Abstract
The current study was performed to investigate the effect of oocyte donor status, including age and body weight, on metaphase II (MII) oocyte recovery using two superovulation methods in cynomolgus monkeys. The use of Method A [recombinant gonadotrophin (75 IU/kg, 3 ×, 3-day intervals) and human chorionic gonadotropin (hCG)] led to great increases in ovary size and the mean number of MII oocytes retrieved in age- and body-weight-dependent manner; in contrast, both the parameters were similar in Method B [recombinant gonadotrophin (60 IU, twice daily, 6 days), recombinant gonadotropin and recombinant human luteinizing hormone (rhLH) (60 IU, twice daily, 3 days), and hCG]. Importantly, Method A showed maximal MII oocyte recovery rate in > 60-month-old or 4.5–5.0-kg female monkeys, whereas Method B was equally effective regardless of the donor age and body weight. These results indicate that superovulatory responses depend on the interaction between oocyte donor status and the superovulation method used in cynomolgus monkeys.
Keywords: Age, Body weight, Cynomolgus monkey, MII oocyte, Superovulation
The necessity of nonhuman primates (NHP) in biomedical research, such as in developmental biology, neuroscience, pathophysiology, and xenotransplantation research, has been increasing because of their marked similarity to humans [1,2,3]. In particular, NHPs are useful in establishing assisted reproductive technologies (ARTs), such as in vitro maturation, in vitro fertilization, and in vitro culture, for efficient production of newborns as well as in studies on human reproduction and fertility [4]. However, development of ARTs in NHPs is constrained because of various issues, such as high cost, long experimental period, and handling difficulties. Thus, to facilitate studies on ART in NHPs, researchers have attempted to maximize the amount of primary experimental materials, including oocytes matured in vivo [5, 6]. Despite numerous reports on optimization of metaphase II (MII) oocyte recovery protocols using female monkeys, the superovulation method in NHPs needs further improvement.
A typical protocol for stimulation of superovulation involves administration of exogenous gonadotropins to obtain a large number of MII oocytes from the ovaries of female monkeys. Many researchers have attempted to increase the yield of MII oocytes by investigating the factors that influence superovulation efficiency. Until recently, most of the studies focused on the development of methodological aspects based on the use of gonadotropin, including the gonadotropin type, dose, administration frequency, and time interval between the doses [7,8,9]. In addition to the optimization of gonadotropin administration protocol, several reports demonstrated that the recovery rate of MII oocytes is greatly influenced by the status of female monkeys. In particular, the age of superovulated female monkey appears to be an important parameter for successful superovulatory responses [10]. Furthermore, studies concerning the effect of age on superovulation efficiency in NHPs have predominantly used rhesus monkeys. As the importance of cynomolgus monkeys in biomedical research has been increasing, the effect of donor status in improving the superovulation method in cynomolgus monkeys must also be investigated.
The current study shows that selection of optimal oocyte donor females is important for successful superovulation in cynomolgus monkeys. We investigated the MII oocyte recovery rate in females stimulated using two representative superovulation methods, and found that successful MII oocyte recovery was dependent on the age of the female and its body weight, and varied with the superovulation method used. These findings should lead to the development of a superovulation protocol for recovery of MII oocytes in large quantity in cynomolgus monkeys.
Materials and Methods
Animals
Female cynomolgus monkeys (40–95-months old, having a body weight of 2.5–5.0 kg) exhibiting regular menstrual cycles were selected as oocyte donors. They were caged individually and kept under a controlled environment (20–24°C, 40–60% humidity, and 0800–2000 h light cycle). Vaginal bleeding was checked at least twice per day to detect the onset of menses. Animal care and all experiments were conducted in accordance with the Korea Research Institute of Bioscience and Biotechnology Guidelines for the Care and Use of Laboratory Animals (KRIBB, Approval No. KRIBB-AEC-16067).
Superovulation and oocyte recovery
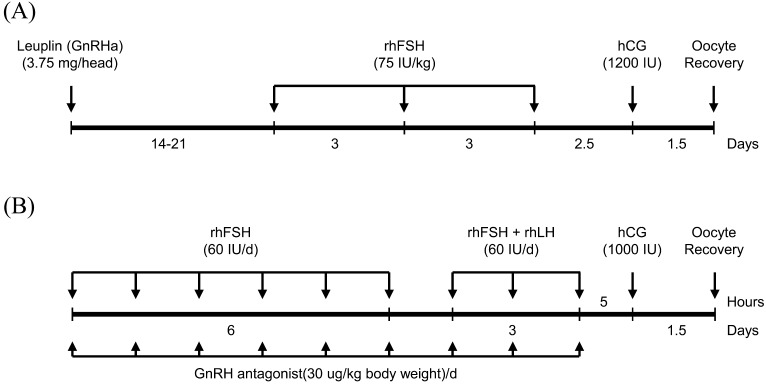
To recover MII oocytes from female monkeys, two representative superovulation methods (Methods A and B) were used. To stimulate superovulation using Method A [7], 3.75-mg leuprorelin acetate (Leuplin; GnRH agonist, Takeda, Japan) was subcutaneously administered to female cynomolgus monkeys on the first day of menstruation. Two to three weeks later, female cynomolgus monkeys were subcutaneously administered 75 IU/kg recombinant human follicle stimulating hormone (rhFSH) three times (Merksereno, NY, USA) at 72-h intervals, followed by intravenous injection of 1200 IU hCG (Sigma, MO, USA), 60 h after the final rhFSH administration. To stimulate superovulation using Method B [11], female monkeys at 1–3 days of menstruation were intramuscularly administered 60 IU rhFSH twice daily for 6 days, and 60 IU rhFSH and 60 IU rhLH (Merck Serono, NY, USA) were administered twice daily for an additional 3 days, followed by intravenous injection of 1,000 IU hCG on the following day. To suppress premature ovulation, a daily injection of 0.25 mg Ganirelix (Organon, Seoul, Korea) was administered beginning on the first day of rhFSH stimulation and was continued until the day of hCG administration (Fig. 1).
Fig. 1.
Schematic illustration of two superovulation methods used in this study.
Oocyte recovery was conducted 36 h after hCG administration in both Methods A and B. Females were anesthetized with an intramuscular injection of 10 mg/kg ketamine (Yuhan, Seoul, Korea), and cumulus oocyte complexes (COCs) were harvested by aspiration with an 18-gauge-needle attached 5-ml syringe containing Tyrode's albumin lactate pyruvate-4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (TALP-HEPES) medium (0.1% polyvinyl alcohol in low-carbonate TALP medium; [12] and 2.5 IU/ml heparin (Sigma).
Nuclear staining
To remove cumulus cells, COCs were treated with 0.1% hyaluronidase in TALP-HEPES medium and were gently pipetted. The oocytes were then washed with modified Connaught Medical Research Laboratories (mCMRL-1066) medium (Invitrogen, CA, USA) containing 10% fetal bovine serum (FBS; Gibco, NY, USA), and developmental stages were visualized under a light or fluorescence microscope after nuclear staining with 5 μg/ml Hoechst 33342 (Sigma) for 10 min at 37°C.
Statistical analysis
All data are expressed as means ± standard deviation (S.D.). Data were analyzed using analysis of variance (ANOVA), followed by Duncan’s multiple range test, using the SAS software (SAS Institute, NC, USA). P-values less than 0.05 were deemed to indicate statistical significance.
Results
Superovulation efficiency in Method A depends on donor age and body weight
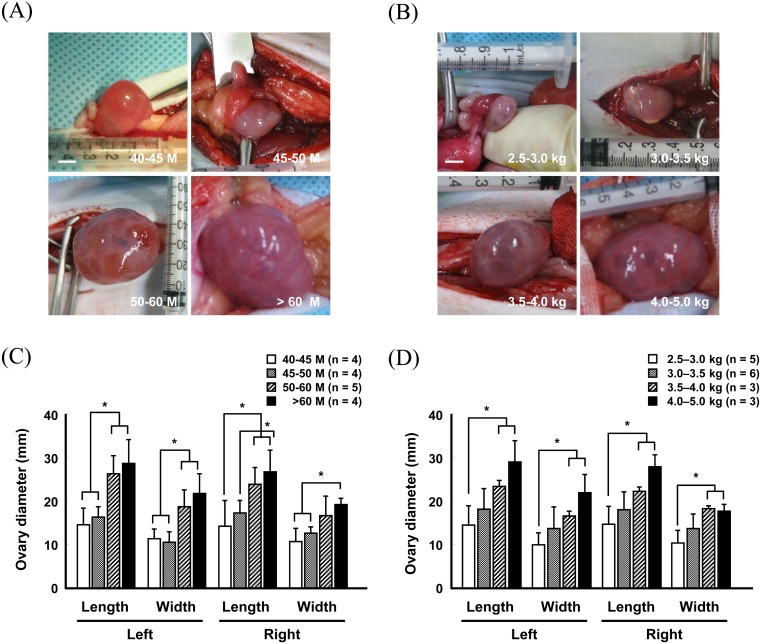
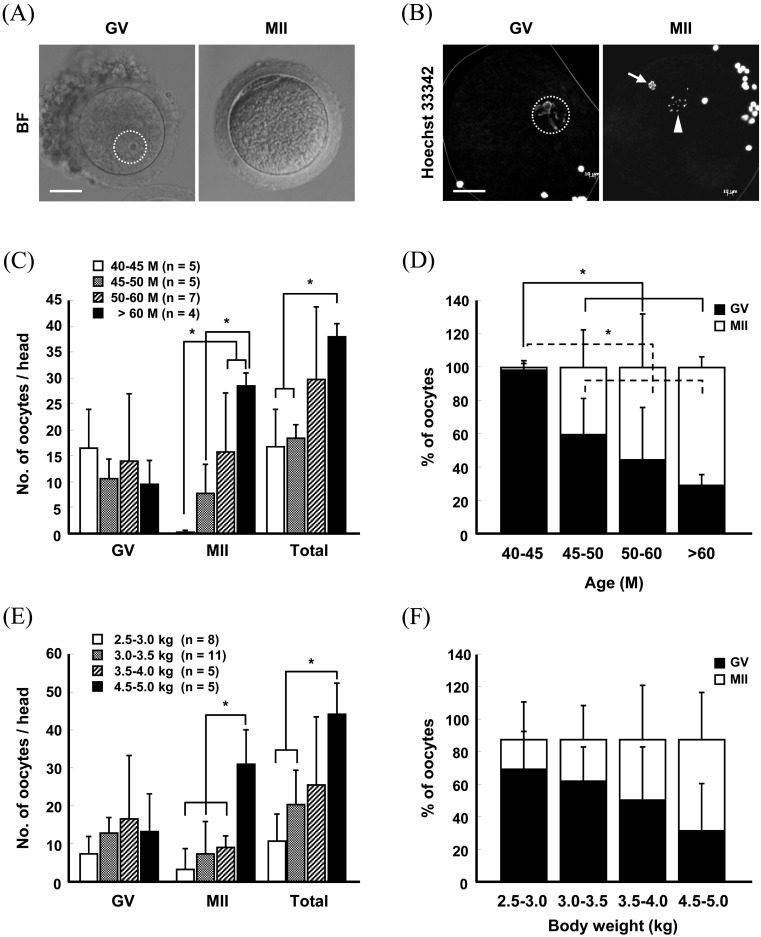
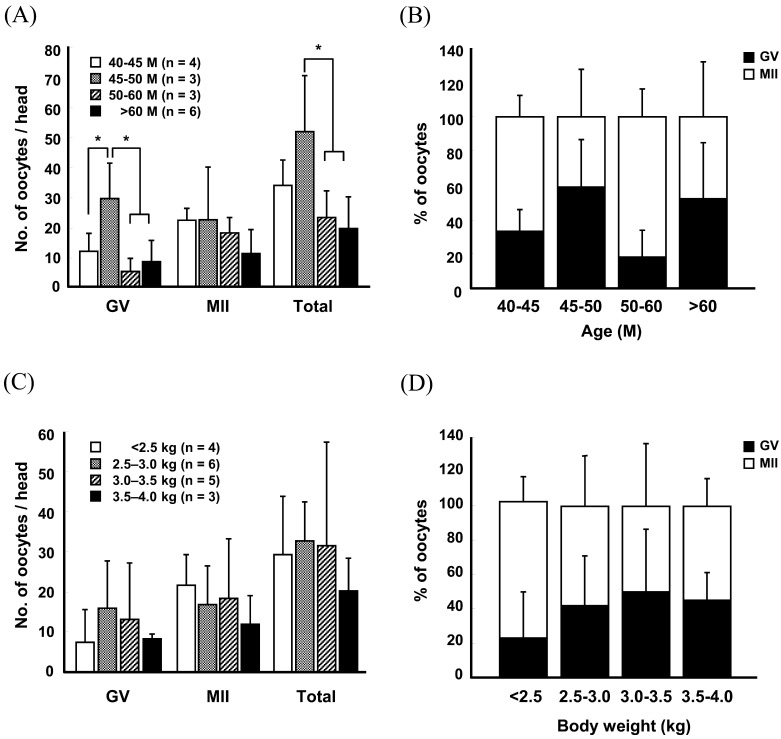
To determine the effects of age and body weight of the oocyte donor on superovulation efficiency, Method A was used to stimulate superovulation in female monkeys, and oocytes were surgically harvested from the ovaries 36 h after hCG administration. Following measurement of ovary size after superovulation, we found that the length and width of the superovulated ovaries in > 60-month-old monkeys were approximately twice those of the ≥ 40-month group (Fig. 2A and 2C) (Supplementary Table 1: online only). Similarly, the ovary size was greatly increased in monkeys with ≥ 4.0-kg body weight compared to those in the ≤ 3.0-kg group (Fig. 2B and 2D) (Supplementary Table 2: online only). The oocytes retrieved from the superovulated ovaries were subsequently directly observed under a light microscope or were subjected to fluorescence microscopy after nuclear staining with Hoechst 33342 to score the number of oocytes at different stages (Fig. 3A and 3B). The recovery rate of MII oocytes was increased in an age- and body weight-dependent manner (Fig. 3C and 3E) (Supplementary Tables 3 and 4: online only). Germinal vesicle (GV) stage oocytes (16.5 ± 7.5) were predominantly harvested from the 40–45-month-old female monkeys, whereas the number (28.5 ± 2.5) and proportion of MII oocytes were highest in > 60-month-old monkeys (Fig. 3C and 3D) (Supplementary Table 3). Similarly, the number and proportion of MII oocytes recovered from the ≥ 4.5-kg body weight females were approximately seven-fold greater than those from the ≤ 3.0-kg group (Fig. 3E and 3F) (Supplementary Table 4). Importantly, the maximum MII oocyte recovery was obtained from the > 60-month and 4.5–5.0-kg body weight groups (Fig. 3C and 3E) (Supplementary Tables 3 and 4), which reached approximately 30/head. However, the other groups yielded ≤ 10/head of MII oocytes (Fig. 3C and 3E) (Supplementary Tables 3 and 4).
Fig. 2.
Changes in ovary size in females of different ages (A and C) and body weights (B and D) after superovulation stimulation by Method A. Scale bar, 5 mm (A and B). Values are presented as the means ± standard deviation (S.D.; * P < 0.05). M, month.
Fig. 3.
Bright- (A) and fluorescence-field (B) images of oocytes recovered from ovaries stimulated by Method A. Scale bar, 25 μm (A and B). The number (C and E) and proportion (D and F) of oocytes retrieved from females of different ages (C and D) and body weights (E and F). Values represent the means ± S.D. (* P < 0.05). BF, bright-field; GV, germinal vesicle; MII, metaphase II.
Lack of correlation between superovulation efficiency and donor status in Method B
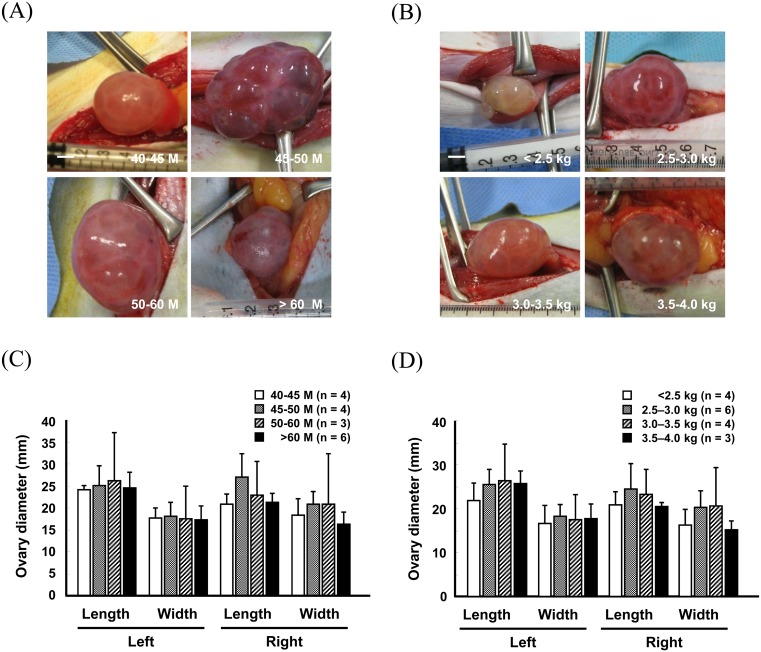
To further define the effect of the superovulation protocol used on the relationship between the donor status and superovulatory responses, female monkeys were stimulated using superovulation Method B, and were subjected to the measurement of ovary size and oocyte recovery rate (Figs. 4 and 5) (Supplementary Tables 5 and 6: online only). Unlike Method A, no relationship was found among the ovary size, and age and body weight of donors in any group (Fig. 4) (Supplementary Tables 5 and 6). Similarly, the number and proportion of MII oocytes did not appear to depend on the age or body weight of female monkeys (Fig. 5A–D) (Supplementary Tables 7 and 8: online only). Moreover, the total number of oocytes recovered was greatly reduced in > 60-month-old monkeys (19.3 ± 10.6) compared to that in the other age groups (40–45 M, 33.8 ± 8.4; 45–50 M, 51.7 ± 18.5; 50–60 M, 23.0 ± 8.9; Fig. 5A) (Supplementary Table 7). Importantly, approximately 20 MII oocytes were harvested per head in all the groups, with the exception of > 60-month-old females (Fig. 5A) (Supplementary Table 7).
Fig. 4.
Changes in ovary size in females of different ages (A and C) and body weights (B and D) after superovulation stimulation by Method B. Scale bar, 5 mm (A and B). Values are presented as the means ± S.D.
Fig. 5.
The number (A and C) and proportion (b and d) of oocytes retrieved from females of different ages (A and B) and body weights (C and D) after stimulation with Method B. Values represent the means ± S.D. (* P < 0.05).
Discussion
NHPs are important laboratory animals for the study of ARTs, such as superovulation, in vitro fertilization, and in vitro culture, because of their marked similarity to humans [5, 6, 9]. Because of ART development in NHPs, generation of transgenic monkeys is feasible [13, 14], which may greatly contribute to biomedical research by providing reliable disease models [15, 16]. For this reason, the results of the present study should facilitates the advancement of ARTs in NHPs, despite several limitations, such as high cost and low efficiency. In particular, the present study demonstrates for the first time that the relationship between donor status and superovulation efficiency varies according to the superovulation method used in cynomolgus monkeys, which might contribute to the optimization of a superovulation protocol in NHP.
Factors that influence superovulation have been explored to maximize the oocyte recovery rate from female monkeys. In fact, superovulation efficiency depends on numerous parameters, such as the dose and type of hormone [6, 8, 17], administration frequency [7,8,9], and the species of monkey [7, 18]. Importantly, recent reports revealed that the age of female monkey greatly influences the superovulation efficiency [6, 10], suggesting that the donor status is an important parameter for successful superovulation. Indeed, 5–10-year-old rhesus monkeys appear to be better superovulatory responders than 3–4- or > 10-year-old animals [19]. In contrast, other studies have shown that ovarian responses to rhFSH decline with age, in female rhesus monkeys [6, 10], possibly due to the elevated basal FSH concentration in serum and reduced the peak estradiol concentration in aged animals. The current study also showed that the recovery rate of MII oocytes was highly dependent on the donor status, including age and body weight. Superovulation efficiency gradually increased in an age- and body-weight-dependent manner in Method A, whereas no significant differences were found in Method B, suggesting that the relationship between donor status and ovarian responses varies according to the superovulation method used. Although the optimal donor status for a high superovulatory response differs among studies, the choice of oocyte donor with regard to body weight and age should be considered an important step for successful MII oocyte recovery in NHPs.
Several studies have demonstrated a close association between the donor age and superovulation protocol used for superovulation in female monkey. Generally, a once- or twice-daily injection of relatively low-dose FSH for 8–9 days has been most frequently used to stimulate the ovarian follicles in monkeys. In juvenile rhesus monkeys, the numbers of responders and retrieved oocytes per head using the daily injection method are greater in females at prepuberty than at menarchy [20]. In addition, 5–10-year-old female monkeys display relatively better superovulatory responses than do 3–4- or > 10-year-old animals [19]. Another report showed that the oocyte recovery rate was reduced in older (16–21 years old) monkeys compared to the younger (5–15 years old) [10]. Although it is difficult to draw a conclusion concerning the optimal age for the introduction of superovulation method, our results suggest that the age of female monkeys should be considered in the daily injection method.
Another type of superovulation method is the administration of relatively high-dose of FSH at an interval of several days. In cynomolgus monkey, three FSH injections at 72-h intervals are almost as effective as the daily injection method [7]. However, unlike the daily injection method, little is known regarding the effect of donor age on the superovulation efficiency of the long-term interval stimulation method in monkeys. In the present study, we found that Method A (long-term interval injection method) was effective in stimulating superovulation in older animals. This is the first description of a donor-age effect on the superovulatory responses following a long-term interval injection method in cynomolgus monkeys. In contrast, no significant differences in superovulation efficiency were observed among the various age groups stimulated by Method B (daily injection method). Interestingly, the mean number of retrieved MII oocytes was relatively high in older females in the same body-weight groups in Method A, whereas it was reversed in Method B (data not shown), suggesting the importance of donor age and the corresponding body weight for optimization of the superovulation method. Based on these data, we propose that major considerations for superovulation in NHPs include donor age and body weight as well as the superovulation method used.
In conclusion, we attempted to establish optimal superovulation conditions regarding donor status, including age and body weight. Following comparison of two representative methods, we found that successful superovulatory responses are highly dependent on the interactions among donor age, body weight, and superovulation method used. These findings will facilitate large-scale production of MII oocytes in cynomolgus monkeys and further development of ARTs in NHPs.
Supplementary
Acknowledgments
This study was supported by grants from the KRIBB Research Initiative Program (KGM4611714, KGM4251723), the Bio & Medical Technology Development Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (MSIP) (No. 2012M3A9B6055362), and the Next-Generation BioGreen 21 Program (No. PJ0099592016), Rural Development Administration, Republic of Korea.
References
- 1.Wolf DP. Assisted reproductive technologies in rhesus macaques. Reprod Biol Endocrinol 2004; 2: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roelfsema PR, Treue S. Basic neuroscience research with nonhuman primates: a small but indispensable component of biomedical research. Neuron 2014; 82: 1200–1204. [DOI] [PubMed] [Google Scholar]
- 3.Kim H, Chee HK, Yang J, Hwang S, Han KH, Kang J, Park JH, Kim JS, Lee SJ, Ock SA, Park MH, Park KS, Lee BC, Cho K, Noh J, Park W, Yun IJ, Ahn C. Outcomes of alpha 1,3-GT-knockout porcine heart transplants into a preclinical nonhuman primate model. Transplant Proc 2013; 45: 3085–3091. [DOI] [PubMed] [Google Scholar]
- 4.Nyachieo A, Spiessens C, Mwenda JM, Debrock S, DHooghe TM. Improving ovarian stimulation protocols for IVF in baboons: lessons from humans and rhesus monkeys. Anim Reprod Sci 2009; 110: 187–206. [DOI] [PubMed] [Google Scholar]
- 5.Vandevoort CA, Baughman WL, Stouffer RL. Comparison of different regimens of human gonadotropins for superovulation of rhesus monkeys: ovulatory response and subsequent luteal function. J In Vitro Fert Embryo Transf 1989; 6: 85–91. [DOI] [PubMed] [Google Scholar]
- 6.Yang S, He X, Hildebrandt TB, Jewgenow K, Goeritz F, Tang X, Zhou Q, Ji W. Effects of rhFSH dose on ovarian follicular response, oocyte recovery and embryo development in rhesus monkeys. Theriogenology 2007; 67: 1194–1201. [DOI] [PubMed] [Google Scholar]
- 7.Shimozawa N, Okada H, Hatori M, Yoshida T, Sankai T. Comparison of methods to stimulate ovarian follicular growth in cynomolgus and African green monkeys for collection of mature oocytes. Theriogenology 2007; 67: 1143–1149. [DOI] [PubMed] [Google Scholar]
- 8.Yang S, He X, Hildebrandt TB, Zhou Q, Ji W. Superovulatory response to a low dose single-daily treatment of rhFSH dissolved in polyvinylpyrrolidone in rhesus monkeys. Am J Primatol 2007; 69: 1278–1284. [DOI] [PubMed] [Google Scholar]
- 9.Yang S, Shen Y, Niu Y, Hildebrandt TB, Jewgenow K, Goeritz F, He X, Zhou Q, Ji W. Effects of rhFSH regimen and time interval on ovarian responses to repeated stimulation cycles in rhesus monkeys during a physiologic breeding season. Theriogenology 2008; 70: 108–114. [DOI] [PubMed] [Google Scholar]
- 10.Schramm RD, Paprocki AM, Bavister BD. Features associated with reproductive ageing in female rhesus monkeys. Hum Reprod 2002; 17: 1597–1603. [DOI] [PubMed] [Google Scholar]
- 11.Wolf DP, Alexander M, Zelinski-Wooten M, Stouffer RL. Maturity and fertility of rhesus monkey oocytes collected at different intervals after an ovulatory stimulus (human chorionic gonadotropin) in in vitro fertilization cycles. Mol Reprod Dev 1996; 43: 76–81. [DOI] [PubMed] [Google Scholar]
- 12.Parrish JJ, Susko-Parrish JL, Leibfried-Rutledge ML, Critser ES, Eyestone WH, First NL. Bovine in vitro fertilization with frozen-thawed semen. Theriogenology 1986; 25: 591–600. [DOI] [PubMed] [Google Scholar]
- 13.Sasaki E, Suemizu H, Shimada A, Hanazawa K, Oiwa R, Kamioka M, Tomioka I, Sotomaru Y, Hirakawa R, Eto T, Shiozawa S, Maeda T, Ito M, Ito R, Kito C, Yagihashi C, Kawai K, Miyoshi H, Tanioka Y, Tamaoki N, Habu S, Okano H, Nomura T. Generation of transgenic non-human primates with germline transmission. Nature 2009; 459: 523–527. [DOI] [PubMed] [Google Scholar]
- 14.Chan AW, Chong KY, Martinovich C, Simerly C, Schatten G. Transgenic monkeys produced by retroviral gene transfer into mature oocytes. Science 2001; 291: 309–312. [DOI] [PubMed] [Google Scholar]
- 15.Yang SH, Cheng PH, Banta H, Piotrowska-Nitsche K, Yang JJ, Cheng EC, Snyder B, Larkin K, Liu J, Orkin J, Fang ZH, Smith Y, Bachevalier J, Zola SM, Li SH, Li XJ, Chan AW. Towards a transgenic model of Huntingtons disease in a non-human primate. Nature 2008; 453: 921–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Niu Y, Shen B, Cui Y, Chen Y, Wang J, Wang L, Kang Y, Zhao X, Si W, Li W, Xiang AP, Zhou J, Guo X, Bi Y, Si C, Hu B, Dong G, Wang H, Zhou Z, Li T, Tan T, Pu X, Wang F, Ji S, Zhou Q, Huang X, Ji W, Sha J. Generation of gene-modified cynomolgus monkey via Cas9/RNA-mediated gene targeting in one-cell embryos. Cell 2014; 156: 836–843. [DOI] [PubMed] [Google Scholar]
- 17.Schuler AM, Westberry JM, Scammell JG, Abee CR, Kuehl TJ, Gordon JW. Ovarian stimulation of squirrel monkeys (Saimiri boliviensis boliviensis) using pregnant mare serum gonadotropin. Comp Med 2006; 56: 12–16. [PubMed] [Google Scholar]
- 18.Marshall VS, Browne MA, Knowles L, Golos TG, Thomson JA. Ovarian stimulation of marmoset monkeys (Callithrix jacchus) using recombinant human follicle stimulating hormone. J Med Primatol 2003; 32: 57–66. [DOI] [PubMed] [Google Scholar]
- 19.Chen Y, Ji S, Niu Y, He X, Xie Y, Tang X, Ji W. Recombinant human gonadotropins for rhesus monkey superovulation: age and the stimulation interval are determinant to the fecundity. J Anim Vet Adv 2011; 10: 3303–3307. [Google Scholar]
- 20.Yang S, He X, Niu Y, Hildebrandt TB, Jewgenow K, Goeritz F, Tang X, Chang Y, Zhou Q, Ji W. Ovarian response to gonadotropin stimulation in juvenile rhesus monkeys. Theriogenology 2009; 72: 243–250. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.