Abstract
The hippocampus is a critical site for alterations that are responsible for age-related changes in memory. Here, we present a relatively novel approach of examining the relationship between memory performance and Glutamate-Glutamine levels using short echo time magnetic resonance spectroscopy (MRS). Specifically, we investigated the relationship between Glx (a composite of glutamate and glutamine) levels in the hippocampus, performance on a word recall task and resting state functional connectivity (RSFC). While there was no overall difference in Glx intensity between young and aging adults, we identified a positive correlation between delayed word-list recall and Glx, bilaterally in older adults, but not in young adults. Collapsed across age, we also discovered a negative relationship between Glx intensity and RSFC between the anterior hippocampus and regions in the subcallosal gyrus, replicating a recent finding by Wagner et al., (2016). These findings demonstrate the possibly utility of Glx in identifying age-related changes in the brain and behavior and provide encouragement that MRS can be useful in predicting age-related decline before any physical abnormalities are present.
Keywords: 3T MRI, aging, MRS, memory, imaging, Glx
1. Introduction
One of the key concerns of older adults is the experience of memory loss, both in the normal course of aging and as it is associated with neural degenerative disease. Many of the types of memory that demonstrate age-related decline are mediated by the hippocampus and surrounding medial temporal lobe structures (Morrison and Baxter, 2012; Small 2001; Yassa et al., 2011a). While there are many laboratory tasks that are designed to tax specific aspects of hippocampal function, standardized neuropsychological tests are broadly available and extensively used in both research and clinical settings. The Rey Auditory Verbal Memory Task (RAVLT; Rey, 1941) has been shown to be highly effective at detecting age-related memory decline (Mitrushina et al., 1991; Schoenberg et al., 2006). In this task, memory is assessed based on the acquisition of a list of 15 unrelated words, followed by free-recall, and repeated over the course of 5 presentations. Participants are then given an interference list of 15 new words, followed by free recall of the original list and another free recall 15 minutes later. Age-related decline has been observed on all recall trials (Mitrushina et al.,1991), with performance on delayed recall correlating with functional scales of memory in those with subjective memory complaints (Estevez-Gonzalez et al., 2003). It is currently used by a number of research groups to identify healthy agers who are particularly “successful” (Mapstone et al., 2014), “super” (Rogalski et al., 2013), or “unimpaired” (Stark et al., 2013) in their memory performance relative to other, healthy participants. Likewise, a delayed word-recall performance on a related task is particularly sensitive to early decline associated with Alzheimer’s disease (Albert 1996).
While the aging brain has been associated with declines in hippocampal volume (Raz et al., 2015), there is strong evidence that this neuronal loss is not simply due to fewer neurons (Burke & Barnes, 2006). Instead, there is evidence of synaptic alterations (Nicholson et al., 2004) and decreases in gene expression (Berchtold et al., 2013) in aging. Likewise, there are neuromodulatory changes in the hippocampus, including decreased cholinergic input to the hippocampus (Sugaya et al., 1998) and a reduction in dopamine that correlates with memory performance (Stemmelin et al., 2000). While these findings have been reporting in rodents, we now have the technology to investigate some of these neurotransmitters in humans using magnetic resonance spectroscopy (MRS). Instead of looking at MRS related to disease, the goal of this work is to link it to behavioral performance.
MRS quantifies the concentration of various metabolites (intermediary and byproducts of metabolism) in the brain, with many of these accessible via conventional proton (1H) imaging. Numerous studies have utilized the efficiency of MRS in providing biological information on cellular/metabolic changes in the aging brain or diseased brain (see Wang et al., 2015 for a review). Specifically, our focus is on Glutamate (Glu), the most abundant metabolite in the brain, a dominant excitatory neurotransmitter. Glutamate is released during neuronal excitation and converted to Glutamine (Gln) with the Glutamate-Glutamine cycle requiring high energy demands. Alterations in concentrations of Glu and Gln have been reported in numerous neurological and psychiatric diseases, such as depression and mood disorders, epilepsy, alcohol and drug abuse, schizophrenia, and neurodegenerative disorders (see Ramadan et al., 2013 for a review).
MRS studies have shown reduced Glu levels in a mouse model of Alzheimer’s disease (AD) (Chen et al., 2012b) and in humans with AD (Rupsingh et al., 2011). They have also shown a decrease in combined Glu and Gln (referred to as Glx) in the cingulate cortices and posterior cingulate gyrus of AD patients (Hattori et al. 2002; Antuono et al. 2001). Little is known about any such alterations in healthy aging or in the hippocampus per se, despite the clear potential for age-related changes in hippocampal activity (Miller et al, 2008; Wilson et al., 2006; Yassa et al., 2011b). In one report, Wagner et al. (2016) investigated the relationship between hippocampal Glx, functional connectivity with the hippocampus, and verbal memory performance (on a different task) in healthy young males. They reported that lower Glx levels were associated with higher functional connectivity of the hippocampus to prefrontal cortex and anterior cingulate. Additionally, they found that Glx concentration in the posterior hippocampus predicted verbal memory performance. These relationships emphasize the utility of MRS as a functional measure of neuronal activity, related to both behavior and brain connectivity.
Given the relationship of Glx to aging and AD, and the possible link to memory performance, the purpose of this study was to investigate age-related changes in Glx levels in the hippocampus using short echo time MRS in young and older adults. We hypothesized that Glx levels would correlate with age-related decline on the RAVLT. Following the findings of Wagner et al. (2016), we also investigated the relationship between Glx and resting state functional connectivity (rs-fMRI) in a subset of our sample for which we were fortunate enough to also have rs-fMRI scans. We hypothesized that we would replicate the negative relationship found between the RSFC anterior hippocampus to regions in the prefrontal cortex and Glx levels in the right anterior hippocampus. These findings would significantly extend those of Wagner et al. (2016) by showing the behavioral relevance of Glx in the hippocampus to healthy aging and link them to the highly-used RAVLT.
2. Materials and Methods
2.1 Participant Recruitment
Forty-one participants, consisting of 21 older (ages 59–84) and 20 younger adults (ages 20–38) were recruited for this study from the community surrounding the University of California in Irvine (UCI). Two participants were removed from the analysis: one young adult was excluded because one of their MRS voxels contained only 20% overlap with the hippocampus (motion between scan preparation and acquisition) and one older adult was excluded for RAVLT scores that fell outside the age-thresholded norms. Thus, we analyzed data from 20 older (8 males, 12 females, mean age of 70 ± 6 years) and 19 younger (8 males, 11 females, mean age of 27 ± 5 years) adults. All participants were right-handed, had no history of neurological disease or psychiatric illness, and had normal or corrected-to-normal vision. Additionally, they all performed within the normal range for their age on standardized neuropsychological testing and scored in the normal to mild range on the Geriatric Depression Scale. All participants gave informed consent approved by the UCI Internal Review Board prior to participation, and were compensated for their time.
Verbal memory was evaluated using the Rey Auditory Verbal Learning Test (RAVLT; Rey, 1941). The RAVLT involved learning a list of 15 unrelated words, repeated 5 times, with a recall test after each administration of the list. Following the 5 presentations, a new interferences list of 15 words was administered, along with a recall task. After the interference list, participants were then asked to recall the original list for an immediate recall test and assessed again 15-minutes later with a delayed recall test.
2.2 MRS Data Acquisition
MRS scans were collected using a 3.0T Philips whole body MRI (Best, the Netherlands), using a 32 channel head coil. High resolution T1-weighted and T2-weighted images were acquired parallel to the hippocampus.
T1-weighted whole-brain anatomical images were acquired using a sagittal magnetization-prepared rapid gradient echo scan (MP-RAGE) at a resolution of 0.75 mm (isotropic), repetition time (TR)/echo time (TE) = 11/4.6 ms, field of view (FOV) = 320 × 264, and flip angle=18°. In addition, a T2-weighted whole-brain anatomical (TR/TE=8/4.6 s) scan was used as an anatomical reference to localize the hippocampal voxel placement for each participant. The rectangular voxel of interest was placed along the long axis of the hippocampus on the sagittal T2 image and contained mainly the anterior hippocampus (Figure 1).
Figure 1.
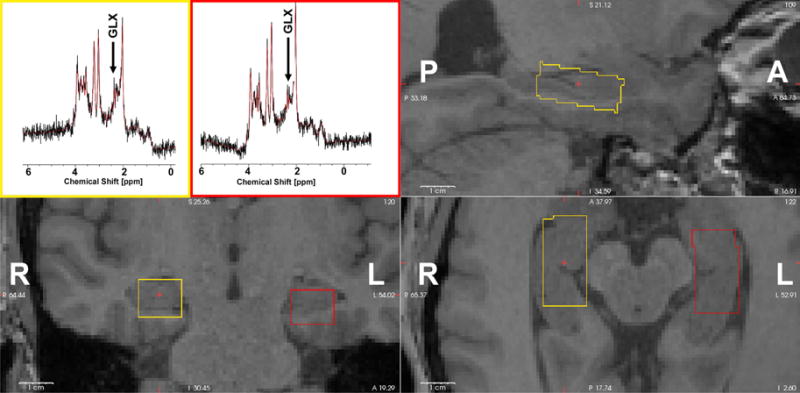
Representative spectra and voxel locations from one participant. Left hippocampus voxel is shown on axial, sagittal and coronal view (yellow). The right hippocampal MRS voxel is shown in red. A-anterior, P-posterior, L-left, R-right.
1H spectroscopy data were acquired using PRESS (TR/TE = 2000/37 ms), from a nominal 2.5×1.5×1.0 cm3 voxel in the left and right hippocampi of all subjects. The acquisition of MRS spectra took about 8 minutes out of a total MR time of 60 min per subject. Voxel dimensions were chosen to maximize the volume while largely contained within the anterior hippocampus of each subject. Hippocampal volumes were calculated using Freesurfer (see MRS Data Analysis section). For the right hippocampus the values ranged between 3.0 cm3 to 3.75 cm3 (mean and SD 3.3 ± 0.6 cm3 in aged subjects and 3.70 ± 0.2 cm3 for young participants). For the left hippocampi the volumes varied between 2.4 cm3 to 4.06 cm3 (mean and SD 3.3 ± 0.6 cm3 in aged subjects and 3.1 ± 0.6 cm3 for young participants). Shimming was accomplished using the iterative VOI method provided by Philips. Only shims below 10 Hz were included in the study. MRS data for five participants were excluded because either of the linewidths (left or right hippocampus) exceeded 10 Hz.
2.3 rs-fMRI Data Acquisition
We were fortunate to have resting-state data from 22 participants (13 young and 9 older adults), collected on a separate day. Echo-planar images were collected on a separate day from the MRS scans using the same scanning equipment. Whole-brain resting state data were acquired with a T2* weighted echo-planar imaging scan, at a resolution of 2.5mm (isotropic), 46 axial slices, TE = 26ms, flip angle = 70°, TR = 2500ms, dynamics = 188. The resting-state scan took 7 minutes 27 seconds, during which time participants were instructed to relax with their eyes open. Using AFNI (http://afni.nimh.nih.gov.afni; Analysis of Functional NeuroImages). The data were aligned to the participant’s MP-RAGE with the script align_epi_anat.py (Saad et al., 2009). Each participant’s structural scan and functional data (statistical maps) were aligned to a model template using ANTS (Advanced Normalization Tools; Avants et al., 2008).
2.4 MRS Data Analysis
Quantitative MRS data was initially analyzed with TARQUIN v.4.3.6 (Wilson et al. 2011), using a non-negative least squares projection to estimate signal amplitudes. Initial point truncation and HSVD water removal was used to reduce baseline interferences (Wilson et al. 2011).
The fractional contributions of gray matter (GM), white matter (WM), and cerebrospinal fluid (CSF) were determined for each voxel by segmenting the T1 weighted images using SPM8 (SPM-Statistical Parametric Mapping 2016; Ashburner and Friston 1997) and MATLAB (the Mathworks, INC. Natick, MA). The fractional tissue contributions were used to correct the estimated metabolite concentrations for partial volume effects (Gussew et al. 2012) using a customized MATLAB code. The partial volume correction required relaxation rates (T1 and T2) of water and metabolites in both WM and GM (Stanisz et al. 2005).
Freesurfer was used to segment the T1-weighted images into hippocampal volumes. The percent of each voxel that overlapped with the hippocampus was calculated and reported in Table 1. The percent of MRS voxel GM consisting of hippocampal tissue was recorded as well. The mean MNI coordinates of each voxel were determined first by warping each anatomical T1 to MNI152 template using Advanced Normalization Tools (ANTs; Avants et al., 2008), and subsequently by applying the warp transform to each voxels 3D binary mask (1mm isotropic resolution). The mean MNI coordinates were calculated from the warped binary mask in MNI space. Hippocampal volumes and ventricular volumes were also calculated on the MP-RAGE for each participant using Freesurfer’s automatic software for volumetric measures (FreeSurfer 2016; Fischl et al. 2002). These volumes were normalized by dividing by total intracranial volume for each participant.
TABLE 1.
Age-group contrasts for RAVLT performance, Glx values, brain volumes with values reported as mean (standard deviation).
| Young | Aging | Independent t-test | |
|---|---|---|---|
| Age [years] | 27 (5) | 70 (6) | |
| Sex [M,F] | 8, 11 | 8, 12 | |
| Behavior | |||
| RAVLT Total | 63 (1.3) | 56 (1.8) | t(37) = 3.14, p<.01 |
| RAVLT Immediate | 13.9 (0.3) | 12.4 (0.5) | t(38) = 2.62, p<.05 |
| RAVLT Delay | 14.1 (0.3) | 12.1 (0.6) | t(38) = 3.01, p<.01 |
| RAVLT Interference | 7.1 (2.0) | 5.6 (2.0) | t(37) = 2.30, p<.05 |
| Metabolites | |||
| Left Glx | 18.4 (4.8) | 16.7 (7.4) | t(37) = 0.67, p=.51 |
| Right Glx | 14.9 (5.6) | 14.0 (4.0) | t(37) = 0.63, p=.53 |
| Volumes | |||
| Left Hippocampus | 0.28% (0.02%) | 0.25% (0.03%) | t(37) = 3.15, p<.01 |
| Right Hippocampus | 0.27% (0.02%) | 0.24% (0.04%) | t(37) = 2.71, p<.05 |
| Ventricular Volume | 1.0% (0.3%) | 2.1% (1.1%) | t(37) = 4.54, p<.01 |
| Left GM fraction | 70% (3%) | 71% (4%) | t(37) = 0.99, p=.32 |
| Right GM fraction | 69% (4%) | 69% (4%) | t(37) = 0.71, p=.48 |
| Left WM fraction | 22% (4%) | 22% (4%) | t(37) = 0.32, p=.75 |
| Right WM fraction | 26% (4%) | 25% (4%) | t(37) = 0.37, p=.71 |
| Left CSF fraction | 8% (2%) | 7% (2%) | t(37) = 1.11, p=.27 |
| Right CSF fraction | 6% (2%) | 5% (2%) | t(37) = 0.61, p=.55 |
| Left Voxel Volume [c.c.] | 3.7 (0.2) | 3.1 (0.6) | t(37) = 3.48, p<.01 |
| Right Voxel Volume [c.c.] | 3.7 (0.2) | 3.2 (0.6) | t(37) = 3.05, p<.01 |
| % of Left Voxel = Hipp | 59% (6%) | 61% (8%) | t(37) = 1.16, p=.25 |
| % of Right Voxel = Hipp | 58% (4%) | 59% (8%) | t(37) = 0.32, p=.75 |
| Left %GM = Hipp | 84% (9%) | 86% (8%) | t(37) = 0.77, p=.44 |
| Right %GM = Hipp | 85% (6%) | 84% (9%) | t(37) = 0.24, p=.81 |
| Avg. MNI Left Voxel [X] | −26 (1) | −27 (1) | |
| Avg. MNI Left Voxel [Y] | −20 (4) | −19 (2) | |
| Avg. MNI Left Voxel [Z] | −18 (2) | −20 (6) | |
| Avg. MNI Right Voxel [X] | 27 (2) | 26 (1) | |
| Avg. MNI Right Voxel [Y] | −19 (3) | −19 (2) | |
| Avg. MNI Right Voxel [Z] | −18 (2) | −19 (2) | |
2.5 rs-fMRI Data Analysis
Data were preprocessed using AFNI and included detrended using 2nd order Legendre polynomials. The time series was then normalized to have zero mean and unit variance and the motion vectors and first derivatives were regressed out of the signal. The first 6 principal components of the variance were computed using CompCor (not including global signal) and regressed out of the signal (Behzadi et al., 2007). The data were then temporally bandpass filtered (0.009 to 0.08 Hz) and TRs with framewise displacement >0.5mm, as well as those one TR before and two TRs after, were removed from analyses by censoring as recommended by Power et al., (2012). Thus, the remaining data for both the young and older adults contained similar, very low levels of motion. Note, all rs-fMRI analyses to identify regions showing reliable connectivity to the hippocampus were done on the entire set of participatns, regardless of age.
We estimated the inherent blur using 3dFWHMx (AFNI) for each participant and used the mean of these values in 3dClustSim1 (AFNI: June 2016) to determine the combined voxel-wise (p<0.005) and cluster-size (nearest-neighbor connectivity of 10 contiguous voxels) thresholds needed to arrive at a multiple-comparison corrected alpha of p < .05.
2.6 RAVLT Data Analysis
Free recall from the 5 acquisition tests in the RAVLT were summed to produce a RAVLT Total Score. In addition, the RAVLT Interference score was calculated (total recalled from the Interference word list administered after these). Finally, we also scored the total recalled from the Immediate Recall and the Delayed Recall, administered 15 minutes later. We used Prism 6 (www.graphpad.com) to calculate unpaired t-tests for between group comparisons and correlations between behavior and Glx values.
3. Results
3.1 Age-related differences in RAVLT performance
One older adult performed more than 2 standard deviations below standardized normative values for his age. As this may represent either exceptionally inattentive performance or potential early clinical impairment, this individual was excluded from further analysis. All other young and older participants performed within 1 standard deviation of the standardized norms for their age.
In the resulting dataset, we examined the effect of aging on the RAVLT using planned independent samples t-tests. As expected, we found that older adults performed worse than young adults on all four measures of the RAVLT: Total Acquisition, Interference, Immediate and Delayed Recall (see Table 1).
3.2 No age-related differences in Glx concentration
Tissue fractions and normalized hippocampal and ventricular volumes for both groups are shown in Table 1. There was no evidence for differences in the proportion of GM, WM, and CSF in our MRS voxels across age groups, reducing potential concerns for artefactual differences in metabolite concentrations resulting from varying partial volume effects across groups. Consistent with a wide range of studies, both left and right hippocampal volume, as well as ventricular volume, were lower in the aging group. Not surprisingly, the average volume of the MRS voxels were also smaller in aged than young participants. However, the average Glx, presented in absolute concentrations (mmol/L), did not differ between the two groups. Thus, while hippocampal atrophy was observed in the aging group, we were still able to obtain a robust measure of Glx.
3.3 Age-related differences in MRS and RA VLT performance
To reduce noise and as we had no a priori hypotheses concerning laterality, we began investigating the relationship between Glx and memory performance by averaging the left and right Glx values for each participant, correlating the result with RAVLT performance. We found significant positive correlations between Glx and RAVLT Total, Immediate and Delayed Recall for the aged group (Figure 2). There was no evidence for such relationship in the young group.
Figure 2.
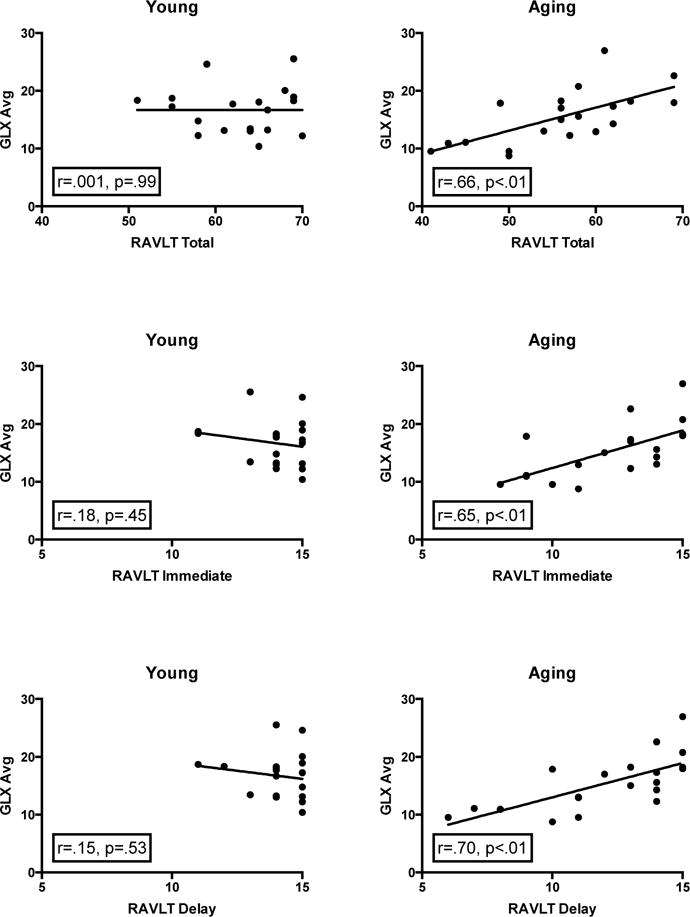
Regression analyses of the average Glx with RAVLT Total Acquisition, Immediate Recall, and Delay Recall memory scores for young and aging participants. The aging group demonstrated a positive relationship between average Glx and each memory measure, yet there is no evidence of this relationship in the young group.
Since MRS was collected from a single, often unilateral voxel, our combination of the two hemispheres’ Glx values in our initial analysis is somewhat atypical. Thus, as a secondary analysis, we analyzed these relationships separately for each hemisphere to determine if the results were consistent and replicable in each. Similar, independent correlations were observed in each hemisphere in the aging group between Glx and RAVLT Total (Left: r = 0.62, p < 0.01; Right: r = 0.39, p = 0.09), Immediate (Left: r = 0.54, p < 0.05; Right: r = 0.52, p < 0.05) and Delayed Recall (Left: r = 0.65, p < 0.01; Right: r = 0.42, p = 0.07). No relationship between Glx and RAVLT performance was observed in the young group (all p’s > 0.5). We note that this lack of a relationship in our anterior hippocampal voxels is consistent with the findings of Wagner et al. (2016). While they observed a relationship between hippocampal Glx and performance on a test that provides a similar measure of memory as the RAVLT Interference score, this relationship was only found for posterior and not anterior hippocampal voxels.
3.4 Relationship between RSFC and Glx
Resting State Functional Connectivity (RSFC) analyses were carried out by correlating the regional signal-time courses extracted from the left and right anterior hippocampus (corresponding to the regions where the MRS scans were collected), to all other voxels in the brain. We applied Fisher z-transformation to the linear correlation maps prior to performing statistical analyses. Due to the reduced number of participants in the young and aging groups who had both MRS and rs-fMRI, we collapsed the young and older adults into a single group.
Using 3dRegAna (AFNI), we tested the relationship between hippocampal RSFC (separately for the left and the right anterior hippocampus) and Glx intensity. There were 14 regions that revealed a relationship between left or right RSFC and Glx concentration presented in Table 2 (multiple-comparison-corrected). Of these, two of the regions (Figure 3) were located in the left subcallosal gyrus, near the orbitofrontal cortex (OFC) location identified by Wagner et al. (2016). In both of these regions, we replicated the Wagner et al. (2016) finding of a negative relationship between Glx concentration and RSFC from the anterior hippocampus to the OFC. Interestingly, while the Wagner group found this relationship in young adult males, we extended these findings in a group of healthy, mixed gender, older and younger adults. We (post hoc) plotted the young and older adults separately in Figure 3 to demonstrate the consistency in both groups but, given our small sample size, we did not contrast them directly. In addition to the two OFC regions that overlapped with those reported by Wagner et al. (2016), we also identified 12 other regions, displaying a mix of positive and negative relationships between Glx intensity and RSFC.
TABLE 2.
Regions that show a relationship between Glx intensity and RSFC with the left and right hippocampus as seed regions. Region names are defined as the focal point in the Tailairach-Tournoux Atlas. Glx refers to the left or right hippocampal seed that showed RSFC to that region. X (right/left), Y (anterior/posterior) and Z (inferior/superior) coordinates given in Tailairach space (TLRC).
| Region Name | Glx | X | Y | Z | Dir. |
|---|---|---|---|---|---|
| Left Subcallosal Gyrus (24) | Left | −13 | 19 | −12 | − |
| Left Subcallosal Gyrus (7) | Right | −20 | 18 | −9 | − |
| Left Superior Frontal Gyrus (11) | Right | −4 | 16 | 55 | − |
| Right Middle Frontal Gyrus (8) | Left | 28 | 24 | 38 | − |
| Left Medial Frontal Gyrus (20) | Right | −4 | 0 | 54 | + |
| Left Postcentral Gyrus (3) | Right | −40 | −11 | 32 | − |
| Right Angular Gyrus (36) | Left | 37 | −58 | 31 | − |
| Left Cuneus (19) | Right | −4 | −77 | 17 | − |
| Left Inferior Parietal Lobe (5) | Right | −55 | −35 | 26 | − |
| Right Lingual Gyrus (6) | Right | 6 | −62 | −3 | − |
| Left Precuneus (5) | Left | −37 | −74 | 36 | − |
| Right Lingual Gyrus (1) | Right | 11 | −86 | −26 | + |
| Right Inferior Occipital (10) | Right | 22 | −89 | 0 | + |
| Right Inferior Occipital Gyrus (15) | Right | 32 | −82 | −3 | + |
| Right Supramarginal Gyrus (8) | Right | 38 | −45 | 24 | + |
| Right Precentral Gyrus (9) | Right | 57 | −14 | 26 | − |
| Right Middle Temporal Gyrus (18) | Right | 50 | −52 | 6 | − |
| Right Cerebellum (29) | Left | 6 | −60 | −27 | + |
| Right Posterior Cingulate (34) | Left | 13 | −54 | 11 | − |
| Left Parahippocampal Gyrus (16) | Right | −27 | −56 | 2 | − |
Figure 3.
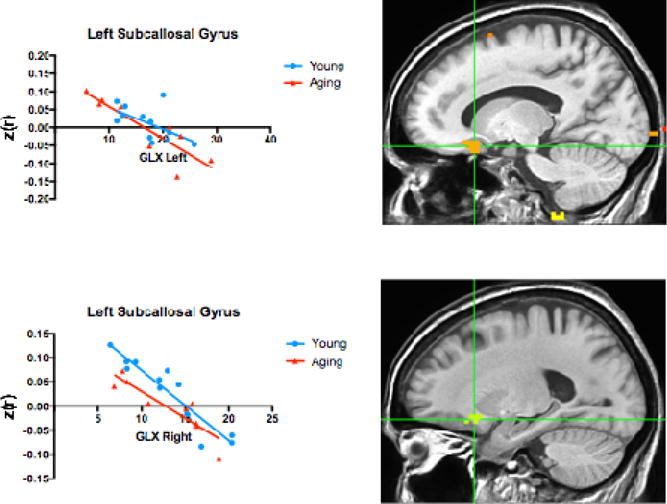
Two regions in the left subcallosal gyrus replicate the negative relationship observed by Wagner et al. (2016) between Glx intensity and RSFC to the right and left anterior hippocampus. The young and aging groups are plotted separately here, but were analyzed as a single group, irrespective of age.
4. Discussion
In this study, we measured Glx (a combined measure of glutamate and glutamine) in the anterior hippocampus of young and older adults. We found that greater Glx concentration in older adults was correlated with higher memory performance in a word recall on the RAVLT, both at encoding and retrieval. Moreso, we showed that this effect was observed in both the left and the right hippocampus, providing an internal replication of this relationship. Interestingly, this relationship was not evident in young subjects.
These findings extend those of Wagner et al. (2016), who reported a positive correlation in young males between hippocampal Glx levels and retroactive interference on a task similar to the RAVLT. Our results are largely consistent and complementary here, as the studies contain both overlapping and independent measures. Briefly, Wagner et al. (2016) observed their Glx — verbal memory correlation in posterior but not anterior Glx measures and only in the Interference memory measure. In addition, they only scanned young males. Our data from the anterior hippocampus failed to show a relationship between Glx and verbal memory consistent with their observations. As we do not have MRS data from the posterior hippocampus, we cannot address whether young individuals show a similar relationship with the Interference score of the RAVLT. While it is by no means clear, there is growing evidence for some form of anterior-posterior distinction in the hippocampus (Strange et al, 2014). For example, activity in the posterior hippocampus has been shown to predict the degree of retroactive interference in a paired-associates task (Kuhl et al., 2010), highlighting the relevance of this region for retroactive interference specifically. We collected data from the anterior hippocampus, which has been associated with novelty detection (Daselaar et al., 2006) and a relationship to the dorsal attention network (Kim, 2015). Thus, the anterior hippocampus may be more sensitive to the recall measures of the RAVLT than to retroactive interference. We caution, however, that such interpretations are entirely speculative and based on reverse inferences at this stage.
We did, however, replicate their basic effect by demonstrating a relationship between hippocampal Glx and RAVLT performance. In our study, we found this relationship in the anterior hippocampus in a heterogeneous group of older adults for several of the verbal learning measures. Note, Wagner et al. (2016) did not test older individuals or women. Thus, we view our results as being consistent with and complementary to those of Wagner et al. (2016) in this regard.
Our observation of a relationship between Glx levels and RAVLT performance in aging adults suggests a possible modulatory effect of glutamate and/or glutamine on memory performance. Higher levels of glutamate in the striatum have been associated with better performance on tests of executive functioning in a group of older adults, provided a potential mechanism for age-related decline in the frontostriatal system (Zahr et al., 2008). N-methyl-D-asparate (NMDA) receptors represent one of the glutamate receptors that are present in high density in the hippocampus and cerebral cortex, even in aging and Alzheimer’s disease (Cotman et al., 1989), but there is mounting evidence that NMDA receptor function declines with age (Barnes et al., 1997). Likewise, an age-related deficiency in NMDA receptor function contributes to impairments in spatial learning and memory in older rodents (Magnusson 1998; Zhao et al., 2009), providing a mechanism for glutamate-related dysfunction in aging that may be contributing to a decline in verbal memory.
As noted earlier, in our current data we are unable to separate the Glu and Gln components of the signal and, like many studies, must combine this into a Glx value. Glutamate and glutamine are complementary, such that glutamate is taken up in the synaptic cleft and then converted to glutamine within the astrocytes. This cycle is rapid and highly dynamic, with Glu located primarily in neurons and Gln located predominately in the glial cells (Ottersen et al., 1992). While we found no age-related decrease in Glx concentration between young and older adults in the hippocampus, other studies have found evidence for an age-related decline in Glu, accompanied by an age-related increase in Gln in the hippocampus (Hadel et al., 2013; Schubert et al., 2004). These opposing effects may have mitigated any age-related difference in overall Glx concentration here, but an underlying age-related imbalance may still account for the relationship with RAVLT performance. Outside of the hippocampus, age-related decreases in glutamate have been reported in the striatum (Zahr et al., 2008; but see Choi et al. (2014) for the opposite pattern), but not the cerebellum (Zahr et al., 2008, 2013), emphasizing the possible regional specificity of these metabolic imbalances. Additionally, the opposing dynamics of the glutamate-glutamine cycle may be completely masked by the combined Glx metric. Future studies using acquisition techniques that can isolate Glu and Gln (e.g., Mega-PRESS sequences) are needed to identify the opposing contributions of these two metabolites.
The relationship between Glu and Gln concentrations in aging is a complicated one. These compounds reflect both neuronal and mitochondrial energy production, with Glu serving as a metabolic precursor of γ-aminobutyric acid (GABA), the main inhibitory neurotransmitter in the cerebral cortex. Mitochondrial dysfunction in normal aging may lead to an increase in Glu (Boumezbeur et al., 2009; Lin & Beal, 2006) and be a major contributor to the underlying cause of AD (Lin et al., 2003). Further, ameliorating this mitochondrial dysfunction has been shown to improve cognitive performance in rodent models of AD (Chen et al., 2012a). Increased in hippocampal Glu levels have also been observed in a number of disorders, including medial temporal lobe epilepsy (Simister et al., 2002, Woermann et al., 1999) and schizophrenia (Kraguljac et al., 2014). Based on these findings, we might have predicted that greater Glu levels would be associated with worse memory performance on the RAVLT in older adults. In contrast, we find that for healthy older adults, greater Glx concentration is associated with greater memory performance. One might speculate that higher Glx values reflect greater regulation of the Glu/Gln cycle, since the combined Glx measure does not allow us to analyze the individual contributions of Glu and Gln to memory performance. Yet, any speculation here must be tempered by the fact that overall Glx concentration did not differ between the older and young adults, therefore the relationship between Glx and memory performance in the older adults may not reflect a global shift in Glu associated with clinical conditions such as schizophrenia, epilepsy, and AD. Notably, in these diseased states, the decrease of Glu is consistently accompanied by a decrease in NAA (Bartha et al., Rupising et al. 2009 and Jessen et al. 2001), signifying neuronal loss or dysfunction. In this study, a decrease in NAA was not observed or expected as healthy aging is not associated with overt neuronal loss in the hippocampus Burke & Barnes, 2006).
In this study, we also replicated another finding reported by Wagner et al. (2016) of a negative relationship between hippocampal Glx in the anterior hippocampus and the RSFC from the anterior hippocampus to regions in the orbitofrontal cortex (OFC). The OFC is one of the regions of the default mode network (DMN), a network of highly interconnected brain structures that show synchronous low-frequency fluctuations during a resting state condition (Raichle et al., 2001). Previous correlations between Glx and nodes of the DMN have been observed, particularly with increased glutamate levels in the anterior cingulate cortex (Enzi et al., 2012) and the posterior cingulate cortex (Duncan et al., 2013; Hu et al., 2013). Thus, the inter-regional synchrony in the DMN may be modulated, at least in part, by the excitation-inhibition balances based on the ratio of Glu and Gln.
In contrast to the findings of Wagner et al. (2016), it is worth noting that we also found both negative and positive relationships between Glx and RSFC between the anterior hippocampus and other regions outside of the DMN, including regions in the parahippocampal gyrus, parietal cortex, and cerebellum. Only a few studies have investigated the relationship between glutamate levels and task-induced BOLD signal or RSFC in humans. Using MRS in the dorsal anterior cingulate cortex, Falkenberg et al. (2012) categorized young healthy adults into high and low glutamate individuals and showed that those with low glutamate levels showed an increased BOLD response during a task that demanded low cognitive control, whereas individuals with high glutamate levels showed the opposite effect. Similarly, a positive relationship between RSFC and glutamate levels in the anterior cingulate cortex have been shown in young adults. These findings emphasize an interaction between glutamate levels and the activation levels of these regions, though they have been limited to very specific regions of interest.
Glutamate, as an excitatory neurotransmitter, may result in high levels of RSFC in regions that are task-activated, while a reduction in this glutamatergic activity, or a counteraction of it by an inhibitory neurotransmitter such as GABA, may lead to task-induced deactivation. These relationships are highly dependent on the task and the regions involved in the task, but reflect the utility of these metabolites as functional measures of neuronal activity. In this study, the rs-fMRI data was collected during “rest”, which is a cognitively uncontrolled condition that results in high BOLD activity in the hippocampus (Stark & Squire, 2001). The complex relationship between how glutamate levels in the hippocampus modulates rest-based RSFC is beyond the scope of this investigation. Instead, we report these relationships to 1) report on a replication with Wagner et al. (2016), 2) demonstrate a relationship between Glx and RSFC across many regions, and 3) reveal the complexity of this relationship across regions. With a larger sample size or multiple MRS voxels, it would be interesting for future studies to investigate any age-related modulation of these relationships.
This study was limited by a small sample size for the rs-fMRI dataset, which prevented us from examining any age-related differences in the relationship between Glx and resting state activity. We were also limited in our ability to contrast Glx and network relationships because we collected MRS from only the anterior hippocampus. In future studies, collecting MRS voxels from the anterior and posterior hippocampus, or from other memory-related regions such as the parahippocampal gyrus, would provide an opportunity to compare the relationship between Glx and functional activity. Finally, since Glx is a composite measure, we are unable to distinguish the individual contributions of glutamate and glutamine to the behavior or the brain activity. The use of a MEGA-PRESS sequence may be able to differentiate these two metabolites in the future. GABAergic inhibition plays a key role in glutamatergic excitation (McCormic et al. 1989), and the balance of is essential to consider with respect to hippocampal function and memory. In MRS, the Glutamate and GABA peaks however overlap, making it difficult to separate those metabolites without the use of J-editing spectral techniques. We were unable to investigate the effects of GABA here since special sequences are needed to resolve this peak but would like to address the role of GABA in future work.
5. Conclusions
We report that decreases in Glx in the anterior hippocampus were accompanied by decreased performance in verbal memory in aged adults. These preliminary findings suggest a potential age-related modulatory role of glutamate and/or glutamine in the hippocampal regulation of memory. Future studies are needed to focus on how a dysregulation of the excitation-inhibition balance in the hippocampus may contribute to age-related memory decline.
Highlights.
Correlation between Glx and verbal memory performance in older adults, but not younger adults
Glx in the anterior hippocampus is related to resting state fMRI in several regions
Glx may provide an age-related modulatory role in the hippocampal regulation of memory
Acknowledgments
The authors would like to thank Samantha Rutledge for her assistance with data collection and Derek Huffman and Veronique Boucquey for assistance with preprocessing the resting state functional connectivity data. Funding for this project was provided in part by a grant from the National Institutes on Aging R01-AG034613 and R21-AG053040.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
3dClustSim was updated in May 2015 to address an error in underestimating multiple corrections, resulting in an overestimation of significance for a given cluster (Eklund et al., 2016). Here, we have used the updated version of 3dClustSim, which corrected this error.
References
- Albert MS. Cognitive and neurobiologic markers of early Alzheimer’s disease. Proc Natl Acad Sci USA. 1996;93:13547–51. doi: 10.1073/pnas.93.24.13547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antuono PG, Jones JL, Wang Y, Li SJ. Decreased glutamate + glutamine in Alzheimer’s Disease detected in vivo with (1)H-MRS at 0.5 T. Neurology. 2001;56:737–42. doi: 10.1212/wnl.56.6.737. 2001. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston K. Multimodal image coregistration and partitioning: a unified framework. NeuroImage. 1997;6(3):209–17. doi: 10.1006/nimg.1997.0290. [DOI] [PubMed] [Google Scholar]
- Avants BB, Epstein CL, Grossman M, Gee JC. Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Medical Image Analysis. 2008;12:26–41. doi: 10.1016/j.media.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes CA, Rao G, Shen J. Age-related decrease in the N-methyl-D-aspartateR-mediate excitatory postsynaptic potential in the hippocampal region CA1. Neurobiology of Aging. 1997;18:445–52. doi: 10.1016/s0197-4580(97)00044-4. [DOI] [PubMed] [Google Scholar]
- Barth C, Villringer A, Sacher J. Sex hormones affect neurotransmitters and shape the adult female brain during hormonal transition periods. Frontiers in Neuroscience. 2015;9:37. doi: 10.3389/fnins.2015.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boumezbeur F, Mason GF, de Graaf RA, Behar KL, Cline GW, Shulman GI, Rothman DL, Peterson KF. Altered brain mitochondrial metabolism in healthy aging as assessed by in vivo magnetic resonance spectroscopy. Journ of Cere Blood Flow and Metab. 2009;30:211–21. doi: 10.1038/jcbfm.2009.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behzadi Y, Restom K, Liau J, Liu TT. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage. 2007;37:90–101. doi: 10.1016/j.neuroimage.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke SN, Barnes CA. Neural plasticity in the ageing brain. Nat Rev Neurosci. 2006;7:30–40. doi: 10.1038/nrn1809. [DOI] [PubMed] [Google Scholar]
- Berchtold NC, Coleman PD, Cribbs DH, Rogers J, Gillen DL, Cotman CW. Synaptic genes are extensively downregulated across multiple brain regions in normal human aging and Alzheimer’s disease. Neurobiol Aging. 2013;34:1653–61. doi: 10.1016/j.neurobiolaging.2012.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Yoo SE, Na R, Liu Y, Ran Q. Cognitive impairment and increased Aβ levels induced by paraquat exposure are attenuated by enhanced removal of mitochondrial H(2)O(2) Neurobiol Aging. 2012;33(2):432e 15–26. doi: 10.1016/j.neurobiolaging.2011.01.008. [DOI] [PubMed] [Google Scholar]
- Chen SQ, Cai Q, Shen YY, Wang PJ, Teng GJ, Zhang W, Zang FC. Age-related changes in brain metabolites and cognitive function in APP/PS1 transgenic mice. Behav Brain Res. 2012b;235(1):1–6. doi: 10.1016/j.bbr.2012.07.016. [DOI] [PubMed] [Google Scholar]
- Choi IY, Lee P, Wang WT, Hui D, Wang X, Brooks WM, Michaelis EK. Metabolism changes during aging in the hippocampus and striatum of Glud1 (Glutamate Dehydrogenase 1) transgenic mice. Neurochem Res. 2014;39(3):446–55. doi: 10.1007/s11064-014-1239-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotman CW, Geddes JW, Bridges RJ, Monaghan DT. N-methyl-D-aspartate receptios and Alzheimer’s disease. Neurobiology of Aging. 1989;10(5):603–05. doi: 10.1016/0197-4580(89)90144-9. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Fleck MS, Cabeza R. Triple dissociation in the medial temporal lobes: recollection, familiarity, and novelty. Journal of Neurophysiology. 2006;96(4):1902–11. doi: 10.1152/jn.01029.2005. [DOI] [PubMed] [Google Scholar]
- Duncan NW, Wiebking C, Tiret B, Marjanska M, Hayes DJ, Lyttleton O, Doyon J, Northoff G. Glutamate concentrations in the medial prefrontal cortex predicts resting-state cortical-subcortical functional connectivity in humans. PLOS One. 2013;8(4):e60312. doi: 10.1371/journal.pone.0060312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund A, Nichols TE, Knutsson H. Cluster failure: Why fMRI inferences for spatial extent has inflated false-positive rates. Proc Natl Acad Sci USA. 2016;113(28):7900–5. doi: 10.1073/pnas.1602413113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enzi B, Duncan NW, Kaufmann J, Tempelmann C, Wiebking C, Northoff G. Glutamate modulates resting state activity in the perigenual anterior cingulate cortex — a combined fMRI-MRS study. Neuroscience. 2012;227:102–09. doi: 10.1016/j.neuroscience.2012.09.039. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale A. Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341–55. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- FreeSurfer. 2016 Accessed March 3. http://surfer.nmr.mgh.harvard.edu/
- Gussew A, Erdtel M, Hiepe P, Rzanny R, Reichenbach JR. Absolute quantitation of brain metabolites with respect to heterogeneous tissue compositions in (1)H-MR spectroscopic volumes. Magma. 2012;25(5):321–33. doi: 10.1007/s10334-012-0305-z. 2012. [DOI] [PubMed] [Google Scholar]
- Hadel S, Wirth C, Rapp M, Gallinat J, Schubert F. Effects of age and sex on the concentrations of glutamate and glutamine in the human brain. Journal of Magnetic Resonance Imaging. 2013;38:1480–7. doi: 10.1002/jmri.24123. [DOI] [PubMed] [Google Scholar]
- Hattori N, Abe K, Sakoda S, Sawada T. Proton MR spectroscopic study at 3 Tesla on glutamate/glutamine in Alzheimer’s Disease. Neuroreport. 2002;13:183–86. doi: 10.1097/00001756-200201210-00041. [DOI] [PubMed] [Google Scholar]
- Hu Y, Chen X, Gu H, Yang Y. Resting-state glutamate and GABA concentrations predict task-induced deactivation in the default mode network. Journal of Neuroscience. 33(47):18566–73. doi: 10.1523/JNEUROSCI.1973-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. Encoding and retrieval along the long axis of the hippocampus and their relationships with dorsal attention and default mode networks: the HERNET model. Hippocampus. 2015;25(4):500–10. doi: 10.1002/hipo.22387. [DOI] [PubMed] [Google Scholar]
- Kraguljac NV, White DM, Hadley J, Reid MA, Lahti AC. Hippocampal-parietal dysconnectivity and glutamate abnormalities in unmedicated patients with schizophrenia. Hippocampus. 2014;24(12):1524–32. doi: 10.1002/hipo.22332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl bA, Shah AT, DuBrow S, Wagner AD. Resistance to forgetting associated with hippocampus-mediated reactivation during new learning. Nature Neuroscience. 2010;13(4):501–06. doi: 10.1038/nn.2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–95. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- Lin AP, Shic F, Enriquez C, Ross BD. Reduced glutamate transmission in patients with Alzheimer’s disease — in vivo 13C magnetic resonance spectroscopy study. Mag Res Mater in Phys, Bio and Med. 2003;16:29–42. doi: 10.1007/s10334-003-0004-x. [DOI] [PubMed] [Google Scholar]
- Magnusson KR. Aging of glutamate receptors: correlations between binding and spatial memory performance in mice. Mechanisms of Ageing and Development. 1998;104(3):227–48. doi: 10.1016/s0047-6374(98)00076-1. [DOI] [PubMed] [Google Scholar]
- Mapstone M, Cheema AK, Fiandaca MS, Zhong X, Mhyre TR, MacArthur LH, Hall WJ, Fisher SG, Peterson DR, Haley JM, Nazar MD, Rich SA, Berlau DJ, Peltz CB, Tan MT, Kawas CH, Federhoff HJ. Plasma phospholipids identify antecedent memory impairment in older adults. Nature Medicine. 2014;4:415–8. doi: 10.1038/nm.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marjanska M, Auerbach EJ, Valabregue R, Van de Moortele PF, Adriany G, Garwood M. Localized 1H NMR spectroscopy in different regions of human brain in vivo at 7 T: T2 relaxation times and concentrations of cerebral metabolites. NMR Biomed. 2012;25:332–39. doi: 10.1002/nbm.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SL, Celone K, DePeau K, Diamond E, Dickerson BC, Rentz D, Pihlajamaki M, Sperling RA. Age-related memory impairment associated with loss of parietal deactivation but preserved hippocampal activation. Proc Natl Acad Sci USA. 2008;105:2181–6. doi: 10.1073/pnas.0706818105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitrushina M, Satz P, Chervinksy A, D’Elia L. Performance of four age groups of normal elderly on the Rey Auditory-Verbal Learning Test. J Clin Psych. 1991;47(3):351–7. doi: 10.1002/1097-4679(199105)47:3<351::aid-jclp2270470305>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Nicholson DA, Yoshida R, Berry RW, Gallagher M, Geinisman Y. Reduction in size of perforated postsynaptic densities in hippocampal axospinous synapses and age-related spatial learning impairments. J Neurosci. 2004;24:7648–53. doi: 10.1523/JNEUROSCI.1725-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottersen O, Zhang N, Walberg F. Metabolic compartmentation of glutamate and glutamine: morphological evidence obtained by quantitative immunocytochemistry in rat cerebellum. Neuroscience. 1992;46:519–34. doi: 10.1016/0306-4522(92)90141-n. [DOI] [PubMed] [Google Scholar]
- Power JD, Snyder AZ, Schlagger BL, Peterson SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–54. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98(2):676–82. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadan S, Lin A, Stanwell P. Glutamate and glutamine: a review of in vivo MRS in the human brain. NMR Biomed. 2013;26(12) doi: 10.1002/nbm.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Dahle C, Gerstorf D, Acker JD. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb Cortex. 2005;15:1676–89. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Rey A. L’examen psychologique dans les cas d’encephalopathie traumatique. Archives of Psychology. 1941;28:286–340. [Google Scholar]
- Rogalski EJ, Gefen T, Shi J, Samimi M, Bigio E, Weintraub S, Geula C, Mesulam MM. Youthful memory capacity in old brains: Anatomic and genetic clues from the Northwestern SuperAging Project. Journal of Cognitive Neuroscience. 2013;25(1):29–36. doi: 10.1162/jocn_a_00300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupsingh R, Borrie M, Smith M, Wells JL, Bartha R. Reduced hippocampal glutamate in Alzheimer disease. Neurobiol Aging. 2011;32(5):802–10. doi: 10.1016/j.neurobiolaging.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Saad ZS, Glen DR, Chen G, Beauchamp MS, Desai R, Cox RW. A new method for improving functional-to-structural MRI alignment using local Pearson correlation. Neuroimage. 2009;44:839–48. doi: 10.1016/j.neuroimage.2008.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenberg MR, Dawson KA, Duff K, Patton D, Scott JG, Adams RL. Test performance and classification statistics for the Rey Auditory Verbal Learning Test in selected clinical samples. Archives of Clinical Neuropsychology. 2006;21(7):693–703. doi: 10.1016/j.acn.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Schubert F, Gallinat J, Seifert F, Rinneberg H. Glutamate concentrations in human brain using single voxel proton magnetic resonance spectroscopy at 3 Tesla. NeuroImage. 2004;21(4):1762–71. doi: 10.1016/j.neuroimage.2003.11.014. 2004. [DOI] [PubMed] [Google Scholar]
- Simister RJ, Woermann FG, McLean MA, Bartlett PA, Barker GJ, Duncan JS. A short-echo-time proton magnetic resonance spectroscopic imaging study of temporal lobe epilepsy. Epilepsia. 2002;43(9):12021–31. doi: 10.1046/j.1528-1157.2002.50701.x. [DOI] [PubMed] [Google Scholar]
- Small SA. Age-related memory decline: Current concepts and future directions. Neurological Review. 2001;58(3):360–4. doi: 10.1001/archneur.58.3.360. [DOI] [PubMed] [Google Scholar]
- SPM Statistical Parametric-Mapping. 2016 Accessed March 3. http://www.fil.ion.ucl.ac.uk/spm/
- Stanisz GJ, Odrobina EE, Pun J, Escaravage M, Graham SJ, Bronskill MJ, Henkelman RM. T1, T2 relaxation and magnetization transfer in tissue at 3T. Magnetic Resonance in Medicine. 2005;54(3):507–12. doi: 10.1002/mrm.20605. [DOI] [PubMed] [Google Scholar]
- Stark CEL, Squire LR. When zero is not zero: The problem of ambiguous baseline condition in fMRI. Proc Natl Acad Sci USA. 2001;98:12760–6. doi: 10.1073/pnas.221462998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark SM, Yassa MA, Lacy JW, Stark CEL. A task to assess behavioral pattern separation (BPS) in humans: Data from healthy aging and mild cognitive impairment. Behavioral Neuroscience. 2013;51:2442–9. doi: 10.1016/j.neuropsychologia.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stemmelin J, Lazarus C, Cassel S, Kelche C, Cassel JC. Immunohistochemical and neurochemical correlates of learning deficits in aged rats. Neuroscience. 2000;24:275–89. doi: 10.1016/s0306-4522(99)00561-8. [DOI] [PubMed] [Google Scholar]
- Strange BA, Witter MP, Lein ES, Moser EI. Functional organization of the hippocampal longitudinal axis. Nature Reviews Neuroscience. 2014;15(10):655–69. doi: 10.1038/nrn3785. [DOI] [PubMed] [Google Scholar]
- Sugaya KRG, Personett D, Robbins M, Kent C, Bryan D, Skiba E, Gallagher M, McKinney M. Septo-hippocampal cholinergic and neurotrophin markers in age-induced cognitive decline. Neurobiol Aging. 1998;19:351–61. doi: 10.1016/s0197-4580(98)00072-4. [DOI] [PubMed] [Google Scholar]
- Wagner G, Gussew A, Köhler S, de la Cruz F, Smesny S, Reichenbach JR, Bär KJ. Resting state functional connectivity of the hippocampus along the anterior-posterior axis and its association with glutamatergic metabolism. Cortex. 2016;81:104–17. doi: 10.1016/j.cortex.2016.03.022. [DOI] [PubMed] [Google Scholar]
- Wang H, Tan L, Wang HF, Liu Y, Yin RH, Wang WY, Chang XL, Jiang T, Yu JT. Magnetic resonance spectroscopy in Alzheimer’s disease: Systematic review and meta-analysis. Journal of Alzheimer’s Disease. 2015;46(4):1049–70. doi: 10.3233/JAD-143225. [DOI] [PubMed] [Google Scholar]
- Wilson IA, Gallagher M, Eichenbaum H, Tanila H. Neurocognitive aging: Prior memories hinder new hippocampal encoding. 2006;29:662–70. doi: 10.1016/j.tins.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M, Reynolds G, Kauppinen RA, Arvanitis TN, Peet AC. A constrained least-squares approach to the automated quantitation of in vivo (1)H magnetic resonance spectroscopy data. Magnetic Resonance Medicine. 2011;65:1–12. doi: 10.1002/mrm.22579. [DOI] [PubMed] [Google Scholar]
- Woermann FG, McLean MA, Bartlett PA, Parker GJ, Duncan JS. Short-echo-time single-voxel 1H magnetic resonance spectroscopy in magnetic resonance imaging negative temporal lobe epilepsy: different biochemical profile compared with hippocampal sclerosis. Ann Neurol. 1999;45(3):369–76. doi: 10.1002/1531-8249(199903)45:3<369::aid-ana13>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Yassa MA, Mattfeld AT, Stark SM, Stark CEL. Age-related memory deficits linked to circuit-specific disruptions in the hippocampus. Proc Natl Acad Sci USA. 2011a;108(21):8873–8. doi: 10.1073/pnas.1101567108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Lacy JW, Stark SM, Albert MS, Gallagher M, Stark CE. Pattern separation deficits associated with increased hippocampal CA3 and dentate gyrus activity in nondemented older adults. Hippocampus. 2011b;21(9):968–79. doi: 10.1002/hipo.20808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahr NM, Mayer D, Pfefferbaum A, Sullivan EV. Low striatal glutamate levels underlie cognitive decline in the elderly: Evidence from in vivo molecular spectroscopy. Cerebral Cortex. 2008;18(10):2241–50. doi: 10.1093/cercor/bhm250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahr NM, Mayer D, Rohlfing T, Chanraud S, Gu M, Sullivan EV, Pfefferbaum A. In vivo glutamate measured with MR spectroscopy: behavioral correlates in aging. Neurobiol Aging. 2013;34(4):1265–76. doi: 10.1016/j.neurobiolaging.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Rosenke R, Kronemann D, Brim B, Das SR, Dunah AW, Magnusson KR. The effects of aging on N-methyl-D-aspartate receptor subunits in the synaptic membrane and relationships to long-term spatial memory. Behavioral Neuroscience. 2009;162(4):933–45. doi: 10.1016/j.neuroscience.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]


