by the Intramural Research Program of the National Institute of Environmental Health Sciences (NIEHS), National Institutes of Health (NIH). This article may be the work product of an employee or group of employees of the NIEHS, NIH; however, the statements, opinions or conclusions contained therein do not necessarily represent the statements, opinions or conclusions of NIEHS, NIH or the United States government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Altenburger R, Nendza M, Schuurmann G. Mixture toxicity and its modeling by quantitative structure-activity relationships. Environ Toxicol Chem. 2003;22:1900–1915. doi: 10.1897/01-386. [DOI] [PubMed] [Google Scholar]
- Ankley GT, Brooks BW, Huggett DB, Sumpter JP. Repeating history: pharmaceuticals in the environment. Environ Sci Technol. 2007;41:8211–8217. doi: 10.1021/es072658j. [DOI] [PubMed] [Google Scholar]
- Arbuckle TE, Davis K, Marro L, Fisher M, Legrand M, LeBlanc A, Gaudreau E, Foster WG, Choeurng V, Fraser WD, Grp MS. Phthalate and bisphenol A exposure among pregnant women in Canada - Results from the MIREC study. Environ Int. 2014;68:55–65. doi: 10.1016/j.envint.2014.02.010. [DOI] [PubMed] [Google Scholar]
- Axelstad M, Christiansen S, Boberg J, Scholze M, Jacobsen PR, Isling LK, Kortenkamp A, Hass U. Mixtures of endocrine-disrupting contaminants induce adverse developmental effects in preweaning rats. Reproduction. 2014;147:489–501. doi: 10.1530/REP-13-0447. [DOI] [PubMed] [Google Scholar]
- Barr DB, Silva MJ, Kato K, Reidy JA, Malek NA, Hurtz D, Sadowski M, Needham LL, Calafat AM. Assessing human exposure to phthalates using monoesters and their oxidized metabolites as biomarkers. Environ Health Perspect. 2003;111:1148–1151. doi: 10.1289/ehp.6074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter CE, Pappas S, Abel MT, Kendall RJ. Organochlorine pesticides, lead, and mercury in northern bobwhite (Colinus virginianus) and scaled quail (Callipepla squamata) from the Rolling Plains ecoregion of Texas and Oklahoma. Environ Toxicol Chem. 2015;34:1505–1510. doi: 10.1002/etc.2917. [DOI] [PubMed] [Google Scholar]
- Becker K, Goen T, Seiwert M, Conrad A, Pick-Fuss H, Muller J, Wittassek M, Schulz C, Kolossa-Gehring M. GerES IV: phthalate metabolites and bisphenol A in urine of German children. Int J Hyg Environ Health. 2009;212:685–692. doi: 10.1016/j.ijheh.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Beko G, Weschler CJ, Langer S, Callesen M, Toftum J, Clausen G. Children's phthalate intakes and resultant cumulative exposures estimated from urine compared with estimates from dust ingestion, inhalation and dermal absorption in their homes and daycare centers. PLoS One. 2013;8:e62442. doi: 10.1371/journal.pone.0062442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beverly BE, Lambright CS, Furr JR, Sampson H, Wilson VS, McIntyre BS, Foster PM, Travlos G, Gray LE., Jr Simvastatin and dipentyl phthalate lower ex vivo testicular testosterone production and exhibit additive effects on testicular testosterone and gene expression via distinct mechanistic pathways in the fetal rat. Toxicol Sci. 2014;141:524–537. doi: 10.1093/toxsci/kfu149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blount BC, Silva MJ, Caudill SP, Needham LL, Pirkle JL, Sampson EJ, Lucier GW, Jackson RJ, Brock JW. Levels of seven urinary phthalate metabolites in a human reference population. Environ Health Perspect. 2000;108:979–982. doi: 10.1289/ehp.00108979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boobis AR, Ossendorp BC, Banasiak U, Hamey PY, Sebestyen I, Moretto A. Cumulative risk assessment of pesticide residues in food. Toxicol Lett. 2008;180:137–150. doi: 10.1016/j.toxlet.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Calafat AM, Brock JW, Silva MJ, Gray LE, Jr, Reidy JA, Barr DB, Needham LL. Urinary and amniotic fluid levels of phthalate monoesters in rats after the oral administration of di(2-ethylhexyl) phthalate and di-n-butyl phthalate. Toxicology. 2006;217:22–30. doi: 10.1016/j.tox.2005.08.013. [DOI] [PubMed] [Google Scholar]
- Carruthers CM, Foster PM. Critical window of male reproductive tract development in rats following gestational exposure to di-n-butyl phthalate. Birth Defects Res B Dev Reprod Toxicol. 2005;74:277–285. doi: 10.1002/bdrb.20050. [DOI] [PubMed] [Google Scholar]
- CDC. National Health and Nutrition Examination Survey Data (NHANES), Years sampled 2005–2006. Hyattsville, MD: Centers for Disease Control and Prevention (CDC) Department of Health and Human Services; 2012. [Google Scholar]
- Sciences, D.f.H., editor. CHAP. Report to the US Consumer Product Safety Commission by the Chronic Hazard Advisory Panel (CHAP) on phthalates and phthalate alternatives. Bethesda, MD: U.S. Consumer Product Safety Commission; 2014. p. 111. [Google Scholar]
- Christiansen S, Scholze M, Dalgaard M, Vinggaard AM, Axelstad M, Kortenkamp A, Hass U. Synergistic disruption of external male sex organ development by a mixture of four antiandrogens. Environ Health Perspect. 2009;117:1839–1846. doi: 10.1289/ehp.0900689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewalque L, Charlier C, Pirard C. Estimated daily intake and cumulative risk assessment of phthalate diesters in a Belgian general population. Toxicol Lett. 2014;231:161–168. doi: 10.1016/j.toxlet.2014.06.028. [DOI] [PubMed] [Google Scholar]
- Durhan EJ, Lambright CS, Makynen EA, Lazorchak J, Hartig PC, Wilson VS, Gray LE, Ankley GT. Identification of metabolites of trenbolone acetate in androgenic runoff from a beef feedlot. Environ Health Perspect. 2006;114(Suppl 1):65–68. doi: 10.1289/ehp.8055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards SW, Tan YM, Villeneuve DL, Meek ME, McQueen CA. Adverse Outcome Pathways-Organizing Toxicological Information to Improve Decision Making. J Pharmacol Exp Ther. 2016;356:170–181. doi: 10.1124/jpet.115.228239. [DOI] [PubMed] [Google Scholar]
- Enke U, Schleussner E, Palmke C, Seyfarth L, Koch HM. Phthalate exposure in pregnant women and newborns - the urinary metabolite excretion pattern differs distinctly. Int J Hyg Environ Health. 2013;216:735–742. doi: 10.1016/j.ijheh.2013.01.006. [DOI] [PubMed] [Google Scholar]
- Eskenazi B, Bradman A, Castorina R. Exposures of children to organophosphate pesticides and their potential adverse health effects. Environ Health Perspect. 1999;107(Suppl 3):409–419. doi: 10.1289/ehp.99107s3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray LE, Jr, Barlow NJ, Howdeshell KL, Ostby JS, Furr JR, Gray CL. Transgenerational effects of di (2-ethylhexyl) phthalate in the male CRL:CD(SD) rat: added value of assessing multiple offspring per litter. Toxicol Sci. 2009;110:411–425. doi: 10.1093/toxsci/kfp109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray LE, Ostby J, Furr J, Wolf CJ, Lambright C, Parks L, Veeramachaneni DN, Wilson V, Price M, Hotchkiss A, Orlando E, Guillette L. Effects of environmental antiandrogens on reproductive development in experimental animals. Hum Reprod Update. 2001;7:248–264. doi: 10.1093/humupd/7.3.248. [DOI] [PubMed] [Google Scholar]
- Greco W, Unkelbach H, H P, Sühnel J, Kundi M, Bodeker W. Consensus on concepts and terminology for combined action assessment: the Saariselkä agreement. Arch Complex Environ Stud. 1992;4:60–65. [Google Scholar]
- Hannas BR, Lambright CS, Furr J, Evans N, Foster PM, Gray EL, Wilson VS. Genomic biomarkers of phthalate-induced male reproductive developmental toxicity: a targeted RT-PCR array approach for defining relative potency. Toxicol Sci. 2012;125:544–557. doi: 10.1093/toxsci/kfr315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann C, Uhl M, Weiss S, Koch HM, Scharf S, Konig J. Human biomonitoring of phthalate exposure in Austrian children and adults and cumulative risk assessment. Int J Hyg Environ Health. 2015;218:489–499. doi: 10.1016/j.ijheh.2015.04.002. [DOI] [PubMed] [Google Scholar]
- Hass U, Scholze M, Christiansen S, Dalgaard M, Vinggaard AM, Axelstad M, Metzdorff SB, Kortenkamp A. Combined exposure to anti-androgens exacerbates disruption of sexual differentiation in the rat. Environ Health Perspect. 2007;115(Suppl 1):122–128. doi: 10.1289/ehp.9360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Health Canada. Ottawa, Ontario, Canada: Health Canada; 2016. Health Canada's Response to the Commissioner of the Environment and Sustainable Development 2015 Audit on Pesticide Safety : Pest Management Regulatory Agency's Approach to Assessing Cumulative Effects of Pesticides. [Google Scholar]
- Hela DG, Lambropoulou DA, Konstantinou IK, Albanis TA. Environmental monitoring and ecological risk assessment for pesticide contamination and effects in Lake Pamvotis, northwestern Greece. Environ Toxicol Chem. 2005;24:1548–1556. doi: 10.1897/04-455r.1. [DOI] [PubMed] [Google Scholar]
- Hosokawa S, Murakami M, Ineyama M, Yamada T, Koyama Y, Okuno Y, Yoshitake A, Yamada H, Miyamoto J. Effects of procymidone on reproductive organs and serum gonadotropins in male rats. J Toxicol Sci. 1993;18:111–124. doi: 10.2131/jts.18.111. [DOI] [PubMed] [Google Scholar]
- Hotchkiss AK, Parks-Saldutti LG, Ostby JS, Lambright C, Furr J, Vandenbergh JG, Gray LE., Jr A mixture of the "antiandrogens" linuron and butyl benzyl phthalate alters sexual differentiation of the male rat in a cumulative fashion. Biol Reprod. 2004;71:1852–1861. doi: 10.1095/biolreprod.104.031674. [DOI] [PubMed] [Google Scholar]
- Hotchkiss AK, Rider CV, Furr J, Howdeshell KL, Blystone CR, Wilson VS, Gray LE., Jr In utero exposure to an AR antagonist plus an inhibitor of fetal testosterone synthesis induces cumulative effects on F1 male rats. Reprod Toxicol. 2010;30:261–270. doi: 10.1016/j.reprotox.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howdeshell KL, Furr J, Lambright CR, Rider CV, Wilson VS, Gray LE., Jr Cumulative effects of dibutyl phthalate and diethylhexyl phthalate on male rat reproductive tract development: altered fetal steroid hormones and genes. Toxicol Sci. 2007;99:190–202. doi: 10.1093/toxsci/kfm069. [DOI] [PubMed] [Google Scholar]
- Howdeshell KL, Rider CV, Wilson VS, Furr JR, Lambright CR, Gray LE., Jr Dose Addition Models Based on Biologically Relevant Reductions in Fetal Testosterone Accurately Predict Postnatal Reproductive Tract Alterations by a Phthalate Mixture in Rats. Toxicol Sci. 2015;148:488–502. doi: 10.1093/toxsci/kfv196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howdeshell KL, Rider CV, Wilson VS, Gray LE., Jr Mechanisms of action of phthalate esters, individually and in combination, to induce abnormal reproductive development in male laboratory rats. Environ Res. 2008a;108:168–176. doi: 10.1016/j.envres.2008.08.009. [DOI] [PubMed] [Google Scholar]
- Howdeshell KL, Wilson VS, Furr J, Lambright CR, Rider CV, Blystone CR, Hotchkiss AK, Gray LE., Jr A mixture of five phthalate esters inhibits fetal testicular testosterone production in the Sprague-Dawley rat in a cumulative, dose-additive manner. Toxicol Sci. 2008b;105:153–165. doi: 10.1093/toxsci/kfn077. [DOI] [PubMed] [Google Scholar]
- Hughes IA, Acerini CL. Factors controlling testis descent. Eur J Endocrinol. 2008;159(Suppl 1):S75–S82. doi: 10.1530/EJE-08-0458. [DOI] [PubMed] [Google Scholar]
- Isling LK, Boberg J, Jacobsen PR, Mandrup KR, Axelstad M, Christiansen S, Vinggaard AM, Taxvig C, Kortenkamp A, Hass U. Late-life effects on rat reproductive system after developmental exposure to mixtures of endocrine disrupters. Reproduction. 2014;147:465–476. doi: 10.1530/REP-13-0448. [DOI] [PubMed] [Google Scholar]
- Jaspers VL, Covaci A, Voorspoels S, Dauwe T, Eens M, Schepens P. Brominated flame retardants and organochlorine pollutants in aquatic and terrestrial predatory birds of Belgium: levels, patterns, tissue distribution and condition factors. Environ Pollut. 2006;139:340–352. doi: 10.1016/j.envpol.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Jobling S, Tyler CR. Introduction: The ecological relevance of chemically induced endocrine disruption in wildlife. Environ Health Perspect. 2006;114(Suppl 1):7–8. doi: 10.1289/ehp.8046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K, Silva MJ, Wolf C, Gray LE, Needham LL, Calafat AM. Urinary metabolites of diisodecyl phthalate in rats. Toxicology. 2007;236:114–122. doi: 10.1016/j.tox.2007.04.009. [DOI] [PubMed] [Google Scholar]
- Kelce WR, Monosson E, Gamcsik MP, Laws SC, Gray LE., Jr Environmental hormone disruptors: evidence that vinclozolin developmental toxicity is mediated by antiandrogenic metabolites. Toxicol Appl Pharmacol. 1994;126:276–285. doi: 10.1006/taap.1994.1117. [DOI] [PubMed] [Google Scholar]
- Kendall R, Lacher T, Cobb G, Cox S. Wildlife Toxicology: Emerging Contaminant and Biodiversity Issues. Boca Raton, FL: CRC Press, Taylor & Francis Group; 2010. p. 322. [Google Scholar]
- Kluwe WM. Overview of phthalate ester pharmacokinetics in mammalian species. Environ Health Perspect. 1982;45:3–9. doi: 10.1289/ehp.82453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch HM, Bolt HM, Angerer J. Di(2-ethylhexyl)phthalate (DEHP) metabolites in human urine and serum after a single oral dose of deuterium-labelled DEHP. Arch Toxicol. 2004;78:123–130. doi: 10.1007/s00204-003-0522-3. [DOI] [PubMed] [Google Scholar]
- Koch HM, Wittassek M, Bruning T, Angerer J, Heudorf U. Exposure to phthalates in 5–6 years old primary school starters in Germany--a human biomonitoring study and a cumulative risk assessment. Int J Hyg Environ Health. 2011;214:188–195. doi: 10.1016/j.ijheh.2011.01.009. [DOI] [PubMed] [Google Scholar]
- Kolok AS, Snow DD, Kohno S, Sellin MK, Guillette LJ., Jr Occurrence and biological effect of exogenous steroids in the Elkhorn River, Nebraska, USA. Sci Total Environ. 2007;388:104–115. doi: 10.1016/j.scitotenv.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Kolpin DW, Furlong ET, Meyer MT, Thurman EM, Zaugg SD, Barber LB, Buxton HT. Pharmaceuticals, hormones, and other organic wastewater contaminants in U.S. streams, 1999–2000: a national reconnaissance. Environ Sci Technol. 2002;36:1202–1211. doi: 10.1021/es011055j. [DOI] [PubMed] [Google Scholar]
- Kortenkamp A, Faust M. Combined exposures to anti-androgenic chemicals: steps towards cumulative risk assessment. Int J Androl. 2010;33:463–474. doi: 10.1111/j.1365-2605.2009.01047.x. [DOI] [PubMed] [Google Scholar]
- Kumagai J, Hsu SY, Matsumi H, Roh JS, Fu P, Wade JD, Bathgate RA, Hsueh AJ. INSL3/Leydig insulin-like peptide activates the LGR8 receptor important in testis descent. J Biol Chem. 2002;277:31283–31286. doi: 10.1074/jbc.C200398200. [DOI] [PubMed] [Google Scholar]
- Lioy PJ, Hauser R, Gennings C, Koch HM, Mirkes PE, Schwetz BA, Kortenkamp A. Assessment of phthalates/phthalate alternatives in children's toys and childcare articles: Review of the report including conclusions and recommendation of the Chronic Hazard Advisory Panel of the Consumer Product Safety Commission. J Expo Sci Environ Epidemiol. 2015;25:343–353. doi: 10.1038/jes.2015.33. [DOI] [PubMed] [Google Scholar]
- Marie C, Vendittelli F, Sauvant-Rochat MP. Obstetrical outcomes and biomarkers to assess exposure to phthalates: A review. Environ Int. 2015;83:116–136. doi: 10.1016/j.envint.2015.06.003. [DOI] [PubMed] [Google Scholar]
- Maruya KA, Dodder NG, Sengupta A, Smith DJ, Lyons JM, Heil AT, Drewes JE. Multimedia screening of contaminants of emerging concern (CECS) in coastal urban watersheds in southern California (USA) Environ Toxicol Chem. 2016;35:1986–1994. doi: 10.1002/etc.3348. [DOI] [PubMed] [Google Scholar]
- McIntyre BS, Barlow NJ, Foster PM. Androgen-mediated development in male rat offspring exposed to flutamide in utero: permanence and correlation of early postnatal changes in anogenital distance and nipple retention with malformations in androgen-dependent tissues. Toxicol Sci. 2001;62:236–249. doi: 10.1093/toxsci/62.2.236. [DOI] [PubMed] [Google Scholar]
- Metzdorff SB, Dalgaard M, Christiansen S, Axelstad M, Hass U, Kiersgaard MK, Scholze M, Kortenkamp A, Vinggaard AM. Dysgenesis and histological changes of genitals and perturbations of gene expression in male rats after in utero exposure to antiandrogen mixtures. Toxicol Sci. 2007;98:87–98. doi: 10.1093/toxsci/kfm079. [DOI] [PubMed] [Google Scholar]
- Mitro SD, Johnson T, Zota AR. Cumulative Chemical Exposures During Pregnancy and Early Development. Curr Environ Health Rep. 2015;2:367–378. doi: 10.1007/s40572-015-0064-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mylchreest E, Wallace DG, Cattley RC, Foster PM. Dose-dependent alterations in androgen-regulated male reproductive development in rats exposed to Di(n-butyl) phthalate during late gestation. Toxicol Sci. 2000;55:143–151. doi: 10.1093/toxsci/55.1.143. [DOI] [PubMed] [Google Scholar]
- National Academies of Science. Phthalates and Cumulative Risk Assessment: The Task Ahead. Washington, DC: National Academies Press; 2008. [PubMed] [Google Scholar]
- Nellemann C, Dalgaard M, Lam HR, Vinggaard AM. The combined effects of vinclozolin and procymidone do not deviate from expected additivity in vitro and in vivo. Toxicol Sci. 2003;71:251–262. doi: 10.1093/toxsci/71.2.251. [DOI] [PubMed] [Google Scholar]
- Orton F, Ermler S, Kugathas S, Rosivatz E, Scholze M, Kortenkamp A. Mixture effects at very low doses with combinations of anti-androgenic pesticides, antioxidants, industrial pollutant and chemicals used in personal care products. Toxicol Appl Pharmacol. 2014;278:201–208. doi: 10.1016/j.taap.2013.09.008. [DOI] [PubMed] [Google Scholar]
- Orton F, Rosivatz E, Scholze M, Kortenkamp A. Competitive androgen receptor antagonism as a factor determining the predictability of cumulative antiandrogenic effects of widely used pesticides. Environ Health Perspect. 2012;120:1578–1584. doi: 10.1289/ehp.1205391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostby J, Kelce WR, Lambright C, Wolf CJ, Mann P, Gray LE., Jr The fungicide procymidone alters sexual differentiation in the male rat by acting as an androgen-receptor antagonist in vivo and in vitro. Toxicol Ind Health. 1999;15:80–93. doi: 10.1177/074823379901500108. [DOI] [PubMed] [Google Scholar]
- Rider CV, Furr J, Wilson VS, Gray LE., Jr A mixture of seven antiandrogens induces reproductive malformations in rats. Int J Androl. 2008;31:249–262. doi: 10.1111/j.1365-2605.2007.00859.x. [DOI] [PubMed] [Google Scholar]
- Rider CV, Furr JR, Wilson VS, Gray LE., Jr Cumulative effects of in utero administration of mixtures of reproductive toxicants that disrupt common target tissues via diverse mechanisms of toxicity. Int J Androl. 2010;33:443–462. doi: 10.1111/j.1365-2605.2009.01049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rider CV, LeBlanc GA. An integrated addition and interaction model for assessing toxicity of chemical mixtures. Toxicol Sci. 2005;87:520–528. doi: 10.1093/toxsci/kfi247. [DOI] [PubMed] [Google Scholar]
- Rider CV, Wilson VS, Howdeshell KL, Hotchkiss AK, Furr JR, Lambright CR, Gray LE., Jr Cumulative effects of in utero administration of mixtures of "antiandrogens" on male rat reproductive development. Toxicol Pathol. 2009;37:100–113. doi: 10.1177/0192623308329478. [DOI] [PubMed] [Google Scholar]
- RIVM. Report EFSA-RIVM Symposium, Rijksinstituut voor Volksgezondheid en Milieu. Bilthoven, The Netherlands: RIVM (Dutch National Institute for Public Health and the Environment); 2016. [Google Scholar]
- Sathyanarayana S, Calafat AM, Liu F, Swan SH. Maternal and infant urinary phthalate metabolite concentrations: are they related? Environ Res. 2008a;108:413–418. doi: 10.1016/j.envres.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathyanarayana S, Karr CJ, Lozano P, Brown E, Calafat AM, Liu F, Swan SH. Baby care products: possible sources of infant phthalate exposure. Pediatrics. 2008b;121:e260–e268. doi: 10.1542/peds.2006-3766. [DOI] [PubMed] [Google Scholar]
- Sexton K. Cumulative risk assessment: an overview of methodological approaches for evaluating combined health effects from exposure to multiple environmental stressors. Int J Environ Res Public Health. 2012;9:370–390. doi: 10.3390/ijerph9020370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe RM, Skakkebaek NE. Testicular dysgenesis syndrome: mechanistic insights and potential new downstream effects. Fertil Steril. 2008;89:e33–e38. doi: 10.1016/j.fertnstert.2007.12.026. [DOI] [PubMed] [Google Scholar]
- Silva E, Rajapakse N, Kortenkamp A. Something from "nothing"--eight weak estrogenic chemicals combined at concentrations below NOECs produce significant mixture effects. Environ Sci Technol. 2002;36:1751–1756. doi: 10.1021/es0101227. [DOI] [PubMed] [Google Scholar]
- Silva MJ, Furr J, Preau JL, Jr, Samandar E, Gray LE, Calafat AM. Identification of potential biomarkers of exposure to di(isononyl)cyclohexane-1,2-dicarboxylate (DINCH), an alternative for phthalate plasticizers. J Expo Sci Environ Epidemiol. 2012;22:204–211. doi: 10.1038/jes.2011.43. [DOI] [PubMed] [Google Scholar]
- Silva MJ, Furr J, Samandar E, Preau JL, Jr, Gray LE, Needham LL, Calafat AM. Urinary and serum metabolites of di-n-pentyl phthalate in rats. Chemosphere. 2011;82:431–436. doi: 10.1016/j.chemosphere.2010.09.052. [DOI] [PubMed] [Google Scholar]
- Silva MJ, Hilton D, Furr J, Gray LE, Preau JL, Calafat AM, Ye X. Quantification of tetrabromo benzoic acid and tetrabromo phthalic acid in rats exposed to the flame retardant Uniplex FPR-45. Arch Toxicol. 2016;90:551–557. doi: 10.1007/s00204-015-1489-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva MJ, Kato K, Gray EL, Wolf C, Needham LL, Calafat AM. Urinary metabolites of di-n-octyl phthalate in rats. Toxicology. 2005;210:123–133. doi: 10.1016/j.tox.2005.01.012. [DOI] [PubMed] [Google Scholar]
- Silva MJ, Reidy JA, Herbert AR, Preau JL, Jr, Needham LL, Calafat AM. Detection of phthalate metabolites in human amniotic fluid. Bull Environ Contam Toxicol. 2004;72:1226–1231. doi: 10.1007/s00128-004-0374-4. [DOI] [PubMed] [Google Scholar]
- Silva MJ, Reidy JA, Preau JL, Samandar E, Needham LL, Calafat AM. Measurement of eight urinary metabolites of di(2-ethylhexyl) phthalate as biomarkers for human exposure assessment. Biomarkers. 2006;11:1–13. doi: 10.1080/13547500500382868. [DOI] [PubMed] [Google Scholar]
- Solecki R, Stein B, Frische T, Matezki S, Wogram J, Streloke M. Paradigm shift in the risk assessment of cumulative effects of pesticide mixtures and multiple residues to humans and wildlife: German proposal for a new approach. J Verbrauch Lebensm. 2014;9:329–331. [Google Scholar]
- Teitelbaum SL, Britton JA, Calafat AM, Ye X, Silva MJ, Reidy JA, Galvez MP, Brenner BL, Wolff MS. Temporal variability in urinary concentrations of phthalate metabolites, phytoestrogens and phenols among minority children in the United States. Environ Res. 2008;106:257–269. doi: 10.1016/j.envres.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Teuschler LK, Rice GE, Wilkes CR, Lipscomb JC, Power FW. A feasibility study of cumulative risk assessment methods for drinking water disinfection by-product mixtures. J Toxicol Environ Health A. 2004;67:755–777. doi: 10.1080/15287390490428224. [DOI] [PubMed] [Google Scholar]
- Tyler CR, Jobling S, Sumpter JP. Endocrine disruption in wildlife: a critical review of the evidence. Crit Rev Toxicol. 1998;28:319–361. doi: 10.1080/10408449891344236. [DOI] [PubMed] [Google Scholar]
- US Congress. Food Quality Protection Act of 1996. Public Law 104–107; United States Congress; August 3, 1996; 1996. pp. 1–50. [Google Scholar]
- US EPA. Office of Pesticide Programs Office of Prevention Pesticides and Toxic Substances. Washington, DC: United States Environmental Protection Agency; 2002. Guidance on cumulative risk assessment of pesticide chemicals that have a common mechanism of toxicity; pp. 1–90. [Google Scholar]
- US EPA. Superfund Risk Assessment. Arlington, VA: National Center for Environmental Assessment, Office of Research and Development, United States Environmental Protection Agency; 2015. [Google Scholar]
- Vinggaard AM, Joergensen EC, Larsen JC. Rapid and sensitive reporter gene assays for detection of antiandrogenic and estrogenic effects of environmental chemicals. Toxicol Appl Pharmacol. 1999;155:150–160. doi: 10.1006/taap.1998.8598. [DOI] [PubMed] [Google Scholar]
- Woodruff TJ, Zota AR, Schwartz JM. Environmental chemicals in pregnant women in the United States: NHANES 2003–2004. Environ Health Perspect. 2011;119:878–885. doi: 10.1289/ehp.1002727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C, Huang X, Witter JD, Spongberg AL, Wang K, Wang D, Liu J. Occurrence of pharmaceuticals and personal care products and associated environmental risks in the central and lower Yangtze river, China. Ecotoxicol Environ Saf. 2014;106:19–26. doi: 10.1016/j.ecoenv.2014.04.029. [DOI] [PubMed] [Google Scholar]
- Ye X, Pierik FH, Angerer J, Meltzer HM, Jaddoe VW, Tiemeier H, Hoppin JA, Longnecker MP. Levels of metabolites of organophosphate pesticides, phthalates, and bisphenol A in pooled urine specimens from pregnant women participating in the Norwegian Mother and Child Cohort Study (MoBa) Int J Hyg Environ Health. 2009;212:481–491. doi: 10.1016/j.ijheh.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarean M, Keikha M, Poursafa P, Khalighinejad P, Amin M, Kelishadi R. A systematic review on the adverse health effects of di-2-ethylhexyl phthalate. [accessed 21 October 2016];Environ Sci Pollut Res Int. 2016 doi: 10.1007/s11356-016-7648-3. [DOI] [PubMed] [Google Scholar]