Abstract
Background & Aims
Little is known about outcomes of patients with ulcerative colitis with low-grade dysplasia (UC-LGD). We estimated the incidence of and risk factors for progression to colorectal cancer (CRC) in cohorts of patients with UC-LGD who underwent surveillance (surveillance cohort), and the prevalence of dysplasia-related findings among patients who underwent colectomy for UC-LGD (surgical cohort).
Methods
We performed a systematic literature review through June 1, 2016 to identify cohort studies of adults with UC-LGD. We estimated pooled incidence rates of CRC and risk factors associated with dysplasia progression in surveillance cohorts, and prevalence of synchronous advanced neoplasia (CRC and/or high-grade dysplasia) in surgical cohorts.
Results
In 14 surveillance cohort studies of 671 patients with UC-LGD (52 developed CRC), the pooled annual incidence of CRC was 0.8% (95% CI, 0.4–1.3); the pooled annual incidence of advanced neoplasia was 1.8% (95% CI, 0.9–2.7). Risk of CRC was higher when LGD was diagnosed by expert gastrointestinal pathologist (1.5%) than by community pathologists (0.2%). Factors significantly associated with dysplasia progression were concomitant primary sclerosing cholangitis (OR, 3.4; 95% CI, 1.5–7.8), invisible dysplasia (vs visible dysplasia; OR, 1.9; 95% CI, 1.0–3.4), distal location (vs proximal location; OR, 2.0; 95% CI, 1.1–3.7) and multifocal dysplasia (vs unifocal dysplasia; OR, 3.5; 95% CI, 1.5– 8.5). In 12 surgical cohort studies of 450 patients who underwent colectomy for UC-LGD, 34 patients had synchronous CRC (pooled prevalence, 17%; 95% CI, 8–33).
Conclusion
In a systematic review of the literature, we found that among patients with UC-LGD under surveillance, the annual incidence of progression to CRC was 0.8%; differences in rates of LGD diagnosis varied with pathologists' level of expertise. Concomitant primary sclerosing cholangitis, invisible dysplasia, distal location, and multifocal LGD are high-risk features associated with dysplasia progression.
Keywords: PSC, proliferation, cancer, colectomy, inflammatory bowel diseases
Introduction
The risk of colorectal cancer (CRC) is 2-5 times higher in patients with ulcerative colitis (UC) than the general population, and it is major cause of morbidity and mortality among these individuals.1-4 In contrast to sporadic CRC, which arises from 1-2 foci of dysplastic changes and follows a well recognized adenoma-carcinoma sequence, colitis-associated CRC results from a field change effect with multi-focal genetic alterations that do not follow the typical adenoma-carcionma sequence of events.5 Neoplasia development in long-standing UC progresses from non-dysplastic mucosa, to visible or invisible low-grade dysplasia (LGD), high-grade dysplasia (HGD) to carcinoma. Periodic surveillance for colorectal neoplasia is recommended for patients with long-standing UC (or those with associated primary sclerosing cholangitis [PSC]), and during surveillance, pooled prevalence of LGD is 9.4%.6-8
While management of non-dysplastic UC (continued periodic surveillance) or UC-HGD (colectomy or endoscopic resection with intensive surveillance) is well accepted, management of UC-LGD is controversial – it is unclear whether these patients should continue surveillance or proceed to surgery, especially in case of non-visible or non-endoscopically resectable dysplasia.8-11 This is, in part, due to limited understanding of the natural history of UC-LGD, with regard to rate and risk factors for progression to HGD and/or CRC and presence of synchronous CRC. In a systematic review of 7 studies in patients with UC-LGD published over a decade ago, the estimated annual incidence of progression to CRC was 1.4% and to advanced neoplasia (CRC and/or HGD) was 3.0%.6 However, there was no synthesis of risk factors associated with dysplasia progression (which may help risk stratify patients to avoid potential over-surveillance in a subset of low-risk patients, and under-surveillance in a subset of high-risk patients), and no assessment of dysplastic findings at colectomy specimens in patients with UC-LGD who opted to undergo surgery (and hence, no assessment of possibility of synchronous, potentially missed, CRC).
In this updated systematic review and meta-analysis, we estimated the (a) incidence and risk factors for progression to colorectal cancer (CRC) and/or high-grade dysplasia (HGD) in patients who continued surveillance after UC-LGD diagnosis (surveillance cohorts), and (b) prevalence and degree of dysplasia in the surgical specimen among patients who underwent colectomy for UC-LGD (surgical cohorts). With the increasing uptake of advanced dysplasia detection techniques like chromoendoscopy with higher rates of detecting LGD, these data would enable a more personalized approach to management of UC-LGD and aid shared-decision making for these patients.
Methods
We followed an a priori protocol registered at the International Prospective Register of Systematic Reviews (PROSPERO CRD42016033500), and followed the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines.12
Study Selection
To address the two primary aims of this review, two sets of inclusion criteria were used. To estimate rate and risk factors associated with progression of LGD, we included cohort studies in (a) adults with UC-LGD identified during colonoscopy (visible or non-visible, resectable or non-resectable), (b) who continued periodic colonoscopic surveillance (had at least one follow-up colonoscopy after the initial LGD diagnosis), and (c) specified number of patients with UC-LGD who developed CRC and/or HGD, along with the total person-years or mean/median follow-up for the subset of patients with UC-LGD. These studies formed the surveillance cohort. We excluded: (a) case-control studies, cross-sectional studies and case series, (b) studies which did not specify the number of patients with UC-LGD who developed CRC and/or HGD, (c) studies which did not report follow-up duration, or (d) studies which selectively reported only outcome after adenoma-like or polypoïd lesion.
To assess dysplasia-related findings on colectomy in patients with UC-LGD, we included cohort studies or case series (>1 patient) in (a) adults with UC-LGD identified during colonoscopy (visible or non-visible, resectable or non-resectable), (b) who underwent colectomy at time of first diagnosis of UC-LGD (without any subsequent colonoscopy), and (c) specified number of patients who were diagnosed with no dysplasia, LGD, HGD and CRC in the surgical specimen (worst finding), with at least 75% follow-up (i.e., dysplasia-related findings reported for at least 75% of patients who underwent surgery for UC-LGD). These studies formed the surgical cohort. Studies in which dysplasia related findings were reported for <75% of cohort, and where findings for HGD and CRC were not separately reported (i.e., where HGD and CRC were grouped as advanced neoplasia) were excluded. In case of multiple publications from the same cohort, data from the most recent comprehensive report were included.
Search Strategy
A systematic literature search of multiple electronic databases was conducted from inception to November 30, 2015, in adults with no language restrictions; this search was updated on June 1, 2016. The databases included: Ovid Medline, Ovid EMBASE, Ovid Cochrane Central Register of Controlled Trials, Ovid Cochrane Database of Systematic Reviews, Web of Science, and Scopus. The search strategy was designed and conducted by an experienced medical librarian with input from the study's investigators, using controlled vocabulary supplemented with keywords, for studies on dysplasia in UC. The details of the search strategy are reported in the Supplementary Appendix. In addition, conference abstracts (Digestive Disease Week, United European Gastroenterology Week, American College of Gastroenterology annual meeting, Advances in Inflammatory Bowel Diseases meeting organized by the Crohn's and Colitis Foundation of America, and European Crohn's and Colitis Organization annual meeting) from 2012 to 2015, as well as bibliography of the selected articles and review articles on the topic were manually searched for additional studies, with no language restrictions. Two reviewers (MF and PSD) independently assessed the title and abstract of studies identified in the primary search for inclusion, and the full text of remaining articles were examined to determine whether they met inclusion criteria. Any discrepancy in article selection was resolved by consensus, and in discussion with a third reviewer (SS). A reviewer (MF) contacted the primary study authors as needed for additional data or missing information.
Data abstraction and definition
Two authors (MF and PSD) independently abstracted data on: (a) study characteristics: primary author, time period of study/year of publication, country of origin, study setting (population-based or referral center); (b) UC-related characteristics: total number patients with UC (if unavailable, total number of IBD patients) and UC-LGD, duration of follow-up (total person-years of follow-up after diagnosis of UC-LGD), number of biopsy specimens taken during surveillance; (c) demographic characteristics: age at UC diagnosis/LGD diagnosis, disease duration, sex, familial history of colorectal neoplasia, extent of UC, concomitant primary sclerosing cholangitis (PSC), smoking status, use of UC-related medications; (d) dysplasia-related characteristics: visible vs. non-visible dysplasia, unifocal vs. multifocal, size and location (distal vs. proximal) of dysplastic lesion, associated stricture, and pathological confirmation of LGD (single or consensus of pathologists, expert vs. community); (e) outcomes: number of patients with UC-LGD who developed HGD and/or CRC (and associated clinical and dysplasia-related characteristics).
For the surgical cohort, dysplasia-related findings on pathological specimens were abstracted. For analysis, “indefinite for dysplasia” was considered equivalent to no dysplasia. Non-visible dysplasia was defined by an absence of documented endoscopic abnormalities. Visible dysplasia was defined as DALM (dysplasia associated lesion or mass), ALM (adenoma-like mass), raised or endoscopically visible flat dysplasia.
Quality assessment
The quality of included studies was assessed using a scale derived from the Newcastle-Ottawa scale for cohort studies, and has been used in a similar study on risk of progression of Barrett's esophagus with LGD.13 This quality score consisted of 7 questions: representative of the average adult in the community, large cohort size, definite histological confirmation of LGD, adequate follow-up of cohort for outcome to occur, clear information on duration of follow-up of patients with UC-LGD, attrition rate, definite information on progression of UC-LGD (Supplementary Appendix). A score of ≥6, 4-5 and ≤3 was considered suggestive of high-, medium- and low-quality study.
Outcomes Assessed
For the surveillance cohort, the primary outcome was the incidence rate (IR) of CRC, and the secondary outcome was incidence of composite outcome of advanced neoplasia (CRC and/or HGD). A priori hypotheses to explain potential heterogeneity in the incidence of CRC among different observational studies included location of study (North America vs. Europe), and whether diagnosis of LGD was confirmed by single vs. consensus of pathologists. Sensitivity analyses were performed to assess stability of findings after excluding (a) low-quality studies and (b) studies in which >50% patients had concomitant PSC was also performed.
In order to identify risk factors associated with progression of UC-LGD to advanced neoplasia, we systematically reviewed UC-related (age at UC diagnosis, sex, disease duration, presence of concomitant PSC) and dysplasia-related factors (age at LGD diagnosis, invisible vs. visible dysplasia, unifocal vs. multifocal dysplasia, one-time vs. persistent LGD, location of dysplasia) associated with progression of UC-LGD to advanced neoplasia. We also qualitatively reviewed the prognosis of CRC diagnosed during surveillance of patients with UC-LGD.
For the surgical cohort, the primary outcome of interest was the proportion of patients with no dysplasia (or indefinite for dysplasia), LGD, HGD and CRC in the surgical specimen after colectomy for UC-LGD.
Statistical Analysis
The summary measure for the surveillance cohort was pooled incidence rate (and 95% confidence interval [CI]), and was estimated using the random effects model proposed by DerSimonian and Laird.14 To identify risk factors associated with progression of dysplasia, we pooled maximally adjusted odds ratio (OR; to account for confounding variables), where reported, using random-effects model. For the surgical cohort, the summary measure was the pooled and weighted prevalence of different dysplasia-related findings. We assessed heterogeneity using the I2 statistic. Values of <30%, 30%-59%, 60%-75%, and >75% were classified as low, moderate, substantial, and considerable heterogeneity, respectively.15 Between-study sources of heterogeneity were assessed using subgroup analyses defined above. A p-value for differences between subgroups of <0.10 was considered statistically significant. Publication bias was assessed quantitatively using Egger's regression test (publication bias considered present if p≤0.10), and qualitatively, by visual inspection of funnel plots.16-17 All analyses were performed using Comprehensive Meta-Analysis software, version 2 (Biostat, Englewood, NJ).
Results
Of 3609 unique studies identified using our search strategy, 14 surveillance cohorts and 12 surgical cohorts were included. Forty-four studies were excluded, primarily because of insufficient data to calculate incidence rate of progression of dysplasia in patients with UC-LGD (Figure 1).
Figure 1. Study selection flow sheet.
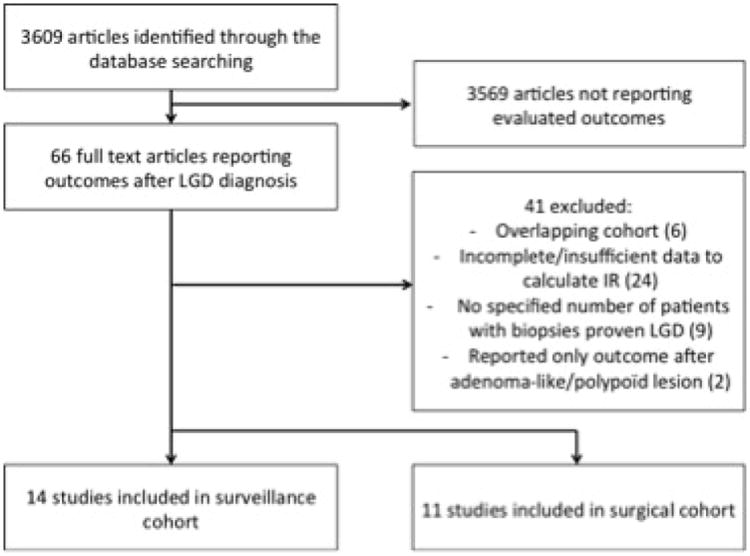
[Abbreviations: LGD, Low-grade dysplasia; IR, incidence rate]
Surveillance Cohorts
Characteristics and Quality of Included Studies
Fourteen studies, reporting on 671 individuals with UC-LGD with total 4,238 patient years of follow-up formed the surveillance cohort.7,18-30 The characteristics of these studies are summarized in Table 1. Seven studies were performed in Europe, and seven in North America. All studies, except one, were single, referral-center studies. An expert pathologist in 11 cohorts confirmed LGD, whereas for 3 studies, a community pathologist alone diagnosed LGD. Associated factors with progression from LGD to advanced neoplasia were available in six studies.7,18-20,23,25 The quality of the included studies is shown in Supplementary Table 1. There was no high-quality study; four studies were classified as low-quality.
Table 1. Characteristics of included studies on risk of dysplasia progression in surveillance cohorts of patients with ulcerative colitis with low-grade dysplasia.
| First Author | Country, City | Study period | Pathology confirmation | Patients with UC-LGD | Median Follow-up (y) | Duration of UC, mean (y) | PSC (n) | Median colonoscopy per patient (n) | Incident HGD (n) | Incident CRC (n) |
|---|---|---|---|---|---|---|---|---|---|---|
| Choi, 2015 | UK, London | 1993-2012 | 2 expert pathologists | 172 | 4 | 23 | 10 | 3 | 13 | 20 |
| Eaton, 2013 | USA, Rochester | 1993-2011 | 2 expert pathologists | 26 | 1 | 23 | 26 | - | 0 | 4 |
| Goldstone, 2011 | USA, New York City | 1994-2006 | 2 expert pathologists | 121 | 3.1 | 19.4 | 0 | 4 | 8 | 7 |
| Navaneethan, 2013 | USA, Cleveland | 1998-2011 | 2 expert pathologists | 102 | 3 | 12 | 0 | 5 | 3 | 2 |
| Venkatesh, 2013 | USA, Cleveland | 1996-2011 | 2 expert pathologists | 10 | 2.8 | 11.5 | 10 | 5 | 1 | 2 |
| Zisman, 2012 | USA, Seattle | 1987-2002 | 2 expert pathologists | 42 | 3.9 | 17.9 | 11 | - | 6 | 2 |
| van Schaik, 2011 | Netherlands | 1990-2006 | 2 expert pathologists | 25* | 2 | - | - | - | 4 | 7 |
| Pekow, 2010 | USA, Chicago | 1994-2008 | 2 expert pathologists | 28 | 4.2 | 20.8 | 3 | 3 | 1 | 1 |
| Jess, 2007 | USA, Rochester | 1940-2001 | 1 expert pathologist | 6 | 17.8 | - | 2 | 3 | 0 | 0 |
| Lim, 2003 | UK, Leeds | 1978-2000 | Community pathologist only | 29 | 17 | - | - | - | 0 | 3 |
| Fusco, 2012 | Germany, Grünheide | 1999-2002 | 2 expert pathologists | 2 | 5.7 | - | - | 5,5 | 0 | 1 |
| Lynch, 1994 | UK, Leeds | 1978-1990 | Community pathologist only | 40 | 5.6 | 17.7 | - | 5 | 0 | 1 |
| Rozen, 1995 | Israel, Tel Aviv | 1976-1994 | 1 expert pathologist | 8 | 3 | 9.5 | - | 2 | 1 | 1 |
| Befrits, 2002 | Sweden, Stockholm | 1976-1998 | Community pathologist only | 49 UC, 11 CD | 10 | - | 10 | 3 | 2 | 1 |
includes both patients with ulcerative colitis and Crohn's disease
Abbreviations: CD, Crohn's Disease; CRC, Colorectal cancer; IBD, Inflammatory Bowel Disease; LGD, Low-grade dysplasia; HGD, High-grade dysplasia; PSC, Primary Sclerosing Cholangitis; UK, United-Kingdom; USA, United States of America; UC, Ulcerative colitis
Incidence of Colorectal Cancer and Advanced Neoplasia
On meta-analysis of 14 studies in 671 patients with UC-LGD under surveillance, 52 patients developed CRC. The pooled incidence rate of CRC was 0.8 per 100-patient year follow-up (95% CI, 0.4-1.3), with substantial heterogeneity across studies (I2=65%) (Figure 2A). Similarly, the pooled incidence of advanced neoplasia was 1.8 per 100-patient year (95% CI, 0.9-2.7), with considerable heterogeneity (I2=82%) (Figure 2B). Five studies reported rates of progression to advanced neoplasia in patients with non-visible (‘flat’) LGD and endoscopically visible dysplasia (DALM/ALM lesions), separately.19,20,21,23,24 On meta-analysis, pooled IR of advanced neoplasia in patients with non-visible LGD was 6.1 per 100-patient year follow-up (95% CI, 0.9-11.4), and with endoscopically visible dysplasia was 1.0 per 100-patient year follow-up (95% CI, 0-2.1).
Figure 2.
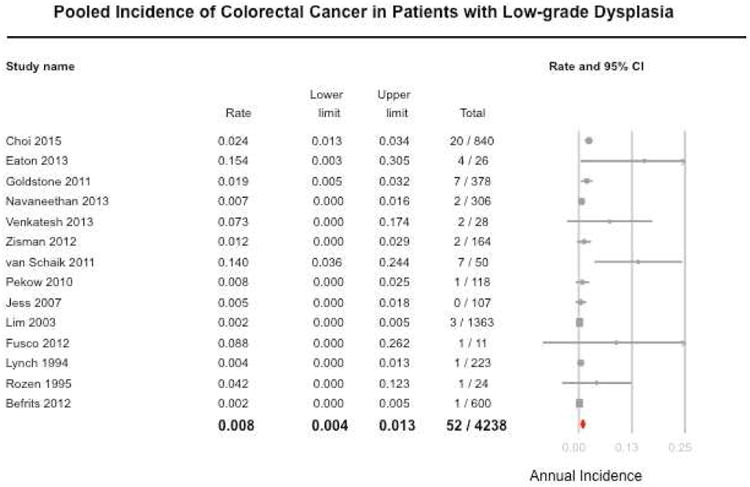

Pooled incidence rate (and 95% confidence interval) of progression to (A) colorectal cancer and (B) advanced neoplasia (colorectal cancer or high-grade dysplasia) in patients with ulcerative colitis with low-grade dysplasia.
In exploring potential sources of heterogeneity, the risk of progression to CRC was significantly lower in studies in which LGD was diagnosed by community pathologists without expert confirmation (IR, 0.2 per 100 patient-years; 95% CI, 0.0-0.4; 3 studies); the corresponding incidence in studies with confirmation by expert pathologists was 1.5% (95% CI, 0.6-2.4; 10 studies) [p-interaction=0.006]. There were no significant differences in IR of CRC between studies conducted in Europe (IR, 0.7; 95% CI, 0.1-1.4; 7 studies) vs. North America (IR, 1.0; 95% CI, 0.3-1.7; 7 studies) [p-interaction=0.54]. Only one study reported a potential beneficial effect between receipt of chromoendoscopy and lower risk of progression to advanced neoplasia in patients with LGD (HR, 0.5; 95% CI, 0.3 – 1.0).7
On sensitivity analysis, the incidence of CRC was similar to the primary estimate after excluding low-quality studies (IR, 1.0; 95% CI, 0.3-1.6; 10 studies) and after excluding studies with high proportion of patients with concomitant PSC (IR, 0.8; 95% CI, 0.3-1.3; 12 studies). After excluding two studies with very high observed rate of progression, including one study performed exclusively in patients with ‘flat’ LGD,7,23 a more conservative IR of progression to CRC was 0.4 per 100 patient-years (95% CI, 0.1-0.8) and advanced neoplasia was 1.2 per 100 patient-years (95% CI, 0.5-1.9). Due to considerable heterogeneity, assessment of publication bias was unreliable.
Risk Factors Associated with Progression to Advanced Neoplasia
Six studies reported various UC- and dysplasia-related factors associated with progression of UC-LGD to advanced neoplasia.7,18-20,23,25 Where ≥2 studies reported on same risk factor, meta-analysis was performed and is summarized in Table 2. Concomitant PSC (OR, 3.4; 95% CI, 1.5-7.8), invisible dysplasia (vs. visible dysplasia) (OR, 1.9; 95% CI, 1.0-3.4), distal dysplasia location (vs. proximal to splenic flexure) (OR, 2.0; 95% CI, 1.1-3.7) and multifocal dysplasia (vs. unifocal dysplasia) (OR, 3.5; 95% CI, 1.5-8.5) were significantly associated with progression to advanced neoplasia. There was no significant association between age at diagnosis of UC or LGD, sex and disease duration. Persistent or metachronous LGD (vs. incident LGD) was also not independently associated with increased risk of progression to advanced neoplasia (OR, 1.4; 95% CI, 0.6-3.3). In one study, dysplastic lesion >1cm (hazard ratio [HR], 3.8; 95% CI, 1.5-13.4) and previous history of ‘indefinite for dysplasia’ (HR, 2.8; 95% CI, 1.2-6.5) was associated with increased risk of progression to advanced neoplasia.7
Table 2.
Ulcerative-colitis and dysplasia-related risk factors associated with progression of dysplasia in patients with low-grade dysplasia.
| Outcome | Number of studies | Pooled OR with CI 65% | P value |
|---|---|---|---|
| Ulcerative colitis-related characteristics | |||
| Age at LGD diagnosis (per unit) | 3 | 0.99 (0.96-1.02) | 0.53 |
| Age at UC diagnosis (per unit) | 2 | 1.00 (0.96-1.03) | 0.86 |
| Disease Duration (per unit) | 3 | 0.99 (0.96-1.02) | 0.42 |
| Male Sex | 2 | 1.10 (0.57-2.12) | 0.77 |
| Concomitant PSC | 3 | 3.42 (1.51-7.78) | <0.01 |
| Dysplasia-related characteristics | |||
| Invisible dysplasia (vs visible dysplasia) | 6 | 1.87 (1.04-3.36) | 0.04 |
| Distal location (vs proximal) | 4 | 2.01 (1.10-3.65) | 0.02 |
| Multifocal dysplasia | 3 | 3.54 (1.47-8.52) | <0.01 |
| Persistent dysplasia (vs. Incident LGD) | 2 | 1.36 (0.56-3.31) | 0.50 |
Abbreviations: LGD, Low-grade dysplasia; OR, Odds ratio; PSC, Primary sclerosing cholangitis; UC, Ulcerative colitis
Surgical Cohorts
Advanced Neoplasia in Patients Undergoing Colectomy for UC-LGD
We identified 12 surgical cohort studies of 450 patients who underwent colectomy for LGD.7, 21, 22, 28, 31-37 Overall, findings at time of surgery were: no dysplasia, 37% (95% CI, 29-47; 197/450; I2=42%), LGD, 34% (95% CI, 30-39; 153/450; I2=0%) or advanced neoplasia, 30% (95% CI, 21-41; 97/450; I2=59%).
At surgery, synchronous CRC was identified in 34 patients (17%, 95% CI, 8-33; I2=77%). In studies published before 2000, rate of synchronous CRC at time of surgery was significantly higher (33%; 95% CI, 20-50) as compared to studies published after 2000 (11%; 95% CI, 4-29) [p-interaction=0.04].
Outcome after Colorectal Cancer Diagnosis
Eight studies reported outcome after diagnosis of 12 CRC.21-23,27-31 Among these, 11 patients underwent surgery with curative intent. Overall, two patients died during follow-up, both related to CRC – one patient died due to metastatic CRC, and another following recurrence of CRC after initial curative surgery.
Discussion
The management of UC-LGD is challenging due to a limited understanding of its natural history with regard to rate of metachronous or synchronous CRC, as well as risk factors associated with progression of dysplasia. In this systematic review of 14 surveillance cohorts and 12 surgical cohorts, we made several important observations. First, among patients with UC-LGD undergoing surveillance, the annual incidence of CRC is approximately 0.8% (95% CI, 0.4-1.3); rates of progression to CRC were higher when LGD was diagnosed by at least one expert gastrointestinal pathologist (IR, 1.5%; 95% CI, 0.6-2.4) as compared to a community pathologist without expert confirmation (IR, 0.2%; 95% CI, 0.0-0.4). Second, dysplastic lesions that were multifocal, invisible, located in the distal colon, or those detected in patients with concomitant PSC, had the higher risk of progression to advanced neoplasia, and variable distribution of these risk factors in included surveillance cohorts may also explain observed heterogeneity. In particular, in patients with invisible dysplasia, annual incidence of progression to advanced neoplasia was 6.1% (95% CI, 0.9-11.4), and with endoscopically visible dysplasia was 1.0% (95% CI, 0-2.1). Finally, we observed that among patients with UC-LGD undergoing colectomy, advanced neoplasia is observed in approximately 30% of patients, whereas in 70% patients, either no dysplasia, ‘indefinite for dysplasia’ or LGD is identified; the risk of identifying synchronous CRC was significantly higher in older studies (published in 1990s, 33%) as compared to more contemporary cohorts (published in 2000s, 11%). Together, these findings comprehensively inform clinical practice on the natural history of UC-LGD and will facilitate shared decision-making regarding intensive endoscopic surveillance vs. early colectomy in patients with UC-LGD.
The observed annual incidence of CRC (0.8%; 95% CI, 0.4-1.3) and advanced neoplasia (1.8%; 95% CI, 0.9-2.7) was lower than observed in the previous systematic review (1.4% and 3.0%, respectively); the previous review included only 7 studies, primarily conducted in the 1990s before widespread uptake of surveillance for CRC, and predated advanced dysplasia detection techniques.6 We identified 8 additional studies since the publication of the last review, and observed lower rates of progression to CRC and advanced neoplasia in our meta-analysis. This might be a true finding with an actual decrease in rate of dysplasia progression with widespread use of disease-modifying therapy that control inflammation better and hence, decrease risk of dysplasia progression, lead-time bias with higher rates of LGD detection at low-risk of progression with surveillance exams and advanced dysplasia detection techniques, or may be attributed to publication bias (with unreported studies) in the earlier meta-analysis. In contrast to UC-LGD, estimated annual incidence of CRC in unselected patients with non-dysplastic UC is 0.3%,38 and between 0.017% and 0.041% in the general CRC screening population.39, 40
We observed wide variability in rates of progression to CRC across studies. This could be explained by two potential reasons. First, the studies were conducted in diverse clinical practices with wide variability in interpretation of LGD diagnosis. In studies where LGD was diagnosed only by a community pathologist, rates of progression to CRC was lower, as compared to studies with expert pathological confirmation. Analogous to LGD in patients with Barrett's esophagus, UC-LGD may be overcalled by community pathologists, such that these patients are intrinsically at lower risk of progression to CRC.13,41,42 In a Dutch pathology registry study, on re-review of 70 patients initially diagnosed as having flat LGD by three expert pathologists, the diagnosis of flat LGD was confirmed in only 21 patients (30%); in 29 patients (41%), the diagnosis was downgraded to indefinite for dysplasia, in 17 patients (24%) to no dysplasia, and in 3 patients (5%) to non-IBD-related dysplasia.23 While the rate of progression to advanced neoplasia in patients originally classified as having ‘flat LGD’ was 19%, this rate increased to 44% on restricting to patients confirmed as having LGD by three expert pathologists. Second, there may be referral bias wherein expert centres may be more likely providing care to patients at highest risk of dysplasia progression.
An apparent discrepancy was observed in rates of metachronous CRC (from surveillance cohorts; annual IR, 0.8%) and synchronous CRC (from surgical cohorts; 17%). We hypothesize that these differences may be due to (a) differences in patient population (systematic difference in patients with LGD who undergo surveillance, and those who undergo surgery, where physicians may be intuitively referring patients deemed to be at high risk of progression to surgery, and selectively including low-risk LGD patients in surveillance), (b) differences in time period (majority of surgical cohorts were published before 2000 and the majority of surveillance cohorts were published after 2000, and with regular use of surveillance colonoscopies and advanced dysplasia detection techniques in recent times may enable early identification of LGD at low risk of harboring synchronous advanced neoplasia), or to (c) potential missed or interval CRCs. In surveillance cohorts, we assumed that risk of CRC increases linearly with time. It is possible that most patients who are diagnosed with CRC after a diagnosis of LGD develop CRC within 1-2 years of LGD diagnosis, and that these cancers were probably ‘missed’ at the original colonoscopy, rather than truly being incident cancers, akin to observations in patients with Barrett's esophagus, in which we estimated that about 25% of esophageal cancers in patients with BE develop within 2 years of initial BE diagnosis.43 In a study from the Netherlands on magnitude of interval CRCs in patients with IBD, the investigators observed that while the annual incidence of LGD in patients with IBD is 5.2%, about 1.3% patients developed interval CRC, possibly due to inadequate colonoscopy, inadequate surveillance intervals, inadequate dysplasia management or true biologic interval CRC.44
The strengths of this systematic review include: (a) comprehensive and systematic literature search with well-defined inclusion criteria; (b) quantitatively and qualitatively studying all aspects of dysplasia progression in patients with UC-LGD including incidence, risk factors and outcomes of metachronous CRC and advanced neoplasia, and risk of synchronous CRC and advanced neoplasia; (c) sub-group and sensitivity analyses to evaluate the stability of findings and identify potential factors responsible for inconsistencies; and (d) rigorous quality assessment of studies.
Our study has several limitations. First, significant heterogeneity was observed in the pooled estimate of incidence of dysplasia progression. We explored and identified potential explicit study-related (study setting, location, time period, whether diagnosis was confirmed by expert gastrointestinal pathologist) and implicit patient-related factors (UC- and dysplasia-related potential risk factors) that may be contributing to this heterogeneity. Second, studies did not consistently report the frequency of endoscopic surveillance, use of advanced dysplasia detection techniques as chromoendoscopy, and numbers of random and/or targeted biopsies taken in the surveillance cohorts.45 Similarly, for surgical cohorts, there was incomplete reporting in terms of all dysplastic findings, resulting in exclusion of some studies with incomplete information. In assessing risk factors, several studies only provided univariate analysis due to limited number of events. Some potentially important risk factors such as disease extent, associated stricture, familial history of CRC or impact of IBD therapies and associated endoscopic and/or histologic remission on dysplasia progression, couldn't be evaluated due to insufficient data. Third, there was variability in study quality especially with regard to duration of follow-up, attrition rate, specific reporting of follow-up in a subset of patients with UC-LGD. Moreover, most of included studies were performed in referral centers, and these tend to overestimate CRC risk as compared to population-based studies.46 In fact, we did not identify any high quality study on this topic due to the aforementioned factors in this review. Finally, the analyses were done assuming that incidence rate is constant over time, which may not be accurate.
Implications for Clinical Practice
Recently, the SCENIC consensus statements have proposed an individualized approach to surveillance in patients with UC-LGD.8 Based on our observations, we would recommend early repeat colonoscopy (within 6m), preferably with chromoendoscopy, in patients diagnosed with LGD, particularly ‘invisible’ dysplasia, due to potential missed CRCs in these patients. Given low rates of progression to advanced neoplasia in patients with endoscopically visible lesions, we agree with the SCENIC consensus statements, that in patients with visible non-polypoid dysplastic lesions which are amenable to endoscopic resection, surveillance colonoscopy may be suggested rather than colectomy. Finally, in patients with invisible dysplasia, we propose risk-stratification to identify patients at low- and high-risk of dysplasia progression, based on presence of absence of risk factors associated with dysplasia progression (concomitant primary sclerosing cholangitis, multifocal LGD, previous indefinite for dysplasia and distal location). Choi and colleagues estimated risk of dysplasia progression based on number of risk factors, and observed a significant increase in risk if multiple risk factors were present. Hence, in patients with multiple high-risk features, particularly those who may be difficult to survey (multiple pseudopolyps, poor compliance, ongoing active disease, etc.), early colectomy may be advisable. However, there are significant differences in patients' and physicians' willingness to undergo colectomy for dysplasia risk, and hence, shared decision-making is recommended.47
In conclusion, we estimate that the annual rate of progression to CRC or to advanced neoplasia in patients with UC-LGD under surveillance is approximately 0.8% (95% CI, 0.4-1.3) and 1.8% (95% CI, 0.9-2.7), respectively, and this rate may be variable depending on whether diagnosis of LGD was confirmed by an expert gastrointestinal pathologist or not. Concomitant PSC, invisible dysplasia, distal colonic location and multifocal LGD are potential high-risk features associated with progression to advanced neoplasia. Among patient undergoing colectomy for UC-LGD, about 17% may have synchronous CRC, and this rate appears to have decreased over time potentially due to regular surveillance and advanced dysplasia detection techniques that enable early LGD diagnosis. Prospective, population-based observational cohort studies in patients with an expert pathologist-confirmed LGD are warranted to better understand the natural history of UC-LGD.
Supplementary Material
Supplementary Table 1. Quality of included studies based on a modification of the Newcastle-Ottawa Scale for cohort studies.
Table 3.
Surgical Cohort–Characteristics of studies reporting dysplasia-related findings in patients with UC-LGD undergoing colectomy.
| Authors, year of publication | Country, City | Study period | Pathology diagnosis | Colectomy for UC-LGD | Definitive pathology on colectomy | ||||
|---|---|---|---|---|---|---|---|---|---|
| No dysplasia or indefinite for dysplasia (n, %) | LGD (n, %) | HGD (n, %) | CRC (n, %) | Advanced neoplasia (HGD or CRC) (n, %) | |||||
| Murphy, 2014 | USA, multicentric | 1993-2012 | 2 expert pathologists | 220 | 105 (48%) | 74 (34%) | 36 (16%) | 5 (2%) | 41 (18%) |
| Kiran, 2014 | USA, Cleveland | 1984-2007 | 2 expert pathologists | 136 | 70 (51%) | 46 (34%) | 16 (12%) | 4 (3%) | 20 (15%) |
| Hata, 2003 | Japan, Tokyo | 1979-2001 | Community pathologist only | 3 | 1 (33%) | 0 | 1 (33%) | 1 (33%) | 2 (66%) |
| Löfberg R, 1990 | Sweden, Stockholm | 1973-1988 | 1 expert pathologist | 4 | 1 (25%) | 3 (75%) | 0 | 0 | 0 |
| Nugent WF, 1991 | USA, Burlington | 1974-1986 | 1 expert pathologist | 4 | 1 (25%) | 1 (25%) | 0 | 2 (50%) | 2 (50%) |
| Woolrich AJ, 1992 | USA, New York | 1977-1987 | 2 expert pathologists | 2 | 0 | 2 (100%) | 0 | 0 | 0 |
| Lindberg B, 1996 | Sweden, Hundinge | 1974-1993 | 2 expert pathologists | 16 | 4 (25%) | 4 (25%) | 0 | 5 (31%) | 5 (31%) |
| Connell WR, 1994 | UK, London | 1971-1990 | 2 expert pathologists | 13 | 4 (30%) | 2 (15%) | 2 (15%) | 5 (38%) | 7 (54%) |
| Choi CH, 2015 | UK, London | 1993-2012 | 2 expert pathologists | 36 | 9 (25%) | 13 (36%) | 5 (14%) | 9 (25%) | 14 (39%) |
| Venkatesh PG, 2013 | USA, Cleveland | 1996-2011 | 2 expert pathologists | 2 | 1 (50%) | 1 (50%) | 0 | 0 | 0 |
| isman TL, 2012 | USA, Seattle | 1987-2002 | 2 expert pathologists | 6 | 1 (16%) | 2 (33%) | 2 (33%) | 1 (16%) | 3 (50%) |
| Fusco V, 2012 | Germany, Grünheide | 1999-2002 | 2 expert pathologists | 8 | 0 | 5 (62%) | 1 (12%) | 2 (24%) | 3 (37%) |
Abbreviations: CD, Crohn's disease; CRC, Colorectal cancer; HGD, High-grade dysplasia; LGD, Low-grade dysplasia PSC, Primary sclerosing cholangitis; UC, Ulcerative Colitis; UK, United-Kingdom; USA, United States of America;
Acknowledgments
Dr. Fumery is supported by the French Society of Gastroenterology (SNFGE, bourse Robert Tournut). Dr. Dulai is supported by the NIDDK training grant 5T32DK007202. Dr. Singh is supported by the NIH/NLM training grant T15LM011271 and the American College of Gastroenterology Junior Faculty Development Award and Crohn's and Colitis Foundation of American Career Development Award.
Footnotes
Disclosures: None of the other authors have any relevant financial disclosures.
- Study concept and design: MF, PSD, SS
- Acquisition of data: MF, PSD, LJP, SS
- Analysis and interpretation of data: MF, SS
- Drafting of the manuscript: MF, SS
- Critical revision of the manuscript for important intellectual content: PSD, LJP, SG, SR, WJS
- Approval of the final manuscript: MF, PSD, LJP, SG, SR, WJS, SS
- Guarantor of the article: SS
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bewtra M, Kaiser LM, TenHave T, Lewis JD. Crohn's disease and ulcerative colitis are associated with elevated standardized mortality ratios: a meta-analysis. Inflamm Bowel Dis. 2013;19:599–613. doi: 10.1097/MIB.0b013e31827f27ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jess T, Rungoe C, Peyrin-Biroulet L. Risk of colorectal cancer in patients with ulcerative colitis: a meta-analysis of population-based cohort studies. Clin Gastroenterol Hepatol. 2012;10:639–45. doi: 10.1016/j.cgh.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 3.Beaugerie L, Itzkowitz SH. Cancers Complicating Inflammatory Bowel Disease. N Engl J Med. 2015;373:195. doi: 10.1056/NEJMc1505689. [DOI] [PubMed] [Google Scholar]
- 4.Bernstein CN, Nugent Z, Targownik LE, Singh H, Lix LM. Predictors and risks for death in a population-based study of persons with IBD in Manitoba. Gut. 2015;64:1403–11. doi: 10.1136/gutjnl-2014-307983. [DOI] [PubMed] [Google Scholar]
- 5.Ullman TA, Itzkowitz SH. Intestinal inflammation and cancer. Gastroenterology. 2011;140:1807–16. doi: 10.1053/j.gastro.2011.01.057. [DOI] [PubMed] [Google Scholar]
- 6.Thomas T, Abrams KA, Robinson RJ, Mayberry JF. Meta-analysis: cancer risk of low-grade dysplasia in chronic ulcerative colitis. Aliment Pharmacol Ther. 2007;25:657–68. doi: 10.1111/j.1365-2036.2007.03241.x. [DOI] [PubMed] [Google Scholar]
- 7.Choi CH, Ignjatovic-Wilson A, Askari A, et al. Low-grade dysplasia in ulcerative colitis: risk factors for developing high-grade dysplasia or colorectal cancer. Am J Gastroenterol. 2015;110:1461–71. doi: 10.1038/ajg.2015.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laine L, Kaltenbach T, Barkun A, et al. SCENIC international consensus statement on surveillance and management of dysplasia in inflammatory bowel disease. Gastroenterology. 2015;148:639–651. doi: 10.1053/j.gastro.2015.01.031. [DOI] [PubMed] [Google Scholar]
- 9.Bernstein CN. Ulcerative colitis with low-grade dysplasia. Gastroenterology. 2004;127:950–6. doi: 10.1053/j.gastro.2004.07.026. [DOI] [PubMed] [Google Scholar]
- 10.Van Assche G, Dignass A, Bokemeyer B, et al. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 3: special situations. J Crohns Colitis. 2013;7:1–33. doi: 10.1016/j.crohns.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 11.Bernstein CN, Weinstein WM, Levine DS, Shanahan F. Physicians' perceptions of dysplasia and approaches to surveillance colonoscopy in ulcerative colitis. Am J Gastroenterol. 1995;90:2106–14. [PubMed] [Google Scholar]
- 12.Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singh S, Manickam P, Amin AV, et al. Incidence of esophageal adenocarcinoma in Barrett's esophagus with low-grade dysplasia: a systematic review and meta-analysis. Gastrointest Endosc. 2014;79:897–909. doi: 10.1016/j.gie.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 14.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 15.Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines: 7. Rating the quality of evidence--inconsistency. J Clin Epidemiol. 2011;64:1294–302. doi: 10.1016/j.jclinepi.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 16.Egger M, Smith GD, Phillips AN. Meta-analysis: principles and procedures. BMJ. 1997;315:1533–7. doi: 10.1136/bmj.315.7121.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Easterbrook PJ, Berlin JA, Gopalan R, Matthews DR. Publication bias in clinical research. Lancet. 1991;337:867–72. doi: 10.1016/0140-6736(91)90201-y. [DOI] [PubMed] [Google Scholar]
- 18.Eaton JE, Smyrk TC, Imam M, et al. The fate of indefinite and low-grade dysplasia in ulcerative colitis and primary sclerosing cholangitis colitis before and after liver transplantation. Aliment Pharmacol Ther. 2013;38:977–87. doi: 10.1111/apt.12469. [DOI] [PubMed] [Google Scholar]
- 19.Goldstone R, Itzkowitz S, Harpaz N, Ullman T. Progression of low-grade dysplasia in ulcerative colitis: effect of colonic location. Gastrointest Endosc. 2011;74:1087–93. doi: 10.1016/j.gie.2011.06.028. [DOI] [PubMed] [Google Scholar]
- 20.Navaneethan U, Jegadeesan R, Gutierrez NG, et al. Progression of low-grade dysplasia to advanced neoplasia based on the location and morphology of dysplasia in ulcerative colitis patients with extensive colitis under colonoscopic surveillance. J Crohns Colitis. 2013;7:e684–91. doi: 10.1016/j.crohns.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 21.Venkatesh PG, Jegadeesan R, Gutierrez NG, Sanaka MR, Navaneethan U. Natural history of low grade dysplasia in patients with primary sclerosing cholangitis and ulcerative colitis. J Crohns Colitis. 2013;7:968–73. doi: 10.1016/j.crohns.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 22.Zisman TL, Bronner MP, Rulyak S, et al. Prospective study of the progression of low-grade dysplasia in ulcerative colitis using current cancer surveillance guidelines. Inflamm Bowel Dis. 2012;18:2240–6. doi: 10.1002/ibd.22912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Schaik FD, Oldenburg B, Offerhaus GJ, et al. Role of immunohistochemical markers in predicting progression of dysplasia to advanced neoplasia in patients with ulcerative colitis. Inflamm Bowel Dis. 2012;18:480–8. doi: 10.1002/ibd.21722. [DOI] [PubMed] [Google Scholar]
- 24.Pekow JR, Hetzel JT, Rothe JA, et al. Outcome after surveillance of low-grade and indefinite dysplasia in patients with ulcerative colitis. Inflamm Bowel Dis. 2010;16:1352–6. doi: 10.1002/ibd.21184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jess T, Loftus EV, Jr, Velayos FS, et al. Risk factors for colorectal neoplasia in inflammatory bowel disease: a nested case-control study from Copenhagen county, Denmark and Olmsted county, Minnesota. Am J Gastroenterol. 2007;102:829–36. doi: 10.1111/j.1572-0241.2007.01070.x. [DOI] [PubMed] [Google Scholar]
- 26.Lim CH, Dixon MF, Vail A, Forman D, Lynch DA, Axon AT. Ten year follow up of ulcerative colitis patients with and without low grade dysplasia. Gut. 2003;52:1127–32. doi: 10.1136/gut.52.8.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fusco V, Ebert B, Weber-Eibel J, et al. Cancer prevention in ulcerative colitis: long-term outcome following fluorescence-guided colonoscopy. Inflamm Bowel Dis. 2012;18:489–95. doi: 10.1002/ibd.21703. [DOI] [PubMed] [Google Scholar]
- 28.Lynch DA, Lobo AJ, Sobala GM, Dixon MF, Axon AT. Failure of colonoscopic surveillance in ulcerative colitis. Gut. 1993;34:1075–80. doi: 10.1136/gut.34.8.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rozen P, Baratz M, Fefer F, Gilat T. Low incidence of significant dysplasia in a successful endoscopic surveillance program of patients with ulcerative colitis. Gastroenterology. 1995;108:1361–70. doi: 10.1016/0016-5085(95)90683-5. [DOI] [PubMed] [Google Scholar]
- 30.Befrits R, Ljung T, Jaramillo E, Rubio C. Low-grade dysplasia in extensive, longstanding inflammatory bowel disease: a follow-up study. Dis Colon Rectum. 2002;45:615–20. doi: 10.1007/s10350-004-6255-4. [DOI] [PubMed] [Google Scholar]
- 31.Murphy J, Kalkbrenner KA, Pemberton JH, et al. Dysplasia in ulcerative colitis as a predictor of unsuspected synchronous colorectal cancer. Dis Colon Rectum. 2014;57:993–8. doi: 10.1097/DCR.0000000000000172. [DOI] [PubMed] [Google Scholar]
- 32.Kiran RP, Ahmed Ali U, Nisar PJ, et al. Risk and location of cancer in patients with preoperative colitis-associated dysplasia undergoing proctocolectomy. Ann Surg. 2014;259:302–9. doi: 10.1097/SLA.0b013e31828e7417. [DOI] [PubMed] [Google Scholar]
- 33.Hata K, Watanabe T, Kazama S, et al. Earlier surveillance colonoscopy programme improves survival in patients with ulcerative colitis associated colorectal cancer: results of a 23-year surveillance programme in the Japanese population. Br JCancer. 2003;89:1232–6. doi: 10.1038/sj.bjc.6601247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Löfberg R, Broström O, Karlén P, Tribukait B, Ost A. Colonoscopic surveillance in long-standing total ulcerative colitis--a 15-year follow-up study. Gastroenterology. 1990;99:1021–31. doi: 10.1016/0016-5085(90)90622-8. [DOI] [PubMed] [Google Scholar]
- 35.Nugent FW, Haggitt RC, Gilpin PA. Cancer surveillance in ulcerative colitis. Gastroenterology. 1991;100:1241–8. [PubMed] [Google Scholar]
- 36.Lindberg B, Persson B, Veress B, Ingelman-Sundberg H, Granqvist S. Twenty years' colonoscopic surveillance of patients with ulcerative colitis. Detection of dysplastic and malignant transformation. Scand J Gastroenterol. 1996;3:1195–204. doi: 10.3109/00365529609036910. [DOI] [PubMed] [Google Scholar]
- 37.Connell WR, Lennard-Jones JE, Williams CB, Talbot IC, Price AB, Wilkinson KH. Factors affecting the outcome of endoscopic surveillance for cancer in ulcerative colitis. Gastroenterology. 1994;107:934–44. doi: 10.1016/0016-5085(94)90216-x. [DOI] [PubMed] [Google Scholar]
- 38.Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48:526–35. doi: 10.1136/gut.48.4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.National Cancer Institute. Surveillance, Epidemiology, and Ends results program. http://seer.cancer.gov/statfacts/html/colorect.html.
- 40.International Agency for Research in Cancer. World Health Organization; http://globocan.iarc.fr/Pages/fact_sheets_population.aspx. [Google Scholar]
- 41.Dixon MF, Brown LJR, Gilmour HM, et al. Observer variation in the assessment of dysplasia in ulcerative colitis. Histopathology. 1988;13:385–97. doi: 10.1111/j.1365-2559.1988.tb02055.x. [DOI] [PubMed] [Google Scholar]
- 42.Eaden J, Abrams K, McKay H, et al. Inter-observer variation between general and specialist gastrointestinal pathologists when grading dysplasia in ulcerative colitis. J Pathol. 2001;194:152–7. doi: 10.1002/path.876. [DOI] [PubMed] [Google Scholar]
- 43.Visrodia K, Singh S, Krishnamoorthi R, et al. Magnitude of Missed Esophageal Adenocarcinoma After Barrett's Esophagus Diagnosis: A Systematic Review and Meta-analysis. Gastroenterology. 2016;150:599–607. doi: 10.1053/j.gastro.2015.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mooiweer E, van der Meulen-de Jong AE, Ponsioen CY, et al. Incidence of Interval Colorectal Cancer Among Inflammatory Bowel Disease Patients Undergoing Regular Colonoscopic Surveillance. Clin Gastroenterol Hepatol. 2015;13:1656–61. doi: 10.1016/j.cgh.2015.04.183. [DOI] [PubMed] [Google Scholar]
- 45.Awais D, Siegel CA, Higgins PD. Modelling dysplasia detection in ulcerative colitis: clinical implications of surveillance intensity. Gut. 2009;58:1498–503. doi: 10.1136/gut.2008.169714. [DOI] [PubMed] [Google Scholar]
- 46.Lutgens MW, van Oijen MG, van der Heijden GJ, et al. Declining risk of colorectal cancer in inflammatory bowel disease: an updated meta-analysis of population-based cohort studies. Inflamm Bowel Dis. 2013;19:789–99. doi: 10.1097/MIB.0b013e31828029c0. [DOI] [PubMed] [Google Scholar]
- 47.Siegel CA, Schwartz LM, Woloshin S, et al. When should ulcerative colitis patients undergo colectomy for dysplasia? Mismatch between patient preferences and physician recommendations. Inflamm Bowel Dis. 2010;16:1658–62. doi: 10.1002/ibd.21233. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Quality of included studies based on a modification of the Newcastle-Ottawa Scale for cohort studies.


