Abstract
A randomized double-blind trial with a 7-valent pneumococcal conjugate vaccine was conducted in The Netherlands among 383 children, aged 1 to 7 years, with a history of recurrent acute otitis media. No effect of vaccination on the pneumococcal colonization rate was found. However, a shift in serotype distribution was clearly observed (R. Veenhoven et al., Lancet 361:2189-2195, 2003). We investigated the molecular epidemiology of 921 pneumococcal isolates retrieved from both the pneumococcal vaccine (PV) and control vaccine (CV) groups during the vaccination study. Within individuals a high turnover rate of pneumococcal restriction fragment end labeling genotypes, which was unaffected by vaccination, was observed. Comparison of the genetic structures before and after completion of the vaccination scheme revealed that, despite a shift in serotypes, there was clustering of 70% of the pneumococcal populations. The remaining isolates (30%) were equally observed in the PV and CV groups. In addition, the degree of genetic clustering was unaffected by vaccination. However, within the population genetic structure, nonvaccine serotype clusters with the serotypes 11, 15, and 23B became predominant over vaccine-type clusters after vaccination. Finally, overall pneumococcal resistance was low (14%), and, albeit not significant, a reduction in pneumococcal resistance as a result of pneumococcal vaccination was observed. Molecular surveillance of colonization in Dutch children shows no effect of pneumococcal conjugate vaccination on the degree of genetic clustering and the genetic structure of the pneumococcal population. However, within the genetic pneumococcal population structure, a clear shift toward nonvaccine serotype clusters was observed.
Streptococcus pneumoniae is worldwide one of the major bacterial causes of invasive disease and respiratory tract infections in children. Risk groups for pneumococcal infections are young children, elderly, and immune-deficient patients. Despite adequate antibiotic treatment, morbidity and mortality due to pneumococcal disease remain high (5). Moreover, the increasing (multi)drug resistance among pneumococcal isolates hampers adequate treatment (6, 11, 21, 30). New pneumococcal conjugate vaccines have been shown to be effective against invasive diseases in young children (3). Furthermore, a protective effect against respiratory tract infections such as (recurrent) otitis media has been observed (3, 15). Thus far, the 7-valent pneumococcal conjugate vaccine Prevnar (Wyeth) has been approved by the U.S. Food and Drug Administration and the European Committee on Proprietary Medicinal Products and is recommended by the U.S. Advisory Committee on Immunization Practices for the prevention of invasive diseases in children under 2 years of age. Recommendations are also made for older children at increased risk for invasive disease, such as those with human immunodeficiency virus infection and asplenia and those with increased risk for pneumococcal mucosal disease, such as children with recurrent acute otitis media (1). We recently studied the effect of a 7-valent pneumococcal conjugate vaccine followed by a 23-valent polysaccharide vaccine in children aged 1 to 7 years with a history of recurrent acute otitis media (32). Clinically, no protective effect of the pneumococcal vaccines on recurrence of acute otitis media was found. At the nasopharyngeal level, however, a significant reduction of colonization with vaccine-type pneumococci was found after vaccination, whereas a simultaneous increase in colonization with nonvaccine serotypes was observed (32).
In this study, we investigated the molecular epidemiological dynamics and resistance profiles of the pneumococcal isolates collected from both children in the pneumococcal vaccine (PV) and the hepatitis control vaccine (CV) groups in order to obtain insight into the effect of conjugate vaccination on the genetic pneumococcal population structure.
MATERIALS AND METHODS
Bacterial isolates.
In total, 383 children, aged 1 to 7 years, suffering from recurrent acute otitis media, were enrolled in this double-blind randomized vaccination trial in the period April 1998 to December 2001 (32). One hundred ninety children received a 7-valent pneumococcal conjugate vaccine, either once for children 24 months of age and older (Prevnar; Wyeth Lederle) or twice for children 12 to 24 months of age, followed by a 23-valent pneumococcal polysaccharide vaccine after 6 months for all children (Pneumune; Wyeth Lederle). The 193 control children received, depending on the age, hepatitis B vaccine (Engerix-B; SmithKline Beecham) three times or hepatitis A vaccine (Havrix junior; SmithKline Beecham) twice. Nasopharyngeal cultures were performed at study entry; just before booster vaccination at 7 months; and at 14, 20, and 26 months. An additional nasopharyngeal sample was obtained at the first acute otitis media event after full vaccination. Pneumococcal carriage was observed in around 50% of all children. This carriage rate was maintained in both study groups in the 26-month follow-up period. Thus, no influence on overall colonization was observed during the study. Instead a decline in vaccine serotype carriage was observed in the PV group whereas the nonvaccine serotype carriage increased (32). Nine hundred twenty-one isolates (95%) from 353 out of 383 patients participating in this study were available for further analysis by genotyping and susceptibility testing.
Bacteriological procedures.
Isolation and identification of the S. pneumoniae isolates were performed by standard methods as described previously (32). Susceptibility testing was performed by the agar dilution method (25). Resistance was defined by the breakpoint concentrations for the respective antibiotics as defined by the NCCLS (26). Multidrug resistance was defined as resistance to three or more classes of antimicrobial agents.
Serotyping.
Pneumococci were serotyped by the method of capsular swelling (Quellung reaction) with commercially available antisera (Statens Serum Institute, Copenhagen, Denmark).
RFEL typing.
Pneumococcal strain typing by restriction fragment end labeling (RFEL) was done as described by van Steenbergen et al. (31) and adapted by Hermans et al. (19). Briefly, purified pneumococcal DNA was digested by the restriction enzyme EcoRI. The DNA restriction fragments were end labeled at 72°C with [α-32P]dATP by using DNA polymerase (Goldstar; Eurogentec, Seraing, Belgium). After the radiolabeled fragments were denatured and separated electrophoretically on a 6% polyacrylamide sequencing gel containing 8 M urea, the gel was transferred onto filter paper, vacuum dried (HBI, Saddlebrook, N.Y.), and exposed for variable times at room temperature to ECL hyperfilm (Amersham Laboratories, Amersham, United Kingdom).
Computer-assisted analysis of DNA band patterns.
RFEL autoradiographs were converted to images (Image Master DTS; Pharmacia Biotech, Uppsala, Sweden) and analyzed by computer (Windows Gelcompar software, version 4; Applied Math., Kortrijk, Belgium). DNA fragments were analyzed as described previously (27). For evaluation of the genetic relatedness of the isolates we used the following definitions: (i) isolates of a particular RFEL type are 100% identical by RFEL analysis; (ii) an RFEL cluster represents a group of RFEL types that differ in only one band (approximately >95% genetic relatedness). A RFEL genotype that is found only once in the collection is defined as a unique RFEL type. A RFEL type that is found twice or more, or that is more than 95% related to another RFEL type, is defined as a clustering genotype.
MLST.
The RFEL genotypes of 34 isolates representing different serotypes were investigated by multilocus sequence typing (MLST) analysis. Within the 23 largest clusters, representing 27 RFEL genotypes, the most prevalent serotypes were analyzed. For this purpose, a fully automated method for MLST was used as described previously (20). The MLST types were compared with the global PMEN database (http://spneumoniae.mlst.net/pmen/pmen.asp).
Data analysis.
P values for differences were calculated with the chi-square test using Prism, version 3.00 for Windows (GraphPad Software, San Diego, Calif.).
RESULTS
Nine hundred twenty-one pneumococcal isolates from nasopharyngeal samples of 351 children participating in the study were available for molecular analysis by RFEL. In total, 450 of these 921 pneumococcal isolates were isolated from children in the PV group, whereas 471 isolates were isolated from children in the CV group. Of the 921 isolates, 180 isolates were isolated at the start of the study (time [T] = 0 months; day of [first] conjugate vaccination); 176 isolates were retrieved at 7 months (day of booster vaccination); and 186, 163, and 119 isolates were isolated after completion of the vaccination scheme at 14, 20, and 26 months of study duration, respectively (Table 1). The remaining 97 isolates were retrieved at the first acute otitis media events after full vaccination (>7 months). The serotype distribution of all pneumococcal isolates collected during this study was discussed previously (32). In summary, the contribution of vaccine serotype pneumococci to colonization gradually declined from 46% at study entry to 26% at the end of the study compared to the CV group, in which the contribution of the conjugate vaccine serotypes remained approximately 50% whereas the total pneumococcal carriage rate remained unaffected.
TABLE 1.
Pneumococcal isolates with regard to study group and study phase
| Phase | Total no. of isolates | PV group
|
CV group
|
||
|---|---|---|---|---|---|
| No. of isolates | % Vaccine-type strains | No. of isolates | % Vaccine-type strains | ||
| All | 921 | 451 | 470 | ||
| 0 mo | 180 | 96 | 46 | 84 | 58 |
| 7 mo | 176 | 81 | 41 | 95 | 46 |
| 14 mo | 186 | 84 | 26 | 102 | 40 |
| 20 mo | 163 | 81 | 25 | 82 | 49 |
| 26 mo | 119 | 64 | 26 | 55 | 54 |
| First AOMa | 97 | 45 | 24 | 52 | 44 |
AOM, acute otitis media.
All 921 isolates were characterized by RFEL. We identified 275 different RFEL genotypes representing 1 to 49 isolates per genotype, with an average of 3.35 isolates per genotype. Analysis of the per-patient follow-up revealed a high turnover rate of pneumococcal RFEL genotypes; persistent carriage for at most three consecutive samples (recurrence twice after a 6- to 7-month interval) was found in only 54 out of the 351 children (Table 2). No statistical difference in the rate of persistent carriage between the pneumococcal vaccine group and the control group children was found (15 versus 16%; P = 0.67). In both groups, the majority of the persistent isolates (60%) had nonvaccine serotypes. On three occasions, persistence of a specific genotype was accompanied by a switch in serotype. In one case, colonization with an isolate having one particular genotype which is closely related to the MLST type displaying serotype 15 was followed 6 months later by colonization with an isolate having a second genotype of serotype 19A. Another 6 months later the initial genotype with a capsular switch to serotype 19A was identified. In the second case a switch from serotype 14 to serotype 8 within the same genotype was observed after a 6-month interval. The third case represented a capsular switch from serotype 6A to serotype 19F, observed after a 6-month interval.
TABLE 2.
Pneumococcal colonization dynamics of the 351 children according to study and age groupa
| Group, age range, and patient no. | Type of pneumococci carried at:
|
|||||
|---|---|---|---|---|---|---|
| Mo 0 | Mo 7 | Mo 14 | Mo 20 | Mo 26 | First AOMb event | |
| PV | ||||||
| 12-24 mo | ||||||
| 1 | 1 | 1 | 1 | 0 | ||
| 2 | 1 | 1 | 1 | 1 | 1 | |
| 3 | 1 | 1 | ||||
| 4 | 1 | 1 | ||||
| 5 | 1 | 1 | 1 | 1 | ||
| 6 | 1 | 1 | 1 | |||
| 7 | 1 | 0 | ||||
| 8 | 0 | 0 | 0 | 0 | 1 | |
| 9 | 1 | |||||
| 10 | 1 | |||||
| 11 | 0 | 1 | 0 | |||
| 12 | 0 | 0 | ||||
| 13 | 1 | 0 | 1 | |||
| 14 | 1 | 1 | 0 | |||
| 15 | 1 | 1 | 0 | |||
| 16 | 1 | |||||
| 17 | 0 | 0 | 1 | |||
| 18 | 0 | |||||
| 19 | 1 | 0 | 1 | 1 | ||
| 20 | 0 | 1 | ||||
| 21 | 0 | 1 | ||||
| 22 | 0 | 0 | 1 | 0 | ||
| 23 | 0 | 1 | 1 | |||
| 24 | 0 | 0 | ||||
| 25 | 1 | 1 | 0 | |||
| 26 | 0 | 1 | ||||
| 27 | 1 | 1 | ||||
| 28 | 1 | 1 | 1 | 1 | ||
| 29 | 1 | 0 | 1 | 1 | ||
| 30 | 0 | 0 | 1 | |||
| 31 | 0 | 1 | ||||
| 32 | 1 | |||||
| 33 | 1 | 1 | 1 | 1 | ||
| 34 | 0 | 0 | 1 | 0 | 1 | |
| 35 | 0 | 1 | ||||
| 36 | 0 | 0 | 0 | |||
| 37 | 0 | 1 | 1 | 1 | 1 | |
| 38 | 0 | 0 | 1 | 1 | 1 | 1 |
| 39 | 1 | 1 | 1 | |||
| 40 | 1 | |||||
| 41 | 1 | 1 | 1 | |||
| 42 | 1 | |||||
| 43 | 1 | |||||
| 44 | 0 | |||||
| 45 | 0 | 0 | 1 | 1 | ||
| 46 | 1 | 1 | 1 | |||
| 47 | 1 | |||||
| 48 | 1 | 1 | ||||
| 49 | 1 | 1 | ||||
| 50 | 1 | 1 | ||||
| 51 | 1 | 0 | 1 | 0 | ||
| 52 | 0 | 0 | ||||
| 53 | 1 | 0 | 1 | 0 | ||
| 54 | 0 | |||||
| 55 | 1 | 1 | 1 | 1 | ||
| 56 | 0 | 1 | ||||
| 57 | 0 | 1 | ||||
| 58 | 1 | 1 | 1 | |||
| 59 | 0 | |||||
| 60 | 0 | 1 | 1 | |||
| 61 | 1 | 0 | ||||
| 62 | 1 | 1 | ||||
| 63 | 1 | 1 | ||||
| 64 | 0 | |||||
| 65 | 1 | |||||
| 66 | 1 | 1 | 1 | |||
| 67 | 0 | 1 | 1 | |||
| 68 | 0 | 0 | ||||
| 69 | 0 | |||||
| 70 | 1 | |||||
| 71 | 0 | 1 | ||||
| 72 | 0 | 1 | 1 | 1 | ||
| 73 | 1 | 0 | ||||
| 74 | 1 | 0 | ||||
| 2-7 yr | ||||||
| 75 | 0 | 1 | 0 | 0 | 1 | |
| 76 | 1 | 1 | ||||
| 77 | 0 | |||||
| 78 | 0 | 1 | ||||
| 79 | 1 | |||||
| 80 | 0 | 0 | 0 | 0 | 1 | |
| 81 | 1 | 0 | 1 | |||
| 82 | 1 | 0 | 1 | |||
| 83 | 1 | 0 | ||||
| 84 | 1 | 1 | 1 | |||
| 85 | 1 | 0 | ||||
| 86 | 1 | 1 | 1 | 1 | ||
| 87 | 1 | 0 | 1 | |||
| 88 | 1 | 1 | 1 | 1 | 1 | |
| 89 | 1 | 1 | 1 | |||
| 90 | 1 | |||||
| 91 | 1 | 1 | ||||
| 92 | 0 | |||||
| 93 | 0 | 1 | 1 | 1 | ||
| 94 | 1 | |||||
| 95 | 1 | 1 | 1 | |||
| 96 | 1 | |||||
| 97 | 1 | 1 | ||||
| 98 | 0 | |||||
| 99 | 1 | 1 | 1 | 1 | ||
| 100 | 0 | 1 | 1 | 0 | ||
| 101 | 0 | 0 | 0 | |||
| 102 | 1 | 0 | 1 | 1 | ||
| 103 | 1 | 0 | ||||
| 104 | 0 | |||||
| 105 | 0 | 0 | 0 | |||
| 106 | 1 | 1 | 1 | |||
| 107 | 1 | 0 | ||||
| 108 | 0 | 0 | ||||
| 109 | 1 | 1 | ||||
| 110 | 1 | 1 | 1 | |||
| 111 | 0 | 1 | ||||
| 112 | 1 | 1 | ||||
| 113 | 0 | 1 | 1 | 1 | 0 | |
| 114 | 1 | |||||
| 115 | 1 | 0 | ||||
| 116 | 1 | 1 | 1 | |||
| 117 | 1 | 0 | 0 | 0 | ||
| 118 | 1 | 1 | 1 | 1 | ||
| 119 | 1 | 1 | 1 | |||
| 120 | 1 | 1 | 0 | |||
| 121 | 1 | 1 | 1 | |||
| 122 | 0 | 0 | ||||
| 123 | 1 | 1 | 1 | 1 | 1 | |
| 124 | 1 | 0 | 1 | 1 | 1 | |
| 125 | 0 | 1 | ||||
| 126 | 0 | 0 | 0 | 1 | ||
| 127 | 1 | 1 | ||||
| 128 | 0 | 1 | 1 | 1 | 1 | |
| 129 | 1 | 1 | ||||
| 130 | 0 | 1 | 0 | 1 | 1 | |
| 131 | 1 | |||||
| 132 | 1 | 1 | 0 | 0 | ||
| 133 | 1 | 1 | ||||
| 134 | 1 | 1 | 1 | 0 | 1 | |
| 135 | 0 | 1 | ||||
| 136 | 1 | 1 | 1 | |||
| 137 | 1 | 1 | 0 | 0 | ||
| 138 | 0 | 1 | ||||
| 139 | 0 | 1 | ||||
| 140 | 1 | 1 | 1 | |||
| 141 | 1 | 1 | 0 | |||
| 142 | 1 | 1 | 1 | 1 | 1 | |
| 143 | 1 | 1 | 1 | 0 | ||
| 144 | 1 | 0 | ||||
| 145 | 1 | 1 | 1 | |||
| 146 | 1 | 1 | ||||
| 147 | 0 | 1 | 1 | |||
| 148 | 0 | 0 | ||||
| 149 | 0 | 1 | ||||
| 150 | 1 | 1 | 1 | |||
| 151 | 1 | |||||
| 152 | 0 | 1 | ||||
| 153 | 0 | 1 | ||||
| 154 | 1 | 0 | ||||
| 155 | 1 | |||||
| 156 | 1 | 0 | ||||
| 157 | 1 | 1 | ||||
| 158 | 1 | |||||
| 159 | 1 | 1 | 1 | |||
| 160 | 1 | 0 | 0 | 1 | ||
| 161 | 1 | 1 | 1 | |||
| 162 | 0 | 0 | 1 | 0 | 1 | |
| 163 | 1 | 0 | 0 | |||
| 164 | 0 | 1 | ||||
| 165 | 0 | |||||
| 166 | 1 | 0 | 1 | |||
| 167 | 0 | 0 | ||||
| 168 | 1 | 1 | ||||
| 169 | 1 | 0 | ||||
| 170 | 1 | |||||
| 171 | 1 | 0 | ||||
| CV | ||||||
| 12-24 mo | ||||||
| 1 | 0 | 0 | 0 | 0 | ||
| 2 | 0 | 0 | 0 | 1 | ||
| 3 | 0 | |||||
| 4 | 1 | 1 | 1 | |||
| 5 | 0 | 0 | 0 | 1 | ||
| 6 | 0 | 0 | ||||
| 7 | 0 | 1 | 0 | |||
| 8 | 0 | 1 | 1 | 0 | ||
| 9 | 1 | 0 | ||||
| 10 | 1 | 0 | ||||
| 11 | 1 | 1 | ||||
| 12 | 1 | 0 | 0 | 0 | ||
| 13 | 1 | 1 | 0 | 0 | ||
| 14 | 0 | |||||
| 15 | 0 | 0 | ||||
| 16 | 0 | 1 | 0 | 1 | 1 | |
| 17 | 0 | 1 | 0 | |||
| 18 | 1 | 1 | 0 | |||
| 19 | 1 | 1 | 1 | 1 | 0 | |
| 20 | 1 | 0 | ||||
| 21 | 0 | 1 | 1 | |||
| 22 | 0 | |||||
| 23 | 0 | 0 | ||||
| 24 | 1 | 0 | 1 | |||
| 25 | 1 | |||||
| 26 | 1 | 1 | ||||
| 27 | 1 | 0 | ||||
| 28 | 1 | 1 | 1 | 0 | 1 | 1 |
| 29 | 1 | 0 | 1 | 0/1 | ||
| 30 | 0 | 1 | 1 | |||
| 31 | 1 | 0 | 0 | |||
| 32 | 1 | 0 | 1 | 0 | 1 | |
| 33 | 0 | |||||
| 34 | 1 | 1 | ||||
| 35 | 0 | |||||
| 36 | 0 | 0 | ||||
| 37 | 0 | 1 | 0 | |||
| 38 | 0 | 0 | 1 | 0 | 0 | 1 |
| 39 | 1 | |||||
| 40 | 0 | 0 | 0/1 | |||
| 41 | 0 | 1 | 0 | 1 | 0 | |
| 42 | 1 | 0 | 0 | 1 | ||
| 43 | 1 | 1 | 0 | 1 | ||
| 44 | 0 | 0 | 0 | |||
| 45 | 0 | 1 | 0 | |||
| 46 | 0 | 0 | 1 | 0 | 0 | 0 |
| 47 | 0 | 0 | 0 | 0 | 0 | |
| 48 | 1 | 1 | 0 | 0 | ||
| 49 | 1 | |||||
| 50 | 0 | 0 | ||||
| 51 | 0 | 1 | ||||
| 52 | 1 | |||||
| 53 | 0 | |||||
| 54 | 0 | 1 | 0 | |||
| 55 | 1 | 0 | 0/1 | |||
| 56 | 0 | 0 | 0 | 0 | 0 | |
| 57 | 0 | |||||
| 58 | 0 | |||||
| 59 | 1 | |||||
| 60 | 0 | 1 | ||||
| 61 | 0 | 0 | 1 | |||
| 62 | 0 | |||||
| 63 | 1 | 0 | 0 | 0 | ||
| 64 | 1 | |||||
| 65 | 0 | 1 | ||||
| 66 | 1 | 0 | 1 | |||
| 67 | 0 | |||||
| 68 | 0 | 1 | ||||
| 69 | 1 | 1 | ||||
| 70 | 1 | 1 | 1 | |||
| 71 | 0 | 0 | 0 | |||
| 72 | 1 | 1 | ||||
| 73 | 0 | 0 | 0 | 0 | ||
| 74 | 1 | |||||
| 75 | 1 | 0 | ||||
| 76 | 1 | 0 | ||||
| 2-7 yr | ||||||
| 77 | 0 | 1 | 0 | 1 | ||
| 78 | 1 | |||||
| 79 | 0 | |||||
| 80 | 1 | |||||
| 81 | 1 | |||||
| 82 | 1 | 0 | 0 | |||
| 83 | 1 | 0 | ||||
| 84 | 0 | 0 | 0 | 1 | 1 | |
| 85 | 0 | 0 | 1 | |||
| 86 | 1 | 0 | ||||
| 87 | 1 | 0 | 1 | 1 | ||
| 88 | 0 | 1 | ||||
| 89 | 1 | 0 | 0 | |||
| 90 | 1 | 0 | 1 | |||
| 91 | 1 | 1 | 1 | |||
| 92 | 1 | 0 | ||||
| 93 | 1 | 1 | 0 | |||
| 94 | 1 | |||||
| 95 | 1 | 1 | 0 | 1 | ||
| 96 | 0 | 1 | 0 | 1 | ||
| 97 | 0 | 1 | 1 | |||
| 98 | 1 | 1 | 1 | |||
| 99 | 0 | 1 | 1 | 1 | ||
| 100 | 1 | 0 | ||||
| 101 | 0 | |||||
| 102 | 0 | 0 | 0 | 0 | ||
| 103 | 1 | |||||
| 104 | 1 | 0 | 1 | 0 | ||
| 105 | 1 | |||||
| 106 | 1 | 0 | 1 | |||
| 107 | 0 | 1/0 | ||||
| 108 | 1 | 1 | 0/1 | 0/0 | ||
| 109 | 1 | 1 | ||||
| 110 | 0 | 0 | ||||
| 111 | 0 | |||||
| 112 | 1 | |||||
| 113 | 0 | |||||
| 114 | 0 | 1 | ||||
| 115 | 0 | |||||
| 116 | 0 | 0 | 0 | 0 | ||
| 117 | 1 | 0 | 1 | |||
| 118 | 1 | 0 | ||||
| 119 | 0 | 1 | 1 | 0 | ||
| 120 | 1 | |||||
| 121 | 0 | |||||
| 122 | 1 | 1 | ||||
| 123 | 0 | 0 | ||||
| 124 | 0 | 0 | 1 | 1 | 1 | |
| 125 | 0 | 1 | 0 | 1 | ||
| 126 | 0 | 1 | 0 | |||
| 127 | 1 | 0 | ||||
| 128 | 1 | 0 | 0 | 0 | ||
| 129 | 1 | 1 | 1 | |||
| 130 | 1 | 1 | ||||
| 131 | 0 | 1 | 1 | |||
| 132 | 1 | 1 | 1 | 1 | ||
| 133 | 0 | 1 | 1 | 1 | ||
| 134 | 0 | 0 | 0 | |||
| 135 | 0 | 0 | 0 | |||
| 136 | 0 | 1 | ||||
| 137 | 1 | 0 | 0 | |||
| 138 | 0 | |||||
| 139 | 1 | 1 | 1 | 1 | ||
| 140 | 0 | 1 | 1 | 1 | 1 | 0 |
| 141 | 0 | 1 | 1 | |||
| 142 | 1 | 1 | 0 | 0 | ||
| 143 | 0 | |||||
| 144 | 1 | 1 | 1 | 1 | ||
| 145 | 0 | |||||
| 146 | 0 | 0 | 1 | 1 | ||
| 147 | 1 | 1 | ||||
| 148 | 1 | 0 | 1 | |||
| 149 | 0 | |||||
| 150 | 1 | 1 | 1 | |||
| 151 | 0 | |||||
| 152 | 0 | |||||
| 153 | 0 | 1 | 0 | |||
| 154 | 1 | 1 | ||||
| 155 | 0 | 1 | ||||
| 156 | 1 | 1 | ||||
| 157 | 1 | |||||
| 158 | 0 | 1 | 1 | 0 | ||
| 159 | 0 | 1 | 0 | 1 | ||
| 160 | 0 | |||||
| 161 | 1 | 0 | ||||
| 162 | 1 | 1 | 0 | |||
| 163 | 0 | |||||
| 164 | 0 | 1 | ||||
| 165 | 0 | 1 | 1 | |||
| 166 | 0 | 0 | 0 | 1 | ||
| 167 | 1 | 1 | 1 | |||
| 168 | 0 | 1 | ||||
| 169 | 1 | 1 | ||||
| 170 | 1 | |||||
| 171 | 1 | 1 | 1 | |||
| 172 | 1 | |||||
| 173 | 0 | 0 | 1 | |||
| 174 | 1 | 0 | ||||
| 175 | 1 | |||||
| 176 | 1 | 0 | ||||
| 177 | 0 | |||||
| 178 | 1 | 1 | 1 | |||
Boldface indicates persistent carriage. 1, vaccine-type pneumococci; 0, nonvaccine-type pneumococci. Values separated by shills indicate the results for two different isolates cultured.
AOM, acute otitis media.
We analyzed the genetic relatedness of the pneumococcal isolates retrieved at the start of the study and 14 months after the initial vaccination from both study groups. The 180 pneumococci isolated at the start of the study displayed 93 RFEL genotypes, representing 52 unique RFEL genotypes and 30 clusters (128 isolates), with an average cluster size of 4.3. The 186 isolates isolated 14 months after the start of the study displayed 105 RFEL genotypes, representing 54 unique RFEL genotypes and 29 clusters (132 isolates), with an average cluster size of 4.6. Close homology (≥95% genetic relatedness) among 70% of the isolates was found at both time points (T = 0 months and T = 14 months). The remaining RFEL genotypes equally represented strains from either PV or CV children (49 and 51%, respectively). The four most predominant clusters at the start of the study were cluster I (7.8% of all isolates; serotypes 6A and 6B), cluster II (7.3%; serotype 14), cluster III (4.5%; serotype 23F), and cluster IV (4.5%; serotype 9V). These clusters were still predominant 14 months after vaccination, though slightly reduced in size (4.8, 4.3, 4.3, and 2.2%, respectively) and mainly observed in CV group children (Fig. 1). In addition, five minor clusters observed in the initial phase of the study, cluster A (2.8% of all isolates; serotype 11), cluster B (1.1%; serotype 11), cluster C (2.8%; serotype 15), cluster D (2.8%; serotype 16), and cluster E (1.7%; serotype 23B), became predominant clusters 14 months after vaccination with prevalences of 4.8, 4.3, 4.3, 5.4, and 3.8%, respectively. The first two clusters, which had 85% homology, were predominantly present in PV children (89 and 63%, respectively) (Fig. 1).
FIG. 1.
Population RFEL structure of the 180 and 186 pneumococcal isolates retrieved before vaccination (T = 0 months) and after pneumococcal conjugate vaccination (T = 14 months), respectively. Levels of genetic relatedness are depicted as percentages. The serotypes and numbers of isolates per genotype are shown in bars for the two periods separately. The contributions of PV children and CV children are shown in grey and black, respectively. I to IV, predominant clusters at the initial phase of the study; A to E, emerging clusters after conjugate vaccination. Clusters consisting of two or more RFEL genotypes are in parentheses. x, clusters of one RFEL genotype.
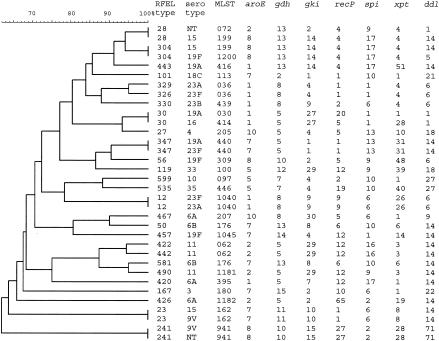
We investigated 34 isolates representing different serotypes by MLST analysis. Within the 23 largest clusters, representing 27 RFEL genotypes, the most prevalent serotypes were analyzed (Fig. 2). We found no PMEN clones in our collection of pneumococci. However, we observed close homology with several of the PMEN clones, i.e., three single-locus variants of the PMEN clones France 9V-3 (ST 162), Tennessee 23F-4 (ST 439), and Portugal 19F-21 (ST 1045) and one double-locus variant of the PMEN clone Hungary 19A-16 (ST 176). In addition, we observed two new MLST genotypes (ST 1181 and ST 1182). In general, the observed homology by RFEL genotyping was confirmed by MLST, except for RFEL genotype 028, for which RFEL genotyping was demonstrated to be less discriminatory than MLST analysis (Fig. 2).
FIG. 2.
RFEL dendrogram of the 34 MLST sequence types observed within the 29 largest RFEL clusters.
We determined the MICs of penicillin, cotrimoxazole, tetracycline, erythromycin, rifampin, vancomycin, and ciprofloxacin for 919 isolates. Resistance to at least one antibiotic was found for 128 pneumococcal isolates (14%). High-level resistance to penicillin, cotrimoxazole, tetracycline, erythromycin, and ciprofloxacin was found in 2 (0.2%), 37 (4.0%), 28 (3.0%), 29 (3.2%), and 2 (0.2%) isolates, respectively. Furthermore, intermediate resistance to penicillin and cotrimoxazole was found in 8 (0.9%) and 61 (6.6%) isolates, respectively. Intermediate resistance to tetracycline, erythromycin, and cefotaxime was seen only occasionally (Table 3). No cases of resistance to rifampin and vancomycin were identified. In Table 4, the resistance profiles and the associated rates, serotypes, and numbers of RFEL genotypes are listed. In total, 21 different resistance profiles were observed. We observed (intermediate) resistance to a single drug in 99 isolates (10.7% of all isolates), dual resistance in 17 isolates (1.8%), and multidrug resistance (resistance to three or more antibiotics) in 12 isolates (1.3%).
TABLE 3.
Antibiotic resistance rates for the S. pneumoniae collection
| Drug | No. of resistant strains | % Of total | No. of intermediately resistant strains | % Of total |
|---|---|---|---|---|
| Penicillin | 2 | 0.2 | 8 | 0.9 |
| Cotrimoxazole | 37 | 4.0 | 61 | 6.6 |
| Tetracycline | 28 | 3.0 | 5 | 0.5 |
| Erythromycin | 29 | 3.2 | 1 | 0.1 |
| Ciprofloxacin | 2 | 0.2 | 0 | |
| Cefotaxime | 0 | 2 | 0.2 |
TABLE 4.
S. pneumoniae antibiotic resistance profiles, profile rates, and numbers of genotypes and their serotype distribution
| Resistance profilea | No. of strains (%) | No. of genotypes | Serotype(s) |
|---|---|---|---|
| Co(I) | 53 (5.7) | 29 | 3/7/8/10/11/12/14/18C/18F/6A/6B/9A/9V/19A/19F/23F/31/33D/NTb |
| Co | 25 (2.7) | 16 | 6A/6B/9V/18C/21/23F/NT/34 |
| T | 7 (0.8) | 6 | 3/19C/19F |
| T(I) | 4 (0.4) | 4 | 23F/18C/11/38 |
| T(I)E | 1 (0.1) | 1 | 33 |
| Tci | 1 (0.1) | 1 | 19F |
| TE | 7 (0.8) | 6 | 9N/19F |
| E | 8 (0.9) | 6 | 11/14/15/33 |
| E(I) | 1 (0.1) | 1 | 23B |
| Co(I)E | 2 (0.2) | 2 | 6A/8 |
| Co(I)T | 1 (0.1) | 1 | 6B |
| Co(I)TE | 1 (0.1) | 1 | 6B |
| CoT | 2 (0.2) | 2 | 23A/19F |
| CoTE | 5 (0.5) | 4 | 6A/6B |
| P(I) | 1 (0.1) | 1 | 11 |
| P(I)CoCf(I) | 1 (0.1) | 1 | 14 |
| P(I)Co(I)ECf(I) | 1 (0.1) | 1 | 6B |
| P(I)CoTE | 3 (0.3) | 3 | 19F/6B/14 |
| PCo(I) | 1 (0.1) | 1 | 15 |
| P(I)Co(I) | 2 (0.2) | 2 | 14/23F |
| PCoTE | 1 (0.1) | 1 | 6B |
| PE | 1 (0.1) | 1 | 6A |
| Total | 128 (13.8) |
Co, cotrimoxazole; P, penicillin; T, tetracycline; E, erythromycin; Cf, cefotaxime; Ci, ciprofloxacin; (I), intermediate resistance.
NT, nontypeable.
To evaluate the effect of vaccination on pneumococcal resistance, we compared resistance rates before full vaccination (samples at study entry and at the 7-month point) and after full vaccination (samples at 14, 20, and 26 months). In the PV children resistance declined from 17.5% before full vaccination to 11.8% after full vaccination, whereas in the CV children resistance was stable (14.5 and 14.3% before and after full vaccination, respectively). This difference did not reach statistical significance. We also evaluated the serotype distribution among the resistant isolates. Fifty-seven percent of all resistant isolates displayed vaccine serotypes. In addition, 10 out of the 12 multidrug-resistant isolates (83%) were vaccine serotype isolates, whereas the remaining two isolates displayed the cross-reactive serotype 6A.
DISCUSSION
Between 1998 and 2002 a large randomized, double-blind vaccination trial with a 7-valent pneumococcal conjugate vaccine followed by a 23-valent polysaccharide vaccine was performed with 383 children, aged 1 to 7 years, with a history of recurrent acute otitis media. Surprisingly, no beneficial effect on the frequency of acute otitis media after pneumococcal vaccination or on the overall colonization rate of S. pneumoniae was observed. However, a shift of vaccine-type pneumococci to nonvaccine-type pneumococci was observed among nasopharyngeal colonization isolates. Emerging nonconjugate vaccine serotypes were serotypes 11, 15, and 16 (32). We questioned whether this shift occurred within specific RFEL genotypes or whether replacement with genetically different strains took place. If the latter was true, we wondered if these different RFEL genotypes were equally capable of horizontal dissemination and whether they represented comparable antibiotic resistance profiles.
Therefore, we analyzed the 921 pneumococcal isolates retrieved from 351 of the 383 participating children. We observed 275 different RFEL genotypes, representing 106 genetic clusters and 75 unique RFEL genotypes. Analyzing the per-patient follow-up revealed few episodes of persistent carriage. This implies that pneumococcal colonization is a dynamical process with a high turnover rate of colonizing strains. No effect of vaccination on the limited rate of persistent strains was found. This was to be expected, because the majority of the persistent strains in both the PV and CV groups represented nonvaccine serotypes.
Remarkably, in three cases of persistent carriage, a serotype switch was observed. However, one could argue whether consecutive colonization with the serotype 15 and serotype 19A variants of a strain closely related to MLST 199 suggests the recruitment of a second isolate with identical genotype rather than a capsular switch. However, in contrast to the situation observed in many countries, including the United States, this genotype is not very common in The Netherlands (3%), and a 19A serotype variant had not been observed before. Although a strain switch cannot be excluded, we believe that these data are suggestive of a capsular switch. So far, this phenomenon, in vivo, has been reported only twice, by Barnes et al. (2) and Sluijter et al. (27). Indirect proof for a capsular switch was previously shown by other investigators, who demonstrated the true recombinational exchanges at the capsular locus (7-9, 24). One might argue that our observations are events enhanced by conjugate vaccination due to the induction of a selective immunological pressure. Indeed, the serotype 15-to-19A switch was observed in a PV child, but no conjugate vaccine-type pneumococci were involved. The two additional cases (serotype 6A-to-19F and 14-to-8 switches) were observed in CV children. Although our data support the theory that serotype switching is a natural process which can be observed occasionally within an individual, a large number of data will be required to study the impact of conjugate vaccination on this process.
Comparison of the genetic structures of the pneumococci isolated at study entry and at 14 months after pneumococcal conjugate vaccination showed 70% homology among the pneumococcal isolates at the two time points. The nonoverlapping isolates were equally distributed among PV and CV children. Furthermore, initially predominant clusters displaying vaccine serotypes had been partially replaced by nonvaccine serotype clusters after vaccination, which displayed a similar capability to spread horizontally. Though replacement by nonvaccine serotypes as a result of increasing age has been proven to occur (12), we observed this shift significantly more often in children who received the pneumococcal vaccines, indicating that this process is enhanced by vaccination.
Our most predominant clusters represented multiple vaccine and nonvaccine serotypes. Since the observed genetic homology was confirmed by MLST analysis, our data suggest that a large number of recombinational events at the capsular loci have occurred within these clusters. This is in line with previous data from the United States and Latin America, where the major (resistant) clones also show multiple serotypes as a result of capsular serotype switch (9, 10, 16, 22, 29). Wolf et al. (33) have shown that these events occur even more often in susceptible pneumococcal clones, which is in line with our findings.
Our data support the hypothesis that serotype replacement observed after conjugate vaccination does not directly indicate a shift in the genetic structure of the pneumococcal population. Shifts toward and the predominance of nonvaccine serotype variants are likely to occur within genetic clusters displaying both vaccine and nonvaccine serotypes. However, MLST analysis of the most predominant clusters of our collection of pneumococcal strains showed the presence of several new genotypes and the absence of PMEN homologous clones. Therefore, this collection of strains might not be representative and predictive for countries where multidrug-resistant clones are predominantly present.
To evaluate whether vaccination will have an effect on the presence of antibiotic resistance, we determined the antibiotic resistance profiles of all 921 isolates. Susceptibility testing of 919 of the 921 pneumococcal isolates was performed for penicillin, cotrimoxazole, tetracycline, erythromycin, rifampin, vancomycin, cefotaxime, and ciprofloxacin. In agreement with previous studies performed in The Netherlands, the overall resistance is low (14% of the isolates) compared to that found in other European countries (4, 13, 14, 17, 18). Penicillin resistance was found rarely in our study. In contrast, we most frequently observed resistance to cotrimoxazole, tetracycline, and erythromycin. We compared our data with a previous study performed in The Netherlands, where 10,489 clinical pneumococcal isolates have been tested for drug susceptibility (14). Compared to results from this study (reference year, 1999), we noted a higher incidence of cotrimoxazole resistance (4.4 versus 10.6%) and a lower incidence of tetracycline resistance (3.5 versus 6.6%). Both observations can be explained by the age difference between the study groups; our study was performed with children under 7 years of age, whereas the surveillance study represented all age groups, including adults. Tetracyclines, routinely given to adults, are contraindicated in children whereas cotrimoxazole is often the first-choice treatment.
In a comparison of our study population to the surveillance study population, we found equal percentages of (intermediate) resistance to a single drug (77% of the resistant isolates), comparable levels of dual resistance (13 and 19%, respectively), and significantly higher levels of multidrug resistance (9 and 4%, respectively; P < 0.01). We hypothesize that our children might select for multidrug-resistant strains because of higher antibiotic consumption, which is in accordance with previous findings (23, 28).
In addition, we analyzed changes in the incidence of pneumococcal resistance. In this respect, we compared levels of resistance among the pneumococcal isolates between the initial phases of the study (before full vaccination) and the postvaccination phases. Although a decline in resistance from 17.5 to 11.8% was seen, this was not statistically significant. Because of the low resistance rates, no subsidiary analysis could be performed for the separate sample dates. Therefore, we analyzed the serotype distribution among the resistant isolates; this analysis showed that the serotype distribution for 57% of the resistant isolates is comparable to the overall serotype distribution. However, all multidrug-resistant isolates had vaccine types or cross-reactive serotypes. Although resistance is low among S. pneumoniae in The Netherlands, these data imply that vaccination with the 7-valent conjugate vaccine may reduce pneumococcal resistance in the population, particularly multidrug resistance.
In conclusion, pneumococcal conjugate vaccination did not induce a shift in the population-based structure of the pneumococci or decrease their tendency to spread horizontally. Our observations combined with the vaccine efficacy data of Veenhoven et al. (32) suggest that pneumococcal conjugate vaccination is not very useful for prevention of pneumococcal colonization in children above 1 year of age. Moreover, we strongly advise continuous and close monitoring of the pneumococcal genetic structure in areas with a conjugate vaccination policy.
Acknowledgments
We thank C. P. Elzenaar (National Institute for Public Health and the Environment, Bilthoven, The Netherlands), J. Bruin (Regional Laboratory of Public Health, Haarlem, The Netherlands), and N. Lemmens-den Toom (Department of Medical Microbiology and Infectious Diseases, Erasmus MC, Rotterdam, The Netherlands) for their technical assistance. Furthermore, we express our gratitude to E. P. IJzerman (Regional Laboratory of Public Health, Haarlem, The Netherlands) for microbiological advice.
This study was sponsored by the Sophia Foundation for Medical Research, The Netherlands (grant 268), and the Dutch Science Foundation (grant SGO-Inf. 005).
REFERENCES
- 1.Advisory Committee on Immunization Practices. 2000. Preventing pneumococcal disease among infants and young children. Recommendations of the Advisory Committee on Immunization Practices (ACIP). Morb. Mortal. Wkly. Rep. Recomm. Rep. 49:1-35. [PubMed] [Google Scholar]
- 2.Barnes, D. M., S. Whittier, P. H. Gilligan, S. Soares, A. Tomasz, and F. W. Henderson. 1995. Transmission of multidrug-resistant serotype 23F Streptococcus pneumoniae in group day care: evidence suggesting capsular transformation of the resistant strain in vivo. J. Infect. Dis. 171:890-896. [DOI] [PubMed] [Google Scholar]
- 3.Black, S., H. Shinefield, B. Fireman, E. Lewis, P. Ray, J. R. Hansen, L. Elvin, K. M. Ensor, J. Hackell, G. Siber, F. Malinoski, D. Madore, I. Chang, R. Kohberger, W. Watson, R. Austrian, K. Edwards, et al. 2000. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Pediatr. Infect. Dis. J. 19:187-195. [DOI] [PubMed] [Google Scholar]
- 4.Bogaert, D., M. N. Engelen, A. J. M. Timmers-Reker, K. P. Elzenaar, P. G. H. Peerbooms, R. A. Coutinho, R. de Groot, and P. W. M. Hermans. 2001. Pneumococcal carriage in children in The Netherlands: a molecular epidemiological study. J. Clin. Microbiol. 39:3316-3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Butler, J. C., E. D. Shapiro, and G. M. Carlone. 1999. Pneumococcal vaccines: history, current status, and future directions. Am. J. Med. 107:69S-76S. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. 1997. Surveillance for penicillin-nonsusceptible Streptococcus pneumoniae—New York City, 1995. JAMA 277:1585-1586. [PubMed] [Google Scholar]
- 7.Coffey, T. J., M. Daniels, M. C. Enright, and B. G. Spratt. 1999. Serotype 14 variants of the Spanish penicillin-resistant serotype 9V clone of Streptococcus pneumoniae arose by large recombinational replacements of the cpsA-pbp1a region. Microbiology 145:2023-2031. [DOI] [PubMed] [Google Scholar]
- 8.Coffey, T. J., C. G. Dowson, M. Daniels, J. Zhou, C. Martin, B. G. Spratt, and J. M. Musser. 1991. Horizontal transfer of multiple penicillin-binding protein genes, and capsular biosynthetic genes, in natural populations of Streptococcus pneumoniae. Mol. Microbiol. 5:2255-2260. [DOI] [PubMed] [Google Scholar]
- 9.Coffey, T. J., M. C. Enright, M. Daniels, J. K. Morona, R. Morona, W. Hryniewicz, J. C. Paton, and B. G. Spratt. 1998. Recombinational exchanges at the capsular polysaccharide biosynthetic locus lead to frequent serotype changes among natural isolates of Streptococcus pneumoniae. Mol. Microbiol. 27:73-83. [DOI] [PubMed] [Google Scholar]
- 10.Coffey, T. J., M. C. Enright, M. Daniels, P. Wilkinson, S. Berron, A. Fenoll, and B. G. Spratt. 1998. Serotype 19A variants of the Spanish serotype 23F multiresistant clone of Streptococcus pneumoniae. Microb. Drug Resist. 4:51-55. [DOI] [PubMed] [Google Scholar]
- 11.Crook, D. W., and B. G. Spratt. 1998. Multiple antibiotic resistance in Streptococcus pneumoniae. Br. Med. Bull. 54:595-610. [DOI] [PubMed] [Google Scholar]
- 12.Dagan, R., N. Givon-Lavi, O. Zamir, M. Sikuler-Cohen, L. Guy, J. Janco, P. Yagupsky, and D. Fraser. 2002. Reduction of nasopharyngeal carriage of Streptococcus pneumoniae after administration of a 9-valent pneumococcal conjugate vaccine to toddlers attending day care centers. J. Infect. Dis. 185:927-936. [DOI] [PubMed] [Google Scholar]
- 13.de Neeling, A. J., and R. V. V. E. Milieu. 1999. Resistentiepeiling in 9 Nederlandse streeklaboratoria in 1995 en 1998. National Institute of Public Health and the Environment Bilthoven, The Netherlands.
- 14.de Neeling, A. J., B. P. Overbeek, A. M. Horrevorts, E. E. Ligtvoet, and W. G. Goettsch. 2001. Antibiotic use and resistance of Streptococcus pneumoniae in The Netherlands during the period 1994-1999. J. Antimicrob. Chemother. 48:441-444. [DOI] [PubMed] [Google Scholar]
- 15.Eskola, J., T. Kilpi, A. Palmu, J. Jokinen, J. Haapakoski, E. Herva, A. Takala, H. Kayhty, P. Karma, R. Kohberger, G. Siber, P. H. Makela, S. Lockhart, and M. Eerola. 2001. Efficacy of a pneumococcal conjugate vaccine against acute otitis media. N. Engl. J. Med. 344:403-409. [DOI] [PubMed] [Google Scholar]
- 16.Gherardi, G., C. G. Whitney, R. R. Facklam, and B. Beall. 2000. Major related sets of antibiotic-resistant pneumococci in the United States as determined by pulsed-field gel electrophoresis and pbp1a-pbp2b-pbp2x-dhf restriction profiles. J. Infect. Dis. 181:216-229. [DOI] [PubMed] [Google Scholar]
- 17.Gruneberg, R. N., and D. Felmingham. 1996. Results of the Alexander Project: a continuing, multicenter study of the antimicrobial susceptibility of community-acquired lower respiratory tract bacterial pathogens. Diagn. Microbiol. Infect. Dis. 25:169-181. [DOI] [PubMed] [Google Scholar]
- 18.Hermans, P. W., M. Sluijter, K. Elzenaar, A. van Veen, J. J. Schonkeren, F. M. Nooren, W. J. van Leeuwen, A. J. de Neeling, B. van Klingeren, H. A. Verbrugh, and R. de Groot. 1997. Penicillin-resistant Streptococcus pneumoniae in the Netherlands: results of a 1-year molecular epidemiologic survey. J. Infect. Dis. 175:1413-1422. [DOI] [PubMed] [Google Scholar]
- 19.Hermans, P. W. M., M. Sluijter, T. Hoogenboezem, H. Heersma, A. van Belkum, and R. de Groot. 1995. Comparative study of five different DNA fingerprint techniques for molecular typing of Streptococcus pneumoniae strains. J. Clin. Microbiol. 33:1606-1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jefferies, J., S. C. Clarke, M. A. Diggle, A. Smith, C. Dowson, and T. Mitchell. 2003. Automated pneumococcal MLST using liquid-handling robotics and a capillary DNA sequencer. Mol. Biotechnol. 24:303-308. [DOI] [PubMed] [Google Scholar]
- 21.Klugman, K. P. 1996. Epidemiology, control and treatment of multiresistant pneumococci. Drugs 52:42-46. [DOI] [PubMed] [Google Scholar]
- 22.Lipsitch, M., J. K. Dykes, S. E. Johnson, E. W. Ades, J. King, D. E. Briles, and G. M. Carlone. 2000. Competition among Streptococcus pneumoniae for intranasal colonization in a mouse model. Vaccine 18:2895-2901. [DOI] [PubMed] [Google Scholar]
- 23.McCormick, A. W., C. G. Whitney, M. M. Farley, R. Lynfield, L. H. Harrison, N. M. Bennett, W. Schaffner, A. Reingold, J. Hadler, P. Cieslak, M. H. Samore, and M. Lipsitch. 2003. Geographic diversity and temporal trends of antimicrobial resistance in Streptococcus pneumoniae in the United States. Nat. Med. 9:424-430. [DOI] [PubMed] [Google Scholar]
- 24.Meats, E., A. B. Brueggemann, M. C. Enright, K. Sleeman, D. T. Griffiths, D. W. Crook, and B. G. Spratt. 2003. Stability of serotypes during nasopharyngeal carriage of Streptococcus pneumoniae. J. Clin. Microbiol. 41:386-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.National Committee for Clinical Laboratory Standards. 1997. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 4th ed. Approved standard M7-A4. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 26.NCCLS. 2002. Performance standards for antimicrobial susceptibility testing, vol. 19, no. 1. Twelfth informational supplement. M100-S12. NCCLS, Wayne, Pa.
- 27.Sluijter, M., H. Faden, R. de Groot, N. Lemmens, W. H. F. Goessens, A. van Belkum, and P. W. M. Hermans. 1998. Molecular characterization of pneumococcal nasopharynx isolates collected from children during their first 2 years of life. J. Clin. Microbiol. 36:2248-2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soriano, F., and V. Rodriguez-Cerrato. 2002. Pharmacodynamic and kinetic basis for the selection of pneumococcal resistance in the upper respiratory tract. J. Antimicrob. Chemother. 50(Suppl. C):51-58. [DOI] [PubMed] [Google Scholar]
- 29.Spratt, B. G., and B. M. Greenwood. 2000. Prevention of pneumococcal disease by vaccination: does serotype replacement matter? Lancet 356:1210-1211. [DOI] [PubMed] [Google Scholar]
- 30.Tomasz, A. 1997. Antibiotic resistance in Streptococcus pneumoniae. Clin. Infect. Dis. 24(Suppl. 1):S85-S88. [DOI] [PubMed] [Google Scholar]
- 31.van Steenbergen, T. J., S. D. Colloms, P. W. Hermans, J. de Graaff, and R. H. Plasterk. 1995. Genomic DNA fingerprinting by restriction fragment end labeling. Proc. Natl. Acad. Sci. USA 92:5572-5576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Veenhoven, R., D. Bogaert, C. Uiterwaal, C. Brouwer, H. Kiezebrink, J. Bruin, P. Hermans, R. de Groot, W. Kuis, G. Rijkers, A. Schilder, and L. Sanders. 2003. Effect of pneumococcal vaccine followed by polysaccharide pneumococcal vaccine on recurrent acute otitis media. Lancet 361:2189-2195. [DOI] [PubMed] [Google Scholar]
- 33.Wolf, B., L. C. Rey, S. Brisse, L. B. Moreira, D. Milatovic, A. Fleer, J. J. Roord, and J. Verhoef. 2000. Molecular epidemiology of penicillin-resistant Streptococcus pneumoniae colonizing children with community-acquired pneumonia and children attending day-care centres in Fortaleza, Brazil. J. Antimicrob. Chemother. 46:757-765. [DOI] [PubMed] [Google Scholar]