Abstract
The aim of this study was to investigate the effect of different heparin concentrations in the course of sexed in vitro fertilization (IVF), on bovine embryonic development and development to term following embryo transfer (ET). With a total of 9156 oocytes for IVF, sorted as well as unsorted sperm from four bulls had different heparin requirements for achieving the highest rate of development in vitro. However, when optimal heparin concentrations were used (40 to 80 µg/ml), the performance of X-sorted sperm (0.3 × 106/ml/IVF droplet) from all four bulls, as judged by blastocyst development (Bulls A, B, C, and D: 25.2, 19.7, 25.1, and 9.8%, respectively), was significantly increased, and the blastocyst rate was comparable to that observed with unsorted sperm at certain heparin concentrations within the four bulls. We determined that near-optimal blastocyst development was possible with sorted sperm from all four bulls, when a heparin concentration of 40 µg/ml was used. Pregnancy rates at d 70 post ET ranged from 39.1 to 40.3% (P > 0.05), and the calving rates ranged from 34.4 to 35.1% (P > 0.05), when heparin was used at a concentration of 10 μg/ml (n = 236), 20 μg/ml (n = 189), and 40 μg/ml (n = 305), respectively. Our study demonstrates that, although the sorted sperm of different bulls performed optimally over a range of heparin concentrations, a generally accepted heparin concentration of 40 µg/ml can be set for sexed IVF. This improvement is beneficial when sexed embryo production by ovum pickup and IVF is an essential component of genetic breeding programs.
Keywords: Bovine, Embryos, Embryo transfer, In vitro fertilization (IVF), Sorted sperm
The successful use of sex-sorted sperm for producing progeny has been one of the greatest advancements in cattle breeding programs. In vitro fertilization (IVF) is a feasible and potentially efficient approach to increase the fertilization efficiency of sex-sorted sperm [1,2,3], especially when used in conjunction with ovum pick up (OPU) technology, which has been widely used to produce genetically valuable offspring from elite female donors [4, 5]. Separation of X- and Y-chromosome-bearing sperm by flow cytometry [6, 7], and their subsequent use to fertilize bovine oocytes in vitro, has resulted in cattle offspring with a sex-sorting accuracy of 95% [8]. A lower number of sorted sperm (1–2 × 106/straw) is commercially available for artificial insemination [9], when compared to conventionally unsorted sperm (15–20 × 106/straw) for artificial insemination in cows/heifers [10] and for IVF [2, 8, 11]. However, low yields of developmentally competent and transferable embryos have been associated with sorted sperm, resulting from physiological anomalies, the sorting process [7], and/or inefficient fertilization procedures [2, 3, 12]. Furthermore, the rate of blastocyst development has been reported to be lower with sorted sperm than with unsorted conventional sperm [3].
Mammalian sperm are incapable of fertilization unless the capacitation process occurs and is followed by the acrosome reaction [13,14,15]. Sperm capacitation is a biochemical modification that spermatozoa must undergo within the female reproductive tract, enabling them to bind to the zona pellucida. At this point, the acrosome reaction allows the sperm to break down the coat of the ovum permitting fertilization to occur [16]. Capacitation can occur in vitro in the absence of reproductive tract fluids with the use of compounds such as a calcium ionophore or progesterone; however, glycosaminoglycans (GAGs) are known to be the most effective at inducing the acrosome reaction in epididymal and ejaculated bovine sperm [15,16,17]. Heparin, the most highly sulfated GAG, is the most potent inducer of bovine sperm capacitation, and possibly the acrosome reaction, in vitro [15, 17, 18]. Following the pioneering work of Parrish et al. [19], heparin has been shown to affect the efficiency of capacitation [20] as well as fertilization [21,22,23]. Improved IVF results have been accomplished by modifying both heparin and sperm concentrations in the fertilization medium [12, 15, 24]. The “bull effect” during IVF is believed to be due to differences in the effect of heparin on sperm capacitation for individual bulls during fertilization [3, 20]. Xu et al. [8] reported that flow cytometry affected the in vitro fertility of sorted sperm in a bull-specific manner, although this was not a significant factor for all bulls. It is also important to determine whether the sorting process affects abnormal fertilization, such as polyspermy [25]. Flow-sorted sperm has been shown to be partially capacitated; therefore, optimization of heparin and sperm concentrations is required for the satisfactory fertilization of bovine oocytes with sorted sperm in vitro [2, 3, 12]. However, whether higher concentrations of heparin would promote efficient pre-implantation and term development of sexed IVF embryos for most bulls has not yet been explored.
The objective of this study was to test the effect of a range of heparin concentrations on pre-implantation (0 to 100 µg/ml), and then term (10, 20, and 40 µg/ml) development of bovine oocytes fertilized by sorted X-sperm in vitro and following embryo transfer (ET) into recipients, respectively. Systematic criteria, including the status of polyspermic fertilization, cleavage, blastocyst development, and pregnancy/calving rate after ET, were examined to improve the efficiency of sexed IVF in cattle. Subsequently, we wished to determine a general heparin concentration suitable for commercially available sexed sperm cells from a majority of bulls in the large-scale in vitro production of sex-predetermined embryos with oocytes, either via the OPU process [5] or from slaughterhouse ovaries [8]. Finally, the potential of development to term following ET of vitrified sexed embryos into synchronized recipients was also determined.
Materials and Methods
All animal experiments were approved by the Animal Care and Use Committee of Nanjing Normal University. All chemicals were purchased from Sigma Aldrich (St. Louis, MO, USA) unless otherwise noted. All oocytes and resultant embryos were cultured in specified media at 39°C in 5% CO2 and humidified air (unless otherwise noted).
Sorted X-chromosome-enriched Holstein sperm
Flow cytometry was used to sort X-sperm from four Holstein bulls (Bulls A-D) using XY™ sorting procedures in the sorting facilities at Sexing Technologies (Navasota, TX, USA), and straws were commercially available for in vitro fertilization. Bulls were selected on the basis of their genetic merit and availability, without evaluation of in vitro fertility. The identification numbers for these four bulls were: A, 44HO314; B, 203HO466; C, 203HO583; and D, 203HO589. Briefly, freshly ejaculated semen samples were stained with HEPES-BSA extender containing 112 μM Hoechst 33342 for 45 min at 34.5°C, followed by food coloring (FD & C#40) (Sensient Colors, Norfolk, UK) for 5 min to stain damaged sperm [6, 8]. X- and Y-chromosome- bearing sperm were sorted into different tubes based on their 3.8% inherent difference in DNA content using wavelengths of 351 and 364 nm, with an average flow rate of 25,000 to 30,000 sperm cells per second. Approximately 2.0 × 106 sorted sperm were packaged in 0.25-ml straws, frozen on racks in a liquid nitrogen vapor phase (–160°C), then stored in liquid nitrogen. The purity of sorted X-sperm was 90 ± 3% based on resort analysis.
Bovine oocyte maturation in vitro
Cumulus-oocyte-complexes (COCs) used in this study (n = 9156) were recovered from Holstein slaughterhouse ovaries by aspiration of 2–8-mm antral follicles [8]. Briefly, COCs were selected and washed in Dulbecco’s PBS (D-PBS; Invitrogen, Grand Island, NY, USA) supplemented with 0.1% polyvinyl alcohol. Selected COCs were matured in groups of 20–25, in drops of 75 μl Medium 199 (M199, Invitrogen) containing Earle’s salts, 100 mg/ml L-glutamine, 2.2 g/l sodium bicarbonate, and 25 mM HEPES, supplemented with 7.5% (v/v) fetal bovine serum (FBS) (Hyclone, Logan, UT, USA) along with 0.5 μg/ml ovine follicle stimulating hormone (FSH; NIDDK, Los Angeles, CA, USA), 5.0 μg/ml ovine luteinizing hormone (LH; NIDDK), and 1.0 μg/ml estradiol. The maturation medium was covered with mineral oil, and COCs were cultured for 22–24 h. COCs with well-expanded cumulus layers at the end of maturation were selected for IVF with unsorted and sorted X-sperm.
In vitro fertilization
Frozen Holstein X-sperm from the four bulls were used for IVF in Brackett and Oliphant (BO) medium [26], according to previously described procedures [8]. Briefly, straws containing semen were thawed for 10 sec in a 37°C water bath after 10–15 sec of gentle shaking in air. Spermatozoa were washed in 10 ml of BO medium containing 3 mg/ml bovine serum albumin (BSA) and 10 mM caffeine. The washed sperm pellet was re-suspended in BO washing solution at a minimum concentration of 0.6 × 106/ml as determined previously in our laboratory [8]. Matured COCs were washed in BO fertilization medium containing 6 mg/ml BSA and various concentrations of heparin (0–200 μg/ml) according to the experimental design, and 50 oocytes were allocated into a 50-μl droplet with the same fertilization medium. A volume of 50 μl sperm suspension was added to each oocyte-containing fertilization droplet at a final sperm concentration of 0.3 × 106/ml [8]. According to the experimental design, each 100-µl fertilization drop (with 50 COCs) contained a different amount of heparin, at final concentrations of 0, 2.5, 5, 10, 20, 40, 60, 80, or 100 μg/ml. Oocytes and washed sperm were incubated for 6 h under the culture conditions described above. Unsorted conventional frozen sperm from the same four Holstein bulls was used for fertilization as controls. The procedures for IVF using unsorted sperm were the same as those described above, with the same final sperm concentration of 0.3 × 106/ml per fertilization droplet.
In vitro embryo culture
Fertilized oocytes were cultured in modified CR1aa medium [27], consisting of 114.7 mM NaCl, 3.1 mM KCl, 26.2 mM NaHCO3, 1 mM L-glutamine, 0.4 mM sodium pyruvate, and 5.5 mM hemicalcium L-lactate, supplemented with 1X MEM (#M7145) and 1X BME (#B6766) amino acids. Presumptive zygotes were randomly allocated to modified CR1aa cultures containing 6 mg/ml BSA under 5% CO2, 5% O2, and 90% N2, at 39°C, and incubated for 2 days. Cumulus cells from COCs were separated and cultured in 7.5% FBS M199 for 2 days to form a confluent monolayer, and were then washed three times with 10% FBS CR1 medium. Cleaved embryos were co-cultured with a monolayer of cumulus cells, as described above, in fresh 10% FBS CR1 medium under 5% CO2 humidified air at 39°C for an additional 5 days. Embryo development stage was evaluated on d 2 (d 0, just after IVF, 2–8-cell stage), and d 7 (blastocyst) of culture. Blastocyst stage embryos were graded according to International Embryo Transfer Society (IETS) standards.
Evaluation of polyspermic fertilization
Twenty-four-hours following IVF, a proportion of oocytes was fixed in 4% formaldehyde D-PBS for at least 48 h, followed by four thorough washes in D-PBS-PVA to remove the formaldehyde fixative. Washed oocytes were stained with 10 μg/ml Hoechst 33342 and the degree of polyspermy was evaluated by fluorescence microscopy. Oocytes were judged to be normally fertilized when one male and one female pronucleus (2PN) were visualized within the oocyte cytoplasm. Polyspermic fertilization was reported whenever two or more male pronuclei were observed (> 2PN). When decondensed sperm head(s) and/or metaphase were observed within the oocyte cytoplasm, the oocytes were considered to be abnormally fertilized, and were excluded from subsequent analyses [25].
Vitrification of pre-selected female embryos
Embryos were cryopreserved by modified droplet vitrification (Vitrification and Warming Kit, Renova Life, College Park, MD, USA) [8, 28]. Briefly, embryos were treated with 0.25% trypsin (Gibco, Grand Island, NY, USA) for 1 min. Embryos with intact zona pellucida were then washed six times in isotonic embryo washing and holding medium (EWH, Renova Life). Vitrification solutions and warming media were prepared using basal D-PBS (oocyte washing plus, OWP, Renova Life). To implement the vitrification protocol, the following solutions were prepared: (1) embryo washing solution consisting of 7.5% FBS (SH30070.03, Hyclone) in D-PBS, supplemented with 100 IU/ml penicillin and 100 µg/ml streptomycin; (2) embryo basal solution, prepared using 15% FBS D-PBS; (3) vitrification solution, consisting of basal solution supplemented with 2.3 M dimethylsulphoxide (DMSO), 2.7 M ethylene glycol (EG), and sucrose (Vitrification Kit, Renova Life). The following steps were performed: (1) embryos were incubated for 5 min in washing solution, followed by incubation in basal solution and holding solution for 3 min at 39°C; (2) embryos were then washed in a 20-µl vitrification solution drop, (3) subsequently each embryo was drawn up into a separate glass pipette in a 2-µl drop of vitrification solution (Fisher Scientific, Pittsburgh, PA, USA), and then directly dropped into liquid nitrogen. Vitrified embryo droplets were transferred into pre-labeled cryovials (Corning, Corning, NY, USA) cooled in liquid nitrogen, sealed, and finally stored in liquid nitrogen.
Embryo transfer of female blastocysts
Vitrified IVF embryos were warmed in 0.28 M sucrose in basal medium (warming solution) at 39°C, and rehydrated in 0.18 M sucrose basal medium (rehydration solution) for 5 min at 39°C (Warming Kit, Renova Life). Embryos were washed in basal solution, and then in embryo transfer medium (Embryo washing and holding, EWH, Renova Life) supplemented with 3 mg/ml BSA at 39°C.
Chinese Native Yellow cattle and Holstein cattle were chosen as recipient breeds and housed at several Chinese farms. Recipients were chosen from either heifers and/or cows according to criteria such as age, health status, reproductive soundness (cyclic animals), breeding history, size and weight, as well as the farm’s nutritional management. Recipients were pooled and synchronized by a regime of two injections of prostaglandin F2α (Lutalyse, Upjohn, Kalamazoo, MI, USA; 25 mg/injection, i.m.) at 11-day intervals. Corpus luteum (CL) regression followed by estrus usually occurred approximately 48–72 h later. The onset of estrus in recipients was monitored closely, and standing heat was considered as d 0. On d 7 following estrus, recipients were selected by either palpation per rectum or ultrasound monitoring to verify the presence and the size of the CL. Blastocysts (one embryo/straw) were loaded into 0.25-ml French straws containing EWH. A single embryo was deposited non-surgically into the uterine horn ipsilateral to the ovary with a functional CL. Pregnancy was determined by palpation per rectum on d 70 after transfer.
Statistical analyses
The proportions of embryos from various treatments that successfully reached cleavage, blastocyst stage, and that subsequently determined pregnancy and calving within each experiment were determined and data were subjected to arc sine transformation. Transformed data were then analyzed by one-way ANOVA (General Linear Model, SPSS 11.0, Chicago, IL, USA). A P value < 0.05 was considered significant.
Results
Effect of heparin concentrations on the prevalence of polyspermy in IVF with sorted sperm
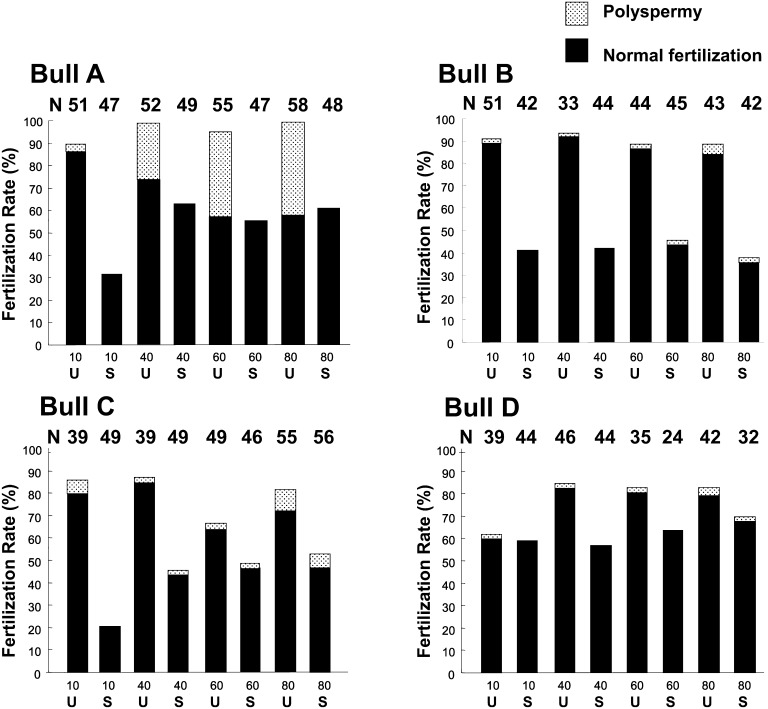
Polyspermy was determined by fluorescence microscopy after oocyte fixation and staining (n = 1541) with 10 µg/ml Hoechst 33342, 18 h following IVF. As shown in Fig. 1, the fertilization rates by sorted sperm from bulls A, B, C and D, as judged by 2PN within the oocytes, were lower than those by unsorted conventional sperm in each treatment group. With Bulls A and C, increased heparin concentration enhanced the fertilization rate, which was in contrast to Bulls B and D. Oocytes fertilized by X-sorted sperm showed a shorter range of polyspermic fertilization, which differed from that observed in conventional unsorted sperm (Fig. 1).
Fig. 1.
The effect of heparin concentration on fertilization status in IVF with bovine X-sorted sperm cells. Heparin concentrations ranged from 10 to 80 µg/ml during the IVF process. U, unsorted control; S, sorted sperm.
Cleavage rates of oocytes fertilized with sexed sperm at different heparin concentrations
A total of 7615 oocytes were matured for IVF, and fertilized oocytes were subsequently cultured in vitro. Cleavage rates were indicative of bull-specific differences when sorted and unsorted sperm were used, although for three of the bulls (A, B, and D), rates with sorted and unsorted sperm were similar (P > 0.05) at lower heparin concentrations (Table 1). However, with increasing heparin concentrations (10–20 to 100 μg/ml), cleavage rates were significantly higher with sorted sperm from Bull A (40.7–57.4%), Bull B (36.3–47.4%), and Bull D (25.1–30.6%) when compared to those at lower heparin concentrations (P < 0.05). A similar trend was observed with unsorted controls when the heparin concentration was increased in these three bulls (Table 1). Variation in heparin concentration, ranging from 0 to 100 μg/ml, did not affect the rate of cleavage for Bull C when unsorted sperm cells were used (P > 0.05), while sorted sperm required a minimum heparin concentration of 10 μg/ml (Table 1) to attain a significantly higher rate of cleavage (38.5–48.4% vs. 10.9–19.4%, respectively, P < 0.05). Finally, for Bull C and Bull D, cleavage rates with sorted sperm were always lower when compared to their unsorted controls, with all the tested heparin concentrations ranging from 0 to 100 μg/ml (Table 1).
Table 1. Effect of heparin concentration on cleavage after IVF using sorted and unsorted sperm.
| Heparin treatment (µg/ml) |
Cleavage (%) at 44–48 h post IVF (mean ± SEM) |
|||||||
| Bull A |
Bull B |
Bull C |
Bull D |
|||||
| Sorted | Unsorted | Sorted | Unsorted | Sorted | Unsorted | Sorted | Unsorted | |
| 0 | 10.2 ± 1.2 a | 12.1 ± 2.1 a | 23.2 ± 5.3 a | 21.3 ± 4.5 a | 10.9 ± 3.2 a | 61.9 ± 6.6 a | 9.1 ± 4.5 a | 23.5 ± 2.3 a |
| 2.5 | 18.8 ± 1.8 a | 13.9 ± 6.9 a | 24.9 ± 3.2 a | 20.1 ± 2.4 a | 16.5 ± 3.4 a | 65.9 ± 8.9 a | 15.6 ± 4.3 a | 23.3 ± 3.4 a |
| 5 | 24.2 ± 3.2 a | 18.3 ± 5.7 a | 28.6 ± 4.5 a | 19.7 ± 0.7 a | 19.4 ± 6.6 a | 58.9 ± 5.7 a | 19.1 ± 6.3 a | 28.9 ± 3.9 a |
| 10 | 40.7 ± 6.3 b | 31.9 ± 3.6 b | 36.3 ± 8.1 b | 20.6 ± 5.5 a | 48.4 ± 3.3 b | 61.5 ± 1.5 a | 18.3 ± 4.3 a | 31.4 ± 4.5 a,b |
| 20 | 45.3 ± 1.2 b | 47.4 ± 3.4 c | 33.9 ± 3.1 b | 50.2 ± 2.3 b | 47.7 ± 4.5 b | 63.9 ± 3.5 a | 28.1 ± 2.5 b | 37.6 ± 3.2 b |
| 40 | 52.5 ± 5.5 b | 61.3 ± 3.4 d | 47.4 ± 6.5 b | 48.2 ± 6.7 b | 54.1 ± 7.2 b | 66.7 ± 1.5 a | 25.6 ± 3.3 b | 50.9 ± 4.8 c |
| 60 | 52.8 ± 4.5 b | 67.1 ± 5.5 d | 34.6 ± 8.4 b | 49.2 ± 6.6 b | 47.2 ± 4.3 b | 79.5 ± 7.5 a | 28.9 ± 3.4 b | 50.8 ± 4.5 c |
| 80 | 57.4 ± 5.4 b | 65.3 ± 4.5 d | 42.7 ± 3.2 b | 53.9 ± 4.5 b | 42.1 ± 8.6 b | 71.1 ± 6.7 a | 25.1 ± 4.5 b | 53.4 ± 3.9 c |
| 100 | 49.2 ± 11 b | 56.7 ± 3.2 d | 39.2 ± 4.5 b | 48.9 ± 7.2 b | 38.5 ± 4.5 b | 71.9 ± 5.3 a | 30.6 ± 5.4 b | 58.3 ± 4.5 c |
Different letters (a, b, c, d) within the same column were significantly different, P < 0.05.
Blastocyst development of sexed embryos following fertilization with different heparin concentrations
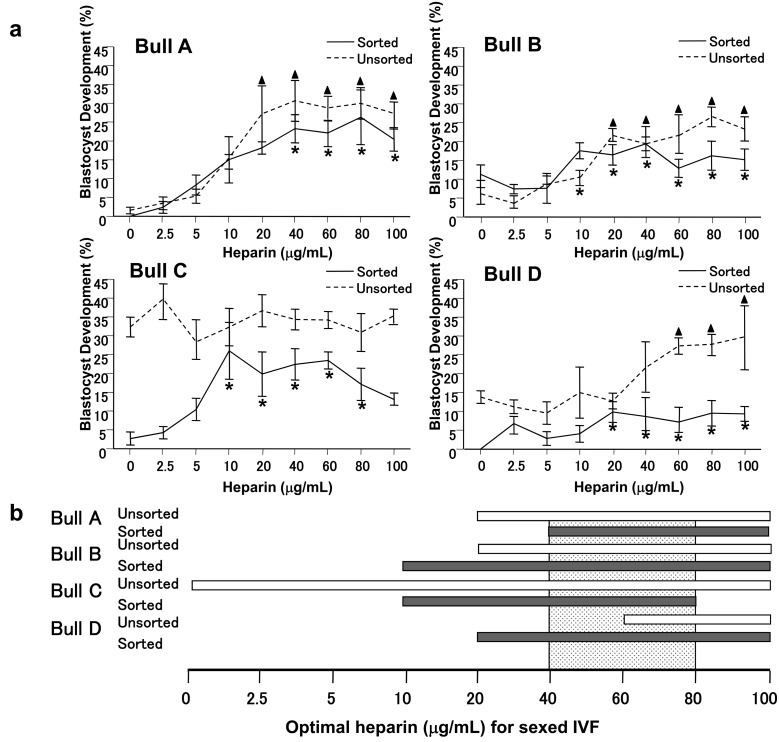
Blastocyst development derived from the use of sorted, as well as unsorted sperm, from all four bulls on fertilized oocytes is shown in Fig. 2. A pair-wise comparison of sorted and unsorted sperm within the same bull (A, B, C, D) showed that heparin requirements for optimal IVF were highly variable among the four bulls tested. Heparin was required to achieve optimal blastocyst development in vitro when sorted X-sperm cells from the four bulls were tested, especially with the highest blastocyst rates in bulls A (25.2%), B (19.7%), and C (25.1%) (Fig. 2). Although the blastocyst yield was low in Bull D, rising from a concentration of 5 to 20 μg/ml of heparin, blastocyst development increased from 2.8 to 9.8% (P < 0.05), respectively, although development was not improved when the heparin concentration was further increased from 20 to 100 μg/ml (P > 0.05). A similar and significant promoting effect for heparin concentration on blastocyst development was observed for three of the four bulls, with the highest rates in Bull A (31.1%), Bull B (26.1%), and Bull D (29.5%), when oocytes were fertilized with unsorted sperm. Bull C was exceptional in that any increase in heparin concentration did not affect blastocyst development (28.5–39.8%, P > 0.05) (Fig. 2). In addition, Bull A and Bull B had a similar IVF fertility rate with both sexed and non-sexed sperm, while for Bull C and Bull D, the use of non-sexed sperm resulted in higher fertility when compared to sexed sperm. However, with increasing heparin concentrations, the significance of the fertility difference was lost (Bull C at 10 μg/ml, Bull D at 20 μg/ml) as shown in Fig. 2.
Fig. 2.
Bull-specific effect of different heparin concentrations on blastocyst development post-IVF with X-sorted sperm. Embryo development to the blastocyst stage was recorded on d 7 after IVF. (a) The significantly higher rate of blastocyst development is labeled as ▲ (unsorted) and * (sorted) in the same bull, respectively. Significance is indicated as non-overlapping error bars at the same heparin concentration by a direct comparison between sorted and unsorted group. As shown in Bull A, sorted sperm gave rise to a significantly higher rate of blastocyst development when the heparin concentration was increased to 40 µg/ml. By pair-wise comparison, there was no significant difference in blastocyst development between X-sorted sperm and conventional controls after increasing the heparin concentration during IVF. Sorted sperm from Bull B showed a significant increase in blastocyst development when heparin concentration was increased to 10 µg/ml and higher. The highest blastocyst rates in unsorted IVF compared to sorted groups were found when the heparin concentration was higher than 60 µg/ml (60–100 µg/ml). Sorted sperm cells from Bull C gave lower results when compared to the unsorted controls during IVF; however, a comparable blastocyst development rate was reached between groups of sorted and unsorted sperm in Bull C with a heparin concentration of 10 µg/ml. A similar tendency was observed for Bull D (sorted vs. unsorted). Nevertheless, in Bull D, a significant increase in blastocyst development was observed when the heparin concentration was increased to 20 µg/ml, and remained level with the rate of development even when heparin was increased from 20 to 100 µg/ml. Solid line, sorted sperm; dotted line, unsorted control. (b) Deduced heparin concentration for optimally sexed IVF was 40 µg/ml.
Determination of a heparin concentration suitable for satisfactory IVF with sorted X-sperm
The performance of sperm from the four tested bulls on IVF fertility, with regards pre-implantation development, was compared and detailed results are provided below and in Fig. 2. Sorted sperm were observed to promote increased embryo development within a range of heparin concentrations, and by optimizing the heparin concentration, the optimal in vitro blastocyst development of sexed IVF could be increased to levels comparable to that observed with unsorted sperm controls within the same bull. The optimal heparin concentrations deduced for promoting comparable blastocyst development (P > 0.05) during sexed IVF for each bull ranged from 40 to 100 μg/ml (Bull A), 10 to 100 μg/ml (Bull B), 10 to 80 μg/ml (Bull C), and 20 to 100 μg/ml (Bull D). Thus, overlapping optimal heparin concentrations for sexed IVF with the four bulls ranged between 40 and 80 μg/ml. Therefore, on the basis of the results obtained in this study, we asserted that satisfactory sexed IVF with a heparin concentration of 40 µg/ml could represent a generally accepted concentration with sorted X-sperm for the majority, and possibly all, bulls.
Similarly, for unsorted sperm controls, a higher rate of blastocyst development was recorded when heparin was increased to various concentrations for all bulls (Bull A, 20–100 µg/ml; Bull B, 20–100 µg/ml; Bull D, 60–100 µg/ml) with the exception of Bull C, which did not exhibit any heparin requirement and showed similar blastocyst development over a range of heparin concentrations from 0 to 100 µg/ml. By recapitulating the pair-wise comparison within each bull between sorted and unsorted sperm to be used for IVF, Bulls A and C required higher concentrations of heparin for an optimal sexed IVF outcome, when compared to unsorted sperm. Conversely, the remaining two bulls (B and D) required lower heparin concentrations for sexed IVF when compared to unsorted sperm controls.
Term development of vitrified and sexed female embryos
Embryo transfer was performed on Chinese farms to evaluate the in vivo developmental potential of sexed, vitrified IVF embryos (Table 2). A total of 730 vitrified embryos produced by sorted sperm from three bulls (A, B, and C) were pooled for use in an ET experiment, while embryos derived from Bull D were not used for ET due to the resultant low embryo yield. A total of 236 (heparin 10 µg/ml, served as a routine IVF control), 189 (heparin 20 µg/ml, IVF treatment), and 305 (heparin 40 µg/ml, IVF treatment) sexed embryos were transferred (one embryo per recipient). Following ET, a total of 291 pregnancies were determined on d 70 post ET. The pregnancy rates were similar among the control and treatment groups: 39.8% (n = 94), 39.1% (n = 74), and 40.3% (n = 123), with heparin concentrations of 10, 20, and 40 µg/ml, respectively (P > 0.05) (Table 2).
Table 2. In vivo developmental competence of sex-sorted in vitro fertilized (IVF) embryos obtained using different concentrations of heparin.
| Embryo type* | No. embryos | No. recipients | Embryo transfer results (mean ± SEM) |
|
| % pregnancies ** | % calving | |||
| Heparin 10 | 236 | 236 | 39.8 ± 5.6 a | 34.7 ± 3.3 a |
| Heparin 20 | 189 | 189 | 39.1 ± 4.3 a | 34.4 ± 4.5 a |
| Heparin 40 | 305 | 305 | 40.3 ± 3.8 a | 35.1 ± 3.2 a |
Same letters within columns were not significantly different (P > 0.05). Sexed embryos derived using different concentrations of heparin during IVF were vitrified on day 7 after culture in vitro. After warming, a single embryo was transferred non-surgically into each synchronized recipient. * Heparin 10, 20, and 40: embryos were derived from sexed IVF with heparin concentrations of 10, 20, and 40 µg/ml. Routine IVF with a heparin concentration of 10 µg/ml [8] served as an ET control for Heparin 20 and 40 µg/ml. ** Pregnancy of recipients was determined by palpation per rectum on d 70 post transfer.
Following normal-length pregnancies, 254 live Holstein calves were born, of which 232 were female (91.3%). Calving rates were also similar among the three different heparin concentrations tested (34.7%, n = 82, heparin 10 µg/ml, IVF control vs. 34.4%, n = 65, heparin 20 µg/ml vs. 35.1%, n = 107, heparin 40 µg/ml) (P > 0.05) (Table 2). Most calves appeared normal and healthy, three had physical defects and two died within 1 h of birth. From the 291 pregnancies detected at d 70, a total of 37 pregnancies were lost up to term, with the miscarriage rate being similar among the three groups (4.7–5.2%, P > 0.05).
Discussion
The present study clearly demonstrated a significant effect of heparin concentration on blastocyst development after IVF using sorted sperm from all bulls tested. Sexed embryos derived from IVF using a heparin concentration of 40 µg/ml and sorted sperm are not detrimental to fetal and term development after ET. The pioneering work of Parrish [15, 17, 19] on the function of heparin in capacitation and fertilization in cattle established the importance of this molecule for cattle IVF and subsequent embryo development. The excellent sexed IVF results reported by Lu and Seidel [12] showed that the efficiency of fertilization with sorted sperm cells can be improved when optimal concentrations of heparin and sperm cells are used in a bull-to-bull manner [3]. This finding was also confirmed in the present study, in which heparin significantly promoted the development of bovine IVF embryos fertilized by sex-sorted sperm in a bull-dependent manner. Previous studies on other species have also found that males can increase variation in the developmental potential of embryos [29]. An optimized heparin concentration for sexed IVF, leading to high rates of blastocyst development, has been reported and is found within a wide range among bulls [2, 3, 30][REMOVED HYPERLINK FIELD]. In the present study, we tested the effect of nine different heparin concentrations (0 to 100 µg/ml) on the performance of sex-sorted and unsorted sperm from four different bulls. The addition of heparin had a dose-dependent effect on the cleavage rate of sorted sperm from all four bulls, as reported previously by other groups [17, 20, 31, 32]. Only a few studies have compared the cleavage rates of sorted and unsorted sperm from the same bulls, and for this reason, an important goal of our study was to conduct a pair-wise comparison between sorted and unsorted sperm for each individual bull. Merton et al. [33] reported that sorting had no effect on cleavage rate but that blastocyst development was significantly affected. However, these results were based on observations from a single bull. Lu et al. [11] reported a lower cleavage rate for sorted-fresh sperm than for unsorted-fresh sperm, although there was no difference in the cleavage rates between the two categories of sperm. In that study, frozen-thawed sperm was separated into live and dead fractions by Percoll gradient prior to the experiment [12, 34]. Although the effect of such a procedure on cleavage and early embryo development is not clear [35, 36], Mendes et al. [31] achieved a higher cleavage and embryo development rate using Percoll-treated sperm as compared to sperm prepared by BSA centrifugation.
A few studies have directly compared embryo development in vitro using sorted and unsorted sperm from the same bull, and a lower blastocyst development rate with sorted sperm as compared to unsorted sperm cells has been reported [7, 11, 33], with the rate of blastocyst development with sorted sperm being approximately 70% that of the unsorted controls. Conversely, in our previous studies [8, 30] the use of sorted sperm from most of the bulls studied resulted in a similar development rate as observed for their unsorted counterpart. In another study, sorted sperm from three out of nine bulls resulted in a rate of late-stage embryo development similar to that reported for unsorted sperm from a good IVF bull [8]. However, in that last study, the heparin concentration was fixed at 10 µg/ml, without the possibility of comparison between different heparin concentrations.
The results of the present study demonstrate and confirm that different heparin concentrations are necessary for achieving the highest rate of blastocyst development with sorted sperm in a bull-dependent manner [8, 30]. In addition, in the present study, unsorted sperm from two of the four bulls required a lower heparin concentration than their unsorted controls to achieve optimal blastocyst development. This finding indicates that partial capacitation might have taken place in sperm during the sorting process. It has been proposed that when sperm cells are prepared and sorted by flow cytometry, they are subjected to an environment that may affect their capacitation [6]. Seidel [3, 12] and King [2] confirmed that the sorting process might result in a partial membrane change or “damage” to sperm cells, rendering them partially capacitated and thus requiring less heparin during IVF to obtain a maximal level of blastocyst development. However, it should be noted that wide variability in response to heparin supplementation in the IVF procedure is reported, and this is also true in the present study, both from sorted and unsorted control sperm from individual bulls. Such variability regarding the effect of heparin on sorted sperm and their capacity to induce optimal embryo development has to be considered, and is in conflict to the partial capacitation hypothesis [2, 12], and the reason for this interesting pattern is unknown. It is tempting to speculate that stress during the sorting process may affect the membrane properties of sperm cells of some bulls, such that the membrane rearrangement of the sperm cell is so dramatic that the capacitation switches into a heparin-dependent mode. The results presented in this study strongly support a significant effect, and requirement, for heparin to be used with sorted sperm in IVF.
In this study, by optimizing heparin concentration, in vitro blastocyst development with sorted sperm from the bulls could be increased to a level comparable to the highest blastocyst rate obtained from the use of unsorted sperm. This finding corroborates the observations of Lu and Seidel [12], and Blondin et al. [2], by comparing different sperm and heparin concentrations, which highlights the need to optimize both parameters for each individual bull in order to yield a similar blastocyst rate from both sorted and unsorted sperm in IVF. However, the results of our study also revealed that a heparin range of 0–10 µg/ml is not sufficient to deduce the ideal heparin requirement for IVF in all bulls; therefore, we included an expanded range comprising nine heparin concentrations from 0 to 100 µg/ml. With this approach, we were able to show that different heparin concentrations were needed for each of the bulls studied.
In addition, for the four bulls with sorted sperm that responded to heparin optimization, the range of heparin concentrations tested resulted in optimal or sub-optimal blastocyst development, and for all ranges, a heparin concentration of 40 µg/ml was found to be optimal (Fig. 2). We previously performed IVF with sex-sorted sperm in more than 20 Holstein bulls and one Brahman bull (Bos indicus), and obtained optimal blastocyst development when heparin concentration was set at 40 µg/ml for sexed IVF (Du et al., unpublished data). Therefore, the successful heparin concentration used in our previous work closely matched the results obtained in the present study, and a heparin concentration of 40 µg/ml was then set as an optimal value for sorted sperm cells from most Holstein bulls used for IVF.
In the present study, and in contrast that by Lu and Seidel [12] in which a longer sperm-oocyte co-incubation period was used, polyspermy was not affected by increased heparin concentration. It is likely that different IVF procedures influence polyspermy itself. In fact, in contrast to the protocol used in this study, where only a 6-h sperm-oocyte co-incubation was used, polyspermy was significantly increased from 4 to 13% when the period of sperm cells and oocyte co-incubation was prolonged from 4 to 18 h [3]. On the other hand, Blondin et al. [2] clearly showed that the reduced in vitro fertility observed when using sorted sperm is not caused by the presence of a higher percentage of sperm cells with defective DNA. In fact, sperm-cell sorting by flow cytometry itself eliminates sperm cells with compromised DNA. This assumption implies that pre-selected sperm (either X or Y sperm) with a high purity (> 90%) would not increase the chance of polyspermic fertilization when the heparin concentration is increased in sexed IVF. Therefore, IVF with sorted sperm cells can be considered a consistent and reliable procedure for embryo production and subsequent ET practice to rapidly increase the number of offspring of high genetic merit on a large scale [30, 37].
The modified droplet vitrification method developed by our group, resulted in a satisfactory and reliable outcome with sexed IVF bovine embryos [8], in Wagyu beef embryos in vivo [8, 28], cloned bovine embryos [38], and mouse embryos [39]. In this study, the pregnancy/implantation rate of 39.1–40.3% derived from IVF with heparin at a concentration of 20–40 µg/ml was not different to that observed in the routine IVF controls (heparin 10 µg/ml), and was similar to that reported in our previous studies with embryos produced through sexed and non-sexed IVF [8]. Our results have shown that the modified droplet vitrification protocol can be used to cryopreserve sexed IVF embryos with minimal damage, resulting in in vivo developmental competence post warming and ET. Furthermore, it appears from our results that a heparin concentration within the range of 20–40 µg/ml during IVF is not detrimental to fetal development post ET.
In this study, the ratio of female calves from newborns was 91.3%, which is comparable with the ratio of X-sperm cells obtained during the sorting procedure (90 ± 3%). The percentage of pregnancies that were lost from sexed IVF embryos (4.7–5.2%), was similar to findings from our previous field ET studies. A very limited number of born calves had physical defects, whereas all the other calves appeared normal and in good health. These findings suggest that the sperm sorting processes does not adversely affect embryo development and the implantation efficiency, or cause any apparent genetic disorders in the newborns.
In conclusion, differential heparin concentrations are required to achieve the highest rate of in vitro blastocyst development using sorted and unsorted sperm from the tested bulls. Sorted sperm could be used to achieve the highest rate of in vitro embryo development using an optimal heparin concentration with some bulls. A heparin concentration of 40 µg/ml consistently resulted in optimal performance for all bulls tested, and ET outcomes support that such concentration is not detrimental to fetal development post ET.
Acknowledgments
We sincerely appreciate the technical support and assistance of Colleen Shaffer, Alicia Shefler, and Terra Kilmer. This study was supported by the USDA Cooperative State Research, Education, and Extension Service (CSREES, Grant Number 2006-03069) to FD; a Premium Science and Technology Foundation of Jiangsu Province of China grant; partial support from the National Natural Science Foundation of China (NSFC, Grant Number 31340041, 31471388); Specialized Research Fund for Doctoral Program of Higher Education (SRFDP, grant No. 20133207110004), Priority Academic Program Development of Jiangsu Higher Education Institutions, and Major Program of Natural Science Research of Jiangsu Higher Education Institutions of China (Grant Number 14KJA180003) to FD.
References
- 1.Wheeler MB, Rutledge JJ, Fischer-Brown A, VanEtten T, Malusky S, Beebe DJ. Application of sexed semen technology to in vitro embryo production in cattle. Theriogenology 2006; 65: 219–227. [DOI] [PubMed] [Google Scholar]
- 2.Blondin P, Beaulieu M, Fournier V, Morin N, Crawford L, Madan P, King WA. Analysis of bovine sexed sperm for IVF from sorting to the embryo. Theriogenology 2009; 71: 30–38. [DOI] [PubMed] [Google Scholar]
- 3.Barceló-Fimbres M, Campos-Chillón LF, Seidel GE., Jr. In vitro fertilization using non-sexed and sexed bovine sperm: sperm concentration, sorter pressure, and bull effects. Reprod Domest Anim 2011; 46: 495–502. [DOI] [PubMed] [Google Scholar]
- 4.Senatore EM, Xu J, Suárez Novoa MV, Gong G, Lin T, Bella A, Moreno JF, Mannino ME, Tian X, Presicce GA, Wu SC, Du F. Improved in vitro development of OPU-derived bovine (Bos taurus) embryos by group culture with agarose-embedded helper embryos. Theriogenology 2010; 74: 1643–1651. [DOI] [PubMed] [Google Scholar]
- 5.Pontes JH, Silva KC, Basso AC, Rigo AG, Ferreira CR, Santos GM, Sanches BV, Porcionato JP, Vieira PH, Faifer FS, Sterza FA, Schenk JL, Seneda MM. Large-scale in vitro embryo production and pregnancy rates from Bos taurus, Bos indicus, and indicus-taurus dairy cows using sexed sperm. Theriogenology 2010; 74: 1349–1355. [DOI] [PubMed] [Google Scholar]
- 6.Schenk JL, Suh TK, Cran DG, Seidel GE., Jr. Cryopreservation of flow-sorted bovine spermatozoa. Theriogenology 1999; 52: 1375–1391. [DOI] [PubMed] [Google Scholar]
- 7.Cran DG, Johnson LA, Polge C. Sex preselection in cattle: a field trial. Vet Rec 1995; 136: 495–496. [DOI] [PubMed] [Google Scholar]
- 8.Xu J, Guo Z, Su L, Nedambale TL, Zhang J, Schenk J, Moreno JF, Dinnyés A, Ji W, Tian XC, Yang X, Du F. Developmental potential of vitrified holstein cattle embryos fertilized in vitro with sex-sorted sperm. J Dairy Sci 2006; 89: 2510–2518. [DOI] [PubMed] [Google Scholar]
- 9.Seidel GE., Jr. Sexing mammalian spermatozoa and embryosstate of the art. J Reprod Fertil Suppl 1999; 54: 477–487. [PubMed] [Google Scholar]
- 10.Nadir S, Saacke RG, Bame J, Mullins J, Degelos S. Effect of freezing semen and dosage of sperm on number of accessory sperm, fertility, and embryo quality in artificially inseminated cattle. J Anim Sci 1993; 71: 199–204. [DOI] [PubMed] [Google Scholar]
- 11.Lu KH, Cran DG, Seidel GE., Jr. In vitro fertilization with flow-cytometrically-sorted bovine sperm. Theriogenology 1999; 52: 1393–1405. [DOI] [PubMed] [Google Scholar]
- 12.Lu KH, Seidel GE., Jr. Effects of heparin and sperm concentration on cleavage and blastocyst development rates of bovine oocytes inseminated with flow cytometrically-sorted sperm. Theriogenology 2004; 62: 819–830. [DOI] [PubMed] [Google Scholar]
- 13.Austin CR. Activation and the correlation between male and female elements in fertilization. Nature 1951; 168: 558–559. [DOI] [PubMed] [Google Scholar]
- 14.Chang MC. Fertilizing capacity of spermatozoa deposited into the fallopian tubes. Nature 1951; 168: 697–698. [DOI] [PubMed] [Google Scholar]
- 15.Parrish JJ, Susko-Parrish J, Winer MA, First NL. Capacitation of bovine sperm by heparin. Biol Reprod 1988; 38: 1171–1180. [DOI] [PubMed] [Google Scholar]
- 16.Parrish JJ. Bovine in vitro fertilization: in vitro oocyte maturation and sperm capacitation with heparin. Theriogenology 2014; 81: 67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parrish JJ, Susko-Parrish JL, First NL. Effect of heparin and chondroitin sulfate on the acrosome reaction and fertility of bovine sperm in vitro. Theriogenology 1985; 24: 537–549. [DOI] [PubMed] [Google Scholar]
- 18.Fukui Y. Effect of follicle cells on the acrosome reaction, fertilization, and developmental competence of bovine oocytes matured in vitro. Mol Reprod Dev 1990; 26: 40–46. [DOI] [PubMed] [Google Scholar]
- 19.Parrish JJ, Parrish JL, First NL. Effect of swim-up separation and heparin pretreatment of frozen-thawed spermatozoa on in vitro fertilization of bovine oocytes. Biol Reprod 1984; 30(Suppl 1): 112.6696958 [Google Scholar]
- 20.Parrish JJ, Susko-Parrish JL, Leibfried-Rutledge ML, Critser ES, Eyestone WH, First NL. Bovine in vitro fertilization with frozen-thawed semen. Theriogenology 1986; 25: 591–600. [DOI] [PubMed] [Google Scholar]
- 21.First NL, Parrish JJ. Sperm maturation and in vitro fertilization. Proc 11th Int Cong Anim Reprod AI1988; 5: 161−168.
- 22.Leibfried-Rutledge ML, Critser ES, Parrish JJ, First NL. In vitro maturation and fertilization of bovine oocytes. Theriogenology 1989; 31: 61–74. [Google Scholar]
- 23.Lu KH, Gordan I. Effect of heparin on the capacitation of frozen-thawed bovine spermatozoa used in the in vitro fertilization of oocytes matured in vitro. Proc 11th Int Cong Anim Reprod AI1988; 5: 161−168.
- 24.First NL, Parrish JJ. Preparation of sperm for in vitro fertilization. Proc Symp Application of Egg and Embryo Technologies to Domestic Animals1991; 33−35.
- 25.Fukui Y, Sonoyama T, Mochizuki H, Ono H. Effects of heparin dosage and sperm capacitation time on in vitro fertilization and cleavage of bovine oocytes matured in vitro. Theriogenology 1990; 34: 579–591. [DOI] [PubMed] [Google Scholar]
- 26.Brackett BG, Bousquet D, Boice ML, Donawick WJ, Evans JF, Dressel MA. Normal development following in vitro fertilization in the cow. Biol Reprod 1982; 27: 147–158. [DOI] [PubMed] [Google Scholar]
- 27.Rosenkrans CF, Jr, Zeng GQ, MCNamara GT, Schoff PK, First NL. Development of bovine embryos in vitro as affected by energy substrates. Biol Reprod 1993; 49: 459–462. [DOI] [PubMed] [Google Scholar]
- 28.An L, Ling PP, Zhu X, Liu Y, Zhang F, Ma X, Xu B, Wang Y, Du Z, Yang L, Xue F, Bella A, Presicce GA, Du F. Successful vitrification of in vivo embryos collected from superovulated Japanese Black cattle (Wagyu). Reprod Domest Anim 2016; 51: 255–261. [DOI] [PubMed] [Google Scholar]
- 29.Miao DQ, Liang B, Wang JZ, Wang HL, Cui W, Liu Y, Tan JH. Fertilization in vitro with spermatozoa from different mice increased variation in the developmental potential of embryos compared to artificial parthenogenetic activation. Mol Reprod Dev 2009; 76: 239–245. [DOI] [PubMed] [Google Scholar]
- 30.Xu J, Chaubal SA, Du F. Optimizing IVF with sexed sperm in cattle. Theriogenology 2009; 71: 39–47. [DOI] [PubMed] [Google Scholar]
- 31.Mendes JO, Jr, Burns PD, De La Torre-Sanchez JF, Seidel GE., Jr. Effect of heparin on cleavage rates and embryo production with four bovine sperm preparation protocols. Theriogenology 2003; 60: 331–340. [DOI] [PubMed] [Google Scholar]
- 32.Parrish JJ, Krogenaes A, Susko-Parrish JL. Effect of bovine sperm separation by either swim-up or Percoll method on success of in vitro fertilization and early embryonic development. Theriogenology 1995; 44: 859–869. [DOI] [PubMed] [Google Scholar]
- 33.Merton JS, Haring RM, Stap J, Hoebe RA, Aten JA. Effect of flow cytometrically sorted frozen thawed semen on success rates of in vitro bovine embryo production. Theriogenology 1997; 47: 295. [Google Scholar]
- 34.Pellegrino CA, Morotti F, Untura RM, Pontes JH, Pellegrino MF, Campolina JP, Seneda MM, Barbosa FA, Henry M. Use of sexed sorted semen for fixed-time artificial insemination or fixed-time embryo transfer of in vitro-produced embryos in cattle. Theriogenology 2016; 86: 888–893. [DOI] [PubMed] [Google Scholar]
- 35.Dode MAN, Rodovalho NC, Ueno VG, Fernandes CE. The effect of sperm preparation and co-incubation time on in vitro fertilization of Bos indicus oocytes. Anim Reprod Sci 2002; 69: 15–23. [DOI] [PubMed] [Google Scholar]
- 36.Seidel JGE, Leipold SD, Shawki H. Preparation of bovine sperm for in vitro fertilization by swim-up or centrifugation through percoll or BSA. Theriogenology 1995; 43: 319. [Google Scholar]
- 37.Morotti F, Sanches BV, Pontes JH, Basso AC, Siqueira ER, Lisboa LA, Seneda MM. Pregnancy rate and birth rate of calves from a large-scale IVF program using reverse-sorted semen in Bos indicus, Bos indicus-taurus, and Bos taurus cattle. Theriogenology 2014; 81: 696–701. [DOI] [PubMed] [Google Scholar]
- 38.Du F, Shen PC, Xu J, Sung LY, Jeong BS, Lucky Nedambale T, Riesen J, Cindy Tian X, Cheng WT, Lee SN, Yang X. The cell agglutination agent, phytohemagglutinin-L, improves the efficiency of somatic nuclear transfer cloning in cattle (Bos taurus). Theriogenology 2006; 65: 642–657. [DOI] [PubMed] [Google Scholar]
- 39.An L, Chang S, Hu Y, Li Y, Xu B, Zhang F, Yang L, Presicce GA, Du F. Efficient cryopreservation of mouse embryos by modified droplet vitrification (MDV). Cryobiology 2015; 71: 70–76. [DOI] [PubMed] [Google Scholar]