Abstract
The understanding, diagnosis and treatment of retinopathy of prematurity (ROP) have changed in the last seventy years since the original description of retrolental fibroplasia associated with high oxygenation. It is now recognized that ROP differs in appearance world-wide and as ever smaller and younger premature infants survive. New methods are being evaluated to image the retina, diagnose severe ROP, and determine windows of time for treatment to save eyes and improve visual and neural outcomes. New treatments to promote physiologic retinal vascular development, vascular repair, and inhibit vasoproliferation by regulating proteins involved in vascular endothelial growth factor, insulin-like growth factor, or erythropoietin signaling. Reducing excessive oxidative/nitrosative stress and understanding progenitor cells and neurovascular and glial vascular interactions are being studied.
Keywords: retinopathy of prematurity, angiogenesis, vasoproliferation, oxygen, vascular endothelial growth factor, vascular development, oxidation or oxidative, severe ROP, erythropoietin, insulin like growth factor 1
I. Historical Context of ROP: Early Understanding of Complications of Preterm Birth
Retinopathy of prematurity (ROP) was first described in the US as retrolental fibroplasia (RLF) by Dr. Stewart Clifford in 1940(141) and later carefully studied and reported by Dr. Theodore Terry in 1946.(168) Although much has changed in neonatal care and in our understanding and treatment of ROP, ROP remains a leading cause of childhood blindness worldwide.(54) It is helpful to have a historical perspective of preterm birth and its complications when reviewing studies and progress in ROP management.
In the 1870’s, the development of the first incubator for premature infants was attributed to Stephane Tarnier.(19) At that time, preterm infant mortality and survival were hidden within an overall higher rate of infant mortality than today, mainly from respiratory distress, hypothermia, weight loss, or infection. As the understanding of the needs of the preterm infant increased, advances were made that included developing nurseries for infants, improving incubators that provided supplemental oxygen, and emphasizing maternal bonding with infants, including educating and supporting mothers pre- and perinatally.(19) Still, appropriate thermal environments and the ability to regulate and monitor oxygen had not yet been fully developed when RLF manifested.
II. Advancements in Understanding the Pathophysiology of ROP
A. Role of Oxygen Supplementation
1. High Oxygen Supplementation: The First Epidemic of ROP
Incubators used 100% inspired oxygen at the time RLF manifested as the appearance of a white pupil, likely fibrovascular tissue abutting the posterior lens capsule, sometimes with an associated retinal detachment. Then, observation of the peripheral retina was not universally performed. Ultrasonography had not been developed, and the indirect ophthalmoscope was introduced and popularized later.(150) It is likely some cases of RLF represented macular dragging with retrolental fibrosis, and others may have represented various degrees of retinal detachment, now classified as Stages 4 or 5 ROP. There also may have been cases involving persistence of the hyaloidal circulation. Thus, the pathogenesis of RLF was largely unknown. (It wasn’t until 1984, some 30 years after the description of RLF, that the International Classification of ROP published early appearances of ROP(1)) After observations and experimental work by Michaelson and Ashton(15), and a report by Campbell(31), a collaborative study compared unrestricted and restricted oxygen therapy in infants less than 1500 g and found restricted oxygen reduced RLF.(141) Arnall Patz(126) was a pioneer and performed a clinical trial that also provided evidence that high oxygen at birth was a cause of RLF.(126) Patz credits Szewczyk with recognizing that the abrupt change from high to low oxygen without acclimatization led to retinal vascular damage from low oxygen. Szewczyk(166) noted that when infants were moved out of high oxygen, retinal vessels became dilated and tortuous, and this may have represented early descriptions of Plus disease. Other investigators, including Silverman, Reese, King, and Owens contributed to the understanding and classification of early ROP.(138) Oxygen use was curtailed, and RLF virtually disappeared. As additional technologic advances were made with improved incubators and oxygen monitoring and regulation, younger and smaller premature infants survived, and ROP re-emerged in the 1970’s.(12) This upsurge is sometimes referred to as the “second epidemic”. Today, in countries that have insufficient resources for prenatal and perinatal care and to regulate oxygen, ROP has emerged in larger infants(154, 155) termed the “third epidemic”.(55) This new epidemic may, however, represent a repeat of history, related to use of unregulated high oxygen and reduced resources to provide optimal perinatal and prenatal care.(54, 131)
2. Other Oxygen Stresses: Importance of Experimental Models of Oxygen-induced Retinopathy
Early studies evaluated the role of oxygen (15, 126) in newborn animals that vascularized their retinas after birth and found that 70 to 80% inspired oxygen delivered continuously for at least 4 days to healthy kittens no older than 12 days of age caused “vaso-obliteration” of the newly formed capillaries.(15) Upon return to ambient air, the kittens experienced “vasoproliferation” from retinal vessels into the vitreous. These descriptions from early models of “oxygen-induced retinopathy” (OIR) began the two-phase hypothesis of effects of high oxygen on retinal vascular development. During the second epidemic of ROP when methods were available to examine the retina, it became recognized that ROP also manifested as a delay in physiologic retinal vascular development followed by changes at the junction between the vascularized and avasculari regions of the retina (See III, B. Classification of ROP and Severe ROP). Later, often 2 to 3 months after birth, the retinal vessels became dilated and tortuous, and vasoproliferation developed into the vitreous at the junctions.(1) When these younger and smaller infants in whom oxygen was regulated developed ROP, it became apparent that stresses besides high oxygen at birth were also important.
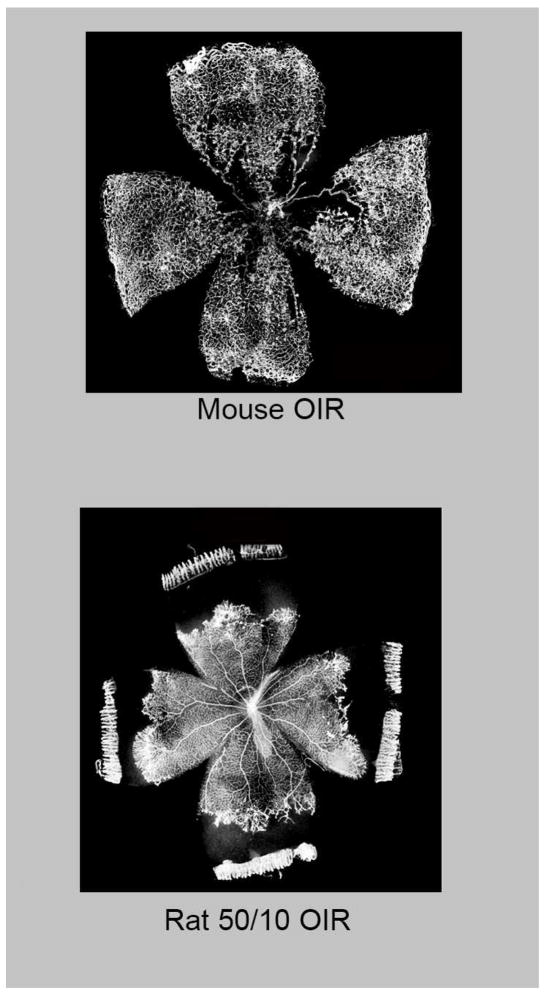
Experimental and clinical evidence demonstrates that fluctuations in oxygenation are associated with the development of features of severe ROP.(40, 128, 205) A model of fluctuations in oxygenation in newborn rats recreates first peripheral avascular retina followed by vasoproliferation at the junction of vascularized and avascular retina (Figure 1), similar to human ROP. The inspired oxygen levels in the model (50% and 10% inspired oxygen) translate to arterial oxygen levels similar to those in human severe ROP.(40) The rat pups also experience reduced postnatal growth, which is linked to severe human ROP.(100) These features make the rat model of fluctuating oxygen (50/10 oxygen induced retinopathy [OIR] model) the most representative of human ROP.(75) The mouse OIR model is useful to study mechanisms of disease through the use of genetically modified mice exposed to first high oxygen, which causes vaso-obliteration of already developed capillaries in the central retina, followed by room air exposure that leads to vasoproliferation into the vitreous at the junctions of vascularized and avascular central retina (Figure 1).(159) The mouse OIR model is helpful to study molecular mechanisms and recaptures the relative differences in oxygen exposure that premature infants experience when they move from the in utero environment to ambient air. The mouse OIR may also recreate a retinal picture similar to the clinical form of severe ROP, aggressive posterior ROP (See III, B. Classification of ROP and Severe ROP). Another well-known OIR model uses newborn beagles in which there is delayed physiologic retinal vascular development and vasoproliferation. The beagle puppy eyes are closer in size to human preterm infant eyes, and this translational aspect of the model is useful when testing pharmaceutical agents being considered for human ROP. OIR models are used to understand the pathophysiology of ROP and assess the effects of potential treatments.(67)
Figure 1.
Retinal flat mounts of oxygen-induced retinopathy: (Top) Mouse and (Bottom) Rat. (courtesy of James Gilman, CRA, FOPS)
B. Other Factors in ROP Pathophysiology
1. Avascular Retinal Area Stimulates Vasoproliferation
Retinal vascularization continues after birth in humans; thus, premature infants have areas of incomplete peripheral retinal vascularization. Peripheral avascular retina can be an indicator of degree of prematurity. Clinical studies have described an association between large peripheral avascular retina and severe ROP.(52, 149) These observations support the hypothesis put forth by Ashton(14) in the 1950’s that avascular retina becomes hypoxic after a preterm infant is moved from high supplemental oxygen to room air and that the ensuing retinal hypoxia triggers the release of angiogenic factors that cause aberrant intravitreal vasoproliferation. Studies reported many different angiogenic factors induce intravitreal vasoproliferation, including vascular endothelial growth factor (VEGF(13)), placental growth factor,(156) insulin-like growth factor-1 (IGF-1(180)), or erythropoietin (EPO),(32) as examples.
Iin the individual preterm infant, however, precise phases do not occur as they do in experimental OIR models, and a concern exists that inhibiting angiogenesis to treat vasoproliferation might lead to avascular retina that would continue to stimulate unwanted vasoproliferation.(33) In addition, each of these and other angiogenic factors when given early during exposure to high oxygen or oxygen fluctuations in experimental OIR models reduced avascular retina and inhibited vasoproliferation,(13, 32, 78, 156, 180) suggesting that timing of delivery or inhibition was critical. Furthermore, in experimental models, angiogenic inhibitors often failed to completely abolish vasoproliferation even when given at established controlled time points in OIR models, suggesting that other pathways were involved in the complexity of OIR and ROP.
Therefore, methods to enhance physiologic retinal vascular development were also studied in experimental models. Early investigators looked into the effects of antioxidants, including vitamins C and E,(127) and found them effective in reducing avascular retina but not always effective in reducing vasoproliferation in OIR models.(119, 127, 145) Inhibitors of NADPH oxidase were found to reduce avascular retina potentially by inhibiting endothelial apoptosis,(145) but only reduced vasoproliferation if the hypoxic stimulus for angiogenesis was minimized when pups were placed into supplemental oxygen.(146) Omega-3 fatty acids produced protective compounds that inhibited vasoproliferation.(39) Prolyl hydroxylase inhibitors stabilize hypoxia inducible factors that translocate to the nucleus, bind to DNA, and facilitate transcription of genes, including angiogenic factors, VEGF, angiopoietins and erythropoietin. In OIR models, these inhibitors were effective in reducing avascular retina in Phase I and vasoproliferation in Phase II OIR models by stabilizing both eye and liver HIF-1.(83, 153, 172) Although some prolyl hydroxylase inhibitors are being tested in clinical trials for anemia in adults, more studies are needed to assess effects of these agents in development, because increased HIF isoform expression occurs.(94)
Deficiency of thrombospondin 1, a natural inhibitor of angiogenesis, increased physiologic retinal vascularization and reduced the sensitivity of retinal vessels to high oxygen induced vaso-obliteration, but did not reduce vasoproliferation. This study suggests that other factors, besides hypoxia related ones, can be involved in aberrant vasoproliferation, at least experimentally.(190)
2. Disordered Developmental Endothelial Cell Growth into Vitreous Instead of Retina: Regulating Overactive VEGF Signaling and Guidance Molecules
The early hypothesis that ischemic retina produced angiogenic factors driven by hypoxia does not explain why blood vessel growth would extend into the vitreous rather than into the retina. Several lines of inquiry into glial- or neural- effects on endothelial cells have addressed this question. Studies provided evidence that hypoxia- or oxidative-induced factors in glial or neural cells mediated overactive signaling of VEGF through VEGF receptor 2 (VEGF2) in endothelial cells. Overactive VEGFR2 then triggered downstream signaling events that disoriented endothelial cell divisions and enabled cells to grow outside the plane of the retina rather than within the retina.(89, 186, 206) Several stresses increase VEGF or VEGFR2 signaling, including fluctuations in oxygenation,(110) reactive oxygen species generated through NADPH oxidase,(187) or interaction with other receptors such as the erythropoietin receptor.(201) Activation of the transcription factor, STAT3, in endothelial cells was also triggered by VEGF(21) and contributed to vasoproliferation.(29) The heat shock protein alphaB crystallin regulated VEGF secretion and may play a role in VEGF-induced angiogenesis.(95) Inhibition of overexpressed Mueller cell VEGF was sufficient to enable physiologic orientation of endothelial cells and inhibit vasoproliferation without adversely affecting physiologic retinal vascular development.(18, 186) Taken together, the evidence supports the hypothesis that over activated VEGF signaling in endothelial cells overwhelms processes to enable normal, ordered intraretinal vascular development and that restoring, but not abolishing, VEGF signaling to a physiologic level would both inhibit vasoproliferation and facilitate physiologic retinal vascular development.(67)
Hypoxia-induced neuronal production of guidance molecules, such as(92) semaphorin 3A mediated through cytokine IL-1beta, can repel endothelial cell growth from the retina and into the vitreous. Semaphorin 3E signaling through plexin D1 restored VEGF induced disoriented growth, (53) whereas semaphorin 3C inhibited pathologic vasoproliferation through neuropilin-1 and plexinD1 receptors (200). Ganglion cells were shown to be involved in experimental disordering of angiogenesis.(91) (147)
3. Poor Postnatal Growth and Reduced Physiologic Retinal Vascular Development
Several investigators reported the association between poor infant growth, large peripheral avascular areas of retina and increased risk of ROP.(100) Insulin like growth factor 1 (IGF-1) is important in fetal growth. Much of IGF-1 comes from the maternal circulation via the placenta during the third trimester. When an infant is born prematurely before the third trimester of gestation, maternal factors are not available, and the infant is not able to make IGF-1 to the degree that a full term infant would. Low serum IGF-1 has been associated with reduced postnatal growth in premature (101) infants and also with increased risk of severe ROP(79). Experimental evidence has been supportive in that in IGF-1 deficient mice, the avascular retina is increased, analogous to human preterm infants at risk for severe ROP(80). Mechanistic studies suggest that IGF-1 and growth hormone are important in stabilizing the retinal vasculature and reducing the effect of hyperoxia on endothelial cell death.(158) Clinical trials are ongoing to address the hypothesis that systemic IGF-1 infusion to levels expected in preterm infants reduces ROP (see IV. Current Clinical Trials, E. IGF-1).
4. Reduced Oxidative Reserve, Increased Oxidative/Nitrosative Stress
Oxidative stress occurs when there is an imbalance between the generation and quenching of reactive oxygen species and has long been implicated in the pathogenesis of ROP. In the premature infant and in ROP, changes in oxygen, light and metabolism have all been linked to increased oxidative stress. The setting of preterm labor and preterm birth can reduce oxidative reserve and present greater oxidative stress compared to that in a full-term birth.(28) Premature infants are also believed to have lower capacity to make their own antioxidant enzymes(69) and are believed to consume more glutathione, which is important in the nonenzymatic reserve in red blood cells. Studies, however, do not support the use of certain antioxidants like n-acetyl cysteine in premature infants.(162) Some antioxidants like vitamin E have shown efficacy, but there were also risks of complications, such as sepsis, and vitamin E is not recommended.(24)
The disappointment from clinical studies to date reflects the complexity of effects from “oxidative stress”. Oxygen and light generate external oxidative compounds that damage lipid rich membranes, such as photoreceptor outer segments. Antioxidants work by becoming less potent pro-oxidants, which in sufficient levels, theoretically, also can be damaging. Besides tissue damaging effects from external oxidative species, there are also important effects from intracellular reactive oxygen species, which act as signaling effectors. Crosstalk among inflammatory, metabolic and angiogenic pathways trigger pathologic or physiologic intracellular oxidative signaling mechanisms in different cell types.(188) In addition, retinal development must be considered. Photoreceptors are highly metabolically active and require oxygen but develop at different times and regions in the retina.(161) Normally, their peripheral development may drive physiologic angiogenesis toward the ora serrata, but with additional stresses lead to pathologic events.(9, 188) All these factors make the analysis of effects from oxidative stress challenging.
Evidence has accumulated that NADPH oxidase is important in experimental OIR models, by generating reactive oxygen species that interfere with vascularization of the peripheral retina and by mediating later vasoproliferation into the vitreous.(146) Several isoforms of NADPH oxidase have been implicated, including NOX1,(194) NOX2(145, 204) and NOX4;(187) however, NADPH oxidase is the key enzyme in leukocytes to fight invading microorganisms, so inhibiting NADPH oxidase may have unwanted consequences.
Hyperoxia-induced apoptosis can occur through nitrosative stress from peroxynitrite.(25, 59, 88) Peroxynitrite forms from nitric oxide in the presence of high concentrations of superoxide radical. Arginase 2, an enzyme that breaks down arginine, is implicated in peroxynitrite-mediated neuro-glial injury(118) and vaso-obliteration in OIR.(165) Nitric oxide, however, can also have beneficial effects on endothelial cells and as a vasodilator. The transcription factor, Nrf2, induces antioxidant protection against hyperoxia-induced endothelial loss in OIR(174) and increases avascular retina. More studies are needed to assess the effect of Nrf2 in development.
Antioxidants may be effective in quenching external reactive oxygen species, but may not be able to access those that trigger pathologic intracellular signaling effectors. Experimental studies have deepened our understanding of the role of oxidative and nitrosative compounds on ROP and show promise for future therapies. A balance or specific targeting of oxidative/nitrosative effects is needed. More study is warranted.(188)
5. Role of Light
Light could lead to the generation of oxygen radicals and be linked to ROP;(140) however, photoreceptor metabolism increases during darkness. Clinical trials (see IV. Current Clinical Trials, D. Light) have not found an association with post-natal light and ROP;(140) however, experimental studies performed on mice in utero provide evidence that light transmitted through the mother’s abdomen has an effect on retinal vascular development of the fetus during a time corresponding to the first trimester in humans.(136) The effects appeared to be from a fetal light-response pathway in which retinal neurons and angiogenesis were regulated by melanopsin expression and not from a maternal factor. Experimental studies provide links between the neurotrophic factor, brain-derived neurotrophic factor (BDNF), light-induced effects on ganglion cell maturation, and ROP. BDNF is important in ganglion cell maturation and is reduced in mice reared in the dark(58, 196). Several studies reported reduced circulating BDNF in extremely low birth weight infants, who developed ROP(77, 135), including in a study of extremely low birth weight infants enrolled through the US Neonatal Research Network.(163) Using genetically tested blood spots from infants in the same cohort, variants in the intronic region of the gene BDNF were found in association with severe ROP in extremely low birth weight infants.(74)
III. Change in Clinical Concepts, Diagnosis and Management in ROP
A. Phases of OIR vs. Human ROP
OIR models are useful in controlled experimentation to investigate disease mechanisms, but human infants do not have discrete phases of avascular retina or vasoproliferation at reproducible times.(67, 75) OIR models are also limited in that they do not use premature animals. Nonetheless, the phases in OIR models can be related to human ROP as Phase I = Stages 1–2 ROP and Phase II = Stage 3 ROP with Plus disease (severe ROP). In human ROP, there is an additional “Phase III” that includes fibrovascular changes with retinal detachment, Stages 4 and 5.(67) Neither the mouse nor rat OIR models recapitulate retinal detachment in ROP. The beagle model more closely associates with Stage 4 ROP in that it develops retinal folds.(111)
B. Classification of ROP and Severe ROP
1. International Classification of ROP
At the time RLF was described, the ability to see the peripheral fundus was limited. The indirect ophthalmoscope was developed by Charles Schepens and Oleg Pomerantzeff and popularized in the 1950’s- 60’s after the initial reports of RLF.(150, 151) The awareness of the effect of high, uncontrolled inspired oxygen at birth and the ability to see the peripheral fundus led to the recognition that earlier stages of ROP existed before RLF. In 1984, the International Classification of ROP (ICROP) developed a classification for acute ROP with five Stages (Figures 2, 3, 4), three Zones and the presence or absence of Plus disease(1) (Table 1). Cicatricial findings were also classified.(191)
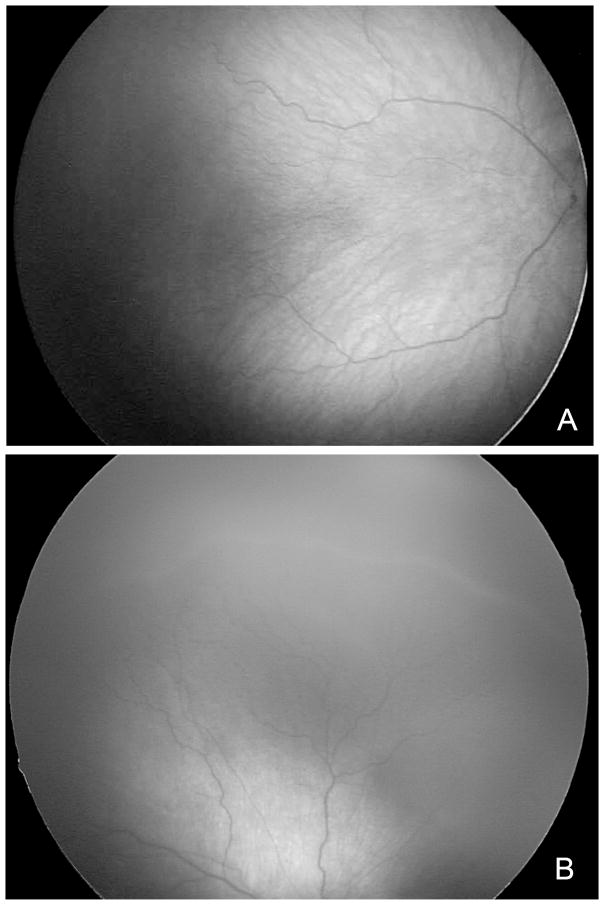
Figure 2.
(A) Stage 1 ROP has a white line at the junction of vascularized and avascularized retina. (B) Stage 2 ROP demonstrates a ridge at the junction of vascularized and avascularized retina. (provided by Cyrie Fry, CRA, OCT-C)
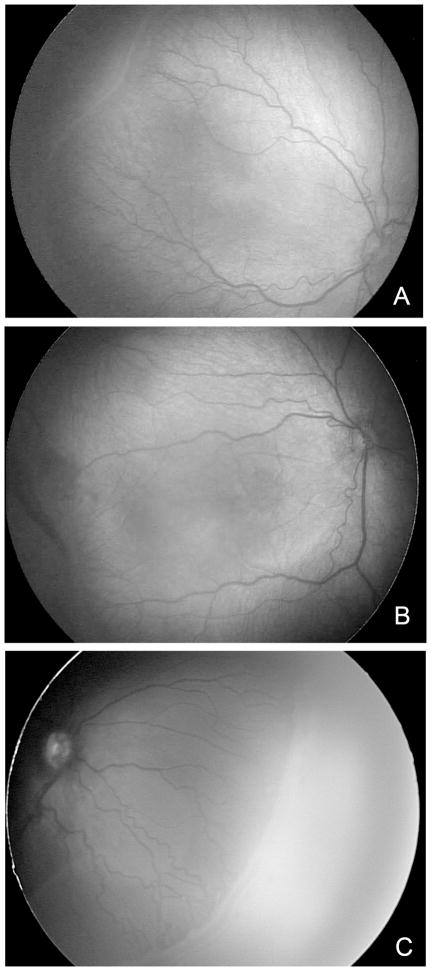
Figure 3.
Forms of Severe ROP (A). Stage 2 ROP with Plus disease; (B) Stage 3 ROP in posterior Zone II appears as flat neovascularization at the junction of vascular and avascular retina; (C) Stage 3 ROP in peripheral severe ROP in Zone 2 at the junction in peripheral severe ROP. (provided by Cyrie Fry, CRA, OCT-C)
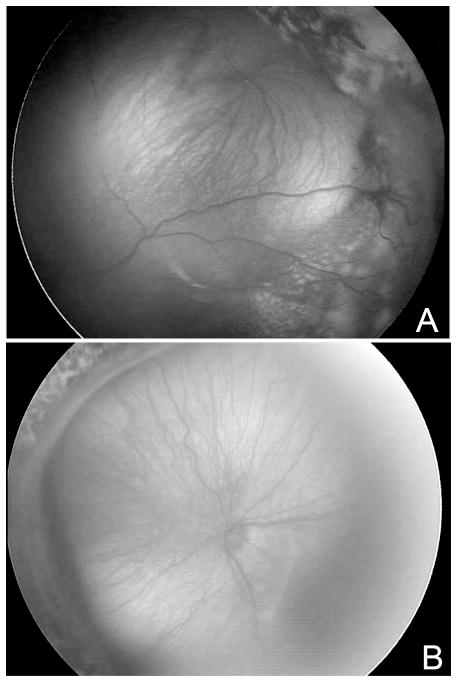
Figure 4.
(A) Exudative Stage 4 ROP in left eye; (B) Tractional Stage 4 ROP shown nasally in left eye. (provided by Cyrie Fry, CRA, OCT-C)
Table 1.
Definitions of Clinically-Important ROP
| Severe ROP | Diagnosis | Zone | Stage | Plus disease |
|---|---|---|---|---|
| a. Threshold | Clinical exam | I or II | 3 (5 contiguous or 8 total clock hours) | 4 quadrants |
| b. Type 1 | Clinical exam | I | Any ROP with Plus disease | 2 quadrants |
| I | Stage 3 ROP without Plus disease | 2 quadrants | ||
| II | Stage 2 or 3 with Plus disease | 2 quadrants | ||
| Referral-Warranted ROP (in either eye) | Contact imaging | I | Any Stage | |
| I or II | Stage 3 ROP | |||
| I or II | 2 quadrants |
The Stages of ROP proceed from incomplete vascularization (no Stage) to descriptions of the retinal appearance at the junction between the vascularized and avascular retina: a faint line in Stage 1, a 3-dimensional ridge in Stage 2 (Figure 2), and “extraretinal neovascularization” (i.e., intravitreal vasoproliferation) that grows from the ridge into the vitreous in Stage 3 (Figure 3). Stage 4 ROP (Figure 4) describes partial retinal detachment with 4a not involving the macula and 4b involving the macula. Stage 5 ROP refers to total retinal detachment and may have peripheral attached retina, particularly if ablative treatment, such as with cryotherapy or laser, had been delivered.. The zones of ROP were defined. Zone I was a circle centered on the optic nerve with a radius equal to twice the distance between the optic nerve and the fovea; Zone II was a circle centered on the optic nerve with a radius equal to the distance between the optic nerve and nasal ora serrata; and Zone III was the temporal crescent remaining. (132)
Plus disease was a descriptive term indicating dilation and tortuosity of retinal arterioles and veins,(125) but now retinal images and computer assessment of tortuosity and dilation of vessels provides the ability to longitudinally measure and evaluate changes. Several investigators have developed software to measure Plus disease automatically, and this ability may improve accuracy and efficiency to determine referral-warranted ROP or assess progress or regression following treatment of severe ROP.(7, 16) Video imaging has also been shown useful in assessing Plus disease.(30) Plus disease was considered a high flow condition related to a temporal vascular shunt and has since been associated with increased activation of the VEGFR2 signaling pathway, which causes tortuosity and widening of the retinal vessels.(72)
ROP was classified by Stage, Zone, extent of Stage, for example extraretinal neovascularization in Stage 3, and the presence and location of Plus disease and drawn on paper examination sheet.
2. Definition of Severe ROP
Stage 3 ROP and Plus disease are features of severe ROP (Figure 3). Severe ROP has been defined at different levels of risk of an unwanted outcome. Initially, severe ROP was defined as threshold ROP, a composite of retinal features that predicted a 50% risk of an unfavorable outcome (e.g. retinal detachment, dragging, retrolental fibrosis, blindness). The Cryotherapy for Retinopathy of Prematurity Study (CRYO-ROP) defined threshold ROP as: Plus disease in all 4 quadrants in Zone I or II with Stage 3 ROP of 5 contiguous or 8 total clock hours (Table 1). Although threshold ROP in Zone II indicated about a 50% risk of a bad outcome, poor outcomes were much more frequent in Zone I, reported in over 80%.(149) This recognition of poor outcomes in Zone I ROP led to the definition of aggressive posterior ROP (APROP), in which incomplete vascularization into Zone I or posterior Zone II occurred, followed by severe ROP manifesting mainly with Plus disease and flat intravitreal neovascularization. Flat neovascularization has a different appearance from Stage 3 in Zone II (Figure 3B), in that vasoproliferation can barely be identified as growing into the vitreous at the junction of the vascularized and avascular retina. Infants developed APROP at young post-gestational or post-menstrual ages (33–35 weeks) and had rapidly progressive ROP without the typical progression from Stage 1 to Stage 2 ROP before the development of severe ROP. (The post-gestational age or post- menstrual age is defined as the sum of the gestational and chronologic ages of the infant in weeks [/content/114/1362.full.html]). This differed from peripheral severe ROP(64) in that APROP also quickly progressed to Stages 4 and 5 ROP often with a week or two of the initial examinations.(177) In addition to APROP, the definition of pre-plus disease was added to the classification to indicate tortuosity and dilation of vessels that did not meet the severity needed for plus disease.(5)
Partly because of APROP, there was interest in defining an earlier level of ROP severity at which intervention be considered. Using mathematical models of risk,(62) high-risk prethreshold ROP predicted the risk of an unfavorable outcome as 15%(45, 62) and was studied as an outcome in the Early Treatment for ROP trial (ETROP). The ETROP found value in ablative treatment (laser preferable to cryotherapy) to peripheral avascular retina in high-risk prethreshold ROP. It should be clarified that the ETROP committee redefined guidelines to provide a clinically reasonable method to characterize high-risk prethreshold ROP at the bedside. This new composite of features was termed Type 1 ROP(45) and was defined as: Zone I, any Stage with Plus disease, Zone I, Stage 3 without Plus disease, Zone II, Stage 2 or 3 with Plus disease. Plus disease only had to be present in 2 quadrants (Table 1).
Post-gestational age (the sum of chronologic and gestational ages in weeks) tended to better predict the peak occurrence of severe ROP than gestational age or birth weight. Type 1 ROP peaked at 35 weeks post-gestational age,(45) and threshold ROP at approximately 37 weeks post-gestational age.(149)
3. Classification and Screening Based on Retinal Imaging
a. Photographic Imaging and Referral-Warranted ROP
As technology advanced, the ability to obtain wide-angle images of infant retinas using contact imaging became available. Several early studies reported on the role of contact imaging for screening ROP.(6, 20, 50) The PhotoROP study included several sites in which examinations were performed with indirect ophthalmoscopy at the bedside to classify severity of ROP. Images also were obtained, transmitted to a central reading center and reviewed in a masked fashion. Agreement was found between diagnoses oat the bedside and from image analysis, and also showed no major difference in timing of “clinically significant ROP”, diagnosed from images, and type 1 ROP.(6, 20) In a subsequent report, the presence of Stage 3 ROP, ROP in Zone I, or Plus disease were collectively termed as “referral warranted” ROP to indicate the need to have an infant evaluated by a trained ophthalmologist for possible treatment.(47) Telemedicine approaches have proved successful in rural areas of Montana where there are not ophthalmologists locally to screen premature infants.(192) It is also being studied to manage the world-wide problem of increasing number of premature infants requiring screening for ROP with insufficient numbers of local ophthalmologists trained to screen and treat severe ROP.(54, 183) The eROP study provided strong support for the validity of remote evaluation of referral-warranted ROP and found that use of contact camera imaging to obtain images by non-physicians and transmission of images to a reading center read by trained non-physician readers was reasonably reliable to detect serious ROP. (42, 134) For features of referral warranted ROP (Table 1), eROP reported intergrader agreement as 0.43 for Zone I disease, 0.57 for Plus disease and 0.67 for Stage 3 ROP. (6) eROP also determined predictors for development of referral warranted ROP after controlling for gestational age and very low birth weight as pre-Plus, retinal hemorrhage, Stage 2 or mechanical ventilation at first examination.(202)
The Kamataka Internet Assisted Diagnosis of Retinopathy of Prematurity study (KIDROP) screened 81 neonatal units of underserved rural India using an indigenously developed tele-ROP model and successfully screened infants for ROP(183). KIDROP represents a standardized telemedicine approach to ROP. Current guidelines of the The American Academy of Ophthalmology published guidelines in 2015.(49) Since then several publications from e-ROP have provided high-level evidence in considering telemedicine for ROP, including that image characteristics at 34 weeks post-menstrual age predicted severe ROP and may be useful once the approach has been validated.(203)
Several other approaches have been developed to capture images of the fundus, including the use of IPhones. Although useful as adjuncts for bedside examinations, these methods are not recommended in place of examinations by qualified ophthalmologists until validity has been tested and demonstrated.
b. Spectral domain OCT
Structural features determined by spectral domain optical coherence tomography (sd-OCT) correspond to histologic reports of premature and developing infant retinas.(81, 175, 183) Typically, inner retinal layers remain and can be retained into later years,(61) similar to that seen in foveal hypoplastic conditions like albinism. The photoreceptor layer is also thinner or absent at early post-gestational ages and develops later.(176) Cysts within the inner layers of the retina have been reported,(181) before or after intravitreal anti-VEGF treatment.(44) In a study of infants in India, cysts peaked at about 37 weeks post-gestational age and were associated with hyperopia. Vision development was slowed compared to a control group of preterm infants without cysts but improved by one year when the cysts resolved.(184) Further studies in larger populations are required to determine if the short-term effect of cysts on visual acuity will affect later vision or refractive errors. Longitudinal evaluations of optic nerve development showed associations between large optic nerve cups and lower Bayley neurocognitive testing scores.(171) Further study is warranted.
C. Screening and Treatment of ROP
1. Screening
Evidence-based screening guidelines are in place for most developed or developing nations and are often based on gestational age, birth weight, and sometimes criteria for infant sickness. Several predictive models have been developed based on infant growth, birth weight, serum IGF-1 and other factors in order to reduce the burden of screening infants for severe ROP. Serum IGF-1 levels were not found to be useful to exclude infants from screening in part because of variations based on race and other factors, and the difficulty in determining a threshold between 31 and 33 weeks post-gestational age that would enable one to exclude infants from screening.(137) Although several studies were performed in the US and throughout the world assessing algorithms that included infant weight gain, only one study was deemed to provide sufficient evidence.(87) The American Academy of Ophthalmology concluded that additional research was needed to optimize ROP predictive model development before models were used widely to reduce the burden of ROP screening examinations(87).
Telemedicine approaches, such as those described above, suggest that screening for referral-warranted ROP is reasonable and also determined predictors of referral warranted ROP.(192, 202)Once a diagnosis of “referral-warranted” ROP is made, however, resources and protocols for transport and treatment of infants must be in place.
2. Treatment of Severe ROP
Initially, xenon arc photocoagulation was reported as a treatment for acute proliferative ROP;(116) however, this method required a direct ophthalmoscopic delivery system that was difficult to use even in the best situation of clear media and well-dilating pupil without the persistence of the tunica vasculosa lentis. CRYO-ROP reported that cryotherapy for threshold ROP significantly reduced an unfavorable anatomic outcome and improved visual development compared to no treatment.(2, 3) At the time of CRYO-ROP, there was no commercial system widely available to deliver laser to the eye through indirect delivery systems. Indirect laser delivery systems were available for ETROP, which found that treatment with laser (or cryotherapy) in type 1 ROP significantly improved outcomes compared to standard care of threshold ROP.(45) This trial also included a greater percentage of Zone I eyes, approximately 40% compared to relatively few in CRYO-ROP, which may have reflected eyes with APROP.
Cryotherapy has been less commonly used for severe ROP since the advent of indirect laser delivery systems in the late 1980’s. Cryotherapy may cause more inflammation, which is involved in the pathogenesis of ROP,(82) and incur poorer outcomes than laser treatment. Cryotherapy is associated with a small loss of visual field in children.(133) Several single site or small-scale studies suggest that myopia is less severe in laser-treated than cryotherapy-treated eyes (10). Myopia appeared mainly to be due to increased lens thickness and not to longer axial length(38). The American Academy of Ophthalmology recommends laser be performed for severe ROP whenever possible and that technology be obtained to deliver laser by indirect delivery system as an optimal therapy to cryotherapy for severe ROP.(157)
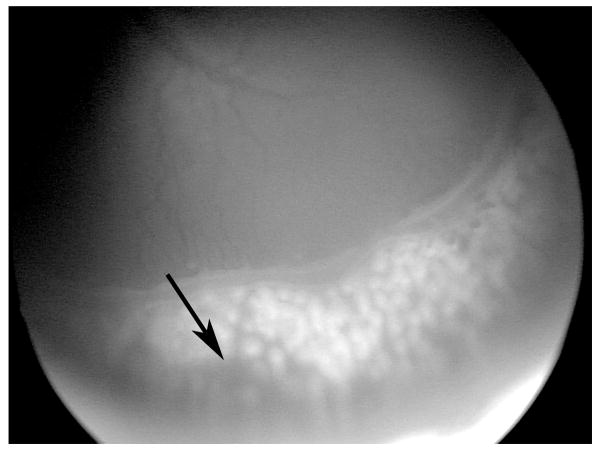
The standard of care for ROP now is laser ablation to the peripheral avascular retina for severe ROP. Anesthesia can be administered by endotracheal intubation or with sedation without general anesthesia in the operating room or in the neonatal nursery(148). Spots should be of moderate intensity and approximately ½ to 1 spot size apart, extending anteriorly from the ridge to the ora serrata. The use of contact imaging is helpful to detect skip areas (Figure 5) that can occur in part from the “slope” created by scleral depression that hides areas of untreated retina and expand sufficiently with ocular growth to stimulate angiogenesis.
Figure 5.
Contact imaging following laser can be helpful to identify skip areas, where there is no laser. (courtesy of James Gilman, CRA, FOPS)
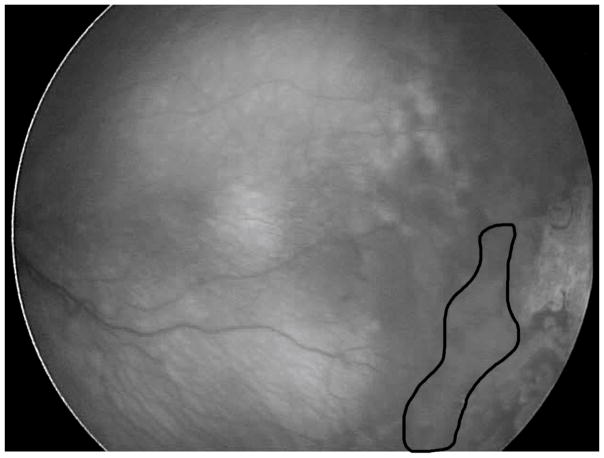
A two-staged laser treatment can safely cause regression with less risk of vitreous hemorrhage in eyes with flat neovascularization.(182) First, laser is applied to the avascular retina up to the flat neovascularization. Once regression of occurs, additional laser is then placed in the newly created avascular bed where the flat neovascularization had been present in order to prevent subsequent neovascularization(182) (Figure 6).
Figure 6.
Outlined area shows where subsequent laser was performed after initial treatment that led to regression of flat neovascularization. Staged laser up to flat neovascularization in APROP avoids vitreous hemorrhage. Later with regression, additional laser is necessary to prevent subsequent vasoproliferation. (courtesy of James Gilman, CRA, FOPS)
Anti-VEGF agents are currently considered for eyes with Zone I, Stage 3, and plus disease and are being evaluated for type 1 ROP(48) and for agent, dose and some safety outcomes (Pediatric Eye Disease Investigative Group, NEI/NIH, NCT02390531 and RAINBOW study, Novartis, NCT02375971). See IV. B.
3. Surgical Treatment of ROP
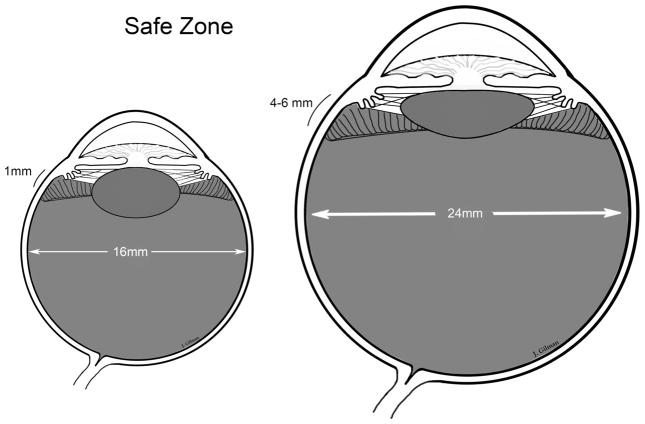
Early studies focused on reattachment of the retina in Stage 5 ROP, and although it was technically possible to reattach retinas, visual results were often limited. Now all efforts are to prevent Stage 5 ROP with lens-sparing vitrectomy when progressive Stage 4 ROP is determined. Since there are risks in operating on preterm infant eyes, determining the “window of surgical opportunity” is important. The region to enter the eye safely so as not to injure the retina or lens is about 1 mm posterior to the limbus (Figure 7).(104) The firm vitreoretinal adhesion in infant eyes increases the risk of an inoperable retinal detachment if a retinal tear occurs. It is also important to enter the vitreous cavity perpendicularly to avoid injuring the lens, which is necessary for visual development. Progression of ROP can lead to recurrent vascular activity with retinal detachment, which reduces surgical success.(63) Therefore, it is important to intervene to prevent progressive stage 4 ROP without adding unnecessary risk to the infant eye.(73) An experienced surgical team includes the pediatric retinal surgeon, surgical assistant, anesthesiologist, nurses and technicians well-versed in infant vitreoretinal surgery.
Figure 7.
The preterm infant eye is smaller than the adult with <1mm zone to enter the vitreous cavity without causing a retinal break or injury to the lens. (courtesy of James Gilman, CRA, FOPS)
When progressive stage 4 ROP develops, a lens-sparing vitrectomy can be effective to prevent stage 5 ROP(120) and preserve the lens for visual rehabilitation of the infant. Although scleral buckling plays a role, lens-sparing vitrectomy has been reported to be more effective in addressing surgical goals in Zone I or Zone II progressive Stage 4 ROP.(71) Several features have been associated with progressive Stage 4 ROP, including two or more of the following: 6 or more clock hours of ridge elevation, recurrent or persistent Plus disease in 2 quadrants, and vitreous condensation or haze.(73) Also important are the presence of vitreous hemorrhage (86) or vitreous organization(37). It is helpful to determine the angle between the posterior retinal veins, with increasingly acute angles suggestive of peripheral traction. Vascularly active ROP is treated to reduce activity(198) before proceeding with surgery to improve outcomes.(63)
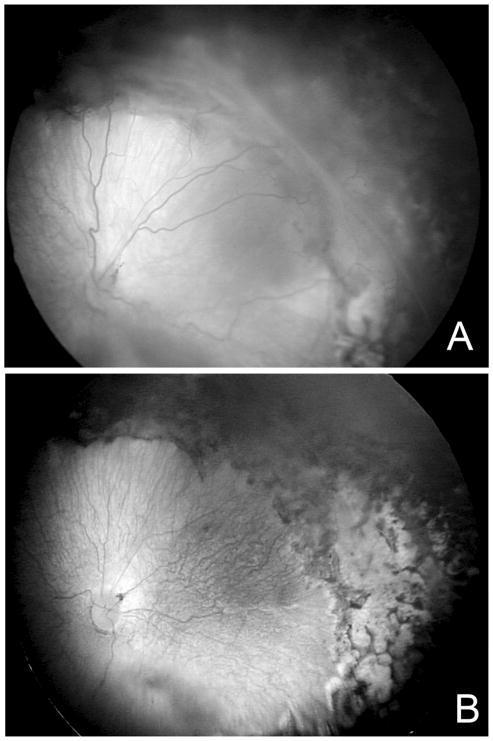
The goals of surgery are to release vitreoretinal tractional components extending between the ridge and the anterior eye, the peripheral retina extending to the ora serrata, the optic nerve and the ridge creating “circumferential” traction. Retinotomies are avoided. Two or three port vitrectomies are successfully used. Complete removal of the vitreous is not attempted. An air bubble is placed into the vitreous cavity to maintain globe shape during closure of the sclerotomies, but forceful fluid-air exchange to reattach the retina is avoided. Once vitreous tractional forces are released, the retina reattaches over time (Figure 8).
Figure 8.
(A) Stage 4A–B ROP shows elevated retina involving the temporal arcade; (B) Following lens-sparing vitrectomy and removal of vitreo-retinal tractional components without retinotomy, the retina settles back. (courtesy of James Gilman, CRA, FOPS)
Late vitreous hemorrhage can occur in patients with regressed ROP.(143) These can resolve spontaneously, but retinal breaks should be ruled out or treated.
D. Changes in Philosophy of ROP Management: From Preventing Stage 5 ROP to Preventing Severe ROP
The goal in the CRYO-ROP study was to prevent Stages 4 and 5 ROP, i.e., retinal detachment. Despite improvement with cryotherapy, infants with Zone I ROP and APROP had poor outcomes. Treatment for less severe ROP was recommended after the ETROP trial. However, infants born under 500 g birth weight were also at high risk of strabismus, nystagmus, high myopia and abnormal retinal structure.(193) Astigmatism and strabismus persisted through 6 years of age.(43, 178) The early treatment group had better visual acuity outcomes than the conventional treatment group, but visual acuity outcomes were less than 20/40 in over 60% of early treated eyes at 6 years.(56) These findings support the importance of long-term vision care of premature infants. Future efforts should focus not only on preventing retinal detachment, but also on preventing severe ROP. One area of current interest is in targeting effects from overactive VEGFR2 signaling to reorient disordered developmental angiogenesis.(67) Greater understanding of neuro- and glial vascular effects are important to understand interactions and crosstalk between cell types that are important in angiogenic and neuronal development. Also, future studies are needed to assess effects from prematurity or perinatal stresses on ROP and on visual development potentially independent of ROP severity.
E. Changes in ROP Phenotype World-wide: The “Third Epidemic” of ROP
The first epidemic of ROP occurred because of unregulated oxygen use. As smaller infants of younger gestational ages survived, the second epidemic of ROP was recognized. Now, a “third epidemic” has been described in countries with sufficient resources to save premature infants (68), but insufficient resources to provide optimal prenatal and perinatal care and regulate and monitor oxygen.(54) Several believe the new “epidemic” reflects a repeat in history and other factors like genetic differences. In developed countries, the risk of blindness from ROP is <10%, but can be as high as 40% in developing countries(130) where larger infants of older gestational ages have severe ROP.(155) This last observation highlights the importance of developing specific ROP screening criteria for individual regions throughout the world.
Another consideration regarding differences in ROP phenotypes is that of comparing effects from new treatments in different patient groups. Intravitreal anti-VEGF treatment to growth-restricted infants of younger developmental ages, as seen in the US, may have adverse effects on organ development and more safety concerns than in infants of larger body weights and older developmental ages (see also IV. Current Clinical Trials).
IV. Current Clinical Trials
A. Studies on Oxygen Saturation
The level of oxygenation safe for infants and to reduce ROP was readdressed after recognition that stresses besides high oxygen at birth were associated with severe ROP. The STOP-ROP (Supplemental Therapeutic Oxygen to Prevent ROP) study was designed to test the effect of increased oxygenation in an effort to offset later hypoxia from avascular retina. STOP-ROP found no increase in threshold ROP when premature infants with prethreshold ROP were maintained at 96–99% oxygen saturation (SaO2) compared to 89–94% SaO2.(4) Subsequently, several small scale studies suggested low oxygen saturation targets reduced ROP risk.(179) From these studies, the multicenter study, Surfactant, Positive Airway Pressure, Pulse Oximetry Randomized Trial (SUPPORT) was performed to compare intubation and surfactant vs. continuous positive airway pressure (CPAP) on a number of outcomes, including ROP. Infants were assigned to target oxygen saturations of 85–89% vs. 91–95% SaO2.(51) ROP occurred less often in the low oxygen saturation group, but mortality was increased. There was variability in infant survival among the centers of SUPPORT, but the Benefits of Oxygen Saturation Targeting Study II (BOOST-II) in the UK and Australia tested the same oxygen targets and found a greater survival among infants at the higher oxygen saturation range.(167) The Canadian Oxygen Trial, however, did not find any differences in ROP or mortality in infants assigned to either oxygen saturation range.(152) There are differences in the infants enrolled in COT vs. SUPPORT or BOOST-II. Now many neonatologists are concerned with risking infant survival by lowering oxygen saturation targets as a strategy to manage ROP. Some believe that fluctuations in oxygenation are more important in causing severe ROP than are absolute targets.(76) In keeping to the strict range of target oxygen saturations, FiO2 might be adjusted frequently, and this practice may lead to swings in oxygen saturation in infants.
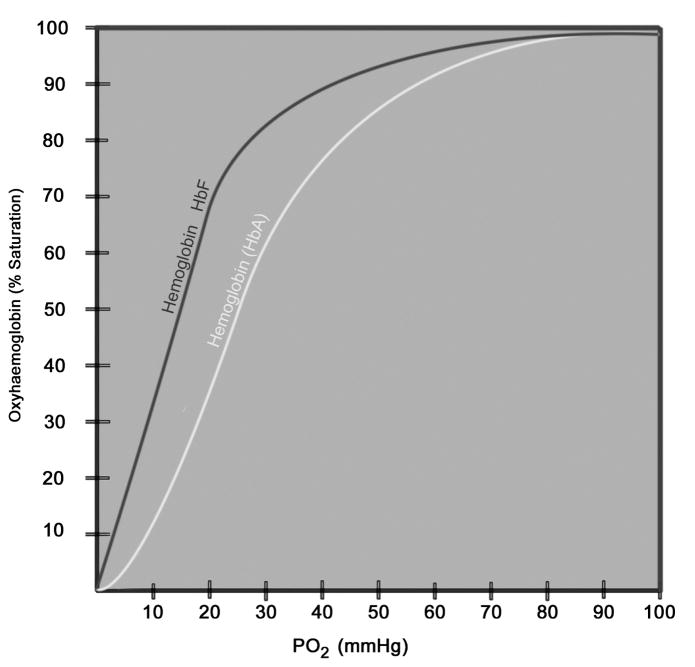
It is difficult to regulate oxygenation because of the inability to determine oxygen level within retinal tissue in the individual premature infant. Currently oxygenation is approximated by blood oxygen saturation (SaO2), which is a ratio of the amount of oxygen bound to hemoglobin in red blood cells to the oxygen carrying capacity of that hemoglobin. SaO2 is represented in a sigmoidal curve, known as the oxyhemoglobin dissociation curve (Figure 9). SaO2 is affected by factors such as temperature, pH, CO2, the amount of oxygen exchange at the lungs, and the type of hemoglobin (e.g. adult vs. fetal(70)). Compared to adult hemoglobin, fetal hemoglobin has higher affinity for oxygen, but is less likely to release oxygen because it requires a lower tissue PaO2 for release. Fetal hemoglobin persists until about 6 months after birth and adult hemoglobin is produced. When preterm infants require blood transfusions for anemia of prematurity, they are transfused with adult hemoglobin. These situations can also affect SaO2. At greater than 60 mm Hg arterial oxygen (PaO2), the oxyhemoglobin dissociation curve becomes flat meaning that at more than 90% SaO2, arterial oxygen content is hard to determine and can be at high levels that are damaging to newly formed blood vessels. Neonatologists strive to maintain SaO2 along the slope of the curve. Difference in the extent of retinal vascularization of individual infant eyes affects retinal oxygen content, and this is only assumed by the extent of inner plexus vascularization. The deep retinal plexus develops after the inner plexus, but is not visible on clinical examination, and retinal hypoxia may be greater in the deeper layers of the retina than expected given the extent of inner retinal vascularization. The preterm infant’s circulation also shunts venous and arterial blood, which reduces oxygen content.
Figure 9.
Oxyhemoglobin dissociation curve. Fetal hemoglobin (HbF) has a curve to the left of adult hemoglobin (HbA). (courtesy of James Gilman, CRA, FOPS)
There remains no consensus among neonatologists on optimal oxygenation targets for overall infant health and to reduce ROP. In the SUPPORT study, there was variability of overall survival among various NICUs, suggesting differences in treatment interventions, genetics of the infant samples, or other characteristics of the populations of premature infants.(11) One report of a post-hoc analysis of the data from the SUPPORT study found that survival was strongly associated with infants appropriate for gestational age (AGA) regardless of whether oxygenation targets were in the high or low oxygen saturation ranges. In contrast, infants small for gestational age (SGA) had twice the mortality rate in the low vs. the high oxygen saturation groups. Severe ROP was significantly reduced in AGA infants in the low oxygen saturation group, but this was not the case for SGA infants. The post-hoc analysis generated an interesting hypothesis as to why differences might have been seen in SUPPORT vs. other studies.(185) The Canadian Oxygen Trial did not find the same outcomes as did SUPPORT or BOOSTII,(122) but infants with pulmonary hypertension may have been excluded and different oxygen saturation monitors may have been used in COT compared to SUPPORT and BOOST, which used Masimo devices. There is also the ongoing concern that the actual tissue oxygen content or that delivered to tissue may vary across neonatal intensive care units due to errors in probe placement, accuracy of pulse oximeters, differences in adult vs. fetal hemoglobin content and extent of vascularized retina among individual infants.(70, 97) There may also be effects due to differences in altitude between NICUs, which affects barometric pressure and ambient oxygen content. A meta-analysis of 5 studies concluded that the quality of evidence was low to support conclusions that liberal oxygen use was associated with lower mortality before discharge without a difference in morbidities, including ROP.(106) Based on variability, individual neonatal intensive care units may need to adjust oxygen requirements to their own needs. Center variability is partly due to differences in patient characteristics and center intervention, but does not account for all the variability in mortality across centers.(11) Ophthalmologists remain concerned that high oxygen extremes increases the number of infants with severe ROP,(107) and neonatologists are concerned that lowering oxygen may reduce infant survival.(106)
B. Anti-VEGF Studies
Evidence suggests that regulating VEGF signaling through VEGF receptors can restore physiologic homeostasis and permit ordered developmental intraretinal angiogenesis thereby reducing hypoxic peripheral avascular retina, which drives disordered angiogenesis, when the infant is removed from high supplemental oxygen.(72, 206) Inhibition of VEGF also reduces intravitreal vasoproliferation.(89, 186) Therefore, inhibiting VEGF signaling is plausible not only to reorder developmental angiogenesis(206) to prevent vasoproliferation and facilitate physiologic retinal vascular development, but also to reduce the need for destructive therapies like laser or cryotherapy. Restoring VEGF signaling to physiologic levels locally in the retina would seem an ideal treatment.
However, preclinical data support the substantial concerns with the use of anti-VEGF treatment in ROP. First, VEGF is important in the development of the retinal vasculature and other tissue beds (60, 164) and in homeostasis of developed vascular beds and of glia and neurons.(89, 144) Inhibiting VEGF may inhibit physiologic angiogenesis and retinal development and health.(89, 115, 144, 170, 195) It is difficult to modulate the dose of VEGF inhibition in the individual preterm infant eye. One dose of bevacizumab, an anti-VEGF agent, led to persistent avascular retina and recurrent disease (85, 160), even 2.5 years later. (160–163, 166–168) (2, 10, 64, 67, 83, 149, 166) (2, 10, 64, 67, 83, 149, 166) (2, 10, 64, 67, 83, 149, 166) (2, 10, 64, 67, 83, 149, 166) (2, 10, 64, 67, 83, 149, 166) (2, 10, 64, 67, 83, 149, 166) (2, 10, 64, 67, 83, 149, 166) (2, 10, 64, 67, 83, 149, 166) (2, 10, 64, 67, 83, 149, 166) The direct comparison of anti-VEGF treatment between premature infant and adult eyes also suggests that ¼ the dose in vitreous in the infant eye would still be much less diluted in the many fold smaller infant blood volume compared to the adult.(17, 66) VEGF is important to kidney, brain, and lung development as well as to retinal health.(60) Inhibiting VEGF thus raises concerns about developing organs in the premature infant.(60) Systemic VEGF levels can be reduced for up to 2 months following a single intravitreal injection of 0.625 mg bevacizumab in a premature infant eye.(197) In adult diabetic patients, imposed monthly anti-VEGF therapy for diabetic macular edema was associated with increased risks of hypertension and vascular events,(26) but effects from inhibition of VEGF are difficult to distinguish from those associated with prematurity. A study provided evidence that infants treated with bevacizumab had worse cognitive scores than those treated with laser, but the authors acknowledged the study was limited by being retrospective, non-randomized and because infants treated with bevacizumab tended to be sicker with more health problems.(114) Other studies have not found differences in cognitive outcomes of infants treated with anti-VEGF vs. laser. All studies are limited by sample size and differences in characteristics of preterm infants confounding comparison. Reduction of VEGF does not regulate overproduction of other hypoxia-induced angiogenic factors, such as angiopoietins or erythropoietin, and experimental evidence shows recurrent intravitreal vasoproliferation occurs in association with upregulation of other angiogenic pathways when capillary density was reduced following treatment with an intravitreal neutralizing antibody to VEGF.(109) Perhaps OCT angiography, adapted for preterm infant eyes, can provide greater insight into effects of prematurity and treatment on the deep capillary plexus.
Despite concerns, a number of relatively small series and some clinical trials have been performed testing the role of various anti-VEGF agents on treatment of severe ROP. The phenotype of ROP varies and in some developing countries, ROP occurs in more developmentally mature and larger infants.(155) The same dose would be diluted more in the systemic circulation in the larger infants. Controlled clinical trials accounting for developmental age, weight, and other factors in premature infants are needed. Comparisons between studies are not valid.
The Bevacizumab Eliminates the Angiogenic Threat of Retinopathy of Prematurity (BEAT-ROP) study was the first clinical trial that compared anti-VEGF therapy with bevacizumab to laser treatment for stage 3+ ROP,(113) a more severe ROP than type 1 ROP. BEAT-ROP reported that inhibiting VEGF reduced Stage 3 ROP and also permitted some physiologic retinal vascular development.(113) Infants, not eyes, were enrolled to reduce possible crossover effects and were treated with 0.625 mg in 0.025 mL given as an intravitreal injection to one or both eyes. Infants with Zone I or posterior Zone II ROP were reported to have better outcomes defined as not requiring retreatment by 54 weeks post-gestational age. In anti-VEGF-treated infants, retreatment occurred in 4% compared to 22% of laser treated infants. (It should be remembered that treatment of flat neovascularization, which occurs in Zone I and posterior Zone II APROP, is often staged to reduce the risk of hemorrhage(182))
Five years after BEAT-ROP, a cohort of 11 patients (22 eyes) treated with intravitreal bevacizumab and 17 patients (32 eyes) with laser showed recurrences in 3 eyes after bevacizumab and 1 eye after laser. The average refraction of bevacizumab- treated eyes was −2.4 D at 22.4 months post-gestational age and of laser-treated eyes was −5.3D at 37.1 months post-gestational age. A later study from a single site reported on recurrences after intravitreal bevacizumab until 65 weeks adjusted age and included 75 infants from BEAT-ROP and newly diagnosed infants with APROP or posterior Zone II severe ROP. Recurrences were seen in 8.3% of 241 infants and occurred in 90% at a mean of 52 weeks (45 to 55 weeks) on average 16.2 weeks after treatment.(112) Other studies have mixed reports with only some suggesting that myopia after anti-VEGF treatment is less compared to after laser treatment, and may reflect different populations of infants, types of agents and doses used.(35) Recurrences of severe ROP have been reported after bevacizumab or ranibizumab. Myopia does not appear to be due to increased axial length(36).
Data from carefully designed and executed clinical trials are needed before recommendations are made as to whether anti-VEGF should be broadly considered for type 1 ROP, and if so, what agents, doses, and follow up plans are needed. The dose may not correctly be assumed to the same in all infants with severe ROP because of differences in blood/eye volumes between large vs. growth restricted infants and potential different needs of growth factors on ocular and systemic health in more developed vs. less-developed infants. Currently, the recommendations provided by the American Academy of Ophthalmology and American Academy of Pediatrics (48) are for careful informed consent since no anti-VEGF agent is approved for premature infant eyes. In cases in which corneal, lens, or vitreous opacities preclude treatment with laser, bevacizumab is to be considered for Zone I, Stage 3+ ROP but not Zone II ROP. Follow up should continue until the full extent of the retina can be examined and deemed vascularized. A log of infants treated and dates of treatment is important and clear communication between treating ophthalmologist and neonatologist is essential upon transfer or discharge of the infant. Injection is safer when given less than 1 mm posterior to the limbus to avoid injuring the retina and lens, but injections were given 2.5 mm posterior to the limbus in the BEAT-ROP study, and retinal detachments from injections were not distinguished from those due to progression of ROP in the study. (113)
Currently at least two multicenter trials are underway for forms of severe ROP, one by the Pediatric Eye Disease Investigator Group (PEDIG, NCT029390531) through National Eye Institute to assess the lowest effective dose of bevacizumab given intravitreally and another through Novartis (RAINBOW; NCT02375971) to compare laser to two doses of ranibizumab in 0.05 mL.
C. Antioxidants
Antioxidant studies have not been as promising as hoped in preventing or treating ROP. NADPH oxidase inhibitors have been beneficial in experimental OIR models.(145, 146) NADPH oxidase, however, also has important effects on fighting infection in the immune-compromised premature infant. Some antioxidants, such as vitamin E, showed benefit in reducing retinopathy, but the safety profile was not adequate.(24) Other antioxidants may not access intracellular signaling.
Lutein/zeaxanthin may reduce oxidative stress and theoretically provide a source of macular pigment in developing photoreceptors. The studies currently have not shown an effect, but may require increased sample size and refinement of studies based on doses needed and timing of intervention.(108) N-acetyl cysteine was tested compared to control, but no effect was found in reducing ROP severity.(162) It is important when assessing outcomes in clinical trials to assess whether infant populations and outcomes, eg., ROP vs. severe ROP, were similar among trials.
D. Light
The LIGHT-ROP clinical trial tested the hypothesis that increased light to premature infants postnatally would affect severity of ROP. At birth, infants less than 1251 g were either randomized to wear goggles that reduced visible light 97% and ultraviolet light 100% or control until 31 weeks post-gestational age or 4 weeks after birth when the first eye examination would be performed.(140) There was no effect on ROP severity by reducing light. A subsequent Cochrane review also showed no effect on ROP from light exposure during the first 7 postnatal days (90).
Since the LIGHT-ROP study, other intriguing experimental studies provided evidence that prenatal light limits retinal ganglion cell number, hypoxia and VEGFA overexpression, thus permitting hyaloidal regression and retinal vascular development to ensue in orderly fashion. The mechanism appeared to be melanopsin dependent.(136) A study in human preterm infants then followed and was conducted in Cincinnati, which is located in a region of the world in which day length varies depending on the time of year. The hypothesis was that infants in gestation during longer daylight would have more normal retinal vascular development and, therefore, less severe ROP. A retrospective multiple logistic regression analysis of 343 premature infants born smaller than 1251 g found that higher average day length significantly reduced the likelihood of having severe ROP.(199) Further study is warranted.
E. IGF-1
IGF-1 is important to the growth of the infant in utero and is supplied largely by the mother through the placenta. An infant born prematurely is incapable of producing IGF-1 and has lower circulating levels than do full-term infants.(99) A Phase II clinical trial to study infused recombinant IGF-1 and IGF binding protein 3 (IGFBP-3) did not reach the primary outcome to reduce severe ROP but did show reduction in severity of secondary outcomes, including bronchopulmonary dysplasia and severe intraventricular hypertension (Shire, NCT01096784). Infusion of recombinant IGF-1 and IGF binding protein 3 (IGFBP-3) can increase serum concentrations in human preterm infants.(98) A continuation study is ongoing (Shire, Section D, NCT01096784; PEDAL, NCT02386839).
F. Erythropoietin and Derivatives
Early studies using the hormone, erythropoietin (EPO), to treat anemia of prematurity found associations of EPO use with severe ROP.(27) Later, experimental evidence suggested that timing of EPO administration made a difference in that EPO given early to reduce avascular retina in Phase 1 OIR was not associated with severe retinopathy, but if inhibited during Phase 2, OIR also reduced retinopathy.(8, 34) In another OIR study, during conditions of high retinal VEGF expression, the EPO receptor interacted with and overactivated VEGF receptor 2 in endothelial cells to cause intravitreal vasoproliferation.(201) Therefore, there was concern that EPO and derivatives may increase the risk of developing severe ROP.(117)
Extreme prematurity is associated with neurodevelopmental delays and severe ROP.(142) There is now renewed interest in the neuro- and tissue protective functions of EPO and derivatives, such as darbepoietin.(9, 23, 105, 124, 142) Use of EPO in preterm infants reduced later cognitive impairment and has been proposed for other diseases with neuropathy, including diabetes mellitus.(189) A recent clinical trial showed no increase (or decrease) in severe ROP in infants treated early in life with a modified EPO, darbepoietin, compared to control,(121) but the number of infants with ROP was small. Altogether, the findings support additional study to understand the effects of EPO on EPO and VEGF receptor signaling experimentally and to assess results from several carefully designed and executed clinical trials on neurocognitive development and ROP. The Preterm Epo Neuroprotection Trial (PENUT, NCT01378273) is testing EPO in preterm infants.(93)
G. Areas of Controversy
1. Inositol
Several studies, including a Cochrane Report, suggested that the essential compound, inositol, was valuable in reducing ROP.(84) A recent clinical trial (NCT01954082) to test the use of inositol to control ROP, however, has been stopped because of safety concerns (clinicaltrials/gov).
2. Hypothermia
Experimental evidence suggests that hypothermia reduces brain damage as well as retinal gliosis associated with perinatal hypoxia(139). Clinical trials have demonstrated benefits from 3 days of cooling to 33–34 degrees C within 6 hours of perinatal asphyxia, but have excluded infants born less than 36 weeks gestation(169).
Neonatal hypothermia is associated with higher mortality and morbidity. There is considerable concern about hypothermia, and a call to develop a standardized approach to collect and analyze hypothermia data has been made(102). One recent study from the Canadian Neonatal Network determined the lowest rates of the composite outcome of severe neurological injury, severe ROP, necrotizing enterocolitis, bronchopulmonary dysplasia, and nosocomial infection was an admission temperature of 36.8 degrees C with a range of 36.5 and 37.2 degrees C.(103)
V. Future Directions
A. Genetics and Epigenetics
There have been a number of small-scale candidate gene studies to assess genetic variants in association with ROP, but there is no consensus as to genetic variants associated with ROP. This lack of consensus can exist because of differences in genetic pools, the abilities to accurately diagnose ROP and severe ROP, and in resources and management of preterm infants. Studies varied as to populations, gestational ages and birth weights of infants enrolled and whether genotype associations were with ROP or treatment-requiring ROP (i.e. severe ROP). The severity of ROP is an important consideration because much ROP regresses spontaneously; however, even non-severe ROP may be associated with refractive errors and visual impairment.(56) Genetic variants in VEGF, EPAS1 (both associated with hypoxia regulation) in members of the WNT signaling pathway (important in development and the disease familial exudative vitreoretinopathy (FEVR))(46, 96) and SOD (encodes superoxide dismutase, an antioxidant enzyme) have been reported in association with any ROP.(74) Members of the WNT pathway have been most commonly found in association with severe ROP. Known mutations of FEVR were associated with intrauterine growth restriction and severe ROP in preterm infants of older gestational ages, suggesting that the presence of FEVR mutations increases the risk of preterm birth and severe ROP in infants who would not have been predicted to develop severe ROP based on degree of prematurity.(41) In a large candidate gene study of blood from 1000 extremely low birth weight infants enrolled in the Neonatal Research Network, intronic variants in the gene that encodes brain-derived neurotrophic factor (BDNF) were highly associated with severe ROP compared to either no ROP or non-severe ROP, (68, 74) supporting a link between vascular and neural pathways in extreme prematurity and ROP.
ROP has been associated with multiple gestations, assisted reproductive technology, both suggesting a genetic link. Genetically identical twins, however, do not always develop the same severity of ROP. Also, ROP is affected by external factors, such as oxidative stress, nutrition, and oxygen exposure, but not universally in all premature infants. Oxidative stress, nutrition, sex differences and changes in development may affect gene expression through the acetylation and methylation of DNA and have been proposed to help explain unpredictable effects of external and genetic factors on ROP.(22, 75) These observations support a hypothesis that epigenetic modifications by perinatal factors affect gene expression and may predispose an infant to severe ROP or an infant genetically predisposed to ROP not to manifest retinopathy. More study into these factors may help explain the tremendous variability in ROP severity among premature infants.
B. Stem Cells/Progenitor Cells
Various stem cell or progenitor cells to repair damaged retinal vasculature (57, 123) have been studied in experimental models of OIR. Endothelial colony forming cells (ECFCs), a form of circulating stem cell/progenitor cell, can be harvested from umbilical cord blood, bone marrow or peripheral blood. ECFCs have been shown to home into regions of vascular injury induced by changes in tissue oxygenation, such as in regions adjacent to avascular retina in OIR.(129) Although stem and progenitor cells are being explored in other human diseases, their role in human ROP have yet to be determined. This line of inquiry is an exciting one to promote physiologic vascularization in order to reduce the hypoxic stimulus from broad areas of avascular retina once an infant is removed from high supplemental oxygen but warrants more study.(173)
C. Metabolism in the Developing Retina
Retinal photoreceptors are highly metabolically active. As photoreceptor transduction and connections with other retinal neurons develop, retinal metabolism increases.(9) Rods differentiate after cones and this differentiation proceeds to the peripheral retina. The oxygen demand is believed to drive physiologic retinal angiogenesis, but depending on other stresses, can result in disordered intravitreal vasoproliferation. Changes during development may help to explain cases of stuttering ROP with regression and newly formed Stage 3 disease.(65) Such cases are more common now as ever younger and more premature infants survive.
VI. Conclusion
Since the original observations of retrolental fibroplasia, many changes in technology, science, and clinical medicine have improved our understanding of the pathogenesis of retinopathy of prematurity. Technical advances in the ability to regulate environmental conditions of the premature infant have improved the survival of extremely premature infants. Scientific techniques to understand the molecular mechanisms involved in pathophysiology provide insight into the normal and pathologic developmental angiogenesis and are now exploring the role of neurovascular interactions in cognitive function and angiogenesis. Important clinical trials have provided information as to the role of oxygenation, screening and classification, and methods of treatment of severe ROP. It has become increasingly recognized that ROP differs worldwide and that there is a need to consider tailored screening and treatment approaches. Treatment of ROP remains an area of study. Targeted therapies to reduce aberrant vasoproliferation and facilitate physiologic retinal vascular development without harming the developing infant are needed.
VII. Methods of Literature Review
Pub Med Searches were performed and included articles from 1946 through 2016 for the terms “Retinopathy of Prematurity”, “Retrolental Fibroplasia”, and “Oxygen-Induced Retinopathy”. Abstracts were reviewed from all languages. Articles were read from English sources. From the abstracts, articles were reviewed that addressed areas of diagnosis, external or environmental stresses involved in the pathophysiology, classification, screening, treatment of severe ROP, genetics, oxygen or oxygenation, clinical studies. Specific studies regarding premature infant care and historical context of incubators were searched using Google.
Footnotes
VIII Disclosure
The author reports no proprietary or commercial inteerst in an product mentioned or concept discussed in this article. The author is Principal Investigator for NEI R01 grants EY015130 and EY017011 and a grant from March of Dimes, 6-FY13-75.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.An international classification of retinopathy of prematurity. The Committee for the Classification of Retinopathy of Prematurity. Archives of ophthalmology. 1984 Aug;102(8):1130–4. doi: 10.1001/archopht.1984.01040030908011. Epub 1984/08/01. eng. [DOI] [PubMed] [Google Scholar]
- 2.Multicenter trial of cryotherapy for retinopathy of prematurity. Preliminary results. Cryotherapy for Retinopathy of Prematurity Cooperative Group. Arch Ophthalmol. 1988 Apr;106(4):471–9. doi: 10.1001/archopht.1988.01060130517027. Epub 1988/04/01. eng. [DOI] [PubMed] [Google Scholar]
- 3.Multicenter trial of cryotherapy for retinopathy of prematurity: Snellen visual acuity and structural outcome at 51/2 years after randomization. Archives of Ophthalmology. 1996;114:417–24. doi: 10.1001/archopht.1996.01100130413008. [DOI] [PubMed] [Google Scholar]
- 4.Supplemental Therapeutic Oxygen for Prethreshold Retinopathy of Prematurity (STOP-ROP), a randomized, controlled trial. I: primary outcomes. Pediatrics. 2000 Feb 1;105(2):295–310. doi: 10.1542/peds.105.2.295. [DOI] [PubMed] [Google Scholar]
- 5.The International Classification of Retinopathy of Prematurity revisited. Arch Ophthalmol. 2005 Jul;123(7):991–9. doi: 10.1001/archopht.123.7.991. Epub 2005/07/13. Eng. [DOI] [PubMed] [Google Scholar]
- 6.The photographic screening for retinopathy of prematurity study (photo-ROP). Primary outcomes. Retina. 2008 Mar;28(3 Suppl):S47–54. doi: 10.1097/IAE.0b013e31815e987f. Epub 2009/02/12. eng. [DOI] [PubMed] [Google Scholar]
- 7.Abbey AM, Besirli CG, Musch DC, et al. Evaluation of Screening for Retinopathy of Prematurity by ROPtool or a Lay Reader. Ophthalmology. 2016 Feb;123(2):385–90. doi: 10.1016/j.ophtha.2015.09.048. Epub 2015/12/19. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aher SM, Ohlsson A. Early versus late erythropoietin for preventing red blood cell transfusion in preterm and/or low birth weight infants. Cochrane Database Syst Rev. 2006;(3):CD004865. doi: 10.1002/14651858.CD004865.pub2. Epub 2006/07/21. eng. [DOI] [PubMed] [Google Scholar]
- 9.Akula JD, Hansen RM, Martinez-Perez ME, Fulton AB. Rod photoreceptor function predicts blood vessel abnormality in retinopathy of prematurity. Invest Ophthalmol Vis Sci. 2007 Sep;48(9):4351–9. doi: 10.1167/iovs.07-0204. Epub 2007/08/29. eng. [DOI] [PubMed] [Google Scholar]
- 10.Al-Ghamdi A, Albiani DA, Hodge WG, Clarke WN. Myopia and astigmatism in retinopathy of prematurity after treatment with cryotherapy or laser photocoagulation. Can J Ophthalmol. 2004 Aug;39(5):521–5. doi: 10.1016/s0008-4182(04)80142-x. Epub 2004/10/20. eng. [DOI] [PubMed] [Google Scholar]
- 11.Alleman BW, Bell EF, Li L, et al. Individual and center-level factors affecting mortality among extremely low birth weight infants. Pediatrics. 2013 Jul;132(1):e175–84. doi: 10.1542/peds.2012-3707. Epub 2013/06/12. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allen MC, Donohue PK, Dusman AE. The limit of viability--neonatal outcome of infants born at 22 to 25 weeks’ gestation. N Engl J Med. 1993 Nov 25;329(22):1597–601. doi: 10.1056/NEJM199311253292201. Epub 1993/11/25. eng. [DOI] [PubMed] [Google Scholar]
- 13.Alon T, Hemo I, Itin A, et al. Vascular endothelial growth factor acts as a survival factor for newly formed retinal vessels and has implications for retinopathy of prematurity. Nat Med. 1995 Oct;1(10):1024–8. doi: 10.1038/nm1095-1024. Epub 1995/10/01. eng. [DOI] [PubMed] [Google Scholar]
- 14.Ashton N, Ward B, Serpell G. Role of oxygen in the genesis of retrolental fibroplasia; a preliminary report. Br J Ophthalmol. 1953;37(9):513–20. doi: 10.1136/bjo.37.9.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ashton N, Ward B, Serpell G. Effect of oxygen on developing retinal vessels with particular reference to the problem of retrolental fibroplasia. Br J Ophthalmol. 1954 Jul;38(7):397–432. doi: 10.1136/bjo.38.7.397. Epub 1954/07/01. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ataer-Cansizoglu E, Bolon-Canedo V, Campbell JP, et al. Computer-Based Image Analysis for Plus Disease Diagnosis in Retinopathy of Prematurity: Performance of the “i-ROP” System and Image Features Associated With Expert Diagnosis. Translational vision science & technology. 2015 Nov;4(6):5. doi: 10.1167/tvst.4.6.5. Epub 2015/12/09. Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Avery RL. Bevacizumab (Avastin) for retinopathy of prematurity: wrong dose, wrong drug, or both? Journal of AAPOS : the official publication of the American Association for Pediatric Ophthalmology and Strabismus/American Association for Pediatric Ophthalmology and Strabismus. 2012 Feb;16(1):2–4. doi: 10.1016/j.jaapos.2011.11.002. Epub 2012/01/13. eng. [DOI] [PubMed] [Google Scholar]
- 18.Bai Y, Ma J-x, Guo J, et al. Mueller cell-derived VEGF is a significant contributor to retinal neovascularization. The Journal of Pathology. 2009;219(4):446–54. doi: 10.1002/path.2611. [DOI] [PubMed] [Google Scholar]
- 19.Baker JP. The incubator and the medical discovery of the premature infant. J Perinatol. 2000 Jul-Aug;20(5):321–8. doi: 10.1038/sj.jp.7200377. Epub 2000/08/02. eng. [DOI] [PubMed] [Google Scholar]
- 20.Balasubramanian M, Capone A, Jr, Hartnett ME, et al. The Photographic Screening for Retinopathy of Prematurity Study (Photo-ROP): study design and baseline characteristics of enrolled patients. Retina. 2006 Sep;26(7 Suppl):S4–10. doi: 10.1097/01.iae.0000244291.09499.88. Epub 2006/09/02. eng. [DOI] [PubMed] [Google Scholar]
- 21.Bartoli M, Platt DH, Lemtalsi T, et al. VEGF differentially activates STAT3 in microvascular endothelial cells. The FASEB Journal. 2003 Jun 17;:02–1084fje. doi: 10.1096/fj.02-1084fje. [DOI] [PubMed] [Google Scholar]
- 22.Baserga M, Kaur R, Hale MA, et al. Fetal growth restriction alters transcription factor binding and epigenetic mechanisms of renal 11beta-hydroxysteroid dehydrogenase type 2 in a sex-specific manner. American journal of physiology Regulatory, integrative and comparative physiology. 2010 Jul;299(1):R334–42. doi: 10.1152/ajpregu.00122.2010. Epub 2010/04/30. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brines M, Cerami A. Discovering erythropoietin’s extra-hematopoietic functions: biology and clinical promise. Kidney Int. 2006 Jul;70(2):246–50. doi: 10.1038/sj.ki.5001546. Epub 2006/06/02. eng. [DOI] [PubMed] [Google Scholar]
- 24.Brion LP, Bell EF, Raghuveer TS. Vitamin E supplementation for prevention of morbidity and mortality in preterm infants. Cochrane Database Syst Rev. 2003;(4):CD003665. doi: 10.1002/14651858.CD003665. Epub 2003/10/30. eng. [DOI] [PubMed] [Google Scholar]
- 25.Brooks SE, Gu X, Samuel S, et al. Reduced severity of oxygen-induced retinopathy in eNOS-deficient mice. Investigative Ophthalmology & Visual Science. 2001;42:222–8. [PubMed] [Google Scholar]
- 26.Brown DM, Nguyen QD, Marcus DM, et al. Long-term outcomes of ranibizumab therapy for diabetic macular edema: the 36-month results from two phase III trials: RISE and RIDE. Ophthalmology. 2013 Oct;120(10):2013–22. doi: 10.1016/j.ophtha.2013.02.034. Epub 2013/05/28. eng. [DOI] [PubMed] [Google Scholar]
- 27.Brown MS, Baron AE, France EK, Hamman RF. Association between higher cumulative doses of recombinant erythropoietin and risk for retinopathy of prematurity. Journal of American Association for Pediatric Ophthalmology and Strabismus. 2006 Apr;10(2):143–9. doi: 10.1016/j.jaapos.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 28.Buhimschi IA, Buhimschi CS, Pupkin M, Weiner CP. Beneficial impact of term labor: Nonenzymatic antioxidant reserve in the human fetus. American Journal of Obstetrics and Gynecology. 2003 Jul;189(1):181–8. doi: 10.1067/mob.2003.357. [DOI] [PubMed] [Google Scholar]
- 29.Byfield G, Budd S, Hartnett ME. The role of supplemental oxygen and JAK/STAT signaling in intravitreous neovascularization in a ROP rat model. Invest Ophthalmol Vis Sci. 2009 Jul;50(7):3360–5. doi: 10.1167/iovs.08-3256. Epub 2009/03/07. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cabrera MT, Freedman SF, Hartnett ME, et al. Real-time, computer-assisted quantification of plus disease in retinopathy of prematurity at the bedside. Ophthalmic surgery, lasers & imaging retina. 2014 Nov-Dec;45(6):542–8. doi: 10.3928/23258160-20141118-09. Epub 2014/11/26. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Campbell K. Intensive oxygen therapy as a possible cause of retrolental fibroplasia. A clinical approach. Medical Journal of Australia. 1951;2:48. [PubMed] [Google Scholar]
- 32.Chen J, Connor KM, Aderman CM, Smith LE. Erythropoietin deficiency decreases vascular stability in mice. J Clin Invest. 2008 Feb;118(2):526–33. doi: 10.1172/JCI33813. Epub 2008/01/26. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen J, Connor KM, Aderman CM, et al. Suppression of Retinal Neovascularization by Erythropoietin siRNA in a Mouse Model of Proliferative Retinopathy. Investigative Ophthalmology & Visual Science. 2009 Mar 1;50(3):1329–35. doi: 10.1167/iovs.08-2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen J, Smith LE. A double-edged sword: erythropoietin eyed in retinopathy of prematurity. J AAPOS. 2008 Jun;12(3):221–2. doi: 10.1016/j.jaapos.2008.02.001. Epub 2008/04/09. eng. [DOI] [PubMed] [Google Scholar]
- 35.Chen SN, Lian I, Hwang YC, et al. Intravitreal anti-vascular endothelial growth factor treatment for retinopathy of prematurity: comparison between Ranibizumab and Bevacizumab. Retina. 2015 Apr;35(4):667–74. doi: 10.1097/IAE.0000000000000380. Epub 2014/12/03. eng. [DOI] [PubMed] [Google Scholar]
- 36.Chen YH, Chen SN, Lien RI, et al. Refractive errors after the use of bevacizumab for the treatment of retinopathy of prematurity: 2-year outcomes. Eye (London, England) 2014 Sep;28(9):1080–6. doi: 10.1038/eye.2014.172. quiz 7. Epub 2014/08/12. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coats DK. Retinopathy of prematurity: involution, factors predisposing to retinal detachment, and expected utility of preemptive surgical reintervention. Trans Am Ophthalmol Soc. 2005;103:281–312. Epub 2006/10/24. eng. [PMC free article] [PubMed] [Google Scholar]
- 38.Connolly BP, Ng EY, McNamara JA, et al. A comparison of laser photocoagulation with cryotherapy for threshold retinopathy of prematurity at 10 years: part 2. Refractive outcome. Ophthalmology. 2002 May;109(5):936–41. doi: 10.1016/s0161-6420(01)01015-6. Epub 2002/05/03. eng. [DOI] [PubMed] [Google Scholar]
- 39.Connor KM, SanGiovanni JP, Lofqvist C, et al. Increased dietary intake of [omega]-3-polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nat Med. 2007 Jul;13(7):868–73. doi: 10.1038/nm1591. print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cunningham S, Fleck BW, Elton RA, Mclntosh N. Transcutaneous oxygen levels in retinopathy of prematurity. Lancet. 1995;346:1464–5. doi: 10.1016/s0140-6736(95)92475-2. [DOI] [PubMed] [Google Scholar]
- 41.Dailey WA, Gryc W, Garg PG, Drenser KA. Frizzled-4 Variations Associated with Retinopathy and Intrauterine Growth Retardation: A Potential Marker for Prematurity and Retinopathy. Ophthalmology. 2015 Sep;122(9):1917–23. doi: 10.1016/j.ophtha.2015.05.036. Epub 2015/06/30. eng. [DOI] [PubMed] [Google Scholar]
- 42.Daniel E, Quinn GE, Hildebrand PL, et al. Validated System for Centralized Grading of Retinopathy of Prematurity: Telemedicine Approaches to Evaluating Acute-Phase Retinopathy of Prematurity (e-ROP) Study. JAMA ophthalmology. 2015 Jun;133(6):675–82. doi: 10.1001/jamaophthalmol.2015.0460. Epub 2015/03/27. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Davitt BV, Quinn GE, Wallace DK, et al. Astigmatism progression in the early treatment for retinopathy of prematurity study to 6 years of age. Ophthalmology. 2011 Dec;118(12):2326–9. doi: 10.1016/j.ophtha.2011.06.006. Epub 2011/08/30. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dubis AM, Subramaniam CD, Godara P, et al. Subclinical macular findings in infants screened for retinopathy of prematurity with spectral-domain optical coherence tomography. Ophthalmology. 2013 Aug;120(8):1665–71. doi: 10.1016/j.ophtha.2013.01.028. Epub 2013/05/16. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Early Treatment for Retinopathy of Prematurity Cooperative Group. Revised indications for the treatment of retinopathy of prematurity: results of the early treatment for retinopathy of prematurity randomized trial. Archives of Ophthalmology. 2003 Dec 1;121(12):1684–94. doi: 10.1001/archopht.121.12.1684. [DOI] [PubMed] [Google Scholar]
- 46.Ells A, Guernsey DL, Wallace K, et al. Severe retinopathy of prematurity associated with FZD4 mutations. Ophthalmic Genet. 2010 Mar;31(1):37–43. doi: 10.3109/13816810903479834. Epub 2010/02/10. eng. [DOI] [PubMed] [Google Scholar]
- 47.Ells AL, Holmes JM, Astle WF, et al. Telemedicine approach to screening for severe retinopathy of prematurity: a pilot study. Ophthalmology. 2003 Nov;110(11):2113–7. doi: 10.1016/S0161-6420(03)00831-5. Epub 2003/11/05. eng. [DOI] [PubMed] [Google Scholar]
- 48.Fierson WM. Screening examination of premature infants for retinopathy of prematurity. Pediatrics. 2013 Jan;131(1):189–95. doi: 10.1542/peds.2012-2996. Epub 2013/01/02. eng. [DOI] [PubMed] [Google Scholar]
- 49.Fierson WM, Capone A., Jr Telemedicine for evaluation of retinopathy of prematurity. Pediatrics. 2015 Jan;135(1):e238–54. doi: 10.1542/peds.2014-0978. Epub 2014/12/31. Eng. [DOI] [PubMed] [Google Scholar]
- 50.Fijalkowski N, Zheng LL, Henderson MT, et al. Stanford University Network for Diagnosis of Retinopathy of Prematurity (SUNDROP): five years of screening with telemedicine. Ophthalmic surgery, lasers & imaging retina. 2014 Mar-Apr;45(2):106–13. doi: 10.3928/23258160-20140122-01. Epub 2014/01/22. eng. [DOI] [PubMed] [Google Scholar]
- 51.Finer NN, Carlo WA, Walsh MC, et al. Early CPAP versus surfactant in extremely preterm infants. N Engl J Med. 2010 May 27;362(21):1970–9. doi: 10.1056/NEJMoa0911783. Epub 2010/05/18. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Flynn JT. Retinopathy of prematurity. Pediatric Clinics of North America. 1987;34(6):1487–516. doi: 10.1016/s0031-3955(16)36370-2. [DOI] [PubMed] [Google Scholar]
- 53.Fukushima Y, Okada M, Kataoka H, et al. Sema3E-PlexinD1 signaling selectively suppresses disoriented angiogenesis in ischemic retinopathy in mice. J Clin Invest. 2011 May;121(5):1974–85. doi: 10.1172/JCI44900. Epub 2011/04/21. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gilbert C. Retinopathy of prematurity: A global perspective of the epidemics, population of babies at risk and implications for control. Early Human Development. 2008 Feb;84(2):77–82. doi: 10.1016/j.earlhumdev.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 55.Gilbert C, Rahi J, Eckstein M, et al. Retinopathy of prematurity in middle-income countries. Lancet. 1997;350:12–4. doi: 10.1016/S0140-6736(97)01107-0. [DOI] [PubMed] [Google Scholar]
- 56.Good WV, Hardy RJ, Dobson V, et al. Final visual acuity results in the early treatment for retinopathy of prematurity study. Arch Ophthalmol. 2010 Jun;128(6):663–71. doi: 10.1001/archophthalmol.2010.72. Epub 2010/04/14. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Grant MB, May WS, Caballero S, et al. Adult hematopoietic stem cells provide functional hemangioblast activity during retinal neovascularization. Nature Medicine. 2002;8(6):607–12. doi: 10.1038/nm0602-607. [DOI] [PubMed] [Google Scholar]
- 58.Grishanin RN, Yang H, Liu X, et al. Retinal TrkB receptors regulate neural development in the inner, but not outer, retina. Mol Cell Neurosci. 2008 Jul;38(3):431–43. doi: 10.1016/j.mcn.2008.04.004. Epub 2008/05/31. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gu X, El-Remessy AB, Brooks SE, et al. Hyperoxia induces retinal vascular endothelial cell apoptosis through formation of peroxynitrite. Am J Physiol Cell Physiol. 2003 Sep;285(3):C546–54. doi: 10.1152/ajpcell.00424.2002. Epub 2003/05/09. eng. [DOI] [PubMed] [Google Scholar]
- 60.Haigh JJ. Role of VEGF in organogenesis. Organogenesis. 2008 Oct;4(4):247–56. doi: 10.4161/org.4.4.7415. Epub 2009/04/02. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hammer DX, Iftimia NV, Ferguson RD, et al. Foveal fine structure in retinopathy of prematurity: an adaptive optics Fourier domain optical coherence tomography study. Invest Ophthalmol Vis Sci. 2008 May;49(5):2061–70. doi: 10.1167/iovs.07-1228. Epub 2008/01/29. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hardy RJ, Palmer EA, Schaffer DB, et al. Outcome-based management of retinopathy of prematurity. Multicenter Trial of Cryotherapy for Retinopathy of prematurity Cooperative Group. Journal of AAPOS : the official publication of the American Association for Pediatric Ophthalmology and Strabismus/American Association for Pediatric Ophthalmology and Strabismus. 1997 Mar;1(1):46–54. doi: 10.1016/s1091-8531(97)90023-9. Epub 1997/03/01. eng. [DOI] [PubMed] [Google Scholar]
- 63.Hartnett ME. Features associated with surgical outcome in patients with stages 4 and 5 retinopathy of prematurity. Retina. 2003;23(3):322–9. doi: 10.1097/00006982-200306000-00006. [DOI] [PubMed] [Google Scholar]
- 64.Hartnett ME. Studies on the pathogenesis of avascular retina and neovascularization into the vitreous in peripheral severe retinopathy of prematurity (an american ophthalmological society thesis) Transactions of the American Ophthalmological Society. 2010;108:96–119. [PMC free article] [PubMed] [Google Scholar]
- 65.Hartnett ME. Retinopathy of prematurity: a template for studying retinal vascular disease. In: Chalupa JSWaLM., editor. The New Visual Neurosciences. Translational Visual Neuroscience. Vol. 1. Cambridge MA: MIT Press; 2014. pp. 1483–502. [Google Scholar]
- 66.Hartnett ME. Vascular endothelial growth factor antagonist therapy for retinopathy of prematurity. Clin Perinatol. 2014 Dec;41(4):925–43. doi: 10.1016/j.clp.2014.08.011. Epub 2014/12/03. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hartnett ME. Pathophysiology and mechanisms of severe retinopathy of prematurity. Ophthalmology. 2015 Jan;122(1):200–10. doi: 10.1016/j.ophtha.2014.07.050. Epub 2014/12/03. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hartnett ME, Cotten CM. Genomics in the neonatal nursery: Focus on ROP. Semin Perinatol. 2015 Dec;39(8):604–10. doi: 10.1053/j.semperi.2015.09.007. Epub 2015/10/20. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hartnett ME, DeAngelis MM. Studies on Retinal and Choroidal Disorders. In: Armstrong D, editor. Studies on Retinal and Choroidal Disorders. 1. New York: Humana Press; 2012. pp. 559–84. [Google Scholar]
- 70.Hartnett ME, Lane RH. Effects of oxygen on the development and severity of retinopathy of prematurity. J AAPOS. 2013 Jun;17(3):229–34. doi: 10.1016/j.jaapos.2012.12.155. Epub 2013/06/26. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hartnett ME, Maguluri S, Thompson HW, McColm JR. Comparison of retinal outcomes after scleral buckle or lens-sparing vitrectomy for stage 4 retinopathy of prematurity. Retina. 2004 Oct;24(5):753–7. doi: 10.1097/00006982-200410000-00011. Epub 2004/10/20. eng. [DOI] [PubMed] [Google Scholar]
- 72.Hartnett ME, Martiniuk D, Byfield G, et al. Neutralizing VEGF decreases tortuosity and alters endothelial cell division orientation in arterioles and veins in a rat model of ROP: relevance to plus disease. Invest Ophthalmol Vis Sci. 2008 Jul;49(7):3107–14. doi: 10.1167/iovs.08-1780. Epub 2008/04/02. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hartnett ME, McColm JR. Retinal features predictive of progression to stage 4 ROP. Retina. 2004;24(2):237–41. doi: 10.1097/00006982-200404000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hartnett ME, Morrison MA, Smith S, et al. Genetic variants associated with severe retinopathy of prematurity in extremely low birth weight infants. Invest Ophthalmol Vis Sci. 2014 Oct;55(10):6194–203. doi: 10.1167/iovs.14-14841. Epub 2014/08/15. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hartnett ME, Penn JS. Mechanisms and Management of Retinopathy of Prematurity. New England Journal of Medicine. 2012 Dec 26;367(26):2515–26. doi: 10.1056/NEJMra1208129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hauspurg AK, Allred EN, Vanderveen DK, et al. Blood Gases and Retinopathy of Prematurity: The ELGAN Study. Neonatology. 2011;99(2):104–11. doi: 10.1159/000308454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hellgren G, Willett K, Engstrom E, et al. Proliferative retinopathy is associated with impaired increase in BDNF and RANTES expression levels after preterm birth. Neonatology. 2010;98(4):409–18. doi: 10.1159/000317779. Epub 2010/11/11. eng. [DOI] [PubMed] [Google Scholar]
- 78.Hellstrom A, Carlsson B, Niklasson A, et al. IGF-I is critical for normal vascularization of the human retina. Journal of Clinical Endocrinology Metabolism. 2002 Jul 1;87(7):3413–6. doi: 10.1210/jcem.87.7.8629. [DOI] [PubMed] [Google Scholar]
- 79.Hellstrom A, Engstrom E, Hard AL, et al. Postnatal serum insulin-like growth factor I deficiency is associated with retinopathy of prematurity and other complications of premature birth. Pediatrics. 2003 Nov;112(5):1016–20. doi: 10.1542/peds.112.5.1016. Epub 2003/11/05. eng. [DOI] [PubMed] [Google Scholar]
- 80.Hellstrom A, Perruzzi C, Ju M, et al. Low IGF-I suppresses VEGF-survival signaling in retinal endothelial cells: direct correlation with clinical retinopathy of prematurity. Proc Natl Acad Sci U S A. 2001 May 8;98(10):5804–8. doi: 10.1073/pnas.101113998. Epub 2001/05/02. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hendrickson AE, Yuodelis C. The morphological development of the human fovea. Ophthalmology. 1984;91(6):603–12. doi: 10.1016/s0161-6420(84)34247-6. [DOI] [PubMed] [Google Scholar]
- 82.Higgins RD, Mendelsohn AL, DeFeo MJ, et al. Antenatal dexamethasone and decreased severity of retinopathy of prematurity. Archives of Ophthalmology. 1998;116:601–5. doi: 10.1001/archopht.116.5.601. [DOI] [PubMed] [Google Scholar]
- 83.Hoppe G, Yoon S, Gopalan B, et al. Comparative systems pharmacology of HIF stabilization in the prevention of retinopathy of prematurity. Proc Natl Acad Sci U S A. 2016 May 3;113(18):E2516–25. doi: 10.1073/pnas.1523005113. Epub 2016/04/20. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Howlett A, Ohlsson A. Inositol for respiratory distress syndrome in preterm infants. Cochrane Database Systematic Reviews. 2000;2000(4):CD000366. doi: 10.1002/14651858.CD000366. [DOI] [PubMed] [Google Scholar]
- 85.Hu J, Blair MP, Shapiro MJ, et al. Reactivation of retinopathy of prematurity after bevacizumab injection. Arch Ophthalmol. 2012 Aug;130(8):1000–6. doi: 10.1001/archophthalmol.2012.592. Epub 2012/04/12. eng. [DOI] [PubMed] [Google Scholar]
- 86.Hutcheson KA, Nguyen AT, Preslan MW, et al. Vitreous hemorrhage in patients with high-risk retinopathy of prematurity. Am J Ophthalmol. 2003 Aug;136(2):258–63. doi: 10.1016/s0002-9394(03)00190-9. Epub 2003/07/31. eng. [DOI] [PubMed] [Google Scholar]
- 87.Hutchinson AK, Melia M, Yang MB, et al. Clinical Models and Algorithms for the Prediction of Retinopathy of Prematurity: A Report by the American Academy of Ophthalmology. Ophthalmology. 2016 Apr;123(4):804–16. doi: 10.1016/j.ophtha.2015.11.003. Epub 2016/02/03. eng. [DOI] [PubMed] [Google Scholar]
- 88.Ishida S, Yamashiro K, Usui T, et al. Leukocytes mediate retinal vascular remodeling during development and vaso-obliteration in disease. Nat Med. 2003 Jun;9(6):781–8. doi: 10.1038/nm877. Epub 2003/05/06. eng. [DOI] [PubMed] [Google Scholar]
- 89.Jiang Y, Wang H, Culp D, et al. Targeting Muller cell-derived VEGF164 to reduce intravitreal neovascularization in the rat model of retinopathy of prematurity. Invest Ophthalmol Vis Sci. 2014 Feb;55(2):824–31. doi: 10.1167/iovs.13-13755. Epub 2014/01/16. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jorge EC, Jorge EN, El Dib RP. Early light reduction for preventing retinopathy of prematurity in very low birth weight infants. The Cochrane database of systematic reviews. 2013;(8):CD000122. doi: 10.1002/14651858.CD000122.pub2. Epub 2013/08/08. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Joyal JS, Nim S, Zhu T, et al. Subcellular localization of coagulation factor II receptor-like 1 in neurons governs angiogenesis. Nat Med. 2014 Oct;20(10):1165–73. doi: 10.1038/nm.3669. Epub 2014/09/14. eng. [DOI] [PubMed] [Google Scholar]
- 92.Joyal JS, Sitaras N, Binet F, et al. Ischemic neurons prevent vascular regeneration of neural tissue by secreting semaphorin 3A. Blood. 2011 Jun 2;117(22):6024–35. doi: 10.1182/blood-2010-10-311589. Epub 2011/03/01. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Juul SE, Mayock DE, Comstock BA, Heagerty PJ. Neuroprotective potential of erythropoietin in neonates; design of a randomized trial. Maternal health, neonatology and perinatology. 2015;1:27. doi: 10.1186/s40748-015-0028-z. Epub 2015/01/01. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kaelin WG, Jr, Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell. 2008 May 23;30(4):393–402. doi: 10.1016/j.molcel.2008.04.009. Epub 2008/05/24. eng. [DOI] [PubMed] [Google Scholar]
- 95.Kase S, He S, Sonoda S, et al. alphaB-crystallin regulation of angiogenesis by modulation of VEGF. Blood. 2010 Apr 22;115(16):3398–406. doi: 10.1182/blood-2009-01-197095. Epub 2009/12/22. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kondo H, Kusaka S, Yoshinaga A, et al. Genetic variants of FZD4 and LRP5 genes in patients with advanced retinopathy of prematurity. Mol Vis. 2013;19:476–85. Epub 2013/02/27. eng. [PMC free article] [PubMed] [Google Scholar]
- 97.Lakshminrusimha S, Manja V, Mathew B, Suresh GK. Oxygen targeting in preterm infants: a physiological interpretation. J Perinatol. 2015 Jan;35(1):8–15. doi: 10.1038/jp.2014.199. Epub 2014/10/31. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ley D, Hansen-Pupp I, Niklasson A, et al. Longitudinal infusion of a complex of insulin-like growth factor-I and IGF-binding protein-3 in five preterm infants: pharmacokinetics and short-term safety. Pediatr Res. 2013 Jan;73(1):68–74. doi: 10.1038/pr.2012.146. Epub 2012/10/26. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liegl R, Hellstrom A, Smith LE. Retinopathy of prematurity: the need for prevention. Eye and Brain. 2016;(8):91–102. doi: 10.2147/EB.S99038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lofqvist C, Andersson E, Sigurdsson J, et al. Longitudinal Postnatal Weight and Insulin-like Growth Factor I Measurements in the Prediction of Retinopathy of Prematurity. Archives of Ophthalmology. 2006 Dec 1;124(12):1711–8. doi: 10.1001/archopht.124.12.1711. [DOI] [PubMed] [Google Scholar]
- 101.Lofqvist C, Hansen-Pupp I, Andersson E, et al. Validation of a new retinopathy of prematurity screening method monitoring longitudinal postnatal weight and insulinlike growth factor I. Arch Ophthalmol. 2009 May;127(5):622–7. doi: 10.1001/archophthalmol.2009.69. Epub 2009/05/13. eng. [DOI] [PubMed] [Google Scholar]
- 102.Lunze K, Bloom DE, Jamison DT, Hamer DH. The global burden of neonatal hypothermia: systematic review of a major challenge for newborn survival. BMC Med. 2013;11:24. doi: 10.1186/1741-7015-11-24. Epub 2013/02/02. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lyu Y, Shah PS, Ye XY, et al. Association between admission temperature and mortality and major morbidity in preterm infants born at fewer than 33 weeks’ gestation. JAMA pediatrics. 2015 Apr;169(4):e150277. doi: 10.1001/jamapediatrics.2015.0277. Epub 2015/04/07. eng. [DOI] [PubMed] [Google Scholar]
- 104.Maguire AM, Trese MT. Lens-sparing vitreoretinal surgery in infants. Arch Ophthalmol. 1992 Feb;110(2):284–6. doi: 10.1001/archopht.1992.01080140140042. Epub 1992/02/01. eng. [DOI] [PubMed] [Google Scholar]
- 105.Maldonado RS, O’Connell R, Ascher SB, et al. Spectral-domain optical coherence tomographic assessment of severity of cystoid macular edema in retinopathy of prematurity. Arch Ophthalmol. 2012 May;130(5):569–78. doi: 10.1001/archopthalmol.2011.1846. Epub 2012/01/11. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Manja V, Lakshminrusimha S, Cook DJ. Oxygen saturation target range for extremely preterm infants: a systematic review and meta-analysis. JAMA pediatrics. 2015 Apr;169(4):332–40. doi: 10.1001/jamapediatrics.2014.3307. Epub 2015/02/11. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Manley BJ, Kuschel CA, Elder JE, et al. Higher Rates of Retinopathy of Prematurity after Increasing Oxygen Saturation Targets for Very Preterm Infants: Experience in a Single Center. J Pediatr. 2016 Jan;168:242–4. doi: 10.1016/j.jpeds.2015.10.005. Epub 2015/11/10. eng. [DOI] [PubMed] [Google Scholar]
- 108.Manzoni P, Guardione R, Bonetti P, et al. Lutein and zeaxanthin supplementation in preterm very low-birth-weight neonates in neonatal intensive care units: a multicenter randomized controlled trial. Am J Perinatol. 2013 Jan;30(1):25–32. doi: 10.1055/s-0032-1321494. Epub 2012/07/10. eng. [DOI] [PubMed] [Google Scholar]
- 109.McCloskey M, Wang H, Jiang Y, et al. Anti-VEGF Antibody Leads to Later Atypical Intravitreous Neovascularization and Activation of Angiogenic Pathways in a Rat Model of Retinopathy of Prematurity. Invest Ophthalmol Vis Sci. 2013;54(3):2020–6. doi: 10.1167/iovs.13-11625. Epub 2013/03/02. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.McColm JR, Geisen P, Hartnett ME. VEGF isoforms and their expression after a single episode of hypoxia or repeated fluctuations between hyperoxia and hypoxia: Relevance to clinical ROP. Molecular Vision. 2004;10:512–20. [PMC free article] [PubMed] [Google Scholar]
- 111.McLeod DS, D’Anna SA, Lutty GA. Clinical and histopathologic features of canine oxygen-induced proliferative retinopathy. Investigative Ophthalmology Visual Science. 1998 Sep 1;39(10):1918–32. [PubMed] [Google Scholar]
- 112.Mintz-Hittner HA, Geloneck MM, Chuang AZ. Clinical Management of Recurrent Retinopathy of Prematurity after Intravitreal Bevacizumab Monotherapy. Ophthalmology. 2016 Sep;123(9):1845–55. doi: 10.1016/j.ophtha.2016.04.028. Epub 2016/06/01. Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mintz-Hittner HA, Kennedy KA, Chuang AZ. Efficacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurity. N Engl J Med. 2011 Feb 17;364(7):603–15. doi: 10.1056/NEJMoa1007374. Epub 2011/02/18. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Morin J, Luu TM, Superstein R, et al. Neurodevelopmental Outcomes Following Bevacizumab Injections for Retinopathy of Prematurity. Pediatrics. 2016 Apr;137(4) doi: 10.1542/peds.2015-3218. Epub 2016/06/01. eng. [DOI] [PubMed] [Google Scholar]
- 115.Myers AC, Lovestam Adrian M, Bruun A, et al. Retinal function and morphology in rabbit after intravitreal injection of VEGF inhibitors. Curr Eye Res. 2012 May;37(5):399–407. doi: 10.3109/02713683.2011.611609. Epub 2012/04/19. eng. [DOI] [PubMed] [Google Scholar]
- 116.Nagata M. Therapeutic possibility in retrolental fibroplasia in the premature infant with light coagulation. Ganka Ophthalmology. 1968 Oct;10(10):719–27. Epub 1968/10/01. jpn. [PubMed] [Google Scholar]
- 117.Nakano M, Satoh K, Fukumoto Y, et al. Important role of erythropoietin receptor to promote VEGF expression and angiogenesis in peripheral ischemia in mice. Circ Res. 2007 Mar 16;100(5):662–9. doi: 10.1161/01.RES.0000260179.43672.fe. Epub 2007/02/13. eng. [DOI] [PubMed] [Google Scholar]
- 118.Narayanan SP, Suwanpradid J, Saul A, et al. Arginase 2 deletion reduces neuroglial injury and improves retinal function in a model of retinopathy of prematurity. PLoS One. 2011;6(7):e22460. doi: 10.1371/journal.pone.0022460. Epub 2011/08/04. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Niesman MR, Johnson KA, Penn JS. Therapeutic effect of liposomal superoxide dismutase in an animal model of retinopathy of prematurity. Neurochemical Research. 1997;22(5):597–605. doi: 10.1023/a:1022474120512. [DOI] [PubMed] [Google Scholar]
- 120.Nudleman E, Robinson J, Rao P, et al. Long-term outcomes on lens clarity after lens-sparing vitrectomy for retinopathy of prematurity. Ophthalmology. 2015 Apr;122(4):755–9. doi: 10.1016/j.ophtha.2014.11.004. Epub 2015/01/21. eng. [DOI] [PubMed] [Google Scholar]
- 121.Ohls RK, Christensen RD, Kamath-Rayne BD, et al. A Randomized, Masked, Placebo-Controlled Study of Darbepoetin Alfa in Preterm Infants. Pediatrics. 2013 Jun 17; doi: 10.1542/peds.2013-0143. Epub 2013/06/19. Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Owen L, Hartnett ME. Current Concepts of Oxygen Management and ROP. Journal of Ophthalmic & Vision Research. 2014 Jan;2014(January 2014):94–100. [PMC free article] [PubMed] [Google Scholar]
- 123.Park TS, Bhutto I, Zimmerlin L, et al. Vascular progenitors from cord blood-derived induced pluripotent stem cells possess augmented capacity for regenerating ischemic retinal vasculature. Circulation. 2014 Jan 21;129(3):359–72. doi: 10.1161/CIRCULATIONAHA.113.003000. Epub 2013/10/29. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Patel S, Ohls RK. Darbepoetin Administration in Term and Preterm Neonates. Clin Perinatol. 2015 Sep;42(3):557–66. doi: 10.1016/j.clp.2015.04.016. Epub 2015/08/08. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Patz A. The role of oxygen in retrolental fibroplasia. Trans Am Ophthalmol Soc. 1968;66:940–85. [PMC free article] [PubMed] [Google Scholar]
- 126.Patz A, Eastham A, Higginbotham DH, Kleh T. Oxygen studies in retrolental fibroplasia. II. The production of the microscopic changes of retrolental fibroplasia in experimental animals. Am J Ophthalmol. 1953 Nov;36(11):1511–22. Epub 1953/11/01. eng. [PubMed] [Google Scholar]
- 127.Penn JS. Oxygen-induced retinopathy in the rat. Vitamins C and E as potential therapies. Investigative Ophthalmology & Visual Science. 1992;33:1836–45. [PubMed] [Google Scholar]
- 128.Penn JS, Tolman BL, Lowery LA. Variable oxygen exposure causes preretinal neovascularisation in the newborn rat. Investigative Ophthalmology & Visual Science. 1993;34:576–85. [PubMed] [Google Scholar]
- 129.Prasain N, Lee MR, Vemula S, et al. Differentiation of human pluripotent stem cells to cells similar to cord-blood endothelial colony-forming cells. Nat Biotechnol. 2014 Nov;32(11):1151–7. doi: 10.1038/nbt.3048. Epub 2014/10/13. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Quinn GE. Retinopathy of prematurity blindness worldwide: phenotypes in the third epidemic. Eye and Brain. 2016;(8):31–6. doi: 10.2147/EB.S94436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Quinn GE, Barr C, Bremer D, et al. Changes in Course of Retinopathy of Prematurity from 1986 to 2013: Comparison of Three Studies in the United States. Ophthalmology. 2016 Jul;123(7):1595–600. doi: 10.1016/j.ophtha.2016.03.026. Epub 2016/04/17. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Quinn GE, Dobson V, Biglan A, et al. Correlation of retinopathy of prematurity in fellow eyes in the cryotherapy for retinopathy of prematurity study. The Cryotherapy for Retinopathy of Prematurity Cooperative Group. Archives of Ophthalmology. 1995;113(4):469–73. doi: 10.1001/archopht.1995.01100040089032. [DOI] [PubMed] [Google Scholar]
- 133.Quinn GE, Dobson V, Hardy RJ, et al. Visual fields measured with double-arc perimetry in eyes with threshold retinopathy of prematurity from the Cryotherapy for Retinopathy of Prematurity Trial. Ophthalmology. 1996;103:1432–7. doi: 10.1016/s0161-6420(96)30487-9. [DOI] [PubMed] [Google Scholar]
- 134.Quinn GE, Ying GS, Daniel E, et al. Validity of a telemedicine system for the evaluation of acute-phase retinopathy of prematurity. JAMA ophthalmology. 2014 Oct;132(10):1178–84. doi: 10.1001/jamaophthalmol.2014.1604. Epub 2014/06/28. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rao R, Mashburn CB, Mao J, et al. Brain-derived neurotrophic factor in infants <32 weeks gestational age: correlation with antenatal factors and postnatal outcomes. Pediatr Res. 2009 May;65(5):548–52. doi: 10.1203/PDR.0b013e31819d9ea5. Epub 2009/02/05. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Rao S, Chun C, Fan J, et al. A direct and melanopsin-dependent fetal light response regulates mouse eye development. Nature. 2013 Feb 14;494(7436):243–6. doi: 10.1038/nature11823. Epub 2013/01/22. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Reddy MA, Patel HI, Karim SM, et al. Reduced utility of serum IGF-1 levels in predicting retinopathy of prematurity reflects maternal ethnicity. Br J Ophthalmol. 2016 Apr;100(4):501–4. doi: 10.1136/bjophthalmol-2015-307234. Epub 2015/08/26. eng. [DOI] [PubMed] [Google Scholar]
- 138.Reese AB, Stepanik J. Cicatricial stage of retrolental fibroplasia. Am J Ophthalmol. 1954 Sep;38(3):308–16. doi: 10.1016/0002-9394(54)90846-6. Epub 1954/09/01. Eng. [DOI] [PubMed] [Google Scholar]
- 139.Rey-Funes M, Dorfman VB, Ibarra ME, et al. Hypothermia prevents gliosis and angiogenesis development in an experimental model of ischemic proliferative retinopathy. Invest Ophthalmol Vis Sci. 2013 Apr;54(4):2836–46. doi: 10.1167/iovs.12-11198. Epub 2013/03/09. eng. [DOI] [PubMed] [Google Scholar]
- 140.Reynolds JD, Hardy RJ, Kennedy KA, et al. Lack of efficacy of light reduction in preventing retinopathy of prematurity. New England Journal of Medicine. 1998;338:1572–6. doi: 10.1056/NEJM199805283382202. [DOI] [PubMed] [Google Scholar]
- 141.Robertson AF. Reflections on errors in neonatology: I. The “Hands-Off” years, 1920 to 1950. J Perinatol. 2003 Jan;23(1):48–55. doi: 10.1038/sj.jp.7210842. Epub 2003/01/31. eng. [DOI] [PubMed] [Google Scholar]
- 142.Rothman AL, Tran-Viet D, Gustafson KE, et al. Poorer neurodevelopmental outcomes associated with cystoid macular edema identified in preterm infants in the intensive care nursery. Ophthalmology. 2015 Mar;122(3):610–9. doi: 10.1016/j.ophtha.2014.09.022. Epub 2014/12/03. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ruth A, Hutchinson AK, Baker Hubbard G. Late vitreous hemorrhage in patients with regressed retinopathy of prematurity. Journal of AAPOS : the official publication of the American Association for Pediatric Ophthalmology and Strabismus/American Association for Pediatric Ophthalmology and Strabismus. 2008 Apr;12(2):181–5. doi: 10.1016/j.jaapos.2007.09.008. Epub 2007/12/18. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Saint-Geniez M, Maharaj AS, Walshe TE, et al. Endogenous VEGF is required for visual function: evidence for a survival role on muller cells and photoreceptors. PLoS One. 2008;3(11):e3554. doi: 10.1371/journal.pone.0003554. Epub 2008/11/04. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Saito Y, Geisen P, Uppal A, Hartnett ME. Inhibition of NAD(P)H oxidase reduces apoptosis and avascular retina in an animal model of retinopathy of prematurity. Molecular Vision. 2007 Jun 12;13:840–53. [PMC free article] [PubMed] [Google Scholar]
- 146.Saito Y, Uppal A, Byfield G, et al. Activated NAD(P)H Oxidase from Supplemental Oxygen Induces Neovascularization Independent of VEGF in Retinopathy of Prematurity Model. Investigative Ophthalmology Visual Science. 2008 Apr 1;49(4):1591–8. doi: 10.1167/iovs.07-1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sapieha P, Sirinyan M, Hamel D, et al. The succinate receptor GPR91 in neurons has a major role in retinal angiogenesis. Nat Med. 2008 Oct;14(10):1067–76. doi: 10.1038/nm.1873. Epub 2008/10/07. eng. [DOI] [PubMed] [Google Scholar]
- 148.Sato Y, Oshiro M, Takemoto K, et al. Multicenter observational study comparing sedation/analgesia protocols for laser photocoagulation treatment of retinopathy of prematurity. J Perinatol. 2015 Nov;35(11):965–9. doi: 10.1038/jp.2015.112. Epub 2015/09/12. eng. [DOI] [PubMed] [Google Scholar]
- 149.Schaffer DB, Palmer EA, Plotsky DF, et al. Prognostic factors in the natural course of retinopathy of prematurity. Ophthalmology. 1993;100:230–7. doi: 10.1016/s0161-6420(93)31665-9. [DOI] [PubMed] [Google Scholar]
- 150.Schepens CL. A new ophthalmoscope demonstration. Trans Am Acad Ophthalmol Otolaryngol. 1947;51:298–301. [PubMed] [Google Scholar]
- 151.Schepens CL, Hartnett ME, Hirose T. Schepens’ Retinal Detachment and Allied Diseases. 2. Boston: Butterworth-Heinemann; 2000. [Google Scholar]
- 152.Schmidt B, Whyte RK, Asztalos EV, et al. Effects of targeting higher vs lower arterial oxygen saturations on death or disability in extremely preterm infants: a randomized clinical trial. JAMA. 2013 May 22;309(20):2111–20. doi: 10.1001/jama.2013.5555. [DOI] [PubMed] [Google Scholar]
- 153.Sears JE, Hoppe G, Ebrahem Q, Anand-Apte B. Prolyl hydroxylase inhibition during hyperoxia prevents oxygen-induced retinopathy. Proceedings of the National Academy of Sciences. 2008 Dec 16;105(50):19898–903. doi: 10.1073/pnas.0805817105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Shah P, Narendran V, Kalpana N, Gilbert C. Severe retinopathy of prematurity in big babies in India: History repeating itself? Indian J Pediatr. 2009;76(8):801–4. doi: 10.1007/s12098-009-0175-1. [DOI] [PubMed] [Google Scholar]
- 155.Shah PK, Narendran V, Kalpana N. Aggressive posterior retinopathy of prematurity in large preterm babies in South India. Arch Dis Child Fetal Neonatal Ed. 2012 Sep;97(5):F371–5. doi: 10.1136/fetalneonatal-2011-301121. Epub 2012/05/23. eng. [DOI] [PubMed] [Google Scholar]
- 156.Shih SC, Ju M, Liu N, Smith LEH. Selective stimulation of VEGFR-1 prevents oxygen-induced retinal vascular degeneration in retinopathy of prematurity. Journal of Clinical Investigation. 2003 Jul 1;112(1):50. doi: 10.1172/JCI17808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Simpson JL, Melia M, Yang MB, et al. Current role of cryotherapy in retinopathy of prematurity: a report by the American Academy of Ophthalmology. Ophthalmology. 2012 Apr;119(4):873–7. doi: 10.1016/j.ophtha.2012.01.003. Epub 2012/03/03. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Smith LEH, Kopchick JJ, Chen W, et al. Essential role of growth hormone in ischemia-induced retinal neovascularization. Science. 1997;276:1706–9. doi: 10.1126/science.276.5319.1706. [DOI] [PubMed] [Google Scholar]
- 159.Smith LEH, Wesolowski E, McLellan A, et al. Oxygen induced retinopathy in the mouse. Investigative Ophthalmology & Visual Science. 1994;35(1):101–11. [PubMed] [Google Scholar]
- 160.Snyder LL, Garcia-Gonzalez JM, Shapiro MJ, Blair MP. Very Late Reactivation of Retinopathy of Prematurity After Monotherapy With Intravitreal Bevacizumab. Ophthalmic Surg Lasers Imaging Retina. 2016 Mar;47(3):280–3. doi: 10.3928/23258160-20160229-12. Epub 2016/03/18. eng. [DOI] [PubMed] [Google Scholar]
- 161.Soetikno BT, Yi J, Shah R, et al. Inner retinal oxygen metabolism in the 50/10 oxygen-induced retinopathy model. Scientific reports. 2015;5:16752. doi: 10.1038/srep16752. Epub 2015/11/19. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Soghier LM, Brion LP. Cysteine, cystine or N-acetylcysteine supplementation in parenterally fed neonates. Cochrane Database Syst Rev. 2006;(4):CD004869. doi: 10.1002/14651858.CD004869.pub2. Epub 2006/10/21. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sood BG, Madan A, Saha S, et al. Perinatal systemic inflammatory response syndrome and retinopathy of prematurity. Pediatr Res. 2010 Apr;67(4):394–400. doi: 10.1203/PDR.0b013e3181d01a36. Epub 2009/12/25. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Stone J, Itin A, Alon T, et al. Development of retinal vasculature is mediated by hypoxia-induced vascular endothelial growth factor (VEGF) expression by neuroglia. Journal of Neuroscience. 1995;15:4738–47. doi: 10.1523/JNEUROSCI.15-07-04738.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Suwanpradid J, Rojas M, Behzadian MA, et al. Arginase 2 deficiency prevents oxidative stress and limits hyperoxia-induced retinal vascular degeneration. PLoS One. 2014;9(11):e110604. doi: 10.1371/journal.pone.0110604. Epub 2014/11/07. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Szewczyk TS. Retrolental fibroplasia: etiology and prophylaxis; a preliminary report. Am J Ophthalmol. 1951 Dec;34(12):1649–50. doi: 10.1016/0002-9394(51)90028-1. Epub 1951/12/01. eng. [DOI] [PubMed] [Google Scholar]
- 167.Tarnow-Mordi W, Stenson B, Kirby A, et al. Outcomes of Two Trials of Oxygen-Saturation Targets in Preterm Infants. N Engl J Med. 2016 Feb 25;374(8):749–60. doi: 10.1056/NEJMoa1514212. Epub 2016/02/11. eng. [DOI] [PubMed] [Google Scholar]
- 168.Terry TL. Retrolental fibroplasia. J Pediatr. 1946 Dec;29(6):770–3. doi: 10.1016/s0022-3476(46)80009-x. Epub 1946/12/01. eng. [DOI] [PubMed] [Google Scholar]
- 169.Thoresen M. Hypothermia after perinatal asphyxia: selection for treatment and cooling protocol. J Pediatr. 2011 Feb;158(2 Suppl):e45–9. doi: 10.1016/j.jpeds.2010.11.013. Epub 2011/02/02. eng. [DOI] [PubMed] [Google Scholar]
- 170.Tokunaga CC, Mitton KP, Dailey W, et al. Effects of anti-VEGF treatment on the recovery of the developing retina following oxygen-induced retinopathy. Invest Ophthalmol Vis Sci. 2014 Mar;55(3):1884–92. doi: 10.1167/iovs.13-13397. Epub 2014/02/20. eng. [DOI] [PubMed] [Google Scholar]
- 171.Tong AY, El-Dairi M, Maldonado RS, et al. Evaluation of optic nerve development in preterm and term infants using handheld spectral-domain optical coherence tomography. Ophthalmology. 2014 Sep;121(9):1818–26. doi: 10.1016/j.ophtha.2014.03.020. Epub 2014/05/09. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Trichonas G, Lee TJ, Hoppe G, et al. Prolyl hydroxylase inhibition during hyperoxia prevents oxygen-induced retinopathy in the rat 50/10 model. Invest Ophthalmol Vis Sci. 2013 Jul;54(7):4919–26. doi: 10.1167/iovs.13-12171. Epub 2013/06/14. eng. [DOI] [PubMed] [Google Scholar]
- 173.Tring TL, Calzi SL, Shaw LC, et al. Promoting vascular repair in the retina: can stem/progenitor cells help? Eye and Brain. 2016;(8):113–22. doi: 10.2147/EB.S94451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Uno K, Prow TW, Bhutto IA, et al. Role of Nrf2 in retinal vascular development and the vaso-obliterative phase of oxygen-induced retinopathy. Exp Eye Res. 2010 Apr;90(4):493–500. doi: 10.1016/j.exer.2009.12.012. Epub 2010/01/13. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Vajzovic L, Hendrickson AE, O’Connell RV, et al. Maturation of the human fovea: correlation of spectral-domain optical coherence tomography findings with histology. Am J Ophthalmol. 2012 Nov;154(5):779–89. e2. doi: 10.1016/j.ajo.2012.05.004. Epub 2012/08/18. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Vajzovic L, Rothman AL, Tran-Viet D, et al. Delay in retinal photoreceptor development in very preterm compared to term infants. Invest Ophthalmol Vis Sci. 2015 Feb;56(2):908–13. doi: 10.1167/iovs.14-16021. Epub 2015/01/15. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Vander JF, Handa J, McNamara JA, et al. Early treatment of posterior retinopathy of prematurity: a controlled trial. Ophthalmology. 1997 Nov;104(11):1731–5. doi: 10.1016/s0161-6420(97)30034-7. discussion 5–6. Epub 1997/12/31. eng. [DOI] [PubMed] [Google Scholar]
- 178.VanderVeen DK, Bremer DL, Fellows RR, et al. Prevalence and course of strabismus through age 6 years in participants of the Early Treatment for Retinopathy of Prematurity randomized trial. Journal of AAPOS : the official publication of the American Association for Pediatric Ophthalmology and Strabismus/American Association for Pediatric Ophthalmology and Strabismus. 2011 Dec;15(6):536–40. doi: 10.1016/j.jaapos.2011.07.017. Epub 2011/12/14. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Vanderveen DK, Mansfield TA, Eichenwald EC. Lower Oxygen Saturation Alarm Limits Decrease the Severity of Retinopathy of Prematurity. Journal of American Association for Pediatric Ophthalmology and Strabismus. 2006 Oct;10(5):445–8. doi: 10.1016/j.jaapos.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 180.Vanhaesebrouck S, Daniels H, Moons L, et al. Oxygen-induced retinopathy in mice: amplification by neonatal IGF-I deficit and attenuation by IGF-I administration. Pediatr Res. 2009 Mar;65(3):307–10. doi: 10.1203/PDR.0b013e3181973dc8. Epub 2008/12/19. eng. [DOI] [PubMed] [Google Scholar]
- 181.Vinekar A, Avadhani K, Sivakumar M, et al. Understanding clinically undetected macular changes in early retinopathy of prematurity on spectral domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2011 Jul;52(8):5183–8. doi: 10.1167/iovs.10-7155. Epub 2011/05/10. eng. [DOI] [PubMed] [Google Scholar]
- 182.Vinekar A, Jayadev C, Mangalesh S, et al. Comparing the outcome of single versus multiple session laser photoablation of flat neovascularization in zone 1 aggressive posterior retinopathy of prematurity: A Prospective Randomized Study. Retina. 2015 Oct;35(10):2130–6. doi: 10.1097/IAE.0000000000000604. Epub 2015/05/23. eng. [DOI] [PubMed] [Google Scholar]
- 183.Vinekar A, Jayadev C, Mangalesh S, et al. Role of tele-medicine in retinopathy of prematurity screening in rural outreach centers in India - a report of 20,214 imaging sessions in the KIDROP program. Semin Fetal Neonatal Med. 2015 Oct;20(5):335–45. doi: 10.1016/j.siny.2015.05.002. Epub 2015/06/21. eng. [DOI] [PubMed] [Google Scholar]
- 184.Vinekar A, Mangalesh S, Jayadev C, et al. Macular edema in Asian Indian premature infants with retinopathy of prematurity: Impact on visual acuity and refractive status after 1-year. Indian J Ophthalmol. 2015 May;63(5):432–7. doi: 10.4103/0301-4738.159879. Epub 2015/07/04. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Walsh MC, Di Fiore JM, Martin RJ, et al. Association of Oxygen Target and Growth Status With Increased Mortality in Small for Gestational Age Infants: Further Analysis of the Surfactant, Positive Pressure and Pulse Oximetry Randomized Trial. JAMA pediatrics. 2016 Mar 1;170(3):292–4. doi: 10.1001/jamapediatrics.2015.3794. Epub 2016/01/10. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Wang H, Smith GW, Yang Z, et al. Short hairpin RNA-mediated knockdown of VEGFA in Muller cells reduces intravitreal neovascularization in a rat model of retinopathy of prematurity. The American journal of pathology. 2013 Sep;183(3):964–74. doi: 10.1016/j.ajpath.2013.05.011. Epub 2013/08/27. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Wang H, Yang Z, Jiang Y, Hartnett ME. Endothelial NADPH oxidase 4 mediates vascular endothelial growth factor receptor 2-induced intravitreal neovascularization in a rat model of retinopathy of prematurity. Mol Vis. 2014;20:231. [PMC free article] [PubMed] [Google Scholar]
- 188.Wang H, Zhang SX, Hartnett ME. Signaling pathways triggered by oxidative stress that mediate features of severe retinopathy of prematurity. JAMA Ophthalmol. 2013 Jan;131(1):80–5. doi: 10.1001/jamaophthalmol.2013.986. Epub 2013/01/12. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Wang Q, Pfister F, Dorn-Beineke A, et al. Low-dose erythropoietin inhibits oxidative stress and early vascular changes in the experimental diabetic retina. Diabetologia. 2010 Jun;53(6):1227–38. doi: 10.1007/s00125-010-1727-7. Epub 2010/03/27. eng. [DOI] [PubMed] [Google Scholar]
- 190.Wang S, Wu Z, Sorenson CM, et al. Thrombospondin-1-deficient mice exhibit increased vascular density during retinal vascular development and are less sensitive to hyperoxia-mediated vessel obliteration. Dev Dyn. 2003 Dec;228(4):630–42. doi: 10.1002/dvdy.10412. Epub 2003/12/04. eng. [DOI] [PubMed] [Google Scholar]
- 191.Watzke RC, Robertson JE, Jr, Palmer EA, et al. Photographic grading in the retinopathy of prematurity cryotherapy trial. Arch Ophthalmol. 1990 Jul;108(7):950–5. doi: 10.1001/archopht.1990.01070090052038. Epub 1990/07/01. eng. [DOI] [PubMed] [Google Scholar]
- 192.Weaver DT, Murdock TJ. Telemedicine detection of type 1 ROP in a distant neonatal intensive care unit. Journal of AAPOS : the official publication of the American Association for Pediatric Ophthalmology and Strabismus/American Association for Pediatric Ophthalmology and Strabismus. 2012 Jun;16(3):229–33. doi: 10.1016/j.jaapos.2012.01.007. Epub 2012/06/12. eng. [DOI] [PubMed] [Google Scholar]
- 193.Wheeler DT, Dobson V, Chiang MF, et al. Retinopathy of prematurity in infants weighing less than 500 grams at birth enrolled in the early treatment for retinopathy of prematurity study. Ophthalmology. 2011 Jun;118(6):1145–51. doi: 10.1016/j.ophtha.2010.09.031. Epub 2011/01/08. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Wilkinson-Berka J, Deliyanti D, Rana I, et al. NADPH oxidase, NOX1, mediates vascular injury in ischemic retinopathy. Antioxid Redox Signal. 2013 Sep 22; doi: 10.1089/ars.2013.5357. Epub 2013/09/24. Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Wittstrom E, Holmberg H, Hvarfner C, Andreasson S. Clinical and electrophysiologic outcome in patients with neovascular glaucoma treated with and without bevacizumab. Eur J Ophthalmol. 2012 Jul-Aug;22(4):563–74. doi: 10.5301/ejo.5000089. Epub 2011/12/06. eng. [DOI] [PubMed] [Google Scholar]
- 196.Wong KY. A retinal ganglion cell that can signal irradiance continuously for 10 hours. J Neurosci. 2012 Aug 15;32(33):11478–85. doi: 10.1523/JNEUROSCI.1423-12.2012. Epub 2012/08/17. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Wu WC, Lien R, Liao PJ, et al. Serum levels of vascular endothelial growth factor and related factors after intravitreous bevacizumab injection for retinopathy of prematurity. JAMA ophthalmology. 2015 Apr;133(4):391–7. doi: 10.1001/jamaophthalmol.2014.5373. Epub 2015/01/09. eng. [DOI] [PubMed] [Google Scholar]
- 198.Xu Y, Zhang Q, Kang X, et al. Early vitreoretinal surgery on vascularly active stage 4 retinopathy of prematurity through the preoperative intravitreal bevacizumab injection. Acta Ophthalmol. 2013 Jun;91(4):e304–10. doi: 10.1111/aos.12055. Epub 2013/05/08. eng. [DOI] [PubMed] [Google Scholar]
- 199.Yang MB, Rao S, Copenhagen DR, Lang RA. Length of day during early gestation as a predictor of risk for severe retinopathy of prematurity. Ophthalmology. 2013 Dec;120(12):2706–13. doi: 10.1016/j.ophtha.2013.07.051. Epub 2013/10/22. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Yang WJ, Hu J, Uemura A, et al. Semaphorin-3C signals through Neuropilin-1 and PlexinD1 receptors to inhibit pathological angiogenesis. EMBO Mol Med. 2015 Oct;7(10):1267–84. doi: 10.15252/emmm.201404922. Epub 2015/07/22. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Yang Z, Wang H, Jiang Y, Hartnett ME. VEGFA Activates Erythropoietin Receptor and Enhances VEGFR2-Mediated Pathological Angiogenesis. The American journal of pathology. 2014 Mar 4; doi: 10.1016/j.ajpath.2013.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Ying GS, Quinn GE, Wade KC, et al. Predictors for the development of referral-warranted retinopathy of prematurity in the telemedicine approaches to evaluating acute-phase retinopathy of prematurity (e-ROP) study. JAMA ophthalmology. 2015 Mar;133(3):304–11. doi: 10.1001/jamaophthalmol.2014.5185. Epub 2014/12/19. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Ying GS, VanderVeen D, Daniel E, et al. Risk Score for Predicting Treatment-Requiring Retinopathy of Prematurity (ROP) in the Telemedicine Approaches to Evaluating Acute-Phase ROP Study. Ophthalmology. 2016 Oct;123(10):2176–82. doi: 10.1016/j.ophtha.2016.06.037. Epub 2016/08/06. Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Yokota H, Narayanan SP, Zhang W, et al. Neuroprotection from retinal ischemia/reperfusion injury by NOX2 NADPH oxidase deletion. Invest Ophthalmol Vis Sci. 2011;52(11):8123–31. doi: 10.1167/iovs.11-8318. Epub 2011/09/16. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.York JR, Landers S, Kirby RS, et al. Arterial Oxygen Fluctuation and Retinopathy of Prematurity in Very-Low-Birth-Weight Infants. J Perinatol. 2004 Jan 22;24(2):82–7. doi: 10.1038/sj.jp.7211040. online. [DOI] [PubMed] [Google Scholar]
- 206.Zeng G, Taylor SM, McColm JR, et al. Orientation of endothelial cell division is regulated by VEGF signaling during blood vessel formation. Blood. 2007 Feb 15;109(4):1345–52. doi: 10.1182/blood-2006-07-037952. [DOI] [PMC free article] [PubMed] [Google Scholar]