Abstract
bile acid malabsorption (BAM). BAs are passively absorbed to a different extent along the mammalian colon, so that levels are lower in the feces than in proximal colon. Our aim was to explore associations among total, primary, and secretory BA in stool and colonic transit in patients with irritable bowel syndrome-diarrhea (IBS-D) without overt BAM.
Methods
In a cross-sectional observational study of 116 patients with IBS-D recruited from local communities in Minnesota, we measured total and individual main fecal BA excretion, fecal fat and fecal weight over 48 hrs, fasting serum levels of C4 (surrogate for BA synthesis), and overall colonic transit by scintigraphy (geometric center at 24 hrs and 48 hrs). Patients without overt BAM were assigned to groups based on total fecal BA level below 2337 µmole/48 hrs (n=86) or serum levels of C4 below 47.1 ng/ml (n=91). We used Spearman correlations to test study hypotheses with correction for 14 correlations tested (P<.0036). Data from 30 healthy volunteers were used as controls.
Results
Patients with IBS-D who had increased or normal total BA excretion in stool or BA synthesis had higher stool proportions of primary BAs (especially chenodeoxycholate), compared with healthy controls. In patients with IBS-D without overt BAM (normal 48 hr total fecal BA or serum C4), there were significant positive correlations between total fecal BA, fecal primary and secretory BA, fecal weight, and increased geometric center at 24 and 48 hrs (P<.0036). Normal and slightly increased levels of total fecal BA have greatest effects on colonic transit at 48 hrs.
Conclusion
In the absence of overt BAM, the total, primary and secretory BAs in stool contribute to the acceleration of colonic transit and fecal weight in the diarrhea of patients with IBS-D.
Keywords: CDCA, lithocholate, intestine, clinical study
INTRODUCTION
Bile acids (BA) are amphiphatic molecules having hydrophobic and hydrophilic regions. They are secreted by the liver into the small intestine in response to feeding to ensure assimilation of lipids and lipid-soluble vitamins. The liver secretes a primary BA pool [chenodeoxycholic acid (CDCA) and cholic acid (CA)] that is 95% actively reabsorbed in the terminal ileum, with the rest undergoing deconjugation and dehydroxylation by gut microbiota into secondary BA [lithocholic acid (LCA) and deoxycholic acid (DCA)] in the colon, where they are either passively absorbed or excreted in the feces1. BA act on many types of nuclear and membrane bound receptors2. There is a delicate equilibrium between gut microbiota, BA pool size/composition and the human diet3.
Approximately 30% of patients with diarrhea-predominant irritable bowel syndrome (IBS-D) patients have bile acid malabsorption (BAM)4. In the remaining IBS-D patients without overt BAM, the role of BAs in the pathophysiology of diarrhea is uncertain. In a previous study, we showed an increased proportion of primary BAs (CDCA and CA) in feces of IBS-D patients compared with healthy controls, regardless of their total fecal BA excretion5, and this increased proportion of primary BAs in feces in IBS-D has been replicated6. The proportion of the secondary BA, DCA (which is also secretory), in feces of IBS-D patients was not significantly different from the proportion in healthy controls.5
The concentrations of BAs in the cecum of healthy subjects are unclear. In an autopsy study conducted within 24 hours of death, Hamilton et al.7 estimated the concentrations of the secretory BAs (DCA and CDCA), were respectively 200 and 60 µmoles/L, and LCA and CA were respectively 156 and 36 µmoles/L. However, Hamilton et al. acknowledged that postmortem changes or the intake of antibiotics prior to death may have influenced the estimated cecal BA profile and concentrations.
Older studies, based predominantly on enzymatic assays, had estimated that daily total BA excretion in stool in healthy volunteers and patients with idiopathic diarrhea8 was similar (mean ~0.5 mmoles/day or ~1000 µmoles/48 hrs), with a higher proportion of primary BAs in stool in idiopathic diarrhea. In the studies of individual stool samples from 3 patients with ileal resection9, the fecal excretion of BAs was expressed per gram of stool, and the calculated concentration of CDCA exceeded 10 mmol/L in individual stools9. In recent years, liquid chromatography with mass spectrometry has increased the accuracy of BA measurements in serum or stool10, 11. In our prior study5, we estimated that the total fecal BA concentration was 3.02±0.45 mmoles/L in 28 healthy volunteers and 6.08±0.67 mmoles/L in 62 unselected patients with IBS-D. Although the proportion of secretory BAs in stool in both groups was ~60%, there were higher concentrations in IBS-D patients.
In the cleansed rabbit colon12, there was passive absorption of ~75% of perfused CDCA through colonic mucosa. In human colonic perfusion studies, Mekhjian et al.13 showed that CA was minimally absorbed, whereas ~33% of DCA and ~50% of CDCA were absorbed in the colon. LCA is not absorbed in the human colon, as it is extremely insoluble14. These data suggest that the concentrations of BAs entering the colon may be up to 2- or 3-fold higher than the concentrations of BAs in stool. However, the higher volume of water in the right colon15 than in stool and the potential for colonic secretion in response to the secretory BAs may actually reduce the concentrations of the BAs. Although it is very difficult to predict the concentrations of BAs in the proximal colon, it is conceivable that patients with diarrhea without definite BAM or ileal resection may have sufficiently high concentrations to stimulate colonic fluid secretion16 and induce colonic propulsive motility17.
Our overall hypothesis is that lower concentrations of fecal BAs in patients with overt BAM may cause diarrhea. This hypothesis is based on several observations: 1.0 and 2.5 mM CDCA induced colonic secretion in rabbit colon18; 1 mM CDCA induced prolonged propagated contractions in humans; and administration (in a delayed-release capsule) of 500 mg or 1000 mg CDCA accelerated colonic transit in healthy controls and patients with irritable bowel syndrome-constipation (IBS-C)19, 20. Our specific hypothesis is that, in IBS-D patients without overt BAM, there is an association between stool BA profile [total and proportion of primary (CDCA and CA) and secretory BAs (CDCA and DCA)] and colon transit and fecal weight.
The primary aim of this study was to explore associations between total fecal BAs, proportions of primary and secretory BAs, and colonic transit measured at 24 hrs and 48 hrs in IBS-D patients with normal total fecal BA excretion and normal BA synthesis. A secondary aim was to explore associations of those fecal BA measurements with fecal weight in IBS-D patients without overt BAM.
METHODS
Study Design
In this cross-sectional, observational study in patients or healthy volunteers participating in research studies in the last eight years5, 10, 21, we appraised bowel functions, total fecal BAs, percentages of fecal BA species, fecal fat and colonic transit in a convenience sample of 116 patients with IBS-D (by Rome III criteria). Patients for this study were recruited from local communities in southeastern Minnesota to avoid tertiary referral bias. Eligibility criteria, methods of selection, periods of recruitment, and data collection were included in prior publications5, 10, 21. We identified patients with IBS-D without evidence of BAM, based on normal fecal total BA excretion or normal synthesis. The data of some of these patients have been published previously5, 10, 21; stool frequency and stool consistency data had been collected during a 2-week period that overlapped with the fecal collections and transit measurements. We used a standard validated bowel pattern daily diary including the Bristol stool form scale, which has been published previously22. However, the current study focuses on an analysis of the relationship between BA species and colonic transit, and focuses on IBS-D without overt BAM.
Measurement of Quantitative Traits
Quantitative traits were measured by methods that have been used extensively and validated in our laboratory: fasting serum C4, total and main fecal BA excretion, fecal fat excretion, fecal weight, and scintigraphic colonic transit23–28. These are described briefly in Supplemental Material.
Statistical Analysis
Bowel function, total and individual fecal BAs, fecal fat and colonic transit were compared in IBS-D subgroups without overt BAM, identified by fecal BA excretion <2,337 µmoles per 48 hrs or by serum C4 <47.1 ng/ml, the upper limit of normal range in our lab21, 23, 29. Data from 30 healthy volunteers, who were previously recruited by advertisement at Mayo Clinic and screened for bowel symptoms by using a brief questionnaire5, were used to compare fecal BA measurements in IBS-D and healthy controls. Patients with missing data were excluded from analysis.
We used Spearman correlation coefficients (Rs) to assess associations (based on ranks) between fecal BA measurements and colonic transit, and fecal weight. The α significance level for associations of BA measurements and colonic transit, as well as fecal weight was adjusted to 0.0036 (for 14 correlations tested). As a form of sensitivity analysis, we also appraised correlations based on fecal BA excretion using the 95th percentile from a healthy volunteer study, which was 2619 µmoles per 48 hrs, to ascertain whether the correlations detected were confirmed.
In addition, we constructed a curve for total fecal BAs and colonic transit by fitting simple models to localized subsets of data to describe the variation in data point by point, using SAS® ODS graphics (https://support.sas.com/rnd/app/ODSGraphics/TipSheet_ODSGraphics.pdf). This is termed LOESS (locally weighted scatterplot smoothing), a statistical method developed by Cleveland et al.30 The smoothing value was selected automatically by the program, that is, 2/number of observations. The LOESS option in SGPLOT from SAS does not create a residual plot.
All analyses were performed using SAS® (SAS Version 9.3, Cary, NC).
RESULTS
Demographics and Phenotype
Table 1A and B summarizes demographics, fasting serum C4, total fecal BAs, proportions of primary (CDCA and CA) and secretory (CDCA and DCA) BAs out of total fecal BAs, fecal fat and colonic transit (GC24, GC48) of the group of 116 IBS-D patients. Complete data were available in 110 patients and were tabulated for those with either one or both tests being normal or abnormal. Reasons for lack of availability were technical problems with assay or patient choice. Subgroups were based on fecal BA excretion and BA synthesis (fasting serum C4). The patients (Table 1B) with higher fecal BA excretion and fasting serum C4 (n=36) compared to those with normal values (n=74) had significantly higher BMI (32.0±1.5 compared to 25.6 + 0.5 kg/m2 respectively, p<0.001).
Table 1.
| A. Data on demographics, bowel habits and quantitative traits of subgroups based on either total fecal BA excretion per 48 hrs or fasting serum C4 (mean ± SE) | ||||
|---|---|---|---|---|
| Total fecal BAs ≥2337 µmoles per 48 hrs |
Total fecal BAs <2337 µmoles per 48 hrs |
Serum C4 ≥47.1 ng/ml | Serum C4 <47.1 ng/ml | |
| N | 24 | 86 | 19 | 91 |
| Age, years | 39.0±2.4 | 42.0±1.2 | 46.7±2.7 | 40.2±1.2 |
| BMI, kg/m2 | 32.0±1.5 | 26.6±0.6 | 31.7±2.1 | 26.9±0.6 |
| # bowel movements/day | 2.22±0.24 | 1.51±0.10 | 1.90±0.22 | 1.62±0.11 |
| Mean stool consistency (BSFS) | 4.61±0.19 | 3.91±0.12 | 4.33±0.28 | 4.01±0.12 |
| Fecal fat (g/24 hrs) | 11.4±8.9 | 6.5±0.6 | 7.8±1.3 | 7.6±0.7 |
| Total stool weight (g/48 hrs) | 557.7±55.4 | 256.8±16.9 | 357.1±43.7 | 316.3±24.3 |
| Total fecal BA excretion (µmoles/48 hrs) |
4839±744 | 728.8±69 | 2573±627 | 1428±248 |
| Total fecal BA concentration (mM) | 9.6±1.1 | 3.2±0.3 | 8.2±1.6 | 3.9±0.4 |
| % fecal CDCA and CA | 16.9±4.1 | 4.5±0.9 | 8.8±2.5 | 6.9±1.4 |
| % fecal CDCA and DCA | 61.3±1.9 | 55.0±1.7 | 64.7±2.2 | 54.7±1.6 |
| Serum C4, ng/mL | 37.7.±4.6 | 25.5±2.5 | 71.0±4.9 | 19.2±1.1 |
| Colonic transit GC24 | 3.2±0.2 | 2.5±0.1 | 3.1±0.3 | 2.5±0.1 |
| Colonic transit GC48 | 4.5±0.2 | 3.7±0.1 | 4.4±0.3 | 3.8±0.1 |
| AC emptying t1/2(hr) | 13.5±1.8 | 16.1±0.9 | 13.6±2.3 | 15.9±0.9 |
| B. Data for patients who had normal or abnormal values of both total fecal BA excretion per 48 hrs or fasting serum C4 | ||
|---|---|---|
| Total BAs ≥2337 µmoles/48 hrs and serum C4>47.1 ng/mL |
Total BAs <2337 µmoles/48 hrs and serum C4<47.1 ng/mL |
|
| N | 36 | 74 |
| Age, years | 39.0±2.4 | 41.1±1.3 |
| BMI, kg/m2 | 32.0±1.5 | 25.6±0.5 |
| # bowel movements/day | 2.22±0.24 | 1.41±0.10 |
| Mean stool consistency (BSFS) | 4.61±0.19 | 3.81±0.13 |
| Fecal fat (g/24h) | 11.4±8.9 | 6.3±0.6 |
| Total stool weight (g/48h) | 557.7±55.4 | 246.2±17.7 |
| Total fecal BA excretion (µmoles/48h) | 4839±744 | 656.1±68.8 |
| Total fecal BA concentration (mM) | 9.6±1.1 | 3.0±0.3 |
| % fecal CDCA and CA | 16.9±4.1 | 4.0±0.9 |
| % fecal CDCA and DCA | 61.3±1.9 | 53.3±1.8 |
| Serum C4, ng/mL | 49.4±4.8 | 17.8±1.1 |
| Colonic transit GC24 | 3.2±0.2 | 2.4±0.1 |
| Colonic transit GC48 | 4.5±0.2 | 3.6±0.1 |
| AC emptying t1/2(hr) | 13.5±1.8 | 16.4±1.0 |
BAs, bile acids; BMI, body mass index; BSFS, Bristol stool form scale; CA, cholic acid; CDCA, chenodeoxycholic acid; DCA, deoxycholic acid; GC, geometric center; AC, ascending colon
Concentrations of Total and Main Fecal BA
Table 2 shows concentrations of total and main fecal BAs in the 91 IBS-D patients with normal serum C4 (<47.1 ng/ml), in the 86 patients with total fecal BAs (<2337 µmoles per 48h), and data from 30 healthy controls studied in our laboratory and previously published in the literature5. There were (as expected, given the selection of patients for this analysis) no differences in total fecal BAs or percent of individual BAs between the 86 patients with total fecal BAs (<2337 µmoles per 48 hrs) and the 91 patients with serum C4 (<47.1 ng/ml). Seventy-four patients had both total fecal BA excretion <2337 µmoles per 48 hrs and serum C4 <47.1 ng/ml (Table 1B). Although the total fecal BA in the 30 healthy controls was not significantly different compared to the two IBS-D groups, there was 4.6-fold or 8-fold higher estimated CDCA concentration and 3.9-fold or 8.3-fold higher CA concentration in the two IBS-D groups compared to healthy controls. In contrast, the fold differences for estimated DCA and LCA concentrations were all <1.5-fold relative to healthy controls.
Table 2.
Concentrations of total and estimated concentrations of main fecal BAs in IBS-D and healthy controls (mean ± SE)
| IBS-D with total fecal BA <2337µmoles/48h |
IBS-D with serum C4 <47.1 ng/ml |
IBS-D total fecal BAs <2337 µmoles / 48 hrs and serum C4 <47.1 ng/ml |
Healthy controls | |||||
|---|---|---|---|---|---|---|---|---|
| N= | 86 | 91 | 74 | 30 | ||||
| % of total fecal BAs |
Concentration in stool (mM)* |
% of total fecal BAs |
Concentration in stool (mM)* |
% of total fecal BAs |
Concentration in stool (mM)* |
% of total fecal BAs |
Concentration in stool (mM)* |
|
| Total fecal BAs | 3.2±0.3 | 3.9±0.4 | 3.0±0.3 | 3.0±0.5 | ||||
| CDCA | 2.19±0.4 | 0.07 | 3.05±0.5 | 0.12 | 2.02±0.5 | 0.06 | 0.5±0.1 | 0.015 |
| CA | 2.33±0.5 | 0.07 | 3.88±0.9 | 0.15 | 2.00±0.5 | 0.06 | 0.6±0.2 | 0.018 |
| LCA | 41.14±1.9 | 1.30 | 39.62±1.9 | 1.52 | 43.30±2.0 | 1.29 | 39.0±2.8 | 1.17 |
| DCA | 52.76±1.7 | 1.67 | 51.6±1.6 | 1.99 | 51.3±1.9 | 1.54 | 59.2±2.7 | 1.78 |
| UDCA | 1.58±0.3 | 0.05 | 1.82±0.3 | 0.07 | 1.37±0.3 | 0.04 | 0.7±0.3 | 0.021 |
Concentrations of individual bile acids were based on mean percent of total fecal BAs multiplied by mean concentration of total fecal BAs actually measured in stool.
BAs, bile acids; IBS-D, diarrhea-predominant irritable bowel syndrome; CA, cholic acid; CDCA, chenodeoxycholic acid; DCA, deoxycholic acid; LCA, lithocholic acid; UDCA, ursodeoxycholic acid
Correlations of Fecal BA and Fecal Weight in IBS-D Subgroup without BAM
A significant correlation was observed between total fecal BAs and fecal weight (Rs=0.72, p<0.0001) in the group with normal BA synthesis (serum C4<47.1 ng/ml). Correlations between these quantitative measurements were also present in the group with normal BA excretion (total fecal BAs <2337 µmoles per 48 hrs) with Rs=0.57, p<0.0001.
Correlations of Fecal BA and Colonic Transit in IBS-D without BAM Based on Normal Serum C4
In the subgroup (91/116) of IBS-D patients with normal BA synthesis (serum C4 <47.1 ng/ml), several significant associations were present (Table 3), consistent with moderate strength correlations. Colonic transit GC24 and GC48 were significantly associated with fecal total BAs and fecal primary BAs (all p<0.0036). The correlation between colonic transit GC48 and proportion of secretory BAs (CDCA and DCA) was borderline significant (p=0.0062, above the threshold of 0.0036).
Table 3.
Univariate associations (Spearman correlation values) for total fecal BAs, proportions of primary (CDCA and CA) and secretory (CDCA and DCA) fecal BAs, and colonic transit at 24 (GC24) and 48 (GC48) hours in IBS-D patients with normal BA synthesis, that is, serum C4 <47.1 ng/ml
| Colonic transit GC24 |
Colonic transit GC48 |
|
|---|---|---|
| Total fecal BA excretion (µmoles per 48 hrs) | 0.36a | 0.48a |
| % fecal primary BAs: CDCA and CA | 0.32a | 0.45a |
| % fecal secretory BAs: CDCA and DCA | 0.23b | 0.29c |
p<0.0036;
p=0.03;
p=0.0062
Correlations of Fecal BA and Colonic Transit in IBS-D without BAM Based on Normal Total Fecal BA Excretion
In the subgroup (86/116) of IBS-D patients with normal BA excretion (total fecal BA <2337 µmoles per 48 hrs), several significant associations were observed (Table 4). Colonic transit at 48 hrs was significantly associated with fecal total BAs, fecal primary BAs and fecal secretory BAs (all p<0.0036). However, no significant associations were present with those fecal BA measurements and colonic transit at 24 hours. When the data were analyzed for the 89/116 patients with total fecal BA excretion <2619 µmoles per 48 hrs, all the significant correlations were confirmed.
Table 4.
Univariate associations (Spearman correlation values) of total fecal BAs, proportions of primary (CDCA and CA) and secretory (CDCA and DCA) fecal BAs, and colonic transit at 24 (GC24) and 48 (GC48) hours in IBS-D patients with normal fecal BA excretion, that is, total fecal BAs <2337 µmoles per 48 hours
| Colonic transit GC24 | Colonic transit GC48 | |
|---|---|---|
| Total fecal BAs (µmoles per 48 hrs) | 0.15b | 0.34a |
| % fecal primary BAs: CDCA and CA | 0.16c | 0.41a |
| % fecal secretory BAs: CDCA and DCA | 0.21d | 0.36a |
p<0.0036;
p=0.1769;
p=0.1337;
p=0.0476
Characterization of Relationships between BA and Colonic Transit Shown by LOESS Analysis
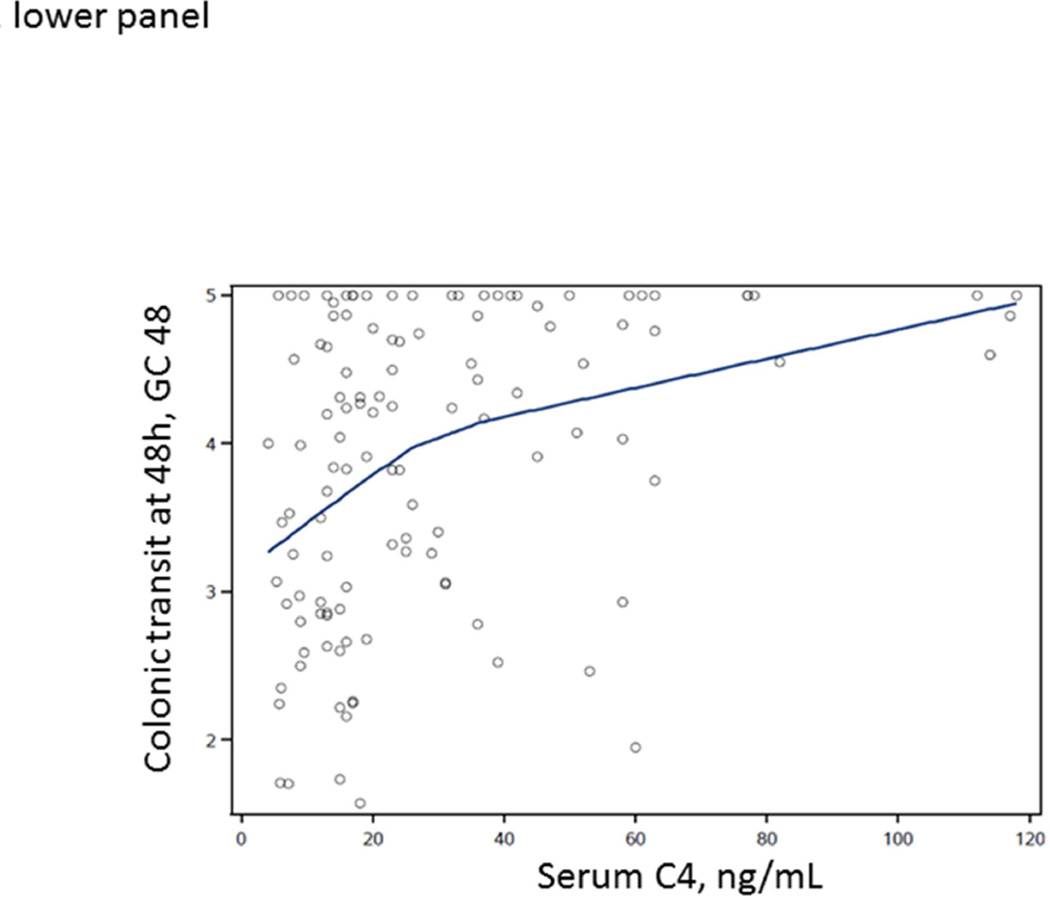
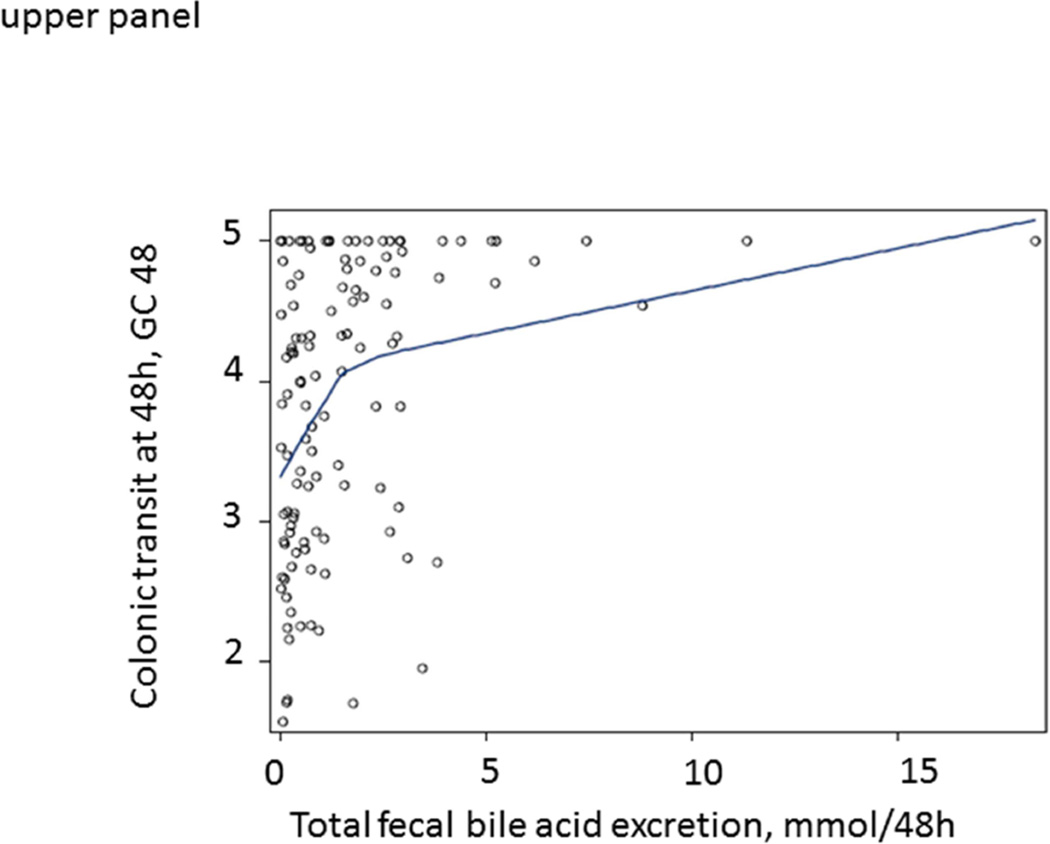
LOESS curves were generated to visualize the associations between total fecal BAs or serum C4 and GC48 in the entire patient cohort (≥2237 and <2337 µmoles/48 hrs, or >47.1 and <47.1 ng/mL), without specifying a regression function. In Figure 1, the LOESS curves show a steep slope for ‘normal’ values of total fecal BAs or serum C4, but they flatten above the upper limit of normal range (approximately at 2400 µmoles per 48 hrs for total fecal BAs and at approximately 40 ng/mL for serum C4). The curves suggest that, within the normal range of BA excretion, there is an important impact of fecal BA excretion or BA synthesis (for which C4 is a surrogate) on colonic transit GC48.
Figure 1.
Relationship between total fecal BAs (upper panel) or fasting serum C4 (lower panel) and colonic transit at 48 hours (GC48) in all IBS-D patients, illustrated by construction of LOESS curves. Note the deflection of the curve near the upper limit of normal range of total fecal BAs (approximately at 2400 µmoles per 48 hours)
DISCUSSION
Our study shows that, in IBS-D patients without overt BAM, total fecal BAs is significantly correlated with fecal weight. In addition, increased total fecal BAs and proportions of primary and secretory BAs are associated with colonic transit at 24 and 48 hrs. Furthermore, as illustrated in Figure 1, among IBS-D patients without overt BAM, fecal BAs impact colonic transit, as shown in the more vertical part of the LOESS curves in contrast to the more horizontal relationship between fecal total BAs and colonic transit among those with overt BAM. Overall, these results suggest that total fecal BA levels that do not represent overt BAM, and alterations in the fecal BA profile, are associated with colonic transit and increased fecal weight in IBS-D patients.
BAs have been ingested for centuries in Oriental civilizations to relieve constipation.31–33 Delivery of BAs to the colon (CDCA in an ileocolonic release capsule) accelerated colonic transit in healthy controls and patients with IBS-C19, 20. Compared to healthy volunteers from our previous study5, IBS-D patients without elevated BA excretion or synthesis in the current study had increased total fecal BAs and primary BAs (including elevated CDCA), as well as increased absolute concentrations of secretory BAs (see Table 2).
BAs may affect either colonic secretion or motility. The infusion of BAs (CDCA and DCA) in the cecum of healthy volunteers inhibited absorption and induced secretion of water and electrolytes [sodium (Na+) and chloride (Cl−)]34, effects associated with di-α hydroxy BA chemistry35–37. In fact, our study shows that the proportions of fecal CDCA and DCA (both di-α hydroxy BA) are significantly associated with increased colonic transit. CDCA has been considered the main secretagogue9, 38; in IBS-D, our previous studies showed that the proportion of DCA in stool was on average 56% (similar to the proportion in healthy controls), and the proportion of CDCA was ~2%. Nevertheless, the concentration of CDCA in feces of IBS-D patients is higher than in healthy controls and may promote secretion5.
The effects of CDCA and DCA on colonic motility may result from increased phasic pressure activity or from the motor responses secondary to colonic secretion. Goy et al8 showed that total fecal BA levels in idiopathic diarrhea were similar to those of healthy controls, but the patients with diarrhea had increased fecal primary BAs (of which CDCA is secretory and CA is non-secretory), and there were higher fecal water and (non-significantly) higher fecal weight, fecal Na+ and potassium (K+). However, the latter changes were not significant because of a type II statistical error, since, with the observed variance in fecal weight, ~65 patients (rather than the 12 studied) per group would be required for the observed difference in fecal weight to be statistically significant.
The effects of BAs leading to acceleration of colonic transit may also reflect effects on colonic motility. Kirwan et al.39 found correlations between increased fecal primary BAs and colonic motility index in patients with chronic diarrhea. Infusion of CDCA and supraphysiological concentrations (15 mM) of DCA in the sigmoid of IBS patients40 stimulated colonic motility. Falconer et al.41 found that 3–30 mM DCA elicited colonic motor activity in the rabbit sigmoid, whereas similar amounts of CA did not. More recently, Bampton and colleagues17 concluded that rectal infusion of CDCA (~2 mM) is a potent stimulus for propagating pressure waves in the proximal colon in humans.
Molecular mechanisms of BA-induced motility are attributed to the G-protein coupled BA receptor 1 (GPBAR1, also known as TGR5). The membrane bound receptor, TGR542, also mediates the effects of BAs on glucose homeostasis (via GLP-1 release by intestinal enteroendocrine cells), inflammation, and BA synthesis and excretion2. Poole et al. demonstrated TGR5 on neurons of the submucosal and myenteric plexus in the murine small bowel and colon, chiefly in nitric oxide synthase (NOS) positive inhibitory neurons (>80% TGR5 positive)43. TGR5 plays a role in the peristaltic reflex evoked by BAs in the colon in a mouse model44.
BA-induced secretion and colonic motility are not mutually exclusive effects. Thus, increased secretion with greater intraluminal volume can act as a mechanical stimulus of motility. Accelerated transit may lead to greater BA excretion in stool (lack of time to reabsorb BAs by passive, diffusion-related mechanisms) and, possibly, to altered fecal BA profile (lack of time for bacterial dihydroxylation) with higher proportion of primary BAs. Proof of the importance of accelerated transit on BA kinetics is demonstrated by the effects of loperamide in patients with chronic radiation enteritis and chronic diarrhea; loperamide resulted in slowing transit and more BA absorption45, 46.
Strengths and Limitations
Our study included a large sample which made it possible to perform advanced statistical analyses such as the LOESS method to support the hypotheses about the biological effects of relatively smaller perturbations of BA homeostasis than have been observed in overt BAM. The correlations reported are statistically significant, even with correction for multiple correlations, with an alpha level of 0.0036. The in vivo measurements with extensively validated techniques take into account the complex, integrative physiology modulating the effects of BAs.
A limitation of our study is that fecal electrolytes and fecal microbiome were not studied. Therefore, we cannot appraise the potential role of dysbiosis in the altered fecal BA pool composition. A second limitation is that, despite refined and accurate measurement techniques of fecal individual BAs, it is difficult to deduce the precise BA concentrations and composition delivered into the cecum that actually influenced the colonic secretion and motility7.
CONCLUSION
In summary, our study shows that total fecal BAs and the fecal BA profile are significantly associated with fecal weight and colonic transit in IBS-D patients without overt BAM; the data are consistent with moderate strength correlations. There is a range of BA excretion in patients with IBS-D.. Our data suggest a mechanistic role for borderline high BA synthesis or excretion and an altered BA profile in the diarrhea in some patients with IBS, with increased proportions of primary BAs or secretory BAs, particularly the CDCA content of stool. Future studies will test this hypothesis by examining the effects of BA sequestrants, FGF-19 analogs or FXR agonists, in patients with IBS-D without overt BAM. The impact on clinical practice in the foreseeable future is that measurement of BA kinetics may identify patients with functional diarrhea in the absence of overt BAM in whom therapy may be directed at restoring normal BA synthesis or excretion.
Supplementary Material
Acknowledgments
The authors thank Mrs. Cindy Stanislav for excellent secretarial assistance.
Grant support: Dr. Camilleri is supported by NIH grant R01-DK92179.
ABBREVIATIONS USED
- AC
ascending colon
- BA
bile acids
- BAM
bile acid malabsorption
- C4
serum 7α-OH-4--cholesten-3-one
- CA
cholic acid
- CDCA
chenodeoxycholic acid
- Cl−
chloride ion
- CT
colonic transit
- DCA
deoxycholic acid
- GC
geometric center
- IBS
irritable bowel disease
- IBS-C
irritable bowel disease-constipation
- IBS-D
irritable bowel disease-diarrhea
- K+
potassium ion
- LCA
lithocholic acid
- Na+
sodium ion
- NOS
nitric oxide synthase
- TGR5/GpBAR1
G protein coupled bile acid receptor1
- SE
standard error of the mean
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The authors have no conflicts of interests.
Authors’ contributions:
M. Camilleri: study concept and design; analysis and interpretation of data; drafting and finalizing the manuscript; study supervision
Cédric Peleman: Analysis of electronic medical records; co-authorship of the manuscript
Irene Busciglio: patient recruitment and care
Duane Burton: colonic transit measurements
Leslie Donato: lab medicine supervision of measurements related to bile acids; co-authorship
Alan Zinsmeister: Biostatistician and co-authorship
Reference List
- 1.Ridlon JM, Kang DJ, Hylemon PB. Bile salt biotransformations by human intestinal bacteria. J Lipid Res. 2006;47(2):241–259. doi: 10.1194/jlr.R500013-JLR200. [DOI] [PubMed] [Google Scholar]
- 2.Bunnett NW. Neuro-humoral signalling by bile acids and the TGR5 receptor in the gastrointestinal tract. J Physiol. 2014;592(14):2943–2950. doi: 10.1113/jphysiol.2014.271155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ridlon JM, Kang DJ, Hylemon PB, Bajaj JS. Bile acids and the gut microbiome. Curr Opin Gastroenterol. 2014;30(3):332–338. doi: 10.1097/MOG.0000000000000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Slattery SA, Niaz O, Aziz Q, Ford AC, Farmer AD. Systematic review with meta-analysis: the prevalence of bile acid malabsorption in the irritable bowel syndrome with diarrhoea. Aliment Pharmacol Ther. 2015;42(1):3–11. doi: 10.1111/apt.13227. [DOI] [PubMed] [Google Scholar]
- 5.Shin A, Camilleri M, Vijayvargiya P, et al. Bowel functions, fecal unconjugated primary and secondary bile acids, and colonic transit in patients with irritable bowel syndrome. Clin Gastroenterol Hepatol. 2013;11(10):1270–1275. doi: 10.1016/j.cgh.2013.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duboc H, Rainteau D, Rajca S, et al. Increase in fecal primary bile acids and dysbiosis in patients with diarrhea-predominant irritable bowel syndrome. Neurogastroenterol Motil. 2012;24(6):513–517. doi: 10.1111/j.1365-2982.2012.01893.x. [DOI] [PubMed] [Google Scholar]
- 7.Hamilton JP, Xie G, Raufman JP, et al. Human cecal bile acids: concentration and spectrum. Am J Physiol Gastrointest Liver Physiol. 2007;293(1):G256–G263. doi: 10.1152/ajpgi.00027.2007. [DOI] [PubMed] [Google Scholar]
- 8.Goy JA, Eastwood MA, Mitchell WD, Pritchard JL, Smith AN. Fecal characteristics contrasted in the irritable bowel syndrome and diverticular disease. Am J Clin Nutr. 1976;29(12):1480–1484. doi: 10.1093/ajcn/29.12.1480. [DOI] [PubMed] [Google Scholar]
- 9.Mitchell WD, Findlay JM, Prescott RJ, Eastwood MA, Horn DB. Bile acids in the diarrhoea of ileal resection. Gut. 1973;14(5):348–353. doi: 10.1136/gut.14.5.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Camilleri M, Busciglio I, Acosta A, et al. Effect of increased bile acid synthesis or fecal excretion in irritable bowel syndrome-diarrhea. Am J Gastroenterol. 2014;109(10):1621–1630. doi: 10.1038/ajg.2014.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vijayvargiya P, Camilleri M, Shin A, Saenger A. Methods for diagnosis of bile acid malabsorption in clinical practice. Clin Gastroenterol Hepatol. 2013;11(10):1232–1239. doi: 10.1016/j.cgh.2013.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Camilleri M, Murphy R, Chadwick VS. Pharmacological inhibition of chenodeoxycholate-induced fluid and mucus secretion and mucosal injury in the rabbit colon. Dig Dis Sci. 1982;27(10):865–869. doi: 10.1007/BF01316567. [DOI] [PubMed] [Google Scholar]
- 13.Mekhjian HS, Phillips SF, Hofmann AF. Colonic absorption of unconjugated bile acids: perfusion studies in man. Dig Dis Sci. 1979;24(7):545–550. doi: 10.1007/BF01489324. [DOI] [PubMed] [Google Scholar]
- 14.Gu JJ, Hofmann AF, Ton-Nu HT, Schteingart CD, Mysels KJ. Solubility of calcium salts of unconjugated and conjugated natural bile acids. J Lipid Res. 1992;33(5):635–646. [PubMed] [Google Scholar]
- 15.Debongnie JC, Phillips SF. Capacity of the human colon to absorb fluid. Gastroenterology. 1978;74(4):698–703. [PubMed] [Google Scholar]
- 16.Thaysen EH, Pedersen L. Idiopathic bile acid catharsis. Gut. 1976;17(12):965–970. doi: 10.1136/gut.17.12.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bampton PA, Dinning PG, Kennedy ML, Lubowski DZ, Cook IJ. The proximal colonic motor response to rectal mechanical and chemical stimulation. Am J Physiol Gastrointest Liver Physiol. 2002;282(3):G443–G449. doi: 10.1152/ajpgi.00194.2001. [DOI] [PubMed] [Google Scholar]
- 18.Camilleri M, Murphy R, Chadwick VS. Dose-related effects of chenodeoxycholic acid in the rabbit colon. Dig Dis Sci. 1980;25(6):433–438. doi: 10.1007/BF01395507. [DOI] [PubMed] [Google Scholar]
- 19.Odunsi-Shiyanbade ST, Camilleri M, McKinzie S, et al. Effects of chenodeoxycholate and a bile acid sequestrant, colesevelam, on intestinal transit and bowel function. Clin Gastroenterol Hepatol. 2010;8(2):159–165. doi: 10.1016/j.cgh.2009.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rao AS, Wong BS, Camilleri M, et al. Chenodeoxycholate in females with irritable bowel syndrome-constipation: a pharmacodynamic and pharmacogenetic analysis. Gastroenterology. 2010;139(5):1549–1558. 1558. doi: 10.1053/j.gastro.2010.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wong BS, Camilleri M, Carlson P, et al. Increased bile acid biosynthesis is associated with irritable bowel syndrome with diarrhea. Clin Gastroenterol Hepatol. 2012;10(9):1009–1015. doi: 10.1016/j.cgh.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zinsmeister AR, Burton D, Camilleri M. Pharmacodynamic and clinical endpoints for functional colonic disorders: statistical considerations. Dig Dis Sci. 2013;58(2):509–518. doi: 10.1007/s10620-012-2369-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Camilleri M, Nadeau A, Tremaine WJ, et al. Measurement of serum 7alpha-hydroxy-4-cholesten-3-one (or 7alphaC4), a surrogate test for bile acid malabsorption in health, ileal disease and irritable bowel syndrome using liquid chromatography-tandem mass spectrometry. Neurogastroenterol Motil. 2009;21(7):e734–e743. doi: 10.1111/j.1365-2982.2009.01288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galman C, Arvidsson I, Angelin B, Rudling M. Monitoring hepatic cholesterol 7alpha-hydroxylase activity by assay of the stable bile acid intermediate 7alpha-hydroxy-4-cholesten-3-one in peripheral blood. J Lipid Res. 2003;44(4):859–866. doi: 10.1194/jlr.D200043-JLR200. [DOI] [PubMed] [Google Scholar]
- 25.Sauter GH, Munzing W, von RC, Paumgartner G. Bile acid malabsorption as a cause of chronic diarrhea: diagnostic value of 7alpha-hydroxy-4-cholesten-3-one in serum. Dig Dis Sci. 1999;44(1):14–19. doi: 10.1023/a:1026681512303. [DOI] [PubMed] [Google Scholar]
- 26.Brydon WG, Nyhlin H, Eastwood MA, Merrick MV. Serum 7 alpha-hydroxy-4-cholesten-3-one and selenohomocholyltaurine (SeHCAT) whole body retention in the assessment of bile acid induced diarrhoea. Eur J Gastroenterol Hepatol. 1996;8(2):117–123. doi: 10.1097/00042737-199602000-00005. [DOI] [PubMed] [Google Scholar]
- 27.Tagliacozzi D, Mozzi AF, Casetta B, et al. Quantitative analysis of bile acids in human plasma by liquid chromatography-electrospray tandem mass spectrometry: a simple and rapid one-step method. Clin Chem Lab Med. 2003;41(12):1633–1641. doi: 10.1515/CCLM.2003.247. [DOI] [PubMed] [Google Scholar]
- 28.Deiteren A, Camilleri M, Bharucha AE, et al. Performance characteristics of scintigraphic colon transit measurement in health and irritable bowel syndrome and relationship to bowel functions. Neurogastroenterol Motil. 2010;22(4):415–423. e95. doi: 10.1111/j.1365-2982.2009.01441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Altman DG, Bland JM. Statistics notes: the normal distribution. BMJ. 1995;310(6975):298. doi: 10.1136/bmj.310.6975.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cleveland WS, Devlin SJ. Locally weighted regression: an approach to regression analysis by local fitting. Journal of the American Statistical Association. 1988;83(403):596–610. [Google Scholar]
- 31.Thorner RS. The effect of exclusion of the bile upon gastrointestinal motility. Am J Roentgenol Radium Ther Nucl Med. 1955;74(6):1096–1122. [PubMed] [Google Scholar]
- 32.Hofmann AF, Hagey LR. Key discoveries in bile acid chemistry and biology and their clinical applications: history of the last eight decades. J Lipid Res. 2014;55(8):1553–1595. doi: 10.1194/jlr.R049437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang DQ, Carey MC. Therapeutic uses of animal biles in traditional Chinese medicine: an ethnopharmacological, biophysical chemical and medicinal review. World J Gastroenterol. 2014;20(29):9952–9975. doi: 10.3748/wjg.v20.i29.9952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mekjian HS, Phillips SF, Hofmann AF. Colonic secretion of water and electrolytes induced by bile acids: perfusion studies in man. J Clin Invest. 1971;50(8):1569–1577. doi: 10.1172/JCI106644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chadwick VS, Gaginella TS, Carlson GL, Debongnie JC, Phillips SF, Hofmann AF. Effect of molecular structure on bile acid-induced alterations in absorptive function, permeability, and morphology in the perfused rabbit colon. J Lab Clin Med. 1979;94(5):661–674. [PubMed] [Google Scholar]
- 36.Gordon SJ, Kinsey MD, Magen JS, Joseph RE, Kowlessar OD. Structure of bile acids associated with secretion in the rat cecum. Gastroenterology. 1979;77(1):38–44. [PubMed] [Google Scholar]
- 37.Keely SJ, Scharl MM, Bertelsen LS, Hagey LR, Barrett KE, Hofmann AF. Bile acid-induced secretion in polarized monolayers of T84 colonic epithelial cells: Structure-activity relationships. Am J Physiol Gastrointest Liver Physiol. 2007;292(1):G290–G297. doi: 10.1152/ajpgi.00076.2006. [DOI] [PubMed] [Google Scholar]
- 38.Hofmann AF. Bile acid malabsorption caused by ileal resection. Arch Intern Med. 1972;130(4):597–605. [PubMed] [Google Scholar]
- 39.Kirwan WO, Smith AN, Mitchell WD, Falconer JD, Eastwood MA. Bile acids and colonic motility in the rabbit and the human. Gut. 1975;16(11):894–902. doi: 10.1136/gut.16.11.894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taylor I, Basu P, Hammond P, Darby C, Flynn M. Effect of bile acid perfusion on colonic motor function in patients with the irritable colon syndrome. Gut. 1980;21(10):843–847. doi: 10.1136/gut.21.10.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Falconer JD, Smith AN, Eastwood MA. The effects of bile acids on colonic motility in the rabbit. Q J Exp Physiol Cogn Med Sci. 1980;65(2):135–144. doi: 10.1113/expphysiol.1980.sp002497. [DOI] [PubMed] [Google Scholar]
- 42.Maruyama T, Miyamoto Y, Nakamura T, et al. Identification of membrane-type receptor for bile acids (M-BAR) Biochem Biophys Res Commun. 2002;298(5):714–719. doi: 10.1016/s0006-291x(02)02550-0. [DOI] [PubMed] [Google Scholar]
- 43.Poole DP, Godfrey C, Cattaruzza F, et al. Expression and function of the bile acid receptor GpBAR1 (TGR5) in the murine enteric nervous system. Neurogastroenterol Motil. 2010;22(7):814–818. doi: 10.1111/j.1365-2982.2010.01487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alemi F, Poole DP, Chiu J, et al. The receptor TGR5 mediates the prokinetic actions of intestinal bile acids and is required for normal defecation in mice. Gastroenterology. 2013;144(1):145–154. doi: 10.1053/j.gastro.2012.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Valdes OR, den Hartog JF, Hoefnagel C, Taal B. Effect of loperamide and delay of bowel motility on bile acid malabsorption caused by late radiation damage and ileal resection. Eur J Nucl Med. 1991;18(5):346–350. doi: 10.1007/BF02285463. [DOI] [PubMed] [Google Scholar]
- 46.Yeoh EK, Horowitz M, Russo A, Muecke T, Robb T, Chatterton BE. Gastrointestinal function in chronic radiation enteritis--effects of loperamide-N-oxide. Gut. 1993;34(4):476–482. doi: 10.1136/gut.34.4.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.