Recombinant DNA technology has revolutionized the production and use of a number of therapeutic proteins, including cytokines and cellular growth factors. These proteins have similar or identical biologic activities as their natural counterparts and are particularly useful for treating patients who are deficient in these cytokines or growth factors because of congenital abnormalities, certain viral or toxicologic pathologies, or cancer chemotherapy. However, in some instances, the use of recombinant therapeutic proteins or proteins contained in certain viral delivery vehicles has led to the production of antibodies (Abs) that can neutralize protein function (12, 22, 27, 41, 46, 73, 86, 91, 92). Therefore, the production of Abs is a potentially limiting factor for the successful clinical use of recombinant therapeutic proteins (72).
Ab responses to therapeutic proteins may impact the safety, efficacy, and pharmacokinetic and pharmacodynamic parameters of therapeutic proteins (18, 46, 54, 73, 97). For example, complexes formed between Abs and the therapeutic agents they recognize can adversely affect the pharmacokinetic or pharmacodynamic characteristics of recombinant proteins by limiting or altering the volume of distribution in vivo (55). Neutralizing Abs can bind to the portions of drug molecules involved in receptor binding or cell activation, thereby blocking the therapeutic effect of the drug (98). In addition, neutralizing Abs formed against a recombinant protein may also inhibit the activity of the corresponding endogenous factor, resulting in a failure to respond to that factor (10). Rarely, Abs may be part of an allergic response that could potentially lead to inflammation, skin reactions, or other adverse events (20, 94).
Therefore, the development and use of sensitive and specific assays to identify and measure Abs against therapeutic proteins is important to successful clinical use of these agents. In addition to measuring high levels (titers) of specific Abs, some assays possess a level of sensitivity sufficient to detect low levels of naturally occurring Abs or auto-Abs.
This article reviews the different types of Ab assays that have been used to measure and characterize Abs specific to certain recombinant therapeutic proteins, with emphasis on recombinant human erythropoietin (EPO), as well as the benefits, disadvantages, and limitations of the different assays used for screening and diagnostic purposes.
Ab-MEDIATED PURE RED BLOOD CELL APLASIA
A recent example of the negative impact of Abs on recombinant protein therapy is the increased prevalence of Ab-mediated pure red blood cell aplasia (PRCA). Patients with Ab-mediated PRCA present with a rapid onset of EPO resistance, followed by severe decreases in blood hemoglobin levels and reticulocyte counts (i.e., erythroblastopenia). Ab-mediated PRCA arises in a small number of patients receiving recombinant human EPO for the treatment of anemia associated with chronic kidney disease (CKD). It is etiologically distinct from more common forms of PRCA caused by certain toxins, viral infections, malignancies, or congenital abnormalities.
Prior to 1998, there were few reports of Abs in patients treated with recombinant EPO. Patients do not generally produce anti-EPO Abs because of the immunologic similarity between recombinant and endogenous EPO and the maintenance of immunologic tolerance to self proteins (73). In recent years, the number of cases of Ab-mediated PRCA in patients with CKD has increased substantially and in most cases is associated with subcutaneous (SC) administration of epoetin alfa formulated without human serum albumin stabilizer (Eprex, Ortho Biologics LLC, Manati, P.R.) (28). Table 1 lists the currently available number of confirmed Ab-mediated PRCA cases worldwide for five currently approved recombinant erythropoietic agents.
TABLE 1.
Reported number of Ab-mediated PRCA cases worldwide by product use in patients with CKD
| PRCA cases by single-product use | Eprexa (epoetin alfa) | Epogen/Procrit (epoetin alfa) | NeoRecormonb (epoetin beta) | Aranesp (darbepoetin alfa) |
|---|---|---|---|---|
| Report through date (mo-day-yr) (reference) | 12-31-03 (45) | 4-31-04 (1) | 12-31-02 (82) | 4-31-04 (1) |
| No. of confirmed PRCA cases | 180 | 6 | 8 | 0 |
Also distributed and sold under brand names Erypo, Epopen, Epoxitin, and Globuren.
Also distributed and sold under brand names Recopen and Recormon.
Several different immunologic assays have been used to measure and characterize Abs against EPO. However, there is currently no consensus on which assay is considered best for screening patients or for diagnostic purposes, because each assay uses different technology for measuring Abs and possesses different levels of sensitivity, ease of use, and potential clinical relevance.
SENSITIVITY AND SPECIFICITY OF Ab ASSAYS
There is a critical need to understand the sensitivity, specificity, and utility of assays that measure anti-EPO Abs in patients with CKD-associated anemia and to reach consensus on a standard assay method. A standard assay may prove useful as an early diagnostic tool for patients developing Ab-mediated PRCA and for monitoring their disease progression and response to treatment. The terms sensitivity and specificity are used to describe the characteristics of Ab assays. Sensitivity refers to the ability of an assay to detect very small concentrations of Abs in a test sample. A highly sensitive assay is more likely to detect low levels of specific Abs in a cohort of patients and therefore has a low probability for producing false-negative findings. The referred relative sensitivities of different assays vary, according to (i) the practical limitations imposed by the types and affinities of Abs in serum samples and internal standards (58), (ii) the type of detection system employed (e.g., radiometric, colorimetric, or chemiluminescent) (66, 80), (iii) the method of analysis (e.g., capture assay, direct or indirect assay, or competitive inhibition assay) (2, 76), (iv) the presence of inhibitory substances such as immune complexes, cross-reactive antigens, or heterophilic Abs in serum samples (7, 32, 50), (v) the nature and concentration of specific target proteins (EPO, in this case) and their epitopes (5, 34, 66), and (vi) the range of Ab concentrations that each assay can measure.
Thus, the practical or perceived sensitivity of each assay is generally more conservative than the actual or real sensitivity, which often lies outside of the range of statistical confidence for calculating Ab concentrations. Therefore, the practical (lower) limit of detection is the lowest threshold at which Ab measurements can be made within the design of each assay system. In addition, assay sensitivity is affected strongly by the relative affinity of different Abs for a particular target protein or antigen. For example, high-affinity immunoglobulin G (IgG) Abs bind to antigens more tightly than the low-affinity IgM and IgG Abs that are typically produced in the early stages of Ab maturation. Naturally occurring Abs, which can interfere with assay results, tend to have low affinity and are present at low concentrations, because there is little or no active immunization (80).
Specificity refers to the ability of an assay to detect only those Abs directed against a specific target protein or ligand. The specificity of each assay is a product of the biochemical mechanics of Abs binding to the test ligand and the subsequent detection method used. Therefore, specificity is largely influenced by the nature and type of Abs in patients' samples, the presence of confounding serum factors, and the target ligands (antigens) that are used in the procedure. A highly specific assay has a very low probability of measuring false positives resulting from nonspecific binding of Abs or cross-recognition of antigenically related proteins or molecular mimics. Most assays use blocking reagents that reduce or eliminate nonspecific Ab binding.
Ideally, a useful Ab assay possesses both high sensitivity and high specificity, but in practical experience, a highly sensitive assay suitable for screening large numbers of samples may not have the level of specificity required to prevent samples from being falsely identified as having Abs present. Comparative analyses of different types of assays have often shown wide variability in their relative sensitivity and specificity for various diagnostic purposes (23, 25).
TYPES OF Ab ASSAYS
A number of different assays have been used to measure Abs specific to recombinant proteins. These assays are generally grouped into two major categories: (i) binding Ab assays, which measure the binding capacity of Abs to proteins; and (ii) neutralizing Ab assays, which measure the ability of Abs to neutralize a biological effect of the protein in biological systems (bioassays). There are several types of binding Ab assays, including enzyme-linked immunosorbent assays (ELISAs), radioimmunoprecipitation (RIP) assays, and the BIAcore biosensor assay. In contrast, only bioassays characterize neutralizing Abs.
ELISA
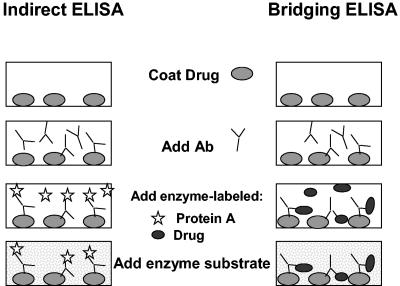
ELISAs colorimetrically detect Abs bound to protein antigens either directly or indirectly. Variations of these assays can improve sensitivity and/or specificity (39, 42, 60). For example, indirect ELISAs detect primary (e.g., serum sample) Abs that bind to target proteins immobilized on plastic wells by using enzyme-linked secondary Abs, such as goat anti-human Ig Abs, and enzyme substrates that change color in the presence of enzyme-labeled Abs. The intensity of substrate color change is proportional to the amount of enzyme-linked Abs present in the sample wells and correspondingly the amount of Abs that bound previously to the plastic-immobilized protein. Properly optimized indirect ELISAs generally give low background readings and high levels of sensitivity and specificity because confounding results from nonspecific Ab binding and interfering serum factors (e.g., rheumatoid factors) are reduced or eliminated (40). Some indirect ELISAs employ the use of avidin-biotin complexes between Abs and protein antigens to increase assay sensitivity (15, 74).
Bridging ELISAs reduce background readings by using fewer amplification steps in the procedure and the requirement for two specific binding events for the target drug, which increases specificity of the assay. The bridging ELISA takes advantage of the two antigen binding sites on each Ab molecule (Ab bivalency) that allows Abs to form a bridge between (i.e., cross-link) drug immobilized on plastic wells with enzyme-labeled drug that is added in the detection step (61). In this assay, the unlabeled drug is first immobilized onto the plastic wells, and optimally diluted Abs in a test serum sample are allowed to bind, followed by the usual washing step to remove unbound Abs. Enzyme-labeled drug is then added to complete the bridge, and a colorimetric substrate is then added to visually detect the presence of specific Abs.
Schematic examples of indirect and bridging ELISAs are shown in Fig. 1, in which an enzyme-linked, anti-Ig (secondary) Ab amplifies the signal for detecting serum (primary) Abs that have bound to immobilized proteins. A version of the indirect ELISA was used by Urra and colleagues to characterize anti-EPO Abs in a patient with EPO-resistant PRCA (87).
FIG. 1.
Schematic illustrations of indirect and bridging ELISA testing procedures for measuring Abs against protein drugs (e.g., EPO). In the indirect ELISA, the protein drug is coated onto the wells of plastic assay plates prior to the addition of serum samples. Any specific Abs that are bound to the immobilized protein are subsequently detected with enzyme-linked, anti-Ig Abs (monoclonal or polyclonal) and enzyme substrate. In the bridging ELISA shown here, the unlabeled protein drug is first coated onto the wells, serum Abs are allowed to bind to the immobilized drug, and Abs are then detected with enzyme-labeled drug plus enzyme substrate. The bridging ELISA depicted here relies on the ability of bivalent Abs (IgG) to bridge the immobilized drug with enzyme-labeled drug in solution.
RIP
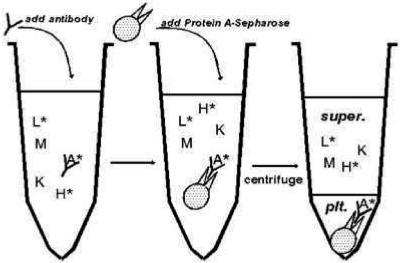
In contrast to ELISAs, which utilize plastic wells as the solid support for Ab testing, RIP assays measure Ab binding to cognate antigens in a fluid phase. Thus, anti-cytokine Abs may be detected and quantified with radiolabeled proteins and precipitating agents, such as protein A-Sepharose or protein G-Sepharose, which bind to the Fc portion of Abs and are used to collect antigen-Ab complexes by centrifugation (5). An illustrated example of a typical RIP testing platform is shown in Fig. 2. This assay was used by Prabhakar and colleagues to characterize the anti-EPO Abs in a patient with EPO-resistant PRCA (69).
FIG. 2.
RIP testing procedure for measuring Abs against a ligand (protein). This method uses fluid-phase binding of sample Abs in a patient's serum with a specific ligand (e.g., radiolabeled EPO [A*]) alone or in a mixture of proteins) (step 1), the subsequent binding of protein A-Sepharose or protein G-Sepharose beads that attach to ligand-Ab complexes (step 2), and the collection of beads by centrifugation (step 3). This assay method can incorporate competitive free (nonradiolabeled) ligand to test the specificity of Abs for individual proteins or peptides. Reproduced from reference 36 with permission.
BIAcore
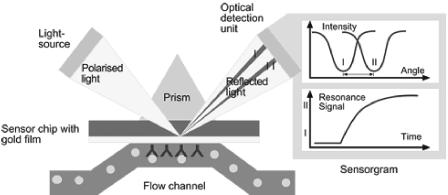
The BIAcore immunoassay uses surface plasmon resonance to optically measure Abs that bind to target antigens immobilized on a special dextran-coated glass surface (Fig. 3). The signal in the BIAcore assay directly increases as the mass of protein (Abs) accumulates on the surface of the sensor chip. The BIAcore immunoassay has been used to characterize autoimmune Abs in patients with systemic lupus erythematosus (38), myasthenia gravis, thymoma (59), autoimmune dilated cardiomyopathy (63), Wegener's granulomatosis (89), and human immunodeficiency virus infection (88). The BIAcore assay has also been used to characterize Abs against a humanized mouse monoclonal Ab used for treating colon cancer (71), pegylated alfa interferon 2b (84), and anti-EPO Abs in normal individuals and patients receiving one or more EPOs (77-79).
FIG. 3.
The principle of optical detection of Ab binding to surfaces of sensor chips in a BIAcore immunoassay device. Binding of Abs to a sensor chip is detected by the internal reflection of incident, polarized light off the chip surface. The angle of internal reflectance is dependent on the mass of Abs binding to the chip surface. Reproduced with permission from BIAcore, Inc.
Bioassays
Bioassays are the only assays that can quantitatively measure neutralizing activity against therapeutic proteins. Ab-mediated neutralization of recombinant proteins depends on the use of cultured cells (primary or recombinant cell lines) that respond physiologically to native proteins. Alteration of protein structure or blocking of receptor binding sites or other functional sites by Abs can inhibit cell responses to cytokines and other therapeutic proteins (83). For this reason, bioassays are useful for measuring the immunogenic potential of proteins expressed in different cells (93) and studying the binding stoichiometries of Abs with their target proteins (31).
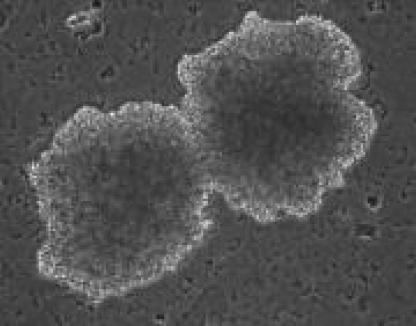
A number of different bioassays have been used to quantify EPO-neutralizing Abs, including EPO-stimulated growth of erythroid precursors in plasma-clot cultures of mononuclear blood cells or bone marrow cells (13, 52) and proliferation of the EPO-dependent human erythroleukemia cell line, UT-7 (13). An example of erythroid colonies growing in culture is shown in Fig. 4. The usefulness of immortalized cell lines lies in the ability to culture these cells over extended periods of time before cell death occurs. Proliferation of such cells can be quantified by using tritiated thymidine to assess nucleic acid synthesis and tetrazolium dyes to assess metabolic activity (33, 96). In addition, genetic variants of cell lines can be produced and cultured for the purposes of biochemical or immunochemical studies. For example, a genetic variant of the UT-7 cell line transfected with the wild-type c-mpl thrombopoietin receptor gene or gene mutants was used to map the functional domains on the thrombopoietin receptor protein responsible for cellular proliferation and megakaryocyte differentiation (85). Similar work on the EPO receptor was done by Harris and colleagues using the murine myeloid cell line 32D transfected with full-length or truncated human EPO receptor (37). The UT-7 cell line and a murine erythroleukemia cell line, HCD57, were used to map the receptor binding domains of EPO (8). The HCD57 cells, which are susceptible to apoptosis during EPO withdrawal, have been used to study gene regulation of programmed cell death in erythroid cells (44). In vitro studies with the UT-7 cell line have elucidated the cytotoxic mechanism of human parvovirus B19 on erythroid cells that is induced through cellular apoprosis mediated by the nonstructural viral protein NS1 (64). Another human erythroleukemia cell line, TF-1, that expresses a truncated EPO receptor, was used to study transcription factor activation of erythroid cell growth and differentiation following EPO binding (17); retroviral transfection of this cell line with a fully functional EPO receptor restored normal transcription factor activation.
FIG. 4.
Photomicrograph of “burst” human erythroid colonies forming in vitro in the presence of EPO and other growth factors. Reproduced from reference 65 with permission. Erythroid colony assays can be performed with blood or bone marrow-derived cells.
Practical aspects of Ab assays
Each Ab assay has its own characteristic advantages and disadvantages based on technical difficulty, intra- and interassay variability (i.e., precision), sensitivity for measuring Abs, specificity for the target protein or ligand, susceptibility to false-positive or false-negative results, and cost. As a result, the use of different Ab assays in various studies may confound the comparative interpretation of results because of the differences in the assays' sensitivity and specificity. This can cause confusion for nephrologists and immunologists monitoring the clinical course of Ab-mediated PRCA. The comparative strengths and weaknesses of the various Ab assays discussed in this review are listed in Table 2.
TABLE 2.
Strengths and weaknesses of various antibody assaysa
| Assay type | Advantage(s) | Disadvantages | Susceptibility to false-positive or false-negative results |
|---|---|---|---|
| RIP | Highly sensitive (29); common usage, wide experience; multiple parameters (more limited than ELISA); inexpensive; versatile and/or different types (90) | Radiometric (exposure and/or waste); rapid decay of radiolabeled antigens; nonautomated procedure requiring centrifugation or microfiltration; not easily scaled up; may not detect all Ab isotypes, especially IgM | Yes; susceptible to interference with IgM rheumatoid factor or high backgrounds with some sera (30) |
| ELISA | Highly sensitive (76); common usage, wide experience; multiple parameters (e.g., cross-titrations); scalable and/or automated, nonradiometric; Ab isotype analysis (81); inexpensive; versatile and/or different types | High background readings in some assays; toxic enzyme substrate reagents; does not always measure low-affinity Abs | Yes; susceptible to interference with IgM rheumatoid factors (false positives) or naturally occurring Abs (40, 53); competition among Ab isotypes can hamper isotype analysis (false negatives) (34); nonspecific binding can cause high background and reduce sensitivity |
| BIAcore | Real time (kinetic) binding and competitive analyses (88) of both low- and high-affinity Abs and isotypes (78); epitope mapping (88); ligand purification analysis (51); Ab affinity measurements (62); semiautomated; sequential testing of samples on single-sensor chip | High cost; poor access to instrument; lack of expertise among researchers | No/yes; remarkably nonsusceptible to contaminating matrices and interfering factors (21, 70) |
| Bioassays | Only measures neutralizing Abs | Only moderately sensitive; can be made automated (35); growth-dependent assays (e.g., primary cells) can be time consuming (days) (52); different cell lines used among labs (e.g., EPO-dependent cell lines) (8, 12, 78); not easily scalable | Yes; serum-neutralizing factors arising from existing morbidities could result in false positives for neutralizing Abs against therapeutic proteins (57, 59); serum factors other than Abs can result in false positives, so bioassay results must be interpreted in light of immunoassay results |
Numbers in parentheses are references.
ASSAYS USED TO MEASURE Abs AGAINST PROTEIN BIOLOGICALS
Various immunologic assays have been used to measure or characterize Abs against recombinant therapeutic proteins. These include assays for Abs against EPO, interferon alfa, beta interferon (β-IFN), granulocyte-macrophage colony-stimulating factor (GM-CSF), streptokinase, thrombopoietin, and others. Table 3 lists examples of assays used to measure naturally occurring or acquired Abs against a variety of cytokines and growth factors, and their relative levels of sensitivity. This table shows wide variability in assay sensitivity (i.e., limits of detection) and concordance between assays, depending on the application. No single assay is predominant over others, and each assay is tailored to individual needs by researchers. The sensitivity of some ELISAs is very good (e.g., 5 ng of Abs/ml) and reaches or approaches the sensitivity of many RIP assays. However, in practical situations, ELISAs tend to have more moderate sensitivity against relatively high background readings. Furthermore, the ELISA may take 2 days to perform, if plates are incubated overnight at 4°C at some step in the procedure. The RIP assay can be performed in a single day, provided the technicians have access to the proper commercial reagent standards. It is the most sensitive of all the assays. However, radioactivity must be counted in each sample tube with a gamma radiation counter. The BIAcore assay is fully automated and therefore requires very little technical assistance, except for the required dilution of serum samples and preparation of the sensor chips (79). In addition, the BIAcore is very fast, with a single analysis including loading and final washout taking only 8 to 10 min to perform. The BIAcore assay is probably the most versatile Ab assay in terms of speed, specificity, sensitivity, ability to characterize binding Abs (i.e., Ab isotypes and relative binding affinities), and ability to regenerate the sensor surface for repeated assays (79). Bioassays are the only assays capable of measuring the neutralizing capacity of Abs, but they are labor intensive, requiring days of cultivation for cells to proliferate and microscopic quantification of the erythroid colonies growing in culture.
TABLE 3.
Representative assays used to measure Abs against human therapeutic proteinsa
| Antigen | Antibodies | Assay method | SL of assay | Results |
|---|---|---|---|---|
| HuIFNγR (26) | Mouse anti-HuIFNR MAbs | RIP; indirect ELISA and cell binding assay; bioassay for inhibition of IFN antiviral activity | Bioassay and ELISA: 1.0-7.0 μg of anti-IFN Abs (for Abs binding IFNγR+ cells)/ml | Six MAbs bound to IFNR+ cells, inhibited 125I-labeled γ-IFN binding, inhibited antiviral activity of γ-IFN |
| Recombinant HuIFNα-2a (Roferon-A) (39) | Patients' sera (various treatments) | RIP; bioassay for inhibition of IFN antiviral activity; ELISA (bead-based assay) | RIP and ELISA: 5 ng of IgG/ml of serum; bioassay: 100 IFN NU (50% viral inhibition endpoint) | Excellent correlation (93%) between ELISA and RIP; antiviral bioassay least sensitive |
| Recombinant HuIFNα-2a (Roferon-A) (42) | Patients' sera (various treatments) | ELISA (bead-based assay); competitive commercial RIP; bioassay for inhibition of IFN antiviral activity | ELISA: 5 ng of IgG/ml of serum; RIP: 50 NU of Abs (1 NU per 1 IFN U); Bioassay-100 IFN NU | Poor correlation between ELISA and RIP (48-50% false negatives) or bioassay (100% false negatives) |
| Islet cells (type 1 diabetes mellitis) (3) | Patients' sera (diabetes- related auto-Abs) | Two commercial ELISA kits; RIP using PEG-mediated precipitation; indirect immunofluorescence assay | ELISA: 1,500 mU Glutamic acid decarboxylase, none specified for second ELISA test; RIP: 5 mU of bound insulin/ml | No correlation among assays; 14-50% false-negatives with ELISAs; 98% sensitivity with RIP assay |
| Pooled human serum IgG preparations (75) | Natural anti-cytokine Abs (GM-CSF, IL-5, IL-10) | Protein G affinity chromatography | SL, ≥0.1 μmol of cytokine/mol of Ab | Commercial pooled human IgG preparations contain natural anti-GM-CSF Abs at highest concentration (0.24-5.0 μmol/mol of Ab), followed by anti-IL-5 (1.3 μmol/mol of Ab) and anti-IL-10 (0.12-0.4 μmol/mol of Ab) |
| TPO (4) | Serum from cancer patient treated with pegylated TPO | Solid-phase RIP; bioassay for neutralizing Abs with Mpl-transfected 32D murine cells | RIP: twofold increase in binding of radiolabeled TPO over baseline; bioassay: 50% growth inhibition against 250 pg of TPO/ml in culture | Emergence of binding Abs (RIP) after sixth SC administration of pegylated TPO and neutralizing Abs after seventh administration |
| TPO (54) | Sera from 2 previously healthy volunteers and 1 cancer patient treated with pegylated TPO | RIP (protein A-, protein G-agarose beads) | SL, ≥75 ng/ml of Abs | Anti-TPO Abs detected after second or third SC administration of pegylated TPO in healthy subjects and after multiple administrations (>20) in the cancer patient |
| Recombinant streptokinase (58) | Patients' sera (myocardial infarction) | Indirect ELISA | Titer, ≤1,750 for IgG1 and 4,200 for IgG | Similar results for assays measur:ing IgG and IgG1 Abs (54-59% anti-streptokinase positive) |
| Recombinant streptokinase (11) | Plasma from 30 healthy volunteers, 60 patients treated with streptokinase for myocardial infarction (7-10 days and 12 and 24 mo), and 12 patients with raised Abs | Indirect ELISA; fibrin plate lysis assay; clot lysis assay for streptokinase neutralization | Not specified | ELISA and neutralization assay results were positively correlated (P < 0.001); neutralization preceded ELISA Abs (IgG); by day 10, all patients had elevated IgG and neutralizing Ab levels; Abs persisted for 24 mo in 75% of patients |
| Recombinant HuGM-CSF (2 Escherichia coli-derived proteins) (91, 93) | Sera from patients with colorectal carcinoma treated with either of two E. coli-derived rHuGM-CSF products | Indirect ELISA for binding Abs, bioassay for neutralizing Abs (TF-1 cells), and Western blotting | SLs of assays not specified; 500 ng/well (5 μg/ml) of rHuGM-CSF coated onto ELISA plates; bioassay measured vol of sera required to neutralize 1 IU of WHO International Standard GM-CSF (88/646) | Total of 47 of 58 patient sera recognized rHuGM-CSF at 500 ng/well (5 μg/ml) in ELISA; 9 of 58 sera (various vol) contained neutralizing Abs; Western blots showed heterogeneity of GM-CSF proteins from E. coli, yeast, and CHO cells |
| Recombinant HuGM-CSF (E. coli-derived protein) (86) | Sera from patients with colorectal carcinoma, metastatic carcinoma, or myeloma treated with high or low-dose E. coli-derived rHuGM-CSF plus tumor antigens or idiotype Ab (myeloma patients) | Indirect ELISA for binding Abs, bioassay for neutralizing Abs (TF-1 cells), and Western blotting | SLs of assays not specified; 500 ng/well (5 μg/ml) of rHuGM-CSF coated onto ELISA plates; bioassay measured volumes of sera required to neutralize 1 IU of WHO International Standard GM-CSF (88/646) | Administration of high-dose rHuGM-CSF (425-500 μg/day for 10 days) plus tumor antigens resulted in production of anti-GM-CSF Abs in 11 of 20 (55%) metastatic carcinoma patient samples; low-dose rHuGM-CSF (75-80 μg/day for 4 days) plus tumor antigens resulted in Ab production in only 4 of 25 (16%) patients with myeloma or colorectal carcinoma; no neutralizing Abs were detected; binding Abs did not interfere with clinical benefits or rHuGM-CSF therapy |
| Recombinant human IL-2 (41) | Sera from patients with colorectal carcinoma treated with either of two E. coli-derived rHuIL-2 products | Indirect ELISA, bioassay for neutralizing Abs (CTLL-2 cells), Western blotting | SLs of assays not specified; 25 ng/well (250 ng/ml) of HuIL-2 coated onto ELISA plates; bioassay measured volume of sera required to neutralize 0.1 IU of rHuIL-2 (2 IU/ml) | Total of 10 of 19 patients developed anti-IL-2 Abs, 1 patient developed neutralizing Abs; Western blots showed Abs recognized native-form IL-2 and two E. coli-derived IL-2 products; neutralizing Abs also cross-neutralized different IL-2 preparations in the CTLL-2 bioassay |
| bFGF (99) | MAb 48.1 (anti-bFGF) capture Ab | BIAcore detection of bFGF in various diluents | 5.65 ng of bFGF/ml of buffered saline | Human serum, bovine serum albumin, Pluronic F127 surfactant, and carboxymethylcellulose did not interfere with binding |
| Humanized anti-IL-5 mouse Ab (95) | Mouse sera | BIAcore | 1 μg of antihuman/mouse chimeric Abs/ml | Assay permits detection of serum levels of injected chimeric Abs and any Abs produced against chimeric Abs |
| HIV-1 gp160 peptides (88) | Polyclonal human sera | BIAcore and ELISA | SL, 5 ng/ml with both assays | Linear range of Ab binding greater with BIAcore than with conventional ELISA |
Abbreviations: MAbs, monoclonal Abs; IL-5, interleukin-5; PEG, polyethylene glycol; SL, sensitivity limit; HIV, human immunodeficiency virus; rHu, recombinant human HuIFNγR, human α-IFN receptor; bFGF, basic fibroblast growth factor; NU, neutralizing units; TPO, thrombopoietin. Numbers in parentheses are references.
ASSAYS FOR MEASURING Abs AGAINST RECOMBINANT EPO
Prior to cloning of the human EPO gene, there were limited amounts of purified natural (urinary) EPO available, and investigators often used functional cellular assays or in vivo bioassays to measure anti-EPO Abs.(47-49, 68) Following the successful cloning and expression of EPO in 1985 (43, 56), simpler in vitro assays for serum EPO levels and binding Abs against EPO were developed (19).
Early reports of Ab-mediated PRCA and development of resistance to EPO increased interest in the potential immunogenicity of EPO and the induction of autoimmunity against endogenous EPO. Studies were carried out to address the immunogenicity of EPO in larger groups of patients with renal anemia and the occurrence of natural anti-EPO Abs in normal, healthy volunteers by different Ab assays. These studies are listed and described briefly in Table 4. These data reveal wide variance in the sensitivity of different assays, with RIP and bioassays used most often. Quantitative results with ELISA have not been described to a great extent in the literature, possibly because of poor sensitivity, specificity, or both. Generally, there appears to be excellent concordance between RIP and different bioassays for anti-EPO Abs. The BIAcore assay technology has only been used recently to measure anti-EPO Abs.
TABLE 4.
Assays used to measure anti-EPO Abs in single-arm or controlled studies of patients with Ab-mediated PRCAa
| No. of serum samples, PRCA or dialysis | Assay method(s) (SL) | Results | |
|---|---|---|---|
| 40 (plus 40 control sera) (16) | Indirect ELISA (EPO-coated wells) (SL, 1:10 serum dilution against 80 ng of EPO/ml) | Controls, negative; dialysis, 67% (27/40) positive with low-affinity Abs | |
| 13 (EPO-resistant PRCA) (14) | RIP (protein G) (SL, 200 mU of EPO/ml of serum); bioassay for neutralizing Abs with normal bone marrow cells (SL, ≥50 IU EPO/ml of serum) | 100% concordance between RIP and neutralizing Ab assays (13/13); RIP Ab positive (range: 3-86 IU epoetin alfa bound/ml); all sera had neutralizing Abs against epoetin alfa | |
| 8 (EPO-resistant PRCA) (78) | RIP (protein A) (SL, 10 ng of Abs/ml; ELISA (bridging assay) (SL, 780 ng of Abs/ml); BIAcore 3000 (SL, 390 ng of Abs/ml); bioassay for neutralizing Abs using EPO-transfected mouse cell line (SL, 900 ng of Abs/ml) | Excellent concordance in Ab positivity (100%) for all eight patients using RIP, BIAcore, and bioassay, good concordance with Ab concentrations; two sera tested Ab-negative by ELISA but were positive by all other assays; BIAcore assay determined the predominant Ab isotypes | |
| 1,531 CKD patients from 5 Canadian centers treated with epoetin alfa (96) | RIP (protein A) (SL, ≥0.9% of total radioactive EPO bound by a 1:20 serum dilution; 0.4%-0.9% EPO bound = borderline positive); bioassay for neutralizing Abs (UT-7 cell line) (SL, ≥20% neutralization in first 2 serum dilutions) | Only 4 patients had anti-EPO Abs, three borderline positive at indicated SL; no neutralizing Abs detected | |
| 1,502 patients from multicenter European darbepoetin alfa trial (9) | RIP (protein A) (SL, 10 ng of Abs/ml) | No patients reported with clinical signs of Ab-mediated PRCA |
SL, sensitivity limit of assay. Numbers in parentheses are reference numbers.
The following is a brief summary of the literature on the various types and results of assays used to measure anti-EPO Abs or inhibitory factors in renal anemia patients with PRCA.
ELISA
ELISAs were used to measure EPO binding Abs in two early reports of an epoetin-treated, hemodialysis patient who had become EPO resistant (67, 87): the 1997 report by Urra and colleagues also compared serum Ab titers in an Ab-positive patient to sera from 30 hemodialysis patients and 10 healthy control subjects. In these reports, titers were determined for the serum from the EPO-resistant patient with renal anemia and compared to fixed amounts of epoetin alfa and epoetin beta in an ELISA with a peroxide-labeled, anti-human secondary Ab for detection of EPO binding Abs (67, 87). A single negative control serum (1 patient) was used in the first study, while sera from the 10 hemodialysis patients and 30 normal patients served as negative controls for the second study. Test results were expressed in optical absorbance units. The PRCA serum, which neutralized EPO in a bioassay, reduced binding of the positive standard serum in the ELISA test by 42 to 75%, depending on the dilution of serum tested and which epoetin was used to coat wells. Sera from the 30 normal donors and 10 hemodialysis patients were negative for anti-EPO Abs.
An indirect ELISA was used by Weber and colleagues to demonstrate the presence of anti-EPO Abs in a patient with Ab-mediated PRCA who had received multiple recombinant EPOs (20, 94). Abs in serum samples collected during the period of EPO resistance were shown to recognize each of the three recombinant EPOs this patient received—epoetin alfa, epoetin beta, and darbepoetin alpha. Specificity of the assay was confirmed by homologous and heterologous displacement (i.e., competition) with the various recombinant proteins (20, 94).
Swanson and colleagues recently developed a bridging ELISA for detecting anti-EPO Abs (78). Sera from 8 of 13 patients with Ab-mediated PRCA, originally reported by Casadevall and colleagues (14), were analyzed by the bridging ELISA, RIP, the BIAcore 3000 immunoassay system, and a bioassay with a murine cell line transfected with the human EPO receptor (78). The sensitivity limits of these four assays for anti-EPO serum Abs were reported to be, in decreasing order, 10 ng of EPO/ml (RIP), 390 ng of EPO/ml (BIAcore), 600 ng of EPO/ml (ELISA), and 900 ng of EPO/ml (bioassay). The bridging ELISA detected anti-EPO Abs in six of the eight PRCA sera, while the other three assays successfully detected Abs in all eight sera tested. These investigators concluded that the two Ab-negative sera with the bridging ELISA represented false-negative results and, consequently, this assay was not used to quantify anti-EPO Abs.
RIP
The first description of an RIP assay for measuring anti-EPO Abs was provided by Bergrem and colleagues in 1993 (6), who used a modified version of the RIP assay for serum EPO described by Egrie and colleagues in 1987 (24). This assay demonstrated the presence of circulating, anti-EPO binding Abs in an EPO-refractory patient with renal anemia at a high titer of 400 (arbitrary) radioimmunoassay units (RUs). Scatchard analysis calculated the plasma EPO-binding capacity to be 32 pmol of EPO/ml of plasma (100 IU of EPO/ml of plasma). The lower limit of detection was given as 10 RUs.
Prabhakar and colleagues used a similar RIP assay to quantitate EPO binding Abs in a hemodialysis patient who had become resistant to EPO (69). Four months after EPO treatment was discontinued, anti-EPO Abs were present at a serum titer of 1:50 (i.e., 50 IU of EPO bound per ml of serum) and decreased slowly to a titer of <1:10 by 24 months following cessation of EPO treatments. Of interest, this patient had disproportionately elevated serum EPO levels during the time when serum Abs were detected. The sensitivity limits of this RIP assay were not specified either in this study (69) or the original Egrie article (24), but estimates from the published data indicated that a titer of 1:50 or higher was meaningful (about two times above the background).
Casadevall and colleagues analyzed serum from a transfusion-dependent elderly patient with severe anemia by a modified RIP assay for anti-EPO Abs (13). Scatchard analysis with the RIP assay demonstrated a single class of high-affinity anti-EPO Abs in this patient's serum (dissociation constant, 430 ± 80 pM). An indirect ELISA demonstrated the presence of IgG Abs but not IgM Abs in this serum. The binding capacity of the patient's serum Abs was calculated to be about 2.7 IU of EPO/ml of serum. Thus, even relatively small amounts of serum Abs could apparently neutralize most of the circulating EPO in vivo. Serum Abs from this patient effectively neutralized the proliferation of EPO-dependent UT-7 cells in a bioassay.
Casadevall and colleagues later used RIP and bioassays to screen a cohort of 13 renal anemia patients who became EPO resistant during their course of treatment with one or more EPOs (14). The relative binding capacities of the patients' sera ranged from 4 to 86 IU of EPO/ml of serum. The lower limit of detection was calculated to be 200 mIU of EPO/ml of serum. All of these patients had EPO-neutralizing Abs in their serum, as determined by a bioassay. Thus, 100% concordance between the two assays was achieved with these sera.
BIAcore
The BIAcore surface plasmon resonance assay system has proven useful for many biochemical and immunologic purposes, including epitope mapping, kinetic binding, Ab-ligand affinity measurements, and product purity analysis (Table 3). However, it has just recently been used to test for the presence of anti-EPO Abs in patients' sera. Results of the BIAcore analysis of 8 of 13 PRCA sera by Swanson (78) showed that this assay was equivalent to RIP and a bioassay for quantifying anti-EPO Abs (in micrograms per milliliter) in individual serum samples, thus demonstrating excellent concordance among assay methods. Ab isotype analysis by BIAcore qualitatively demonstrated the predominant presence of the IgG4 and IgG1 Ab subclasses in these patients' sera. The BIAcore assay also provided Ab dissociation data, which confirmed the specificity of Abs for epoetin alfa (78).
Bioassays
In 1967, Krantz and colleagues characterized a serum factor that inhibited erythropoiesis in patients with EPO-resistant PRCA by using an in vitro culture system of autologous bone marrow cells (47-49). The inhibitor substance, shown to be serum IgG, halted new heme synthesis by cultured autologous bone marrow erythroid cells and produced Ab-dependent cellular cytotoxicity (lysis) of erythroid cells. In serum from one patient, this IgG factor bound to erythroblast nuclei (47).
In 1975, Peschle and colleagues first demonstrated the presence of neutralizing anti-EPO Abs in the serum of a renal anemia patient with PRCA with a polycythemic mouse model of erythropoiesis (68). These investigators coined the terms PRCA type A and type B to distinguish between red blood cell aplasia associated with serum inhibition of erythroid colony formation and red blood cell binding (type A) but not EPO neutralization, and serum neutralization of EPO without red blood cell binding (type B). The limit of sensitivity of the in vivo mouse assay was not determined in this study.
Casadevall and colleagues tested serum obtained from patients with confirmed Ab-mediated PRCA for the presence of EPO-neutralizing Abs (14). Using bone marrow cells from healthy donors, they showed that all PRCA serum samples effectively inhibited proliferation of bone marrow cultures in vitro, thus demonstrating 100% concordance between this bioassay and results with RIP. In this study, the EPO-dependent UT-7 cell line was used to further characterize neutralizing Abs obtained from one of the patients. Similar bioassays with autologous bone marrow cells and the UT-7 cell line were used earlier by Casadevall and colleagues to demonstrate the presence of EPO-neutralizing Abs in a patient with confirmed Ab-mediated PRCA, and the RIP assay was used to confirm the bioassay results (13).
INTERPRETATION OF Ab TESTING RESULTS
Researchers have used a number of different immunologic assays to detect the presence of anti-EPO Abs in patients with Ab-mediated PRCA. Generally, Abs associated with Ab-mediated PRCA are high affinity in nature and recognize distinct epitopes on the protein portion of the EPO molecule (14). Although anti-EPO Abs can be detected by the different assay methods, there are no universal reagent standards for the various assays, and there is currently no single assay that is considered superior to the others. Furthermore, the assay most often used to measure anti-EPO Abs, the RIP assay, is used in various laboratory conditions and formats, making it difficult to directly compare results from different laboratories. A similar situation exists for ELISA, in that researchers use different reagents, incubation times, and enzyme substrates to develop the plates.
Tables 2, 3, and 4 show that there are large differences in the sensitivities and specificities of different assays. In the case of Ab-mediated PRCA, ELISA and RIP assays measure binding Abs that may or may not be correlated with neutralizing Abs, and the production of binding Abs does not always parallel the production of neutralizing Abs in amplitude or time to appearance. Data from one study indicated that RIP and EPO-neutralization assays are highly correlated (100%), but the study size was quite small (8 patients) (79).
The utility of the RIP assay for screening large numbers of patients was shown by Wu and colleagues (96), who found only three borderline Ab-positive patients and one low-titer Ab patient in a cohort of over 1,500 EPO-treated renal anemia patients. In this study, the RIP titers appeared to correspond to the severity of symptoms of PRCA; that is, there was no evident PRCA in the three borderline-positive patients and a generally mild and reversible EPO-refractory anemia in the 1 Ab-positive patient. The RIP assay can also be used to determine Ab binding affinities (14).
Results with the ELISA are less clear. In a controlled study, the ELISA detected low-affinity Abs in a relatively high percentage of EPO-treated patients (16). Since these low-affinity Abs did not correlate with any disease symptoms, the ELISA used in this study may have limited clinical utility as a screening assay for PRCA. In a later study, a bridging ELISA did not detect anti-EPO Abs in two of eight patients with confirmed Ab-mediated PRCA (78); this result may be related to the poor ability of at least some ELISA to detect lower-affinity, anti-EPO Abs.
Bioassays are essential for detecting neutralizing Abs or other factors that can functionally inhibit cytokine or growth factor activity and may be the most clinically relevant Ab assay for measuring neutralizing capability of Abs against EPO. However, neutralizing Ab assays may not be suitable for rapid screening of large patient groups, because of their lack of sensitivity and length of time required to culture erythroid cells (7 to 14 days). However, the use of continuously growing EPO-dependent cell lines will overcome this problem.
The BIAcore immunoassay has been used successfully to measure high-affinity, anti-EPO Abs in the sera of patients with Ab-mediated PRCA, and these Abs appeared to correlate well with the presence of EPO-neutralizing Abs. The ability of the BIAcore assay to readily determine Ab concentration and binding affinities may ultimately prove useful in differentiating low-affinity from high-affinity Abs and their relative contribution to the development of Ab-mediated PRCA.
Currently, there are no recommendations for which assay(s) should be used as the standard method for screening patients for anti-EPO Abs or which should be used as the definitive diagnostic method. To date, diagnosis of Ab-mediated PRCA remains dependent on results from bone marrow examination plus demonstration of anti-EPO Abs by BIAcore, RIP, or ELISA. The question of which patients should be tested remains unresolved, because there is no published case definition of Ab-mediated PRCA. An association between the presence of baseline anti-EPO Abs and increased risk for developing Ab-mediated PRCA has not been demonstrated.
Demographically, patients receiving human serum albumin-free formulations of the Eprex brand of epoetin alfa by the SC route appear to have the greatest probability of developing Ab-mediated PRCA (28). However, the relative risks for developing Ab-mediated PRCA with the use of any of the commercially available EPO preparations (e.g., epoetin beta [NeoRecormon], epoetin alfa [Epogen], or darbepoetin alfa [Aranesp]) are very low. Most published studies present Ab data on patients already diagnosed with PRCA or on patients in whom the clinical signs of PRCA are emerging. Currently, there are no longitudinal data for Ab responses before and during the course of EPO therapy in anemia patients. Therefore, a clear need exists for standardization of Ab testing in patients with developing or full-blown Ab-mediated PRCA.
Other issues that remain to be addressed include which patients should be tested for Abs and when, how often they should be tested, which assay results are the most clinically relevant to the development of Ab-mediated PRCA and its resolution, and what factors impact cost and reimbursement. Reaching consensus on these issues will facilitate effective diagnosis and treatment of patients with this serious disorder.
REFERENCES
- 1.Amgen, Inc. Amgen statement on pure red cell aplasia. [Online.] www.amgen.com/clinicians/prca.html.
- 2.Arranz, O., J. Ara, R. Rodriguez, L. Quinto, J. Font, E. Mirapeix, and A. Darnell. 2001. Comparison of anti-PR3 capture and anti-PR3 direct ELISA for detection of antineutrophil cytoplasmic antibodies (ANCA) in long-term clinical follow-up of PR3-ANCA-associated vasculitis patients. Clin. Nephrol. 56:295-301. [PubMed] [Google Scholar]
- 3.Baron, E. J., D. E. Weber, and L. G. Weide. 1996. Lack of agreement among two commercial enzyme-linked immunosorbent antibody assays and a conventional immunofluorescence-based method for detecting islet cell autoantibodies. Clin. Diagn. Lab. Immunol. 3:429-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Basser, R. L., E. O'Flaherty, M. Green, M. Edmonds, J. Nichol, D. M. Menchaca, B. Cohen, and C. G. Begley. 2002. Development of pancytopenia with neutralizing antibodies to thrombopoietin after multicycle chemotherapy supported by megakaryocyte growth and development factor. Blood 99:2599-2602. [DOI] [PubMed] [Google Scholar]
- 5.Bendtzen, K., M. B. Hansen, C. Ross, and M. Svenson. 2000. Detection of autoantibodies to cytokines. Mol. Biotechnol. 14:251-261. [DOI] [PubMed] [Google Scholar]
- 6.Bergrem, H., B. G. Danielson, K. U. Eckardt, A. Kurtz, and M. Stridsberg. 1993. A case of antierythropoietin antibodies following recombinant human erythropoietin treatment, p. 265-275. In P. Sciqalla (ed.), Erythropoietin: molecular physiology and clinical application. Marcel Dekker, New York, N.Y.
- 7.Black, J. B., T. F. Schwarz, J. L. Patton, K. Kite-Powell, P. E. Pellett, S. Wiersbitzky, R. Bruns, C. Muller, G. Jager, and J. A. Stewart. 1996. Evaluation of immunoassays for detection of antibodies to human herpesvirus 7. Clin. Diagn. Lab. Immunol. 3:79-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boissel, J. P., W. R. Lee, S. R. Presnell, F. E. Cohen, and H. F. Bunn. 1993. Erythropoietin structure-function relationships. Mutant proteins that test a model of tertiary structure. J. Biol. Chem. 268:15983-15993. [PubMed] [Google Scholar]
- 9.Bommer, J., and J. Wagner. 2003. Safety of Aranesp (darbepoetin alfa) in dialysis patients with renal anaemia—results of a German mulitcentre study in 1502 patients. Nephrol. Dial. Transplant. 18(Suppl. 4):165. [Google Scholar]
- 10.Bonfield, T. L., M. S. Kavuru, and M. J. Thomassen. 2002. Anti-GM-CSF titer predicts response to GM-CSF therapy in pulmonary alveolar proteinosis. Clin. Immunol. 105:342-350. [DOI] [PubMed] [Google Scholar]
- 11.Buchalter, M. B., G. Suntharalingam, I. Jennings, C. Hart, R. J. Luddington, R. Chakraverty, S. K. Jacobson, P. L. Weissberg, and T. P. Baglin. 1992. Streptokinase resistance: when might streptokinase administration be ineffective? Br. Heart J. 68:449-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Casadevall, N. 2002. Antibodies against rHuEPO: native and recombinant. Nephrol. Dial. Transplant. 17(Suppl. 5):42-47. [DOI] [PubMed] [Google Scholar]
- 13.Casadevall, N., E. Dupuy, P. Molho-Sabatier, G. Tobelem, B. Varet, and P. Mayeux. 1996. Autoantibodies against erythropoietin in a patient with pure red-cell aplasia. N. Engl. J. Med. 334:630-633. [DOI] [PubMed] [Google Scholar]
- 14.Casadevall, N., J. Nataf, B. Viron, A. Kolta, J. J. Kiladjian, P. Martin-Dupont, P. Michaud, T. Papo, V. Ugo, I. Teyssandier, B. Varet, and P. Mayeux. 2002. Pure red-cell aplasia and antierythropoietin antibodies in patients treated with recombinant erythropoietin. N. Engl. J. Med. 346:469-475. [DOI] [PubMed] [Google Scholar]
- 15.Cassano, W. F. 1989. Murine monoclonal anti-avidin antibodies enhance the sensitivity of avidin-biotin immunoassays and immunohistologic staining. J. Immunol. Methods 117:169-174. [DOI] [PubMed] [Google Scholar]
- 16.Castelli, G., A. Famularo, C. Semino, A. M. Machi, A. Ceci, G. Cannella, and G. Melioli. 2000. Detection of anti-erythropoietin antibodies in haemodialysis patients treated with recombinant human-erythropoietin. Pharmacol. Res. 41:313-318. [DOI] [PubMed] [Google Scholar]
- 17.Chretien, S., P. Varlet, F. Verdier, S. Gobert, J. P. Cartron, S. Gisselbrecht, P. Mayeux, and C. Lacombe. 1996. Erythropoietin-induced erythroid differentiation of the human erythroleukemia cell line TF-1 correlates with impaired STAT5 activation. EMBO J. 15:4174-4181. [PMC free article] [PubMed] [Google Scholar]
- 18.Conlon, K. C., W. J. Urba, J. W. Smith II, R. G. Steis, D. L. Longo, and J. W. Clark. 1990. Exacerbation of symptoms of autoimmune disease in patients receiving alpha-interferon therapy. Cancer 65:2237-2242. [DOI] [PubMed] [Google Scholar]
- 19.Cotes, P. M., and D. R. Bangham. 1966. The international reference preparation of erythropoietin. Bull. W. H. O. 35:751-760. [PMC free article] [PubMed] [Google Scholar]
- 20.Crombet, T., O. Torres, V. Rodriguez, A. Menendez, A. Stevenson, M. Ramos, F. Torres, R. Figueredo, I. Veitia, N. Iznaga, R. Perez, and A. Lage. 2001. Phase I clinical evaluation of a neutralizing monoclonal antibody against epidermal growth factor receptor in advanced brain tumor patients: preliminary study. Hybridoma 20:131-136. [DOI] [PubMed] [Google Scholar]
- 21.Crooks, S. R., G. A. Baxter, M. C. O'Connor, and C. T. Elliot. 1998. Immunobiosensor—an alternative to enzyme immunoassay screening for residues of two sulfonamides in pigs. Analyst 123:2755-2757. [DOI] [PubMed] [Google Scholar]
- 22.Dai, Y., E. M. Schwarz, D. Gu, W. W. Zhang, N. Sarvetnick, and I. M. Verma. 1995. Cellular and humoral immune responses to adenoviral vectors containing factor IX gene: tolerization of factor IX and vector antigens allows for long-term expression. Proc. Natl. Acad. Sci. USA 92:1401-1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dayan, P., F. Ahmad, J. Urtecho, M. Novick, P. Dixon, D. Levine, and S. Miller. 2002. Test characteristics of the respiratory syncytial virus enzyme-linked immunoabsorbent assay in febrile infants < or = 60 days of age. Clin. Pediatr. (Philadelphia) 41:415-418. [DOI] [PubMed] [Google Scholar]
- 24.Egrie, J. C., P. M. Cotes, J. Lane, R. E. Gaines Das, and R. C. Tam. 1987. Development of radioimmunoassays for human erythropoietin using recombinant erythropoietin as tracer and immunogen. J. Immunol. Methods 99:235-241. [DOI] [PubMed] [Google Scholar]
- 25.Field, P. R., J. L. Mitchell, A. Santiago, D. J. Dickeson, S. W. Chan, D. W. Ho, A. M. Murphy, A. J. Cuzzubbo, and P. L. Devine. 2000. Comparison of a commercial enzyme-linked immunosorbent assay with immunofluorescence and complement fixation tests for detection of Coxiella burnetii (Q fever) immunoglobulin M. J. Clin. Microbiol. 38:1645-1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garotta, G., L. Ozmen, M. Fountoulakis, Z. Dembic, A. P. van Loon, and D. Stuber. 1990. Human interferon-gamma receptor. Mapping of epitopes recognized by neutralizing antibodies using native and recombinant receptor proteins. J. Biol. Chem. 265:6908-6915. [PubMed] [Google Scholar]
- 27.Ge, Y., S. Powell, M. Van Roey, and J. G. McArthur. 2001. Factors influencing the development of an anti-factor IX (FIX) immune response following administration of adeno-associated virus-FIX. Blood 97:3733-3737. [DOI] [PubMed] [Google Scholar]
- 28.Gershon, S. K., H. Luksenburg, T. R. Cote, and M. M. Braun. 2002. Pure red-cell aplasia and recombinant erythropoietin. N. Engl. J. Med. 346:1584-1586. [DOI] [PubMed] [Google Scholar]
- 29.Giovanella, L., and L. Ceriani. 2002. High-sensitivity human thyroglobulin (hTG) immunoradiometric assay in the follow-up of patients with differentiated thyroid cancer. Clin. Chem. Lab. Med. 40:480-484. [DOI] [PubMed] [Google Scholar]
- 30.Griffiths, P. D., and H. O. Kangro. 1984. A user's guide to the indirect solid-phase radioimmunoassay for the detection of cytomegalovirus-specific IgM antibodies. J. Virol. Methods 8:271-282. [DOI] [PubMed] [Google Scholar]
- 31.Grossberg, S. E., Y. Kawade, M. Kohase, H. Yokoyama, and N. Finter. 2001. The neutralization of interferons by antibody. I. Quantitative and theoretical analyses of the neutralization reaction in different bioassay systems. J. Interferon Cytokine Res. 21:729-742. [DOI] [PubMed] [Google Scholar]
- 32.Gussin, H. A., K. L. Russo, and M. Teodorescu. 2000. Effect of circulating immune complexes on the binding of rheumatoid factor to histones. Ann. Rheum. Dis. 59:351-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hammerling, U., R. Kroon, T. Wilhelmsen, and L. Sjodin. 1996. In vitro bioassay for human erythropoietin based on proliferative stimulation of an erythroid cell line and analysis of carbohydrate-dependent microheterogeneity. J. Pharm. Biomed. Anal. 14:1455-1469. [DOI] [PubMed] [Google Scholar]
- 34.Hansen, K. 1994. Lyme neuroborreliosis: improvements of the laboratory diagnosis and a survey of epidemiological and clinical features in Denmark 1985-1990. Acta Neurol. Scand. Suppl. 151:1-44. [PubMed] [Google Scholar]
- 35.Hansen, M. B., C. Ross, and K. Berg. 1990. A sensitive antiviral neutralization bioassay for measuring antibodies to interferons. J. Immunol. Methods. 127:241-248. [DOI] [PubMed] [Google Scholar]
- 36.Hansen, P. J. Analysis of proteins by immunoprecipitation. [Online.] http://www.dps.ufl.edu/hansen/protocols/imp98.prt.htm.
- 37.Harris, K. W., X. J. Hu, S. Schultz, M. O. Arcasoy, B. G. Forget, and N. Clare. 1998. The distal cytoplasmic domain of the erythropoietin receptor induces granulocytic differentiation in 32D cells. Blood 92:1219-1224. [PubMed] [Google Scholar]
- 38.Hellmich, B., E. Csernok, H. Schatz, W. L. Gross, and A. Schnabel. 2002. Autoantibodies against granulocyte colony-stimulating factor in Felty's syndrome and neutropenic systemic lupus erythematosus. Arthritis Rheum. 46:2384-2391. [DOI] [PubMed] [Google Scholar]
- 39.Hennes, U., W. Jucker, E. A. Fischer, T. Krummenacher, A. V. Palleroni, P. W. Trown, S. Linder-Ciccolunghi, and M. Rainisio. 1987. The detection of antibodies to recombinant interferon alfa-2a in human serum. J. Biol. Stand. 15:231-244. [DOI] [PubMed] [Google Scholar]
- 40.Hennig, C., L. Rink, U. Fagin, W. J. Jabs, and H. Kirchner. 2000. The influence of naturally occurring heterophilic anti-immunoglobulin antibodies on direct measurement of serum proteins using sandwich ELISAs. J. Immunol. Methods 235:71-80. [DOI] [PubMed] [Google Scholar]
- 41.Hjelm Skog, A. L., M. Wadhwa, M. Hassan, B. Gharizadeh, C. Bird, P. Ragnhammar, R. Thorpe, and H. Mellstedt. 2001. Alteration of interleukin 2 (IL-2) pharmacokinetics and function by IL-2 antibodies induced after treatment of colorectal carcinoma patients with a combination of monoclonal antibody 17-1A, granulocyte macrophage colony-stimulating factor, and IL-2. Clin. Cancer Res. 7:1163-1170. [PubMed] [Google Scholar]
- 42.Itri, L. M., M. Campion, R. A. Dennin, A. V. Palleroni, J. U. Gutterman, J. E. Groopman, and P. W. Trown. 1987. Incidence and clinical significance of neutralizing antibodies in patients receiving recombinant interferon alfa-2a by intramuscular injection. Cancer 59:668-674. [DOI] [PubMed] [Google Scholar]
- 43.Jacobs, K., C. Shoemaker, R. Rudersdorf, S. D. Neill, R. J. Kaufman, A. Mufson, J. Seehra, S. S. Jones, R. Hewick, E. F. Fritsch, et al. 1985. Isolation and characterization of genomic and cDNA clones of human erythropoietin. Nature 313:806-810. [DOI] [PubMed] [Google Scholar]
- 44.Jacobs-Helber, S. M., A. Wickrema, M. J. Birrer, and S. T. Sawyer. 1998. AP1 regulation of proliferation and initiation of apoprosis in erythropoietin-dependent erythroid cells. Mol. Cell. Biol. 18:3699-3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Johnson & Johnson. Summary of PRCA case reports. [Online.] http://www.jnj.com/news/jnj_news/1021024_095632.htm.
- 46.Koren, E., L. A. Zuckerman, and A. R. Mire-Sluis. 2002. Immune responses to therapeutic proteins in humans—clinical significance, assessment and prediction. Curr. Pharm. Biotechnol. 3:349-360. [DOI] [PubMed] [Google Scholar]
- 47.Krantz, S. B., and V. Kao. 1967. Studies on red cell aplasia. I. Demonstration of a plasma inhibitor to heme synthesis and an antibody to erythroblast nuclei. Proc. Natl. Acad. Sci. USA 58:493-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Krantz, S. B., and V. Kao. 1969. Studies on red cell aplasia. II. Report of a second patient with an antibody to erythroblast nuclei and a remission after immunosuppressive therapy. Blood 34:1-13. [PubMed] [Google Scholar]
- 49.Krantz, S. B., W. H. Moore, and S. D. Zaentz. 1973. Studies on red cell aplasia. V. Presence of erythroblast cytotoxicity in G-globulin fraction of plasma. J. Clin. Investig. 52:324-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kuroki, M., Y. Matsumoto, F. Arakawa, M. Haruno, M. Murakami, M. Kuwahara, H. Ozaki, T. Senba, and Y. Matsuoka. 1995. Reducing interference from heterophilic antibodies in a two-site immunoassay for carcinoembryonic antigen (CEA) by using a human/mouse chimeric antibody to CEA as the tracer. J. Immunol. Methods 180:81-91. [DOI] [PubMed] [Google Scholar]
- 51.Lackmann, M., T. Bucci, R. J. Mann, L. A. Kravets, E. Viney, F. Smith, R. L. Moritz, W. Carter, R. J. Simpson, N. A. Nicola, K. Mackwell, E. C. Nice, A. F. Wilks, and A. W. Boyd. 1996. Purification of a ligand for the EPH-like receptor HEK using a biosensor-based affinity detection approach. Proc. Natl. Acad. Sci. USA 93:2523-2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lacombe, C., N. Casadevall, O. Muller, and B. Varet. 1984. Erythroid progenitors in adult chronic pure red cell aplasia: relationship of in vitro erythroid colonies to therapeutic response. Blood 64:71-77. [PubMed] [Google Scholar]
- 53.Leon, A. D., M. Tellez Araiza, J. Arellano Garcia, and E. Martinez-Cordero. 2002. Interference by rheumatoid factor activity in the detection of antiavian antibodies in pigeon breeders disease. Clin. Exp. Med. 2:59-67. [DOI] [PubMed] [Google Scholar]
- 54.Li, J., C. Yang, Y. Xia, A. Bertino, J. Glaspy, M. Roberts, and D. J. Kuter. 2001. Thrombocytopenia caused by the development of antibodies to thrombopoietin. Blood 98:3241-3248. [DOI] [PubMed] [Google Scholar]
- 55.Liebe, V., M. Bruckmann, K. G. Fischer, K. K. Haase, M. Borggrefe, and G. Huhle. 2002. Biological relevance of anti-recombinant hirudin antibodies—results from in vitro and in vivo studies. Semin. Thromb. Hemost. 28:483-490. [DOI] [PubMed] [Google Scholar]
- 56.Lin, F. K., S. Suggs, C. H. Lin, J. K. Browne, R. Smalling, J. C. Egrie, K. K. Chen, G. M. Fox, F. Martin, Z. Stabinsky, et al. 1985. Cloning and expression of the human erythropoietin gene. Proc. Natl. Acad. Sci. USA 82:7580-7584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Linardaki, G. D., K. A. Boki, A. Fertakis, and A. G. Tzioufas. 1999. Pure red cell aplasia as presentation of systemic lupus erythematosus: antibodies to erythropoietin. Scand. J. Rheumatol. 28:189-191. [DOI] [PubMed] [Google Scholar]
- 58.Lynch, M., B. L. Pentecost, W. A. Littler, and R. A. Stockley. 1996. The distribution of antibodies to streptokinase. Postgrad. Med. J. 72:290-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Meager, A., M. Wadhwa, P. Dilger, C. Bird, R. Thorpe, J. Newsom-Davis, and N. Willcox. 2003. Anti-cytokine autoantibodies in autoimmunity: preponderance of neutralizing autoantibodies against interferon-alpha, interferon-omega and interleukin-12 in patients with thymoma and/or myasthenia gravis. Clin. Exp. Immunol. 132:128-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Millett, B., A. M. Sullivan, M. Morimoto, and G. Parsons. 1994. A third generation immunoassay for tumor necrosis factor alpha. BioTechniques 17:1166-1171. [PubMed] [Google Scholar]
- 61.Mimms, L., A. Goetze, S. Swanson, M. Floreani, B. Edwards, J. Macioszek, G. Okasinski, and W. Kiang. 1989. Second generation assays for the detection of antibody to HBsAg using recombinant DNA-derived HBsAg. J. Virol. Methods 25:211-231. [DOI] [PubMed] [Google Scholar]
- 62.Mobini, R., M. Fu, G. Wallukat, Y. Magnusson, A. Hjalmarson, and J. Hoebeke. 2000. A monoclonal antibody directed against an autoimmune epitope on the human beta1-adrenergic receptor recognized in idiopathic dilated cardiomyopathy. Hybridoma 19:135-142. [DOI] [PubMed] [Google Scholar]
- 63.Mobini, R., A. Staudt, S. B. Felix, G. Baumann, G. Wallukat, J. Deinum, H. Svensson, A. Hjalmarson, and M. Fu. 2003. Hemodynamic improvement and removal of autoantibodies against beta(1)-adrenergic receptor by immunoadsorption therapy in dilated cardiomyopathy. J. Autoimmun. 20:345-350. [DOI] [PubMed] [Google Scholar]
- 64.Moffatt, S., N. Yaegashi, K. Tada, N. Tanaka, and K. Sugamura. 1998. Human parvovirus B19 nonstructural (NS1) protein induces apoprosis in erythroid lineage cells. J. Virol. 72:3018-3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Moore, M. Developmental hematopoiesis. [Online.] http://www.mskcc.org/mskcc/html/10645.cfm.
- 66.Nice, E., J. Layton, L. Fabri, U. Hellman, A. Engstrom, B. Persson, and A. W. Burgess. 1993. Mapping of the antibody- and receptor-binding domains of granulocyte colony-stimulating factor using an optical biosensor. Comparison with enzyme-linked immunosorbent assay competition studies. J. Chromatogr. 646:159-168. [DOI] [PubMed] [Google Scholar]
- 67.Peces, R., M. de la Torre, R. Alcazar, and J. M. Urra. 1996. Antibodies against recombinant human erythropoietin in a patient with erythropoietin-resistant anemia. N. Engl. J. Med. 335:523-524. [DOI] [PubMed] [Google Scholar]
- 68.Peschle, C., A. M. Marmont, G. Marone, A. Genovese, G. F. Sasso, and M. Condorelli. 1975. Pure red cell aplasia: studies on an IgG serum inhibitor neutralizing erythropoietin. Br. J. Haematol. 30:411-417. [DOI] [PubMed] [Google Scholar]
- 69.Prabhakar, S. S., and T. Muhlfelder. 1997. Antibodies to recombinant human erythropoietin causing pure red cell aplasia. Clin. Nephrol. 47:331-335. [PubMed] [Google Scholar]
- 70.Rasooly, A. 2001. Surface plasmon resonance analysis of staphylococcal enterotoxin B in food. J. Food Prot. 64:37-43. [DOI] [PubMed] [Google Scholar]
- 71.Ritter, G., L. S. Cohen, C. Williams, Jr., E. C. Richards, L. J. Old, and S. Welt. 2001. Serological analysis of human anti-human antibody responses in colon cancer patients treated with repeated doses of humanized monoclonal antibody A33. Cancer Res. 61:6851-6859. [PubMed] [Google Scholar]
- 72.Rosenberg, A. S. 2003. Immunogenicity of biological therapeutics: a hierarchy of concerns. Dev. Biol. (Basel) 112:15-21. [PubMed] [Google Scholar]
- 73.Schellekens, H. 2002. Immunogenicity of therapeutic proteins: clinical implications and future prospects. Clin. Ther. 24:1720-1740. [DOI] [PubMed] [Google Scholar]
- 74.Suter, M., and J. E. Butler. 1986. The immunochemistry of sandwich ELISAs. II. A novel system prevents the denaturation of capture antibodies. Immunol. Lett. 13:313-316. [DOI] [PubMed] [Google Scholar]
- 75.Svenson, M., M. B. Hansen, C. Ross, M. Diamant, K. Rieneck, H. Nielsen, and K. Bendtzen. 1998. Antibody to granulocyte-macrophage colony-stimulating factor is a dominant anti-cytokine activity in human IgG preparations. Blood 91:2054-2061. [PubMed] [Google Scholar]
- 76.Svojanovsky, S. R., K. L. Egodage, J. Wu, M. Slavik, and G. S. Wilson. 1999. High sensitivity ELISA determination of taxol in various human biological fluids. J. Pharm. Biomed. Anal. 20:549-555. [DOI] [PubMed] [Google Scholar]
- 77.Swanson, S. J. 2004. Detection of baseline anti-erythropoietin antibodies in patients with chronic kidney disease, using the BIA core immunoassay. Presented at the 41st Congress of the European Renal Association and European Dialysis and Transplant Association, Lisbon, Portugal, July 17, 2003.
- 78.Swanson, S. J. 2003. New technologies for the detection of antibodies to therapeutic proteins, p. 127-133. In A. R. Mire-Sluis (ed.), Immunogenicity of therapeutic biological products, vol. 112. Karger, Basel, Switzerland. [PubMed] [Google Scholar]
- 79.Swanson, S. J., J. Ferbas, P. Mayeux, and N. Casadevall. 2004. Evaluation of methods to detect and characterize antibodies against recombinant human erythropoietin. Nephron. Clin Pract. 96:c88-c95. [DOI] [PubMed] [Google Scholar]
- 80.Swanson, S. J., S. J. Jacobs, D. Mytych, C. Shah, S. R. Indelicato, and R. W. Bordens. 1999. Applications for the new electrochemiluminescent (ECL) and biosensor technologies. Dev. Biol. Stand. 97:135-147. [PubMed] [Google Scholar]
- 81.Swedler, W., J. Wallman, C. J. Froelich, and M. Teodorescu. 1997. Routine measurement of IgM, IgG, and IgA rheumatoid factors: high sensitivity, specificity, and predictive value for rheumatoid arthritis. J. Rheumatol. 24:1037-1044. [PubMed] [Google Scholar]
- 82.Swissmedic. 2003. Nouvel effet indésirable grave mais rare de l’érythropoïétine recombinante. Swissmedic medical update letter. [Online.] http://www.swissmedic.ch/en/laien/overall.asp?lang=2&theme=0.00062.00004.00001&theme_id=810&news_id=2588&page=1.
- 83.Sytkowski, A. J., and K. A. Donahue. 1987. Immunochemical studies of human erythropoietin using site-specific anti-peptide antibodies. Identification of a functional domain. J. Biol. Chem. 262:1161-1165. [PubMed] [Google Scholar]
- 84.Takacs, M. A., S. J. Jacobs, R. M. Bordens, and S. J. Swanson. 1999. Detection and characterization of antibodies to PEG-IFN-α2b using surface plasmon resonance. J. Interferon Cytokine Res. 19:781-789. [DOI] [PubMed] [Google Scholar]
- 85.Takatoku, M., M. Kametaka, R. Shimizu, Y. Miura, and N. Komatsu. 1997. Identification of functional domains of the human thrombopoietin receptor required for growth and differentiation of megakaryocytic cells. J. Biol. Chem. 272:7259-7263. [DOI] [PubMed] [Google Scholar]
- 86.Ullenhag, G., C. Bird, P. Ragnhammar, J. E. Frodin, K. Strigard, O. I. A., R. Thorpe, H. Mellstedt, and M. Wadhwa. 2001. Incidence of GM-CSF antibodies in cancer patients receiving GM-CSF for immunostimulation. Clin. Immunol. 99:65-74. [DOI] [PubMed] [Google Scholar]
- 87.Urra, J. M., M. de la Torre, R. Alcazar, and R. Peces. 1997. Rapid method for detection of anti-recombinant human erythropoietin antibodies as a new form of erythropoietin resistance. Clin. Chem. 43:848-849. [PubMed] [Google Scholar]
- 88.VanCott, T. C., L. D. Loomis, R. R. Redfield, and D. L. Birx. 1992. Real-time biospecific interaction analysis of antibody reactivity to peptides from the envelope glycoprotein, gp160, of HIV-1. J. Immunol. Methods 146:163-176. [DOI] [PubMed] [Google Scholar]
- 89.Van Der Geld, Y. M., P. C. Limburg, and C. G. Kallenberg. 1999. Characterization of monoclonal antibodies to proteinase 3 (PR3) as candidate tools for epitope mapping of human anti-PR3 autoantibodies. Clin. Exp. Immunol. 118:487-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vyse, A. J., W. A. Knowles, B. J. Cohen, and D. W. Brown. 1997. Detection of IgG antibody to Epstein-Barr virus viral capsid antigen in saliva by antibody capture radioimmunoassay. J. Virol. Methods 63:93-101. [DOI] [PubMed] [Google Scholar]
- 91.Wadhwa, M., C. Bird, P. Dilger, R. Gaines-Das, and R. Thorpe. 2003. Strategies for detection, measurement and characterization of unwanted antibodies induced by therapeutic biologicals. J. Immunol. Methods 278:1-17. [DOI] [PubMed] [Google Scholar]
- 92.Wadhwa, M., C. Bird, J. Fagerberg, R. Gaines-Das, P. Ragnhammar, H. Mellstedt, and R. Thorpe. 1996. Production of neutralizing granulocyte-macrophage colony-stimulating factor (GM-CSF) antibodies in carcinoma patients following GM-CSF combination therapy. Clin. Exp. Immunol. 104:351-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wadhwa, M., A. L. Skog, C. Bird, P. Ragnhammar, M. Lilljefors, R. Gaines-Das, H. Mellstedt, and R. Thorpe. 1999. Immunogenicity of granulocyte-macrophage colony-stimulating factor (GM-CSF) products in patients undergoing combination therapy with GM-CSF. Clin. Cancer Res. 5:1353-1361. [PubMed] [Google Scholar]
- 94.Weber, G., J. Gross, A. Kromminga, H. H. Loew, and K. U. Eckardt. 2002. Allergic skin and systemic reactions in a patient with pure red cell aplasia and anti-erythropoietin antibodies challenged with different epoetins. J. Am. Soc. Nephrol. 13:2381-2383. [DOI] [PubMed] [Google Scholar]
- 95.Wong, R. L., D. Mytych, S. Jacobs, R. Bordens, and S. J. Swanson. 1997. Validation parameters for a novel biosensor assay which simultaneously measures serum concentrations of a humanized monoclonal antibody and detects induced antibodies. J. Immunol. Methods 209:1-15. [DOI] [PubMed] [Google Scholar]
- 96.Wu, G., B. Nathoo, D. Mendelssohn, S. Pandeya, and P. Tam. 2003. Antibody to erythropoietin surveillance study in 5 Ontario renal centres. Nephrol. Dial. Transplant. 18(Suppl. 4):162. [Google Scholar]
- 97.Zang, Y. C., D. Yang, J. Hong, M. V. Tejada-Simon, V. M. Rivera, and J. Z. Zhang. 2000. Immunoregulation and blocking antibodies induced by inter-feron beta treatment in MS. Neurology 55:397-404. [DOI] [PubMed] [Google Scholar]
- 98.Zhong, D., E. L. Saenko, M. Shima, M. Felch, and D. Scandella. 1998. Some human inhibitor antibodies interfere with factor VIII binding to factor IX. Blood 92:136-142. [PubMed] [Google Scholar]
- 99.Zhu, G., B. Yang, and R. N. Jennings. 2000. Quantitation of basic fibroblast growth factor by immunoassay using BIAcore 2000. J. Pharm. Biomed. Anal. 24:281-290. [DOI] [PubMed] [Google Scholar]