Abstract
Hepatocellular carcinoma (HCC) and intrahepatic cholangiocarcinoma (ICC) often present at stages where patients have limited treatment options. Use of selective internal radiation therapy (SIRT) with yttrium-90 (Y-90) resin microspheres has progressed as data increasingly speak to its utility in patients with both intermediate and late stage disease in these cancers. In anticipation of the pending completion of several prospective randomized controlled multicenter studies exploring the use of Y-90 resin microspheres in primary liver cancers, this article outlines mechanisms involved in SIRT administration and reviews key efficacy and safety data that are currently available in the literature involving use of this therapy in both HCC and ICC.
Keywords: Hepatocellular carcinoma (HCC), intrahepatic cholangiocarcinoma (ICC), yttrium-90 resin microspheres, SIR-Spheres, radioembolization
Introduction
Hepatocellular carcinoma (HCC) and intrahepatic cholangiocarcinoma (ICC) are complex primary liver malignancies with symptoms that often do not appear until the cancer has progressed to an intermediate or advanced stage. As a result, curative treatment with surgical resection or liver transplantation is usually no longer an option for many of these patients. The decision as to what course of treatment to then provide is one that depends on several factors including patient’s performance status, comorbidities, tumor location, liver tumor burden, and liver function reserve. The National Comprehensive Cancer Network (NCCN) Guidelines state that treatment options for intermediate to advanced patients include systemic therapy, locoregional therapy, clinical trials, or best supportive care (1). Advances in locoregional treatments are continually being made to downstage these malignancies so that patients become eligible for resection or liver transplantation. The usual clinical practice for treating HCC or ICC is assessment by a hepatologist or an oncologist for systemic chemotherapy, after a surgeon confirms that the tumor is unresectable. With unresectable liver cancer, other members of a multi-disciplinary healthcare team are sometimes consulted only after the disease has proven refractory to systemic chemotherapy; patients are then provided alternative locoregional therapies such as external beam radiation therapy, thermal ablation, or transarterially directed therapies (1). Several locoregional options are playing an increasingly important role as studies confirm their utility in the treatment of hepatic malignancies when used alone or in combination with other therapies across different disease stages. Of particular interest in this review is the use of locoregional therapy with yttrium-90 (Y-90) resin selective internal radiation therapy (SIRT) for treatment of HCC and ICC.
SIRT
SIRT, also known as radioembolization, is a minimally invasive, image-guided procedure that delivers intra-arterial brachytherapy to cancer in the liver. SIRT achieves cell death with a high dose of selectively targeted radiation to the tumor. In contradistinction to transarterial chemoembolization (TACE), which utilizes a combination of chemotherapy and ischemic insult to achieve tumor cell death, beta particle radiation in SIRT serves as the cytotoxic mechanism. As a locoregional therapy, SIRT is distinct from external beam radiation therapy in which the radiosensitive nature of the liver often limits the amount of radiation that patients are able to receive. SIRT is performed with millions of tiny beta-emitting Y-90 microspheres infused through a microcatheter positioned within the hepatic artery (Figure 1). With a median diameter of 32.5 microns, the resin microspheres penetrate deep within the tumor parenchyma and embed at the level of the arterioles. These microspheres emit high-energy, low-penetration radiation to the tumor while sparing much of the healthy liver parenchyma. The average penetration depth of the beta radiation is 2.5 mm. SIRT may be used on a subset of patients, including those with a life expectancy of at least 3 months with unresectable hepatic primary or metastatic cancer, and tumor burden primarily in the liver (2-4).
Figure 1.
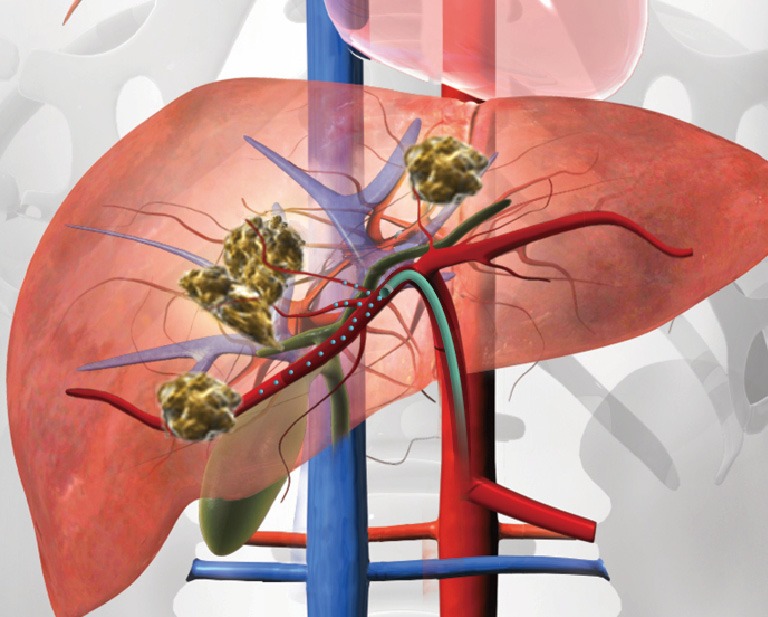
SIRT delivery of Y-90 microspheres to primary hepatic tumors. Illustration of multifocal bi-lobar HCC with selective catheterization of the right hepatic artery for SIRT treatment. SIRT, selective internal radiation therapy; HCC, hepatocellular carcinoma.
Two commercially available Y-90 medical devices are currently available: TheraSphere (BTG International, London, United Kingdom) and SIR-Spheres Resin Microspheres (Sirtex Medical, Sydney, Australia).
There are several major prospective, randomized, controlled multicenter studies involving Y-90 resin microspheres that are currently active (Table 1). These trials include comparison of Y-90 resin microspheres to sorafenib for the treatment of advanced HCC in the (SARAH) trial (NCT01482442) and the SIRveNIB trial (NCT01135056). The SORAMIC Study (NCT01126645), another prospective, multicenter, multi-national (European) RCT will assess whether overall survival (OS) of patients with HCC can be improved by combining sorafenib with Y-90 resin microspheres vs. sorafenib alone. In ICC, (NCT01798147) will determine whether treatment with SIR-Spheres is superior to TACE. In the anticipation of pending results from these and other studies, this article will focus on the use of Y-90 resin microspheres in these cancers.
Table 1. Ongoing studies.
| Study | Indication | Study design | Treatment arm | Primary endpoint | Anticipated completion |
|---|---|---|---|---|---|
| SIRveNIB (NCT01135056) | HCC (intermediate—advanced) | RCT multicenter | Sorafenib | Survival | Not available |
| SIR Spheres | |||||
| SARAH (NCT01482442) | HCC (advanced) | RCT multicenter | Sorafenib | Survival | By early 2017 |
| SIR Spheres | |||||
| SORAMIC (NCT01126645) | HCC (early-advanced) | RCT multicenter | Sorafenib | Survival | Early 2018 |
| SIR Spheres > sorafenib | |||||
| STOCS (NCT02167711) | ICC (advanced) | Single arm, multicenter | SIR Spheres > | Survival | Late 2016 |
| Gemcitabine + cisplatin | |||||
| SIRT-TACE-CCC (NCT01798147) | ICC (advanced) | RCT single-center | TACE | PFS | Late 2016 |
| SIR Spheres |
Sources: http://www.sirtex.com/media/35508/clinical-investigation-programme-964-ea-0115.pdf; www.clinicaltrials.gov. HCC, hepatocellular carcinoma.
SIRT MAA hepatic arterial mapping procedure
Y-90 has a long history of clinical use with data in the literature back to the 1960s supporting its utility in primary and metastatic liver tumors (5). In March 2002, Y-90 resin microspheres were initially approved for the treatment of inoperable tumors from primary colorectal cancer metastatic to the liver.
While preparing patients for treatment with Y-90 resin microspheres, an initial planning angiography is performed 1–2 weeks prior to treatment day. SIRT is a potent therapy, which requires an interventional radiologist to appropriately recognize key vascular structures leading to extrahepatic organs, and to embolize these vessels before administering the Y-90 microspheres. Non-target extrahepatic deposition of radioembolic spheres poses the potential risk of gastrointestinal or pulmonary accumulation of the microsphere (5). A thorough evaluation of abdominal arterial anatomy is performed during the planning study, which includes hepatic arterial mapping, precautionary non-target vessel embolization, and administration of macroaggregate albumin (MAA), which serves to mimic microspheres deposition. These steps minimize the potential for non-target clinical toxicities, including gastric ulceration, pancreatitis, skin irritation, and pulmonary edema/radiation pneumonitis (6). The celiac angiogram helps assess hepatic anatomy, including such variants as a replaced or accessory left hepatic artery, separate origins of the medial and lateral segmental branches of the left hepatic artery, and potential parasitization of other vessels that may supply hepatic tumors. Celiac artery angiography also allows for evaluation of the gastroduodenal arcade. A superior mesenteric artery (SMA) arteriogram is performed to identify replaced or accessory hepatic arteries. High power injection rates are used during these arteriograms to maximally opacify the arteries. This provides a supraphysiologic flow, stimulating the retrograde flow and reflux that may occur with embolization procedures. All branch vessels felt to be at risk for reflux that could lead to non-target radioembolization on the subsequent treatment day receive prophylactic embolization with permanent intra-arterial occlusion devices, most commonly metallic coils. The most common sites of prophylactic embolization are the gastroduodenal and right gastric arteries, in order to prevent reflux of radioembolic particles into the stomach, pancreas, and bowel. Various other vessels that may be embolized during the MAA hepatic arterial mapping study are the falciform, supraduodenal, retroduodenal, left inferior phrenic, accessory left gastric, and inferior esophageal arteries (5,7). A very small risk of radiation cholecystitis exists, and the cystic artery is embolized by some interventional radiologists on a case by case basis. After thorough examination and prophylactic embolization, the final step of the MAA hepatic arterial mapping study is the administration of Tc99m labeled MAA particles followed by a nuclear medicine scan, which quantifies the accumulation of MAA particles in the liver and percent of shunting to the lungs. Lung shunt fractions, which allow for pulmonary exposure below 30 Gy in a single treatment and 50 Gy cumulative, are deemed acceptable to continue with SIRT.
Efficacy of Y-90 resin microspheres
Numerous studies have been conducted to test the efficacy of Y-90 resin microspheres when used in each stage of liver malignancy (Table 2).
Table 2. Y-90 Resin efficacy in HCC and ICC.
| Tumor type | Author | Type of Trial | Treatment | N | Stage | Response | Survival |
|---|---|---|---|---|---|---|---|
| HCC | Kim; Am J Clin Oncol 2015 | Prospective, multiple centers | Y-90 resin | 40 | BCLC 0: 1 (2.5%); BCLC A: 18 (45.0%); BCLC B: 19 (47.5%); BCLC C: 2 (5.0%) | Modified RECIST, n (%) | Overall 3-y survival rate =75%; 3-y survival rate in patients with BCLC B =50% |
| Month 3: CR =4 (10.0); PR =19 (47.5); SD =15 (37.5); PD =2 (5.0); Overall =40 (100.0); response rate: 23/40 (57.5); disease control (DC) rate: 38/40 (95.0) | |||||||
| Month 6: CR =4 (11.1); PR =19 (52.8); SD =7 (19.4); PD =6 (16.7); Overall =36 (100.0); response rate: 23/36 (63.9); disease control (DC) rate: 30/36 (83.3) | |||||||
| Kolligs; Liver Int 2015 | Prospective, open-label, multicenter randomized-controlled pilot study (SIRTACE) | Y-90 resin, n=13 or TACE, n=15 | 28 patients with unresectable HCC | Y-90 resin, n (%): BCLC A, 5 (38.5); BCLC B, 5 (38.5); BCLC C, 3 (23.0) | Y-90 resin: PR 30.8%; disease control rate 76.9% | Y-90 resin: PFS =3.6 mo (95% CI: 2.3–6.2); 6 mo survival rate =69.2%; 1-y survival rate =46.2% |
|
| TACE, n (%): BCLC A, 4 (26.7); BCLC B, 8 (53.3); BCLC C, 3 (20.0) | TACE: PR 13.3%; disease control rate 73.3% | TACE: PFS =3.7 mo (95% CI: 1.6–11.0); 6 mo survival rate =86.7%; 1-y survival rate =66.7% |
|||||
| Lau; Br J Cancer 1994 | Prospective, single center | Y-90 resin | 18 | Unresectable HCC (BCLC stage not provided) | PR, n=8 (44.4%); SD, n=8 (44.4%); PD, n=2 (11.1%) | Median OS (P=0.005): >120 Gy =12.9 mo; <120 Gy =6.0 mo |
|
| Lau; Int J Rad Onc 1998 | Prospective, single center | Y-90 | 71 | Unresectable HCC without extrahepatic disease (BCLC stage not provided) | CR (AFP levels), n=31 (67%); PR (AFP levels) n=10 (22%) | Median OS =9.4 mo | |
| Saxena; Intl J Surg 2014 | Retrospective, single center | Y-90 resin | 45 patients | Unresectable HCC | CR, 1/40 (3%); PR, 18 (45%); SD, 11 (22%); PD, 10 (25%) | Median OS =27.7 mo. Survival rate: 6-mo =74%; 1-y =62%; 2-y =50%; 3-yr =26% |
|
| Strigari; J Nucl Med 2010 | Prospective, single arm, single center | Y-90 resin | 73 | Measurable unresectable HCC | Tumor control probability 73% per EASL and 55% per RECIST | NR | |
| Chow; PLoS One 2014 | Open-label, single arm, investigator initiated, multiple centers, phase 2 | Y-90 resin followed by sorafenib | 29 patients | BCLC B, n=11; BCLC C, n=18 | Best ORR (RECIST v1.0.) 7/28 patients (25%; 95% CI, 11–45%): CR, 2 (7%); PR, 5 (18%); SD, 15 (54%); PD, 5 (18%) | Median PFS: BCLC B =15.2 mo (95% CI: 4.6– not reached); BCLC C= 6.5 mo (95% CI =3.5–9.1) |
|
| Overall disease control rate: 79% (95% CI, 59–92%). Disease control rate in BCLC B: 100%; disease control rate in BCLC C: 65% |
Median OS: BCLC B =20.3 mo (95% CI =10.9– not reached); BCLC C =8.6 mo (95% CI: 5.6–14.2) |
||||||
| D’Avalo; Hepatogastr oenterology 2009 | Retrospective, 2-arm, single center | Y-90 resin or control (conventional, experimental, or no treatment) | Y-90 resin, n=35; control, n=43 | Y-90 resin arm: BCLC B, 51%; BCLC C, 46% | NR | Y-90 Resin: median OS =16 mo (P<0.05); 1-y survival rate =67%; 2-y survival rate =36% |
|
| Control: median OS =8 mo; 1-y survival rate =35%; 2-y survival rate =3% |
|||||||
| Gabrielson; Front Oncol 2015 | Retrospective, single center | Y-90 resin | 27 patients | BCLC B, 3 (11%); BCLC C, 24 (89%) | Three-month disease control rate: target lesions, 63%; whole liver, 52% | Median liver-PFS =2.5 mo (95% CI: 1.9–3.1); median OS =11.7 mo (95% CI: 6.6–16.8) |
|
| Golfieri; J Hepatol 2013 | Retrospective, two-arm, multiple centers (ENRY) | Y-90 resin: (I) elderly patients (≥70 y); (II) younger patients (<70 y) | 325 patients: ≥70 y, 128 patients; <70 y, 197 patients | BCLC, n (%): elderly: BCLC A, 21 (16.4); BCLC B, 35 (27.3); BCLC C, 72 (56.3). Younger: BCLC A, 31 (15.7); BCLC B, 52 (26.4); BCLC C, 111 (56.3); BCLC D, 3 (1.5) |
NR | Median OS (P=0.94); elderly =14.5 mo (95% CI: 10.6–16.8); younger =12.8 mo (95% CI: 10.8–17.9) |
|
| Gramenzi; Liver Int 2015 | Retrospective, single center | Y-90 resin or sorafenib | 137 patients: sorafenib 74; Y-90 resin 63 | Y-90 resin: BCLC B 41%; BCLC C 59% | Y-90 resin: CR, 12 (14.3%); PR, 34 (53.9%); SD, 12 (14.3%); DC, 58 (92%) | Y-90 resin: OS =13.2 mo (95% CI: 6.1–20.2); 1-y survival rate =51.8%; 2-y survival rate =27.8%; 3-y survival rate =21.6% |
|
| Sorafenib: BCLC B 53%; BCLC C 47% | Sorafenib: CR not observed; PR, 7 (9.5%); SD, 31 (41.9%); DC, 38 (51.4%) | Sorafenib: OS =14.4 mo (95% CI: 4.3–24.5); 1-y survival rate =52.1%; 2-y survival rate =29.3%; 3-y survival rate=14.7% |
|||||
| Iñarrairaegui; J Vasc Interv Radiol 2010 | Retrospective, single center | Y-90 resin | 25 | Unresectable HCC and compromised portal flow (BCLC stage not provided) | Global response: controlled disease achieved in 66.7% of patients at 2 months and 50% of patients at 6 months | Median OS =10 mo (95% CI: 6.6–13.3 mo) | |
| ICC | Camacho; J Vasc Interv Radiol 2014 | Prospective, single center | Y-90 resin | 21 | Unresectable mass-like ICC refractory to standard chemotherapy | Overall mRECIST at 3 months: CR =12.5%; PR =25.0%; SD =12.5%; PD =50.0% | Median OS =16.3 mo (95% CI: 7.2–25.4 mo) |
| Filippi; Nucl Med Biol 2015 | Prospective, single arm, single center | Y-90 resin | 18 | Unresectable and chemotherapy- refractory ICC | NR | Median OS =14.8 mo (95% CI: 12.6–17.1 mo) | |
| Haug; Eur J Nucl Med Mol Imaging 2011 | Retrospective, single arm, single center | Y-90 resin | 26 | Unresectable ICC | PR, 5 (22%); SD, 15 (65%); PD, 3 (13%) | Median OS =11.7 mo | |
| Hoffman; Cardiovasc Interventional Radiology 2012 | Retrospective, single arm, single center | Y-90 resin | 33 | Unresectable cholangiocellular carcinoma or chemotherapy-refractory liver metastases from cholangiocellular carcinoma | RECIST after 3 months: PR, n=12 (36.4%); SD, n=17 (51.5%); PD, n=5 (15.2%) | Median OS =22 mo (95% CI: 7.9–29.4) | |
| Rafi; Cardiovasc Intervent Radiol 2013 | Prospective, single arm, single center | Y-90 resin | 19 | Unresectable, standard chemorefractory ICC | Tumor response at 3 months: CR, 0 (0%); PR, 2 (11%); SD, 13 (68%); PD, 4 (21%) | Median OS =11.3 mo (95% CI: 3.1–19.6) | |
| Saxena; Ann Surg Oncol 2010 | Prospective, single arm, single center | Y-90 resin | 25 | Unresectable ICC | PR, 6 (24%); SD, 11 (48%); PD, 5 (20%); 1 patient with PR was downstaged to resection after treatment | Median OS =9.3 mo; 6-mo survival rate =56%; 1-y survival rate =40%; 2-y survival rate =27%; 3-y survival rate =13% |
|
| Soydal; Ann Nucl Med 2016 | Retrospective | Y-90 resin | 16 | Unresectable ICC | Response to treatment 3 months after Y-90, n=5 patients (30%) | Median OS =9.6 mo (95% CI: 5.1–14.2 mo) |
HCC, hepatocellular carcinoma; ICC, intrahepatic cholangiocarcinoma; BCLC, Barcelona clinic liver cancer; CR, complete responses; PR, partial responses; SD, stable disease; OS, overall survival.
Early-stage disease
HCC
Techniques and procedures are continually being improved upon so that HCC can be diagnosed and treated at an early stage. Partial hepatectomy or liver transplantation following tumor downstaging with SIRT using Y-90 resin microspheres may provide the possibility of long-term survival in a select group of patients who would otherwise have limited treatment options. In one study of 21 UNOS T3 stage HCC patients, 29% (n=6) were downstaged and went on to have surgical resection or liver transplantation after treatment with Y-90 resin microspheres. More importantly, 75% of the patients experienced long-term survival at 3 years. These results were considered comparable to survival in patients with early-stage disease who were treated with ablation, surgery, or transplantation at the time of diagnosis (8). At the 2015 Americas Hepato-Biliary Association meeting, the post SIR-Spheres surgery study (P4S) was reviewed and it demonstrated that following SIRT, successful surgical hepatectomy or transplantation was achieved with a good safety profile and positive outcomes. Over half of the 100 patients in this study consisted of HCC and ICC patients (9). There has also been a case study that describes a cirrhotic patient who underwent extended right hepatectomy for a large HCC after transarterial SIRT and right portal vein embolization. With this approach, the cancer was being treated while also driving hypertrophy of the untreated contralateral disease-free left lobe of the liver. The result was a 53% reduction in tumor volume and compensatory hypertrophy in the contralateral liver. This study also suggests that SIRT with Y-90 resin microspheres before major hepatectomy may be safe and effective (10). The same effect was shown in another review of 15 patients of varied cancer types, including HCC, where notable hypertrophy of the future liver remnant occurred in unresectable liver cancer following SIRT with resin microspheres (11).
ICC
In patients with early stage ICC, 5-year survival for patients who are treated with surgical resection can range from 30–40% (12). Rates of recurrence can be substantial in this population. A multicenter retrospective examination of 301 patients with early stage ICC who received surgical resection suggests that more than 50% of patients with ICC will experience recurrence, with the most common intrahepatic only and the second most common concomitant intra- and extrahepatic recurrence of their disease post-resection. Patients with larger tumors (≥5 cm), microvascular invasion, and lymph node metastases are more likely to have their disease recur (13). Currently the optimal treatment once the cancer has recurred is unclear (12), and additional studies on whether SIRT with Y-90 resin microspheres is optimal in patients with recurrent as well as de novo disease are needed.
Reports on the use of Y-90 resin microspheres in patients with early stage ICC are limited. Whitney et al. were able to downstage 4 patients for surgery with Y-90 resin microspheres; 2 of whom were diagnosed with ICC. One patient with ICC received Y-90 resin microspheres as her first locoregional therapy, following standard neoadjuvant chemotherapy; whereas a second patient received Y-90 resin microspheres to downstage disease that recurred less than a year after initial resection. After being treated with Y-90 resin microspheres, both patients were downstaged to successful surgery. They exhibited no signs of liver dysfunction and were disease free at 9 and 18 months post-resection, respectively, at the time of the initial publication (14).
Unresectable disease
Intermediate to advanced stage HCC (ineligible for surgery)
Patients with intermediate stage HCC comprise a diverse population with respect to the extent of tumor burden, liver function, and the array of available treatment options (15). The following studies explore use of Y-90 resin microspheres as monotherapy, compared to TACE or sorafenib, and as sequential therapy with sorafenib in patients with intermediate stage disease. Not surprisingly, these studies reveal that Barcelona clinic liver cancer (BCLC) staging designation can be an important predictor of clinical effectiveness on Y-90 resin microspheres treatment outcomes as with traditional therapies.
In an investigation of Y-90 resin microspheres in 40 Korean patients, Kim et al. found that at 3 months the overall response rate and disease control rate (DCR) was 57.5% and 95%, respectively. DCR represents the sum of complete responses (CR), partial responses (PR), and stable disease (SD). Of those patients, 90% were Child-Pugh-A, 45% BCLC-A, 47.5% BCLC-B, and 90% had no prior therapy. Overall, the 3-year survival was 75%, yet in patients who had BCLC-B stage disease at baseline, 3-year survival was reduced to 50% (16). SIRTACE, an open-label, randomized controlled pilot of 28 European patients (32% BCLC-A, 46% BCLC-B) demonstrated a PR rate of 30.8% with Y-90 resin microspheres and 13.3% with TACE, and a DCR of 76.9% and 73.3%, respectively. Median progression-free survival (PFS) between the two arms was comparable at 3.6 months with Y-90 resin microspheres and 3.7 months with TACE. However, patients in the TACE cohort were administered an average of 3.4 treatments with TACE compared to a single administration with Y-90 resin microspheres to achieve the reported outcomes (17).
The earliest investigations that focused on use of Y-90 resin microspheres in patients with intermediate to advanced stage HCC included patients cared for by Lau and colleagues at Prince of Wales Hospital in Hong Kong in the mid to late 1990s. Eighteen patients with inoperable HCC were treated with Y-90 resin microspheres in phase I, and 71 patients in phase II studies. The authors found that these treated HCC patients had manageable toxicity and median survivals from the Phase I and II trials ranged from 5.2 to 12.9 months (18,19). Gramenzi et al. later retrospectively investigated 137 intermediate to advanced stage HCC patients at a single institution in Italy who were administered either Y-90 resin microspheres (n=69; BCLC-B/C =41%/59%) or sorafenib (n=74; BCLC-B/C 53%/47%). Median OS and 3-year survival rates were comparable for the two therapies in this setting at 14.4 months and 14.7% in sorafenib, and 13.2 months and 21.6% in Y-90 resin microspheres treated patients. BCLC-B patients who were treated with either sorafenib or Y-90 resin microspheres experienced a prolonged median OS of 20.4 and 22.1 months, respectively (20).
An open-label, single arm, investigator-initiated phase II study conducted by Chow et al. at four Asia-Pacific centers evaluated the clinical impact of sequential administration of Y-90 resin microspheres and sorafenib. This investigation of 29 patients (38% BCLC B, 62% BCLC C) who received 400 mg sorafenib BID, 14 days post-Y-90 resin microspheres, further demonstrates the effect of BCLC staging on outcomes. Overall response rate and DCR were 25% and 79% in the total population, yet in patients with BCLC-B disease at baseline they were 46% and 100%. Furthermore, median OS was 20.3 months with BCLC stage B patients compared to 8.6 months with BCLC stage C patients, suggesting that even when used in conjunction with chemotherapy, enhanced outcomes may be observed in patients with earlier stage disease (21). Similarities of OS observed in the Gramenzi and Chow studies, warrant larger prospective investigations exploring the efficacy of sequential Y-90 resin microspheres plus sorafenib, vs. Y-90 resin microspheres alone or sorafenib alone in this setting. The SORAMIC (NCT01126645) Phase II trial based out of the University of Magdeburg, Germany will evaluate BCLC-A-C patients stratified to receive radiofrequency ablation followed by sorafenib or placebo in the local group. The palliative treatment group will receive Y-90 resin microspheres followed by sorafenib or sorafenib alone. Primary outcomes include OS, time to recurrence, and utility of Primovist®-enhanced MRI in this setting. Anticipated enrollment is 375 patients and the study is expected to be completed in 2018 (22). Preliminary data from the planned safety analysis of the first 40 SORAMIC patients who received sorafenib only or Y-90 resin microspheres followed by sorafenib suggest that these therapeutic modalities offer similar tolerability. More robust safety and efficacy data are pending (23).
SIRveNIB (NCT01135056), a Phase III investigation of Y-90 resin microspheres vs. sorafenib in Asia-Pacific patients with locally advanced disease (BCLC-B or BCLC-C without extra-hepatic disease) is expected to share data in the near future as well. The primary outcome for the SIRveNIB trial is OS (24).
Advanced stage HCC
To date, much of the data regarding use of Y-90 resin microspheres in advanced HCC has arisen from investigators in Europe. In 2006, Sangro and colleagues from the Spanish study group at Clinica Universidad de Navarra reported results from the use of Y-90 resin microspheres in 24 consecutive patients who achieved disease control (100%) and disease response rates (23.8%). Sangro et al. highlighted the importance of applying more stringent selection and dosing criteria in patients who have cirrhosis versus those without, in order to mitigate potential toxicity (25).
This team later retrospectively compared the effect of Y-90 resin microspheres on survival to a control group of active treatment and supportive care in 78 patients with unresectable HCC. In this study of patients with BCLC-B & C stage disease, 35 (45%) of patients received Y-90 resin microspheres, 43 (55%) in the control group had either active treatment or supportive care. Included in the actively treated patient group were a few individuals who were treated with TACE and some treated with sorafenib. The authors reported that median survival was significantly higher (16 vs. 8 months) in patients treated with Y-90 resin microspheres vs. the control group. This difference was notable even after adjustments were made for the presence of cirrhosis, bi-lobar or multinodular disease, or vascular invasion (26).
The team at Clinica Universidad de Navarra then examined the use of Y-90 resin microspheres in 25 patients with unresectable HCC and portal vein thrombosis (PVT). The presence of PVT is often a relative contraindication for other catheter-directed locoregional therapies, such as conventional chemoembolization and drug-eluting beads chemoembolization. These authors were able to achieve disease control in 50% of patients at 6 months, and a median survival of 10 months with minimal toxicity (27). In historical controls, the median survival time of untreated HCC patients with PVT is 2.7 months (28).
It is this same group of authors that designed the European Network on Radioembolization with Y-90 resin microspheres (ENRY) study, which reviewed a retrospective database of 325 patients with unresectable HCC across eight European centers. Patients in the study were predominantly BCLC-C (56%). Authors found that while clinical factors such as BCLC stage, the presence of ascites at baseline, and tumor burden may influence survival, age does not. Older (>70 years old) patients receiving Y-90 resin microspheres experienced similar survival as younger patients [median 14.5 (95% CI: 10.6–16.8) vs. 12.8 (95% CI: 10.8–17.9) months] (29).
Case details and imaging of a 66-y/o BCLC-C stage patient with HCC who was successfully treated at our institution are shared in Figure 2. Clinical data in the literature and real world success of Y-90 resin microspheres in advanced HCC prompted the development of the prospective, multicenter Phase III SARAH trial (NCT01482442). This trial is the first prospective investigation directly comparing sorafenib to Y-90 resin microspheres, and has the potential to shift the current treatment paradigm for patients with advanced HCC. A total of 400 patients are expected to be enrolled; with 1:1 randomization for the Y-90 resin microspheres and sorafenib treatment arms. Patients will be further stratified by ECOG score, PVT, and failure of previous chemoembolization. The primary endpoint is OS. Secondary endpoints will include tolerability, 6-month PFS, and response rate as well as quality of life (QOL) and health economics outcomes. Data from this study is expected to be available by early 2017 (30).
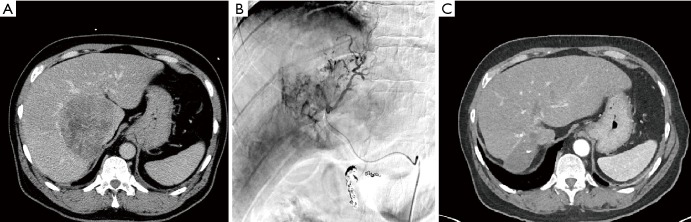
Figure 2.
66-y/o male with 10 cm unresectable HCC, BCLC stage C, that has invaded the inferior vena cava. (A) CT prior to Y-90 resin administration; (B) angiogram on day of Y-90 resin treatment; (C) CT following three Y-90 resin treatments that occurred in January, March, and July of 2010; small area of radiation necrosis at prior site of 10 cm tumor observed. Patient’s baseline AFP was 143,987 ng/mL and decreased to 113,200; 43,295; and 9.5 after the 1st, 2nd and 3rd Y-90 resin treatments, respectively. As of February 2016 the patient’s AFP is 13.2. HCC, hepatocellular carcinoma; BCLC, Barcelona clinic liver cancer; AFP, alpha-fetoprotein.
Unresectable ICC
Y-90 alone and ICC
Long-term survival in patients with ICC is not good, even after surgical resection. Given the poor prognosis of patients with ICC, studies with minimally invasive treatment options such as Y-90 resin microspheres have become more relevant. Between 2004 and 2009, Saxena et al. prospectively assessed use of Y-90 resin microspheres in 25 Australian patients with unresectable ICC. Patients were predominantly ECOG 0 (68%) and ECOG 1 (28%) and had received prior treatment with chemotherapy (72%), resection (40%), and/or TACE/RFA (16%). Median survival for patients with unresectable ICC can range from 12–15 months with conventional therapies (31). Saxena et al. reported median survival of 9.3 months post-Y-90 resin microspheres in a population predominantly made up of patients who had already failed conventional therapy. Patients with ECOG 0 and peripheral tumors experienced a significantly improved survival of 18.3 months (32). In 2013, Rafi et al. published their prospective data in 19 US patients with unresectable chemorefractory ICC, who had also been treated with Y-90 resin microspheres. This population was predominantly ECOG 1 (74%) and survival post-Y-90 resin microspheres was 11.5 months. Interestingly, Rafi and colleagues found no significant difference in survival in patients who entered the study at ECOG 0/1 vs. ECOG 2 (33). We report case details and imaging for a 62-y/o patient with infiltrative unresectable ICC treated with Y-90 resin microspheres at our institution in Figure 3.
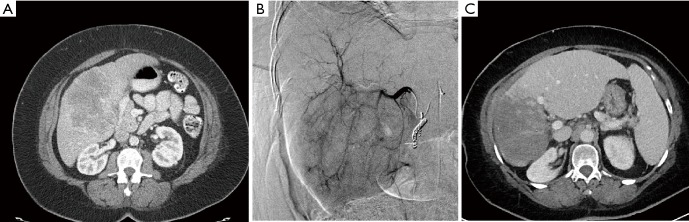
Figure 3.
62 y/o female with 12 cm unresectable, infiltrative ICC, >50% liver involvement. (A) CT prior to Y-90 resin administration; (B) angiogram on day of Y-90 resin treatment. Patient received right lobe SIRT on 11/2012, 12/2012, 8/2013 and 8/2014. She received left lobe medial segment SIRT on 12/2014; (C) CT 3+ years after initial Y-90 resin treatment. Radiation necrosis in prior site of 12 cm ICC. Compensatory hypertrophy left lobe. ICC, intrahepatic cholangiocarcinoma.
Identifying factors that may predict outcomes in unresectable, chemorefractory ICC have been of particular interest for the clinical community. Retrospective investigations published by Hoffmann et al. in 2012 (n=33) and Camacho et al. in 2014 (n=21) in German and American cohorts, respectively, offer varying insights (34). Hoffmann et al. demonstrated significantly improved OS outcomes in patients with ECOG 0 vs. 1 status (29.4 vs. 10 months) and ≤25% tumor volume (26.7 vs. 6 months). Furthermore, patients who exhibited a positive 3-month RECIST disease control response of SD (35.5 months) and PR (17.7 months) experienced significantly longer OS than patients who experienced progressive disease (5.7 months) (35). Camacho reports that patients who demonstrated response according to modified RECIST and EASL criteria at 3 months experienced significantly longer survival, while at 3 months, positive response using older RECIST criteria was not associated with statistically significant correlations to survival (34). Other studies suggest that while FDG avidity may serve as a poor prognostic factor in patients with unresectable ICC, changes in tumor volume on FDG PET/CT could possibly function as a viable marker for survival (36-38).
Y-90 and chemotherapy in ICC
Servajean describes a patient with an unresectable 11 cm ICC mass, and two satellite nodules, which was amenable to resection after successful concomitant use of chemotherapy and Y-90 resin microspheres, and remained alive without evidence of recurrence 1 year post-surgery. This patient was administered four cycles of GEMOX/FOLFIRINOX prior to Y-90 resin microspheres, and later received FOLFIRINOX in conjunction with Y-90 resin microspheres (39). An open-label safety study is currently in progress, to assess concomitant use of gemcitabine, cisplatin, and Y-90 resin microspheres in unresectable ICC. Estimated enrollment is 30 patients. Data are expected to be released in 2018 (40).
QOL
QOL data for Y-90 resin microspheres in HCC and ICC is limited. Kolligs et al. found HR-QOL outcomes to be similar for TACE and Y-90 resin in 18 patients with unresectable HCC involved with the SIRTACE investigation, with no statistically significant difference demonstrated by week 12 in their responses to the FACT-Hep questionnaire (17). Utilizing the EQ-5D index, Chow et al. showed an improved index score compared to the baseline in 18 BCLC stage C patients, over 3 years after recruitment in their study investigating the use of sequential SIRT-sorafenib (21). The planned HR-QOL data that will be released in the SARAH, SORAMIC, and SIRveNIB trials should offer enhanced understandings of the ways in which QOL outcomes are impacted by use of Y-90 resin microspheres in patients with HCC (22,24,40).
Safety of Y-90 resin microspheres
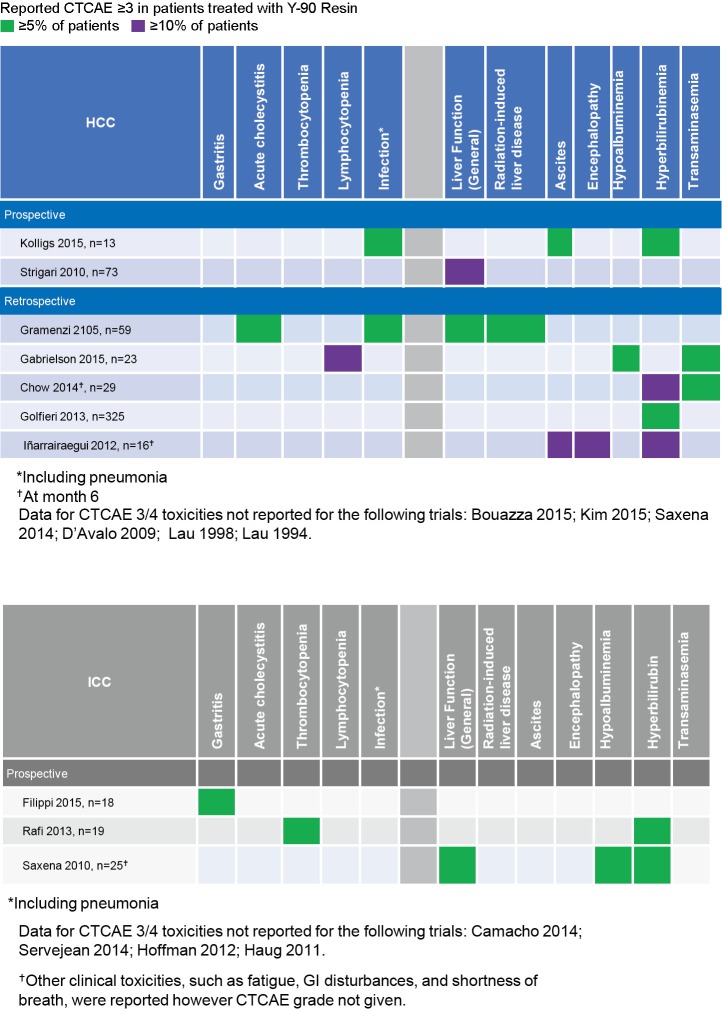
With proper administration, SIRT with Y-90 resin microspheres is a relatively well tolerated procedure. Patients may experience fatigue, fever, or occasionally GI-related symptoms such as nausea/vomiting, abdominal pain, or mild to moderate transient asymptomatic hepatic enzyme level increases (3,29,41). Grade 3–4 adverse events (AEs) are uncommon (41). When they do appear, unless there is a reported post-treatment infection, grade 3–4 AEs are predominantly either localized to the liver or comprise of clinical sequelae related to liver dysfunction (Figure 4).
Figure 4.
Y-90 resin safety in HCC and ICC. HCC, hepatocellular carcinoma; ICC, intrahepatic cholangiocarcinoma.
Gastrointestinal ulcerations have also been reported infrequently with the use of Y-90 resin microspheres and are likely the result of inadequate pre-SIRT embolization of non-target vessels. Experienced interventional radiologists will evaluate and recognize varying vascular anatomy and effectively coil off vessels during the initial pre-treatment hepatic arterial mapping study to ensure safe administration of the Y-90 resin microspheres on the day of the SIRT treatment. GI ulceration risk of less than 2% is seen at experienced high-volume centers of excellence. In a population of patients with both primary and metastatic liver cancers who received a total of 379 Y-90 resin SIRT procedures over the course of 9 years, Rodríguez-Lago et al. describe 6 (1.5%) patients who experienced gastrointestinal ulcerations (42). Amongst the studies overviewing efficacy of Y-90 resin microspheres in HCC and ICC in the sections above, only Golfieri et al. describe patients who experienced GI ulceration. Similar to Rodríguez-Lago et al., Golfieri et al. report 6 (1.8%) cases of GI ulceration amongst their 325 unresectable HCC patients (29).
Radioembolization-induced liver disease (REILD) is a form of liver injury associated with radiation and is one of the most significant potential complications of radiation therapy. REILD is usually identified 1–2 months after treatment, as patients present with jaundice, ascites, and hepatic insufficiency (43). In studies exploring Y-90 resin microspheres use in patients with both primary and metastatic liver cancers, REILD is an uncommon occurrence that has been reported in 0–8% of patients (3,20,29,44,45).
REILD is usually a transient condition and many affected patients will progress towards recovery with supportive measures. Proper radiation dosing, pharmacological prophylaxis with steroids or ursodeoxycholic acid, and patient selection is important since it can be a rare cause of treatment-related death (0.5%) (46,47).
Risk of REILD may increase with whole liver single-session treatments, use of an outdated Empirical formula for radiation dosing, and without appropriate dose-reduction to take into account cirrhosis or prior chemotherapy treatments.
Conclusions
Interventional oncology procedures, including SIRT, have become an essential part of the multi-disciplinary approach to management of liver cancer at most major medical centers. Transarterial Y-90 radioembolization is a promising treatment modality increasingly being used to treat various liver malignancies including HCC and ICC. The clinical data in support of its use has continued to grow over the past decade. This minimally invasive approach is primarily performed in the outpatient setting, which minimizes hospital stay and costs. With proper patient selection which includes patient ECOG performance status not higher than 2 and well-preserved liver function with serum total bilirubin <2 mg/dL (3,6), SIRT procedures boast benefits that outweigh the risks, with increased survival and DCRs, and maintenance of good QOL for patients with an unfortunate prognosis. Side effects are few and include fatigue, abdominal pain, nausea & vomiting. Grade 3–4 AEs such as GI ulcerations and REILD are uncommon. As the body of research with prospective, randomized, controlled multicenter studies with a focus on SIRT oncology procedures continues to grow, a more promising future exists for improved outcomes and survival for patients who have liver cancer with advanced disease and limited treatment options.
Acknowledgements
The authors would like to thank Christine Kuepfer and Eubio Medical Communications for editorial support (funding provided by Sirtex Medical Inc.).
Footnotes
Conflicts of Interest: EA Wang is a training proctor for Sirtex Medical. The other authors have no conflicts of interest to declare.
References
- 1.National Comprehensive Cancer Network. NCCN clinical practice guidelines in Oncology: Hepatobiliary cancers 2015. Report No.: Version 1.2016. [DOI] [PMC free article] [PubMed]
- 2.Yamada R, Kishi K, Sato M, et al. Transcatheter arterial chemoembolization (TACE) in the treatment of unresectable liver cancer. World J Surg 1995;19:795-800. 10.1007/BF00299773 [DOI] [PubMed] [Google Scholar]
- 3.Kennedy A, Nag S, Salem R, et al. Recommendations for radioembolization of hepatic malignancies using yttrium-90 microsphere brachytherapy: a consensus panel report from the radioembolization brachytherapy oncology consortium. Int J Radiat Oncol Biol Phys 2007;68:13-23. 10.1016/j.ijrobp.2006.11.060 [DOI] [PubMed] [Google Scholar]
- 4.SIRTEX. SIR-Spheres microspheres (Yttrium-90 Microspheres) package insert. 2014.
- 5.Salem R, Thurston KG. Radioembolization with 90Yttrium microspheres: a state-of-the-art brachytherapy treatment for primary and secondary liver malignancies. Part 1: Technical and methodologic considerations. J Vasc Interv Radiol 2006;17:1251-78. 10.1097/01.RVI.0000233785.75257.9A [DOI] [PubMed] [Google Scholar]
- 6.Lewandowski RJ, Salem R. Yttrium-90 radioembolization of hepatocellular carcinoma and metastatic disease to the liver. Semin Intervent Radiol 2006;23:64-72. 10.1055/s-2006-939842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lewandowski RJ, Sato KT, Atassi B, et al. Radioembolization with 90Y microspheres: angiographic and technical considerations. Cardiovasc Intervent Radiol 2007;30:571-92. 10.1007/s00270-007-9064-z [DOI] [PubMed] [Google Scholar]
- 8.Iñarrairaegui M, Pardo F, Bilbao JI, et al. Response to radioembolization with yttrium-90 resin microspheres may allow surgical treatment with curative intent and prolonged survival in previously unresectable hepatocellular carcinoma. Eur J Surg Oncol 2012;38:594-601. 10.1016/j.ejso.2012.02.189 [DOI] [PubMed] [Google Scholar]
- 9.Pardo F. Radioembolization of liver metastases. A bridge to surgery 5th international workshop on the treatment of hepatic and lung metastases of colorectal carcinoma November 12-13; Barcelona, Spain 2015. [Google Scholar]
- 10.Bouazza F, Poncelet A, Garcia CA, et al. Radioembolisation and portal vein embolization before resection of large hepatocellular carcinoma. World J Gastroenterol 2015;21:9666-70. 10.3748/wjg.v21.i32.9666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bishay V, Edwards M, Lo G, et al. Hypertrophy of future liver remnant in unresectable liver cancer following radioembolization with resin microspheres. J Vasc Interv Radiol 2015;2:S91 10.1016/j.jvir.2014.12.247 [DOI] [Google Scholar]
- 12.Weber SM, Ribero D, O'Reilly EM, et al. Intrahepatic cholangiocarcinoma: expert consensus statement. HPB (Oxford) 2015;17:669-80. 10.1111/hpb.12441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hyder O, Hatzaras I, Sotiropoulos GC, et al. Recurrence after operative management of intrahepatic cholangiocarcinoma. Surgery 2013;153:811-8. 10.1016/j.surg.2012.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whitney R, Tatum C, Hahl M, et al. Safety of hepatic resection in metastatic disease to the liver after yttrium-90 therapy. J Surg Res 2011;166:236-40. 10.1016/j.jss.2009.05.021 [DOI] [PubMed] [Google Scholar]
- 15.Bruix J, Sherman M, American Association for the Study of Liver Diseases Management of hepatocellular carcinoma: an update. Hepatology 2011;53:1020-2. 10.1002/hep.24199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim DY, Park BJ, Kim YH, et al. Radioembolization With Yttrium-90 Resin Microspheres in Hepatocellular Carcinoma: A Multicenter Prospective Study. Am J Clin Oncol 2015;38:495-501. 10.1097/COC.0b013e3182a78dba [DOI] [PubMed] [Google Scholar]
- 17.Kolligs FT, Bilbao JI, Jakobs T, et al. Pilot randomized trial of selective internal radiation therapy vs. chemoembolization in unresectable hepatocellular carcinoma. Liver Int 2015;35:1715-21. 10.1111/liv.12750 [DOI] [PubMed] [Google Scholar]
- 18.Lau WY, Leung WT, Ho S, et al. Treatment of inoperable hepatocellular carcinoma with intrahepatic arterial yttrium-90 microspheres: a phase I and II study. Br J Cancer 1994;70:994-9. 10.1038/bjc.1994.436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lau WY, Ho S, Leung TW, et al. Selective internal radiation therapy for nonresectable hepatocellular carcinoma with intraarterial infusion of 90yttrium microspheres. Int J Radiat Oncol Biol Phys 1998;40:583-92. 10.1016/S0360-3016(97)00818-3 [DOI] [PubMed] [Google Scholar]
- 20.Gramenzi A, Golfieri R, Mosconi C, et al. Yttrium-90 radioembolization vs sorafenib for intermediate-locally advanced hepatocellular carcinoma: a cohort study with propensity score analysis. Liver Int 2015;35:1036-47. 10.1111/liv.12574 [DOI] [PubMed] [Google Scholar]
- 21.Chow PK, Poon DY, Khin MW, et al. Multicenter phase II study of sequential radioembolization-sorafenib therapy for inoperable hepatocellular carcinoma. PLoS One 2014;9:e90909. 10.1371/journal.pone.0090909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.U.S. National Institutes of Health. Sorafenib and Micro-therapy Guided by Primovist Enhanced MRI in Patients With Inoperable Liver Cancer (SORAMIC): ClinicalTrials.gov; 2015 [updated December 2, 2015; cited 2016 May 7]. Available online: https://clinicaltrials.gov/ct2/show/NCT01126645
- 23.Ricke J, Bulla K, Kolligs F, et al. Safety and toxicity of radioembolization plus Sorafenib in advanced hepatocellular carcinoma: analysis of the European multicentre trial SORAMIC. Liver Int 2015;35:620-6. 10.1111/liv.12622 [DOI] [PubMed] [Google Scholar]
- 24.U.S. National Institutes of Health. Study to Compare Selective Internal Radiation Therapy (SIRT) Versus Sorafenib in Locally Advanced Hepatocellular Carcinoma (HCC) (SIRveNIB): ClinicalTrials.gov; 2014 [updated November 26, 2014; cited 2016 May 7]. Available online: https://clinicaltrials.gov/ct2/show/NCT01135056
- 25.Sangro B, Bilbao JI, Boan J, et al. Radioembolization using 90Y-resin microspheres for patients with advanced hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 2006;66:792-800. 10.1016/j.ijrobp.2006.05.065 [DOI] [PubMed] [Google Scholar]
- 26.D'Avola D, Lñarrairaegui M, Bilbao JI, et al. A retrospective comparative analysis of the effect of Y90-radioembolization on the survival of patients with unresectable hepatocellular carcinoma. Hepatogastroenterology 2009;56:1683-8. [PubMed] [Google Scholar]
- 27.Iñarrairaegui M, Thurston KG, Bilbao JI, et al. Radioembolization with use of yttrium-90 resin microspheres in patients with hepatocellular carcinoma and portal vein thrombosis. J Vasc Interv Radiol 2010;21:1205-12. 10.1016/j.jvir.2010.04.012 [DOI] [PubMed] [Google Scholar]
- 28.Llovet JM, Bustamante J, Castells A, et al. Natural history of untreated nonsurgical hepatocellular carcinoma: rationale for the design and evaluation of therapeutic trials. Hepatology 1999;29:62-7. 10.1002/hep.510290145 [DOI] [PubMed] [Google Scholar]
- 29.Golfieri R, Bilbao JI, Carpanese L, et al. Comparison of the survival and tolerability of radioembolization in elderly vs. younger patients with unresectable hepatocellular carcinoma. J Hepatol 2013;59:753-61. 10.1016/j.jhep.2013.05.025 [DOI] [PubMed] [Google Scholar]
- 30.Vilgrain V, Abdel-Rehim M, Sibert A, et al. Radioembolisation with yttrium‒90 microspheres versus sorafenib for treatment of advanced hepatocellular carcinoma (SARAH): study protocol for a randomised controlled trial. Trials 2014;15:474. 10.1186/1745-6215-15-474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bridgewater J, Galle PR, Khan SA, et al. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol 2014;60:1268-89. 10.1016/j.jhep.2014.01.021 [DOI] [PubMed] [Google Scholar]
- 32.Saxena A, Bester L, Chua TC, et al. Yttrium-90 radiotherapy for unresectable intrahepatic cholangiocarcinoma: a preliminary assessment of this novel treatment option. Ann Surg Oncol 2010;17:484-91. 10.1245/s10434-009-0777-x [DOI] [PubMed] [Google Scholar]
- 33.Rafi S, Piduru SM, El-Rayes B, et al. Yttrium-90 radioembolization for unresectable standard-chemorefractory intrahepatic cholangiocarcinoma: survival, efficacy, and safety study. Cardiovasc Intervent Radiol 2013;36:440-8. 10.1007/s00270-012-0463-4 [DOI] [PubMed] [Google Scholar]
- 34.Camacho JC, Kokabi N, Xing M, et al. Modified response evaluation criteria in solid tumors and European Association for The Study of the Liver criteria using delayed-phase imaging at an early time point predict survival in patients with unresectable intrahepatic cholangiocarcinoma following yttrium-90 radioembolization. J Vasc Interv Radiol 2014;25:256-65. 10.1016/j.jvir.2013.10.056 [DOI] [PubMed] [Google Scholar]
- 35.Hoffmann RT, Paprottka PM, Schön A, et al. Transarterial hepatic yttrium-90 radioembolization in patients with unresectable intrahepatic cholangiocarcinoma: factors associated with prolonged survival. Cardiovasc Intervent Radiol 2012;35:105-16. 10.1007/s00270-011-0142-x [DOI] [PubMed] [Google Scholar]
- 36.Haug AR, Heinemann V, Bruns CJ, et al. 18F-FDG PET independently predicts survival in patients with cholangiocellular carcinoma treated with 90Y microspheres. Eur J Nucl Med Mol Imaging 2011;38:1037-45. 10.1007/s00259-011-1736-x [DOI] [PubMed] [Google Scholar]
- 37.Soydal C, Kucuk ON, Bilgic S, et al. Radioembolization with (90)Y resin microspheres for intrahepatic cholangiocellular carcinoma: prognostic factors. Ann Nucl Med 2016;30:29-34. 10.1007/s12149-015-1026-y [DOI] [PubMed] [Google Scholar]
- 38.Filippi L, Pelle G, Cianni R, et al. Change in total lesion glycolysis and clinical outcome after (90)Y radioembolization in intrahepatic cholangiocarcinoma. Nucl Med Biol 2015;42:59-64. 10.1016/j.nucmedbio.2014.08.011 [DOI] [PubMed] [Google Scholar]
- 39.Servajean C, Gilabert M, Piana G, et al. One case of intrahepatic cholangiocarcinoma amenable to resection after radioembolization. World J Gastroenterol 2014;20:5131-4. 10.3748/wjg.v20.i17.5131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.U.S. National Institutes of Health. 90Y transarterial radioembolization (TARE) plus gemcitabine and cisplatin in unresectable intrahepatic cholangiocarcinoma: ClinicalTrials.gov; 2016 [cited 2016 May 7]. Available online: https://clinicaltrials.gov/ct2/show/NCT02512692
- 41.Piana PM, Gonsalves CF, Sato T, et al. Toxicities after radioembolization with yttrium-90 SIR-spheres: incidence and contributing risk factors at a single center. J Vasc Interv Radiol 2011;22:1373-9. 10.1016/j.jvir.2011.06.006 [DOI] [PubMed] [Google Scholar]
- 42.Rodríguez-Lago I, Carretero C, Herráiz M, et al. Long-term follow-up study of gastroduodenal lesions after radioembolization of hepatic tumors. World J Gastroenterol 2013;19:2935-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lam MG, Louie JD, Iagaru AH, et al. Safety of repeated yttrium-90 radioembolization. Cardiovasc Intervent Radiol 2013;36:1320-8. 10.1007/s00270-013-0547-9 [DOI] [PubMed] [Google Scholar]
- 44.Zarva A, Mohnike K, Damm R, et al. Safety of repeated radioembolizations in patients with advanced primary and secondary liver tumors and progressive disease after first selective internal radiotherapy. J Nucl Med 2014;55:360-6. 10.2967/jnumed.113.127662 [DOI] [PubMed] [Google Scholar]
- 45.Sangro B, Carpanese L, Cianni R, et al. Survival after yttrium-90 resin microsphere radioembolization of hepatocellular carcinoma across Barcelona clinic liver cancer stages: a European evaluation. Hepatology 2011;54:868-78. 10.1002/hep.24451 [DOI] [PubMed] [Google Scholar]
- 46.Sangro B, Gil-Alzugaray B, Rodriguez J, et al. Liver disease induced by radioembolization of liver tumors: description and possible risk factors. Cancer 2008;112:1538-46. 10.1002/cncr.23339 [DOI] [PubMed] [Google Scholar]
- 47.Gil-Alzugaray B, Chopitea A, Iñarrairaegui M, et al. Prognostic factors and prevention of radioembolization-induced liver disease. Hepatology 2013;57:1078-87. 10.1002/hep.26191 [DOI] [PubMed] [Google Scholar]