Abstract
Recent insights into the bioactivation and signaling actions of inorganic, dietary nitrate and nitrite now suggest a critical role for the microbiome in the development of cardiac and pulmonary vascular diseases. Once thought to be the inert, end-products of endothelial-derived nitric oxide (NO) heme-oxidation, nitrate and nitrite are now considered major sources of exogenous NO that exhibit enhanced vasoactive signaling activity under conditions of hypoxia and stress. The bioavailability of nitrate and nitrite depend on the enzymatic reduction of nitrate to nitrite by a unique set of bacterial nitrate reductase enzymes possessed by specific bacterial populations in the mammalian mouth and gut. The pathogenesis of pulmonary hypertension (PH), obesity, hypertension and CVD are linked to defects in NO signaling, suggesting a role for commensal oral bacteria to shape the development of PH through the formation of nitrite, NO and other bioactive nitrogen oxides. Oral supplementation with inorganic nitrate or nitrate-containing foods exert pleiotropic, beneficial vascular effects in the setting of inflammation, endothelial dysfunction, ischemia-reperfusion injury and in pre-clinical models of PH, while traditional high-nitrate dietary patterns are associated with beneficial outcomes in hypertension, obesity and CVD. These observations highlight the potential of the microbiome in the development of novel nitrate- and nitrite-based therapeutics for PH, CVD and their risk factors.
Keywords: Nitric oxide, Nitrate, Nitrite, Microbiome, Cardiovascular disease, Pulmonary hypertension, Inflammation, Nitrated fatty acids
Graphical Abstract
Introduction
The study of the human microbiome has had a profound impact on the perception of human health. Characterization of body site-specific microbial ecosystems has revealed a highly diverse and regional set of microbial community networks that can exert systemic effects on the host through the production of metabolites, shaping the immune response, and influencing host gene expression[1]. Changes in these microbial populations have been linked to many ‘non-infectious’ diseases, such as obesity, diabetes, and cardiovascular disease (CVD). As research progresses and the microbiome is increasingly implicated in a wide range of diseases, the focus of work has shifted to the elucidation of the mechanisms behind such associations and their translation into tangible therapeutic solutions.
Despite an explosion of associative studies involving the microbiome and vascular and cardiovascular diseases, few connections have been identified with mechanistic certainty[2,3]. For example, the contribution of the microbiome in the development of pulmonary hypertension (PH) is emerging as a critical mitigating factor. Deficits in mammalian nitric oxide (NO) signaling have been linked to PH pathogenesis, as well as a host of associated risk factors such as hypertension, obesity, reduced insulin sensitivity and CVD. Further, recent insight into the biochemical reactions and signaling actions of nitrate, nitrite and nitric oxide reveal that the bioavailability of inorganic nitrate and nitrite, the major sources of exogenous NO, is dependent on the metabolic activity of specific oral microbiota. Thus, there is encouraging evidence for the study of microbe-driven, nitrogen oxide pharmacology in the treatment of several forms of PH.
Herein, we discuss a novel interrelationship between mammalian NO biology and microbial control of nitrate and nitrite bioavailability in the development of PH. Our discussion centers on the hypothesis that maintenance of pulmonary and systemic vascular health in mammals in part depends on the enzymatic reduction of dietary nitrates by commensal oral bacteria to vasoactive and anti-inflammatory mediators that provide systemic NO-effects critical under conditions of hypoxemia and stress. We review the evidence supporting a beneficial role of dietary and salivary inorganic nitrogen salts in the oxygen-independent generation of NO and secondary reaction products important for the maintenance of pulmonary vascular health, with a particular focus on the critical role of oral and gut microbial communities in the enzymatic reduction of nitrate to nitrite and the impact of diet and probiotics.
Dysfunctional Nitric Oxide Signaling underlies Pulmonary Hypertension Pathogenesis
Pulmonary Hypertension
Pulmonary hypertension (PH) is a progressive and fatal clinical disorder of the pulmonary circulation characterized by increased pulmonary vascular resistance, elevated pulmonary pressures (mean pulmonary arterial pressures ≥25mmHg), right ventricular overload and ultimately collapse[4,5]. There are a multitude of causes of PH. Many cases are idiopathic (Group I-pulmonary arterial hypertension, PAH), and secondary PH occurs in certain subsets of patients in the setting of left heart dysfunction (Group II) or secondary to advanced lung diseases (Group III)[5,6]. Other conditions such as HIV infection are classified as Group I, but may also have unique attributes[7–11]. Despite the diversity of patients who develop PH, many share a common link to the metabolic syndrome[4,12–14]. For example, obesity, hyperlipidemia, and systemic hypertension are common risk factors for Group I PAH as well as Group II (secondary to left-sided heart failure or PH-heart failure with preserved ejection fraction, HFpEF)[12,15,16], and are also a feature of HIV infection[17,18]. It is therefore likely that on some level, PH can be characterized by similar pathogenic mechanisms.
Nitric Oxide Signaling Dysfunction in Pulmonary Hypertension
NO is an endogenously-produced, lipophilic, and diffusible molecule that exerts a diverse array of critical autocrine and paracrine signaling activities. Chiefly, NO acts directly on smooth muscle cells via activation of soluble guanylyl cyclase leading to formation of the second-messenger, cyclic guanosine monophosphate (cGMP), that in turn promotes smooth muscle relaxation and inhibits both platelet function and vascular smooth muscle cell proliferation and migration[19–21]. Classically, the majority of NO is derived from the oxidation of the essential amino acid, L-arginine by a set of 3 isoforms of the enzyme nitric oxide synthase (NOS) in a reaction requiring oxygen, reduced nicotinamide-adenine dinucleotide phosphate (NADPH), tetrahydrobiopterin (BH4) and other cofactors[20,22]. Importantly, endothelial-derived NO production and function is largely determined by, and integral to the maintenance of a healthy endothelium[23–25].
Pathologically, all types of PH develop via an early, common vasculopathy driven by disproportionate pulmonary vasoconstriction and dramatic remodeling of the endothelium and vessel wall[5,26,27]. The hallmark feature of PAH and other forms of PH is reduced bioavailability of NO signaling and poor endothelial responsiveness to triggers of vasodilatation[28–34]. Similarly, vascular disease, cardiovascular disease (CVD), and metabolic syndrome all share effective loss of NO function[23,35–39]. In PH, the cause of NO signaling loss remains unclear, possibly being related to abnormal endothelial NOS expression[40], impaired NO formation[41–43] and increased NO scavenging by elevated rates of reactive oxygen species (ROS) generation by mitochondria, oxidases, oxygenases and uncoupling of NOS electron transfer reactions[23,44–47]. Given that most efficacious therapies for the treatment of PH involve targeting of NO signaling[48,49], it is clear that pulmonary vascular health is intimately tied to effective NO bioactivity.
The nitrate-nitrite-NO Pathway & Enterosalivary Circulation of Nitrate as ‘Alternate’ Source of NO Signaling
Nitrite, derived from the oxygen- and NOS-independent, microbial enzymatic reduction of dietary nitrate, has been identified as an important secondary source of NO and other bioactive nitrogen oxides[25]. Nitrite metabolism can be activated by hypoxia, low pH and reactions with metalloproteins[50–58]. As with NOS-derived NO, inorganic nitrite demonstrates robust NO-signaling effects[52,53,57,59] and contributes to basal vascular tone[51,55,60,61], blood pressure[62–66], protection from endothelial dysfunction[67–71] and vascular inflammation[72] under conditions of metabolic and inflammatory stress.
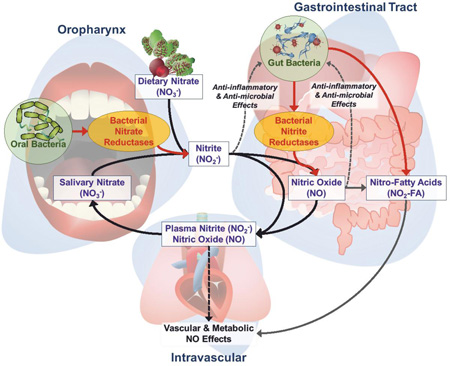
Formation of nitrite and propagation of its downstream NO-signaling effects depends on the oral bacterial reduction of inorganic nitrate by a set of bacterial nitrate reductase enzymes (NaRs) that are largely absent from the human genome[73–78]. In a process referred to as the enterosalivary nitrate circulation, or the nitrate-nitrite-NO pathway, dietary nitrate is swallowed and rapidly absorbed in the proximal gastrointestinal tract (Figure 1)[79–81]. In proportion to the dietary load of nitrate, approximately 25% of total circulating nitrate is actively sequestered into salivary glands and concentrated in saliva up to 20 times that in plasma[73,75,82–88]. Once in the mouth, salivary nitrate undergoes NOS- and oxygen-independent, enzymatic reduction to nitrite. Nitrite is then swallowed where it can undergo a diversity of downstream reactions, including formation of NO and NO-donating species following a) protonation to HNO2 and an array of secondary nitrosating and nitrating species in reactions facilitated by the low pH of the stomach[80,83,89–91], or b) further reduction by enteric bacterial nitrite reductases (NiRs)[74]. These reactions also lead to the generation of electrophilic nitro-fatty acid (NO2-FA) signaling mediators, including NO2-conjugated linoleic acid (NO2-CLA) and S-nitrosothiol derivatives, all of which can be detected at increased concentrations in the systemic circulation following oral consumption of nitrate[72,92–96]. In turn, these reactions and their products can not only instigate salutary NO signaling responses, but also post-translational protein modifications and altered patterns of gene expression. These reactions can beneficially regulate cardio-metabolic function and will be attenuated or abolished by chemical or physical destruction of the oral microbiota[60,75,76,97–99].
Figure 1.
Schematic of the enterosalivary nitrate (NO3−), nitrite (NO2−), nitric oxide (NO), and nitro-fatty acid (NO2-FA) pathways and interactions with the oral and gut microbiome.
Formation of and signal transduction by nitrate-derived nitrite, NO and bioactive nitrogen oxides is not a linear progression to NO and cGMP-dependent signaling, as the reference to the enterosalivary nitrate-nitrite-NO pathway might suggest. There exists a broad range of redox-derived nitrogen oxides that exert both cGMP-dependent and -independent signaling actions as a result of nitrosative-, nitrative- and oxidative-induced post-translational protein modifications and subsequent changes in protein function and gene expression profiles[100–102]. Several aspects of these reactions are addressed below and have been extensively reviewed previously[91,93,96,103–105].
Microbial Nitrate Reduction to Nitrite and NO is Physiologically Important
Given the importance of NO’s effects on the vasculature and the apparent contribution of the enterosalivary nitrate pathway to systemic NO bioavailability, the loss of the ability to reduce nitrate to nitrite and NO is expected to have systemic implications, particularly in situations of systemic or vascular metabolic and inflammatory stress. Extensive evidence supports that microbial bioactivation of nitrate may play a role in the prevention and treatment of systemic vascular pathology and PH.
Blood Pressure & Endothelial Health
An impressive body of preclinical and clinical evidence has demonstrated beneficial blood pressure and vascular effects of nitrate and nitrite supplementation in the form of sodium/potassium salt or concentrated dietary supplement such as beetroot. Studies in Sprague-Dawley rats[78] and normal human volunteers[106] showed that depletion of oral bacterial nitrate reductases by chlorhexidine mouthwash correlated with a 90% decrease in oral nitrite levels (p<0.001) in humans, along with a 25% decrease in plasma levels (p=0.001), and 2–3.5mmHg increase in blood pressure that occurred in proportion to decreased plasma nitrite (R2=0.56, p=0.002)[106]. At least 19 studies in hypertensive, overweight, and other patient populations have been performed, all revealing predictable acute or chronic blood pressure reduction with varying types of nitrate supplementation[107], and that antimicrobial mouthwash abolishes this beneficial effect both acutely and chronically (reviewed in:[108,109]).
From the standpoint of vascular health, endothelial dysfunction has been associated with decreased circulating levels of nitrite[67,110]. Oral supplementation with either nitrite or nitrate increases circulating nitrite levels and prevents and reverses endothelial dysfunction in a dose-dependent manner both in mice[68,69] and in healthy[70,71,97,111,112], hypertensive[113], and obese individuals[114], or in those with hypercholesterolemia[115] and peripheral arterial disease[116]. Further, dietary nitrate and nitrite confer protection from intimal hyperplasia in rodents[117–119], while nitrate supplementation prevents or limits ischemia-reperfusion injury and endothelial dysfunction in both animal[98,120–124] and human[97,125] models of ischemia-reperfusion.
Exercise Capacity & Oxygen Utilization
Microbial reduction of nitrate to nitrite, NO and other bioactive nitrogen oxides may influence more than vascular health alone. Functional resilience to debilitating diseases such as PH may also be conferred by microbes via improved exercise capacity, oxygen utilization, and myocardial function[126]. For example, one study demonstrated that nitrate supplementation offers net decrease in oxygen cost (VO2, mL/kg/min) during moderate and heavy submaximal exercise in healthy adults[127], an effect that over 14 studies have confirmed with reduced VO2 and increased work capacity in response to nitrate during submaximal exercise in healthy individuals[128,129] (reviewed in:[130,131]) and in certain chronic diseases[127,132]. Additionally, in the setting of heart failure and cardiomyopathy, dietary nitrate improves left ventricular function in mice[133] and overall cardiac contractility and exercise performance in humans[134–137], suggesting the critical potential of exploiting the oral microbiome to enhance functional outcomes in patients via mechanisms spanning from improved mitochondrial function to increased vascular and cardiac performance[138].
Pulmonary Hypertension
In terms of PH, there have been no human clinical studies testing the effects of oral nitrate or nitrite, though animal model data support a therapeutic role. Inhaled NO induces vasodilation in PAH and is capable of reversing hypoxic pulmonary vasoconstriction[139–141]. Similarly, nitrite administered via inhalation or infusion is a selective pulmonary vasodilator with increased activity in hypoxia[142–144], and is capable of inhibiting both hypoxia-induced and monocrotaline-induced PH in rats[145]. Delivered via intravenous, inhaled, or oral routes, nitrite reduces pulmonary and right heart pressures and prevents or reverses characteristic vascular remodeling and right ventricular hypertrophy (RVH) in several animal models of PAH[15,117,143,146–149]. In a single experiment using nitrate, dietary supplementation prevented pulmonary vascular remodeling and RVH in both wild-type and endothelial NOS-deficient mice[150], indicating an active role for microbial, nitrate-derived nitrite and NO in the development and progression of PH. Further, in a population of individuals with HFpEF, there was a 14% increase in cardiopulmonary exercise testing performance in 17 patients after a single dose of beetroot juice (12.9 mmol nitrate)[137], and in another study, a 24% improvement in submaximal aerobic endurance with 1 week of daily beetroot juice consumption (6.1 mmol nitrate)[151]. These studies support the clinical utility of nitrate in at least some types of PH.
Current evidence suggests the biological importance of nitrate and its enzymatic and non-enzymatic reaction products in maintenance of vascular health and PH. These data also support the concept that an intricate and dependent relationship exists between mammals and commensal, oral, nitrate-reducing microbiota in maintaining adequate NO signaling. Yet, despite the importance of these microbial populations, relatively little is known about the identity and function of specific bacteria and their effects on nitrate metabolism.
Safety of Inorganic Nitrate and Nitrite
For many years, dietary nitrate and nitrite were considered harmful for regular human consumption due to two prominent health concerns, the development of methemoglobinemia and the potential for carcinogenic effects. Ostensibly, the toxic effects of nitrate and nitrite are driven by the more reactive nitrite after either oral reduction by the enterosalivary pathway or direct consumption. In the acute setting, nitrite can bind to hemoglobin and oxidize the ferrous iron in heme to the ferric state, thereby forming methemoglobin (metHgb) and preventing the binding of oxygen[152]. Methemogobinemia becomes clinically significant when baseline levels of metHgb (1–3%) rise to 5–12%, with cyanosis and fatal toxicity occurring at levels between 30–50%[153]. Early concerns of infant exposures led to current regulations limiting nitrate content in water to 44 mg/L[154,155], although more recent evidence in both infants and adults suggests that exceptionally high concentrations of nitrite (and well beyond the <75mg nitrite necessary to achieve blood pressure effects) would be necessary to pose a significant threat to safety[25,51,72,113,155–158]. Similarly, little evidence exists to suggest that chronic nitrate or nitrite consumption are directly carcinogenic in humans[159,160]. However, in certain settings nitrite can form nitrous acid via the N-nitrosation pathway in the stomach, which then reacts with dietary amines and amides to form powerful alkylating, and carcinogenic, N-nitroso compounds[155,161,162]. Indeed, nitrites in combination with dietary amines or amides, such as in red and cured meats, have been linked to stomach and several other cancers[155,157,160,163–166]. Excitingly, when ingested nitrate and nitrite are rather paired with plant- and fruit-based polyphenols and essential nutrients such as ascorbate, gastric nitrosation is inhibited[162,167,168], suggesting that the mutagenic potential of dietary nitrate and nitrite is likely contextual, depending on the complex interactions between nitrate and nitrite with factors such as the relative content of amines, amides and plant-based, antioxidant substrate[155,169].
The Oral Microbiome and Bacterial Nitrate Reduction
Complexity of the Oral Microbiome
As the body’s threshold to the outside world, the oral cavity is one of the most ecologically dynamic, and most studied, environments in the body. Culture-independent sequencing of the bacterial 16S ribosome has suggested an estimated 50–100 billion bacteria in the mouth, comprised of nearly 700 identified bacterial species[170,171]. Up to 80% of oral bacteria are dominated by about 200 species in the Phyla Firmicutes (esp. Streptococcus, Veillonella, Granulicatella, Gemella spp.) and Proteobacteria (esp. Neisseria, Haemophilus spp.), with contributions from Bacteroidetes, Actinobacteria and Fusobacteria totaling upwards of 95% of all identified oral microbiota (Figure 2)[171–177]. Further diversity exists between the varied micro-ecologies of the mouth[174,178], such as the tooth surface, gingiva, hard and soft palate, and even regionally on the tongue (Figure 2)[173,176,179]. Local variation in site-specific “core” microbial members speaks to the heterogeneity of influences bacterial communities experience, including host factors such as age, diet, geography and oral health[180–182].
Figure 2.
The ‘core’ oral microbiome. Charts represent a general summary of the relative abundances of major bacterial phyla (>1% total microbial abundance) constituting the normal microbiome of the human oral cavity. Individual charts represent regional microbial diversity within the entire oral cavity, saliva and oral wash, dorsum of the tongue, buccal mucosa, subgingiva, and the hard palate. Values presented are summary estimates only, extrapolated and pooled from multiple published analyses[173–175,177,194].
Amid this complexity, an ever-growing number of specific microbial taxa have been associated with both oral and systemic diseases[181,182]. For instance, saccharolytic (sugar metabolizing) bacteria such as Streptococcus and Lactobacillus have been associated with dental caries, while proteolytic (protein metabolizing) bacteria such as Prevotella and Porphyromonas have been associated with periodontitis and halitosis[183]. Associations between atherosclerosis and Bacilllus typhosus were first described over 125 years ago[184], while more recent work has linked Porphyromonas gingivalis to atherosclerosis[185], smoking[186], and several cancers[187]. Yet, individual bacteria do not act in a vacuum, but rather function in interdependent microbial communities whose conglomerate activity can impact oral and systemic health[183,188,189]. Indeed, even the nitrate-nitrite-NO pathway is affected by microbial diversity[190] and oral pathology[191–193]. It is within this context of regional, community function that the importance of oral bacterial nitrate reduction to nitrite, NO and other bioactive nitrogen oxides must be viewed.
Bacterial Nitrate Reduction
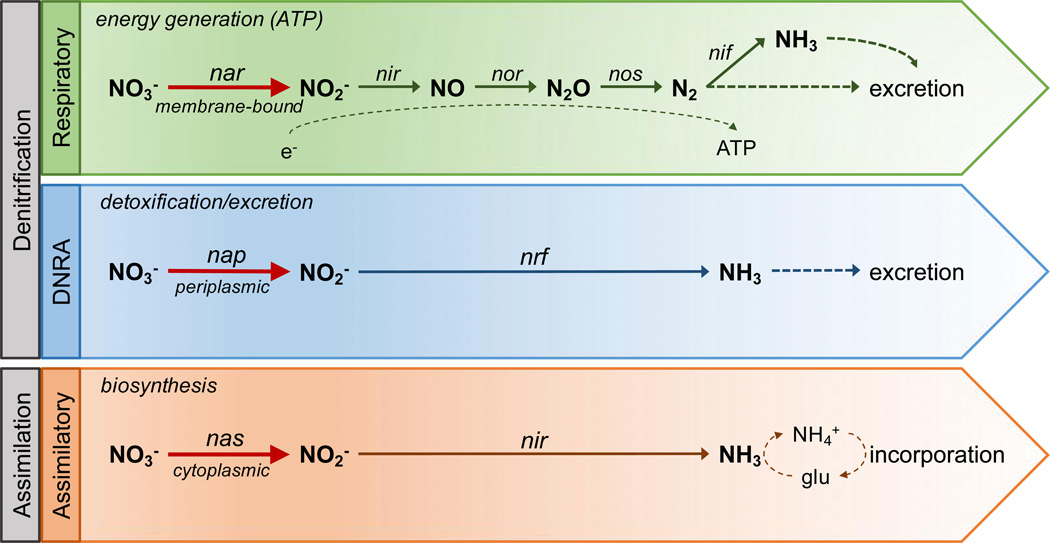
Bacterial metabolism of inorganic nitrogen compounds, ranging from nitrate in its most oxidized state to ammonia in its most reduced, is fundamental to the global/ecological nitrogen cycle. Reduction of nitrate to nitrite by molybdenum-dependent nitrate reductases is the initial and rate-limiting step in anaerobic nitrogen metabolism[77]. Broadly, nitrate reduction is classified into three major pathways (Figure 3)[77,195–197]: 1) Respiratory denitrification, catalyzed by membrane-bound nitrate reductase, nar, or periplasmic nap, is the major electrochemical respiratory pathway for bacterial energy generation under hypoxia via generation of a proton motive force, serving as a two-electron sink in the final step of ATP generation via the respiratory electron transport chain[196]. In this process, nitrite is further reduced to gaseous NO, nitrous oxide, and dinitrogen by a series of reducing enzymes, nitrite reductase, nitric oxide reductase, and nitrous oxide reductase, respectively. Gaseous nitrogen can then either be excreted or, in a subset of bacteria, undergo further reduction via the enzyme nitrogenase to ammonia, in a process called nitrogen fixation. 2) Dissimilatory nitrate reduction to ammonia (DNRA), catalyzed by periplasmic nitrate reductase, nap, is thought to be the major detoxification/excretion pathway for nitrogen compounds in which nitrite is directly converted to ammonia and excreted[198]. Alternatively, dissimilatory nitrate reduction can indirectly participate in ATP generation when coupled with formate oxidation[77,199]. 3) Assimilatory nitrate reduction, catalyzed by cytoplasmic nitrate reductase, nas, is the biosynthetic, anabolic pathway by which nitrite is further reduced to ammonia, which can then undergo ammonium assimilation via production of the amino acid glutamine and incorporated into the bacterial biomass.
Figure 3.
Schematic representation of major bacterial nitrate reduction pathways. Abbreviations: DNRA, dissimilatory nitrate reduction to ammonia; ATP, adenosine triphosphate; NO3−, nitrate; NO2−,nitrite; NO, nitric oxide; N2O, nitrous oxide; N2, dinitrogen; NH3, ammonia; NH4•, ammonium; glu, glutamine; nar, nap, and nas, nitrate reductases; nir, nrf, nitrite reductases; nor, nitric oxide reductase; nos, nitrous oxide reductase; nif, nitrogenase; e−, electrons. Adapted from Sparacino-Watkins et al.[77] with permission of The Royal Society of Chemistry. http://dx.doi.org/10.1039/c3cs60249d.
Numerous bacteria contain genomic nitrate reductase genes and are capable of their expression and of reducing nitrate to nitrite in culture; however, in vivo identification of the major nitrate-reducing bacteria that contribute to the mammalian enterosalivary nitrate pathway is less certain. The bulk of physiologically relevant nitrate reduction occurs via the activity of facultative anaerobic bacteria dwelling in crypts of the posterior dorsum of the tongue[81,200,201]. In both rat and human studies species of Firmicutes (Staphylococcus, Streptococcus and Veillonella) and Actinobacteria (Actinomyces) have been most frequently identified as among the highest nitrate-reducers, while numerous other taxa have also been identified, including Pasteurella, Rothia, Neisseria, Haemophilus, Granulicatella, etc.[190,200–204]. Using nitrate supplementation as selective pressure for bacteria capable of nitrate reduction, several studies found proliferation of Veillonella[205], Streptococcus[204], Neisseria[115], Haemophilus[204], and Rothia species[115], all previously identified as high nitrate reducers. Yet, despite these varied attempts, there remains a high degree of variability in results depending on numerous methodological factors[206], including source and sampling of sample (saliva versus oral wash versus tongue scraping), direct sampling versus culture, and choice of 16S variable region for sequencing.
Also critical to bacterial nitrate reduction is the trans-membrane transport of nitrate and subsequent management of potentially cytotoxic nitrite. Nitrate reductase subtypes are found in locations commensurate with their function: membrane-bound respiratory nar faces the cytoplasm, facilitating movement of protons across the cell wall and thereby creating the proton motive force necessary for ATP generation; membrane-bound dissimilatory/detoxifying nap faces the periplasm, allowing for protective neutralization of excess nitrate before it is transported into the cell; and soluble assimilatory nas moves freely within cytoplasm, close to active sites of biosynthesis. Depending on enzyme location, nitrate transport is thought to occur either by direct, ATP-hydrolyzing, ABC-type transporters in assimilatory nitrate reduction, or via a set of secondary nitrate/nitrite porters (NNPs) of the major facilitator superfamily (MFS), NarK and NarU, in respiratory nitrate reduction [207,208]. Of biological importance, however, two functionally distinct NarK subfamilies are thought to represent the bulk of nitrate-specific transport[208,209]. While the precise mechanism of transport is not yet known, several structural analyses have suggested that transporter NarK functions as an electroneutral nitrate/nitrite antiporter, exchanging periplasmic nitrate one-to-one for cytoplasmic nitrite[208,210], whereas NarU acts as a nitrate/proton symporter, linking nitrate uptake to an existing proton gradient[211,212]. Notably, control of transporter gene expression is linked to the same oxygen- and nitrate-responsive regulatory systems used by the nitrate reductases[207,213], reinforcing the concept that bacterial nitrate reduction is a dynamic process intimately related to both bacterial genetics and local environmental pressures.
Local Factors Influence Bacterial Nitrate Reduction
From the perspective of bacterial survival and the development of targeted therapies, several additional features can influence bacterial nitrate reduction. Microbiota are fundamentally communal organisms and exist in mutualistic, interdependent networks that are crucial to their survival. These networks allow adaptation to host and environmental pressures through the sharing of metabolic products, adhesion and biofilm formation, chemical signaling, quorum sensing, and horizontal gene transfer (reviewed in:[214,215]). Based on genomic content, it is possible for a single bacterial strain to fully reduce nitrate to NO, nitrogen, or ammonia via multiple reductive pathways. However, the presence and expression of nitrate reductase genes is highly variable within and between bacterial species[216]. Demonstrating the complexity of community metabolism, an ex vivo experiment showed increased nitrate consumption in a polymicrobial culture of mixed high- and low-nitrate reducing bacteria challenged with nitrate compared to one of high-nitrate reducers alone[190]. Similarly, there was decreased NO generation in a polymicrobial culture containing NO-consuming bacteria in rats[217]. Thus, alterations in bacterial communities may be more important than changes in single bacterial species, and due consideration must be paid to functional community networks in the design and implementation of targeted therapies[218,219].
Further, bacterial networks and host-associations must include the robust viral and fungal flora that also cohabit the mouth. Bacteriophages share with host bacteria a similar site-specific diversity in the mouth and disease associations such as periodontitis[220,221]. Other viruses, such as HIV, also alter the oral microbiome, mycobiome, and NO-driven disease susceptibility (such as CVD and PH)[222–224]. They also exert synergistic effects with bacteria, as with herpesviruses in the pathogenesis of periodontitis[225]. Fungal populations also share regional diversity and complex metabolic interactions with resident bacteria[226,227] and display multiple disease associations[228]. Importantly, multiple fungal species, including the ubiquitous Candida, contain a fungal nitrate reductase enzyme and are known nitrate-reducers in the environment[229,230]. Both the oral virome and mycobiome thus have the potential to alter bacterial nitrate metabolism through alterations in bacterial populations and their metabolism, direct reduction of nitrate and alterations in metabolic substrate, changing local host and environmental conditions such as stimulating inflammation, and propagation of oral and systemic disease.
Second, protective host factors may exert selective environmental pressures that influence mechanisms of oral microbial metabolism of nitrogen. For instance, nitrite and nitrate are important protective elements of the oral and mucosal immune response, especially in settings of stress and periodontal injury[86,193,231]. Nitrite can exert pH-dependent antimicrobial effects[204,232,233] via formation of reactive secondary nitrogen oxides that can disrupt aerobic respiration[234], inhibit bacterial acid production, and disrupt biofilm formation[235–237]. In settings of low pH and high nitrate and nitrite concentrations, selected bacteria and bacterial communities capable of nitrate reduction may preferentially survive or expand their population[115,205]. Indeed, increased production of the weak base, ammonia via nitrate reduction is a well-known strategy for bacteria to alkalinize their cytoplasm and local environment and protect against the potential toxic effects of high concentrations of nitrate, nitrite and NO[238]. This may relate to the paradoxical association between nitrate-reducing oral bacteria and protection from dental caries[191,205,232,238,239].
Third, it is clear that pH, particularly with regard to stomach acidity, plays an important role in nitrate, nitrite, and NO metabolism. One of the main functions of low gastric pH is to clear pathogenic bacteria before they enter the distal GI tract. Mechanistically, much of the toxicity is due to the non-enzymatic protonation of nitrite to HNO2 and NO and subsequent oxidative and nitrosative stress. A prominent example of such pH-NO and host-microbe dynamics is the well-studied human pathogen Helicobacter pylori. Epidemiologically, H. pylori is linked to peptic ulcer disease, gastric cancer, as well as diabetes, autoimmune disease, chronic inflammation, CVD and endothelial dysfunction[240–245]. To survive the hostile gastric milieu, H. pylori has developed robust mechanisms for altering its local environment on two fronts. First, H. pylori has been shown to inhibit iNOS expression and NO production by the host macrophage[242]. Second, H. pylori can alter stomach pH via expression of urease that allows for the formation of both ammonia and bicarbonate from urea, thus reducing the acidity of the stomach and potentially reducing the nascent pH-dependent formation of NO from salivary nitrite[242,246,247]. Supporting the importance of such mechanisms, a growing body of evidence has linked acid-reducing medications such as proton pump inhibitors and histamine H2 antagonists, with increased risk of enteric infections such as clostridium difficile[248–250] and profound alterations of the gut microbiome[251–253]. Additionally, acid-reduction is known to reduce gastric production of NO[76,90,254] and has recently been shown to attenuate the blood pressure lowering effects of nitrite in healthy adults[255]. These data then suggest that gastric pH may be equally as important in nitrate bioactivation as a viable oral, nitrate reducing microbiome.
Finally, the oxygen content itself in any particular oral or gut microenvironment can profoundly influence microbial mechanisms of respiration and the role of particular nitrate reduction pathways. The vast majority of oral and GI nitrate reducers are facultative anaerobes. From the standpoint of energetics, facultative anaerobes prefer aerobic respiration, although they maintain the capacity to use anaerobic fermentation for growth in anoxic or low oxygen environments, a mechanism dominated by the respiratory nitrate reductive pathway[77]. Indeed, the expression and activation of bacterial nitrate reductases are tightly regulated via oxygen-dependent and nitrate-responsive mechanisms[77,256]. For instance, the respiratory nitrate reductase, nar, expresses two major operons, NarGHI and NarZYW[77,256]. The first, NarGHI, is highly oxygen-responsive, under primary transcriptional control via the oxygen sensor fumarate nitrate reductase regulator (FNR), and appears to be the major contributor to respiratory anaerobic activity[256–258]. Conversely, NarZYW is constitutively expressed at low levels in the aerobic setting and only up-regulated in response to high nitrate concentrations, likely aiding in the transition from aerobic to anaerobic respiration [256,259,260]. Comparatively, while the DNRA nitrate reductase, nap, shares some regulatory elements with nar such as the oxygen-dependent FNR[77,256,261–264], it is notable that nap is maximally expressed under low oxygen and low nitrate conditions, a difference suggested to possibly relate to a selective advantage over nar in the distal GI tract[77,265]. What is clear is that the primary determinants of bacterial respiration are the ambient oxygen tension and nitrate concentration at any given point.
Understanding of the particular conditions that trigger such dramatic shifts in microbial energy production is lso necessary to understand in vivo function. In a recent set of experiments using gut facultative anaerobes, Escherichia coli and several Lactobacillus sp., there was an increase in the expression of nitrate reductase enzymes, an increase in nitrite formation, and a clear growth benefit in the setting of adequate nitrate as oxygen concentration decreases below 4% [233]. Conversely, microbiota isolated from dental plaque were capable of denitrification under aerobic conditions, supporting the idea that biological activity is regulated by multiple forms of nitrate reductase enzymes[192]. Biologically important nitrate reduction, therefore, arises from regional interactions between oxygen tension, nitrate concentration, and microbial genetics.
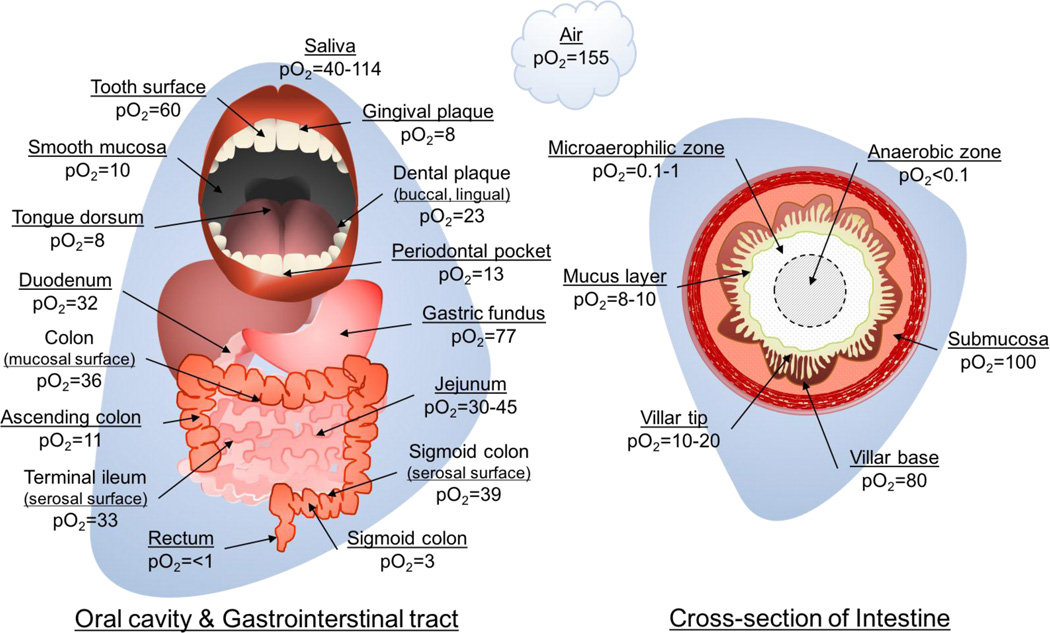
In the mammalian GI tract, several remarkably steep oxygen gradients must be considered in order to understand the functional context of nitrate reductase activity (Figure 4). In the mouth, regions of high oxygen concentration (20–114mmHg, ~3–15%) are generally expected to be in whole saliva, on the tooth surface, and in dental plaque[266]. Conversely, areas that have high nitrate reductase activity such as the dorsum of the tongue, gingival plaque and periodontal pockets demonstrate low relative oxygen concentrations (8–13 mmHg, ~1–2%)[81,193,201,266]. In the gut, two broad oxygen gradients must be considered as well. Longitudinally, oxygen concentrations generally fall, with the highest pO2 in the proximal gut including the gastric fundus (77mmHg; ~10%) and small bowel (30–45mmHg, ~4–6%), and declining through the colon to concentrations of less than 3 mmHg (ρ%) in the sigmoid colon and rectum[267]. Second, diffusion of oxygen from the enteral vasculature through the submucosal tissue and into the intestinal lumen creates an incredibly steep oxygen gradient, ranging from 80–100mmHg (~10–13%) in the submucosa and base of the villi, down to only 8–10mmHg (~1–2%) in the overlying mucus layer and an essentially anaerobic luminal center (Figure 4)[233,267]. Add to this the wide temporal fluctuations in oxygen tension at the mucosal border during the massive hyperemia experienced after meals, and it rapidly becomes clear that the ability to switch metabolism from aerobic to largely nitrate-driven anaerobic is a requirement for microbial survival[267–269]. Considering, however, that despite the capacity of bacteria in the distal GI tract to reduce nitrate[74], it is unlikely they significantly contribute to the enterosalivary circulation given that the majority of nitrate and nitrite are absorbed in the proximal small intestine[233,270].
Figure 4.
Diagram of estimated oxygen concentration at various locations in the oral cavity and gastrointestinal tract. Left figure identifies locations in the oral cavity, stomach, and large and small bowel. Right figure is representative of a segment of bowel in cross-section. pO2, partial pressure of oxygen (mmHg). pO2 estimates from Espey 2013[267] and Hill & Marsh 1989[266].
Many questions remain regarding the mechanisms of microbial nitrate reduction and contributions to enterosalivary-derived nitrite, NO and other bioactive nitrogen oxides. Nitrite is the common early intermediate in all bacterial nitrate-reductive pathways, but it is unlikely that all three mechanisms contribute in equal amounts. In hypoxic conditions, respiratory denitrification is the most energetically favorable process that promotes respiration-dependent growth[77]. The logical assumption would be that in a healthy mouth, ATP-generating respiration would predominate. Conversely, under conditions of host stress or inflammation, selective pressures on the microbiota change, leading to increased nitrate, nitrite and reactive nitrogen oxides, reduced pH, and a hostile environment that may favor the dissimilatory pathway, leading to ammonia production, alkalization, and nitrogen detoxification[271]. Hence, it is likely that different environmental conditions favor different mechanisms of nitrate reduction and their associated microbial communities. Further understanding of this point is crucial to the development of safe and effective therapies that target this pathway.
Dysbiosis of the Microbiome in Pulmonary hypertension & Vascular Disease
The Oral Microbiome and ph
To date, there are no studies that directly link microbial community features of the oral and gut microbiome, nitrate bioavailability, and the development of PH. The critical evidence of such an association, however, rests in the global dependency of enterosalivary nitrate reduction on living oral microbiota to enact its biologic activity[73,99,109]. As reviewed, oral administration of nitrate and nitrite provides dose-dependent improvement in endothelial dysfunction, inflammation and vascular function[71], protection from ischemia-reperfusion injury[97,98,272], and reversal of vascular and ventricular remodeling in several models of PAH[147,150]. Clinically, nitrate supplementation will reliably produce anti-hypertensive effects[107], improved exercise efficiency[127], and improved exercise performance in patients with heart failure and HFpEF[135,137,151]. Based on these findings, there can be little doubt that oral nitrate supplementation exerts profound beneficial vascular effects via microbial-dependent bioactivation in numerous settings closely related to PH and its pathophysiological mechanisms.
From the standpoint of the oral microbiota, there is evidence that particular population changes are associated with pathologies that confer an increased risk of developing PH and other vascular disease, including obesity[273–275], diabetes[276], and insulin resistance[277]. While the mechanistic underpinnings of these effects deserves additional study, it is likely to be transduced in part by NO and the pleiotropic reactions of secondary nitrogen oxides induced by microbial nitrate reduction and downstream reactions. Additional factors could include further gut microbial metabolism of secondary nitrogen oxides, the generation of bioactive fatty acid signaling mediators, and the downstream signaling reactions emanating from a host of other metabolic mediators that could influence PH, especially in metabolic syndrome and obesity[217,233,278,279].
Microbial Communities & Nitrate Bioactivation are Disrupted in Periodontal Disease & Xerostomia
Outside of global shifts in the oral microbiome, two additional aspects of oral health and the microbiome are worth mentioning in relationship to nitrogen oxide metabolism and the development of PH. First, periodontal disease is a condition defined by acute and chronic inflammation of the gingiva that leads to bleeding, reduced clinical attachment, gum recession and eventual tooth loss. Characteristically, disease is accompanied by the transition from symbiotic, facultative microbiota to overgrowth of dysbiotic, anaerobic bacteria together known as the ‘red complex' that reside in the relatively hypoxic subgingival crypts and pockets of the gums[182]. A long-described, yet puzzling and hitherto unproven, association between periodontal disease and CVD[280–282] has been variably attributed to direct vascular entry of these largely nitrate-reducing oral pathogens[283–286], generalized immune activation, systemic inflammation, and endothelial dysfunction, all processes that are improved with periodontal treatment (typically bactericidal chlorhexidine rinses with or without quadrant scaling)[193,287–290]. Interestingly, both salivary nitrate and nitrite concentrations and gingival expression of NO-forming NOS have been found to be increased in periodontal disease, which also tend to increase with disease severity and improve with periodontal treatment[99,193,291,292]. However, it remains uncertain whether increased nitrite derives from oxidation of gingival NOS-derived NO or bacterial reduction of salivary nitrate. What is clear is that inflammation in the localized anaerobic environment of the expanding subgingival crypts, in concert with high salivary nitrate, likely selects for survival and growth of the anaerobic, nitrate-reducing bacteria typical of periodontal disease[193]. Yet, despite these localized, periodontal effects on microbial populations, high salivary nitrite concentrations may also exert antimicrobial effects elsewhere in the oral cavity and gut, possibly disrupting community composition or function of biologically beneficial populations of nitrate-reducing bacteria[204,232–237]. Given these associations and recent links between periodontal disease and PH-associated conditions such as obesity and diabetes[182], it is possible that dysbiosis and systemic inflammation due to periodontal disease and overall oral health may impact or reverse the beneficial effects of physiological enterosalivary nitrate metabolism and promote the development of PH.
An additional and provocative association also exists between dysbiosis of the oral microbiome, PH, and diseases of dysfunctional salivary flow (xerostomia). Nitrate content in saliva is highly regulated[85,86,193,231]. In the setting of salivary gland dysfunction, there is a decrease in salivary nitrate concentration and corollary increase in urinary excretion, thereby reducing total body nitrate content and its enterosalivary circulation[84,193,231,293]. Significantly, several diseases that share both disproportionately high rates of vascular disease and PH, including Sjögren's and systemic sclerosis[294–297], HIV[7,11], and cystic fibrosis[298] also feature dysregulated salivary flow[299–302] and altered oral microbial populations[224,303–306]. While there is no conclusive link between these factors, these associations offer a potentially novel line of investigation.
The Gut Microbiome
Although the major, rate-limiting steps in the nitrate-nitrite-NO pathway occur in mouth, the gut constitutes the nexus of many host-microbe interactions that may impact nitrate recirculation and further reduction to nitrite, NO and other bioactive nitrogen oxides and signaling mediators. Relative to the oral cavity, the gut harbors over 1kg of bacterial biomass and upwards of 1000 different species[307]. Ecological conditions vary immensely along both the length of the GI tract as well as in cross-section from lumen to the mucosal brush-border[173,267,308,309]. Numerous facets of GI microbial community composition and metabolism have been associated with various disease states, including CVD, obesity, diabetes, and features of metabolic syndrome[274,310–313]. There are no studies that directly investigate an association between the gut microbiome, nitrate, and PH. However, several features common to gut dysbiosis, altered NO-signaling, and PH suggest a role in disease including inflammation and NO production.
Inflammation
Inflammation is a prominent feature of PH that may be mediated by the host-microbe interaction[314,315]. Studies suggest that in the gut, greater microbial diversity is associated with protection from inflammation and inflammatory disease[316,317], while conversely, reduced microbial diversity is associated with inflammation, obesity, hypertension, and features of metabolic syndrome[311,313,318,319]. In the injured or diseased gut, inflammation leads to increased production of reactive oxygen species such as superoxide, hydrogen peroxide and hydroxyl radicals and reactive nitrogen oxides such as peroxynitrite, dinitrogen trioxide and nitrogen dioxide[320]. Elevated rates of generation of these oxidative inflammatory mediators can have direct effects on enterocytes and mucosal integrity, impair NO-dependent vascular function, reduce nitrate and nitrite absorption, and exert selective pressure on bacteria by augmenting innate anti-bacterial effects[204,233,321–324]. Consequently, microbial diversity is reduced[325,326] while local and systemic inflammation and decreased NO bioavailability persist, propagating vascular injury and the risk of developing PH.
Recently, the ratio of Firmicutes to Bacteroidetes in the gut has gained increasing acceptance as a barometer of gastrointestinal and systemic health[327,328]. Elevations in the Firmicutes:Bacteroidetes ratio have been associated with a high-fat diet and obesity[274,328,329], irritable bowel syndrome[330], hypertension[331], type 1 diabetes[276], and HIV[332]. Characteristically, dysbiosis of this ratio heralds a shift in microbiota from obligate anaerobes to nitrate-reducing, facultative anaerobes of the Proteobacteria and Firmicutes phyla[333–335]. The typically low-abundance Enterobacteraceae expand in concert with inflammation[336] and degree of dysbiosis of the Firmicutes:Bacteroidetes ratio[333,337,338]. This shift is particularly notable in the context of inflammatory bowel disease, where massive blooms of pathogenic, nitrate-reducing Salmonella and Escherichia coli are seen in response to inflammation[333,334,338,339]. Presumably, in the setting of inflammation, excess nitrite and nitrate are formed along with reactive oxygen radicals and nitrogen oxides through NO oxidation, thereby increasing the amount of substrate available for respiratory denitrification and rapid bacterial growth[323,337]. Similarly, this concept has been proposed in pathognomonic populations of Pseudomonas and other Proteobacteria in patients with cystic fibrosis[323,337], suggesting that bacterial nitrate metabolism in the gut is an important selective factor in pathogenic bacterial survival, thereby acting to disrupt the luminal microenvironment and alter the generation of NO and uptake of nitrate and nitrite. This alteration in turn can promote chronic inflammation and contribute to the impaired NO signaling and inflammation-rich intravascular environment that is a hallmark PH.
NO Production
Although the majority of ingested nitrate and nitrite are absorbed into the circulation in the proximal intestine, commensal gut bacteria are able to directly produce NO and also modify NO production, potentially influencing regional blood flow, mucosal integrity, microbial diversity, and effective nitrate and nitrite uptake. Many bacteria are capable of reducing nitrate to nitrite to NO via the denitrification pathway. For example, both Lactobacilli and Bifidobacteria have been shown to generate NO from nitrite in culture, while Lactobacilli, Escherichia coli and Salmonella typhimurium all increase cecal NO production when supplied with nitrate[217,233,271,278].
Additionally, some bacteria, such as Lactobaciilus[340] and Streptomyces[341], produce NO via a bacterial NOS oxidation of L-arginine, akin to mammalian endothelial NOS function[342]. Both commensal and pathogenic bacteria possess this capability, although it remains unclear how active these mechanisms are in vivo and exactly what benefit this provides to bacteria. From the standpoint of NO signaling and PH, respiratory denitrification of nitrite and NOS-derived NO likely serve beneficial roles in modifying the local microenvironment, and maintaining adequate blood flow and mucus generation. Alternatively, the intriguing hypothesis has been raised that NO formation may relate to pathogen virulence via biosynthesis of toxins, protection from host oxidative damage, or even stimulation of blood vessel formation in tissue that is beneficial to bacterial proliferation[342].
Another avenue for microbial influence on NO production in the gut is via modulation of hydrogen sulfide (H2S) metabolism. H2S is a biologically important thiol that shares significant overlapping signaling activity with NO, reacts with NO-derived reactive species and has been implicated in modulating the pathogenesis of several inflammatory bowel diseases[91,343,344]. At least 50% of intestinal H2S is derived from a set of commensal Proteobacteria, called the sulfate-reducing bacteria (SRB), either by cysteine degradation or a dissimilatory sulfate reduction reaction[345]. H2S directly interfaces with nitrogen oxide reduction and the generation of intestinal NO by serving as an electron donor during nitrite reduction to NO by nitrite reductase, or by reacting with the nitrogen oxide S-nitrosothiol[91,102,346]. Notably, H2S is required for both efficient nitrite reduction to NO in the gut and in NO formation from nitrite in hypoxic systemic tissues[343,346]. Additionally, the major SRB families Desulfovibrionaceae (Desulfovibrio, etc.) and Enterobacteriaceae (Enterobacter, Salmonella, Escherichia, Shigella, etc.), found to be enriched in inflammatory bowel conditions as well as periodontal disease and metabolic syndrome[344,345], overlap significantly with those identified as pathogenic, nitratereducing Proteobacteria in the inflammatory gut and cystic fibrosis lung[323,337]. Indeed, there remains conflicting evidence regarding the precise role of H2S in the gut[344,347]. On one hand, it appears that lower concentrations of endogenously derived H2S exert beneficial cytoprotective, antioxidant and anti-inflammatory actions[344,348–351], while on the other, increased H2S formation by SRB can promote oxidant formation, impair colonic epithelial H2S detoxification and induce cytotoxicity and disruption of intestinal barrier integrity, thereby driving local and systemic inflammation[344,347,352,353]. Certainly, further understanding of the roles that H2S concentration, substrate, and location play in inflammation and nitrate metabolism remain to be more fully elucidated. However, intestinal bacterial metabolism of H2S may represent an additional therapeutic target to modify the bioavailability of nitrite, NO and nitrogen oxides, as well as enteric and systemic inflammation, and potentially vascular injury and PH.
Therapeutic Strategies to Target the Enterosalivary Pathway: Beyond Nitrate Supplementation
With the advent of scientific interest in the microbiome, modulation of the microbiome by prebiotics, probiotics, and antibiotics have gained recent attention as potentially effective avenues for treating a variety of conditions[354–356]. Each strategy seeks to select for a targeted, healthy set of microbiota. Prebiotics, for instance, refers to the use of foods, drugs, or supplements to selectively promote the growth of desired microbiota (Table 1)[357]. Probiotics, refers to the ingestion of live bacteria or bacterial communities in order to recolonize with or select for desired microbiota, while antibiotics refers to the use of anti-microbial drugs to kill unhealthy, or pathogenic bacteria (Table 1)[357]. Many of these targeted strategies have great potential to transition from epidemiological or homeopathic associations to rigorously studied therapeutics for PH and other vascular diseases.
Table 1.
Classifications of potential therapeutic strategies to modulate NO signaling of bacterial nitrate reduction to nitrite, NO and nitrogen oxides in vascular disease and pulmonary hypertension
| Prebiotics | |
| Foods, drugs, or food ingredients (supplements) indigestible by the host that selectively enhance the growth and activity of beneficial bacteria or bacterial communities in the gut[357,358]. | |
| Diet-based | Supplements |
| Probiotics | |
| Live bacteria or polymicrobial cultures that confer benefit on the host by colonizing or otherwise altering existing bacterial populations and their metabolism[357,400,402]. | |
| Strain-specific, single & consortia | Non-specific |
| Antibiotics & Antimicrobial Agents | ||
| Bactericidal drugs or substances that selectively and non-selectively kill or deplete populations of pathogenic bacteria[357]. | ||
| Non-selective | Broad-spectrum | Targeted |
|
||
Abbreviations: DASH, Dietary Approaches to Stop Hypertension; cLA, conjugated linoleic acid; FMT, fecal microbial transplantation; NO, nitric oxide; Ab, antibody
Prebiotic therapy: Dietary Interventions
Attempts to define prebiotics as more than just ‘food’ have led to the definition as, “non-digestible food ingredients that beneficially affect the host by selectively stimulating the growth and/or activity of one or a limited number of bacterial species already resident in the colon”[358]. However, changing one’s diet remains one of the most ubiquitous methods of risk reduction for disease and has potential to alter the microbiome and NO formation in a variety of ways. All dietary manipulations seek to alter systemic metabolic function at some level, though almost no mechanisms of observed effects are completely understood. The current trend in ‘biotic’ therapies is shifting from more generalized dietary recommendations toward more targeted supplementation with or elimination of specific elements of a given diet. Numerous studies have now begun to link dietary features (such as high fat intake, vegetarianism, etc.) and their associated health consequences with changes in the gut microbiome[157,359,360]. Here, the potential relationship between two major dietary strategies, the high fruit and vegetable diet recommended by the Dietary Approaches to Stop Hypertension (DASH) study[361] and the traditional, fatty acid-rich Mediterranean diet[362], and their impact on the enterosalivary nitrate pathway and development of PH and vascular diseases are discussed.
Major epidemiological studies have consistently identified that vegetable- and fruit-rich diets improve every major risk factor for PH and CVD, including blood pressure[361,363,364], ischemic stroke[365], ischemic heart disease[366], obesity, insulin resistance and diabetes[367], and metabolic syndrome[365,366,368,369].
Specifically, green leafy vegetables appear to be most important in conferring these benefits[369]. Green leafy vegetables as well as root vegetables such as beets are known to contain the highest amounts of inorganic nitrate[36,161,370]. In Western countries, a sub-analysis of the DASH study found that nitrate-mediated blood pressure effects were first seen at daily levels of about 160mg of vegetable-based nitrate[361,371], with a typical DASH-compliant diet estimated to contain a range of nitrate from 174–1222mg/day[161]. Unsurprisingly, estimates of average daily nitrate consumption in the U.S. and Europe, regions with high rates of CVD and vascular disease, reach only 40–100mg and 30–180mg, respectively[157,161,372]. Comparing this level of consumption to a study of the high vegetable-containing, traditional Japanese diet, a population with historically low rates of cardiovascular disease, Sobko et al. found the average daily nitrate consumption in Japan to be >1100mg/day for a 60kg person, and associated with increased circulating nitrate and nitrite and with reduced blood pressure[373]. Combining these dietary findings with the numerous studies already reviewed above that demonstrate physiological blood pressure and vascular effects and increased circulating levels of nitrate and nitrite with natural sources of nitrate in beetroot juice[374] and spinach[111], there is compelling evidence that at least some of the beneficial effects of major dietary initiatives are driven by the enterosalivary nitrate pathway.
A second potentially important dietary target in modifying PH risk is the consumption of “healthy” fats in the form of unsaturated fatty acids, such as oleic and linoleic acid[375]. Current dietary investigations have found these fatty acids and dietary nitrate to be concentrated in the traditional Mediterranean diet, a diet with beneficial effects on the development of CVD and its risk factors[362,375–377]. In contrast to the high-nitrate diet of Japan, the Seven Countries Study[378] found lower rates of CVD and cancer with the traditional Mediterranean diet, despite 37% of total daily energy coming from fat, versus only 11% in the traditional Japanese diet[378,379]. Further work has since identified associations in both diets with high content of alpha linoleic acids (ALA), estimated to make up approximately 24% of total energy intake along with oleic acid in the traditional Mediterranean diet[379–381]. Comparatively, U.S. consumption of total polyunsaturated fatty acids and ALA are around 6–7% and 0.6–0.7% of total energy intake, respectively[382,383]. These numbers, along with the relative incidence of CVD and vascular risk factors support the hypothesis of a possible synergistic, diet-mediated NO-effect between inorganic nitrates and unsaturated fatty acids[19]. The specifics of these dietary interventions remain largely speculative and have not been investigated in PH, but could offer a novel strategy for implementation and study.
Mechanistically, synergy between unsaturated fatty acids and ingested nitrate and nitrite may be mediated by the anti-inflammatory effects of electrophilic (electron pair acceptor via Michael addition), nitro-fatty acid (NO2-FA) formation in the stomach. Under low pH, non-enzymatic reduction of nitrite to NO produces an intermediate nitrogen dioxide radical that can react with dietary, unsaturated, electrophilic fatty acids such as the essential FAs, oleic and linoleic acid, to form nitro-conjugated fatty acids and S-nitrosothiol derivatives[93,384]. NO2-FAs exert pleiotropic post-translational, anti-oxidant and anti-inflammatory effects[105,321,385] that include inhibition of vascular smooth muscle proliferation and neointima formation after injury, cardioprotection, improved endothelial function[94,386–390], and activation of downstream NO signaling pathways[385,389,391–394]. In a high-fat diet model of PH, Kelley et al. found that mice supplemented with the NO2-FA, nitro-octadecenoic acid, for 6.5 out of 20 weeks on a high-fat diet had reductions in markers of oxidative stress, inflammation, right ventricular remodeling, and degree of pulmonary pressure elevation seen in mice without NO2-FA supplementation on the same high-fat diet[391]. Further, supplementation with the conjugated, diene-containing FA, conjugated linoleic acid (cLA), has been shown in itself to be beneficial in obesity and insulin sensitivity in humans, exert powerful anti-inflammatory effects in the setting of ischemia-reperfusion[72,105,384,395], and to alter the gut microbiome[396,397]. Given such evidence, it stands to reason that the full physiological impact of the enterosalivary nitrate pathway can involve both direct nitrite/NO-mediated and NO2-FA-mediated vascular responses. Further study of the dietary treatment and prevention of PH and vascular disease must, therefore, include consideration of both the nitrate-nitrite-NO and the nitrate-nitrite-NO-FA pathways.
Probiotics
As understanding of the nuanced bacterial interrelationships within the microbiome becomes more sophisticated, the ability to modify community structure and function through the selective introduction of beneficial bacteria in the form of probiotics will likely become more widespread. That is not to say that the practice of ingesting fermented foods for health and entertainment has not existed since Neolithic times[398], though it has only been a relatively short period that medical science has given serious consideration of this concept[399–401]. Formally, the modern definition of probiotics was set in 2001 as consisting of “live microorganisms which, when administered in adequate amounts, confer a health benefit on the host”[400,402]. In subsequent years, there has been an explosion of work demonstrating clinical benefit of probiotics in modifying cardiovascular risk, inflammatory and infectious bowel disorders, and even ventilator-associated pneumonia (reviewed in:[399,403]). Yet, the mechanisms of these associations continue to remain unclear and their relationship to clinical outcomes unproven.
The goal of probiotic administration is to functionally alter the microbe-host interaction to favorably affect health. Mechanistically, there exists numerous potential avenues to accomplish this goal, including positively or negatively affecting indigenous microbial populations, integration of probiotic microbes into existing communities, altering microbial and/or host metabolism and gene expression, and indirect perturbations to the ambient microenvironment[400]. While human and animal studies have established the ability to successfully populate the oral cavity or gastrointestinal tract with specific strains, combinations of bacteria, or bacteria genetically modified to produce enzymes or metabolites[74,404–407], it is by no means clear that repopulation by probiotic bacteria must occur to achieve its intended effects[309,407]. Given, however, that probiotic therapy exerts a beneficial effect on dental caries, periodontitis, and pharyngitis in the oral cavity[408–411], as well as on obesity, hypertension, insulin resistance, and heart failure via the gut(reviewed:[399,403]), there is clear potential to treat PH with microbial interventions by modifying either oral or gastrointestinal bacteria.
The overwhelming majority of research into probiotics has focused on the lactic acid fermenting bacteria, Bifidobacterium and Lactobacillus, likely due to the widespread historical reliance on fermented dairy products, consistency in manufacturing, and overall safety[399,401]. Indeed, members of these genera are among the only probiotics accepted in Canada and Italy as ones with sufficient evidence to market beneficial health claims[400] and achieve GRAS status (“generally recognized as safe”) in the U.S.[399]. Lactobacillus spp., in particular, appears to offer an enticing target for modulation of NO-effects. At the genus level, Lactobacilli are common and robust bacteria in the mouth and gut, able to tolerate low pH and to generate NO in culture[74,412]. In polymicrobial environments, Lactobacilli engage in advantageous, mutualistic interactions with other bacteria to survive under hypoxia and alter NO generation[217,413], as well as improve inflammation associated with inflammatory bowel conditions and reduce blood pressure in pre-hypertensive and hypertensive patients[401,403,414].
In particular, the commonly available probiotic strain, L. rhamnosus, is capable of reducing nitrate to nitrite[217], producing NO in the gut in response to oral nitrate administration[74,217,278], and to generate and isomerize cLA and exert anti-obesity effects in diet-induced obese mice in a cLA-dependent manner[279,403,415,416]. Functionally, L. rhamnosus mediates physiological effects in the mouth and has good adherence to saliva-coated surfaces[408,417]. It has also been linked to weight reduction, prevention of ischemia-reperfusion injury, and attenuation of cardiac hypertrophy and post-infarction heart failure in rodents[403,418,419], and beneficial effects in obesity and metabolic syndrome in humans[405,420]. Based on these findings, it is certainly plausible that L. rhamnosus or other probiotic bacterial strains, alone or in combination, may be able to improve nitrate and nitrite signaling in both the oral cavity and the gut, thus potentially influencing PH.
Further work on probiotic strains remains promising in the treatment and prevention of PH. Future studies will need not only to identify and isolate new candidate species, but also explore targeted engineering of beneficial strains to enhance their clinical effects.
Antibiotic & Antimicrobial Agents
In current use, antibiotics are blunt tools of mass microbial destruction that can permanently alter the microbiome and predispose to numerous diseases such as obesity and diabetes[421]. Overuse and antibiotic resistance have proliferated[422] to the point that the U.S. Food and Drug Administration has now banned the public sale of certain antibacterial soaps[423]. Recent ecological findings show that even low levels of common antibiotics contaminate groundwater and soil and can disrupt bacterial denitrification[424–426], possibly exerting pressure on nitrate- and NO-signaling through agriculture, human food sources, and even disease pathology. While broad discussion of antibiotics is far beyond the scope of this review, two very speculative concepts related to nitrate-signaling and PH will be briefly mentioned.
First, the logical concern resulting from the finding that vascular health may depend on the oral microbiome is to ask if current oral hygiene practices, particularly the use of mouthwash, could be causing more harm than good. After publication by Ahluwalia et al. that 7 days of chlorhexidine mouthwash significantly raises resting blood pressures in healthy volunteers[106], such questions became the subject of widespread public curiosity[427]. However, the answer remains unclear. Two studies have since shown increased blood pressures after chlorhexidine-containing mouthwash treatment in a population of hypertensive men on antihypertensive therapy[428] and during exercise in healthy volunteers[429], while a recent crossover study in healthy women showed no blood pressure effects with similar treatment[430]. Conversely, epidemiological studies consistently show that in the general population, good oral hygiene (specifically frequent tooth brushing and mouthwash use) protects against development of hypertension and CVD[431–433]. Such incongruities may be in part explained by evidence suggesting that one’s choice in mouthwash (from the strongest bactericidal formulations with chlorhexidine, to weaker ones such as with triclosan or cetylpyridinium, to the widely available and weakest antiseptic formulations) greatly impacts the degree of bacterial nitrate reduction and blood pressure effects[99,429,434], and that brushing with even bactericidal toothpaste exerts little to no effect on bacterial nitrate reduction[434]. Clearly, further targeted studies will be needed to fully understand if regular antimicrobial mouthwash use truly impacts the risk for development of PH and its risk factors.
Second, the ability to design and deliver targeted, bacteria- or community-specific antimicrobial therapies to selectively disrupt or augment oral and gut microbial populations and their nitrate and NO metabolism is a very real future[435]. Nitrite, for instance, disrupts protective biofilm formation by pathogenic bacteria and is currently under investigation as an adjunct to standard antibiotic therapy[235,236]. Potentially, a combination of nitrite-mediated biofilm dispersion and use of an antibiotic-conjugated antibody[436] or bacteriophage[437] with species-selectively may offer a multifaceted approach to target antimicrobial populations in the mouth. Yet, it is the discovery of bacteria-produced, antimicrobial peptides by human commensal bacteria that offer potentially significant informational and therapeutic value[438]. Recently, a Streptococcus mutans-specific peptide was developed and tested in a model of human saliva, where selective depletion of S. mutans triggered community-wide shifts in major nitrate-reducing and non-nitrate-reducing populations[439]. The implications of such a tool could allow greater understanding of bacterial networking and their metabolic interdependencies as well as the development of new tools to study and modify their nitrate-reducing activity.
Conclusions
The breadth of signaling responses instigated by nitrate, nitrite, NO and downstream products of nitrogen oxide reactions uniquely exists at the nexus of NO biology and the human-microbe interface. It is clear that these reactions are highly dependent on, and can be readily altered by, oral and gut bacteria separately or in combination. Whether changes to resident microbiota are beneficial or harmful in PH is unknown, but they could influence response to drug therapy, and augmentation of key bacteria or bacterial functions may improve efficacy of therapies with fewer side effects. There is obvious potential for the development of nitrate- and nitrite-based therapies for cardiovascular disease, PH, and their risk factors, and such studies are currently ongoing. Relationships of the microbiome to PH have never been directly examined, but represent a novel testable and modifiable factor in disease pathogenesis.
Future directions will need to include mechanistic and direct clinical testing of both oral nitrate therapy and microbial-based therapeutics or probiotics in PH. Further work is needed to understand the systemic role that enterosalivary nitrate circulation plays in pulmonary vascular health, onset of disease and its progression. The safety of brief and chronic administration of supplemental nitrate and nitrite in the context of PH and dysfunctional NO signaling must be ascertained[440], as well as the potential to generate formulations that are more bioavailable and efficacious while maintaining the relative paucity of negative effects. From the standpoint of microbial nitrate reduction, deeper insights must be ascertained into the complex relationships between bacteria and their local community, their host, and other factors such as fungi, viruses, and bacteriophages.
Despite these complexities, a rich fund of knowledge already exists in the form of effective PH therapies targeting NO signaling and ongoing clinical testing of inhaled and oral formulations of inorganic nitrate and nitrite, as well as synthetic nitro-fatty acids. The study of probiotics has identified specific bacterial strains and their combinations that are capable of inducing pre-clinical and clinical effects. These and future efforts should shed new light on the impact that metabolic convergence of the microbiome and nitrogen oxides will have on the treatment and prevention of PH, CVD, and other vascular diseases.
Highlights.
The microbiome may influence pulmonary hypertension via nitrogen oxide signaling.
Oral microbiota are necessary for nitrate reduction to vasoactive nitrogen oxides.
Dietary nitrate and nitrite are major sources of exogenous nitrogen oxides.
Dietary nitrate in vegetables exerts pleiotropic, beneficial nitrogen oxide effects.
Diet and probiotic therapy can alter beneficial nitrate and fatty acid metabolism.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Takahashi D, Hase K. Commensal microbiota-derived signals regulate host immune system through epigenetic modifications. Inflammation and Regeneration. 2015;35:129–136. [Google Scholar]
- 2.Tang WHW, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, et al. Intestinal Microbial Metabolism of Phosphatidylcholine and Cardiovascular Risk. N. Engl. J. Med. 2013;368:1575–1584. doi: 10.1056/NEJMoa1109400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013;19:576–585. doi: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lai Y-C, Potoka KC, Champion HC, Mora AL, Gladwin MT. Pulmonary arterial hypertension: the clinical syndrome. Circ. Res. 2014;115:115–130. doi: 10.1161/CIRCRESAHA.115.301146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simonneau G, Gatzoulis MA, Adatia I, Celermajer D, Denton C, Ghofrani A, et al. Updated clinical classification of pulmonary hypertension. J. Am. Coll. Cardiol. 2013;62:D34–D41. doi: 10.1016/j.jacc.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 6.Galiè N, Humbert M, Vachiery J-L, Gibbs S, Lang I, Torbicki A, et al. 2015 ESC/ERS Guidelines for the Diagnosis and Treatment of Pulmonary Hypertension. Rev Esp Cardiol (Engl Ed) 2016;69:177. doi: 10.1016/j.rec.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 7.Morris A, Gingo MR, George MP, Lucht L, Kessinger C, Singh V, et al. Cardiopulmonary function in individuals with HIV infection in the antiretroviral therapy era. Aids. 2012;26:731–740. doi: 10.1097/QAD.0b013e32835099ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.George MP, Champion HC, Gladwin MT, Norris KA, Morris A. Injection drug use as a “second hit” in the pathogenesis of HIV-associated pulmonary hypertension. Am J Respir Crit Care Med. 2012;185:1144–1146. doi: 10.1164/rccm.201204-0609ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tcherakian C, Couderc L-J, Humbert M, Godot V, Sitbon O, Devillier P. Inflammatory mechanisms in HIV-associated pulmonary arterial hypertension. Semin Respir Crit Care Med. 2013;34:645–653. doi: 10.1055/s-0033-1356489. [DOI] [PubMed] [Google Scholar]
- 10.Simon MA, Lacomis CD, George MP, Kessinger C, Weinman R, McMahon D, et al. Isolated right ventricular dysfunction in patients with human immunodeficiency virus. Journal of Cardiac Failure. 2014;20:414–421. doi: 10.1016/j.cardfail.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morse JH, Barst RJ, Itescu S, Flaster ER, Sinha G, Zhang Y, et al. Primary pulmonary hypertension in HIV infection: an outcome determined by particular HLA class II alleles. Am J Respir Crit Care Med. 1996;153:1299–1301. doi: 10.1164/ajrccm.153.4.8616557. [DOI] [PubMed] [Google Scholar]
- 12.Robbins IM, Newman JH, Johnson RF, Hemnes AR, Fremont RD, Piana RN, et al. Association of the metabolic syndrome with pulmonary venous hypertension. Chest. 2009;136:36. doi: 10.1378/chest.08-2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thenappan T, Shah SJ, Gomberg-Maitland M, Collander B, Vallakati A, Shroff P, et al. Clinical characteristics of pulmonary hypertension in patients with heart failure and preserved ejection fraction. Circ Heart Fail. 2011;4:257–265. doi: 10.1161/CIRCHEARTFAILURE.110.958801. [DOI] [PubMed] [Google Scholar]
- 14.Pugh ME, Robbins IM, Rice TW, West J, Newman JH, Hemnes AR. Unrecognized glucose intolerance is common in pulmonary arterial hypertension. J. Heart Lung Transplant. 2011;30:904–911. doi: 10.1016/j.healun.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lai Y-C, Tabima DM, Dube JJ, Hughan KS, Vanderpool RR, Goncharov DA, et al. SIRT3-AMP-Activated Protein Kinase Activation by Nitrite and Metformin Improves Hyperglycemia and Normalizes Pulmonary Hypertension Associated With Heart Failure With Preserved Ejection Fraction. Circulation. 2016;133:717–731. doi: 10.1161/CIRCULATIONAHA.115.018935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burger CD, Foreman AJ, Miller DP, Safford RE, McGoon MD, Badesch DB. Comparison of body habitus in patients with pulmonary arterial hypertension enrolled in the Registry to Evaluate Early and Long-term PAH Disease Management with normative values from the National Health and Nutrition Examination Survey. Mayo Clin. Proc. 2011;86:105–112. doi: 10.4065/mcp.2010.0394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nix LM, Tien PC. Metabolic syndrome, diabetes, and cardiovascular risk in HIV. Curr HIV/AIDS Rep. 2014;11:271–278. doi: 10.1007/s11904-014-0219-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jantarapakde J, Phanuphak N, Chaturawit C, Pengnonyang S, Mathajittiphan P, Takamtha P, et al. Prevalence of metabolic syndrome among antiretroviral-naive and antiretroviral-experienced HIV-1 infected Thai adults. AIDS Patient Care STDS. 2014;28:331–340. doi: 10.1089/apc.2013.0294. [DOI] [PubMed] [Google Scholar]
- 19.Lundberg JO, Gladwin MT, Weitzberg E. Strategies to increase nitric oxide signalling in cardiovascular disease. Nat Rev Drug Discov. 2015;14:623–641. doi: 10.1038/nrd4623. [DOI] [PubMed] [Google Scholar]
- 20.Moncada S, Higgs A. The L-arginine-nitric oxide pathway. N. Engl. J. Med. 1993;329:2002–2012. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- 21.Chatterjee A, Black SM, Catravas JD. Endothelial nitric oxide (NO) and its pathophysiologic regulation. Vascul. Pharmacol. 2008;49:134–140. doi: 10.1016/j.vph.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coggins MP, Bloch KD. Nitric oxide in the pulmonary vasculature, Arteriosclerosis. Thrombosis, and Vascular Biology. 2007;27:1877–1885. doi: 10.1161/ATVBAHA.107.142943. [DOI] [PubMed] [Google Scholar]
- 23.Förstermann U, Münzel T. Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation. 2006;113:1708–1714. doi: 10.1161/CIRCULATIONAHA.105.602532. [DOI] [PubMed] [Google Scholar]
- 24.Moncada S, Higgs EA. The discovery of nitric oxide and its role in vascular biology. British Journal of Pharmacology. 2006;147(Suppl 1):S193–S201. doi: 10.1038/sj.bjp.0706458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lundberg JO, Weitzberg E, Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov. 2008;7:156–167. doi: 10.1038/nrd2466. [DOI] [PubMed] [Google Scholar]
- 26.Galiè N, Palazzini M, Manes A. Pulmonary arterial hypertension: from the kingdom of the near-dead to multiple clinical trial meta-analyses. European Heart Journal. 2010;31:2080–2086. doi: 10.1093/eurheartj/ehq152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sitbon O, Morrell N. Pathways in pulmonary arterial hypertension: the future is here. Eur Respir Rev. 2012;21:321–327. doi: 10.1183/09059180.00004812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gaston B, Drazen JM, Loscalzo J, Stamler JS. The biology of nitrogen oxides in the airways. Am J Respir Crit Care Med. 1994;149:538–551. doi: 10.1164/ajrccm.149.2.7508323. [DOI] [PubMed] [Google Scholar]
- 29.Kaneko FT, Arroliga AC, Dweik RA, Comhair SA, Laskowski D, Oppedisano R, et al. Biochemical reaction products of nitric oxide as quantitative markers of primary pulmonary hypertension. Am J Respir Crit Care Med. 1998;158:917–923. doi: 10.1164/ajrccm.158.3.9802066. [DOI] [PubMed] [Google Scholar]
- 30.Ozkan M, Dweik RA, Laskowski D, Arroliga AC, Erzurum SC. High levels of nitric oxide in individuals with pulmonary hypertension receiving epoprostenol therapy. Lung. 2001;179:233–243. doi: 10.1007/s004080000064. [DOI] [PubMed] [Google Scholar]
- 31.Champion HC, Bivalacqua TJ, Greenberg SS, Giles TD, Hyman AL, Kadowitz PJ. Adenoviral gene transfer of endothelial nitric-oxide synthase (eNOS) partially restores normal pulmonary arterial pressure in eNOS-deficient mice. Proceedings of the National Academy of Sciences. 2002;99:13248–13253. doi: 10.1073/pnas.182225899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hsu LL, Champion HC, Campbell-Lee SA, Bivalacqua TJ, Manci EA, Diwan BA, et al. Hemolysis in sickle cell mice causes pulmonary hypertension due to global impairment in nitric oxide bioavailability. Blood. 2007;109:3088–3098. doi: 10.1182/blood-2006-08-039438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goret L, Tanguy S, Guiraud I, Dauzat M, Obert P. Acute administration of l-arginine restores nitric oxide-mediated relaxation in isolated pulmonary arteries from pulmonary hypertensive exercise trained rats. Eur. J. Pharmacol. 2008;581:148–156. doi: 10.1016/j.ejphar.2007.11.037. [DOI] [PubMed] [Google Scholar]
- 34.Tonelli AR, Haserodt S, Aytekin M, Dweik RA. Nitric oxide deficiency in pulmonary hypertension: Pathobiology and implications for therapy. Pulm Circ. 2013;3:20–30. doi: 10.4103/2045-8932.109911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Napoli C, Ignarro LJ. Nitric oxide and pathogenic mechanisms involved in the development of vascular diseases. Arch. Pharm. Res. 2009;32:1103–1108. doi: 10.1007/s12272-009-1801-1. [DOI] [PubMed] [Google Scholar]
- 36.Kobayashi J, Ohtake K, Uchida H. NO-Rich Diet for Lifestyle-Related Diseases. Nutrients. 2015;7:4911–4937. doi: 10.3390/nu7064911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sansbury BE, Hill BG. Regulation of obesity and insulin resistance by nitric oxide. Free Radic. Biol. Med. 2014;73:383–399. doi: 10.1016/j.freeradbiomed.2014.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rizzo NO, Maloney E, Pham M, Luttrell I, Wessells H, Tateya S, et al. Reduced NO-cGMP signaling contributes to vascular inflammation and insulin resistance induced by high-fat feeding, Arteriosclerosis. Thrombosis, and Vascular Biology. 2010;30:758–765. doi: 10.1161/ATVBAHA.109.199893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Omar SA, Webb AJ, Lundberg JO, Weitzberg E. Therapeutic effects of inorganic nitrate and nitrite in cardiovascular and metabolic diseases. J Intern Med. 2016;279:315–336. doi: 10.1111/joim.12441. [DOI] [PubMed] [Google Scholar]
- 40.Xue C, Johns RA. Endothelial nitric oxide synthase in the lungs of patients with pulmonary hypertension. N. Engl. J. Med. 1995;333:1642–1644. doi: 10.1056/NEJM199512143332416. [DOI] [PubMed] [Google Scholar]
- 41.Demoncheaux EAG, Higenbottam TW, Kiely DG, Wong J-M, Wharton S, Varcoe R, et al. Decreased whole body endogenous nitric oxide production in patients with primary pulmonary hypertension. J. Vasc. Res. 2005;42:133–136. doi: 10.1159/000083502. [DOI] [PubMed] [Google Scholar]
- 42.Black SM, Heidersbach RS, McMullan DM, Bekker JM, Johengen MJ, Fineman JR. Inhaled nitric oxide inhibits NOS activity in lambs: potential mechanism for rebound pulmonary hypertension. Am. J. Physiol. 1999;277:H1849–H1856. doi: 10.1152/ajpheart.1999.277.5.H1849. [DOI] [PubMed] [Google Scholar]
- 43.Xu W, Koeck T, Lara AR, Neumann D, DiFilippo FP, Koo M, et al. Alterations of cellular bioenergetics in pulmonary artery endothelial cells. Proceedings of the National Academy of Sciences. 2007;104:1342–1347. doi: 10.1073/pnas.0605080104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am. J. Physiol. 1996;271:C1424–C1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 45.Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ. Res. 2000;87:840–844. doi: 10.1161/01.res.87.10.840. [DOI] [PubMed] [Google Scholar]
- 46.Grobe AC, Wells SM, Benavidez E, Oishi P, Azakie A, Fineman JR, et al. Increased oxidative stress in lambs with increased pulmonary blood flow and pulmonary hypertension: role of NADPH oxidase and endothelial NO synthase. Am. J. Physiol. Lung Cell Mol. Physiol. 2006;290:L1069–L1077. doi: 10.1152/ajplung.00408.2005. [DOI] [PubMed] [Google Scholar]
- 47.Tabima DM, Frizzell S, Gladwin MT. Reactive oxygen and nitrogen species in pulmonary hypertension. Free Radic. Biol. Med. 2012;52:1970–1986. doi: 10.1016/j.freeradbiomed.2012.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Taichman DB, Ornelas J, Chung L, Klinger JR, Lewis S, Mandel J, et al. Pharmacologic therapy for pulmonary arterial hypertension in adults: CHEST guideline and expert panel report. Chest. 2014;146:449–475. doi: 10.1378/chest.14-0793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ghofrani H-A, Galiè N, Grimminger F, Grünig E, Humbert M, Jing Z-C, et al. Riociguat for the treatment of pulmonary arterial hypertension. N. Engl. J. Med. 2013;369:330–340. doi: 10.1056/NEJMoa1209655. [DOI] [PubMed] [Google Scholar]
- 50.Ignarro LJ, Lippton H, Edwards JC, Baricos WH, Hyman AL, Kadowitz PJ, et al. Mechanism of vascular smooth muscle relaxation by organic nitrates, nitrites, nitroprusside and nitric oxide: evidence for the involvement of S-nitrosothiols as active intermediates. J Pharmacol Exp Ther. 1981;218:739–749. [PubMed] [Google Scholar]
- 51.Cosby K, Partovi KS, Crawford JH, Patel RP, Reiter CD, Martyr S, et al. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat. Med. 2003;9:1498–1505. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- 52.Gladwin MT, Schechter AN, Kim-Shapiro DB, Patel RP, Hogg N, Shiva S, et al. The emerging biology of the nitrite anion. Nat. Chem. Biol. 2005:308–314. doi: 10.1038/nchembio1105-308. [DOI] [PubMed] [Google Scholar]
- 53.Huang Z, Shiva S, Kim-Shapiro DB, Patel RP, Ringwood LA, Irby CE, et al. Enzymatic function of hemoglobin as a nitrite reductase that produces NO under allosteric control. J. Clin. Invest. 2005;115:2099–2107. doi: 10.1172/JCI24650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dalsgaard T, Simonsen U, Fago A. Nitrite-dependent vasodilation is facilitated by hypoxia and is independent of known NO-generating nitrite reductase activities. Am. J. Physiol. Heart Circ. Physiol. 2007;292:H3072–H3078. doi: 10.1152/ajpheart.01298.2006. [DOI] [PubMed] [Google Scholar]
- 55.Dejam A, Hunter CJ, Tremonti C, Pluta RM, Hon YY, Grimes G, et al. Nitrite infusion in humans and nonhuman primates: endocrine effects, pharmacokinetics, and tolerance formation. Circulation. 2007;116:1821–1831. doi: 10.1161/CIRCULATIONAHA.107.712133. [DOI] [PubMed] [Google Scholar]
- 56.Gladwin MT, Kim-Shapiro DB. The functional nitrite reductase activity of the heme-globins. Blood. 2008;112:2636–2647. doi: 10.1182/blood-2008-01-115261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bueno M, Wang J, Mora AL, Gladwin MT. Nitrite signaling in pulmonary hypertension: mechanisms of bioactivation, signaling, and therapeutics. Antioxid. Redox Signal. 2013;18:1797–1809. doi: 10.1089/ars.2012.4833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim-Shapiro DB, Gladwin MT. Mechanisms of nitrite bioactivation. Nitric Oxide. 2014;38:58–68. doi: 10.1016/j.niox.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gladwin MT, Raat NJH, Shiva S, Dezfulian C, Hogg N, Kim-Shapiro DB, et al. Nitrite as a vascular endocrine nitric oxide reservoir that contributes to hypoxic signaling, cytoprotection, and vasodilation. Am. J. Physiol. Heart Circ. Physiol. 2006;291:H2026–H2035. doi: 10.1152/ajpheart.00407.2006. [DOI] [PubMed] [Google Scholar]
- 60.Gladwin MT, Shelhamer JH, Schechter AN, Pease-Fye ME, Waclawiw MA, Panza JA, et al. Role of circulating nitrite and S-nitrosohemoglobin in the regulation of regional blood flow in humans. Proceedings of the National Academy of Sciences. 2000;97:11482–11487. doi: 10.1073/pnas.97.21.11482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lidder S, Webb AJ. Vascular effects of dietary nitrate (as found in green leafy vegetables and beetroot) via the nitrate-nitrite-nitric oxide pathway. Br J Clin Pharmacol. 2013;75:677–696. doi: 10.1111/j.1365-2125.2012.04420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Classen HG, Stein-Hammer C, Thöni H. Hypothesis: the effect of oral nitrite on blood pressure in the spontaneously hypertensive rat. Does dietary nitrate mitigate hypertension after conversion to nitrite? J Am Coll Nutr. 1990;9:500–502. doi: 10.1080/07315724.1990.10720407. [DOI] [PubMed] [Google Scholar]
- 63.Beier S, Classen HG, Loeffler K, Schumacher E, Thöni H. Antihypertensive effect of oral nitrite uptake in the spontaneously hypertensive rat. Arzneimittelforschung. 1995;45:258–261. [PubMed] [Google Scholar]
- 64.Kanematsu Y, Yamaguchi K, Ohnishi H, Motobayashi Y, Ishizawa K, Izawa Y, et al. Dietary doses of nitrite restore circulating nitric oxide level and improve renal injury in L-NAME-induced hypertensive rats. Am. J. Physiol. Renal Physiol. 2008;295:F1457–F1462. doi: 10.1152/ajprenal.00621.2007. [DOI] [PubMed] [Google Scholar]
- 65.Kapil V, Milsom AB, Okorie M, Maleki-Toyserkani S, Akram F, Rehman F, et al. Inorganic nitrate supplementation lowers blood pressure in humans: role for nitrite-derived NO. Hypertension. 2010;56:274–281. doi: 10.1161/HYPERTENSIONAHA.110.153536. [DOI] [PubMed] [Google Scholar]
- 66.Ghosh SM, Kapil V, Fuentes-Calvo I, Bubb KJ, Pearl V, Milsom AB, et al. Enhanced vasodilator activity of nitrite in hypertension: critical role for erythrocytic xanthine oxidoreductase and translational potential. Hypertension. 2013;61:1091–1102. doi: 10.1161/HYPERTENSIONAHA.111.00933. [DOI] [PubMed] [Google Scholar]
- 67.Rassaf T, Heiss C, Hendgen-Cotta U, Balzer J, Matern S, Kleinbongard P, et al. Plasma nitrite reserve and endothelial function in the human forearm circulation. Free Radic. Biol. Med. 2006;41:295–301. doi: 10.1016/j.freeradbiomed.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 68.Stokes KY, Dugas TR, Tang Y, Garg H, Guidry E, Bryan NS. Dietary nitrite prevents hypercholesterolemic microvascular inflammation and reverses endothelial dysfunction. Am. J. Physiol. Heart Circ. Physiol. 2009;296:H1281–H1288. doi: 10.1152/ajpheart.01291.2008. [DOI] [PubMed] [Google Scholar]
- 69.Sindler AL, Fleenor BS, Calvert JW, Marshall KD, Zigler ML, Lefer DJ, et al. Nitrite supplementation reverses vascular endothelial dysfunction and large elastic artery stiffness with aging. Aging Cell. 2011;10:429–437. doi: 10.1111/j.1474-9726.2011.00679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rodriguez-Mateos A, Hezel M, Aydin H, Kelm M, Lundberg JO, Weitzberg E, et al. Interactions between cocoa flavanols and inorganic nitrate: additive effects on endothelial function at achievable dietary amounts. Free Radic. Biol. Med. 2015;80:121–128. doi: 10.1016/j.freeradbiomed.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 71.Lara J, Ashor AW, Oggioni C, Ahluwalia A, Mathers JC, Siervo M. Effects of inorganic nitrate and beetroot supplementation on endothelial function: a systematic review and meta-analysis. Eur J Nutr. 2016;55:451–459. doi: 10.1007/s00394-015-0872-7. [DOI] [PubMed] [Google Scholar]
- 72.Delmastro-Greenwood M, Hughan KS, Vitturi DA, Salvatore SR, Grimes G, Potti G, et al. Nitrite and nitrate-dependent generation of anti-inflammatory fatty acid nitroalkenes. Free Radic. Biol. Med. 2015;89:333–341. doi: 10.1016/j.freeradbiomed.2015.07.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Witter JP, Balish E, Gatley SJ. Distribution of nitrogen-13 from labeled nitrate and nitrite in germfree and conventional-flora rats. Applied and Environmental Microbiology. 1979;38:870–878. doi: 10.1128/aem.38.5.870-878.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sobko T, Reinders CI, Jansson E, Norin E, Midtvedt T, Lundberg JO. Gastrointestinal bacteria generate nitric oxide from nitrate and nitrite. Nitric Oxide. 2005;13:272–278. doi: 10.1016/j.niox.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 75.Lundberg JO, Govoni M. Inorganic nitrate is a possible source for systemic generation of nitric oxide. Free Radic. Biol. Med. 2004;37:395–400. doi: 10.1016/j.freeradbiomed.2004.04.027. [DOI] [PubMed] [Google Scholar]
- 76.Govoni M, Jansson EÅ, Weitzberg E, Lundberg JO. The increase in plasma nitrite after a dietary nitrate load is markedly attenuated by an antibacterial mouthwash. Nitric Oxide. 2008;19:333–337. doi: 10.1016/j.niox.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 77.Sparacino-Watkins C, Stolz JF, Basu P. Nitrate and periplasmic nitrate reductases. Chem Soc Rev. 2014;43:676–706. doi: 10.1039/c3cs60249d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Petersson J, Carlström M, Schreiber O, Phillipson M, Christoffersson G, Jägare A, et al. Gastroprotective and blood pressure lowering effects of dietary nitrate are abolished by an antiseptic mouthwash. Free Radic. Biol. Med. 2009;46:1068–1075. doi: 10.1016/j.freeradbiomed.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 79.van Velzen AG, Sips AMAJ, Schothorst RC, Lambers AC, Meulenbelt J. The oral bioavailability of nitrate from nitrate-rich vegetables in humans. Toxicology Letters. 2008;181:177–181. doi: 10.1016/j.toxlet.2008.07.019. [DOI] [PubMed] [Google Scholar]
- Licht WR, Schultz DS, Fox JG, Tannenbaum SR, Deen WM. Mechanisms for nitrite loss from the stomach. Carcinogenesis. 1986;7:1681–1687. doi: 10.1093/carcin/7.10.1681. [DOI] [PubMed] [Google Scholar]
- 81.Duncan C, Dougall H, Johnston P, Green S, Brogan R, Leifert C, et al. Chemical generation of nitric oxide in the mouth from the enterosalivary circulation of dietary nitrate. Nat. Med. 1995;1:546–551. doi: 10.1038/nm0695-546. [DOI] [PubMed] [Google Scholar]
- 82.Spiegelhalder B, Eisenbrand G, Preussmann R. Influence of dietary nitrate on nitrite content of human saliva: possible relevance to in vivo formation of N-nitroso compounds. Food Cosmet Toxicol. 1976;14:545–548. doi: 10.1016/s0015-6264(76)80005-3. [DOI] [PubMed] [Google Scholar]
- 83.McKnight GM, Smith LM, Drummond RS, Duncan CW, Golden M, Benjamin N. Chemical synthesis of nitric oxide in the stomach from dietary nitrate in humans. Gut. 1997;40:211–214. doi: 10.1136/gut.40.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xia D, Deng D, Wang S. Alterations of nitrate and nitrite content in saliva, serum, and urine in patients with salivary dysfunction. J Oral Pathol Med. 2003;32:95–99. doi: 10.1034/j.1600-0714.2003.00109.x. [DOI] [PubMed] [Google Scholar]
- 85.Qin L, Liu X, Sun Q, Fan Z, Xia D, Ding G, et al. Sialin (SLC17A5) functions as a nitrate transporter in the plasma membrane. 2012;109:13434–13439. doi: 10.1073/pnas.1116633109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jin L, Qin L, Xia D, Liu X, Fan Z, Zhang C, et al. Active secretion and protective effect of salivary nitrate against stress in human volunteers and rats. Free Radic. Biol. Med. 2013;57:61–67. doi: 10.1016/j.freeradbiomed.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Clodfelter WH, Basu S, Bolden C, Dos Santos PC, King SB, Kim-Shapiro DB. The relationship between plasma and salivary NOx. Nitric Oxide. 2015;47:85–90. doi: 10.1016/j.niox.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wagner DA, Schultz DS, Deen WM, Young VR, Tannenbaum SR. Metabolic fate of an oral dose of 15N-labeled nitrate in humans: effect of diet supplementation with ascorbic acid. Cancer Res. 1983;43:1921–1925. [PubMed] [Google Scholar]
- 89.Benjamin N, O'Driscoll F, Dougall H, Duncan C, Smith L, Golden M, et al. Stomach NO synthesis. Nature. 1994;368:502–502. doi: 10.1038/368502a0. [DOI] [PubMed] [Google Scholar]
- 90.Lundberg JO, Weitzberg E, Lundberg JM, Alving K. Intragastric nitric oxide production in humans: measurements in expelled air. Gut. 1994;35:1543–1546. doi: 10.1136/gut.35.11.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fukuto JM, Carrington SJ, Tantillo DJ, Harrison JG, Ignarro LJ, Freeman BA, et al. Small molecule signaling agents: the integrated chemistry and biochemistry of nitrogen oxides, oxides of carbon, dioxygen, hydrogen sulfide, and their derived species. Chem. Res. Toxicol. 2012;25:769–793. doi: 10.1021/tx2005234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Richardson G, Hicks SL, O'Byrne S, Frost MT, Moore K, Benjamin N, et al. The ingestion of inorganic nitrate increases gastric S-nitrosothiol levels and inhibits platelet function in humans. Nitric Oxide. 2002;7:24–29. doi: 10.1016/s1089-8603(02)00010-1. [DOI] [PubMed] [Google Scholar]
- 93.Freeman BA, Baker PRS, Schopfer FJ, Woodcock SR, Napolitano A, d'Ischia M. Nitro-fatty acid formation and signaling. 2008;283:15515–15519. doi: 10.1074/jbc.R800004200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schopfer FJ, Cipollina C, Freeman BA. Formation and signaling actions of electrophilic lipids. Chem. Rev. 2011;111:5997–6021. doi: 10.1021/cr200131e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bondonno CP, Croft KD, Puddey IB, Considine MJ, Yang X, Ward NC, et al. Nitrate causes a dose-dependent augmentation of nitric oxide status in healthy women. Food Funct. 2012;3:522–527. doi: 10.1039/c2fo10206d. [DOI] [PubMed] [Google Scholar]
- 96.Lundberg JO, Weitzberg E. Biology of nitrogen oxides in the gastrointestinal tract. Gut. 2013;62:616–629. doi: 10.1136/gutjnl-2011-301649. [DOI] [PubMed] [Google Scholar]
- 97.Webb AJ, Patel N, Loukogeorgakis S, Okorie M, Aboud Z, Misra S, et al. Acute blood pressure lowering, vasoprotective, and antiplatelet properties of dietary nitrate via bioconversion to nitrite. Hypertension. 2008;51:784–790. doi: 10.1161/HYPERTENSIONAHA.107.103523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hendgen-Cotta UB, Luedike P, Totzeck M, Kropp M, Schicho A, Stock P, et al. Dietary nitrate supplementation improves revascularization in chronic ischemia. Circulation. 2012;126:1983–1992. doi: 10.1161/CIRCULATIONAHA.112.112912. [DOI] [PubMed] [Google Scholar]
- 99.Woessner M, Smoliga JM, Tarzia B, Stabler T, Van Bruggen M, Allen JD. A stepwise reduction in plasma and salivary nitrite with increasing strengths of mouthwash following a dietary nitrate load. Nitric Oxide. 2016;54:1–7. doi: 10.1016/j.niox.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 100.Pereira C, Ferreira NR, Rocha BS, Barbosa RM, Laranjinha J. The redox interplay between nitrite and nitric oxide: From the gut to the brain. Redox Biol. 2013;1:276–284. doi: 10.1016/j.redox.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liu M, Zollbrecht C, Peleli M, Lundberg JO, Weitzberg E, Carlström M. Nitrite-mediated renal vasodilatation is increased during ischemic conditions via cGMP-independent signaling. Free Radic. Biol. Med. 2015;84:154–160. doi: 10.1016/j.freeradbiomed.2015.03.025. [DOI] [PubMed] [Google Scholar]
- 102.Vitturi DA, Minarrieta L, Salvatore SR, Postlethwait EM, Fazzari M, Ferrer-Sueta G, et al. Convergence of biological nitration and nitrosation via symmetrical nitrous anhydride. Nat. Chem. Biol. 2015;11:504–510. doi: 10.1038/nchembio.1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gow AJ, Farkouh CR, Munson DA, Posencheg MA, Ischiropoulos H. Biological significance of nitric oxide-mediated protein modifications. Am. J. Physiol. Lung Cell Mol. Physiol. 2004;287:L262–L268. doi: 10.1152/ajplung.00295.2003. [DOI] [PubMed] [Google Scholar]
- 104.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Delmastro-Greenwood M, Freeman BA, Wendell SG. Redox-dependent anti-inflammatory signaling actions of unsaturated fatty acids. Annu. Rev. Physiol. 2014;76:79–105. doi: 10.1146/annurev-physiol-021113-170341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kapil V, Haydar SMA, Pearl V, Lundberg JO, Weitzberg E, Ahluwalia A. Physiological role for nitrate-reducing oral bacteria in blood pressure control. Free Radic. Biol. Med. 2013;55:93–100. doi: 10.1016/j.freeradbiomed.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gee LC, Ahluwalia A. Dietary Nitrate Lowers Blood Pressure: Epidemiological, Pre-clinical Experimental and Clinical Trial Evidence. Curr. Hypertens. Rep. 2016;18:17. doi: 10.1007/s11906-015-0623-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Siervo M, Lara J, Ogbonmwan I, Mathers JC. Inorganic nitrate and beetroot juice supplementation reduces blood pressure in adults: a systematic review and meta-analysis. J. Nutr. 2013;143:818–826. doi: 10.3945/jn.112.170233. [DOI] [PubMed] [Google Scholar]
- 109.Hobbs DA, George TW, Lovegrove JA. The effects of dietary nitrate on blood pressure and endothelial function: a review of human intervention studies. Nutr Res Rev. 2013;26:210–222. doi: 10.1017/S0954422413000188. [DOI] [PubMed] [Google Scholar]
- 110.Kleinbongard P, Dejam A, Lauer T, Jax T, Kerber S, Gharini P, et al. Plasma nitrite concentrations reflect the degree of endothelial dysfunction in humans. Free Radic. Biol. Med. 2006;40:295–302. doi: 10.1016/j.freeradbiomed.2005.08.025. [DOI] [PubMed] [Google Scholar]
- 111.Liu AH, Bondonno CP, Croft KD, Puddey IB, Woodman RJ, Rich L, et al. Effects of a nitrate-rich meal on arterial stiffness and blood pressure in healthy volunteers. Nitric Oxide. 2013;35:123–130. doi: 10.1016/j.niox.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 112.Lee J-S, Stebbins CL, Jung E, Nho H, Kim J-K, Chang M-J, et al. Effects of chronic dietary nitrate supplementation on the hemodynamic response to dynamic exercise. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015;309:R459–R466. doi: 10.1152/ajpregu.00099.2015. [DOI] [PubMed] [Google Scholar]
- 113.Kapil V, Khambata RS, Robertson A, Caulfield MJ, Ahluwalia A. Dietary nitrate provides sustained blood pressure lowering in hypertensive patients: a randomized, phase 2, double-blind, placebo-controlled study. Hypertension. 2015;65:320–327. doi: 10.1161/HYPERTENSIONAHA.114.04675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Joris PJ, Mensink RP. Beetroot juice improves in overweight and slightly obese men postprandial endothelial function after consumption of a mixed meal. Atherosclerosis. 2013;231:78–83. doi: 10.1016/j.atherosclerosis.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 115.Velmurugan S, Gan JM, Rathod KS, Khambata RS, Ghosh SM, Hartley A, et al. Dietary nitrate improves vascular function in patients with hypercholesterolemia: a randomized, double-blind, placebo-controlled study. Am. J. Clin. Nutr. 2016;103:25–38. doi: 10.3945/ajcn.115.116244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kenjale AA, Ham KL, Stabler T, Robbins JL, Johnson JL, Vanbruggen M, et al. Dietary nitrate supplementation enhances exercise performance in peripheral arterial disease. J. Appl. Physiol. 2011;110:1582–1591. doi: 10.1152/japplphysiol.00071.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Alef MJ, Vallabhaneni R, Carchman E, Morris SM, Shiva S, Wang Y, et al. Nitrite-generated NO circumvents dysregulated arginine/NOS signaling to protect against intimal hyperplasia in Sprague-Dawley rats. J. Clin. Invest. 2011;121:1646–1656. doi: 10.1172/JCI44079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Alef MJ, Tzeng E, Zuckerbraun BS. Nitric oxide and nitrite-based therapeutic opportunities in intimal hyperplasia. Nitric Oxide. 2012;26:285–294. doi: 10.1016/j.niox.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 119.Vavra AK, Havelka GE, Martinez J, Lee VR, Fu B, Jiang Q, et al. Insights into the effect of nitric oxide and its metabolites nitrite and nitrate at inhibiting neointimal hyperplasia. Nitric Oxide. 2011;25:22–30. doi: 10.1016/j.niox.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Webb A, Bond R, McLean P, Uppal R, Benjamin N, Ahluwalia A. Reduction of nitrite to nitric oxide during ischemia protects against myocardial ischemia-reperfusion damage. Proceedings of the National Academy of Sciences. 2004;101:13683–13688. doi: 10.1073/pnas.0402927101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Duranski MR, Greer JJM, Dejam A, Jaganmohan S, Hogg N, Langston W, et al. Cytoprotective effects of nitrite during in vivo ischemia-reperfusion of the heart and liver. J. Clin. Invest. 2005;115:1232–1240. doi: 10.1172/JCI22493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Shiva S, Sack MN, Greer JJ, Duranski M, Ringwood LA, Burwell L, et al. Nitrite augments tolerance to ischemia/reperfusion injury via the modulation of mitochondrial electron transfer. J. Exp. Med. 2007;204:2089–2102. doi: 10.1084/jem.20070198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kumar D, Branch BG, Pattillo CB, Hood J, Thoma S, Simpson S, et al. Chronic sodium nitrite therapy augments ischemia-induced angiogenesis and arteriogenesis. 2008;105:7540–7545. doi: 10.1073/pnas.0711480105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ashmore T, Fernandez BO, Branco-Price C, West JA, Cowburn AS, Heather LC, et al. Dietary nitrate increases arginine availability and protects mitochondrial complex I and energetics in the hypoxic rat heart. J. Physiol. (Lond.) 2014;592:4715–4731. doi: 10.1113/jphysiol.2014.275263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ingram TE, Fraser AG, Bleasdale RA, Ellins EA, Margulescu AD, Halcox JP, et al. Low-dose sodium nitrite attenuates myocardial ischemia and vascular ischemia-reperfusion injury in human models. J. Am. Coll. Cardiol. 2013;61:2534–2541. doi: 10.1016/j.jacc.2013.03.050. [DOI] [PubMed] [Google Scholar]
- 126.Bhushan S, Kondo K, Polhemus DJ, Otsuka H, Nicholson CK, Tao Y-X, et al. Nitrite therapy improves left ventricular function during heart failure via restoration of nitric oxide-mediated cytoprotective signaling. Circ. Res. 2014;114:1281–1291. doi: 10.1161/CIRCRESAHA.114.301475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Pawlak-Chaouch M, Boissière J, Gamelin FX, Cuvelier G, Berthoin S, Aucouturier J. Effect of dietary nitrate supplementation on metabolic rate during rest and exercise in human: A systematic review and a meta-analysis. Nitric Oxide. 2016;53:65–76. doi: 10.1016/j.niox.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 128.Larsen FJ, Weitzberg E, Lundberg JO, Ekblom B. Effects of dietary nitrate on oxygen cost during exercise. Acta Physiol (Oxf) 2007;191:59–66. doi: 10.1111/j.1748-1716.2007.01713.x. [DOI] [PubMed] [Google Scholar]
- 129.Larsen FJ, Weitzberg E, Lundberg JO, Ekblom B. Dietary nitrate reduces maximal oxygen consumption while maintaining work performance in maximal exercise. Free Radic. Biol. Med. 2010;48:342–347. doi: 10.1016/j.freeradbiomed.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 130.Affourtit C, Bailey SJ, Jones AM, Smallwood MJ, Winyard PG. On the mechanism by which dietary nitrate improves human skeletal muscle function. Front. Physiol. 2015;6:211. doi: 10.3389/fphys.2015.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Jones AM. Dietary nitrate supplementation and exercise performance. Sports Med. 2014;44(Suppl 1):S35–S45. doi: 10.1007/s40279-014-0149-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Berry MJ, Justus NW, Hauser JI, Case AH, Helms CC, Basu S, et al. Dietary nitrate supplementation improves exercise performance and decreases blood pressure in COPD patients. Nitric Oxide. 2015;48:22–30. doi: 10.1016/j.niox.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Rammos C, Hendgen-Cotta UB, Totzeck M, Pohl J, Lüdike P, Flögel U, et al. Impact of dietary nitrate on age-related diastolic dysfunction. Eur. J. Heart Fail. 2016;18:599–610. doi: 10.1002/ejhf.535. [DOI] [PubMed] [Google Scholar]
- 134.Coggan AR, Leibowitz JL, Spearie CA, Kadkhodayan A, Thomas DP, Ramamurthy S, et al. Acute Dietary Nitrate Intake Improves Muscle Contractile Function in Patients With Heart Failure: A Double-Blind, Placebo-Controlled, Randomized Trial. Circ Heart Fail. 2015;8:914–920. doi: 10.1161/CIRCHEARTFAILURE.115.002141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Coggan AR, Peterson LR. Dietary Nitrate and Skeletal Muscle Contractile Function in Heart Failure. Curr Heart Fail Rep. 2016;13:158–165. doi: 10.1007/s11897-016-0293-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kerley CP, O’Neill JO, Reddy Bijjam V, Blaine C, James PE, Cormican L. Dietary nitrate increases exercise tolerance in patients with non-ischemic, dilated cardiomyopathy-a double-blind, randomized, placebo-controlled, crossover trial. J. Heart Lung Transplant. 2016;35:922–926. doi: 10.1016/j.healun.2016.01.018. [DOI] [PubMed] [Google Scholar]
- 137.Zamani P, Rawat D, Shiva-Kumar P, Geraci S, Bhuva R, Konda P, et al. Effect of inorganic nitrate on exercise capacity in heart failure with preserved ejection fraction. Circulation. 2015;131:371–380. doi: 10.1161/CIRCULATIONAHA.114.012957. – discussion 380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Larsen FJ, Schiffer TA, Borniquel S, Sahlin K, Ekblom B, Lundberg JO, et al. Dietary inorganic nitrate improves mitochondrial efficiency in humans. Cell Metab. 2011;13:149–159. doi: 10.1016/j.cmet.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 139.Frostell C, Fratacci MD, Wain JC, Jones R, Zapol WM. Inhaled nitric oxide. A selective pulmonary vasodilator reversing hypoxic pulmonary vasoconstriction. Circulation. 1991;83:2038–2047. doi: 10.1161/01.cir.83.6.2038. [DOI] [PubMed] [Google Scholar]
- 140.Pepke-Zaba J, Higenbottam TW, Dinh-Xuan AT, Stone D, Wallwork J. Inhaled nitric oxide as a cause of selective pulmonary vasodilatation in pulmonary hypertension. The Lancet. 1991;338:1173–1174. doi: 10.1016/0140-6736(91)92033-x. [DOI] [PubMed] [Google Scholar]
- 141.Totzeck M, Hendgen-Cotta UB, Luedike P, Berenbrink M, Klare JP, Steinhoff H-J, et al. Nitrite regulates hypoxic vasodilation via myoglobin-dependent nitric oxide generation. Circulation. 2012;126:325–334. doi: 10.1161/CIRCULATIONAHA.111.087155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Blood AB, Schroeder HJ, Terry MH, Merrill-Henry J, Bragg SL, Vrancken K, et al. Inhaled nitrite reverses hemolysis-induced pulmonary vasoconstriction in newborn lambs without blood participation. Circulation. 2011;123:605–612. doi: 10.1161/CIRCULATIONAHA.110.001073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hunter CJ, Dejam A, Blood AB, Shields H, Kim-Shapiro DB, Machado RF, et al. Inhaled nebulized nitrite is a hypoxia-sensitive NO-dependent selective pulmonary vasodilator. Nat. Med. 2004;10:1122–1127. doi: 10.1038/nm1109. [DOI] [PubMed] [Google Scholar]
- 144.Ingram TE, Pinder AG, Bailey DM, Fraser AG, James PE. Low-dose sodium nitrite vasodilates hypoxic human pulmonary vasculature by a means that is not dependent on a simultaneous elevation in plasma nitrite. Am. J. Physiol. Heart Circ. Physiol. 2010;298:H331–H339. doi: 10.1152/ajpheart.00583.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zuckerbraun BS, Shiva S, Ifedigbo E, Mathier MA, Mollen KP, Rao J, et al. Nitrite potently inhibits hypoxic and inflammatory pulmonary arterial hypertension and smooth muscle proliferation via xanthine oxidoreductase-dependent nitric oxide generation. Circulation. 2010;121:98–109. doi: 10.1161/CIRCULATIONAHA.109.891077. [DOI] [PubMed] [Google Scholar]
- 146.Casey DB, Badejo AM, Dhaliwal JS, Murthy SN, Hyman AL, Nossaman BD, et al. Pulmonary vasodilator responses to sodium nitrite are mediated by an allopurinol-sensitive mechanism in the rat. Am. J. Physiol. Heart Circ. Physiol. 2009;296:H524–H533. doi: 10.1152/ajpheart.00543.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Pankey EA, Badejo AM, Casey DB, Lasker GF, Riehl RA, Murthy SN, et al. Effect of chronic sodium nitrite therapy on monocrotaline-induced pulmonary hypertension. Nitric Oxide. 2012;27:1–8. doi: 10.1016/j.niox.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Dias-Junior CAC, Gladwin MT, Tanus-Santos JE. Low-dose intravenous nitrite improves hemodynamics in a canine model of acute pulmonary thromboembolism. Free Radic. Biol. Med. 2006;41:1764–1770. doi: 10.1016/j.freeradbiomed.2006.08.022. [DOI] [PubMed] [Google Scholar]
- 149.Horstman DJ, Frank DU, Rich GF. Prolonged inhaled NO attenuates hypoxic, but not monocrotaline-induced, pulmonary vascular remodeling in rats. Anesth. Analg. 1998;86:74–81. doi: 10.1097/00000539-199801000-00015. [DOI] [PubMed] [Google Scholar]
- 150.Baliga RS, Milsom AB, Ghosh SM, Trinder SL, Macallister RJ, Ahluwalia A, et al. Dietary nitrate ameliorates pulmonary hypertension: cytoprotective role for endothelial nitric oxide synthase and xanthine oxidoreductase. Circulation. 2012;125:2922–2932. doi: 10.1161/CIRCULATIONAHA.112.100586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Eggebeen J, Kim-Shapiro DB, Haykowsky M, Morgan TM, Basu S, Brubaker P, et al. One Week of Daily Dosing With Beetroot Juice Improves Submaximal Endurance and Blood Pressure in Older Patients With Heart Failure and Preserved Ejection Fraction. JACC Heart Fail. 2016;4:428–437. doi: 10.1016/j.jchf.2015.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Doyle MP, Pickering RA, DeWeert TM, Hoekstra JW, Pater D. Kinetics and mechanism of the oxidation of human deoxyhemoglobin by nitrites. J. Biol. Chem. 1981;256:12393–12398. [PubMed] [Google Scholar]
- 153.Cortazzo JA, Lichtman AD. Methemoglobinemia: a review and recommendations for management. J. Cardiothorac. Vasc. Anesth. 2014;28:1043–1047. doi: 10.1053/j.jvca.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 154.Comly HH. Landmark article Sept 8, 1945: Cyanosis in infants caused by nitrates in well-water. By Hunter H. Comly, Jama. 1987;257:2788–2792. [PubMed] [Google Scholar]
- 155.Ahluwalia A, Gladwin M, Coleman GD, Hord N, Howard G, Kim-Shapiro DB, et al. Dietary Nitrate and the Epidemiology of Cardiovascular Disease: Report From a National Heart, Lung, and Blood Institute Workshop. J Am Heart Assoc. 2016;5:e003402–e003411. doi: 10.1161/JAHA.116.003402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Cornblath M, Hartmann AF. Methemoglobinemia in young infants. J. Pediatr. 1948;33:421–425. doi: 10.1016/s0022-3476(48)80200-3. [DOI] [PubMed] [Google Scholar]
- 157.Rathod KS, Velmurugan S, Ahluwalia A. A “green” diet-based approach to cardiovascular health? Is inorganic nitrate the answer? Molecular Nutrition & Food Research. 2016;60:185–202. doi: 10.1002/mnfr.201500313. [DOI] [PubMed] [Google Scholar]
- 158.Hunault CC, van Velzen AG, Sips AJAM, Schothorst RC, Meulenbelt J. Bioavailability of sodium nitrite from an aqueous solution in healthy adults. Toxicology Letters. 2009;190:48–53. doi: 10.1016/j.toxlet.2009.06.865. [DOI] [PubMed] [Google Scholar]
- 159.J.F.W.E. Consultation. Nitrate (and potential endogenous formation of N-nitroso compounds) In: Speijers GJ, van den Brandt PA, editors. Safety Evaluation of Certain Food Additives and Contaminants. Geneva: 2003. http://www.inchem.org/documents/jecfa/jecmono/v50je05.htm [Google Scholar]
- 160.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. IARC monographs on the evaluation of carcinogenic risks to humans. Ingested nitrate and nitrite, and cyanobacterial peptide toxins. IARC Monogr Eval Carcinog Risks Hum. 2010;94:v–vii. 1–412. [PMC free article] [PubMed] [Google Scholar]
- 161.Hord NG, Tang Y, Bryan NS. Food sources of nitrates and nitrites: the physiologic context for potential health benefits. Am. J. Clin. Nutr. 2009;90:1–10. doi: 10.3945/ajcn.2008.27131. [DOI] [PubMed] [Google Scholar]
- 162.Vermeer IT, van Maanen JM. Nitrate exposure and the endogenous formation of carcinogenic nitrosamines in humans. Rev Environ Health. 2001;16:105–116. doi: 10.1515/reveh.2001.16.2.105. [DOI] [PubMed] [Google Scholar]
- 163.Cross AJ, Ferrucci LM, Risch A, Graubard BI, Ward MH, Park Y, et al. A large prospective study of meat consumption and colorectal cancer risk: an investigation of potential mechanisms underlying this association. Cancer Res. 2010;70:2406–2414. doi: 10.1158/0008-5472.CAN-09-3929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ferrucci LM, Sinha R, Ward MH, Graubard BI, Hollenbeck AR, Kilfoy BA, et al. Meat and components of meat and the risk of bladder cancer in the NIH-AARP Diet and Health Study. Cancer. 2010;116:4345–4353. doi: 10.1002/cncr.25463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Sinha R, Park Y, Graubard BI, Leitzmann MF, Hollenbeck A, Schatzkin A, et al. Meat and meat-related compounds and risk of prostate cancer in a large prospective cohort study in the United States. Am. J. Epidemiol. 2009;170:1165–1177. doi: 10.1093/aje/kwp280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Dietrich M, Block G, Pogoda JM, Buffler P, Hecht S, Preston-Martin S. A review: dietary and endogenously formed N-nitroso compounds and risk of childhood brain tumors. Cancer Causes Control. 2005;16:619–635. doi: 10.1007/s10552-005-0168-y. [DOI] [PubMed] [Google Scholar]
- 167.Helser MA, Hotchkiss JH, Roe DA. Influence of fruit and vegetable juices on the endogenous formation of N-nitrosoproline and N-nitrosothiazolidine-4-carboxylic acid in humans on controlled diets. Carcinogenesis. 1992;13:2277–2280. doi: 10.1093/carcin/13.12.2277. [DOI] [PubMed] [Google Scholar]
- 168.Bartsch H, Pignatelli B, Calmels S, Ohshima H. Inhibition of nitrosation. Basic Life Sci. 1993;61:27–44. doi: 10.1007/978-1-4615-2984-2_3. [DOI] [PubMed] [Google Scholar]
- 169.Bartsch H, Ohshima H, Pignatelli B, Calmels S. Human exposure to endogenous N-nitroso compounds: quantitative estimates in subjects at high risk for cancer of the oral cavity, oesophagus, stomach and urinary bladder. Cancer Surv. 1989;8:335–362. [PubMed] [Google Scholar]
- 170.Chen T, Yu W-H, Izard J, Baranova OV, Lakshmanan A, Dewhirst FE. The Human Oral Microbiome Database: a web accessible resource for investigating oral microbe taxonomic and genomic information. Database (Oxford) 2010;2010:baq013–baq013. doi: 10.1093/database/baq013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Dewhirst FE, Chen T, Izard J, Paster BJ, Tanner ACR, Yu W-H, et al. The human oral microbiome. J. Bacteriol. 2010;192:5002–5017. doi: 10.1128/JB.00542-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zaura E, Keijser BJF, Huse SM, Crielaard W. Defining the healthy “core microbiome” of oral microbial communities. BMC Microbiol. 2009;9:259. doi: 10.1186/1471-2180-9-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Segata N, Haake SK, Mannon P, Lemon KP, Waldron L, Gevers D, et al. Composition of the adult digestive tract bacterial microbiome based on seven mouth surfaces, tonsils, throat and stool samples. Genome Biol. 2012;13:R42. doi: 10.1186/gb-2012-13-6-r42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Eren AM, Borisy GG, Huse SM, Mark Welch JL. Oligotyping analysis of the human oral microbiome. 2014;111:E2875–E2884. doi: 10.1073/pnas.1409644111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Bik EM, Long CD, Armitage GC, Loomer P, Emerson J, Mongodin EF, et al. Bacterial diversity in the oral cavity of 10 healthy individuals. The ISME Journal. 2010;4:962–974. doi: 10.1038/ismej.2010.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Aas JA, Paster BJ, Stokes LN, Olsen I, Dewhirst FE. Defining the normal bacterial flora of the oral cavity. J. Clin. Microbiol. 2005;43:5721–5732. doi: 10.1128/JCM.43.11.5721-5732.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Ahn J, Yang L, Paster BJ, Ganly I, Morris L, Pei Z, et al. Oral microbiome profiles: 16S rRNA pyrosequencing and microarray assay comparison. PLoS ONE. 2011;6:e22788. doi: 10.1371/journal.pone.0022788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Xu X, He J, Xue J, Wang Y, Li K, Zhang K, et al. Oral cavity contains distinct niches with dynamic microbial communities. Environmental Microbiology. 2015;17:699–710. doi: 10.1111/1462-2920.12502. [DOI] [PubMed] [Google Scholar]
- 179.Krishnan K, Chen T, Paster BJ. A practical guide to the oral microbiome and its relation to health and disease. Oral Dis. 2016 doi: 10.1111/odi.12509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.McLean JS. Advancements toward a systems level understanding of the human oral microbiome. Frontiers in Cellular and Infection Microbiology. 2014;4:98. doi: 10.3389/fcimb.2014.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Chen H, Jiang W. Application of high-throughput sequencing in understanding human oral microbiome related with health and disease. Front Microbiol. 2014;5:508. doi: 10.3389/fmicb.2014.00508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kumar PS. From focal sepsis to periodontal medicine: a century of exploring the role of the oral microbiome in systemic disease. J. Physiol. (Lond.) 2016 doi: 10.1113/JP272427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Takahashi N. Oral Microbiome Metabolism: From "Who Are They?" to "What Are They Doing?". Journal of Dental Research. 2015;94:1628–1637. doi: 10.1177/0022034515606045. [DOI] [PubMed] [Google Scholar]
- 184.Gilbert A, Lion G. Arterites infectieuses experimentales. Comptes Rendus Hebdomadaires Des Séances Et Mémoires De La Societé De Biologie. 1889;6:583–600. [Google Scholar]
- 185.Ford PJ, Gemmell E, Timms P, Chan A, Preston FM, Seymour GJ. Anti-P. gingivalis response correlates with atherosclerosis. Journal of Dental Research. 2007;86:35–40. doi: 10.1177/154405910708600105. [DOI] [PubMed] [Google Scholar]
- 186.Morris A, Beck JM, Schloss PD, Campbell TB, Crothers K, Curtis JL, et al. Comparison of the respiratory microbiome in healthy nonsmokers and smokers. Am J Respir Crit Care Med. 2013;187:1067–1075. doi: 10.1164/rccm.201210-1913OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Atanasova KR, Yilmaz O. Looking in the Porphyromonas gingivalis cabinet of curiosities: the microbium, the host and cancer association. Mol Oral Microbiol. 2014;29:55–66. doi: 10.1111/omi.12047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Duran-Pinedo AE, Frias-Lopez J. Beyond microbial community composition: functional activities of the oral microbiome in health and disease. Microbes Infect. 2015;17:505–516. doi: 10.1016/j.micinf.2015.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Zaura E, Mira A. Editorial: the oral microbiome in an ecological perspective. Frontiers in Cellular and Infection Microbiology. 2015;5:39. doi: 10.3389/fcimb.2015.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Hyde ER, Andrade F, Vaksman Z, Parthasarathy K, Jiang H, Parthasarathy DK, et al. Metagenomic analysis of nitrate-reducing bacteria in the oral cavity: implications for nitric oxide homeostasis. PLoS ONE. 2014;9:e88645. doi: 10.1371/journal.pone.0088645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Doel JJ, Hector MP, Amirtham CV, Al-Anzan LA, Benjamin N, Allaker RP. Protective effect of salivary nitrate and microbial nitrate reductase activity against caries. Eur. J. Oral Sci. 2004;112:424–428. doi: 10.1111/j.1600-0722.2004.00153.x. [DOI] [PubMed] [Google Scholar]
- 192.Schreiber F, Stief P, Gieseke A, Heisterkamp IM, Verstraete W, de Beer D, et al. Denitrification in human dental plaque. BMC Biol. 2010;8:24. doi: 10.1186/1741-7007-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Sánchez GA, Miozza VA, Delgado A, Busch L. Total salivary nitrates and nitrites in oral health and periodontal disease. Nitric Oxide. 2014;36:31–35. doi: 10.1016/j.niox.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 194.Keijser BJF, Zaura E, Huse SM, van der Vossen JMBM, Schuren FHJ, Montijn RC, et al. Pyrosequencing analysis of the oral microflora of healthy adults. Journal of Dental Research. 2008;87:1016–1020. doi: 10.1177/154405910808701104. [DOI] [PubMed] [Google Scholar]
- 195.Kox M, Jetten M. The nitrogen cycle. Principles of Plant-Microbe Interactions. 2015 [Google Scholar]
- 196.Brittain T, Blackmore R, Greenwood C, Thomson AJ. Bacterial nitrite-reducing enzymes. Eur. J. Biochem. 1992;209:793–802. doi: 10.1111/j.1432-1033.1992.tb17350.x. [DOI] [PubMed] [Google Scholar]
- 197.Maia LB, Moura JJG. How biology handles nitrite. Chem. Rev. 2014;114:5273–5357. doi: 10.1021/cr400518y. [DOI] [PubMed] [Google Scholar]
- 198.Mania D, Heylen K, van Spanning RJM, Frostegård Å. The nitrate-ammonifying and nosZ-carrying bacterium Bacillus vireti is a potent source and sink for nitric and nitrous oxide under high nitrate conditions. Environmental Microbiology. 2014;16:3196–3210. doi: 10.1111/1462-2920.12478. [DOI] [PubMed] [Google Scholar]
- 199.Jepson BJN, Mohan S, Clarke TA, Gates AJ, Cole JA, Butler CS, et al. Spectropotentiometric and structural analysis of the periplasmic nitrate reductase from Escherichia coli. J. Biol. Chem. 2007;282:6425–6437. doi: 10.1074/jbc.M607353200. [DOI] [PubMed] [Google Scholar]
- 200.Li H, Duncan C, Townend J, Killham K, Smith LM, Johnston P, et al. Nitrate-reducing bacteria on rat tongues. Applied and Environmental Microbiology. 1997;63:924–930. doi: 10.1128/aem.63.3.924-930.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Doel JJ, Benjamin N, Hector MP, Rogers M, Allaker RP. Evaluation of bacterial nitrate reduction in the human oral cavity. Eur. J. Oral Sci. 2005;113:14–19. doi: 10.1111/j.1600-0722.2004.00184.x. [DOI] [PubMed] [Google Scholar]
- 202.Smith AJ, Benjamin N, Weetman DA. The microbial generation of nitric oxide in the human oral cavity. Microbial Ecology in …. 1999;11:23–27. [Google Scholar]
- 203.Palmerini CA, Palombari R, Perito S, Arienti G. NO synthesis in human saliva. Free Radic Res. 2003;37:29–31. doi: 10.1080/1071576021000028398. [DOI] [PubMed] [Google Scholar]
- 204.Hyde ER, Luk B, Cron S, Kusic L, McCue T, Bauch T, et al. Characterization of the rat oral microbiome and the effects of dietary nitrate. Free Radic. Biol. Med. 2014;77:249–257. doi: 10.1016/j.freeradbiomed.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 205.Koopman JE, Buijs MJ, Brandt BW, Keijser BJF, Crielaard W, Zaura E. Nitrate and the Origin of Saliva Influence Composition and Short Chain Fatty Acid Production of Oral Microcosms. Microb Ecol. 2016;72:479–492. doi: 10.1007/s00248-016-0775-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Luo T, Srinivasan U, Ramadugu K, Shedden KA, Neiswanger K, Trumble E, et al. Effects of Specimen Collection Methodologies and Storage Conditions on the Short-Term Stability of Oral Microbiome Taxonomy. Applied and Environmental Microbiology. 2016;82:5519–5529. doi: 10.1128/AEM.01132-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Moir JW, Wood NJ. Nitrate and nitrite transport in bacteria. Cell. Mol. Life Sci. 2001;58:215–224. doi: 10.1007/PL00000849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Jia W, Cole JA. Nitrate and nitrite transport in Escherichia coli. Biochem. Soc. Trans. 2005;33:159–161. doi: 10.1042/BST0330159. [DOI] [PubMed] [Google Scholar]
- 209.Goddard AD, Moir JWB, Richardson DJ, Ferguson SJ. Interdependence of two NarK domains in a fused nitrate/nitrite transporter. Mol. Microbiol. 2008;70:667–681. doi: 10.1111/j.1365-2958.2008.06436.x. [DOI] [PubMed] [Google Scholar]
- 210.Zheng H, Wisedchaisri G, Gonen T. Crystal structure of a nitrate/nitrite exchanger. Nature. 2013;497:647–651. doi: 10.1038/nature12139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Jia W, Tovell N, Clegg S, Trimmer M, Cole J. A single channel for nitrate uptake, nitrite export and nitrite uptake by Escherichia coli NarU and a role for NirC in nitrite export and uptake. Biochemical Journal. 2009;417:297–307. doi: 10.1042/BJ20080746. [DOI] [PubMed] [Google Scholar]
- 212.Yan H, Huang W, Yan C, Gong X, Jiang S, Zhao Y, et al. Structure and mechanism of a nitrate transporter. Cell Rep. 2013;3:716–723. doi: 10.1016/j.celrep.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 213.Noji S, Nohno T, Saito T, Taniguchi S. The narK gene product participates in nitrate transport induced in Escherichia coli nitrate-respiring cells. FEBS Letters. 1989;252:139–143. doi: 10.1016/0014-5793(89)80906-8. [DOI] [PubMed] [Google Scholar]
- 214.Jakubovics NS. Talk of the town: interspecies communication in oral biofilms. Mol Oral Microbiol. 2010;25:4–14. doi: 10.1111/j.2041-1014.2009.00563.x. [DOI] [PubMed] [Google Scholar]
- 215.Nobbs AH, Jenkinson HF. Interkingdom networking within the oral microbiome. Microbes Infect. 2015;17:484–492. doi: 10.1016/j.micinf.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.González PJ, Correia C, Moura I, Brondino CD, Moura JJG. Bacterial nitrate reductases: Molecular and biological aspects of nitrate reduction. J. Inorg. Biochem. 2006;100:1015–1023. doi: 10.1016/j.jinorgbio.2005.11.024. [DOI] [PubMed] [Google Scholar]
- 217.Sobko T, Huang L, Midtvedt T, Norin E, Gustafsson LE, Norman M, et al. Generation of NO by probiotic bacteria in the gastrointestinal tract. Free Radic. Biol. Med. 2006;41:985–991. doi: 10.1016/j.freeradbiomed.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 218.Bashan A, Gibson TE, Friedman J, Carey VJ, Weiss ST, Hohmann EL, et al. Universality of human microbial dynamics. Nature. 2016;534:259–262. doi: 10.1038/nature18301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Lee MS, Oh S, Tang H. Characterization of microbial associations in human oral microbiome. Biomed Mater Eng. 2014;24:3737–3744. doi: 10.3233/BME-141202. [DOI] [PubMed] [Google Scholar]
- 220.Ly M, Abeles SR, Boehm TK, Robles-Sikisaka R, Naidu M, Santiago-Rodriguez T, et al. Altered oral viral ecology in association with periodontal disease. mBio. 2014;5:e01133–e01114. doi: 10.1128/mBio.01133-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Wang J, Gao Y, Zhao F. Phage-bacteria interaction network in human oral microbiome. Environmental Microbiology. 2016;18:2143–2158. doi: 10.1111/1462-2920.12923. [DOI] [PubMed] [Google Scholar]
- 222.Cool CD, Rai PR, Yeager ME, Hernandez-Saavedra D, Serls AE, Bull TM, et al. Expression of human herpesvirus 8 in primary pulmonary hypertension. N. Engl. J. Med. 2003;349:1113–1122. doi: 10.1056/NEJMoa035115. [DOI] [PubMed] [Google Scholar]
- 223.Mukherjee PK, Chandra J, Retuerto M, Sikaroodi M, Brown RE, Jurevic R, et al. Oral Mycobiome Analysis of HIV-Infected Patients: Identification of Pichia as an Antagonist of Opportunistic Fungi. PLoS Pathog. 2014;10:e1003996–e1003917. doi: 10.1371/journal.ppat.1003996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Beck JM, Schloss PD, Venkataraman A, Twigg H, Jablonski KA, Bushman FD, et al. Multicenter Comparison of Lung and Oral Microbiomes of HIV-infected and HIV-uninfected Individuals. Am J Respir Crit Care Med. 2015;192:1335–1344. doi: 10.1164/rccm.201501-0128OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Slots J. Herpesviral-bacterial synergy in the pathogenesis of human periodontitis. Curr. Opin. Infect. Dis. 2007;20:278–283. doi: 10.1097/QCO.0b013e3280964da0. [DOI] [PubMed] [Google Scholar]
- 226.Diaz PI, Strausbaugh LD, Dongari-Bagtzoglou A. Fungal-bacterial interactions and their relevance to oral health: linking the clinic and the bench. Frontiers in Cellular and Infection Microbiology. 2014;4:101. doi: 10.3389/fcimb.2014.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Xu H, Dongari-Bagtzoglou A. Shaping the oral mycobiota: interactions of opportunistic fungi with oral bacteria and the host. Current Opinion in Microbiology. 2015;26:65–70. doi: 10.1016/j.mib.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Cui L, Morris A, Ghedin E. The human mycobiome in health and disease. Genome Med. 2013;5 doi: 10.1186/gm467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Gorfer M, Blumhoff M, Klaubauf S, Urban A, Inselsbacher E, Bandian D, et al. Community profiling and gene expression of fungal assimilatory nitrate reductases in agricultural soil. The ISME Journal. 2011;5:1771–1783. doi: 10.1038/ismej.2011.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Shoun H, Fushinobu S, Jiang L, Kim S-W, Wakagi T. Fungal denitrification and nitric oxide reductase cytochrome P450nor. Philos. Trans. R. Soc. Lond., B, Biol. Sci. 2012;367:1186–1194. doi: 10.1098/rstb.2011.0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Lundberg JO. Nitrate transport in salivary glands with implications for NO homeostasis. 2012;109:13144–13145. doi: 10.1073/pnas.1210412109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Silva Mendez LS, Allaker RP, Hardie JM, Benjamin N. Antimicrobial effect of acidified nitrite on cariogenic bacteria. Oral Microbiol. Immunol. 1999;14:391–392. doi: 10.1034/j.1399-302x.1999.140612.x. [DOI] [PubMed] [Google Scholar]
- 233.Tiso M, Schechter AN. Nitrate reduction to nitrite, nitric oxide and ammonia by gut bacteria under physiological conditions. PLoS ONE. 2015;10:e0119712. doi: 10.1371/journal.pone.0119712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Rowe JJ, Yarbrough JM, Rake JB, Eagon RG. Nitrite inhibition of aerobic bacteria. Curr Microbiol. 1979;2:51–54. [Google Scholar]
- 235.Schlag S, Nerz C, Birkenstock TA, Altenberend F, Götz F. Inhibition of staphylococcal biofilm formation by nitrite. J. Bacteriol. 2007;189:7911–7919. doi: 10.1128/JB.00598-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Zemke AC, Shiva S, Burns JL, Moskowitz SM, Pilewski JM, Gladwin MT, et al. Nitrite modulates bacterial antibiotic susceptibility and biofilm formation in association with airway epithelial cells. Free Radic. Biol. Med. 2014;77:307–316. doi: 10.1016/j.freeradbiomed.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Arora DP, Hossain S, Xu Y, Boon EM. Nitric Oxide Regulation of Bacterial Biofilms. Biochemistry. 2015;54:3717–3728. doi: 10.1021/bi501476n. [DOI] [PubMed] [Google Scholar]
- 238.Nascimento MM, Gordan VV, Garvan CW, Browngardt CM, Burne RA. Correlations of oral bacterial arginine and urea catabolism with caries experience. Oral Microbiol. Immunol. 2009;24:89–95. doi: 10.1111/j.1399-302X.2008.00477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Li H, Thompson I, Carter P, Whiteley A, Bailey M, Leifert C, et al. Salivary nitrate--an ecological factor in reducing oral acidity. Oral Microbiol. Immunol. 2007;22:67–71. doi: 10.1111/j.1399-302X.2007.00313.x. [DOI] [PubMed] [Google Scholar]
- 240.Wang F, Liu J, Zhang Y, Lei P. Association of Helicobacter pyloriinfection with chronic obstructive pulmonary disease and chronic bronchitis: a meta-analysis of 16 studies. Infectious Diseases. 2015;47:597–603. doi: 10.3109/00365548.2014.989539. [DOI] [PubMed] [Google Scholar]
- 241.Franceschi F, Tortora A, Gasbarrini G, Gasbarrini A. Helicobacter pyloriand Extragastric Diseases. Helicobacter. 2014;19:52–58. doi: 10.1111/hel.12159. [DOI] [PubMed] [Google Scholar]
- 242.Gobert AP, Wilson KT. The Immune Battle against Helicobacter pylori Infection: NO Offense. Trends in Microbiology. 2016;24:366–376. doi: 10.1016/j.tim.2016.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Danesh J. Coronary heart disease, Helicobacter pylori, dental disease, Chlamydia pneumoniae, and cytomegalovirus: meta-analyses of prospective studies. 1999;138:S434–S437. doi: 10.1016/s0002-8703(99)70270-x. http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=10539843&retmode=ref&cmd=prlinks. [DOI] [PubMed] [Google Scholar]
- 244.Tabata N, Hokimoto S, Akasaka T, Sueta D, Arima Y, Sakamoto K, et al. Helicobacter pyloriseropositivity along with pro-inflammatory interleukin-1 polymorphisms correlated with myocardial infarction. Ctrsc. 2016;17:9–14. [Google Scholar]
- 245.Grabczewska Z, Nartowicz E, Kubica J, Rość D. Endothelial function parameters in patients with unstable angina and infection with Helicobacter pylori and Chlamydia pneumoniae. Eur. J. Intern. Med. 2006;17:339–342. doi: 10.1016/j.ejim.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 246.Briskey D, Tucker PS, Johnson DW, Coombes JS. Microbiota and the nitrogen cycle: Implications in the development and progression of CVD and CKD. Nitric Oxide. 2016;57:64–70. doi: 10.1016/j.niox.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 247.Kuwahara H, Miyamoto Y, Akaike T, Kubota T, Sawa T, Okamoto S, et al. Helicobacter pylori urease suppresses bactericidal activity of peroxynitrite via carbon dioxide production. Infection and Immunity. 2000;68:4378–4383. doi: 10.1128/iai.68.8.4378-4383.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.McDonald EG, Milligan J, Frenette C, Lee TC. Continuous Proton Pump Inhibitor Therapy and the Associated Risk of Recurrent Clostridium difficile Infection. JAMA Intern Med. 2015;175:784–791. doi: 10.1001/jamainternmed.2015.42. [DOI] [PubMed] [Google Scholar]
- 249.Leonard J, Marshall JK, Moayyedi P. Systematic review of the risk of enteric infection in patients taking acid suppression. Am. J. Gastroenterol. 2007;102 doi: 10.1111/j.1572-0241.2007.01275.x. 2047–56– quiz 2057. [DOI] [PubMed] [Google Scholar]
- 250.Janarthanan S, Ditah I, Adler DG, Ehrinpreis MN. Clostridium difficile-associated diarrhea and proton pump inhibitor therapy: a meta-analysis. Am. J. Gastroenterol. 2012;107:1001–1010. doi: 10.1038/ajg.2012.179. [DOI] [PubMed] [Google Scholar]
- 251.Imhann F, Bonder MJ, Vich Vila A, Fu J, Mujagic Z, Vork L, et al. Proton pump inhibitors affect the gut microbiome. Gut. 2016;65:740–748. doi: 10.1136/gutjnl-2015-310376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Clooney AG, Bernstein CN, Leslie WD, Vagianos K, Sargent M, Laserna-Mendieta EJ, et al. A comparison of the gut microbiome between long-term users and non-users of proton pump inhibitors. Aliment Pharmacol Ther. 2016;43:974–984. doi: 10.1111/apt.13568. [DOI] [PubMed] [Google Scholar]
- 253.Freedberg DE, Toussaint NC, Chen SP, Ratner AJ, Whittier S, Wang TC, et al. Proton Pump Inhibitors Alter Specific Taxa in the Human Gastrointestinal Microbiome: A Crossover Trial. Gastroenterology. 2015;149:883–885. e9. doi: 10.1053/j.gastro.2015.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Pinheiro LC, Montenegro MF, Amaral JH, Ferreira GC, Oliveira AM, Tanus-Santos JE. Increase in gastric pH reduces hypotensive effect of oral sodium nitrite in rats. 2012;53:701–709. doi: 10.1016/j.freeradbiomed.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 255.Montenegro MF, Sundqvist ML, Larsen FJ, Zhuge Z, Carlström M, Weitzberg E, et al. Blood Pressure-Lowering Effect of Orally Ingested Nitrite Is Abolished by a Proton Pump Inhibitor. Hypertension. 2016 doi: 10.1161/HYPERTENSIONAHA.116.08081. HYPERTENSIONAHA.116.08081. [DOI] [PubMed] [Google Scholar]
- 256.Hille R, Hall J, Basu P. The Mononuclear Molybdenum Enzymes. Chem. Rev. 2014;114:3963–4038. doi: 10.1021/cr400443z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Rabin RS, Collins LA, Stewart V. In vivo requirement of integration host factor for nar (nitrate reductase) operon expression in Escherichia coli K-12. Proceedings of the National Academy of Sciences. 1992;89:8701–8705. doi: 10.1073/pnas.89.18.8701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Noriega CE, Lin H-Y, Chen L-L, Williams SB, Stewart V. Asymmetric cross-regulation between the nitrate-responsive NarX-NarL and NarQ-NarP two-component regulatory systems from Escherichia coli K-12. Mol. Microbiol. 2010;75:394–412. doi: 10.1111/j.1365-2958.2009.06987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Constantinidou C, Hobman JL, Griffiths L, Patel MD, Penn CW, Cole JA, et al. A reassessment of the FNR regulon and transcriptomic analysis of the effects of nitrate, nitrite, NarXL, and NarQP as Escherichia coli K12 adapts from aerobic to anaerobic growth. J. Biol. Chem. 2006;281:4802–4815. doi: 10.1074/jbc.M512312200. [DOI] [PubMed] [Google Scholar]
- 260.Dong Y, Wang J, Fu H, Zhou G, Shi M, Gao H. A Crp-dependent two-component system regulates nitrate and nitrite respiration in Shewanella oneidensis. PLoS ONE. 2012;7:e51643. doi: 10.1371/journal.pone.0051643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Stewart V. Regulation of nitrate and nitrite reductase synthesis in enterobacteria. Antonie Van Leeuwenhoek. 1994;66:37–45. doi: 10.1007/BF00871631. [DOI] [PubMed] [Google Scholar]
- 262.Stewart V. Nitrate regulation of anaerobic respiratory gene expression in Escherichia coli. Mol. Microbiol. 1993;9:425–434. doi: 10.1111/j.1365-2958.1993.tb01704.x. [DOI] [PubMed] [Google Scholar]
- 263.Sears HJ, Sawers G, Berks BC, Ferguson SJ, Richardson DJ. Control of periplasmic nitrate reductase gene expression (napEDABC) from Paracoccus pantotrophus in response to oxygen and carbon substrates. Microbiology. 2000;146(Pt 11):2977–2985. doi: 10.1099/00221287-146-11-2977. [DOI] [PubMed] [Google Scholar]
- 264.Gavira M, Roldán MD, Castillo F, Moreno-Vivián C. Regulation of nap gene expression and periplasmic nitrate reductase activity in the phototrophic bacterium Rhodobacter sphaeroides DSM158. J. Bacteriol. 2002;184:1693–1702. doi: 10.1128/JB.184.6.1693-1702.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Potter L, Millington P, Griffiths L, Thomas G. Competition between Escherichia coli strains expressing either a periplasmic or a membrane-bound nitrate reductase: does Nap confer a selective advantage during …. Biochem J. 1999 [PMC free article] [PubMed] [Google Scholar]
- 266.Hill MJ, Marsh PD. Human Microbial Ecology. CRC Press; 1989. [Google Scholar]
- 267.Espey MG. Role of oxygen gradients in shaping redox relationships between the human intestine and its microbiota. Free Radic. Biol. Med. 2013;55:130–140. doi: 10.1016/j.freeradbiomed.2012.10.554. [DOI] [PubMed] [Google Scholar]
- 268.Matheson PJ, Wilson MA, Garrison RN. Regulation of intestinal blood flow. J. Surg. Res. 2000;93:182–196. doi: 10.1006/jsre.2000.5862. [DOI] [PubMed] [Google Scholar]
- 269.Cole J. Nitrate reduction to ammonia by enteric bacteria: redundancy, or a strategy for survival during oxygen starvation? FEMS Microbiol. Lett. 1996;136:1–11. doi: 10.1111/j.1574-6968.1996.tb08017.x. [DOI] [PubMed] [Google Scholar]
- 270.Hezel MP, Weitzberg E. The oral microbiome and nitric oxide homoeostasis. Oral Dis. 2015;21:7–16. doi: 10.1111/odi.12157. [DOI] [PubMed] [Google Scholar]
- 271.Vermeiren J, Van de Wiele T, Verstraete W, Boeckx P, Boon N. Nitric oxide production by the human intestinal microbiota by dissimilatory nitrate reduction to ammonium. J. Biomed. Biotechnol. 2009;2009:284718–284710. doi: 10.1155/2009/284718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Cooke JP, Ghebremariam YT. Dietary nitrate, nitric oxide, and restenosis. J. Clin. Invest. 2011;121:1258–1260. doi: 10.1172/JCI57193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Bäckhed F, Manchester JK, Semenkovich CF, Gordon JI. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proceedings of the National Academy of Sciences. 2007;104:979–984. doi: 10.1073/pnas.0605374104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457:480–484. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Turnbaugh PJ, Bäckhed F, Fulton L, Gordon JI. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe. 2008;3:213–223. doi: 10.1016/j.chom.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Wen L, Ley RE, Volchkov PY, Stranges PB, Avanesyan L, Stonebraker AC, et al. Innate immunity and intestinal microbiota in the development of Type 1 diabetes. Nature. 2008;455:1109–1113. doi: 10.1038/nature07336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Bäckhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, et al. The gut microbiota as an environmental factor that regulates fat storage. Proceedings of the National Academy of Sciences. 2004;101:15718–15723. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Sobko T, Reinders C, Norin E, Midtvedt T, Gustafsson LE, Lundberg JO. Gastrointestinal nitric oxide generation in germ-free and conventional rats. Am. J. Physiol. Gastrointest. Liver Physiol. 2004;287:G993–G997. doi: 10.1152/ajpgi.00203.2004. [DOI] [PubMed] [Google Scholar]
- 279.Kishino S, Takeuchi M, Park S-B, Hirata A, Kitamura N, Kunisawa J, et al. Polyunsaturated fatty acid saturation by gut lactic acid bacteria affecting host lipid composition. 2013;110:17808–17813. doi: 10.1073/pnas.1312937110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.DeStefano F, Anda RF, Kahn HS, Williamson DF, Russell CM. Dental disease and risk of coronary heart disease and mortality. Bmj. 1993;306:688–691. doi: 10.1136/bmj.306.6879.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Leng W-D, Zeng X-T, Kwong JSW, Hua X-P. Periodontal disease and risk of coronary heart disease: An updated meta-analysis of prospective cohort studies. J. Cardiol. 2015;201:469–472. doi: 10.1016/j.ijcard.2015.07.087. [DOI] [PubMed] [Google Scholar]
- 282.Pussinen PJ, Könönen E. Oral health: A modifiable risk factor for cardiovascular diseases or a confounded association? European Journal of Preventive Cardiology. 2016;23:834–838. doi: 10.1177/2047487316636506. [DOI] [PubMed] [Google Scholar]
- 283.Ott SJ, El Mokhtari NE, Musfeldt M, Hellmig S, Freitag S, Rehman A, et al. Detection of diverse bacterial signatures in atherosclerotic lesions of patients with coronary heart disease. Circulation. 2006;113:929–937. doi: 10.1161/CIRCULATIONAHA.105.579979. [DOI] [PubMed] [Google Scholar]
- 284.Koren O, Spor A, Felin J, Fåk F, Stombaugh J, Tremaroli V, et al. Human oral, gut, and plaque microbiota in patients with atherosclerosis. 2011;108(Suppl 1):4592–4598. doi: 10.1073/pnas.1011383107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Serra e Silva Filho W, Casarin RCV, Nicolela EL, Passos HM, Sallum AW, Gonçalves RB. Microbial diversity similarities in periodontal pockets and atheromatous plaques of cardiovascular disease patients. PLoS ONE. 2014;9:e109761. doi: 10.1371/journal.pone.0109761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Fåk F, Tremaroli V, Bergström G, Bäckhed F. Oral microbiota in patients with atherosclerosis. Atherosclerosis. 2015;243:573–578. doi: 10.1016/j.atherosclerosis.2015.10.097. [DOI] [PubMed] [Google Scholar]
- 287.Loos BG, Craandijk J, Hoek FJ, Wertheim-van Dillen PM, van der Velden U. Elevation of systemic markers related to cardiovascular diseases in the peripheral blood of periodontitis patients. J. Periodontol. 2000;71:1528–1534. doi: 10.1902/jop.2000.71.10.1528. [DOI] [PubMed] [Google Scholar]
- 288.Seinost G, Wimmer G, Skerget M, Thaller E, Brodmann M, Gasser R, et al. Periodontal treatment improves endothelial dysfunction in patients with severe periodontitis. American Heart Journal. 2005;149:1050–1054. doi: 10.1016/j.ahj.2004.09.059. [DOI] [PubMed] [Google Scholar]
- 289.Tonetti MS, D'Aiuto F, Nibali L, Donald A, Storry C, Parkar M, et al. Treatment of periodontitis and endothelial function. N. Engl. J. Med. 2007;356:911–920. doi: 10.1056/NEJMoa063186. [DOI] [PubMed] [Google Scholar]
- 290.D'Aiuto F, Orlandi M, Gunsolley JC. Evidence that periodontal treatment improves biomarkers and CVD outcomes. J. Periodontol. 2013;84:S85–S105. doi: 10.1902/jop.2013.134007. [DOI] [PubMed] [Google Scholar]
- 291.Parwani SR, Chitnis PJ, Parwani RN. Salivary nitric oxide levels in inflammatory periodontal disease - a case-control and interventional study. J Dent Hyg. 2012;10:67–73. doi: 10.1111/j.1601-5037.2011.00508.x. [DOI] [PubMed] [Google Scholar]
- 292.Wadhwa D, Bey A, Hasija M, Moin S. Determination of levels of nitric oxide in smoker and nonsmoker patients with chronic periodontitis. Of Periodontal &. 2013 doi: 10.5051/jpis.2013.43.5.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Xia DS, Deng DJ, Wang SL. Destruction of parotid glands affects nitrate and nitrite metabolism. Journal of Dental Research. 2003;82:101–105. doi: 10.1177/154405910308200205. [DOI] [PubMed] [Google Scholar]
- 294.Battle RW, Davitt MA, Cooper SM, Buckley LM, Leib ES, Beglin PA, et al. Prevalence of pulmonary hypertension in limited and diffuse scleroderma. Chest. 1996;110:1515–1519. doi: 10.1378/chest.110.6.1515. [DOI] [PubMed] [Google Scholar]
- 295.Bertoni M, Niccoli L, Porciello G, Storri L, Nannini C, Manes A, et al. Pulmonary hypertension in primary Sjögren's syndrome: report of a case and review of the literature. Clin. Rheumatol. 2005;24:431–434. doi: 10.1007/s10067-004-1071-8. [DOI] [PubMed] [Google Scholar]
- 296.Hatron P-Y, Tillie-Leblond I, Launay D, Hachulla E, Fauchais AL, Wallaert B. Pulmonary manifestations of Sjögren's syndrome. Presse Med. 2011;40:e49–e64. doi: 10.1016/j.lpm.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 297.Kobak S, Oksel F, Aksu K, Kabasakal Y. The frequency of sicca symptoms and Sjögren's syndrome in patients with systemic sclerosis. J Rheum Dis. 2013;16:88–92. doi: 10.1111/j.1756-185X.2012.01810.x. [DOI] [PubMed] [Google Scholar]
- 298.Ratjen F. Pulmonary artery hypertension: an underrated disease manifestation in cystic fibrosis? Lancet Respir Med. 2016;4:596–598. doi: 10.1016/S2213-2600(16)30107-2. [DOI] [PubMed] [Google Scholar]
- 299.von Bültzingslöwen I, Sollecito TP, Fox PC, Daniels T, Jonsson R, Lockhart PB, et al. Salivary dysfunction associated with systemic diseases: systematic review and clinical management recommendations. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103(Suppl):S57.e1–S57.e15. doi: 10.1016/j.tripleo.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 300.Navazesh M, Mulligan R, Karim R, Mack WJ, Ram S, Seirawan H, et al. Effect of HAART on salivary gland function in the Women's Interagency HIV Study (WIHS) Oral Dis. 2009;15:52–60. doi: 10.1111/j.1601-0825.2008.01456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.López-Verdín S, Andrade-Villanueva J, Zamora-Perez AL, Bologna-Molina R, Cervantes-Cabrera JJ, Molina-Frechero N. Differences in Salivary Flow Level, Xerostomia, and Flavor Alteration in Mexican HIV Patients Who Did or Did Not Receive Antiretroviral Therapy. AIDS Res Treat. 2013;2013:613278–613276. doi: 10.1155/2013/613278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.da Silva Modesto KB, de Godói Simões JB, de Souza AF, Damaceno N, Duarte DA, Leite MF, et al. Salivary flow rate and biochemical composition analysis in stimulated whole saliva of children with cystic fibrosis. Archives of Oral Biology. 2015;60:1650–1654. doi: 10.1016/j.archoralbio.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 303.Navazesh M, Mulligan R, Pogoda J, Greenspan D, Alves M, Phelan J, et al. The effect of HAART on salivary microbiota in the Women's Interagency HIV Study (WIHS) Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;100:701–708. doi: 10.1016/j.tripleo.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 304.Leung KCM, Leung WK, McMillan AS. Supra-gingival microbiota in Sjögren's syndrome. Clin Oral Invest. 2007;11:415–423. doi: 10.1007/s00784-007-0132-1. [DOI] [PubMed] [Google Scholar]
- 305.Filkins LM, Hampton TH, Gifford AH, Gross MJ, Hogan DA, Sogin ML, et al. Prevalence of streptococci and increased polymicrobial diversity associated with cystic fibrosis patient stability. J. Bacteriol. 2012;194:4709–4717. doi: 10.1128/JB.00566-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Li M, Zou Y, Jiang Q, Jiang L, Yu Q, Ding X, et al. A preliminary study of the oral microbiota in Chinese patients with Sjögren's syndrome. Archives of Oral Biology. 2016;70:143–148. doi: 10.1016/j.archoralbio.2016.06.016. [DOI] [PubMed] [Google Scholar]
- 307.Nicholson JK, Holmes E, Wilson ID. Gut microorganisms, mammalian metabolism and personalized health care. Nat. Rev. Microbiol. 2005;3:431–438. doi: 10.1038/nrmicro1152. [DOI] [PubMed] [Google Scholar]
- 308.Maynard CL, Elson CO, Hatton RD, Weaver CT. Reciprocal interactions of the intestinal microbiota and immune system. Nature. 2012;489:231–241. doi: 10.1038/nature11551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Yasuda K, Oh K, Ren B, Tickle TL, Franzosa EA, Wachtman LM, et al. Biogeography of the intestinal mucosal and lumenal microbiome in the rhesus macaque. Cell Host Microbe. 2015;17:385–391. doi: 10.1016/j.chom.2015.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Serino M, Luche E, Chabo C, Amar J, Burcelin R. Intestinal microflora and metabolic diseases. Diabetes Metab. 2009;35:262–272. doi: 10.1016/j.diabet.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 311.Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490:55–60. doi: 10.1038/nature11450. [DOI] [PubMed] [Google Scholar]
- 312.Serino M, Blasco-Baque V, Nicolas S, Burcelin R. Far from the eyes, close to the heart: dysbiosis of gut microbiota and cardiovascular consequences. Curr Cardiol Rep. 2014;16:540. doi: 10.1007/s11886-014-0540-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Le Chatelier E, Nielsen T, Qin J, Prifti E, Hildebrand F, Falony G, et al. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500:541–546. doi: 10.1038/nature12506. [DOI] [PubMed] [Google Scholar]
- 314.Rabinovitch M, Guignabert C, Humbert M, Nicolls MR. Inflammation and immunity in the pathogenesis of pulmonary arterial hypertension. Circ. Res. 2014;115:165–175. doi: 10.1161/CIRCRESAHA.113.301141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Gomez-Arroyo J, Abbate A, Voelkel NF. Pulmonary arterial hypertension and the Enigma code of smouldering inflammation. Eur. Respir. J. 2016;48:305–307. doi: 10.1183/13993003.00996-2016. [DOI] [PubMed] [Google Scholar]
- 316.Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nature Reviews Immunology. 2009;9:313–323. doi: 10.1038/nri2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Lukens JR, Gurung P, Vogel P, Johnson GR, Carter RA, McGoldrick DJ, et al. Dietary modulation of the microbiome affects autoinflammatory disease. Nature. 2014;516:246–249. doi: 10.1038/nature13788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Cotillard A, Kennedy SP, Kong LC, Prifti E, Pons N, Le Chatelier E, et al. Dietary intervention impact on gut microbial gene richness. Nature. 2013;500:585–588. doi: 10.1038/nature12480. [DOI] [PubMed] [Google Scholar]
- 319.Sun J, Xu W, Wang G. The Role of Intestinal Microflora and Its Possible Mechanisms in Hypertension. Lipid and Cardiovascular Research. 2016 [Google Scholar]
- 320.Szabó C, Ischiropoulos H, Radi R. Peroxynitrite: biochemistry, pathophysiology and development of therapeutics. Nat Rev Drug Discov. 2007;6:662–680. doi: 10.1038/nrd2222. [DOI] [PubMed] [Google Scholar]
- 321.Jädert C, Phillipson M, Holm L, Lundberg JO, Borniquel S. Preventive and therapeutic effects of nitrite supplementation in experimental inflammatory bowel disease. Redox Biol. 2014;2:73–81. doi: 10.1016/j.redox.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Chokshi NK, Guner YS, Hunter CJ, Upperman JS, Grishin A, Ford HR. The role of nitric oxide in intestinal epithelial injury and restitution in neonatal necrotizing enterocolitis. Semin. Perinatol. 2008;32:92–99. doi: 10.1053/j.semperi.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Scales BS, Dickson RP, Huffnagle GB. A tale of two sites: how inflammation can reshape the microbiomes of the gut and lungs. J. Leukoc. Biol. 2016 doi: 10.1189/jlb.3MR0316-106R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Petersson J, Phillipson M, Jansson EÅ, Patzak A, Lundberg JO, Holm L. Dietary nitrate increases gastric mucosal blood flow and mucosal defense. Am. J. Physiol. Gastrointest. Liver Physiol. 2007;292:G718–G724. doi: 10.1152/ajpgi.00435.2006. [DOI] [PubMed] [Google Scholar]
- 325.Moilanen E, Vapaatalo H. Nitric oxide in inflammation and immune response. Ann. Med. 1995;27:359–367. doi: 10.3109/07853899509002589. [DOI] [PubMed] [Google Scholar]
- 326.Stuehr DJ, Gross SS, Sakuma I, Levi R, Nathan CF. Activated murine macrophages secrete a metabolite of arginine with the bioactivity of endothelium-derived relaxing factor and the chemical reactivity of nitric oxide. J. Exp. Med. 1989;169:1011–1020. doi: 10.1084/jem.169.3.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Ley RE, Bäckhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proceedings of the National Academy of Sciences. 2005;102:11070–11075. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 329.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 330.Kassinen A, Krogius-Kurikka L, Mäkivuokko H, Rinttilä T, Paulin L, Corander J, et al. The fecal microbiota of irritable bowel syndrome patients differs significantly from that of healthy subjects. Gastroenterology. 2007;133:24–33. doi: 10.1053/j.gastro.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 331.Yang T, Santisteban MM, Rodriguez V, Li E, Ahmari N, Carvajal JM, et al. Gut dysbiosis is linked to hypertension. Hypertension. 2015;65:1331–1340. doi: 10.1161/HYPERTENSIONAHA.115.05315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Salas JT, Chang TL. Microbiome in human immunodeficiency virus infection. Clin. Lab. Med. 2014;34:733–745. doi: 10.1016/j.cll.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Winter SE, Winter MG, Xavier MN, Thiennimitr P, Poon V, Keestra AM, et al. Host-derived nitrate boosts growth of E. coli in the inflamed gut. Science. 2013;339:708–711. doi: 10.1126/science.1232467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Winter SE, Bäumler AJ. Dysbiosis in the inflamed intestine: chance favors the prepared microbe. Gut Microbes. 2014;5:71–73. doi: 10.4161/gmic.27129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Verdam FJ, Fuentes S, de Jonge C, Zoetendal EG, Erbil R, Greve JW, et al. Human intestinal microbiota composition is associated with local and systemic inflammation in obesity. Obesity (Silver Spring) 2013;21:E607–E615. doi: 10.1002/oby.20466. [DOI] [PubMed] [Google Scholar]
- 336.Biagi E, Nylund L, Candela M, Ostan R, Bucci L, Pini E, et al. Through ageing, and beyond: gut microbiota and inflammatory status in seniors and centenarians. PLoS ONE. 2010;5:e10667. doi: 10.1371/journal.pone.0010667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Vázquez-Torres A, Bäumler AJ. Nitrate, nitrite and nitric oxide reductases: from the last universal common ancestor to modern bacterial pathogens. Current Opinion in Microbiology. 2016;29:1–8. doi: 10.1016/j.mib.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Stecher B, Robbiani R, Walker AW, Westendorf AM, Barthel M, Kremer M, et al. Salmonella enterica serovar typhimurium exploits inflammation to compete with the intestinal microbiota. PLoS Biol. 2007;5:2177–2189. doi: 10.1371/journal.pbio.0050244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Lundberg JO, Hellström PM, Lundberg JM, Alving K. Greatly increased luminal nitric oxide in ulcerative colitis. The Lancet. 1994;344:1673–1674. doi: 10.1016/s0140-6736(94)90460-x. [DOI] [PubMed] [Google Scholar]
- 340.Morita H, Yoshikawa H, Sakata R, Nagata Y, Tanaka H. Synthesis of nitric oxide from the two equivalent guanidino nitrogens of L-arginine by Lactobacillus fermentum. J. Bacteriol. 1997;179:7812–7815. doi: 10.1128/jb.179.24.7812-7815.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Kers JA, Wach MJ, Krasnoff SB, Widom J, Cameron KD, Bukhalid RA, et al. Nitration of a peptide phytotoxin by bacterial nitric oxide synthase. Nature. 2004;429:79–82. doi: 10.1038/nature02504. [DOI] [PubMed] [Google Scholar]
- 342.Sudhamsu J, Crane BR. Bacterial nitric oxide synthases: what are they good for? Trends in Microbiology. 2009;17:212–218. doi: 10.1016/j.tim.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 343.Cortese-Krott MM, Fernandez BO, Kelm M, Butler AR, Feelisch M. On the chemical biology of the nitrite/sulfide interaction. Nitric Oxide. 2015;46:14–24. doi: 10.1016/j.niox.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 344.Singh SB, Lin HC. Hydrogen Sulfide in Physiology and Diseases of the Digestive Tract. Microorganisms. 2015;3:866–889. doi: 10.3390/microorganisms3040866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Carbonero F, Benefiel AC, Alizadeh-Ghamsari AH, Gaskins HR. Microbial pathways in colonic sulfur metabolism and links with health and disease. Front. Physiol. 2012;3:448. doi: 10.3389/fphys.2012.00448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Vermeiren J, Van de Wiele T, Van Nieuwenhuyse G, Boeckx P, Verstraete W, Boon N. Sulfide-and nitrite-dependent nitric oxide production in the intestinal tract. Microb Biotechnol. 2012;5:379–387. doi: 10.1111/j.1751-7915.2011.00320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Guo F-F, Yu T-C, Hong J, Fang J-Y. Emerging Roles of Hydrogen Sulfide in Inflammatory and Neoplastic Colonic Diseases. Front. Physiol. 2016;7:156. doi: 10.3389/fphys.2016.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Liu H, Bai XB, Shi S, Cao YX. Hydrogen sulfide protects from intestinal ischaemia–reperfusion injury in rats. Journal of Pharmacy and Pharmacology. 2009;61:207–212. doi: 10.1211/jpp/61.02.0010. [DOI] [PubMed] [Google Scholar]
- 349.Zhao H, Yan R, Zhou X, Ji F, Zhang B. Hydrogen sulfide improves colonic barrier integrity in DSS-induced inflammation in Caco-2 cells and mice. Immunopharmacol. 2016;39:121–127. doi: 10.1016/j.intimp.2016.07.020. [DOI] [PubMed] [Google Scholar]
- 350.Motta J-P, Flannigan KL, Agbor TA, Beatty JK, Blackler RW, Workentine ML, et al. Hydrogen sulfide protects from colitis and restores intestinal microbiota biofilm and mucus production. Inflammatory Bowel Diseases. 2015;21:1006–1017. doi: 10.1097/MIB.0000000000000345. [DOI] [PubMed] [Google Scholar]
- 351.Hirata I, Naito Y, Takagi T, Mizushima K, Suzuki T, Omatsu T, et al. Endogenous hydrogen sulfide is an anti-inflammatory molecule in dextran sodium sulfate-induced colitis in mice. Dig Dis Sci. 2011;56:1379–1386. doi: 10.1007/s10620-010-1461-5. [DOI] [PubMed] [Google Scholar]
- 352.Arijs I, Vanhove W, Rutgeerts P, Schuit F, Verbeke K, De Preter V. Decreased mucosal sulfide detoxification capacity in patients with Crohn's disease. Inflammatory Bowel Diseases. 2013;19:E70–E72. doi: 10.1097/MIB.0b013e31827e790e. [DOI] [PubMed] [Google Scholar]
- 353.De Preter V, Arijs I, Windey K, Vanhove W, Vermeire S, Schuit F, et al. Decreased mucosal sulfide detoxification is related to an impaired butyrate oxidation in ulcerative colitis. Inflammatory Bowel Diseases. 2012;18:2371–2380. doi: 10.1002/ibd.22949. [DOI] [PubMed] [Google Scholar]
- 354.Tang WHW, Hazen SL. The contributory role of gut microbiota in cardiovascular disease. J. Clin. Invest. 2014;124:4204–4211. doi: 10.1172/JCI72331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Vinjé S, Stroes E, Nieuwdorp M, Hazen SL. The gut microbiome as novel cardio-metabolic target: the time has come! European Heart Journal. 2014;35:883–887. doi: 10.1093/eurheartj/eht467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Knight R. Why microbiome treatments could pay off soon. Nature. 2015;518:S5–S5. doi: 10.1038/518S5a. [DOI] [PubMed] [Google Scholar]
- 357.Kinlay S, Michel T, Leopold JA. The Future of Vascular Biology and Medicine. Circulation. 2016;133:2603–2609. doi: 10.1161/CIRCULATIONAHA.116.023513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Gibson GR, Roberfroid MB. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. Journal of Nutrition. 1995;125:1401–1412. doi: 10.1093/jn/125.6.1401. [DOI] [PubMed] [Google Scholar]
- 359.do Rosario VA, Fernandes R, de EBS, Trindade M. Vegetarian diets and gut microbiota: important shifts in markers of metabolism and cardiovascular disease. Nutrition Reviews. 2016;74:444–454. doi: 10.1093/nutrit/nuw012. [DOI] [PubMed] [Google Scholar]
- 360.Baothman OA, Zamzami MA, Taher I, Abubaker J, Abu-Farha M. The role of Gut Microbiota in the development of obesity and Diabetes. Lipids Health Dis. 2016;15:108. doi: 10.1186/s12944-016-0278-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N. Engl. J. Med. 1997;336:1117–1124. doi: 10.1056/NEJM199704173361601. [DOI] [PubMed] [Google Scholar]
- 362.de Lorgeril M. Mediterranean diet and cardiovascular disease: historical perspective and latest evidence. Curr Atheroscler Rep. 2013;15:370. doi: 10.1007/s11883-013-0370-4. [DOI] [PubMed] [Google Scholar]
- 363.Rouse IL, Beilin LJ, Armstrong BK, Vandongen R. Blood-pressure-lowering effect of a vegetarian diet: controlled trial in normotensive subjects. The Lancet. 1983;1:5–10. doi: 10.1016/s0140-6736(83)91557-x. [DOI] [PubMed] [Google Scholar]
- 364.Lindahl O, Lindwall L, Spångberg A, Stenram A, Ockerman PA. A vegan regimen with reduced medication in the treatment of hypertension. Br. J. Nutr. 1984;52:11–20. doi: 10.1079/bjn19840066. [DOI] [PubMed] [Google Scholar]
- 365.Joshipura KJ, Ascherio A, Manson JE, Stampfer MJ, Rimm EB, Speizer FE, et al. Fruit and vegetable intake in relation to risk of ischemic stroke. Jama. 1999;282:1233–1239. doi: 10.1001/jama.282.13.1233. [DOI] [PubMed] [Google Scholar]
- 366.Hung H-C, Joshipura KJ, Jiang R, Hu FB, Hunter D, Smith-Warner SA, et al. Fruit and vegetable intake and risk of major chronic disease. J. Nat. Cancer Inst. 2004;96:1577–1584. doi: 10.1093/jnci/djh296. [DOI] [PubMed] [Google Scholar]
- 367.Carter P, Gray LJ, Troughton J, Khunti K, Davies MJ. Fruit and vegetable intake and incidence of type 2 diabetes mellitus: systematic review and meta-analysis. Bmj. 2010;341:c4229–c4229. doi: 10.1136/bmj.c4229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Wang X, Ouyang Y, Liu J, Zhu M, Zhao G, Bao W, et al. Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: systematic review and dose-response meta-analysis of prospective cohort studies. Bmj. 2014;349:g4490–g4490. doi: 10.1136/bmj.g4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Joshipura KJ, Hu FB, Manson JE, Stampfer MJ, Rimm EB, Speizer FE, et al. The effect of fruit and vegetable intake on risk for coronary heart disease. Ann. Intern. Med. 2001;134:1106–1114. doi: 10.7326/0003-4819-134-12-200106190-00010. [DOI] [PubMed] [Google Scholar]
- 370.Santamaria P. Nitrate in vegetables: toxicity, content, intake and EC regulation. Journal of the Science of Food and Agriculture. 2006;86:10–17. [Google Scholar]
- 371.Obarzanek E, Moore TJ. Using feeding studies to test the efficacy of dietary interventions: lessons from the Dietary Approaches to Stop Hypertension trial. J Am Diet Assoc. 1999;99:S9–S11. doi: 10.1016/s0002-8223(99)00409-5. [DOI] [PubMed] [Google Scholar]
- 372.Gangolli SD, van den Brandt PA, Feron VJ, Janzowsky C, Koeman JH, Speijers GJ, et al. Nitrate, nitrite and N-nitroso compounds. Eur. J. Pharmacol. 1994;292:1–38. doi: 10.1016/0926-6917(94)90022-1. [DOI] [PubMed] [Google Scholar]
- 373.Sobko T, Marcus C, Govoni M, Kamiya S. Dietary nitrate in Japanese traditional foods lowers diastolic blood pressure in healthy volunteers. Nitric Oxide. 2010;22:136–140. doi: 10.1016/j.niox.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 374.Lundberg JO, Carlström M, Larsen FJ, Weitzberg E. Roles of dietary inorganic nitrate in cardiovascular health and disease. Cardiovasc. Res. 2011;89:525–532. doi: 10.1093/cvr/cvq325. [DOI] [PubMed] [Google Scholar]
- 375.Charles RL, Rudyk O, Prysyazhna O, Kamynina A, Yang J, Morisseau C, et al. Protection from hypertension in mice by the Mediterranean diet is mediated by nitro fatty acid inhibition of soluble epoxide hydrolase. Proc. Natl. Acad. Sci. U.S.a. 2014;111:8167–8172. doi: 10.1073/pnas.1402965111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Martinez-Gonzalez MA, Bes-Rastrollo M, Serra-Majem L, Lairon D, Estruch R, Trichopoulou A. Mediterranean food pattern and the primary prevention of chronic disease: recent developments. Nutrition Reviews. 2009;67(Suppl 1):S111–S116. doi: 10.1111/j.1753-4887.2009.00172.x. [DOI] [PubMed] [Google Scholar]
- 377.Estruch R, Ros E, Salas-Salvadó J, Covas M-I, Corella D, Arós F, et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N. Engl. J. Med. 2013;368:1279–1290. doi: 10.1056/NEJMc1806491. [DOI] [PubMed] [Google Scholar]
- 378.A. Keys. Coronary heart disease in seven countries. 1970. 1997 doi: 10.1016/s0899-9007(96)00410-8. [DOI] [PubMed] [Google Scholar]
- 379.Simopoulos AP. The Mediterranean diets: What is so special about the diet of Greece? The scientific evidence. Journal of Nutrition. 2001;131:3065S–3073S. doi: 10.1093/jn/131.11.3065S. [DOI] [PubMed] [Google Scholar]
- 380.Kafatos A, Verhagen H, Moschandreas J, Apostolaki I, Van Westerop JJ. Mediterranean diet of Crete: foods and nutrient content. J Am Diet Assoc. 2000;100:1487–1493. doi: 10.1016/s0002-8223(00)00416-8. [DOI] [PubMed] [Google Scholar]
- 381.Sandker GW, Kromhout D, Aravanis C, Bloemberg BP, Mensink RP, Karalias N, et al. Serum cholesteryl ester fatty acids and their relation with serum lipids in elderly men in Crete and The Netherlands. Eur J Clin Nutr. 1993;47:201–208. [PubMed] [Google Scholar]
- 382.Kris-Etherton PM, Taylor DS, Yu-Poth S, Huth P, Moriarty K, Fishell V, et al. Polyunsaturated fatty acids in the food chain in the United States. Am. J. Clin. Nutr. 2000;71:179S–188S. doi: 10.1093/ajcn/71.1.179S. [DOI] [PubMed] [Google Scholar]
- 383.Gebauer SK, Psota TL, Harris WS, Kris-Etherton PM. n-3 fatty acid dietary recommendations and food sources to achieve essentiality and cardiovascular benefits. Am. J. Clin. Nutr. 2006;83:1526S–1535S. doi: 10.1093/ajcn/83.6.1526S. [DOI] [PubMed] [Google Scholar]
- 384.Bonacci G, Baker PRS, Salvatore SR, Shores D, Khoo NKH, Koenitzer JR, et al. Conjugated linoleic acid is a preferential substrate for fatty acid nitration. 2012;287:44071–44082. doi: 10.1074/jbc.M112.401356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Baker PRS, Schopfer FJ, O’Donnell VB, Freeman BA. Convergence of nitric oxide and lipid signaling: anti-inflammatory nitro-fatty acids. Free Radic. Biol. Med. 2009;46:989–1003. doi: 10.1016/j.freeradbiomed.2008.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Nadtochiy SM, Zhu QM, Urciuoli W, Rafikov R, Black SM, Brookes PS. Nitroalkenes confer acute cardioprotection via adenine nucleotide translocase. 2015;1(290):30267–30267. doi: 10.1074/jbc.A111.298406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Cole MP, Rudolph TK, Khoo NKH, Motanya UN, Golin-Bisello F, Wertz JW, et al. Nitro-fatty acid inhibition of neointima formation after endoluminal vessel injury. Circ. Res. 2009;105:965–972. doi: 10.1161/CIRCRESAHA.109.199075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Rudolph V, Rudolph TK, Schopfer FJ, Bonacci G, Woodcock SR, Cole MP, et al. Endogenous generation and protective effects of nitro-fatty acids in a murine model of focal cardiac ischaemia and reperfusion. Cardiovasc. Res. 2010;85:155–166. doi: 10.1093/cvr/cvp275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Kansanen E, Bonacci G, Schopfer FJ, Kuosmanen SM, Tong KI, Leinonen H, et al. Electrophilic nitro-fatty acids activate NRF2 by a KEAP1 cysteine 151-independent mechanism. 2011;286:14019–14027. doi: 10.1074/jbc.M110.190710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Kelley EE, Batthyany CI, Hundley NJ, Woodcock SR, Bonacci G, Del Rio JM, et al. Nitro-oleic acid, a novel and irreversible inhibitor of xanthine oxidoreductase. 2008;283:36176–36184. doi: 10.1074/jbc.M802402200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Kelley EE, Baust J, Bonacci G, Golin-Bisello F, Devlin JE, St Croix CM, et al. Fatty acid nitroalkenes ameliorate glucose intolerance and pulmonary hypertension in high-fat diet-induced obesity. Cardiovasc. Res. 2014;101:352–363. doi: 10.1093/cvr/cvt341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Khoo NKH, Rudolph V, Cole MP, Golin-Bisello F, Schopfer FJ, Woodcock SR, et al. Activation of vascular endothelial nitric oxide synthase and heme oxygenase-1 expression by electrophilic nitro-fatty acids. Free Radic. Biol. Med. 2010;48:230–239. doi: 10.1016/j.freeradbiomed.2009.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Cui T, Schopfer FJ, Zhang J, Chen K, Ichikawa T, Baker PRS, et al. Nitrated fatty acids: Endogenous anti-inflammatory signaling mediators. 2006;281:35686–35698. doi: 10.1074/jbc.M603357200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Narala VR, Subramani PA, Narasimha VR, Shaik FB, Panati K. The role of nitrated fatty acids and peroxisome proliferator-activated receptor gamma in modulating inflammation. Immunopharmacol. 2014;23:283–287. doi: 10.1016/j.intimp.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 395.Adkins Y, Kelley DS. Mechanisms underlying the cardioprotective effects of omega-3 polyunsaturated fatty acids. J. Nutr. Biochem. 2010;21:781–792. doi: 10.1016/j.jnutbio.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 396.Marques TM, Wall R, O'Sullivan O, Fitzgerald GF, Shanahan F, Quigley EM, et al. Dietary trans-10, cis-12-conjugated linoleic acid alters fatty acid metabolism and microbiota composition in mice. Br. J. Nutr. 2015;113:728–738. doi: 10.1017/S0007114514004206. [DOI] [PubMed] [Google Scholar]
- 397.Go G-W, Oh S, Park M, Gang G, McLean D, Yang H-S, et al. t10,c12 conjugated linoleic acid upregulates hepatic de novo lipogenesis and triglyceride synthesis via mTOR pathway activation. J. Microbiol. Biotechnol. 2013;23:1569–1576. doi: 10.4014/jmb.1308.08008. [DOI] [PubMed] [Google Scholar]
- 398.McGovern PE, Zhang J, Tang J, Zhang Z, Hall GR, Moreau RA, et al. Fermented beverages of pre- and proto-historic China. Proceedings of the National Academy of Sciences. 2004;101:17593–17598. doi: 10.1073/pnas.0407921102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Gogineni VK, Morrow LE, Gregory PJ. Probiotics: History and Evolution. Journal of Ancient …. 2013 [Google Scholar]
- 400.Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014:506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 401.McFarland LV. From yaks to yogurt: the history, development, and current use of probiotics. Clin. Infect. Dis. 2015;60(Suppl 2):S85–S90. doi: 10.1093/cid/civ054. [DOI] [PubMed] [Google Scholar]
- 402.A. Hotel, J.F.W.E. Consultation. Health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. Córdoba, Argentina: 2001. [Google Scholar]
- 403.Ettinger G, MacDonald K, Reid G, Burton JP. The influence of the human microbiome and probiotics on cardiovascular health. Gut Microbes. 2014;5:719–728. doi: 10.4161/19490976.2014.983775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Ridaura VK, Faith JJ, Rey FE, Cheng J, Duncan AE, Kau AL, et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science. 2013;341:1241214–1241214. doi: 10.1126/science.1241214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Mekkes MC, Weenen TC, Brummer RJ, Claassen E. The development of probiotic treatment in obesity: a review. Benef Microbes. 2014;5:19–28. doi: 10.3920/BM2012.0069. [DOI] [PubMed] [Google Scholar]
- 406.Chen Z, Guo L, Zhang Y, Walzem RL, Pendergast JS, Printz RL, et al. Incorporation of therapeutically modified bacteria into gut microbiota inhibits obesity. J. Clin. Invest. 2014;124:3391–3406. doi: 10.1172/JCI72517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Derrien M, van Hylckama Vlieg JET. Fate, activity, and impact of ingested bacteria within the human gut microbiota. Trends in Microbiology. 2015;23:354–366. doi: 10.1016/j.tim.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 408.Toiviainen A, Jalasvuori H, Lahti E, Gursoy U, Salminen S, Fontana M, et al. Impact of orally administered lozenges with Lactobacillus rhamnosus GG and Bifidobacterium animalis subsp. lactis BB-12 on the number of salivary mutans streptococci, amount of plaque, gingival inflammation and the oral microbiome in healthy adults. Clin Oral Invest. 2015;19:77–83. doi: 10.1007/s00784-014-1221-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Romani Vestman N, Chen T, Lif Holgerson P, Öhman C, Johansson I. Oral Microbiota Shift after 12-Week Supplementation with Lactobacillus reuteri DSM 17938 and PTA 5289;A Randomized Control Trial. PLoS ONE. 2015;10:e0125812. doi: 10.1371/journal.pone.0125812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Terai T, Okumura T, Imai S, Nakao M, Yamaji K, Ito M, et al. Screening of Probiotic Candidates in Human Oral Bacteria for the Prevention of Dental Disease. PLoS ONE. 2015;10:e0128657. doi: 10.1371/journal.pone.0128657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Anusha RL, Umar D, Basheer B, Baroudi K. The magic of magic bugs in oral cavity: Probiotics. J Adv Pharm Technol Res. 2015;6:43–47. doi: 10.4103/2231-4040.154526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Edlund A, Yang Y, Yooseph S, Hall AP, Nguyen DD, Dorrestein PC, et al. Meta-omics uncover temporal regulation of pathways across oral microbiome genera during in vitro sugar metabolism. The ISME Journal. 2015;9:2605–2619. doi: 10.1038/ismej.2015.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Heinken A, Thiele I. Anoxic Conditions Promote Species-Specific Mutualism between Gut Microbes In Silico. Applied and Environmental Microbiology. 2015;81:4049–4061. doi: 10.1128/AEM.00101-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Dong J-Y, Szeto IMY, Makinen K, Gao Q, Wang J, Qin L-Q, et al. Effect of probiotic fermented milk on blood pressure: a meta-analysis of randomised controlled trials. Br. J. Nutr. 2013;110:1188–1194. doi: 10.1017/S0007114513001712. [DOI] [PubMed] [Google Scholar]
- 415.Lee K, Paek K, Lee HY, Park JH, Lee Y. Antiobesity effect of trans-10,cis-12-conjugated linoleic acid-producing Lactobacillus plantarum PL62 on diet-induced obese mice. J. Appl. Microbiol. 2007;103:1140–1146. doi: 10.1111/j.1365-2672.2007.03336.x. [DOI] [PubMed] [Google Scholar]
- 416.Lee H-Y, Park J-H, Seok S-H, Baek M-W, Kim D-J, Lee K-E, et al. Human originated bacteria, Lactobacillus rhamnosus PL60, produce conjugated linoleic acid and show anti-obesity effects in diet-induced obese mice. Biochim. Biophys. Acta. 2006;1761:736–744. doi: 10.1016/j.bbalip.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 417.Meurman JH, Pärnänen P, Kari K, Samaranayake L. Effect of amine fluoride-stannous fluoride preparations on oral yeasts in the elderly: a randomised placebo-controlled trial. Gerodontology. 2009;26:202–209. doi: 10.1111/j.1741-2358.2008.00246.x. [DOI] [PubMed] [Google Scholar]
- 418.Gan XT, Ettinger G, Huang CX, Burton JP, Haist JV, Rajapurohitam V, et al. Probiotic administration attenuates myocardial hypertrophy and heart failure after myocardial infarction in the rat. Circ Heart Fail. 2014;7:491–499. doi: 10.1161/CIRCHEARTFAILURE.113.000978. [DOI] [PubMed] [Google Scholar]
- 419.Zhao B, Sun G, Feng G, Duan W, Zhu X, Chen S, et al. Carboxy terminus of heat shock protein (HSP) 70-interacting protein (CHIP) inhibits HSP70 in the heart. J. Physiol. Biochem. 2012;68:485–491. doi: 10.1007/s13105-012-0161-3. [DOI] [PubMed] [Google Scholar]
- 420.Takemura N, Okubo T, Sonoyama K. Lactobacillus plantarum strain No. 14 reduces adipocyte size in mice fed high-fat diet. Exp. Biol. Med. (Maywood) 2010;235:849–856. doi: 10.1258/ebm.2010.009377. [DOI] [PubMed] [Google Scholar]
- 421.Blaser MJ. Antibiotic use and its consequences for the normal microbiome. Science. 2016;352:544–545. doi: 10.1126/science.aad9358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Neu HC. The crisis in antibiotic resistance. Science. 1992;257:1064–1073. doi: 10.1126/science.257.5073.1064. [DOI] [PubMed] [Google Scholar]
- 423.O.O.T. Commissioner. Press Announcements - FDA issues final rule on safety and effectiveness of antibacterial soaps. 2016 http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm517478.htm.
- 424.DeVries SL, Loving M, Li X, Zhang P. The effect of ultralow-dose antibiotics exposure on soil nitrate and N2O flux. Scientific Reports. 2015;5:16818. doi: 10.1038/srep16818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Yan C, Dinh QT, Chevreuil M, Garnier J, Roose-Amsaleg C, Labadie P, et al. The effect of environmental and therapeutic concentrations of antibiotics on nitrate reduction rates in river sediment. Water Res. 2013;47:3654–3662. doi: 10.1016/j.watres.2013.04.025. [DOI] [PubMed] [Google Scholar]
- 426.Ahmad M, Vithanage M, Kim K, Cho J-S, Lee YH, Joo YK, et al. Inhibitory effect of veterinary antibiotics on denitrification in groundwater: a microcosm approach. Scientific World Journal. 2014;2014:879831–879837. doi: 10.1155/2014/879831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Heid M. You Asked: Should I Use Mouthwash? Time. 2016 http://time.com/4267890/gingivitis-mouthwash-toothpaste/ [Google Scholar]
- 428.Bondonno CP, Liu AH, Croft KD, Considine MJ, Puddey IB, Woodman RJ, et al. Antibacterial mouthwash blunts oral nitrate reduction and increases blood pressure in treated hypertensive men and women. Am. J. Hypertens. 2015;28:572–575. doi: 10.1093/ajh/hpu192. [DOI] [PubMed] [Google Scholar]
- 429.McDonagh STJ, Wylie LJ, Winyard PG, Vanhatalo A, Jones AM. The Effects of Chronic Nitrate Supplementation and the Use of Strong and Weak Antibacterial Agents on Plasma Nitrite Concentration and Exercise Blood Pressure. J Sports Med. 2015;36:1177–1185. doi: 10.1055/s-0035-1554700. [DOI] [PubMed] [Google Scholar]
- 430.Sundqvist ML, Lundberg J, Weitzberg E. Antiseptic mouthwash use and effect on resting metabolic rate and blood pressure among healthy females: a randomized, double-blind, crossover study. The FASEB Journal. 2016 doi: 10.1016/j.niox.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 431.Fujita M, Ueno K, Hata A. Lower frequency of daily teeth brushing is related to high prevalence of cardiovascular risk factors. Exp. Biol. Med. (Maywood) 2009;234:387–394. doi: 10.3181/0809-RM-265. [DOI] [PubMed] [Google Scholar]
- 432.de Oliveira C, Watt R, Hamer M. Toothbrushing, inflammation, and risk of cardiovascular disease: results from Scottish Health Survey. Bmj. 2010;340:c2451–c2451. doi: 10.1136/bmj.c2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Choi HM, Han K, Park Y-G, Park J-B. Associations Among Oral Hygiene Behavior and Hypertension Prevalence and Control: The 2008 to 2010 Korea National Health and Nutrition Examination Survey. J. Periodontol. 2015;86:866–873. doi: 10.1902/jop.2015.150025. [DOI] [PubMed] [Google Scholar]
- 434.van Maanen JM, van Geel AA, Kleinjans JC. Modulation of nitrate-nitrite conversion in the oral cavity. Cancer Detect. Prev. 1996;20:590–596. [PubMed] [Google Scholar]
- 435.Brown ED, Wright GD. Antibacterial drug discovery in the resistance era. Nature. 2016;529:336–343. doi: 10.1038/nature17042. [DOI] [PubMed] [Google Scholar]
- 436.Lehar SM, Pillow T, Xu M, Staben L, Kajihara KK, Vandlen R, et al. Novel antibody-antibiotic conjugate eliminates intracellular S. aureus. Nature. 2015;527:323–328. doi: 10.1038/nature16057. [DOI] [PubMed] [Google Scholar]
- 437.Golkar Z, Bagasra O, Pace DG. Bacteriophage therapy: a potential solution for the antibiotic resistance crisis. J Infect Dev Ctries. 2014;8:129–136. doi: 10.3855/jidc.3573. [DOI] [PubMed] [Google Scholar]
- 438.Zipperer A, Konnerth MC, Laux C, Berscheid A, Janek D, Weidenmaier C, et al. Human commensals producing a novel antibiotic impair pathogen colonization. Nature. 2016;535:511–516. doi: 10.1038/nature18634. [DOI] [PubMed] [Google Scholar]
- 439.Guo L, McLean JS, Yang Y, Eckert R, Kaplan CW, Kyme P, et al. Precision-guided antimicrobial peptide as a targeted modulator of human microbial ecology. Proc. Natl. Acad. Sci. U.S.a. 2015;112:7569–7574. doi: 10.1073/pnas.1506207112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Song P, Wu L, Guan W. Dietary Nitrates, Nitrites, and Nitrosamines Intake and the Risk of Gastric Cancer: A Meta-Analysis. Nutrients. 2015;7:9872–9895. doi: 10.3390/nu7125505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Lundberg JO, Feelisch M, Björne H, Jansson EÅ, Weitzberg E. Cardioprotective effects of vegetables: is nitrate the answer? Nitric Oxide. 2006;15:359–362. doi: 10.1016/j.niox.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 442.Lima ES, Bonini MG, Augusto O, Barbeiro HV, Souza HP, Abdalla DSP. Nitrated lipids decompose to nitric oxide and lipid radicals and cause vasorelaxation. Free Radic. Biol. Med. 2005;39:532–539. doi: 10.1016/j.freeradbiomed.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 443.Churruca I, Fernández-Quintela A, Portillo MP. Conjugated linoleic acid isomers: differences in metabolism and biological effects. Biofactors. 2009;35:105–111. doi: 10.1002/biof.13. [DOI] [PubMed] [Google Scholar]
- 444.Cani PD, Everard A. Talking microbes: When gut bacteria interact with diet and host organs. Molecular Nutrition & Food Research. 2016;60:58–66. doi: 10.1002/mnfr.201500406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Kelly CR, Kahn S, Kashyap P, Laine L, Rubin D, Atreja A, et al. Update on Fecal Microbiota Transplantation 2015: Indications, Methodologies, Mechanisms, and Outlook. Gastroenterology. 2015;149:223–237. doi: 10.1053/j.gastro.2015.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.García-Caballero L, Quintas V, Prada-López I, Seoane J, Donos N, Tomás I. Chlorhexidine substantivity on salivary flora and plaque-like biofilm: an in situ model. PLoS ONE. 2013;8:e83522. doi: 10.1371/journal.pone.0083522. [DOI] [PMC free article] [PubMed] [Google Scholar]