Abstract
Elevated excitability in the hippocampus has emerged as a key contributor to reduced memory function in aging and in cognitive impairment prodromal to Alzheimer’s disease. Here, we investigated the relationship between neural activity and memory in the hippocampus and a connectional cortical network using an aged rat model of individual differences for memory impairment. The expression of cFos was used as a measure of pharmacologically induced neural activity. Aged memory-impaired rats exhibited elevated cFos relative to young adult and aged unimpaired rats in the CA3 subfield of the hippocampus and in several cortical regions including the retrosplenial, parietal, and orbitofrontal cortices. Strong correlations between cFos intensity and task performance across the activated network showed a tight coupling between excitability and cognitive phenotype in aging. Elevated neural excitability extending beyond the hippocampus to interconnected posterior cortex (retrosplenial/parietal) was reduced by treatment with levetiracetam, a therapeutic with behavioral efficacy that has previously translated from rodent models of age-related impairment and Alzheimer’s disease to humans with amnestic mild cognitive impairment.
Keywords: Hippocampal Hyperactivity, Parietal cortex, Retrosplenial cortex, Levetiracetam, Aging, Memory
1. Introduction
Aging is recognized as the greatest risk factor for sporadic, late-onset Alzheimer’s disease, in which progressive memory loss occurs in the earliest symptomatic phase of the disease. Aging itself can be associated with limited memory impairment not only in elderly humans but also in many other species that do not develop Alzheimer’s disease (AD). Memory impairment in aged laboratory animals, including both rodents and non-human primates, is associated with increased neural activity in specific circuits within the hippocampal memory system (Thomé et al., 2016; Wilson et al., 2005; Simkin et al., 2015). Mounting evidence from basic science and clinical studies has also demonstrated that neuronal circuits become hyperactive particularly in early stages of AD (Busche et al., 2012; Busche and Konnerth, 2015). For example, a condition of hippocampal hyperactivity detected using functional magnetic resonance imaging (fMRI) is similarly localized in normal aged humans compared to young adults (Yassa et al., 2011), with much greater activation in prodromal AD such as in patients with amnestic mild cognitive impairment (aMCI; e.g., Bakker et al., 2012; Dickerson et al., 2005; Miller et al., 2008b; Putcha et al., 2011; Yassa et al., 2010). AD pathophysiology is likely coupled with the augmentation of hyperactivity in aMCI relative to age-matched controls as the greatest hippocampal hyperactivity is observed in amyloid-positive patients in the aMCI phase of AD (Huijbers et al., 2015).
An emerging consensus on the functional significance of hippocampal hyperactivity is that this condition contributes to, rather than compensates for, impaired hippocampal processing. Treatments to reduce hyperactivity not only improve performance on memory tasks in aged rats (Koh et al., 2010; 2013), but also have efficacy in transgenic AD mouse models (Sanchez et al., 2012; Shi et al., 2013) and in human clinical studies of patients with aMCI (Bakker et al., 2012; 2015). Specifically, low dose administration of the atypical antiepileptic, levetiracetam (LEV) improves performance of aged rats with memory impairment in hippocampal-dependent tasks (Koh et al., 2010) and reduces firing rates of overactive hippocampal neurons (Robitsek et al., 2015). Therapeutic efficacy with this treatment has also been demonstrated on both behavior and hippocampal physiology in AD models of amyloid overexpression (Devi and Ohno, 2013; Hall et al., 2015; Sanchez et al., 2012; Shi et al., 2013; Suberbielle et al., 2013). Clinical studies in patients diagnosed with aMCI similarly show reductions in task-related fMRI hippocampal activation in a low dose range that concomitantly improves performance on a behavioral task that targets specific hippocampal-dependent computational functions critical for episodic memory (Bakker et al., 2012; 2015).
In addition to the effects of aging on hippocampal circuits, cortical networks functionally connected to the hippocampus demonstrate alterations in both aging and in prodromal AD. In particular, the default mode network (DMN) shows impaired task-related deactivation and altered functional connectivity in older adults (Andrews-Hanna et al., 2007; Buckner et al., 2009; Sperling et al., 2009). Posterior regions of the DMN, which form strong reciprocal connections with the medial temporal lobe (MTL) memory system, are also sites of early amyloid deposition. Whether the effects on the DMN in older adults are due to AD pathology as observed by PET imaging (or even occult pathology that is not detected by those methods) rather than aging itself has been a matter of debate (Sperling et al., 2009). Studies of the homologous connectional anatomy of hippocampal/cortical circuits across species could provide information about the condition of such networks in aging, independent of age-related neurodegenerative disease.
Here, we ask if an age-dependent signature of heightened excitability exists in cortical regions beyond the MTL network in a rat model of cognitive aging in the absence of neurodegeneration or pathological markers of AD. Consistent with the heterogeneity of aging outcomes seen within the human population, outbred Long Evans aged rats exhibit a range of abilities in old age that includes both preserved cognitive function (performance within the range of young rats) and impaired performance on hippocampal-dependent memory tasks (Gallagher et al., 1993). Extensive characterization of aged rats with cognitive impairment has demonstrated hippocampal hyperactivity and MTL circuit deficits analogous to elderly humans with impaired memory function (see reviews by Gallagher et al., 2006; Wilson et al., 2006), providing a model for the study of functional alterations independent of specific disease processes that may co-occur in studies of elderly humans.
Using a pharmacological induction protocol that previously demonstrated an age-dependent elevation in the neural activity marker, cFos, in Long Evans rats (Bucci et al., 1998), we examined neural activity-dependent gene expression in cortical regions of aged rats that were cognitively characterized for intact or impaired memory performance compared with young adults. In addition, in a subset of aged impaired subjects, we examined the consequences of a dose of levetiracetam (LEV) previously shown to be cognitively effective (Koh et al., 2010). Consistent with the prior study by Bucci et al. (1998), we found significantly elevated cFos expression in cortical regions, reflecting heightened induction of neuronal activity in aging. As an extension of that study, behavioral characterization demonstrated that the heightened cortical activation was specifically observed in aged rats with impaired memory, while aged rats with preserved cognitive function did not differ from young adults. Notably, LEV treatment reduced cFos expression in memory-impaired aged rats not only in the hippocampus but also in a subset of the cortical regions that align with the posterior components of the DMN. These data suggest that aging with cognitive impairment is associated with heightened excitability that extends beyond the hippocampus, and that therapeutic reduction of overactivity may encompass a larger scale of hippocampal-cortical network.
2. Methods
2.1 Subjects
Aged, male Long-Evans rats were obtained at 8–9 mo of age from Charles River Laboratories (Raleigh, NC) and housed in a vivarium at Johns Hopkins University until 24–26 mo of age. Young rats obtained from the same source were tested at 6 mo of age. All rats were individually housed at 25°C and maintained on a 12 hr light/dark cycle. Food and water were provided ad libitum. The rats were examined for health and pathogen-free status throughout the study, as well as necropsies at the time of sacrifice. All procedures were approved by the Johns Hopkins University Institutional Animal Care and Use Committee in accordance with the National Institutes of Health directive.
2.2 Behavioral characterization
Young and aged rats were tested for hippocampal-dependent memory performance prior to any pharmacological protocol. That behavioral assessment used a well-established Morris water maze protocol as described in detail elsewhere (Gallagher et al., 1993). Briefly, rats were trained for eight days (three trials per day) to locate a camouflaged escape platform that remained at the same location throughout training in a water maze. Every sixth trial consisted of a probe trial (no escape platform for the first 30s of the trial) that served to assess the development of a spatially localized search. A learning index was generated from the proximity of the rat to the escape platform during probe trials and was used to define impairment in the aged rats. For the learning index, the proximity (distance) between the rat and the platform location was tracked 10 times per second during probe trials. The mean proximity of each probe trial was then multiplied by empirically derived weights so as to favor rapid acquisition (see Gallagher et al., 1993 for details). Low scores reflect a more accurate search indicating better retention of the platform location. A learning index cutoff was used to segregate aged rats into unimpaired (AU, learning index < 240) and impaired (AI, learning index > 240) such that aged unimpaired rats fell within the range of young (Y) normative data collected over many years. Cue training (visible escape platform; six 30s trials) occurred on the last day of training to test for sensorimotor and motivational factors independent of spatial learning. Only rats with successful cue training performance (average scores less than 20s) were included in the present study. After behavioral characterization and cue training, a total of 23 rats, including Y (n = 5), AU (n = 6), and AI (n = 12) subjects, were selected for the study.
2.3 Drug Treatment
2.3.1 Levetiracetam treatment
After behavioral characterization and prior to pharmacological induction of cFos expression, a subset of the AI rats was implanted subcutaneously with osmotic minipumps (ALZET Model 2ML4, Durect, Cupertino, CA) containing either LEV (10 mg/kg/day, n = 5) or vehicle saline (n = 2) for 28 days prior to administration of pilocarpine. AI rats with saline pumps were combined with untreated AI rats into a single group labeled “AI” for all comparisons. The learning index distribution from the behavioral characterization, which occurred prior to LEV treatment, was not different between rats in the AI and AI-LEV groups, t(10) = 1.207, p = 0.255.
2.3.2 Pharmacological induction of neural activity
As in Bucci et al. (1998), a low, sub-convulsive dose of pilocarpine was administered systemically to induce widespread neural activity across the brain, including the hippocampus and cortical structures under study. Pilocarpine, a muscarinic acetylcholine receptor agonist, modulates both inhibitory and excitatory synaptic transmission with a net excitatory effect on brain activity that reflects the condition of the activated networks (Curia et al., 2008; Priel and Albuquerque, 2002). At the time of pharmacological treatment with pilocarpine, all rats were injected with a low, sub-convulsive dose of pilocarpine (25 mg/kg in a volume of 1 ml/kg in vehicle saline) intraperitoneally and left undisturbed in their home cage. Cholinergic peripheral symptoms (tremor and lacrimation) with no overt seizures were assessed by a trained observer; during that time no differences in those symptoms were detected between young and aged rats consistent with previous observations (Bucci et al., 1998). One hour after pilocarpine injection, the rats were anesthetized with isoflourane and perfused transcardially with ice cold 0.1 M phosphate buffered saline, followed by 4% paraformaldehyde in 0.1 M phosphate buffer. Brains were harvested and post-fixed in paraformaldehyde overnight and then in 16% sucrose with 4% paraformaldehyde for another 24 hours.
2.4 cFos in situ hybridization
Induction of neural activity was assessed by cFos mRNA expression measured by quantitative in situ hybridization. Brains were cut coronally at 40 μm using a freezing microtome and processed for mRNA of the immediate-early gene cFos used here as a marker for neuronal activity. Probe generation and in situ hybridization were performed as in Haberman et al. (2011). Briefly, brain sections matched for number and location were hybridized overnight at 60°C in buffer containing a 35S-UTP labeled riboprobe generated using the Maxiscript kit (Ambion) from a 373 bp fragment (nucleotides 670 to 1043 from Genbank sequence NM_022197.2) cloned into pGem7zf+ plasmid. This region of the gene has little to no homology to any other rat gene based on a BLAST search of fragment sequence. Hybridized sections were then extensively washed and mounted onto slides. Mounted, dried sections were then exposed in a phosphorimager cassette. Brain regions of interest were outlined by hand and matched for level along the anterior-posterior axis and quantified, blind to experimental conditions, using ImageQuant (GE Healthcare, PA). Radioactive standards exposed at the same time as the brain sections ensured that section intensity was within the linear range. The intensity level of cFos mRNA labeling of sections for each area of interest was averaged to obtain a single score for each rat. The areas analyzed included orbitofrontal cortex (extending from bregma +2.2 mm to +4.7 mm), frontal cortex (+1.0 mm to +2.7 mm), anterior cingulate cortex (+0.7 mm to +2.7 mm), retrosplenial cortex (−2.3 mm to −3.8 mm), parietal cortex (−2.3 mm to −4.8 mm), CA1 of the hippocampus (−2.3 mm to −5.8 mm), CA3 (−2.8 mm to −4.16 mm) and thalamus (−2.3 mm to −3.8 mm).
2.5 Statistical Analysis
The experiment, including the in situ hybridization, was performed in two batches. Each batch contained Y, AU and AI subjects with AI-LEV rats included only in the second batch. Thus, the data for each region was normalized by z-transformation to combine data across the two batches. The data was back transformed and normalized to Y to express relative differences in gene expression. A one-way ANOVA was used to determine overall group differences in each brain region followed by t-tests for planned comparisons.
3. Results
3.1 Cognitive assessment
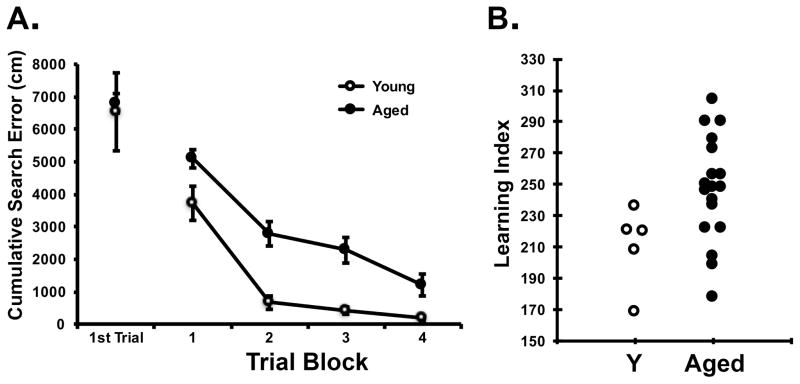
Rats were tested for memory performance using a hidden platform water maze protocol developed in this study population and optimized for sensitivity to detect individual differences in cognitive aging apart from confounds due to physical disability or pathological conditions (Gallagher et al., 1993). Figure 1 shows task performance during training and the learning index distribution of young control (n = 5) and all aged rats (n = 18) used in this study. Prominent age differences are routinely observed during training (Fig 1A). Higher learning index scores signify worse performance by reflecting search at a greater distance from the escape location during memory probe tests (Fig 1B). Learning index scores are the optimal measure of task performance as they integrate spatial search over the course of training, have high test-retest reliability and reveal many measures in the medial temporal lobe system that differentiate cognitive performance. As found repeatedly in this study population (e.g., Gallagher et al., 1993, Rapp and Gallagher, 1996; Haberman et al., 2011) and in the subset of animals used here, aged rats showed a larger range of individual differences in memory performance than young control rats such that some older rats (aged unimpaired, AU) performed on a par with the normative performance of the younger adults (Y) while others (aged impaired, AI) performed outside that range. An overall one-way ANOVA of learning index demonstrated significant differences across age, F(2, 20) = 17.44, p = 0.001. T-tests between groups showed significantly higher learning indexes in AI rats relative to Y, t(15) = 4.535, p = 0.001, and between AI and AU rats, t(16) = 5.161, p = 0.001. The background behavioral characterization of the subjects used in the current experiment is consistent with a long history of data using this rat model with clear behavioral differences between AU and AI subjects that have shown a high degree of test-retest reliability in other studies (Gallagher and Burwell, 1989; Robitsek et al., 2008).
Figure 1. Performance in background characterization.
A. Cumulative search error during training blocks of 5 trials each. This measure reflects the distance of the rat from the escape platform throughout its search, with higher numbers indicating worse performance. Aged rats (black circles) were significantly impaired relative to young (open circles) across training blocks, but not on the first trial which indicates initial, naïve performance. A repeated measures ANOVA confirmed all rats improved with training, [F(3, 63) = 45.214, p = 0.001], and showed a group difference [F(1, 21) = 8.716, p = 0.008] but no interaction. B. Learning index distribution of aged and young rats. Aged unimpaired rats, defined as having a learning index <240, scored within the range of young. Aged impaired rats have learning indexes above 240.
3.2 Pharmacologically induced cFos expression is increased in memory-impaired aged rats
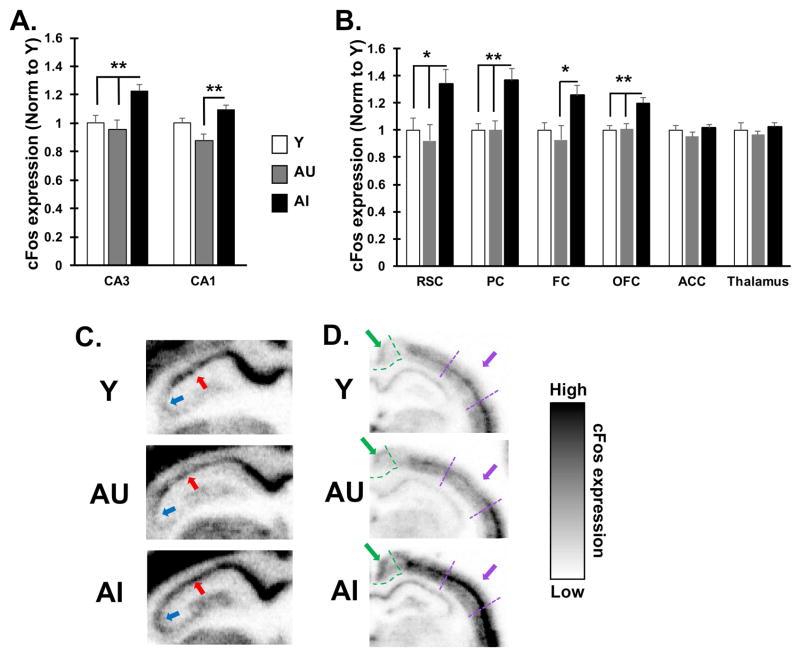
A sub-convulsive dose of pilocarpine was administered to generate widespread neuronal activity without inducing a seizure. As previously described (Bucci et al., 1998), relative to control saline injections, this treatment increases cFos mRNA and numbers of cFos immunoreactive neurons in both young and aged rats, with regionally selective greater increases in drug-treated aged rats compared to young adults. In the current study, behaviorally characterized rats were perfused 1 hour after drug administration, and brains were processed for cFos mRNA, as a measure of neuronal activity, by in situ hybridization. This methodology provides quantitative detection coupled with regional and subregional anatomical analysis. As in Bucci et al. (1998), we examined several cortical regions that functionally interconnect with the hippocampus either directly or indirectly and play important roles in cognitive functions that are impacted by aging. Alongside these cortical regions, the CA3 and CA1 subfields of the hippocampus and the thalamus were examined. Figure 2 shows the relative level of cFos mRNA expression examined in Y, AU, and AI rats.
Figure 2. Pharmacologically induced cFos mRNA is elevated in aged rats with memory impairment.
A–B. Quantification of induced cFos mRNA by in situ hybridization in brain sections of young, AU and AI rats. A. CA3 subfield of the hippocampus shows higher expression in AI rats compared to both AU and Y whereas CA1 subfield expression differs only between aged subgroups (AU and AI). B. Select cortical regions interconnected with the hippocampus also show higher cFos expression in AI rats except in ACC. cFos expression in the thalamus also shows no difference between subject groups. Significant difference across groups was determined by one-way ANOVA. Post-hoc significance was determined by t-test as indicted on the graphs: *p<0.05; **p<0.01. Values represent group means ± SEM. Abbreviations: RSC: retrosplenial cortex; PC: parietal cortex; FC: frontal cortex; OFC: orbitofrontal cortex; ACC: anterior cingulate cortex. C–E. Representative grey scale images of the hippocampus (C) and posterior cortical areas (D) from Y, AU and AI rats after cFos in situ hybridization as detected by phosphorimager. Red arrow points to the CA1 pyramidal cell layer; Blue arrow points to CA3 pyramidal layer. Green outline and arrow define retrosplenial cortex and parietal cortex is delineated by purple lines and arrow.
Because the intrinsic electrophysiological and firing properties of the hippocampus have been subjected to substantial characterization in this study population, we initially examined cFos expression in the CA3 and CA1 subfields (Figure 2A and C). Hippocampal hyperactivity, characterized by higher neuronal firing rates and associated with age-related cognitive impairment, has been localized specifically to the CA3 subfield (Wilson et al., 2003; 2005), which is consistent with the CA3/dentate gyrus localization of greater fMRI activation in human neuroimaging (Yassa et al., 2011). Consistent with those data, cFos induction in CA3 showed an overall significant difference across groups [F(2, 15) = 7.81, p = 0.005], with higher cFos expression in AI rats relative to both Y and AU rats (all p-values < 0.01). No difference was found between Y and AU rats, consistent with data from this model suggesting AU rats are able to maintain excitatory/inhibitory balance (Haberman et al., 2013). A significant overall difference in CA1 [F(2, 15) = 8.44, p = 0.004] was due to lower cFos induction in AU rats relative to the AI subgroup (p = 0.003) but neither aged subgroup differed significantly from Y. This result is consistent with data from electrophysiological recording of neural activity indicating that hyperactivity in the model is localized to CA3 but not CA1 (Wilson et al., 2005). Thus pharmacological induction of cFos expression appears to capture differences in aging phenotypes previously identified in the hippocampus by recording the firing rates and encoding properties of neurons.
Induced cFos expression also differed across groups in several cortical regions (Figure 2B and D) including the retrosplenial cortex [F(2, 15) = 4.54, p = 0.029], posterior parietal cortex [F(2, 15) = 8.612, p = 0.003], the frontal cortex [F(2, 15) = 3.81, p = 0.046] and orbitofrontal cortex, [F(2,15) = 7.81, p = 0.005]. Across these regions, cFos showed a pattern of expression similar to that of CA3 with expression higher in AI rats relative to Y and AU rats. Comparisons between groups revealed significant cFos differences between AI and AU in all 4 cortical regions (all p-values < 0.05). Significant group differences between AI and Y were also found in retrosplenial, parietal and orbitofrontal cortices (all p-values < 0.05), with a statistical trend for a difference in frontal cortex (p = 0.064). In contrast, no overall difference among the groups was observed in either the anterior cingulate cortex [F(2, 15) = 1.183, p = 0.334] or thalamus [F(2, 15) = 0.676, p = 0.523]. Overall these data demonstrate that aged rats with cognitive impairment exhibit regionally selective heightened excitability in response to pharmacological stimulation of neural activity and that age-dependent changes in excitability in rats with cognitive impairment are not limited to the hippocampal system, but also extend to the cortex.
3.3 cFos expression correlates with cognitive performance
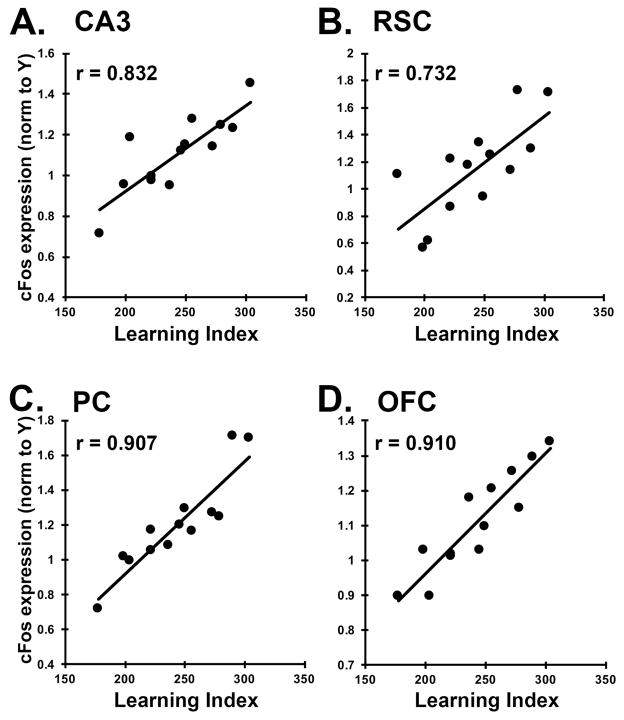
To further examine the relationship between cFos induction and learning, we examined gene expression across the full spectrum of individual differences in the aged rats, in several regions that showed group differences. For each brain region, we plotted the cFos mRNA intensity for each rat against the animal’s learning index (Figure 3). As expected, cFos expression in CA3 correlated significantly with learning index (r = 0.832, p < 0.001). cFos expression was also significantly correlated with learning in the retrosplenial cortex (r = 0.732, p = 0.004), the parietal cortex (r = 0.907, p < 0.001), and the orbitofrontal cortex (r = 0.910, p < 0.001). In all cases, the correlations were in the positive direction, indicating increased cFos induction in rats with greater memory impairment. Correlations were particularly strong in the parietal cortex and orbitofrontal cortex (Fig 3C, D). The correlations between cFos and learning suggest a connection between activity levels in a number of cortical regions and medial temporal lobe dependent behavioral task performance.
Figure 3. cFos expression correlates with behavioral performance in aged rats.
A–D. Average cFos expression intensity for each aged rat (AU and AI), derived from in situ hybridization, was plotted against learning index for each brain region. Pearson r values are indicated in the corner of each graph and all correlations are significant at p < 0.05. Positive correlations indicate higher expression is associated with poorer task performance. A. CA3, B. RSC, retrosplenial cortex, C. PC, parietal cortex, and D. OFC, orbitofrontal cortex.
3.4 Treatment with levetiracetam modulates cFos induction in impaired aged rats
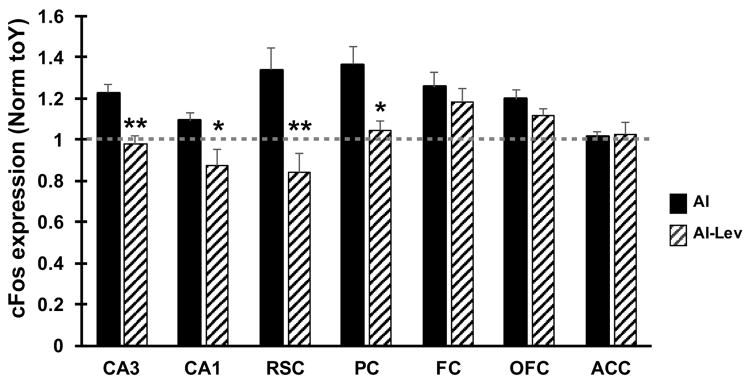
Alongside the subject groups discussed in sections 3.2–3.3, a set of AI rats was treated with LEV (10mg/kg/day) for 28 days via subcutaneous osmotic pump prior to pharmacological induction of cFos. Pilocarpine injections, perfusions, and in situ hybridization of LEV treated AI rats (AI-LEV) were performed concurrently with Y, AU and AI groups as described in the methods (section 2.3). cFos expression levels for AI-LEV rats, relative to AI, are shown in Figure 4. For the CA3, retrosplenial cortex and parietal cortex, induced cFos expression in AI-LEV rats was significantly lower than in AI rats (all p-values < 0.02) resulting in similar expression levels between AI-LEV subjects and Y and AU rats. Induced cFos mRNA in CA1 was also reduced in AI-LEV relative to AI, such AI-LEV cFos levels resembled those of AU. Thus, in these brain regions, LEV treatment normalized pharmacologically induced cFos levels in AI rats. However, in the frontal cortex and orbitofrontal cortex, LEV treatment did not significantly alter cFos induction in these cohorts of subjects, showing no difference between AI-LEV and AI groups. AI-LEV rats showed significantly higher cFos expression in orbitofrontal cortex (p = 0.037) and marginally higher expression in frontal cortex (p=0.061) relative to Y control rats, suggesting that LEV treatment does not significantly alter cFos induction in these regions. Together, these findings suggest that LEV treatment may exhibit regional specificity in its ability to alter heightened excitability in AI rats.
Figure 4. Treatment with levetiracetam modulates cFos induction in impaired aged rats.
Quantification of cFos mRNA of LEV treated AI rats (AI-LEV) is shown alongside values from AI rats (same AI data as shown in Figure 2A and B). The dotted line indicates the average, normalized value for the Y group. LEV treatment significantly reduced cFos induction in hippocampal subfields and posterior cortical structures, but not in frontal cortical areas. *p<0.05 and **p<0.01 compared to AI group using Students t-test. Values represent group means ± SEM. Abbreviations: RSC: retrosplenial cortex; PC: parietal cortex; FC: frontal cortex; OFC: orbitofrontal cortex; ACC: anterior cingulate cortex.
4. Discussion
cFos expression has been validated as a marker of neural activity that can be upregulated in response to behavioral and pharmacological stimuli (Joo et al., 2016). The current findings demonstrate that pharmacological induction of cFos expression is elevated in aged rats across a number of cortical regions, with the increase occurring selectively in aged rats exhibiting an impaired memory phenotype. This outcome is consistent with an earlier report (Bucci et al., 1998) which used the same methodology, and found pilocarpine-induced increased cFos mRNA expression together with increased cFos immunoreactivity in aged 24 months Long-Evans rats as compared to young, 6 month old, adults. Based on the current findings, increased cFos expression is not merely associated with chronological age but occurs in the memory-impaired phenotype in this well-characterized model of neurocognitive aging. Notably, the evidence for increased cFos expression in AI relative to both young and AU rats in the CA3 region of the hippocampus is consistent with the elevation of neural firing rates previously demonstrated by electrophysiological recording methods (Wilson et al., 2003; 2005).
In vivo recordings from ensembles of pyramidal neurons have documented elevated firing rates in memory-impaired aged rats in the CA3 region during exploration of novel and familiar environments (Wilson et al., 2005). In the analysis of spatial encoding, neurons with higher firing rates in aged rats with memory impairment were less able to encode distinctive representations of similar but not identical environments, representing a deficient pattern separation function that is normally critical for episodic memory. Consistent with that evidence, CA3 cFos expression in the current study was significantly higher in AI rats relative to both young and AU. In addition, the tightly coupled association between neuronal activity (encoding) and cognitive status observed in prior electrophysiological recording studies (Wilson et al., 2003; 2005), was also evident in the current study by the strong correlation between increased cFos levels and severity of memory impairment among the behaviorally characterized aged rats. Similar to evidence in rodents and humans (Bakker et al., 2012; 2015; Koh et al., 2010), low dose LEV is efficacious in targeting the hippocampal overactivity localized in the CA3/DG region detected by cFos activation. Those findings support the interpretation that higher cFos expression in AI rats serves as an indicator of heightened neural activity associated with cognitive impairment.
Differences in neural activation determined by cFos expression also extended to a number of cortical regions as a function of the aging cognitive phenotype. Notably, the regions that exhibited such differences are homologous to cortical components of a distributed DMN in the human brain. Recent studies have confirmed the existence of a DMN in rats (Hsu et al., 2016; Lu et al., 2012; for review, see Gozzi and Schwarz, 2016). Specifically, increased cFos expression in AI rats was observed in retrosplenial cortex, posterior parietal cortex, and orbitofrontal cortex, three structures that constitute key components of the rat DMN. In human neuroimaging studies, the DMN is relatively active during rest or when an individual is not engaged in a cognitive task. With task engagement, DMN functional activation and connectivity is normally reduced in favor of task specific networks. Analogous task-dependent network changes have been identified in the DMN of rats (Li et al., 2015), demonstrating potential similarities in the regulatory function of the DMN across species. Importantly in the context of the rodent model used in the current investigation, clinical studies of aging and age-related cognitive decline have reported impaired deactivation of DMN during task engagement (e.g., Hansen et al., 2014; Miller et al., 2008a; Pihlajamäki and Sperling, 2009). While similar studies in aged rodents have not yet been published, our data suggest that heightened age-related neural activation exists within critical components of the DMN and might represent a condition in AI rats contributing to impaired regulation of the DMN.
Systemic pilocarpine, which targets muscarinic cholinergic receptors (mAchRs), induces widespread neural activation; at doses much higher than used in the current investigation, pilocarpine induces seizures. In large-scale networks that differ in the comparison conditions, cFos, as a readout of activation, could reflect direct or indirect pharmacological effects on mAChRs. It is also important to note that neural activation by pilocarpine can occur via mAchRs on both excitatory and inhibitory neurons (Drever et al., 2011), such that a shift in the balance of signaling via mAchRs on inhibitory interneurons, as reported recently in an AD model (Schmid et al., 2016), could heighten excitatory activation of principal neurons within a network. A loss of interneuron integrity as seen in aging could contribute to activation differences in the current study (Speigel et al. 2013, Thome et al., 2016). Although not examined in the current work, Bucci et al (1998) included a control, saline-injected condition that represents baseline gene expression. No differences in cFos expression were observed in that study between age groups suggesting the hyperactivity signature is a phenomenon dependent upon an induction paradigm. Nonetheless, subtle differences between the cognitive aging groups in baseline cFos expression, not detected with the age comparison, could then be subsequently augmented by pilocarpine. Such an observation would not alter the overall implications of this study that altered excitability associated with impairment occurs, not just in the hippocampus, but also across a connected cortical network.
The elevated cFos in aged memory-impaired subjects relative to both young and aged unimpaired confirms much other behavioral and neurobiological evidence for individual differences in neurocognitive aging in this outbred rodent model. With respect to the localization of cortical cFos expression observed specifically in rats with age-related memory impairment, individual differences have also been observed in the effects of aging in humans on task-induced deactivation in subgroups of individuals characterized by their subclinical cognitive decline at midlife (Hansen et al., 2014). In a Danish birth cohort assessed in youth and late midlife, Hansen et al. (2014) report that reduced task-induced deactivation in posterior cortex (i.e., retrosplenial and parietal) was observed in the subgroup of individuals that exhibited decline relative to the subgroup with maintained cognitive abilities. Although the signature of excitability reported here included those regions of the DMN where amyloid pathology by PET imaging first accumulates, alterations in the rodent aging model would suggest that this network is susceptible to effects of aging on the brain apart from even the occult pathology of AD.
By cognitively characterizing our aged subjects prior to pilocarpine, we found that pilocarpine induced cFos expression in AU rats differs very little from young rats. These data support extensive research in this model demonstrating that AU animals maintain critical mechanisms in the hippocampus and elsewhere that support inhibitory processes and synaptic plasticity in MTL memory system (Spiegel et al., 2013), maintaining the integrity of critical circuit functions to support intact cognition. Evidence from both gene expression profiling and electrophysiological studies has further indicated that active maintenance or compensatory mechanisms occur in the AU phenotype (Haberman et al., 2013; Yang et al., 2013). While this prior work has largely focused on the hippocampus, the current data suggest such mechanisms of active maintenance may also extend to the cortex.
An important finding in the current investigation was that treatment of AI rats with LEV reduced cFos elevation not only in the hippocampus but also extended to some cortical regions. One observation in the current study is consistent with prior evidence on the effects of low dose treatment with LEV. At low doses, LEV does not produce a generalized suppression of excitability; for example, no lowering of cFos expression was observed in brain areas where AI rats showed no difference from young adults such as the anterior cingulate cortex. Low dose LEV has similarly been observed to selectively normalize the aberrant conditions associated with aging and AD pathology without altering comparable molecular and electrophysiological properties in controls, such as wild type mice in comparison with human amyloid precursor protein mice (Hall et al., 2015; Sanchez et al., 2012). In that context, it is of further interest that the response to LEV appeared to be limited to hippocampus, retrosplenial cortex, and parietal cortex. While the reason for this selectivity is unclear, it is notable that the posterior cortical regions modified by LEV are early regions of AD pathology within the DMN, and are proposed to be initiators in a cascade of network failure that progressively occurs across the disease spectrum (Jones et al., 2016). In a model based on longitudinal data obtained in the Alzheimer’s Disease Neuroimaging Initiative, the failure in the cortical cascade begins in the posterior default mode network in a load shifting process to frontal regions that transiently serve a compensatory function but subsequently succumb to the progression of AD pathology. Here, the cortical signature of age-related memory impairment, in the absence of AD, is localized in the initiating regions identified in the proposed cascade of network failure, and similar to the hippocampus, is responsive to normalization with LEV treatment.
5. Conclusion
Against the backdrop of hippocampal overactivity that is widely documented in aging and exacerbated with progression to prodromal AD, we provide evidence of increased excitability in cortical structures functionally interconnected with the hippocampus in an aging model without disease neuropathology. The increase in neural activity was limited to memory-impaired aged rats and correlations between cFos and task performance, alongside a reduction of neural activity by a cognitively effective dose of LEV, support the association between increased excitability and cognitive impairment. These findings suggest that age-dependent alterations in neural excitability occur within a larger hippocampal-cortical network and may contribute to disruption of network properties.
Highlights.
A pharmacological stimulus induces cFos, a hippocampal hyperactivity signature
Induced cFos is selectively elevated in the hippocampus of aged memory impaired rats
Hyperactivity extends to a network of cortical regions of aged memory impaired rats
An efficacious drug for hippocampal hyperactivity reduces excess cortical activity
Acknowledgments
This work was supported by National Institute on Aging/National Institutes of Health Grant AG009973-22 to MG.
Footnotes
Disclosure statement:
M.G. is the founder of AgeneBio Incorporated, a biotechnology company that is dedicated to discovery and development of therapies, including Levetiracetam, to treat cognitive impairment in aging. She has a financial interest in the company. The authors (R.P.H., M.T.K. and M.G.) are inventors on Johns Hopkins University intellectual property for indication and use of levetiracetam that is licensed to AgeneBio. Otherwise, M.G. has had no consulting relationships with other public or private entities in the past three years and has no other financial holdings that could be perceived as constituting a potential conflict of interest. Neither M.T.K nor R.P.H. have received financial support or compensation from any individual or corporate entity for research or professional services, and have no financial holdings that could be perceived as constituting a potential conflict of interest. All conflicts of interest are managed by Johns Hopkins University.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andrews-Hanna JR, Snyder AZ, Vincent JL, Lustig C, Head D, Raichle ME, Buckner RL. Disruption of large-scale brain systems in advanced aging. Neuron. 2007;56:924–35. doi: 10.1016/j.neuron.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker A, Albert MS, Krauss G, Speck CL, Gallagher M. Response of the medial temporal lobe network in amnestic mild cognitive impairment to therapeutic intervention assessed by fMRI and memory task performance. Neuroimage Clin. 2015;7:688–98. doi: 10.1016/j.nicl.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker A, Krauss GL, Albert MS, Speck CL, Jones LR, Stark CE, Yassa MA, Bassett SS, Shelton AL, Gallagher M. Reduction of hippocampal hyperactivity improves cognition in amnestic mild cognitive impairment. Neuron. 2012;74:467–474. doi: 10.1016/j.neuron.2012.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucci DJ, Rosen DL, Gallagher M. Effects of age on pilocarpine-induced c-fos expression in rat hippocampus and cortex. Neurobiol Aging. 1998;19:227–32. doi: 10.1016/s0197-4580(98)00051-7. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Sepulcre J, Talukdar T, Krienen FM, Liu H, Hedden T, Andrews-Hanna JR, Sperling RA, Johnson KA. Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer’s disease. J Neurosci. 2009;29:1860–73. doi: 10.1523/JNEUROSCI.5062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busche MA, Chen X, Henning HA, Reichwald J, Staufenbield M, Sakmann B, Konnerth A. Critical role of soluble amyloid-β for early hippocampal hyperactivity in a mouse model of Alzheimer’s disease. Proceedings of the National Academy of Sciences USA. 2012;109:8740–8745. doi: 10.1073/pnas.1206171109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busche MA, Konnerth A. Neuronal hyperactivity – A key defect in Alzheimer’s disease? Bioessays. 2015;37:624–632. doi: 10.1002/bies.201500004. [DOI] [PubMed] [Google Scholar]
- Curia G1, Longo D, Biagini G, Jones RS, Avoli M. The pilocarpine model of temporal lobe epilepsy. J Neurosci Methods. 2008;172:143–57. doi: 10.1016/j.jneumeth.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devi L, Ohno M. Effects of levetiracetam, an antiepileptic drug, on memory impairments associated with aging and Alzheimer’s disease in mice. Neurobiol Learn Mem. 2013;102:7–11. doi: 10.1016/j.nlm.2013.02.001. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Salat DH, Greve DN, Chua EF, Rand-Giovannetti E, Rentz DM, Bertram L, Mullin K, Tanzi RE, Blacker D, Albert MS, Sperling RA. Increased hippocampal activation in mild cognitive impairment compared to normal aging and AD. Neurology. 2005;65:404–11. doi: 10.1212/01.wnl.0000171450.97464.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drever BD, Riedel G, Platt B. The cholinergic system and hippocampal plasticity. Behav Brain Res. 2011;221:505–14. doi: 10.1016/j.bbr.2010.11.037. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Burwell RD. Relationship of age-related decline across several behavioral domains. Neurobiol Aging. 1989;10:691–708. doi: 10.1016/0197-4580(89)90006-7. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Burwell R, Burchinal M. Severity of spatial learning impairment in aging: development of a learning index for performance in the Morris water maze. Behav Neurosci. 1993;107:618–26. doi: 10.1037//0735-7044.107.4.618. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Colantuoni C, Eichenbaum H, Haberman RP, Rapp PR, Tanila H, Wilson IA. Individual differences in neurocognitive aging of the medial temporal lobe. Age. 2006;28:221–33. doi: 10.1007/s11357-006-9017-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozzi A, Schwarz AJ. Large-scale functional connectivity networks in the rodent brain. Neuroimage. 2016;127:496–509. doi: 10.1016/j.neuroimage.2015.12.017. [DOI] [PubMed] [Google Scholar]
- Haberman RP, Colantuoni C, Koh MT, Gallagher M. Behaviorally activated mRNA expression profiles produce signatures of learning and enhanced inhibition in aged rats with preserved memory. PLoS One. 2013;8:e83674. doi: 10.1371/journal.pone.0083674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberman RP, Colantuoni C, Stocker AM, Schmidt AC, Pedersen JT, Gallagher M. Prominent hippocampal CA3 gene expression profile in neurocognitive aging. Neurobiol Aging. 2011;32:1678–92. doi: 10.1016/j.neurobiolaging.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall AM, Throesch BT, Buckingham SC, Markwardt SJ, Peng Y, Wang Q, Hoffman DA, Roberson ED. Tau-dependent Kv4.2 depletion and dendritic hyperexcitability in a mouse model of Alzheimer’s disease. J Neurosci. 2015;35:6221–30. doi: 10.1523/JNEUROSCI.2552-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen NL, Lauritzen M, Mortensen EL, Osler M, Avlund K, Fagerlund B, Rostrup E. Subclinical cognitive decline in middle-age is associated with reduced task-induced deactivation of the brain’s default mode network. Hum Brain Mapp. 2014;35:4488–98. doi: 10.1002/hbm.22489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu LM, Liang X, Gu H, Brynildsen JK, Stark JA, Ash JA, Lin CP, Lu H, Rapp PR, Stein EA, Yang Y. Constituents and functional implications of the rat default mode network. Proc Natl Acad Sci USA. 2016;113:E4541–7. doi: 10.1073/pnas.1601485113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huijbers W, Mormino EC, Schultz AP, Wigman S, Ward AM, Larvie M, Amariglio RE, Marshall GA, Rentz DM, Johnson KA, Sperling RA. Amyloid-β deposition in mild cognitive impairment is associated with increased hippocampal activity, atrophy and clinical progression. Brain. 2015;138:1023–35. doi: 10.1093/brain/awv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo JY, Schaukowitch K, Farbiak L, Kilaru G, Kim TK. Stimulus-specific combinatorial functionality of neuronal c-fos enhancers. Nat Neurosci. 2016;19:75–83. doi: 10.1038/nn.4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DT, Knopman DS, Gunter JL, Graff-Radford J, Vemuri P, Boeve BF, Petersen RC, Weiner MW, Jack CR, Jr Alzheimer’s Disease Neuroimaging Initiative. Cascading network failure across the Alzheimer’s disease spectrum. Brain. 2016;139:547–62. doi: 10.1093/brain/awv338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh MT, Haberman RP, Foti S, McCown TJ, Gallagher M. Treatment strategies targeting excess hippocampal activity benefit aged rats with cognitive impairment. Neuropsychopharmacology. 2010;35:1016–25. doi: 10.1038/npp.2009.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh MT, Rosenzweig-Lipson S, Gallagher M. Selective GABA(A) α5 positive allosteric modulators improve cognitive function in aged rats with memory impairment. Neuropharmacology. 2013;64:145–52. doi: 10.1016/j.neuropharm.2012.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Martin S, Tricklebank MD, Schwarz AJ, Gilmour G. Task-induced modulation of intrinsic functional connectivity networks in the behaving rat. J Neurosci. 2015;35:658–65. doi: 10.1523/JNEUROSCI.3488-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Zou Q, Gu H, Raichle ME, Stein EA, Yang Y. Rat brains also have a default mode network. Proc Natl Acad Sci USA. 2012;109:3979–84. doi: 10.1073/pnas.1200506109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SL, Celone K, DePeau K, Diamond E, Dickerson BC, Rentz D, Pihlajamäki M, Sperling RA. Age-related memory impairment associated with loss of parietal deactivation but preserved hippocampal activation. Proc Natl Acad Sci USA. 2008a;105:2181–6. doi: 10.1073/pnas.0706818105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SL, Fenstermacher E, Bates J, Blacker D, Sperling RA, Dickerson BC. Hippocampal activation in adults with mild cognitive impairment predicts subsequent cognitive decline. J Neurol Neurosurg Psychiatry. 2008b;79:630–5. doi: 10.1136/jnnp.2007.124149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pihlajamäki M, Sperling RA. Functional MRI assessment of task-induced deactivation of the default mode network in Alzheimer’s disease and at-risk older individuals. Behav Neurol. 2009;21:77–91. doi: 10.3233/BEN-2009-0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priel MR, Albuquerque EX. Short-term effects of pilocarpine on rat hippocampal neurons in culture. Epilepsia. 2002;43:40–6. doi: 10.1046/j.1528-1157.2002.043s1040.x. [DOI] [PubMed] [Google Scholar]
- Putcha D, Brickhouse M, O’Keefe K, Sullivan C, Rentz D, Marshall G, Dickerson B, Sperling R. Hippocampal hyperactivation associated with cortical thinning in Alzheimer’s disease signature regions in non-demented elderly adults. J Neurosci. 2011;31:17680–17688. doi: 10.1523/JNEUROSCI.4740-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp PR, Gallagher M. Preserved neuron number in the hippocampus of aged rats with spatial learning deficits. Proc Natl Acad Sci U S A. 1996 Sep 3;93(18):9926–30. doi: 10.1073/pnas.93.18.9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robitsek RJ, Fortin NJ, Koh MT, Gallagher M, Eichenbaum H. Cognitive aging: a common decline of episodic recollection and spatial memory in rats. J Neurosci. 2008;28:8945–54. doi: 10.1523/JNEUROSCI.1893-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robitsek J, Ratner MH, Stewart T, Eichenbaum H, Farb DH. Combined administration of levetiracetam and valproic acid attenuates age-related hyperactivity of CA3 place cells, reduces place field area, and increases spatial information content in aged rat hippocampus. Hippocampus. 2015;25:1541–55. doi: 10.1002/hipo.22474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez PE, Zhu L, Verret L, Vossel KA, Orr AG, Cirrito JR, Devidze N, Ho K, Yu GQ, Palop JJ, Mucke L. Levetiracetam suppresses neuronal network dysfunction and reverses synaptic and cognitive deficits in an Alzheimer’s disease model. Proc Natl Acad Sci USA. 2012;109:E2895–2903. doi: 10.1073/pnas.1121081109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid LC, Mittag M, Poll S, Steffen J, Wagner J, Geis HR, Schwarz I, Schmidt B, Schwarz MK, Remy S, Fuhrmann M. Dysfunction of Somatostatin-Positive Interneurons Associated with Memory Deficits in an Alzheimer’s Disease Model. Neuron. 2016;92:1–12. doi: 10.1016/j.neuron.2016.08.034. [DOI] [PubMed] [Google Scholar]
- Shi JQ, Wang BR, Tian YY, Xu J, Gao L, Zhao SL, Jiang T, Xie HG, Zhang YD. Antiepileptics topiramate and levetiracetam alleviate behavioral deficits and reduce neuropathology in APPswe/PS1dE9 transgenic mice. CNS Neurosci Ther. 2013;19:871–81. doi: 10.1111/cns.12144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simkin D, Hattori S, Ybarra N, Musial TF, Buss EW, Richter H, Oh MM, Nicholson DA, Disterhoft JF. Aging-Related Hyperexcitability in CA3 Pyramidal Neurons Is Mediated by Enhanced A-Type K+ Channel Function and Expression. J Neurosci. 2015;35:13206–18. doi: 10.1523/JNEUROSCI.0193-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Laviolette PS, O’Keefe K, O’Brien J, Rentz DM, Pihlajamaki M, Marshall G, Hyman BT, Selkoe DJ, Hedden T, Buckner RL, Becker JA, Johnson KA. Amyloid deposition is associated with impaired default network function in older persons without dementia. Neuron. 2009;63:178–88. doi: 10.1016/j.neuron.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel AM, Koh MT, Vogt NM, Rapp PR, Gallagher M. Hilar interneuron vulnerability distinguishes aged rats with memory impairment. J Comp Neurol. 2013;521:3508–23. doi: 10.1002/cne.23367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suberbielle E, Sanchez PE, Kravitz AV, Wang X, Ho K, Eilertson K, Devidze N, Kreitzer AC, Mucke L. Physiologic brain activity causes DNA double-strand breaks in neurons, with exacerbation by amyloid-β. Nat Neurosci. 2013;16:613–21. doi: 10.1038/nn.3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomé A, Gray DT, Erickson CA, Lipa P, Barnes CA. Memory impairment in aged primates is associated with region-specific network dysfunction. Mol Psychiatry. 2016;21:1257–62. doi: 10.1038/mp.2015.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson IA, Gallagher M, Eichenbaum H, Tanila H. Neurocognitive aging: prior memories hinder new hippocampal encoding. Trends Neurosci. 2006;29:662–70. doi: 10.1016/j.tins.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson IA, Ikonen S, Gallagher M, Eichenbaum H, Tanila H. Age-associated alterations of hippocampal place cells are subregion specific. J Neurosci. 2005;25:6877–86. doi: 10.1523/JNEUROSCI.1744-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson IA, Ikonen S, McMahan RW, Gallagher M, Eichenbaum H, Tanila H. Place cell rigidity correlates with impaired spatial learning in aged rats. Neurobiol Aging. 2003;24:297–305. doi: 10.1016/S0197-4580(02)00080-5. [DOI] [PubMed] [Google Scholar]
- Yang S, Megill A, Ardiles AO, Ransom S, Tran T, Koh MT, Lee HK, Gallagher M, Kirkwood A. Integrity of mGluR-LTD in the associative/commissural inputs to CA3 correlates with successful aging in rats. J Neurosci. 2013;33:12670–8. doi: 10.1523/JNEUROSCI.1086-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Lacy JW, Stark SM, Albert MS, Gallagher M, Stark CE. Pattern separation deficits associated with increased hippocampal CA3 and dentate gyrus activity in nondemented older adults. Hippocampus. 2011;21:968–79. doi: 10.1002/hipo.20808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Stark SM, Bakker A, Albert MS, Gallagher M, Stark CE. High-resolution structural and functional MRI of hippocampal CA3 and dentate gyrus in patients with amnestic Mild Cognitive Impairment. Neuroimage. 2010;51:1242–52. doi: 10.1016/j.neuroimage.2010.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]