Abstract
From previous cDNA subtraction studies analyzing gene expression in equine endometrium, high lipocalin 2 (LCN2) mRNA expression was found in the gravid endometrium. In the uterus, LCN2 may transport hydrophobic molecules and siderophores with iron, or may form a complex with another protein, however, the expression of uterine LCN2 beyond day 20 of equine pregnancy and its receptor has not been characterized. To study the expression and potential roles of uterine LCN2 from pre-implantation to mid-gestation period, stage-specific endometrial samples were obtained from day 13 (day 0 = ovulation) cyclic and days 13, 19, 25, and 60 to 131 pregnant mares. Expression of LCN2 mRNA increased in day 19 gravid endometrium and was abundant from day 60 onward. The expression of LCN2 mRNA was localized to the glandular epithelium. LCN2 protein was detected in day 25 gravid endometrium and luminal fluid, and the protein was localized to the glandular epithelium and luminal cavity, whereas LCN2 receptor expression was found in luminal and glandular epithelium and trophectoderm throughout the experimental period. The presence of matrix metalloproteinase-9 (MMP9) was also examined because MMP9 is known to form a complex with LCN2. Although MMP9 and LCN2 were both found in luminal fluid from day 25 pregnant uterus, the complex of these proteins was not detected. Localization of the receptor in the trophectoderm suggests that endometrial LCN2 could play a role in carrying small substances from the mother to fetus in the equine species.
Keywords: Endometrium, Equine, Lipocalin 2 (LCN2), LCN2 receptor (SLC22A17), Matrix metalloproteinase-9 (MMP9)
To identify changes in the expression of molecules during the equine peri-implantation period, cDNA subtraction studies with RNA extracted from day 13 cyclic and days 13, 19, and 25 pregnant endometria were performed previously [1]. During the course of these studies, an increase in lipocalin 2 (LCN2) mRNA was found in days 19 and 25 pregnant endometria. This lipocalin, also called neutrophil gelatinase-associated lipocalin (NGAL), was first found in mouse SV-40-infected kidney cells as 24p3 [2], and belongs to the lipocalin super-family with a hydrophobic binding site [3]. LCN2 is known as a multifunctional protein and through its receptor, the recently identified SLC22A17 [4,5,6], LCN2 plays roles in transporting hydrophobic molecules [3], causing apoptosis [7], facilitating cell survival [8], and binding to siderophores with iron [9]. In addition, the uterine expression of lipocalin families, retinol-binding proteins (RBP), salivary lipocalin (SAL1), and P19 lipocalin, has been reported in mice, sows, cows, and mares [10,11,12,13]. Their expressions are localized to luminal and glandular epithelia and the proteins are found in luminal fluid during the late luteal phase and/or pregnancy period. One of their likely roles in the uterus is to carry small molecules to conceptuses through the formation of a complex with their substances. Native expression of LCN2 in the uterus has been reported in mice, where it supports uterotropic growth by increasing iron transportation to these endometrial epithelial cells [14]. The expression of LCN2 in the uterus was also reported in cyclic and early stage of pregnant (earlier than day 20) mares [15]. However, the expression and/or function of uterine LCN2 beyond day 20 of pregnancy have not been characterized in mares.
LCN2 is a 178-amino acid protein which exists in three molecular forms, including a 25-kDa monomer, a 45-kDa homodimer, and a 135-kDa heterodimer in a complex with matrix metalloproteinase 9 (MMP-9) [16, 17]. LCN2 has been characterized as an up-regulated molecule in many pathological conditions including cancer [12, 17]. For the last decade, LCN has been recognized as a molecule that plays a protective role against MMP-9 degradation [10, 16]. MMPs are thought to play central roles in the degradation of extracellular matrix components [18]. Among them, MMP2 and MMP9 are also called gelatinase A and B, respectively, because of their ability to catabolize gelatin [17]. They also degrade collagen IV and laminin, which are the main components of basement membranes [18]. The expression of MMP2 and MMP9 mRNA in the uterus tends to increase at estrus in mice [19, 20]. In addition, abundant expression of these genes is detected during the implantation process in rodents [21, 22]. Conceptuses of rodents invade into the endometrium concurrent with extensive uterine tissue remodeling, and MMP9 is expressed predominantly around the conceptus implantation sites [21]. Although MMP2 mRNA is expressed in the endometrial regions surrounding conceptuses in rats [22], its expression is concentrated around the mesometrial pole in mice [21]. In either case, both MMP2 and MMP9 play an important role in successful implantation of invasive conceptuses [18, 19], but their importance is also recognized in noninvasive conceptuses [23].
Equine trophoblast cells do not invade the endometrium until endometrial cups are formed on day 36 of pregnancy [24]. Thus, MMP degradation of endometrial proteins in the early pregnant mare is not expected. In our previous cDNA subtraction study, along with calcium maintenance factor, Stanniocalcin (STC) transcripts and protein [25], LCN2 was found to be one of the transcripts expressed abundantly in the pregnant uterus [25]. The present study was conducted to determine changes in uterine expression of LCN2 and its receptor from pre-implantation to mid-pregnancy period. The study was extended to find if LCN2 forms a complex with MMP9.
Materials and Methods
Animals and tissue collections
Fourteen clinically healthy Thoroughbred mares showing regular estrous cycles aged 4 to 17 years were maintained at two local farms through arrangements made by Japan Racing Association (JRA) and the Hidaka horse breeders’ association in Urakawa, Hokkaido, Japan. The horses were allowed to graze together each day, and were fed twice daily on a balanced ration of pelleted feed and hay. Two other Thoroughbred mares aged 11 and 23 years were maintained at Obihiro University of Agriculture and Veterinary Medicine and similarly fed. The use of horses was reviewed and approved by the animal care committees at JRA, the University of Tokyo, and Obihiro University of Agriculture and Veterinary Medicine. Ovaries were monitored by rectal palpation and ultrasonography (ECHOPAL, Hitachi, Tokyo, Japan, with a 5.0 to 7.5 MHz changeable probe (EUP-O33J) or HS-101V, Honda, Toyohashi, Japan) [26]. Mares were treated intramuscularly with PGF2α (0.25 mg/mare, Planate; Dainippon Sumitomo Pharma, Osaka, Japan) during the luteal phase. When growing follicles over 3.5 cm in diameter were found, hCG (2,500 IU/mare, GONATROPIN; ASKA Pharmaceutical, Tokyo, Japan) was administered to induce ovulation. Fourteen mares were mated with fertile stallions and two mares were artificially inseminated with fresh semen at the appropriate timing, and the day of ovulation (day 0) was recorded. Through the use of ultrasonography and detection of liquids in fetal membranes, pregnancy was confirmed on day 12.
Uteri from cyclic mares on day 13 and pregnant mares on days 13, 19, 25 (n = 2 each), 60, 83, 88, 96, 120, and 131 (grouped into days 60 to 88 and days 96 to 131) were removed immediately following slaughter at a local abattoir. To obtain a sufficient number (n = 3) of samples at each time point, additional endometrial samples were biopsied from two mares on day 13 cyclic and days 13, 19, and 25 pregnancy (n = 1 each). On day 25 of pregnancy, luminal fluid was taken using an insemination pipet just before biopsy. Five milliliters of 20 mM phosphate buffered saline (PBS, pH 7.2) was injected into the cranial uterine body and immediately aspirated. The fluid was centrifuged at 1,600 × g, at 4°C for 15 min, and the supernatant was stored at –70°C. Uterine horns and body from days 13 to 25 were each divided into three parts and numbered from anterior to posterior, non-gravid (N1–N3) or gravid (G1–G3), representing the absence or presence of a conceptus, respectively. Portions of the uterine body were designated as B1–B3, posterior to anterior, relative to uterine horns (Supplementary Fig. 1: online only). In the experiments involving assay validation, high LCN2 expression was observed in the uterine region, where conceptus fixation occurred (G3). In this study, therefore, uterine tissues and uterine biopsies taken from N3 and G3 regions were used for a series of LCN2 expression studies. A piece of uterine tissue from each of these parts was fixed in 4% paraformaldehyde for immunohistochemistry studies. Days 13, 19, and 25 conceptuses were collected following longitudinal incision of the uterus to open the endometrium and immediately snap-frozen in liquid nitrogen. Additionally, some endometrial tissue pieces from the remaining uteri and from the portion close to umbilical cord were collected on days 60 to 131, frozen immediately in liquid nitrogen, stored at –70°C, and used for subsequent RNA and protein extraction, immunohistochemistry, and/or in situ hybridization.
Real-time PCR
Total RNA was extracted from frozen endometrial tissues, using Isogen (Nippon Gene, Tokyo, Japan). One microgram of total RNA with A260/280 value higher than 1.8 was treated with DNase (Promega, Madison, WI, USA), from which cDNA was synthesized using M-MLV reverse transcriptase (Invitrogen, Carlsbad, CA, USA) with random primers (Life Technologies, Carlsbad, CA, USA) according to the manufacturer’s instructions [27]. The targeted cDNAs were quantified by real-time PCR using an ABI PRISM 7900HT system (Applied Biosystems, Foster City, CA, USA). Nucleotide structures for oligonucleotide primers were designed using the web-based Primer3 software. The primer sequences for LCN2 and GAPDH were: LCN2 (forward, 5'-CTC CTG TGG CTA GGC TTC AC-3' and reverse, 5'-ACC CAC GAC ATA CCA CTT CC-3'), GAPDH (forward, 5'-CAT CCT GGG CTA CAC TGA GG T-3' and reverse, 5'-GTC CAC CAC CCT ATT GCT GT-3') [1, 25]. PCRs were performed using Ex Taq Hot Start version containing SYBR-Green I (TaKaRa, Kyoto, Japan), and the PCR amplification consisted of 40 cycles of denaturation at 95°C for 30 sec, annealing at 60°C for 30 sec, and extension at 72°C for 1 min. The melting curve of samples and no template control were checked to confirm the absence of non-specific reaction. Because endometrial GAPDH expression was stable across the examined period [1, 25], GAPDH was used as a reference gene transcript in this study. The level of each target mRNA relative to GAPDH mRNA indicated that the amplification efficiency of both genes was similar.
In situ hybridization
To generate cRNA probes specific for equine LCN2, respective cDNAs were obtained from equine endometrial RNA using RT-PCR. Oligonucleotide primers used for this PCR amplifications were forward, 5'-CTC CTG TGG CTA GGC TTC AC-3' and reverse, 5'-CTG AAA AAC ACG ATG GCA AA-3' and the product length was 419 bps. The RT-PCR-derived fragments were subcloned into Easy T vectors (Promega), from which digoxigenin (DIG)-labeled cRNA probes were generated using the DIG RNA Labeling Kit (Roche Diagnostics, Basel, Switzerland) following the manufacturer’s protocol.
Frozen tissues were sectioned (10 μm) and mounted onto silan-coated slides and fixed in 4% paraformaldehyde in PBS [28]. Slide sections were pretreated sequentially with 0.2N HCl and 20 μg/ml proteinase K in Tris-HCl (pH 7.6), 4% paraformaldehyde, and 0.2% glycine. The sections were then prehybridized with the solution containing 50% formamide, 5 × SSC, 1 × Denhardt’s, 100 μg/ml heparin, 10 mM DTT, 10% dextran sulfate, and 0.1 mg/ml denatured tRNA and ssDNA, followed by hybridization with DIG-labeled antisense or sense cRNA probes at 45°C for 16 h. After hybridization and washing once with 4 × SSC at 42°C for 20 min, the sections were incubated with RNase-A (10 μg/ml) at 37°C for 30 min, washed twice with 2 × SSC at 65°C for 30 min and then twice with 0.1 × SSC at 65°C for 30 min. The slides were blocked with a blocking reagent (Roche Diagnostics), and incubated with an anti-DIG alkaline phosphatase-conjugated antibody (Roche Diagnostics). The signals were detected with 5-bromo-4-chloro-3-indolyl phosphate and nitro blue tetrazolium (Promega).
Western blot analysis
Protein samples (20 μg) from endometrial lysates in reduced condition and centrifugally concentrated (Microcon YM-3; Millipore, Bedford, MA, USA) proteins from luminal fluid (4 μg) in reduced or non-reduced condition were separated on 12.5% SDS-PAGE and transferred onto nitrocellulose membrane (Immobilon; Millipore) [1, 25, 28]. After blocking with Block Ace (Dainippon Sumitomo Pharma) at room temperature for 1 h, the membranes were incubated with goat anti-human LCN2 antibody (200 ng/ml; AF1757, R&D systems, Minneapolis, MN, USA) or mouse anti-β-actin antibody (30 ng/ml; Sigma-Aldrich, St. Louis, MO, USA) at 4°C for 12 h. After incubation, the membrane was washed three times in TBS-Tween 20 and incubated with horseradish peroxidase-conjugated donkey anti-goat IgG or goat anti-mouse IgG (Amersham Biosciences, Piscataway, NJ, USA) at room temperature for 1 h. Signals were detected using ECL Plus Western Blotting Detection Reagents (GE Healthcare UK, Buckinghamshire, UK).
Immunohistochemistry
Endometrial tissues that had been fixed in 4% paraformaldehyde and embedded in paraffin were sectioned at 6 μm, and mounted onto MAS-coated slides (Matsunami Glass Ind, Osaka, Japan) [28]. Frozen fetal membranes were also sectioned at 6 μm in cryostat, mounted on glass slides, and then fixed in 4% paraformaldehyde. Antigen retrieval was initially performed in a heat-treated (95°C for 5 min) sodium citrate buffer (0.01 M, pH 6.0). The sections were treated with 3% H2O2/methanol at room temperature for 30 min to reduce endogenous peroxidase activity and then in Block Ace at room temperature for 1 h to quench non-specific staining. The sections were incubated with goat anti-human LCN2 antibody (4 μg/ml, AF1757, R&D Systems), goat anti-human SLC22A17 antibody (4 μg/ml, OAEB00639, Aviva Systems Biology, San Diego, CA, USA) or normal goat IgG (4 μg/ml) at 4°C for 12 h. After the primary antibody incubation, the slides were incubated with biotin-conjugated donkey anti-goat IgG (GE Healthcare UK Ltd.) at room temperature for 1 h. Specific signals were visualized using the VECTASTAIN Elite ABC Kit (Vector Laboratories, Burlingame, CA, USA). The tissue sections were counterstained with hematoxylin.
Gelatin zymography
Endometrial protein extracts (10 μg) and luminal fluid (4 μg) samples, incubated in sample buffer without 2-mercaptoethanol at 37°C for 30 min, were separated on 10% SDS-PAGE containing 0.5 mg/ml gelatin at 4°C. The gel was washed twice with 2.5% Triton X-100 for 15 min and three times with gelatinase buffer (50 mM Tris-HCl, 200 mM NaCl, 5 mM CaCl2, 1 μM ZnCl2, and 0.01% NaN3, pH 7.5) for 10 min. The gel was then incubated in gelatinase buffer at 37°C for 16 h. After incubation, the gel was stained with 0.25% Coomassie brilliant blue R-250 and destained until clear bands were visualized.
Statistical analysis
The data are presented as means ± SEM. Results from real-time PCR analysis were subjected to one-way ANOVA using the general linear model procedures (JMP; SAS Institute Japan). The model used in the ANOVA included day, including gravid and non-gravid uterine horns on days 19 and 25, and replicate as sources of variation. When a significant difference based on day of pregnancy or gravid and non-gravid uterine horns was detected (P < 0.05), the data for mRNA amounts were analyzed by Dunnett’s multiple comparison tests vs. C13. Because the degree of LCN2 mRNA expression differs from early (2–3 fold increase vs. C13) to mid-pregnancy (more than 100 fold increase vs. C13), additional analysis was performed separately: early (P13, P19, and P25 vs. C13) and early to mid-pregnancy (P13, P19, P25, P60-88, and P96-131 vs. C13).
Results
Change of LCN2 mRNA in pregnant endometrium
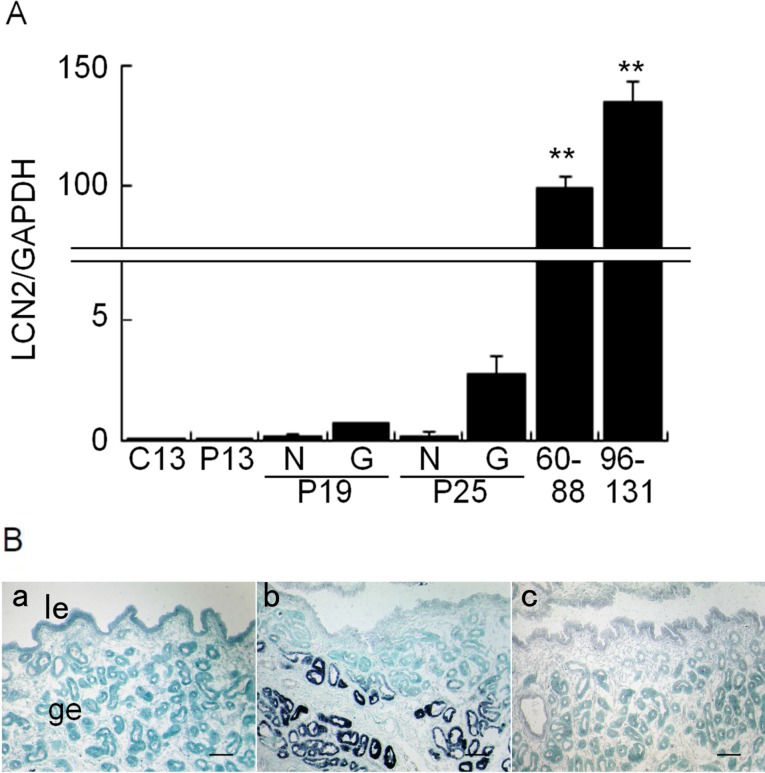
Endometrial LCN2 mRNA levels in RNA extracted from day 13 cyclic and days 13, 19 (N3 and G3), 25 (N3 and G3), 60 to 88, and 96 to 131 pregnant uterine horns were examined by real-time PCR analysis. These uterine tissues corresponded to the region where conceptus fixation/lodging occurred [1]. RNA was extracted from similar regions in day 13 cyclic and pregnant endometria, N3 or G3 of days 19 and 25 pregnant horns, and from the regions close to umbilical cord in days 60 to 131 pregnant samples. Minimal expression of LCN2 mRNA was detected in the uterine endometrium of day 13 cyclic and day 13 pregnant mares (Fig. 1A). Because of the different order of magnitude of endometrial LCN2 mRNA increase between early and mid-pregnancy, separate statistical analyses were performed to analyze those of early pregnancy and those of the entire experimental periods. LCN2 mRNA increased on day 25 and exhibited marked increases in the gravid endometrium on days 60 to 88 and 96 to 131 pregnancy. In situ hybridization was performed to localize LCN2 transcripts in frozen uterine sections prepared from day 13 pregnant and day 25 gravid endometria (Fig. 1B). Whereas endometrial LCN2 mRNA was not detected in day 13 cyclic mares, it was detected in the glandular epithelium, in agreement with the results of real-time PCR.
Fig. 1.
Levels and localization of LCN2 mRNA in the equine uterus during early- to mid-pregnancy. A) Real-time PCR analysis of LCN2 mRNA in the equine uteri (n = 3 each day). In day 13 cyclic (C13) and day 13 pregnant (P13) uteri, RNA was extracted from left (L3) and right (R3) regions of the endometrium. Regardless of uterine samples examined, LCN2 mRNA levels were minimal in the samples from L3 or R3 region, and therefore their average was used as the value for C13 or P13. On day 19 (P19) and day 25 (P25) of pregnancy, RNA was extracted from non-gravid (N3) or gravid (G3) region separately, and the average of three samples was used as LCN2 value for non-gravid or gravid uterine horn. In days 60 to 88 and 96 to 131 pregnancy, RNA was extracted from the region close to umbilical cord, and the average of days 60 to 88 and 96 to 131 LCN2 value were used. Note that the degree of LCN2 mRNA abundance differs between early and mid-pregnant endometrium. When the levels of LCN2 mRNA in the early pregnant endometrium were analyzed separately from those of the entire experimental period, the increase in LCN2 mRNA in days 19 and 25 (vs. C13) endometrium was significant. B) In situ hybridization analysis of LCN2 mRNA in the equine uterus. DIG-labeled days 13 and 25 pregnant mares (a and b), sense LCN2 in day 25 pregnant mare (c). A representative image is shown. le, luminal epithelium; ge, glandular epithelium. Bar = 100 μm. ** P < 0.01.
Change of LCN2 and its receptor expression in pregnant uterus
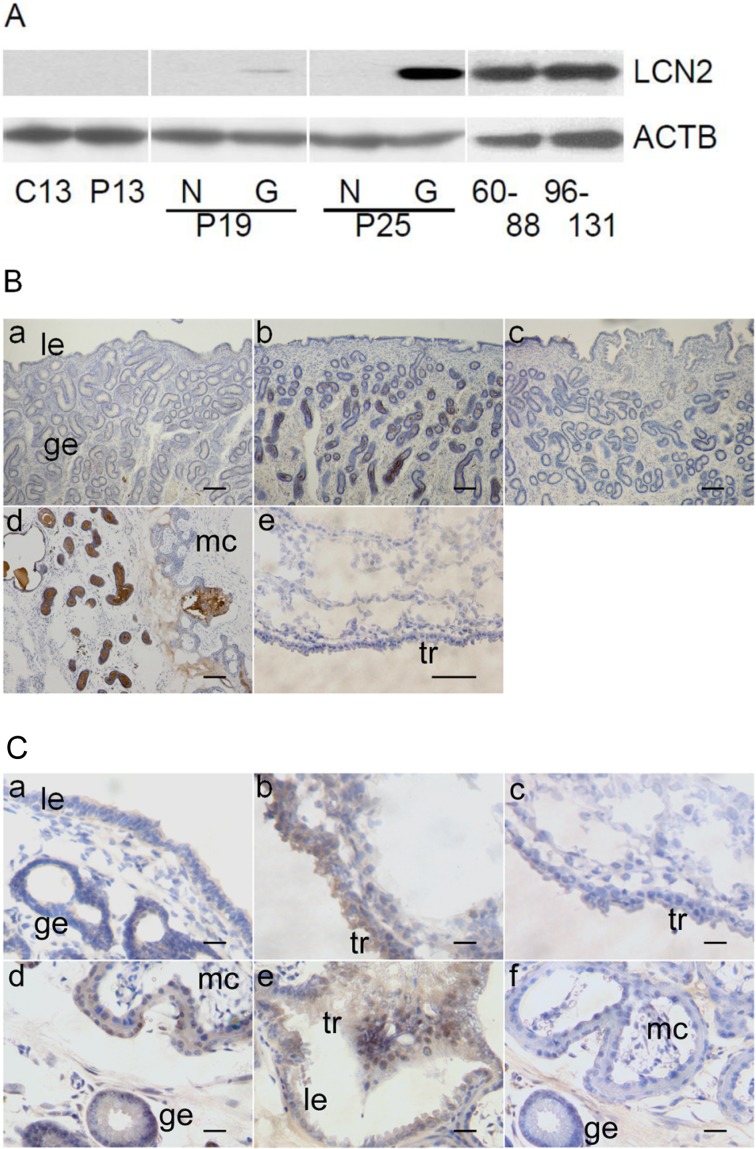
Endometrial and fetal membrane LCN2 in day 13 cyclic and days 13, 19 (N3 and G3), 25 (N3 and G3), 60 to 88, and 96 to 131 pregnancy were examined by western blot analysis (Fig. 2A). Consistent with the result from the quantitative real-time PCR and in situ hybridization analyses, LCN2 expression was found in day 25 gravid endometrium, but not in fetal membranes. A strong signal for LCN2 was also detected in 120 day pregnant endometrium, particularly in the areolae of non-placental regions between microcotyledons. Abundant LCN2 expression was also seen in the 60 to 88 and 96 to 131 pregnant endometria. Immunohistochemical analysis revealed that LCN2 was localized in the glandular epithelium on day 25 (Fig. 2B), agreeing with the results of in situ hybridization. In addition, positive signals for the presence of LCN2 were observed in the luminal cavity of uterine glands of day 25 gravid uterus.
Fig. 2.
Expression and localization of LCN2 in the equine uterus during early- to mid-pregnancy. A) Western blot analysis of LCN2 in the extracts from equine endometrial samples. In day 13 cyclic (C13) and day 13 pregnant (P13) uteri, samples from left (L3) and right (R3) endometrium were pooled and analyzed. In days 19 (P19) and 25 (P25) pregnant uteri, non-gravid (N3) or gravid (G3) endometrium were separately analyzed. In days 60 to 88 and 96 to 131 pregnant uteri, samples were pooled and analyzed. Note that minute and definitive LCN2 expression were seen in days 19 and 25 endometrial extracts, respectively. B) Immunohistochemical analysis of LCN2 in the equine uterine endometrium and fetal membranes. Goat anti-human LCN2 antibody was applied to tissues obtained from day 13 and 25 pregnant endometrium (a and b), normal goat IgG in day 25 pregnant mare (c), day 120 interface of endometrium and chorioallantois (d), and day 25 fetal membrane (e). A representative image is shown. le, luminal epithelium; ge, glandular epithelium. Bar = 100 μm. C) Immunohistochemical analysis of LCN2 receptor, SLC22A17, in the equine endometrium and fetal membranes. Goat anti-human SLC22A17 antibody was used to detect LCN2 receptor in day 19 pregnant endometrium and fetal membrane (a and b), day 120 interface of microcotyledon and areolae (d and e), and normal goat IgG in day 19 fetal membrane and day 120 interface of microcotyledon (c and f). A representative image is shown. le, luminal epithelium; ge, glandular epithelium; mc, microcotyledon; tr, trophectoderm. Bar = 40 μm.
Through the use of immunohistochemistry, the presence of endometrial and fetal membrane LCN2 receptor, SLC22A17, was examined in day 13 cyclic and days 13, 19 (N3 and G3), 25 (N3 and G3), 60 to 88, and 96 to 131 pregnant uteri (Fig. 2C). SLC22A17 expression was detected in luminal and glandular epithelium, as well as in trophectoderm throughout the experimental period.
Expression of MMP2, MMP9, and LCN2 in endometrium and luminal fluid
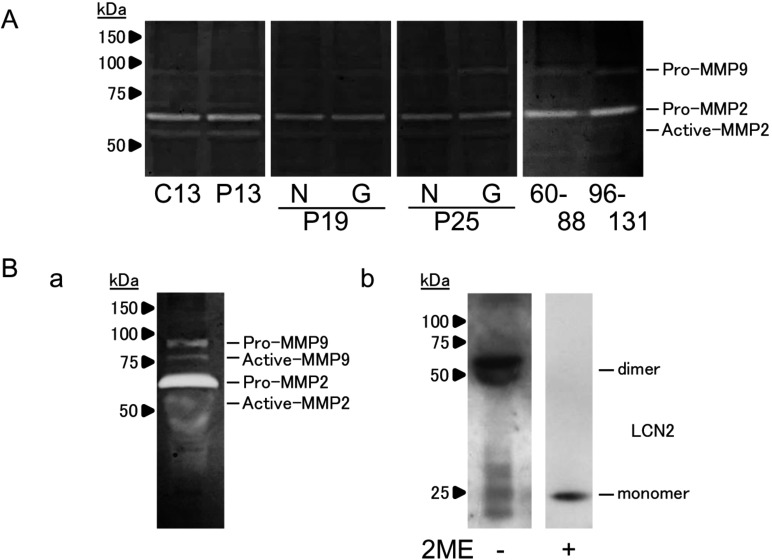
Endometrial MMP2 and MMP9 activities on day 13 cyclic and days 13, 19 (N3 and G3), 25 (N3 and G3), 60 to 88, and 96 to 131 pregnancy were examined by gelatin zymography. Both pro-protein and active forms of MMP2 and pro-MMP9 were found in the endometrium. No extra band above 100 kDa was found in any sample.
Gelatin zymography also revealed both pro-protein and active forms of MMP2 and MMP9 in day 25 luminal fluid. No extra band above 100 kDa was found (Fig. 3Ba), possibly indicating the absence of a heterodimer complex with MMP9. Western blotting of day 25 luminal fluid also revealed that LCN2 homodimer was present under non-reducing condition, whereas LCN2 monomer was found under the reduced PAGE condition (Fig. 3Bb). These results indicated that although sufficient amounts of LCN2 and MMP9 were present in the equine uterus, they were likely to have existed independently without a complex formation.
Discussion
The increase in LCN2 in the endometrial region where conceptus fixation has occurred indicates that endometrial LCN2 expression during early pregnant period, i.e., on days 19 and 25 (Fig. 1 and Fig. 2A), could be enhanced by the conceptus and/or its secretory products. It has been demonstrated that expression of LCN2 is induced by estradiol-17β (E2) in luminal and glandular epithelium of ovariectomized mouse uterus [29], and that progesterone (P4) quenches the stimulatory effect of E2 on LCN2 expression when E2 is used in concert with P4 on ovariectomized mice [29]. We have previously demonstrated the presence of a high concentration of conceptus-derived E2 in the gravid endometrium on day 25 of pregnancy [1]. These and our results suggest that initial increase in endometrial LCN2 expression during the early pregnant period could be due to the local effects of conceptus derived E2.
In this study, LCN2 mRNA was localized in the glandular epithelium (Fig. 1B-b), and the protein was detected in the glandular and luminal epithelial regions, but not in fetal membranes (Fig. 2B). The presence of LCN2 in the luminal fluid (Fig. 3B-b) indicated that the protein was secreted into the uterine cavity. In addition, LCN2 receptor was detected in both endometrial regions and fetal membranes (Fig. 2C). It has been observed that equine placenta begins to develop approximately on day 40, and it is fully developed with placental villi and microcotyledons by day 120 [30]. Furthermore, the placental areolae which absorb secretory products from endometrial glands are present even during late stages of pregnancy [31]. Our finding that strong signal for LCN2 was detected in the areolae of non-placental regions between microcotyledons of 120 pregnant endometrium agrees with those results. A previous study shows that LCN2 may play important roles in the regulation of cell proliferation and survival [8]. Proliferating cells have a higher demand for intracellular iron than quiescent cells, and increased iron availability is essential for the maintenance of cell proliferation, resulting from active synthesis of DNA, cytochromes and other ion containing proteins [32]. In fact, LCN2 expression is found in the proliferative phase of mouse uterus [29] or high-grade carcinoma [11]. LCN2 has also been reported to have the ability to bind ferric siderophores [9]. Therefore, endometrial LCN2, through its receptor, may supply sufficient iron and hydrophobic nutrients required by the conceptus and/or endometrium of pregnant mare at least until mid-pregnancy.
Fig. 3.
MMP2 and MMP9 activity in the equine uterus during early- to mid-pregnancy. (A) Gelatin zymography analysis of MMP2 and MMP9 activity in the extracts from equine endometrial samples. In day 13 cyclic (C13) and day 13 pregnant (P13) uteri, samples from left (L3) or right (R3) endometrium were pooled and analyzed. In days 19 (P19) and 25 (P25) pregnant uteri, samples from non-gravid (N3) or gravid (G3) endometrium were separately analyzed. In days 60 to 88 and 96 to 131 pregnant uteri, three independent samples were mixed and analyzed. (B-a) Gelatin zymography analysis of MMP2 and MMP9 activity in the luminal fluid on day 25 of pregnancy. (B-b) Western blot analysis of LCN2 in the luminal fluid in non-reduced (–ME) and reduced (+ME) conditions on day 25 of pregnancy is also presented. A representative image is shown.
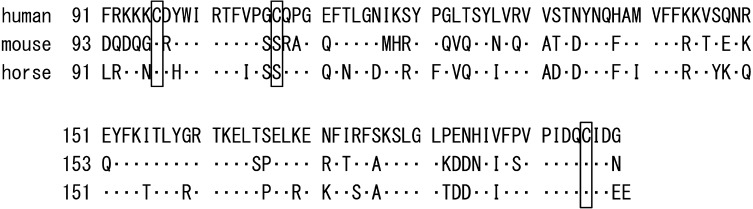
It has been demonstrated that in humans, LCN2 binds to MMP9, resulting in the formation of an approximately 125 kDa complex, which increases MMP9 activity by preventing it from degradation [16]. In the present study, MMP9 activity was found in all endometrial extracts (Fig. 3A) and in luminal fluid (Fig. 3B-a) on day 25 of pregnancy. By immunoblotting, LCN2 was also found in the luminal fluid (Fig. 3B-b). In this study, however, the LCN2-MMP9 complex was not detected even though LCN2 homodimer was detected in non-reduced condition. A lack of LCN2-MMP9 complex formation has been observed in murine granulocytes and tumor tissues [33]. This could be due to the fact that Cys-107, which is required for LCN2-MMP9 complex formation through a disulfide bond, is not conserved in mice. Similar to mice, the LCN2-MMP9 complex was not found in horses, which could be due to the absence of Cys-107 in the equine LCN2 (Fig. 4). The high expression of LCN2 and MMP9 shown in this study and by others [34] suggests that these proteins are required for pregnancy in horses, but these proteins are likely to play their roles independently in the luminal cavity of uterus in pregnant mares.
Fig. 4.
Partial amino acid sequences of LCN2 in humans, mice and horses. C-terminal amino acids sequences of LCN2 in humans, mice and horses in which the presence of cysteine residues was compared. Amino acid residues that are the same as those of humans are represented as dots. Amino acid residues corresponding to cysteine in the human sequence are marked with rectangles in the mouse and horse sequences. Unlike humans, Cys-107 is not present in mouse or horse LCN2.
In summary, an increase in endometrial LCN2 expression was detected during early pregnancy period, tending toward a marked increase by mid-pregnancy, whereas consistent expression of LCN2 receptor was found throughout the experimental period. Although high expression of LCN2 and MMP9 was found, no signs of the LCN2-MMP9 complex in the luminal cavity could be detected, suggesting instead that LCN2 functions with its receptor as a carrier protein for small molecules in the equine uterus.
Supplementary
Acknowledgments
The authors appreciated the dedicated care of the horses at Nakawaki and Haraguchi farms, Urakawa, Hokkaido. The authors would like to thank Mr Robert Moriarty for his critical reading of the manuscripts. This work was supported by research funds from Japan Racing Association (JRA) and by a Grant-in-Aid for Scientific Research (18108004) to KI from the Japan Society for the Promotion of Science (JSPS).
References
- 1.Haneda S, Nagaoka K, Nambo Y, Kikuchi M, Nakano Y, Matsui M, Miyake Y, Macleod JN, Imakawa K. Interleukin-1 receptor antagonist expression in the equine endometrium during the peri-implantation period. Domest Anim Endocrinol 2009; 36: 209–218. [DOI] [PubMed] [Google Scholar]
- 2.Hraba-Renevey S, Türler H, Kress M, Salomon C, Weil R. SV40-induced expression of mouse gene 24p3 involves a post-transcriptional mechanism. Oncogene 1989; 4: 601–608. [PubMed] [Google Scholar]
- 3.Flower DR. The lipocalin protein family: structure and function. Biochem J 1996; 318: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miyamoto T, Asaka R, Suzuki A, Takatsu A, Kashima H, Shiozawa T. Immunohistochemical detection of a specific receptor for lipocalin2 (solute carrier family 22 member 17, SLC22A17) and its prognostic significance in endometrial carcinoma. Exp Mol Pathol 2011; 91: 563–568. [DOI] [PubMed] [Google Scholar]
- 5.Zhang PX, Zhang FR, Xie JJ, Tao LH, Lü Z, Xu XE, Shen J, Xu LY, Li EM. Expression of NGAL and NGALR in human embryonic, fetal and normal adult tissues. Mol Med Rep 2012; 6: 716–722. [DOI] [PubMed] [Google Scholar]
- 6.Cabedo Martinez AI, Weinhäupl K, Lee WK, Wolff NA, Storch B, Żerko S, Konrat R, Koźmiński W, Breuker K, Thévenod F, Coudevylle N. Biochemical and Structural Characterization of the Interaction between the Siderocalin NGAL/LCN2 (Neutrophil Gelatinase-associated Lipocalin/Lipocalin 2) and the N-terminal Domain of Its Endocytic Receptor SLC22A17. J Biol Chem 2016; 291: 2917–2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Devireddy LR, Teodoro JG, Richard FA, Green MR. Induction of apoptosis by a secreted lipocalin that is transcriptionally regulated by IL-3 deprivation. Science 2001; 293: 829–834. [DOI] [PubMed] [Google Scholar]
- 8.Lin HH, Liao CJ, Lee YC, Hu KH, Meng HW, Chu ST. Lipocalin-2-induced cytokine production enhances endometrial carcinoma cell survival and migration. Int J Biol Sci 2011; 7: 74–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goetz DH, Holmes MA, Borregaard N, Bluhm ME, Raymond KN, Strong RK. The neutrophil lipocalin NGAL is a bacteriostatic agent that interferes with siderophore-mediated iron acquisition. Mol Cell 2002; 10: 1033–1043. [DOI] [PubMed] [Google Scholar]
- 10.Kubben FJ, Sier CF, Hawinkels LJ, Tschesche H, van Duijn W, Zuidwijk K, van der Reijden JJ, Hanemaaijer R, Griffioen G, Lamers CB, Verspaget HW. Clinical evidence for a protective role of lipocalin-2 against MMP-9 autodegradation and the impact for gastric cancer. Eur J Cancer 2007; 43: 1869–1876. [DOI] [PubMed] [Google Scholar]
- 11.Seo H, Kim M, Choi Y, Ka H. Salivary lipocalin is uniquely expressed in the uterine endometrial glands at the time of conceptus implantation and induced by interleukin 1 beta in pigs. Biol Reprod 2011; 84: 279–287. [DOI] [PubMed] [Google Scholar]
- 12.Costello LM, O'Boyle P, Godkin JD, Diskin MG, Hynes AC, Morris DG. Retinol-binding protein (RBP), retinol and beta-carotene in the bovine uterus and plasma during the oestrous cycle and the relationship between systemic progesterone and RBP on day 7. Reprod Fertil Dev 2010; 22: 1198–1205. [DOI] [PubMed] [Google Scholar]
- 13.Crossett B, Allen WR, Stewart F. A 19 kDa protein secreted by the endometrium of the mare is a novel member of the lipocalin family. Biochem J 1996; 320: 137–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jessen KA, Satre MA. Mouse retinol binding protein gene: cloning, expression and regulation by retinoic acid. Mol Cell Biochem 2000; 211: 85–94. [DOI] [PubMed] [Google Scholar]
- 15.Merkl M, Ulbrich SE, Otzdorff C, Herbach N, Wanke R, Wolf E, Handler J, Bauersachs S. Microarray analysis of equine endometrium at days 8 and 12 of pregnancy. Biol Reprod 2010; 83: 874–886. [DOI] [PubMed] [Google Scholar]
- 16.Yan L, Borregaard N, Kjeldsen L, Moses MA. The high molecular weight urinary matrix metalloproteinase (MMP) activity is a complex of gelatinase B/MMP-9 and neutrophil gelatinase-associated lipocalin (NGAL). Modulation of MMP-9 activity by NGAL. J Biol Chem 2001; 276: 37258–37265. [DOI] [PubMed] [Google Scholar]
- 17.Lippi G, Meschi T, Nouvenne A, Mattiuzzi C, Borghi L. Neutrophil gelatinase-associated lipocalin in cancer. Adv Clin Chem 2014; 64: 179–219. [DOI] [PubMed] [Google Scholar]
- 18.Salamonsen LA. Role of proteases in implantation. Rev Reprod 1999; 4: 11–22. [DOI] [PubMed] [Google Scholar]
- 19.Rudolph-Owen LA, Hulboy DL, Wilson CL, Mudgett J, Matrisian LM. Coordinate expression of matrix metalloproteinase family members in the uterus of normal, matrilysin-deficient, and stromelysin-1-deficient mice. Endocrinology 1997; 138: 4902–4911. [DOI] [PubMed] [Google Scholar]
- 20.Zhang X, Nothnick WB. The role and regulation of the uterine matrix metalloproteinase system in menstruating and non-menstruating species. Front Biosci 2005; 10: 353–366. [DOI] [PubMed] [Google Scholar]
- 21.Das SK, Yano S, Wang J, Edwards DR, Nagase H, Dey SK. Expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in the mouse uterus during the peri-implantation period. Dev Genet 1997; 21: 44–54. [DOI] [PubMed] [Google Scholar]
- 22.Zhao YG, Xiao AZ, Cao XM, Zhu C. Expression of matrix metalloproteinase -2, -9 and tissue inhibitors of metalloproteinase -1, -2, -3 mRNAs in rat uterus during early pregnancy. Mol Reprod Dev 2002; 62: 149–158. [DOI] [PubMed] [Google Scholar]
- 23.Hirata M, Sato T, Tsumagari M, Shimada A, Nakano H, Hashizume K, Ito A. Differential regulation of the expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases by cytokines and growth factors in bovine endometrial stromal cells and trophoblast cell line BT-1 in vitro. Biol Reprod 2003; 68: 1276–1281. [DOI] [PubMed] [Google Scholar]
- 24.Allen WR, Hamilton DW, Moor RM. The origin of equine endometrial cups. II. Invasion of the endometrium by trophoblast. Anat Rec 1973; 177: 485–501. [DOI] [PubMed] [Google Scholar]
- 25.Kikuchi M, Nakano Y, Nambo Y, Haneda S, Matsui M, Miyake Y, Macleod JN, Nagaoka K, Imakawa K. Production of calcium maintenance factor Stanniocalcin-1 (STC1) by the equine endometrium during the early pregnant period. J Reprod Dev 2011; 57: 203–211. [DOI] [PubMed] [Google Scholar]
- 26.Nambo Y, Nagaoka K, Tanaka Y, Nagamine N, Shinbo H, Nagata S, Yoshihara T, Watanabe G, Groome NP, Taya K. Mechanisms responsible for increase in circulating inhibin levels at the time of ovulation in mares. Theriogenology 2002; 57: 1707–1717. [DOI] [PubMed] [Google Scholar]
- 27.Nagaoka K, Sakai A, Nojima H, Suda Y, Yokomizo Y, Imakawa K, Sakai S, Christenson RK. A chemokine, interferon (IFN)-gamma-inducible protein 10 kDa, is stimulated by IFN-tau and recruits immune cells in the ovine endometrium. Biol Reprod 2003; 68: 1413–1421. [DOI] [PubMed] [Google Scholar]
- 28.McGuire WJ, Imakawa K, Tamura K, Meka CS, Christenson RK. Regulation of endometrial granulocyte macrophage-colony stimulating factor (GM-CSF) in the ewe. Domest Anim Endocrinol 2002; 23: 383–396. [DOI] [PubMed] [Google Scholar]
- 29.Huang HL, Chu ST, Chen YH. Ovarian steroids regulate 24p3 expression in mouse uterus during the natural estrous cycle and the preimplantation period. J Endocrinol 1999; 162: 11–19. [DOI] [PubMed] [Google Scholar]
- 30.Le NT, Richardson DR. The role of iron in cell cycle progression and the proliferation of neoplastic cells. Biochim Biophys Acta 2002; 1603: 31–46. [DOI] [PubMed] [Google Scholar]
- 31.Allen WR, Stewart F. Equine placentation. Reprod Fertil Dev 2001; 13: 623–634. [DOI] [PubMed] [Google Scholar]
- 32.Allen WR, Wilsher S. A review of implantation and early placentation in the mare. Placenta 2009; 30: 1005–1015. [DOI] [PubMed] [Google Scholar]
- 33.Cramer EP, Glenthøj A, Häger M, Juncker-Jensen A, Engelholm LH, Santoni-Rugiu E, Lund LR, Laerum OD, Cowland JB, Borregaard N. No effect of NGAL/lipocalin-2 on aggressiveness of cancer in the MMTV-PyMT/FVB/N mouse model for breast cancer. PLoS ONE 2012; 7: e39646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hofmaier F, Hauck SM, Amann B, Degroote RL, Deeg CA. Changes in matrix metalloproteinase network in a spontaneous autoimmune uveitis model. Invest Ophthalmol Vis Sci 2011; 52: 2314–2320. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.