Abstract
The objective of this study was to compare the cAMP and cGMP levels in cumulus-oocyte complexes (COCs) derived from the middle follicles (MFs, 3−6 mm in diameter) and small follicles (SFs, 1–3 mm in diameter) of pre-pubertal gilts during the first 24-h period of maturation in vitro (IVM). Both cAMP and cGMP levels in MF- and SF-derived oocytes did not change during this period. Although the cAMP levels increased in the COCs at 10 and 20 h after the start of IVM, the levels of cAMP were significantly higher in MF-derived COCs than in SF-derived COCs at 20 h after the start of IVM. On the other hand, the cGMP levels in COCs decreased to basal levels between 10 and 20 h after the start of the IVM, whereas cGMP levels were lower in SF-derived COCs than in MF-derived COCs during the first 10 h. The number of cumulus cells was larger in the MF-derived COCs than in the SF-derived COCs during the first 20-h period of IVM. The estimated cAMP level per cumulus cell at 10 h after the start of the IVM was higher in SF-derived COCs than in MF-derived COCs, whereas the estimated cGMP level per cumulus cell was no different between MF- and SF-derived COCs. From these results, we conclude that cAMP and cGMP levels in COCs, but not in oocytes, drastically change during the first 20-h period of IVM, and that both cAMP and cGMP levels significantly differ between MF- and SF-derived COCs.
Keywords: cAMP, cGMP, Cumulus cells, Maturation in vitro (IVM), Pigs
Since pre-pubertal pigs in many countries are commonly mostly slaughtered, ovaries from pre-pubertal females can be easily obtained for use as a source of gametes for embryo production in vitro. Techniques to produce embryos via in vitro maturation and fertilization (IVM-IVF) of the cumulus-oocyte complexes (COCs) derived from medium follicles (MFs; with 3–6 mm in diameter) of pre-pubertal ovaries have been well-developed [1, 2]. However, the number of COCs from MFs is very limited on the surface of pre-pubertal gilt ovaries since small follicles (SFs) of less than 2 mm in diameter are the majority [3]. Furthermore, the meiotic and developmental competences of oocytes are significantly lower in SF-derived COCs than in MF-derived COCs [4,5,6]. Recently, we demonstrated that the follicular size significantly affects the meiotic and developmental competences. The capability of MF- and SF-derived oocytes to be fertilized and form pronuclei is similar only when mature oocytes containing the first polar body are selected following IVM [6]. Paracrine factors from the oocyte and gap junction-mediated communication seem to be involved in modulating large-scale chromatin structures during the final phase of oocyte maturation and these affect the acquisition of embryonic developmental competence in fully grown bovine oocytes [7]. We have also shown that autocrine factors from cumulus cells beneficially affect the meiotic and developmental competences of oocytes from SFs [8], and that a disruption of gap junction-mediated communication at around 20 h of IVM enhances the meiotic progression of SF-derived oocytes to the metaphase II stage [9]. However, it is still unknown why meiotic competence is lower in oocytes derived from SFs than in those derived from MFs; the differences in the resultant cells are also unknown. Additionally, both cAMP and cGMP are well-known secondary messengers that are involved in gap junction-mediated communications between the cumulus cells and the oocyte to affect oocyte resumption. The cAMP produced in the cumulus cells is transported into the oocyte through the gap junctions [10, 11]. Increased intra-ooplasmic cAMP levels inhibit the activity of maturation-promoting factor (MPF) via the cAMP-dependent protein kinase pathway, which then consequently induces the arrest of oocytes at the germinal vesicle stage [12]. The cumulus cells produce cGMP though the natriuretic peptide precursor type C/natriuretic peptide receptor 2 signal pathway [13], which also negatively affects the rate of cAMP degeneration in oocytes by reducing the activity of phosphodiesterase-3 after being transported through the gap junctions, therefore preventing oocyte resumption during meiosis [13, 14]. The activation of mitogen-activated protein kinases [15, 16] by a gonadotropin-induced rise in cAMP levels [17, 18] results in a reduction in the permeability of the gap junctions between the cumulus foot process and the oocyte. This occurs via the phosphorylation of proteins that are used to construct the gap junction and has been believed to induce meiotic resumption of the oocytes following a reduction in intra-ooplasmic cAMP and cGMP levels [15, 16, 19]. Therefore, the lower meiotic competence of oocytes derived from SFs than of those derived from MFs may be owing to changes in cAMP/cGMP reactions in the COCs/cumulus-free oocytes following gonadotropic stimulation until the breakdown of germinal vesicle during the first half of IVM. In the current study, we examined the cAMP and cGMP levels per COC or oocyte during the first 24-h period of IVM in MF- and SF-derived COCs before, during, and immediately after stimulation by dibutyryl-cAMP and gonadotropins.
Materials and Methods
Chemicals and culture media
NaCl, KCl, KH2PO4, MgCl2·6H2O, CaCl2·2H2O, and gentamicin sulfate were purchased from Nacalai Tesque (Kyoto, Japan). MgSO4·7H2O was purchased from Ishizu Pharmaceutical (Osaka, Japan). Furthermore, eCG (the trade name; Serotropin) and hCG (the trade name; Gonatropin) were purchased from ASKA Pharmaceutical (Tokyo, Japan). Unless otherwise specified, other chemicals were purchased from Sigma Aldrich (St. Louis, MO, USA).
Modified TL-HEPES-PVA medium composed of 114 mM NaCl, 3.2 mM KCl, 2 mM NaHCO3, 0.34 mM NaH2PO4, 10 mM Na-lactate, 0.5 mM MgCl2·6H2O, 2 mM CaCl2·2H2O, 12 mM sorbitol, 10 mM HEPES, 0.2 mM Na-pyruvate, 0.1% (w/v) polyvinyl alcohol (PVA), 25 µg/ml gentamicin sulfate, and 65 µg/ml potassium penicillin G was used for collecting and washing COCs. The basic IVM medium was a BSA-free, chemically-defined, porcine oocyte medium (POM, Research Institute for the Functional Peptides, Yamagata, Japan) supplemented with 50 µM beta-mercaptoethanol (mPOM) [20]. This IVM medium was equilibrated at 39°C in an atmosphere of 5% CO2 overnight prior to use.
In vitro maturation of COCs
Ovaries without any evidence of corpora lutea were collected from slaughtered commercial pre-pubertal gilts at a local abattoir and transported within 1 h to the laboratory at 32–35°C in a 0.9% (w/v) NaCl solution containing 75 µg/ml potassium penicillin G and 50 µg/ml streptomycin sulfate. The COCs were washed three times with the NaCl solution at room temperature and then were aspirated from either the SFs (1–3 mm in diameter) or MFs (3–6 mm in diameter) using a disposable 10 ml syringe and 18-gauge needle, separately stocked into a 50 ml centrifuge tube, and then washed three times with modified TL-HEPES-PVA. Only the COCs with a uniform ooplasm and a compact cumulus cell mass with at least 3 layers were then washed three times with mPOM. Approximately 30–40 COCs from SFs and MFs were separately cultured in 500 µl of mPOM supplemented with 10 IU/ml eCG, 10 IU/ml hCG, and 1 mM dibutyryl cyclic adenosine 3’,5’-monophosphate (dbcAMP) in 4-well culture plates (Thermo Fisher Scientific, Roskilde, Denmark) for 20 h in an atmosphere of 5% CO2 at 39°C [20, 21]. The start of the IVM was considered to be when the COCs were first exposed to the gonadotropins and dbcAMP. The COCs were then washed three times with fresh IVM medium without gonadotropins and dbcAMP, and continued to be cultured in the medium for an additional 4 or 24 h.
Assessment of nuclear maturation
After IVM for 20 and 44 h, the cumulus cells surrounding the oocytes were removed by pipetting in a modified TL-HEPES-PVA medium containing 0.1% (w/v) hyaluronidase. Denuded oocytes were mounted on a glass slide and fixed in an ethanol:acetic acid (3:1, v/v) solution for 48–72 h at room temperature. The meiotic progression of the oocytes (111 oocytes from 3 replications and 393 oocytes from 5 replications at 20 and 44 h after the start of IVM, respectively) was evaluated under a phase contrast microscope at 200 × and 400 × magnifications after staining with 1% (w/v) orcein in 45% (v/v) acetic acid.
Measuring intracellular cAMP and cGMP contents
At 0, 10, 20, and 24 h of the IVM, the COCs from the SFs and MFs were separately transferred into pre-warmed TL-HEPES-PVA. The cumulus cells and zona pellucida were removed from some of the COCs by treating with 0.1% (w/v) hyaluronidase and 0.1% (w/v) protease, respectively, to obtain denuded oocytes. Thirty COCs or denuded oocytes were transferred into microtubes with 10 µl of TL-HEPES-PVA and stored at –80°C until they were assayed. The cAMP and cGMP levels in the COCs (1,680 and 930 COCs, respectively) and oocytes (1,590 and 1,080 oocytes, respectively) were measured using direct cAMP and cGMP enzyme immunoassay kits (Arbor Assays, Ann Arbor, MI, USA) and an iMark microplate reader (Bio-Rad, Hercules, CA, USA) according to the manufacturer’s protocol. Briefly, 30 COCs and denuded oocytes were lysed in sample diluent (250 µl) and vortexed for 10 min at room temperature. After centrifugation at 2,200 × g for 10 min at 4°C, the supernatants (200 µl) were treated with acetylation reagent (10 µl) and then used for the assay. After adding a special plate primer (50 µl) to all wells of a microtiter plate, 50 µl of standards or samples were introduced into each well of the plate. Then, 25 µl of cAMP or cGMP peroxidase conjugate was added to all the wells. The binding reaction was initiated by adding an antibody for cAMP or cGMP (25 µl). After 2 h, the plate was washed and the substrate (100 µl) was added. After a short incubation for 30 min, the stop solution (50 µl) was added and then the intensity of the generated color was detected by using a microplate reader at 450 nm. The cAMP or cGMP concentrations (5–7 and 3–6 samples in each observation point, respectively) were calculated by using the Microplate Manager 6 software (Bio-Rad, Hercules, CA, USA).
To assess the status of the cumulus cells, the cAMP or cGMP levels per cumulus cell were estimated from the amount of cAMP/cGMP in COCs or oocytes along with the number of cumulus cells in a COC as follows:
Estimated amount of cAMP/cGMP per cumulus cell = ([amount of cAMP/cGMP in a COC] – [amount of cAMP/cGMP in a COC]) / [the number of cumulus cells in a COC]
Assessment of the number of cumulus cells in a COC
At 0, 10, and 20 h of IVM, cumulus cells from thirty COCs were separated by treatment with 0.05% (w/v) trypsin in 0.5 mM EDTA solution for 10 min by pipetting. The number of cumulus cells per COC was calculated using a cell counting chamber. The experiments were replicated 5 times.
Statistical analysis
Data from 3–7 replicates were processed by a chi-square test (meiotic progression) or one-way ANOVA (amount of cAMP/cGMP and the number of cumulus cells) using the GraphPad Prism 6 software (GraphPad Software, CA, USA). All data were expressed as mean ± SEM. Findings were considered statistically significant at P < 0.05 and when there was a significant effect in ANOVA. Values were compared by a Bonferroni-type Dunn post-hoc test.
Results
In total, 222 MF- and SF-derived oocytes (111 oocytes from each) from three replicated trials were assessed for meiotic stages at 20 h after the start of the IVM (Table 1). The majority of the oocytes were at the GV-II stage, whereas the percentage was significantly higher in MF-derived (58.7 ± 1.9%) than in SF-derived oocytes (44.1 ± 2.6%). The incidence of oocytes at the GV-0 stage was significantly higher in SF-derived (23.4 ± 5.0%) than in MF-derived oocytes (8.8 ± 1.9%). When the meiotic progression of MF- (198 oocytes from 5 replications) and SF-derived (195 oocytes from 5 replications) oocytes was examined following IVM at 44 h, the percentage of oocytes that reached the metaphase-II stage was significantly higher (P < 0.05) in those from the MFs than in those from the SFs (Table 2). The percentage of oocytes at the GV or metaphase-I stages was significantly higher in SF-derived than in MF-derived oocytes (Table 2).
Table 1. Meiotic stages of MF- and SF-derived oocytes at 20 h after the start of maturation in vitro.
| Origin of COCs | No. of oocytes examined | No. (%) of oocytes at the stage of |
||||||
| GV-0 | GV-I | GV-II | GV-III | GV-IV | Pro M-I | M-I | ||
| MF | 111 | 10 (8.8 ± 1.9) a | 5 (4.6 ± 1.1) | 65 (58.7 ± 1.9) a | 13 (11.8 ± 0.9) | 15 (13.2 ± 2.4) | 1 (1.0 ± 1.0) | 2 (1.8 ± 0.9) |
| SF | 111 | 26 (23.4 ± 5.0) b | 1 (0.9 ± 0.9) | 49 (44.1 ± 2.6) b | 16 (14.4 ± 0.7) | 16 (14.4 ± 5.0) | 3 (2.8 ± 1.6) | 0 |
Data are shown as means ± SEM from three replicated trials. a,b Values with different superscripts within columns are significantly different (P < 0.05). MF, middle follicles; SF, small follicles; COCs, cumulus-oocyte complexes; GV, germinal vesicle; Pro M-I, pro-metaphase-I; M-I, metaphase-I.
Table 2. Meiotic stages of MF- and SF-derived oocytes following in vitro maturation culture for 44 h.
| Origin of COCs | No. of oocytes examined | No. (%) of oocytes at the stage of |
||||
| GV | Pro M-I | M-I | A/T-I | M-II | ||
| MF | 198 | 12 (6.1 ± 2.0) a | 6 (3.0 ± 1.2) | 23 (11.6 ± 2.6) a | 0 | 157 (79.3 ± 4.0) a |
| SF | 195 | 32 (16.4 ± 2.3) b | 12 (6.2 ± 1.3) | 40 (20.6 ± 3.2) b | 5 (2.6 ± 1.4) | 106 (54.2 ± 3.3) b |
Data are shown as means ± SEM from five replicated trials. a,b Values with different superscripts within columns are significantly different (P < 0.05). MF, middle follicles; SF, small follicles; COCs, cumulus-oocyte complexes; GV, germinal vesicle; Pro M-I, pro-metaphase-I; M-I, metaphase-I; A/T-I, anaphase-I and telophase-I; M-II, metaphase-II.
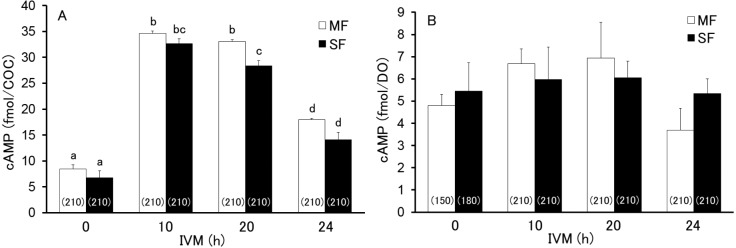
When the levels of cAMP in MF- and SF-derived COCs (1,680 total) and oocytes (1,590 total) were examined during the first 24 h after the start of IVM, the levels of MF- and SF-derived oocytes did not change (P > 0.05) throughout the period and did not differ significantly (P > 0.05) between the groups, although the cAMP levels in MF-derived oocytes displayed an upward trend around 10 h of IVM (Fig. 1B). However, cAMP levels in MF- and SF-derived COCs drastically changed just after the start of IVM, and were significantly higher at 10 and 20 h than at 0 and 24 h after the start of IVM (Fig. 1A). Furthermore, the levels of cAMP were significantly higher (P < 0.05) in MF-derived COCs (33.0 ± 0.5 fmol/COC) than in SF-derived COCs (28.4 ± 1.0 fmol/COC) at 20 h after the start of IVM. However, no significant differences (P > 0.05) in the cAMP levels were observed between MF- and SF-derived COCs at 0, 10, and 24 h after the start of IVM (Fig. 1A).
Fig. 1.
Levels of cAMP in the cumulus-oocyte complex (COC; A) and denuded oocyte (DO; B) derived from middle and small follicles (MFs and SFs, respectively) at 0, 10, 20, and 24 h after the start of maturation in vitro. Different letters indicate statistical significance (P < 0.05, 5–7 replicated trials). The numbers in the parentheses indicate the total number of COCs or DOs assayed.
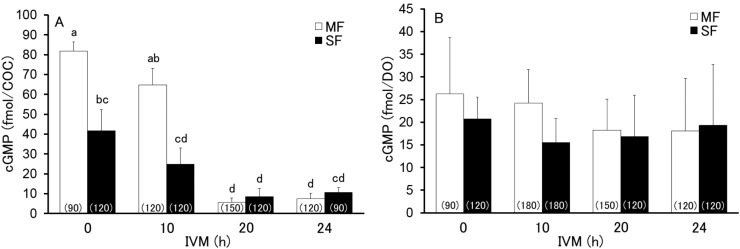
The levels of cGMP in MF- and SF-derived COCs (930 total) and the oocytes (1,080 total) were examined at 0, 10, 20, and 24 h after the start of IVM. As shown in Fig. 2B, the cGMP levels in the oocytes did not changed (P > 0.05) throughout the period and did not differ (P > 0.05) between MF- and SF-derived COCs. However, cGMP levels in both MF- and SF-derived COCs significantly decreased to a basal level between 10 and 20 h after the start of IVM (Fig. 2A), whereas the cGMP levels at 0 and 10 h after the start of IVM were significantly lower (P < 0.05) in SF-derived COCs (41.7 ± 10.6 fmol/COC and 24.8 ± 8.2 fmol/COC, respectively) than in MF-derived COCs (81.8 ± 4.5 fmol/COC and 64.8 ± 8.4 fmol/COC, respectively). There were no significant differences (P > 0.05) in cGMP levels at 20 and 24 h after the start of IVM regardless of the origin of the COCs.
Fig. 2.
Levels of cGMP in cumulus-oocyte complex (COC; A) and denuded oocyte (DO; B) derived from middle and small follicles (MF and SF, respectively) at 0, 10, 20, and 24 h after the start of maturation in vitro. Different letters indicate statistical significance (P < 0.05, 3–6 replicated trials). The numbers in the parentheses indicate the total number of COCs or DOs assayed.
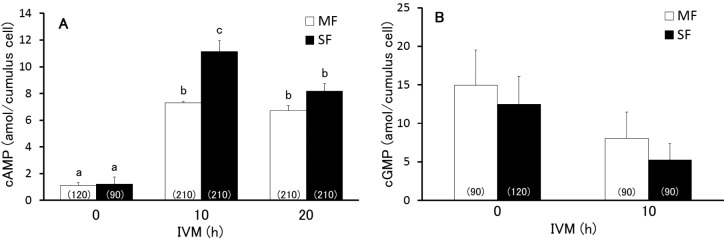
Finally, the number of cumulus cells in MF- or SF-derived COCs was examined at 0, 10, and 20 h after the start of IVM (450 total COCs per group). Throughout this period, the number of cumulus cells was significantly larger (P < 0.05) in COCs derived from MFs than from SFs (Table 3). As shown in Fig. 3A, the estimated cAMP level per cumulus cell was calculated by balancing the cAMP levels in a COC and an oocyte with the number of cumulus cells in a COC. The estimated cAMP levels increased significantly from 0 h (1.1 ± 0.2 amol/cell in MFs and 1.2 ± 0.5 amol/cell in SFs) to 10 h (7.2 ± 0.1 amol/cell in MFs and 11.1 ± 0.8 amol/cell in SFs) and 20 h (6.7 ± 0.4 amol/cell in MFs and 8.2 ± 0.5 amol/cell in SFs) after the start of IVM. Only at 10 h after the start of IVM, the estimated cAMP levels in a cumulus cell from a SF (11.1 ± 0.8 amol) were significantly higher than that from a MF (7.3 ± 0.1 amol). However, the estimated cGMP levels were no different (P > 0.05) between SF-derived (12.5 ± 3.6 amol/cell at 0 h and 5.3 ± 2.1 amol/cell at 10 h) and MF-derived (14.9 ± 4.6 amol/cell at 0 hour and 8.0 ± 3.4 amol/cell at 10 h) cumulus cells, whereas a downward trend (P > 0.05) was observed between 0 and 10 h after the start of IVM (Fig. 3B). The cGMP levels in the cumulus cells at 20 h were estimated to be at basal levels (–3.2 ± 1.4 amol/cell and –3.1 ± 1.8 amol/cell in MF- and SF-derived COCs, respectively).
Table 3. Number of cumulus cells in a MF- and SF-derived cumulus-oocyte complex (COC) at 0, 10 and 20 h after the start of maturation in vitro.
| Origin of COCs | No. of cumulus cells (× 103 cells/COC) |
||
| 0 h | 10 h | 20 h | |
| MF | 3.72 ± 0.08 a | 3.83 ± 0.05 a | 3.88 ± 0.17 a |
| SF | 2.31 ± 0.22 b | 2.40 ± 0.16 b | 2.73 ± 0.15 b |
Data are shown as means ± SEM from five replicated trials. a,b Values with different superscripts within columns are significantly different (P < 0.05). MF, middle follicles; SF, small follicles.
Fig. 3.
Estimated cAMP (A) and cGMP (B) derived from middle and small follicles (MF and SF, respectively) during the first 20-h period of maturation in vitro. Different letters indicate statistical significance (P < 0.05). The numbers in the parentheses indicate the total number of COCs assayed.
Discussion
It is well known that the meiotic competence is significantly lower in COCs collected from SFs than in those collected from MFs [4, 6, 8, 9]. In the present study, we observed that oocytes from MFs were at the metaphase-II stage and those from SFs were at the germinal vesicle or metaphase-I stage. The results presented here are consistent with previously published findings [6, 8, 9].
So far, some reports have shown different results regarding the dynamics of intracellular cAMP levels in MF-derived porcine oocytes during IVM [22−24]. Mattioli et al. [22] reported that the cAMP levels in COCs and oocytes did not change during the first 24 h (at 0, 3, 6, 12, and 24 h time points) after the start of IVM. On the other hand, Shimada and Terada [23] demonstrated that cAMP levels in COCs significantly increased at 4 h, peaked around 20 h, and then was maintained at that level until 48 h after the start of IVM (gonadotropins were supplemented through IVM for 48 h in this experiment). Furthermore, they also showed that the cAMP levels of oocytes increased between 0 and 4 h, peaked around 8 h, and then started to decrease around 16 h before returning to basal levels around 32 h after the start of IVM. Additionally, Bagg et al. [24] reported that even in follicles with similar sizes in diameter, the dynamics in cAMP levels differed between pre-pubertal (no differences between 0 and 22 h of IVM) and post-pubertal gilts (significantly higher after 22 h of IVM). In the present study, we observed that the cAMP levels in MF- and SF-derived oocytes of pre-pubertal gilts did not change significantly during the first 24 h after the start of IVM. However, an upward trend in cAMP levels was observed in MF-derived oocytes after the start of IVM in the presence of dbcAMP (4.8 ± 0.5 fmol/oocyte at 0 h, 6.7 ± 0.7 fmol/oocyte at 10 h, and 6.9 ± 1.6 fmol/oocyte at 20 h). Moreover, we observed that cAMP levels drastically increased in COCs in the presence of gonadotropins and dbcAMP and then decreased again in the absence of these factors. Therefore, changes in cAMP levels in cumulus cells may stimulate functions that consequently contribute to the meiotic resumption of the oocyte.
However, the levels of cAMP were significantly lower in SF-derived COCs than in MF-derived COCs at 20 h after the start of IVM, even in the presence of dbcAMP. To our knowledge, this is the first study to report cAMP dynamics in SF-derived COCs and oocytes, although there was a paper that compared cAMP levels in COCs and oocytes derived from MFs of different sizes (3–8 mm in diameter) [25]. They showed that gap junctions between the oocyte and cumulus cells were maintained longer during IVM in COCs derived from follicles that were 3 mm in diameter instead of those that were 5–8 mm in diameter, and that the extent of cumulus expansion was also lower in COCs derived from smaller follicles [25]. Increased levels of cAMP in cumulus cells induced not only cumulus expansion [26, 27], but also a disruption of the gap junctions in the COCs via the phosphorylation of connexin through the mitogen-activated protein kinase pathway [15, 28]. Therefore, lower levels of cAMP in SF-derived COCs during IVM may affect a lower meiotic competence in the oocytes due to insufficient cumulus expansion and a delayed disruption of gap junctions in the COCs. In fact, we also found here that the meiotic progression of the oocytes was significantly different between the MF- and SF-derived COCs, since more oocytes were at the GV-II stage in MF-derived COCs and at the GV-0 stage in SF-derived COCs at 20 h after the start of IVM when supplemented with gonadotropins and dbcAMP. In a previous study [21], we have also shown that the incidence of oocytes at the GV-II stage increased when MF-derived COCs were cultured with gonadotropins and dbcAMP. A difference in the reaction against gonadotropins and dbcAMP between MF- and SF-derived COCs may be the cause of the observed disparity in cAMP levels and the meiotic progression during the first 20-h period of IVM. Recently, we have also reported that removing cumulus cells from SF-derived COCs 20 h after the start of IVM significantly improved the meiotic competence of the oocytes [9]. Therefore, it may be important to achieve a suitable reaction for SF-derived COCs against gonadotropins and dbcAMP to induce a well-timed disruption of gap junctions between the cumulus cells and the oocyte.
Furthermore, in the present study, we examined the cGMP levels in MF- and SF-derived COCs and oocytes at 0, 10, 20, and 24 h after the start of IVM. To our knowledge, this is also the first paper to report cGMP dynamics in MF- and SF-derived COCs and oocytes during IVM in pigs. We found that levels of cGMP significantly decreased to basal levels in both MF- and SF-derived COCs between 10 and 20 h after the start of IVM in the presence of gonadotropins and dbcAMP. After a hCG injection in vivo, the levels of cGMP have been shown to decrease in mouse ovaries [29] and oocytes [14]. Previous studies have also shown that cGMP levels were significantly reduced in isolated follicles following the addition of luteinizing hormone to the medium [14, 16, 29]. These previous reports in mice are consistent with our current results.
We have also observed that levels of cGMP were significantly lower in SF-derived COCs than in MF-derived COCs at 0 and 10 h after the start of IVM, although the cGMP levels in oocytes did not differ. Production of cGMP via the NPPC/NPR2 pathway has been shown to be accelerated by oocyte-secreting factors and 17β-estradiol [13, 30]. Since intra-follicular 17β-estradiol concentration increases with the growth of the follicle [31], this factor may be affecting the observed difference in cGMP levels between the MF- and SF-derived COCs.
The present study also demonstrated that the number of cumulus cells was significantly lower in SF-derived COCs than in MF-derived COCs through IVM. These results are consistent with previous reports [5, 32]. When the estimated levels of cAMP per cumulus cell were calculated from the current results, the overall dynamics reflected the cAMP levels in COCs. However, no differences between the cAMP levels in MF- and SF-derived cumulus cells were observed within 10 h after the start of the IVM. Furthermore, the estimated cGMP levels did not differ significantly through IVM between the MF- and SF-derived cumulus cells. These results suggest that the total number of cumulus cells per COC may reflect the total cAMP and cGMP levels in COCs as well as the meiotic progress of the oocytes during IVM. Further research is required to investigate whether an increase in the number of cumulus cells surrounding the SF-derived oocyte before or during IVM will improve the meiotic and developmental competence of the oocytes.
Additionally, it is generally believed that cGMP plays a role in COCs to inhibit the decomposition of cAMP by inhibiting phosphodiesterase-3 activity [13, 16], which consequently maintains meiotic arrest at the germinal vesicle stage. On the other hand, the cGMP/protein kinase G pathway is associated with lipid metabolism in adipocytes [33, 34]. Since lipid metabolism in cumulus cells affects the viability and meiotic competence of bovine oocytes [35], the role of cGMP in the meiotic and developmental competences of COCs should be further investigated.
In conclusion, both the levels of cAMP and cGMP in COCs drastically change during the first 20-h period of IVM, and these levels differ significantly between MF- and SF-derived COCs. These differences may significantly affect the meiotic competence of MF- and SF-derived oocytes.
Acknowledgments
The authors thank the Okayama Prefectural Local Meat Wholesale Market for donations of porcine ovaries. This work was supported by Okayama University.
References
- 1.Funahashi H, Day BN. Advances in in vitro production of pig embryos. J Reprod Fertil Suppl 1997; 52: 271–283. [PubMed] [Google Scholar]
- 2.Day BN, Funahashi H. In vitro maturation and fertilization of pig oocytes. In: H. MR, Pursel VG, Norman HD (eds.), Beltsville Symposia in Agricultural Research XX. Biotechnology's role in the genetic improvement of farm animals. Savoy, IL, USA: American Society of Animal Science; 1996: 125−144.
- 3.Morbeck DE, Esbenshade KL, Flowers WL, Britt JH. Kinetics of follicle growth in the prepubertal gilt. Biol Reprod 1992; 47: 485–491. [DOI] [PubMed] [Google Scholar]
- 4.Marchal R, Vigneron C, Perreau C, Bali-Papp A, Mermillod P. Effect of follicular size on meiotic and developmental competence of porcine oocytes. Theriogenology 2002; 57: 1523–1532. [DOI] [PubMed] [Google Scholar]
- 5.Yoon KW, Shin TY, Park JI, Roh S, Lim JM, Lee BC, Hwang WS, Lee ES. Development of porcine oocytes from preovulatory follicles of different sizes after maturation in media supplemented with follicular fluids. Reprod Fertil Dev 2000; 12: 133–139. [DOI] [PubMed] [Google Scholar]
- 6.Kohata C, Izquierdo-Rico MJ, Romar R, Funahashi H. Development competence and relative transcript abundance of oocytes derived from small and medium follicles of prepubertal gilts. Theriogenology 2013; 80: 970–978. [DOI] [PubMed] [Google Scholar]
- 7.Lodde V, Franciosi F, Tessaro I, Modina SC, Luciano AM. Role of gap junction-mediated communications in regulating large-scale chromatin configuration remodeling and embryonic developmental competence acquisition in fully grown bovine oocyte. J Assist Reprod Genet 2013; 30: 1219–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bui TMT, Nguyen KX, Karata A, Ferre P, Tran MT, Wakai T, Funahashi H. The presence of VEGF during the first half of in-vitro maturation improves the meiotic and developmental competences of porcine oocytes from small follicles. Reprod Fertil Dev 2016. (In press). [DOI] [PubMed] [Google Scholar]
- 9.Ferré P, Bui TMT, Wakai T, Funahashi H. Effect of removing cumulus cells from porcine cumulus-oocyte complexes derived from small and medium follicles during IVM on the apoptotic status and meiotic progression of the oocytes. Theriogenology 2016; 86: 1705–1710. [DOI] [PubMed] [Google Scholar]
- 10.Bornslaeger EA, Schultz RM. Regulation of mouse oocyte maturation: effect of elevating cumulus cell cAMP on oocyte cAMP levels. Biol Reprod 1985; 33: 698–704. [DOI] [PubMed] [Google Scholar]
- 11.Salustri A, Petrungaro S, De Felici M, Conti M, Siracusa G. Effect of follicle-stimulating hormone on cyclic adenosine monophosphate level and on meiotic maturation in mouse cumulus cell-enclosed oocytes cultured in vitro. Biol Reprod 1985; 33: 797–802. [DOI] [PubMed] [Google Scholar]
- 12.Bornslaeger EA, Mattei P, Schultz RM. Involvement of cAMP-dependent protein kinase and protein phosphorylation in regulation of mouse oocyte maturation. Dev Biol 1986; 114: 453–462. [DOI] [PubMed] [Google Scholar]
- 13.Zhang M, Su YQ, Sugiura K, Xia G, Eppig JJ. Granulosa cell ligand NPPC and its receptor NPR2 maintain meiotic arrest in mouse oocytes. Science 2010; 330: 366–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vaccari S, Weeks JL, 2nd, Hsieh M, Menniti FS, Conti M. Cyclic GMP signaling is involved in the luteinizing hormone-dependent meiotic maturation of mouse oocytes. Biol Reprod 2009; 81: 595–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Norris RP, Freudzon M, Mehlmann LM, Cowan AE, Simon AM, Paul DL, Lampe PD, Jaffe LA. Luteinizing hormone causes MAP kinase-dependent phosphorylation and closure of connexin 43 gap junctions in mouse ovarian follicles: one of two paths to meiotic resumption. Development 2008; 135: 3229–3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Norris RP, Ratzan WJ, Freudzon M, Mehlmann LM, Krall J, Movsesian MA, Wang H, Ke H, Nikolaev VO, Jaffe LA. Cyclic GMP from the surrounding somatic cells regulates cyclic AMP and meiosis in the mouse oocyte. Development 2009; 136: 1869–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Downs SM, Hunzicker-Dunn M. Differential regulation of oocyte maturation and cumulus expansion in the mouse oocyte-cumulus cell complex by site-selective analogs of cyclic adenosine monophosphate. Dev Biol 1995; 172: 72–85. [DOI] [PubMed] [Google Scholar]
- 18.Shimada M, Nishibori M, Isobe N, Kawano N, Terada T. Luteinizing hormone receptor formation in cumulus cells surrounding porcine oocytes and its role during meiotic maturation of porcine oocytes. Biol Reprod 2003; 68: 1142–1149. [DOI] [PubMed] [Google Scholar]
- 19.Conti M, Hsieh M, Zamah AM, Oh JS. Novel signaling mechanisms in the ovary during oocyte maturation and ovulation. Mol Cell Endocrinol 2012; 356: 65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Akaki Y, Yoshioka K, Noguchi M, Hoshi H, Funahashi H. Successful piglet production in a chemically defined system for in-vitro production of porcine embryos: dibutyryl cyclic amp and epidermal growth factor-family peptides support in-vitro maturation of oocytes in the absence of gonadotropins. J Reprod Dev 2009; 55: 446–453. [DOI] [PubMed] [Google Scholar]
- 21.Funahashi H, Cantley TC, Day BN. Synchronization of meiosis in porcine oocytes by exposure to dibutyryl cyclic adenosine monophosphate improves developmental competence following in vitro fertilization. Biol Reprod 1997; 57: 49–53. [DOI] [PubMed] [Google Scholar]
- 22.Mattioli M, Galeati G, Barboni B, Seren E. Concentration of cyclic AMP during the maturation of pig oocytes in vivo and in vitro. J Reprod Fertil 1994; 100: 403–409. [DOI] [PubMed] [Google Scholar]
- 23.Shimada M, Terada T. Roles of cAMP in regulation of both MAP kinase and p34(cdc2) kinase activity during meiotic progression, especially beyond the MI stage. Mol Reprod Dev 2002; 62: 124–131. [DOI] [PubMed] [Google Scholar]
- 24.Bagg MA, Nottle MB, Grupen CG, Armstrong DT. Effect of dibutyryl cAMP on the cAMP content, meiotic progression, and developmental potential of in vitro matured pre-pubertal and adult pig oocytes. Mol Reprod Dev 2006; 73: 1326–1332. [DOI] [PubMed] [Google Scholar]
- 25.Bagg MA, Nottle MB, Armstrong DT, Grupen CG. Effect of follicle size and dibutyryl cAMP on the cAMP content and gap junctional communication of porcine prepubertal cumulus-oocyte complexes during IVM. Reprod Fertil Dev 2009; 21: 796–804. [DOI] [PubMed] [Google Scholar]
- 26.Dekel N, Kraicer PF. Induction in vitro of mucification of rat cumulus oophorus by gonadotrophins and adenosine 3,5-monophosphate. Endocrinology 1978; 102: 1797–1802. [DOI] [PubMed] [Google Scholar]
- 27.Eppig JJ. Gonadotropin stimulation of the expansion of cumulus oophori isolated from mice: general conditions for expansion in vitro. J Exp Zool 1979; 208: 111–120. [DOI] [PubMed] [Google Scholar]
- 28.Norris RP, Freudzon M, Nikolaev VO, Jaffe LA. Epidermal growth factor receptor kinase activity is required for gap junction closure and for part of the decrease in ovarian follicle cGMP in response to LH. Reproduction 2010; 140: 655–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu X, Xie F, Zamah AM, Cao B, Conti M. Multiple pathways mediate luteinizing hormone regulation of cGMP signaling in the mouse ovarian follicle. Biol Reprod 2014; 91: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang M, Su YQ, Sugiura K, Wigglesworth K, Xia G, Eppig JJ. Estradiol promotes and maintains cumulus cell expression of natriuretic peptide receptor 2 (NPR2) and meiotic arrest in mouse oocytes in vitro. Endocrinology 2011; 152: 4377–4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shores EM, Hunter MG. Immunohistochemical localization of steroidogenic enzymes and comparison with hormone production during follicle development in the pig. Reprod Fertil Dev 1999; 11: 337–344. [DOI] [PubMed] [Google Scholar]
- 32.Schoevers EJ, Colenbrander B, Roelen BAJ. Developmental stage of the oocyte during antral follicle growth and cumulus investment determines in vitro embryo development of sow oocytes. Theriogenology 2007; 67: 1108–1122. [DOI] [PubMed] [Google Scholar]
- 33.Lafontan M, Moro C, Berlan M, Crampes F, Sengenes C, Galitzky J. Control of lipolysis by natriuretic peptides and cyclic GMP. Trends Endocrinol Metab 2008; 19: 130–137. [DOI] [PubMed] [Google Scholar]
- 34.Armani A, Marzolla V, Rosano GM, Fabbri A, Caprio M. Phosphodiesterase type 5 (PDE5) in the adipocyte: a novel player in fat metabolism? Trends Endocrinol Metab 2011; 22: 404–411. [DOI] [PubMed] [Google Scholar]
- 35.Sanchez-Lazo L, Brisard D, Elis S, Maillard V, Uzbekov R, Labas V, Desmarchais A, Papillier P, Monget P, Uzbekova S. Fatty acid synthesis and oxidation in cumulus cells support oocyte maturation in bovine. Mol Endocrinol 2014; 28: 1502–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]