Abstract
Background
Circulating tumor cells (CTCs) expressing AR-V7 protein localized to the nucleus (nuclear-specific) identify metastatic castration-resistant prostate cancer (mCRPC) patients with improved overall survival (OS) on taxane therapy relative to the androgen receptor signaling inhibitors (ARSis) abiraterone acetate, enzalutamide, and apalutamide.
Objective
To evaluate if expanding the positivity criteria to include both nuclear and cytoplasmic AR-V7 localization (“nuclear-agnostic”) identifies more patients who would benefit from a taxane over an ARSi.
Design, setting, and participants
The study used a coss-sectional cohort. Between December 2012 and March 2015, 193 pretherapy blood samples, 191 of which were evaluable, were collected and processed from 161 unique mCRPC patients before starting a new line of systemic therapy because of disease progression at the Memorial Sloan Kettering Cancer Center. The association between two AR-V7 scoring criteria, post-therapy prostate-specific antigen change (PTPC) and OS following ARSi or taxane treatment, was explored. One criterion required nuclear-specific AR-V7 localization, and the other required an AR-V7 signal but was agnostic to protein localization in CTCs.
Outcome measurements and statistical analyses
Correlation of AR-V7 status to PTPC and OS was investigated. Relationships with survival were analyzed using multivariable Cox regression and log-rank analyses.
Results and limitations
A total of 34 (18%) samples were AR-V7-positive using nuclear-specific criteria, and 56 (29%) were AR-V7-positive using nuclear-agnostic criteria. Following ARSi treatment, none of the 16 nuclear-specific AR-V7-positive samples and six of the 32 (19%) nuclear-agnostic AR-V7-positive samples had ≥50% PTPC at 12 wk. The strongest baseline factor influencing OS was the interaction between the presence of nuclear-specific AR-V7-positive CTCs and treatment with a taxane (hazard ratio 0.24, 95% confidence interval 0.078–0.79; p = 0.019). This interaction was not significant when nuclear-agnostic criteria were used.
Conclusions
To reliably inform treatment selection using an AR-V7 protein biomarker in CTCs, nuclear-specific localization is required.
Patient summary
We analyzed outcomes for patients with metastatic castration-resistant prostate cancer on androgen receptor signaling inhibitors and standard chemotherapy. Patients with circulating tumor cells that had AR-V7 protein in the cellular nuclei were very likely to survive longer on chemotherapy, and tests unable to distinguish where the protein is located in the cell are not as predictive of benefit.
Keywords: AR-V7, Circulating tumor cells, Liquid biopsy, Prostate cancer
1. Introduction
The increasing use of molecular profiling has revealed that each round of systemic therapy can change the biologic profile of a patient’s disease, supporting the need for serial analyses before each change in therapy to best inform therapy selection [1]. For this reason, each new line of therapy for progressing metastatic castration-resistant prostate cancer (mCRPC) represents a key clinical treatment decision according to the Prostate Cancer Working Group 3 recommendations [1]. In the context of mCRPC management, a crucial decision is the choice between an androgen receptor signaling inhibitor (ARSi) or taxane-based chemotherapy, both approved and life-prolonging. For this context, therapy-guiding predictive biomarkers are an unmet medical need.
Circulating tumor cells (CTCs) can be obtained from routine phlebotomy samples with minimal patient discomfort, which allows profiling of cells from multiple lesions at once. By contrast, single-site tumor biopsies are invasive, costly, and difficult to repeat [2]. Androgen receptor splice variant 7 (AR-V7) contains a truncated C-terminal region lacking the ligand-binding domain, allowing AR signaling to be activated independent of a ligand. Several groups have recently demonstrated that detection of AR-V7 mRNA in pooled, lysed CTCs from patients with progressing mCRPC [3–5] predicts disease resistance to ARSis [3]. However, mRNA-based methods have yet to identify patients who would live significantly longer if given a taxane when adjusting for preclinical features, such as line of therapy, in multivariate models [6].
How AR splice variants are regulated at the mRNA and protein levels are active areas of research. Studies evaluating AR-V7 in tissue samples typically require nuclear localization of AR-V7 protein to score positive, which cannot be assessed in pooled mRNA samples [7–9]. Several reported tests for AR-V7 in CTCs have used epithelial cell adhesion molecule (EpCAM) enrichment capture followed by AR-V7 mRNA detection in pooled CTC aggregates [3,5,10] or detection of cell-free AR-V7 mRNA transcripts in whole blood [11]. The Epic Sciences platform uses a pathology slide–based, non-selection CTC detection method that allows assessment of AR-V7 protein presence and localization in individual CTCs [12].
In previous work, we validated a CTC AR-V7 protein assay in the context of use to predict response to ARSis or taxanes in patients with progressing mCRPC when a change in therapy was needed [12]. The AR-V7 CTC positivity criteria used in the study required nuclear-specific protein localization [12], similar to previous reports using AR-V7 protein in metastatic biopsy samples [7–9]. The results showed that AR-V7 protein detection frequency in the nucleus of CTCs increased by line of therapy, ranging from 3% for the first line to 31% for the third line or greater. Detection of nuclear-specific AR-V7-positive CTCs before therapy was highly specific for lack of response to ARSis but not to taxanes, and demonstrated a statistically significant interaction between improved overall survival (OS) for patients on taxanes compared to ARSis [12]. The clinical benefit shown supports the clinical utility of the nuclear-specific AR-V7 protein assay in informing decisions to administer a taxane over an ARSi.
Of note, the rate of AR-V7 detection in CTCs using the nuclear-specific criteria was lower than the reported mRNA-based detection methods in comparable patient cohorts, averaging 18% of patients with AR-V7-positive CTCs and 29–55% of patients positive for AR-V7 mRNA transcripts [3,5]. Given the demonstrated importance of the AR-V7 biomarker, analytical and clinical validation of methodologies to measure AR-V7 cannot be understated [13]. However, it was hypothesized that strict nuclear-specific AR-V7 protein scoring in CTCs was too stringent, potentially sacrificing sensitivity to detect more patients who might benefit from taxanes over ARSis. Noting that a proportion of patient samples exhibited predominantly cytoplasmic AR-V7 protein expression, and presumably also expressing AR-V7 mRNA transcripts, we evaluated whether expanding the AR-V7 scoring criteria to include both nuclear and cytoplasmic AR-V7 localization (“nuclear-agnostic”) could identify more patients who would have more favorable outcomes on taxane over ARSi therapy.
2. Patients and methods
2.1. Patient selection
Blood samples from patients with progressing mCRPC who were about to start a new line of ARS or taxane-based therapy were considered. Patient histories are outlined in Table 1. Castration status (serum testosterone <50 ng/dl) was confirmed via a standard blood panel. All patients signed consent to an institutional review board–approved protocol before sample collection.
Table 1.
Patient and sample demographics
| Patient characteristics | All patients | |||
|---|---|---|---|---|
| Number of unique patients | 161 | |||
| Age (yr) | 68 (45–91) | |||
| Gleason score at diagnosis | 8 (5–10) | |||
| Primary treatment | ||||
| Prostatectomy | 77 (48%) | |||
| Radiation | 28 (18%) | |||
| Brachytherapy | 7 (4%) | |||
| None | 49 (30%) | |||
| Sample characteristics | All samples | Pre-ARSi | Pre-taxane | p value a |
| Baseline samples (n) | 193 | 130 b | 63 | |
| Age (yr) | 68 (45–91) | 68.5 (45–87) | 68 (48– 91) | 0.4190 |
| Blood age (h) | 26 (1–78) | 25 (2–78) | 27 (1–51) | 0.2563 |
| Treatment decision, n (%) c | ||||
| First | 67 (34.7) | 56 (43.1) | 11 (17.4) | <0.0001 |
| Second | 50 (25.9) | 40 (30.8) | 10 (15.9) | |
| Third or later | 76 (39.4) | 34 (26.1) | 42 (66.7) | |
| Prior therapy, n (%) d | ||||
| None | 67 (34.7) | 56 (43.1) | 11 (17.5) | <0.0001 |
| ARSi only | 53 (27.5) | 34 (26.2) | 19 (30.1) | |
| Taxane ± other | 10 (5.2% | 10 (7.7) | 0 | |
| ARSi + taxane ± other | 63 (32.6) | 30 (23.0) | 33 (52.4) | |
| Chemotherapy status, n (%) | ||||
| Chemotherapy-naïve | 120 (62) | 90 (69) | 30 (48) | 0.0045 |
| Chemotherapy-exposed | 73 (38) | 40 (31) | 33 (52) | |
| Laboratory results before therapy, median (range) | ||||
| PSA (ng/ml) | 37.7 (0.1–3728.2) | 28.0 (0.1–2454.5) | 99.5 (0.1–3728.2) | <0.0001 |
| Alkaline phosphatase (U/l) | 111 (25–2170) | 99 (25–2170) | 181 (49–1816) | <0.0001 |
| Lactate dehydrogenase (U/l) | 220 (123–1293) | 208 (123–1293) | 251.5 (141–1004) | 0.0006 |
| Hemoglobin (g/dl) | 12.1 (7.0–15.0) | 12.4 (7.0–15.0) | 11.6 (8.2–14.5) | 0.0052 |
| Total CTCs (cells/ml) | 2.38 (0–601.5) | 1.77 (0–441.3) | 4.35 (0–601.5) | 0.0040 |
| Clinical survival data | ||||
| Median follow-up (mo) | 11 (1–30) | 16 (2–29) | 9 (1–30) | <0.001 |
| Death events (n) | 74 | 42 | 32 | 0.016 |
mCRPC = metastatic castration-resistant prostate cancer; ARSi = androgen receptor signaling inhibitor.
The p values are for the Wilcoxon rank-sum test for continuous variables, and Fisher’s exact test for categorical variables.
Two of the 130 pre-ARSi samples were not evaluable, bringing the cohort total to 191 evaluable samples from 161 unique patients.
Only includes standard-of-care life-prolonging therapies and experimental therapies a patient was exposed to after standard androgen deprivation therapy and development of mCRPC disease and before initiation on the baseline therapy.
Prior exposure to life-prolonging therapies in the mCRPC setting. Other therapies included antibody drug conjugate, experimental therapies, and combinations. ARSi therapies included abiraterone acetate, enzalutamide, and apalutamide. Taxane therapies included docetaxel, cabazitaxel, and paclitaxel. A total of 17 patients received both ARSi and taxane therapies for one or more occurrences.
2.2. Post-treatment outcomes
For each treatment course, antitumor effects were assessed as treatment-specific post-therapy prostate-specific antigen (PSA) change (PTPC), as previously described [12]. All patients with “sensitive” PTPC had a ≥50% PSA decline at 12 w, and those with “resistant” PTPC did not achieve a 50% decline. For taxane patients, the maximum PSA decline may occur after 12 wk, in which case the later date was used. OS was calculated from initiation of therapy to death from any cause, with right-censoring for patients alive at last follow-up. For patients who were followed for more than one therapy, samples from all treatment decisions before the last were right-censored, with time calculated from initiation of therapy to date of next draw. The choice of therapy was made by the treating physician without knowledge of AR-V7 status.
2.3. CTC collection
Blood (7.5 ml) was collected in Streck tubes and processed at Memorial Sloan Kettering Cancer Center or shipped to Epic Sciences and processed within 48 h. After red blood cell lysis, approximately 3 000 000 nucleated cells were dispensed onto 10–16 glass microscope slides and placed at −80°C as previously described [14,15]. Sample processing and testing were conducted in laboratories following Clinical Laboratory Improvement Amendments (CLIA) regulations.
2.4. CTC immunofluorescent staining and analysis
CTC identification and characterization were performed as previously described [14,16]. In brief, pathology slides created from all nucleated cells in blood samples from mCRPC patients underwent automated immunofluorescent staining for DNA, cytokeratins (CK), CD45, and AR-V7 (Fig. 1) using a rabbit monoclonal anti-AR-V7 antibody (EPR15656, Abcam, Burlingame, CA, USA). The AR-V7 antibody specificity was comprehensively validated via western blots and single-cell PCR, as well as tissue microarrays (TMAs) containing malignant, tumor-adjacent, and healthy tissue samples, by a third party [12]. Fluorescent microscopes imaged every nucleated object on the slides, and morphology algorithms were used for identification of CTCs among nucleated blood cells on the slides. Classification as a CTC requires an intact nucleus (DNA dye, 4′,6-diamidino-2-phenylindole [DAPI]), lack of CD45 staining (a blood lineage marker), and a distinct morphology from surrounding white blood cells. Clinical laboratory scientists (licensed in California) conducted final quality control of CTC identification and subcellular biomarker localization without knowledge of patient outcome.
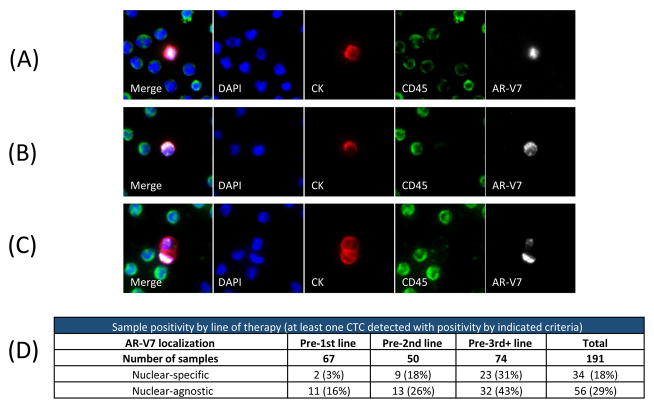
Fig. 1.
AR-V7 protein can be localized to the nucleus and/or cytoplasm of circulating tumor cells (CTCs). Example images show (A) individual CTCs with AR-V7 nuclear-specific localization, (B) CTC with predominantly cytoplasmic AR-V7 staining and (C) CTC cluster with varying AR-V7 expression. (D) Sample-level positivity for these CTCs. DAPI = 4′,6-diamidino-2-phenylindole; CK = cytokeratin.
Two different AR-V7 scoring criteria were used: one requiring nuclear-specific localization of a signal on top of a threshold cellular intensity (nuclear-specific) [12], and a second requiring a threshold signal intensity independent of localization (nuclear-agnostic). CTC images are nonconfocal images of whole cells (not sectioned, like in tissue), and diffuse localization in both cytoplasm and nucleus cannot necessarily be interpreted as a signal from proteins that have entered the nucleus of CTCs. Therefore, the nuclear-specific criterion requires a nondiffuse signal only in the nucleus to be considered positive. Samples with at least one AR-V7-positive CTC that met these criteria were considered AR-V7-positive.
2.5. Statistical analyses
Descriptive statistics were used to summarize patient demographics and clinical characteristics overall, by line of therapy, and by type of drug administered. Wilcoxon rank-sum tests were used to compare treatment groups. Time-to-event outcome measures were evaluated using the Kaplan-Meier method. Differences in time-to-event outcomes between samples negative or positive for AR-V7 scoring criteria were evaluated using the log-rank test, with hazard ratios estimated from univariable and multivariable Cox proportional hazards (PH) regression methods.
Multivariable Cox PH models included pretherapy measures: PSA, alkaline phosphatase, lactate dehydrogenase, hemoglobin levels, line of therapy, type of therapy, and AR-V7 status. Using a best subset selection method based on univariate significance (p < 0.05) and a global score χ2 statistic, the presence of liver and/or lung metastases, patient age, and albumin were excluded from the final model. These are the same model construction criteria previously utilized [12], but with the additional follow-up time and death events, a few of the covariates included changed from the previous model. All statistical tests were two-sided and were performed at the 5% significance level. KNIME [17] was utilized for data consolidation, and all statistical analyses were performed with the R v3.2.0 procedures survival and stats.
3. Results
The cohort included 191 evaluable pretherapy samples, including 128 pre-ARSi and 63 pre-taxane samples, from 161 unique mCRPC patients [12]. Of the unique patients, 130 (80.8%) had a single therapy, 30 (18.6%) had two therapies (60 samples), and one (0.6%) had three therapies. A total of 74 patients succumbed to their disease.
3.1. AR-V7 protein can be localized to the cytoplasm or nucleus of CTCs
AR-V7 protein localization was identified in the nucleus (Fig. 1A), cytoplasm (Fig. 1B), and in single CTCs within CTC clusters (Fig. 1C). The incidence of nuclear-specific AR-V7-positive CTCs and nuclear-agnostic AR-V7-positive CTCs both increased with the line of therapy (Fig. 1D).
3.2. Nuclear-specific AR-V7 localization is required for the specificity of PSA response prediction for patients on ARSi
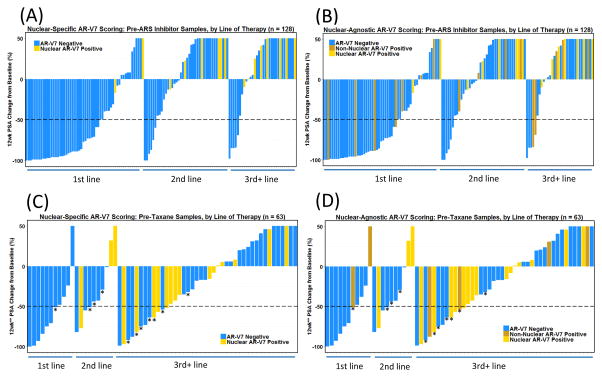
Of the 128 pre-ARSi samples in the cohort, 47 (37%) showed sensitive and 81 (63%) showed resistant PTPC. With the nuclear-specific scoring criterion, zero of 47 (0%) sensitive and 16 of 81 (20%) resistant pre-ARSi samples were AR-V7-positive (Fig. 2A). With the nuclear-agnostic scoring criterion, six of 47 (13%) sensitive and 26 of 81 (32%) resistant pre-ARSi samples were AR-V7-positive (Fig. 2B). Neither scoring criterion showed specificity for sensitive or resistant PTPC on taxane therapy (Fig. 2C, D).
Fig. 2.
Nuclear-specific AR-V7 localization is required for specificity of PTPC for patients on ARSi therapy. Waterfall plots of the percentage change in PSA at 12 wk on therapy, stratified by the number of previous lines of therapy. Each bar represents an individual patient. (A) Pre-ARSi AR-V7 status according to nuclear-specific localization only. (B) Pre-ARSi AR-V7 status according to nuclear-agnostic localization. (C) Pre-taxane AR-V7 status according to nuclear-specific localization only. (D) Pre-taxane AR-V7 status according to nuclear-agnostic localization. PSA = prostate-specific antigen; PTPC = post-therapy PSA change; ARSi = androgen receptor signaling inhibitor. * Longer than 12 wk (see the text).
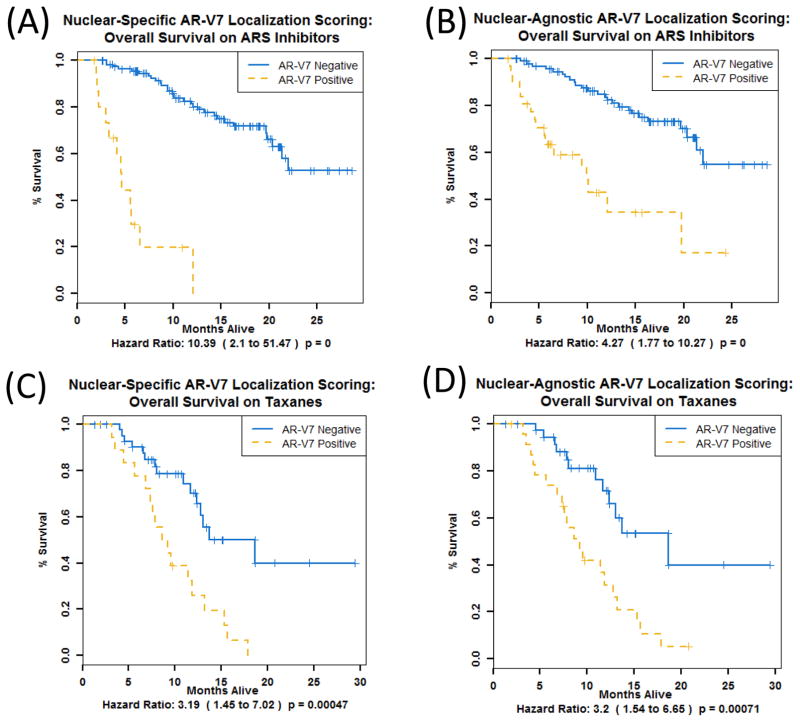
3.3. Nuclear-specific AR-V7 localization improves prognostication of OS for patients on ARSi
Pre-ARSi samples with AR-V7-positive CTCs according to either the nuclear-specific (Fig. 3A) or nuclear-agnostic criterion (Fig. 3B) had less favorable OS compared to AR-V7-negative samples. However, the magnitude of the difference in OS when using nuclear-specific localization was much greater (HR 10.4, p < 0.0001 vs HR 4.3, p < 0.0001) and the median OS was shorter (4.6 vs 10.0 mo). Pre-taxane samples with AR-V7-positive CTCs according to either criterion had poorer OS compared to those without (HR 3.2, p = 0.0005 vs HR 3.2, p = 0.0007) and similar median OS (8.9 vs 9.2 mo).
Fig. 3.
Nuclear-specific AR-V7 localization improves prognostication of overall survival (OS) for patients on ARSi therapy. OS is shown for patient samples stratified by pre-ARSi AR-V7 status (n = 128) determined according to (A) nuclear-specific localization and (B) nuclear-agnostic localization and stratified by pre-taxane AR-V7 status (n = 63) determined according to (C) nuclear-specific localization and (D) nuclear-agnostic localization. ARSi = androgen receptor signaling inhibitor.
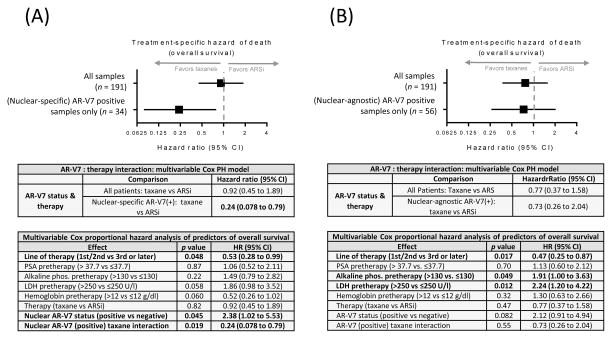
3.4. Nuclear-specific AR-V7 localization is necessary for prediction of treatment-specific reduction in risk of death
A higher proportion of patients receiving taxanes were on their third or later line of therapy compared to those on ARSis (67% vs 25%). Despite this, patients with nuclear-specific AR-V7 positivity had better median survival times on taxane therapy than on ARSis (8.9 vs 4.6 mo).
Multivariate analysis updated from that previously reported [12] incorporating the additional follow-up time and more death events again showed that the interaction between the presence of pretherapy nuclear-specific AR-V7-positive CTCs and the therapy administered was the most significant factor influencing OS. Patients classified as having AR-V7-positive CTCs using the nuclear-specific scoring criterion had a significantly lower risk of death on taxanes (HR 0.24, 95% CI 0.078–0.79; p = 0.019; Fig. 4A). Using an identically constructed multivariate model and changing only the criterion for AR-V7 positivity to nuclear-agnostic, both AR-V7 and the interaction between AR-V7 and therapy lost significance (HR 0.73, 95% CI 0.26–2.04; p = 0.55; Fig. 4B).
Fig. 4.
Nuclear-specific AR-V7 localization is necessary for prediction of a treatment-specific reduction in risk of death. Individual covariates were tested for additive power in predicting outcome using a Cox proportional hazards model. The p values are the result of compensating for the other factors listed. The interaction of therapy and AR-V7 status was further investigated using a multivariable Cox proportional hazards model. The forest plot shows the hazard ratio and 95% confidence interval (CI) for (A) a cohort evaluated for AR-V7 positivity utilizing nuclear-specific localization and (B) a cohort evaluated for positivity utilizing nuclear-agnostic localization. ARSi = androgen receptor signaling inhibitor; PSA = prostate-specific antigen; phos. = phosphatase; LDH = lactate dehydrogenase.
4. Discussion
Within the clinical context of choosing an ARSi versus taxane therapy for progressing mCRPC, most patients prefer the former given the ease of administration and the more favorable safety profile. The decision to choose a cytotoxic drug is therefore not taken lightly. Thus, it is essential that the false positive rate of any therapy-guiding predictive biomarker at this decision point in mCRPC be as low as possible. Here, the defined biomarker was based on detection of nuclear-specific AR-V7 protein in CTCs collected from mCRPC patients before ARSi or taxane treatment [12]. We showed the significance and clinical utility of nuclear-specific versus nuclear-agnostic AR-V7-positive localization in terms of improved specificity of resistant PTPC prediction for patients on ARSis, combined with more favorable survival demonstrated using an alternative, US Food and Drug Administration–approved life-prolonging therapeutic class (taxanes). The results are consistent with the known biology of the AR-V7 splice variant.
In the entire cohort, containing both pre-ARSi and pre-taxane samples, 34 of 191 (18%) were positive for AR-V7 using the nuclear-specific protein criterion, and 56 of 191 (29%) using the nuclear-agnostic scoring criterion. Among the 128 pre-ARSi samples, 16 were AR-V7-positive according to the nuclear-specific and 32 were positive according to the nuclear-agnostic scoring criterion, for which the median survival was 4.6 and 10 mo, respectively (Fig. 3). None of the 16 nuclear-specific AR-V7-positive samples showed sensitive PTPC, in contrast to six of the additional 16 (38%) nuclear-agnostic samples, a false-positive result, relative to the nuclear-specific criterion, that could potentially deny a patient a minimally toxic, safe, life-prolonging therapy (Fig. 2).
Critically, with additional follow-up time and death events, the nuclear-specific AR-V7 protein scoring criterion retained a significant treatment-specific reduction in risk of death for patients on taxanes, and was the most predictive pretreatment clinical feature influencing patient survival (HR 0.24, 95% CI 0.078–0.79; p = 0.019; Fig. 4A). The nuclear-agnostic AR-V7 scoring criterion had less magnitude for prognosticating outcome for patients on ARSi therapy (Fig. 3) and failed to show a treatment-specific interaction (Fig. 4B), indicating that patients considered positive according to this criterion were not predicted to have improved OS on an already-defined alternative therapy (taxanes).
The nuclear-specific AR-V7 protein localization requirement for positivity in studies exploring the relationship between biomarker presence and outcome is based on the recognition that downstream AR signaling does not occur until AR transcriptional elements bind to DNA [7–9]. Full-length AR protein has a nuclear localization sequence motif that is exposed on androgen binding, resulting in conformational changes to the protein that allow nuclear translocation so that DNA binding can occur [18,19]. AR-V7 is truncated after exon 3, and as a result lacks the complete nuclear localization sequence of full-length AR [20,21]. Despite this, the AR-V7 protein does localize to the nucleus of rapidly growing cells in culture [22], in transgenic prostate cancer models [23], and in human solid tumor tissues [3,7–9,24]. Exactly how localization of this truncated protein occurs is an area of active research, but it may be related to the unique C-terminal sequence present in the AR-V7 molecule [22].
The patients included in this study were treated in a routine clinical practice setting with agents that are already FDA approved. Overall, 12% of samples had AR-V7 signal that was not nuclear-specific, a scoring criteria that did not predict resistance to ARSis with the specificity needed at this clinical decision point. Only nuclear-specific AR-V7 protein in CTCs demonstrated therapy-changing clinical utility through both a low false-positive rate (PTPC) and significantly lower risk of death on taxanes (multivariate, OS), which, in combination, justify a change of therapy in this clinical decision.
Our results highlight an important limitation of mRNA-based approaches in CTCs: the inability to determine whether the AR-V7 message has been translated into protein and, if so, whether the protein is present in the nucleus, where it is known to function as an oncogenic driver of tumor growth. A prospective clinical trial testing the predictive capacity of pooled CTC AR-V7 mRNA and nuclear-specific AR-V7 protein in matched samples is ongoing (NCT02269982).
Despite high specificity for predicting resistant PTPC, the presence of nuclear-specific AR-V7 identified only 16 of 81 (20%) of the samples that showed resistant PTPC. This result is not surprising given other mechanisms of resistance to ARSis that have been reported beyond AR-V7, including signaling driven via AR gene mutations [25], reciprocal feedback via PTEN loss [15,26], and the development of a neuroendocrine phenotype of prostate cancer [27]. Biomarker assays that identify other resistance mechanisms will need to be analytically and clinically validated with the same rigor as that applied to AR-V7.
5. Conclusions
Expanding the AR-V7 protein scoring criteria for CTCs from nuclear-specific to include both nuclear and/or cytoplasmic AR-V7 localization (“nuclear-agnostic”) confirmed that nuclear-specific protein localization is required to reliably inform treatment selection between ARSis and taxanes using a CTC AR-V7 biomarker.
Supplementary Material
*Take Home Message.
Expanding the AR-V7 protein scoring criteria for circulating tumor cells (CTCs) from nuclear-specific to include both nuclear and/or cytoplasmic AR-V7 localization (“nuclear-agnostic”) confirmed that nuclear-specific protein localization is required to reliably inform treatment selection between androgen receptor signaling inhibitors and taxanes using a CTC AR-V7 biomarker.
Acknowledgments
Funding/Support and role of the sponsor: Equal distributions of funds from NIH/NCI P50-CA92629 SPORE in Prostate Cancer, NIH/NCI Cancer Center Support Grant P30 CA008748, Department of Defense Prostate Cancer Research Program (PC071610 and PC121111), a Prostate Cancer Foundation Challenge Award, and the David H. Koch Fund for Prostate Cancer Research were used to support the design and conduct of the study; the collection, management, analysis, and interpretation of the data, which took place independently at MSKCC and Epic Sciences to remove any bias; the preparation, review, and approval of the manuscript; and the decision to submit the manuscript for publication.
We would like to thank the patients and their families taking part in this study, and the clinical and laboratory staff at MSKCC and Epic Sciences. We would also like to thank Lee Horiuchi, BS and Aaron Oh, BS from Epic Sciences for technical imaging expertise.
Footnotes
Author contributions: Howard I. Scher had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Scher, Dittamore.
Acquisition of data: Scher, Graf, Schreiber, McLaughlin, Lu, Dittamore.
Analysis and interpretation of data: Scher, Graf, Schreiber, McLaughlin, Lu, Dittamore.
Drafting of the manuscript: Scher, Graf, Dittamore.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Graf, Johnson, Heller.
Obtaining funding: Scher.
Administrative, technical, or material support: Schreiber, McLaughlin, Louw, Dugan.
Supervision: Scher, Dittamore.
Other: None.
Financial disclosures: Howard I. Scher certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Howard I. Scher reports nonfinancial support from Janssen and Medivation, receipt of personal fees from Astellas and Sanofi Aventis, and receipt of research funding from Janssen Diagnostics, Janssen Pharmaceuticals, and Medivation. Ryon P. Graf, David Lu, Jessica Louw, Lyndsey Dugan, Ann Johnson, and Ryan Dittamore are employees of Epic Sciences. The remaining authors have no potential conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Scher HI, Morris MJ, Stadler WM, et al. Trial design and objectives for castration-resistant prostate cancer: updated recommendations from the Prostate Cancer Clinical Trials Working Group 3. J Clin Oncol. 2016;34:1402–18. doi: 10.1200/JCO.2015.64.2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yap TA, Lorente D, Omlin A, Olmos D, de Bono JS. Circulating tumor cells: a multifunctional biomarker. Clin Cancer Res. 2014;20:2553–68. doi: 10.1158/1078-0432.CCR-13-2664. [DOI] [PubMed] [Google Scholar]
- 3.Antonarakis ES, Lu C, Wang H, et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med. 2014;371:1028–38. doi: 10.1056/NEJMoa1315815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakazawa M, Lu C, Chen Y, et al. Serial blood-based analysis of AR-V7 in men with advanced prostate cancer. Ann Oncol. 2015;26:1859–65. doi: 10.1093/annonc/mdv282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Onstenk W, Sieuwerts AM, Kraan J, et al. Efficacy of cabazitaxel in castration-resistant prostate cancer is independent of the presence of AR-V7 in circulating tumor cells. Eur Urol. 2015;68:939–45. doi: 10.1016/j.eururo.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 6.Antonarakis ES, Lu C, Luber B, et al. Androgen receptor splice variant 7 and efficacy of taxane chemotherapy in patients with metastatic castration-resistant prostate cancer. JAMA Oncol. 2015;1:582–91. doi: 10.1001/jamaoncol.2015.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Welti J, Rodrigues DN, Sharp A, et al. Analytical validation and clinical qualification of a new immunohistochemical assay for androgen receptor splice variant-7 protein expression in metastatic castration-resistant prostate cancer. Eur Urol. 2016;70:599–608. doi: 10.1016/j.eururo.2016.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Efstathiou E, Titus M, Wen S, et al. Molecular characterization of enzalutamide-treated bone metastatic castration-resistant prostate cancer. Eur Urol. 2015;67:53–60. doi: 10.1016/j.eururo.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qu Y, Dai B, Ye D, et al. Constitutively active AR-V7 plays an essential role in the development and progression of castration-resistant prostate cancer. Sci Rep. 2015;5:7654. doi: 10.1038/srep07654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Antonarakis ES, Lu C, Chen Y, et al. AR splice variant 7 (AR-V7) and response to taxanes in men with metastatic castration-resistant prostate cancer (mCRPC) J Clin Oncol. 2015;33(Suppl 7):138. [Google Scholar]
- 11.Qu F, Xie W, Nakabayashi M, et al. Association of AR-V7 and prostate specific antigen RNA levels in blood with efficacy of abiraterone acetate and enzalutamide treatment in men with prostate cancer. Clin Cancer Res. doi: 10.1158/1078-0432.CCR-16-1070. In press. http://dx.doi.org/10.1158/1078-0432.CCR-16-1070. [DOI] [PMC free article] [PubMed]
- 12.Scher HI, Lu D, Schreiber NA, et al. Association of AR-V7 on circulating tumor cells as a treatment-specific biomarker with outcomes and survival in castration-resistant prostate cancer. JAMA Oncol. doi: 10.1001/jamaoncol.2016.1828. In press. http://dx.doi.org/10.1001/jamaoncol.2016.1828. [DOI] [PMC free article] [PubMed]
- 13.Antonarakis ES, Scher HI. Do patients with AR-V7-positive prostate cancer benefit from novel hormonal therapies? It all depends on definitions. Eur Urol. doi: 10.1016/j.eururo.2016.08.038. In press. http://dx.doi.org/10.1016/j.eururo.2016.08.038. [DOI] [PubMed]
- 14.Werner SL, Graf RP, Landers ML, et al. Analytical validation and capabilities of the Epic CTC platform: enrichment-free circulating tumour cell detection and characterization. J Circulat Biomarkers. 2015;4:3. doi: 10.5772/60725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Punnoose EA, Ferraldeschi R, Szafer-Glusman E, et al. PTEN loss in circulating tumour cells correlates with PTEN loss in fresh tumour tissue from castration-resistant prostate cancer patients. Br J Cancer. 2015;113:1225–33. doi: 10.1038/bjc.2015.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marrinucci D, Bethel K, Kolatkar A, et al. Fluid biopsy in patients with metastatic prostate, pancreatic and breast cancers. Phys Biol. 2012;9:016003. doi: 10.1088/1478-3975/9/1/016003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mazanetz MP, Marmon RJ, Reisser CB, Morao I. Drug discovery applications for KNIME: an open source data mining platform. Curr Top Med Chem. 2012;12:1965–79. doi: 10.2174/156802612804910331. [DOI] [PubMed] [Google Scholar]
- 18.Ware KE, Garcia-Blanco MA, Armstrong AJ, Dehm SM. Biologic and clinical significance of androgen receptor variants in castration resistant prostate cancer. Endocrine Relat Cancer. 2014;21:T87–103. doi: 10.1530/ERC-13-0470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lamont KR, Tindall DJ. Androgen regulation of gene expression. Adv Cancer Res. 2010;107:137–62. doi: 10.1016/S0065-230X(10)07005-3. [DOI] [PubMed] [Google Scholar]
- 20.Jenster G, Trapman J, Brinkmann AO. Nuclear import of the human androgen receptor. Biochem J. 1993;293:761–8. doi: 10.1042/bj2930761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou ZX, Sar M, Simental JA, Lane MV, Wilson EM. A ligand-dependent bipartite nuclear targeting signal in the human androgen receptor. Requirement for the DNA-binding domain and modulation by NH2-terminal and carboxyl-terminal sequences. J Biol Chem. 1994;269:13115–23. [PubMed] [Google Scholar]
- 22.Watson PA, Chen YF, Balbas MD, et al. Constitutively active androgen receptor splice variants expressed in castration-resistant prostate cancer require full-length androgen receptor. Proc Natl Acad Sci U S A. 2010;107:16759–65. doi: 10.1073/pnas.1012443107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun F, Chen HG, Li W, et al. Androgen receptor splice variant AR3 promotes prostate cancer via modulating expression of autocrine/paracrine factors. J Biol Chem. 2014;289:1529–39. doi: 10.1074/jbc.M113.492140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu R, Lu C, Mostaghel EA, et al. Distinct transcriptional programs mediated by the ligand-dependent full-length androgen receptor and its splice variants in castration-resistant prostate cancer. Cancer Res. 2012;72:3457–62. doi: 10.1158/0008-5472.CAN-11-3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wyatt AW, Azad AA, Volik SV, et al. Genomic alterations in cell-free DNA and enzalutamide resistance in castration-resistant prostate cancer. JAMA Oncol. doi: 10.1001/jamaoncol.2016.0494. In press. http://dx.doi.org/10.1001/jamaoncol.2016.0494. [DOI] [PMC free article] [PubMed]
- 26.Robinson D, Van Allen EM, Wu YM, et al. Integrative clinical genomics of advanced prostate cancer. Cell. 2015;161:1215–28. doi: 10.1016/j.cell.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beltran H, Tomlins S, Aparicio A, et al. Aggressive variants of castration-resistant prostate cancer. Clin Cancer Res. 2014;20:2846–50. doi: 10.1158/1078-0432.CCR-13-3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.