Abstract
The Na/K-ATPase α1 polypeptide supports both ion-pumping and signaling functions. The Na/K-ATPase α3 polypeptide differs from α1 in both its primary structure and its tissue distribution. The expression of α3 seems particularly important in neurons, and recent clinical evidence supports a unique role of this isoform in normal brain function. The nature of this specific role of α3 has remained elusive, because the ubiquitous presence of α1 has hindered efforts to characterize α3-specific functions in mammalian cell systems. Using Na/K-ATPase α1 knockdown pig kidney cells (PY-17), we generated the first stable mammalian cell line expressing a ouabain-resistant form of rat Na/K-ATPase α3 in the absence of endogenous pig α1 detectable by Western blotting. In these cells, Na/K-ATPase α3 formed a functional ion-pumping enzyme and rescued the expression of Na/K-ATPase β1 and caveolin-1 to levels comparable with those observed in PY-17 cells rescued with a rat Na/K-ATPase α1 (AAC-19). The α3-containing enzymes had lower Na+ affinity and lower ouabain-sensitive transport activity than their α1-containing counterparts under basal conditions, but showed a greater capacity to be activated when intracellular Na+ was increased. In contrast to Na/K-ATPase α1, α3 could not regulate Src. Upon exposure to ouabain, Src activation did not occur, yet ERK was activated through Src-independent pathways involving PI3K and PKC. Hence, α3 expression confers signaling and pumping properties that are clearly distinct from that of cells expressing Na/K-ATPase α1.
Keywords: caveolin, extracellular‐signal‐regulated kinase (ERK), Na+/K+-ATPase, phosphatidylinositide 3‐kinase (PI 3‐kinase), Src, signal transduction
the Na/K-ATPase is a ubiquitously expressed membrane protein in mammalian cells. It transports K+ in and Na+ out of the cell at the expense of ATP. This maintains ionic homeostasis and establishes the transmembrane Na+ gradient necessary for normal neuronal activity and also for the Na+-dependent transport of nutrients (32). A functional Na/K-ATPase unit is made up of two subunits, α and β. The α-subunit, a 110 kDa protein, is the catalytic subunit that contains the binding sites for ATP, ions, and cardiotonic steroids (CTS) such as ouabain. In addition to the ubiquitously present α1, three more isoforms of the catalytic subunit have been characterized. These isoforms are encoded by three different but homologous genes (21). The gene for α3 isoform is located on chromosome 19, whereas the genes for α1 and α2 are located on chromosome 1. Unlike α1 and α2, the α3 polypeptide is not subjected to posttranslational processing in the NH2-terminal region (7). Na/K-ATPase α2 is found mainly in muscle and glial cells, α3 mainly in neurons, and α4 expression is restricted to sperm. Across species, α2 and α3 share up to 92 and 96% similarity with α1, respectively (5). At first glance, such high levels of similarity suggest a substantial level of functional redundancy among the α-isoforms. Instead, a plethora of reports on tissue-specific expression and clear isoform-specific regulations by physiological (e.g., development) and pathological (e.g., malignancy) processes have long suggested that they must be serving specific functions (37, 43). In rodents, singularly, the ouabain affinity of α1 is two to three orders of magnitude lower than that of α2 or α3 because of known amino acid substitutions (44). Investigators have therefore been able to use low concentrations of ouabain to single out specific roles served by non-α1 isoforms. By using this approach in selected rodent cells naturally expressing known combination of isoforms, or in artificial systems where an ouabain-resistant isoform is expressed in a ouabain-sensitive recipient cell, isoenzymes-specific ion-pumping features have been identified. For example, Na/K-ATPase α3 has a distinctive low affinity for Na+ compared with the three other isoenzymes, leading to the suggestion that α3 is particularly suitable for the restoration of basal membrane potential after depolarization and could play an essential role in short-term regulation of neuronal function (2). In addition to its well-known ion-pumping function, Na/K-ATPase α1 serves important signal transducing functions (reviewed in Refs. 1, 15, 42, 58, 59). Increasingly, animal studies are pointing to a previously unsuspected and fundamental role of these functions in physiological and pathological processes (8, 9, 12, 14, 24, 46, 56). We have shown that Na/K-ATPase α1 interacts with and regulates Src kinase, and this complex acts as a functional receptor for low concentrations of CTS to activate protein kinase cascades (30, 48). This is particularly important for endogenous CTS to exert their physiological regulation. Structurally, Na/K-ATPase α1 interacts with Src at two sites to form a functional signal transducing receptor-protein complex. Na/K-ATPase α1 also interacts with caveolin-1 in cholesterol-rich invaginations of the plasma membrane called caveolae (35). In caveolae, Na/K-ATPase α1 interacts directly or indirectly with many other proteins such as phosphoinositide 3-kinase (PI3K), epidermal growth factor receptor (EGFR), and phospholipase C (PLC) (17, 63, 64). Unfortunately, the signaling function of other isoforms cannot be examined in cells because of the presence of α1. Consequently, a lot less is known about the signaling function of non-α1-isoforms, although there has been evidence in support of isoform-specific mechanisms (22, 23, 41). Recently, we have developed and used the first mammalian stable cell line devoid of detectable α1 to study the signaling function of α2 (57).
In the present study, we used this mammalian cell system to test whether rat Na/K-ATPase α3 possesses Src-dependent signaling abilities similar to those we have reported for rat α1, or lacks these signaling properties as we have recently shown for rat α2. These observations suggest that the difference in amino acid sequence in NaKtide region may confer the difference in signaling properties of different isoforms of α Na/K-ATPase. To this end, we first generated a new LM-α3-1 cell line, expressing an enzymatically competent Na/K-ATPase α3-containing complex in the absence of any α1 detectable by Western blot analysis. When treated with ouabain, these cells displayed a signaling response that was clearly distinct from that of cells expressing only rat Na/K-ATPase α1 and also distinct from what we have found for α2. Taken together, these data suggest that signaling by Na/K-ATPase is α-isoform specific, which may be an important aspect of the physiological functions they serve and the diseases associated with their mutations.
MATERIALS AND METHODS
Reagents.
The polyclonal anti-Src[pY418] antibody (raised in rabbit; Catalog No. 44660G), cell culture media, fetal bovine serum, trypsin, and Lipofectamine 2000 were from Invitrogen. The QuikChange mutagenesis kit was from Agilent Technologies. The polyclonal anti-Na/K-ATPase α1(raised in mouse; Catalog No. 05-369) and the anti-Na/K-ATPase β1 (clone C464.8) (raised in mouse; Catalog No. 05-382) antibodies as well as the recombinant human Src were from Upstate Biotechnology. The monoclonal anti- Na/K-ATPase α1 antibody (α6F) was from the Developmental Studies Hybridoma Bank at the University of Iowa (raised in chicken). The monoclonal anti-c-Src (B-12) antibody (raised in mouse; Catalog No. sc-8056), polyclonal anti-ERK1/2 antibody (raised in rabbit; Catalog No. sc-94), and all the secondary horseradish peroxidase-conjugated antibodies (goat anti-rabbit, Catalog No. sc-2004; goat anti-mouse, Catalog No. sc-2005) were from Santa Cruz Biotechnology. The polyclonal anti-Na/K-ATPase α3 antibody was from Millipore (raised in rabbit, Catalog No. 06-172). The monoclonal anti-pERK1/2 (raised in rabbit, Catalog No. 9101S), anti-pAkt (raised in rabbit, Catalog No. 9018S), anti-Akt (raised in rabbit, Catalog No. 9272S), and the monoclonal anti-caveolin-1 (raised in rabbit, Catalog No. 3267S) antibodies were purchased from Cell Signaling. Radioactive 86Rb+ was from PerkinElmer Life Science Products. Details on how the 20 mer NaKtide peptide was derived from a sequence in the N domain of Na/K-ATPase α1 and how it was found to be interacting with Src and regulating its activity in a manner that distinguishes it clearly from other Src inhibitors such as PP2 can be found in Ref. 29. All peptides had a purity of more than 95% (as determined by RP-HPLC) and were purchased from HD Biosciences (China). The specific inhibitors GO6983 and PD153035 were from Santa Cruz Biotechnology, LY 29400 from Cell Signaling, and PP2 from Calbiochem.
Expression vectors, gene transfer, and selection.
Rat wild-type α3 cDNA was received from Dr. Blanco (Department of Molecular and Integrative Physiology, Kansas University Medical University) and subcloned into the HindIII and XbaI sites of the eukaryotic expression vector CMV5 (from Addgene) with the aid of a HindIII and XbaI adaptor.
A variant of the rat wild-type α3 isoform was constructed by site-directed mutagenesis (Q108R and N119D) using QuikChange II XL Site-Directed Mutagenesis Kit from Agilent Technologies to obtain an isoform resistant to micromolar concentrations of ouabain as described previously (44). The sequence of the mutant α3 cDNA was confirmed by DNA sequencing (Iowa Institute of Human Genetics).
Generation of α3-expressing stable cell lines.
Gene transfer and selection was performed as previously described (30). Briefly, Na/K-ATPase α1 knockdown PY-17 cells, a stable cell line developed from LLC-PK1 by using siRNA (see Ref. 30 for initial characterization) were used. Cells were cultured in six-well plates and transfected with the α3-containing CMV5 vector. After selection with 3µM ouabain for 1 wk, the surviving ouabain-resistant colonies were collected and individually plated into 96-well plates. In colonies that expanded successfully into stable cell lines, the expression of rat α3 was verified with a specific anti-Na/K-ATPase α3 antibody after cells were cultured in the absence of ouabain for three generations.
Cell Culture, ouabain treatment, and determination of cell growth rate.
Cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM), supplemented with 10% fetal bovine serum, 100 units/ml penicillin, 100 µg/ml streptomycin, and 1 µg/ml puromycin. When cells reached 90–100% confluence, they were serum starved overnight and used for experiments unless otherwise noted. In studies using ouabain, duration of exposure was 10 min in all experiments irrespective of cell line or other pharmacological treatment. When the role of calcium was assessed, cells were preincubated in Krebs-Henseleit (KH) buffer [NaCl (118.0 mM), KCl (4.0 mM), KH2PO4 (1.3 mM), MgSO4 (1.2 mM), ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA) (0.4 mM), NaHCO3 (25 mM), D-glucose (11.0 mM), with a pH of 7.4] either without Ca2+ or with Ca2+ [CaCl2 (1.8 mM) in KH buffer] for 30 min before ouabain treatment.
For cell growth rate determination, cells were plated in 12-well plates (20,000 cells/well), cultured for 24, 48, 72, 96, or 120 h in DMEM + 10% FBS, and counted manually with a hemocytometer.
Immunostaining.
Immunostaining of Na/K-ATPase α3 was performed as previously described (28). Briefly, cells were cultured on coverslips in six-well plates. Once they reached 90–100% confluence, the cells were serum starved overnight. Cells were then washed twice with ice-cold PBS, fixed with prechilled (−20°C) methanol for 15 min, and blocked with PBS containing 1% BSA at room temperature for 30 min before incubation with the primary antibody overnight at 4°C, washed and incubated with Alexa Fluor-conjugated secondary antibody for 2 h at room temperature. The coverslip preparations were then washed, mounted, and visualized with a Leica DMIRE2 microscope (Wetzlar, Germany).
[3H] Ouabain binding.
To measure the surface expression of the endogenous pig Na/K-ATPase, [3H] ouabain binding assay was performed as described (49). Briefly, cells were cultured in 12-well plates until confluent and serum starved overnight. Afterward, the cells were incubated in K+-free Krebs solution (NaCl 142.4 mM, CaCl2 2.8 mM, NaH2PO4 0.6 mM, MgSO4 1.2 mM, glucose 10 mM, Tris 15 mM, pH7.4) for 15 min and then exposed to 200 nM [3H] ouabain for 30 min at 37°C. After incubation, the cells were washed three times with ice-cold K+-free Krebs solution, solubilized in 0.1 M NaOH-0.2% SDS, and counted in a scintillation counter for [3H] ouabain. Nonspecific binding was measured in the presence of 1 mM of unlabeled ouabain and subtracted from total binding. All counts were normalized to protein amount.
Src kinase assay.
NaKtide and other peptides were tested for their regulation of Src phosphorylation. It was measured with in vitro Src kinase assay as described (29). Briefly, purified Src (4.5 U) was incubated with different concentrations of peptides in PBS (137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 2 mM KH2PO4, pH 7.4) for 15 min at 37°C. ATP/Mg2+ (2 mM) was added to initiate autophosphorylation and was allowed to continue for 15 min at 37°C. SDS sample buffer was added to stop the reaction, and then samples were analyzed for Src pY418 by Western blotting.
Western blot analysis.
After indicated treatment, cells were washed with ice-cold PBS and lysed in ice-cold radioimmunoprecipitation buffer (0.25% sodium deoxycholate, 1% Nonidet P-40, 1 mM EDTA, 1 mM PMSF, 1 mM sodium orthovanadate, 1 mM Sodium fluoride, 150 mM NaCl, 50 mM Tris-HCl, pH 7.4, and 1% protease inhibitor cocktail). Cell lysates were centrifuged at 14,000 g for 15 min, supernatants were collected, and the protein content was measured by Lowry’s assay. Equal amounts of protein of each sample were loaded, separated by SDS-PAGE, transferred to an Optitran membrane, and probed with the appropriate antibody. ImageJ 1.46 was used for quantitative analyses of the resulting bands.
Ouabain-sensitive ATPase activity.
Cells were collected by scrapping and homogenized in ice-cold buffer A (150 mM sucrose, 5 mM HEPES, 4 mM EGTA, 0.8 mM dithiothreitol) and briefly sonicated. After centrifugation (800 g, 10 min), the postnuclear fraction was further centrifuged (100,000 g, 45 min) to obtain a crude membrane (30). The crude membrane pellet was resuspended in buffer A, and the protein content was determined. Aliquots of protein were treated with alamethicin (0.1 mg/mg of protein) for 10 min at room temperature and then added to the buffer containing 20 mM Tris (pH 7.4), 1 mM EGTA, 1 mM MgCl2, 25 mM KCl, 100 mM NaCl, 5 mM NaN3. After 15 min preincubation at 37°C, ATP/Mg was added to a final concentration of 2 mM to start the reaction. The reaction was allowed to run for 45 min and was stopped by adding 8% ice-cold trichloroacetic acid. The phosphate generated during ATP hydrolysis was measured by BIOMOL GREEN Reagent (Enzo Life Science). Ouabain-sensitive Na/K-ATPase activity was calculated as the difference between ATPase activity in the presence and in the absence of 1 mM ouabain.
Ouabain-sensitive 86Rb+ influx.
Ouabain-sensitive 86Rb+ was performed to measure Na/K-ATPase-mediated ion transport in situ as previously described (34). Briefly, 90% confluent cells were cultured in 12-well plates and serum starved overnight. Monolayers were washed and incubated in DMEM with or without 1.0 mM ouabain for 10 min at 37°C. 86Rb+ (1 µCi/well) was added for 10 min and the reaction was stopped by washing with ice-cold 0.1 M MgCl2. Trichloroacetic acid soluble 86Rb+ was counted in a Beckman scintillation counter. All counts were normalized to protein amount. To measure the maximal transport function of Na/K-ATPase in cells, 20 µM of monensin was used. Cells were incubated with monensin for 10 min to clamp intracellular Na+, and then 86Rb+ was added to measure the pump activity.
Data analysis.
Data presented are means ± SE of at least three independent experiments, and statistical analysis was performed by Student’s t-test. When more than two groups were compared, one-way ANOVA was performed before comparison of individual groups. Significance was accepted at P < 0.05. When applicable, Km and Vmax values were determined through fitting of the experimental data to the Hill equation by nonlinear regression analysis.
RESULTS
Na/K-ATPase expression in LM-α3–1 cells.
To evaluate the specific enzymatic and signaling functions supported by Na/K-ATPase α3 in a mammalian system, we generated a cell line expressing rat α3 in the absence of a detectable amount of α1. To reach this goal, we used a knockdown and rescue protocol in which α1 knockdown PY-17 cells [a stable cell line derived from pig kidney LLC-PK1 by using siRNA (30)] are stably transfected with a ouabain-insensitive rat Na/K-ATPase α3-expressing plasmid. The transfection and ouabain selection protocols described in materials and methods yielded multiple viable clones, each expressing Na/K-ATPase α3 (data not shown). Because it had the highest expression of Na/K-ATPase α3, Clone 1 was named LM-α3-1 and selected for subsequent studies. In addition, AAC-19 cells were used as control. AAC-19 is a stable cell line previously obtained by rescuing PY-17 cells with a wild-type ouabain-resistant rat α1 as described (30). First, the total amount of α1 polypeptide was assessed by Western blotting analysis of AAC-19, LM-α3-1, and PY-17 cell lysates by using an antibody that recognizes both endogenous (pig) and exogenous (rat) α1 polypeptides. As shown in Fig. 1A, a clear band was typically detected at the expected molecular weight in the AAC-19 lysates, whereas a faint band becoming more clear upon longer exposure time could be detected in PY-17. Under these conditions and upon higher exposure, no α1 signal was detected in LM-α3-1 lysates, suggesting that the residual level of pig α1 was even lower in LM-α3-1 than in PY-17 cells. To further assess the level of residual endogenous α1 expression in LM-α3-1, if any, we took advantage of the fact that surface expression of the pig (endogenous) ouabain-sensitive Na/K-ATPase α1, but not that of the ouabain-insensitive rat isoforms, could be accurately measured by 3H-ouabain binding studies. These studies revealed that 3H-ouabain binding in LM-α3-1 was barely detectable (Fig. 1E). Indeed, the number of counts was less than half of the number detected in PY-17. Based on the estimated number of sites in PY-17 [14 ± 4 104/cell (31)], there would be less than 7 × 104 sites in LM-α3-1, a number which is below the variation observed in the number of ouabain binding sites in the parent line LLC-PK1 [85 ± 8 104/cell (31)], hence our conclusion that there is virtually no expression of Na/K-ATPase α1 in LM-α3-1.
Fig. 1.
Generation of stable cell lines expressing Na/K-ATPase α3. Whole cell lysates from AAC-19, LM-α3-1, and PY-17 were separated by SDS-PAGE and analyzed by Western blot for the expression of Na/K-ATPase α1 (A), Na/K-ATPase α3 (B), and Na/K-ATPase β1 (C). A representative Western blot is shown. Cells were immunostained with anti-Na/K-ATPase α3 primary and Alexa Fluor-conjugated secondary antibody (D). The scale bar represents 25 µM (n = 3–6). *P < 0.05, ** P < 0.01 compared with AAC-19. E: 3H ouabain binding. Cells were treated for 2 µM ouabain for 30 min and then assayed for ouabain binding as described in materials and methods. Quantification of data is shown as means ± SE from at least three separate experiments.
As expected, Na/K-ATPase α3 was detected in LM-α3-1 lysates but not in AAC-19 or PY-17 (Fig. 1B). Like rat Na/K-ATPase α1 in AAC-19, expression of rat Na/K-ATPase α3 in LM-α3-1 was able to rescue the expression of glycosylated β1 that is altered in PY-17 (Fig. 1C). Also consistent with the ability of α3 to form a functional Na/K-ATPase in association with the endogenous β1 polypeptide in LM-α3-1, immunostaining as described in materials and methods revealed that the Na/K-ATPase α3 polypeptide was efficiently transported and expressed at the cell surface in LM-α3-1 (Fig. 1D). Consistent with Western blotting and 3H ouabain binding assays, we could not detect any signal following immunostaining targeting the α1 polypeptide in LM-α3-1. Taken together, these findings indicated that the expressed α3 was fully capable of assembling with β1 into a functional Na/K-ATPase.
Enzymatic properties of Na/K-ATPase α3 in LM-α3-1 cells.
Ouabain-sensitive ATPase activity and 86Rb+ influx were compared in AAC-19 and LM-α3-1 cells. As shown in Fig. 2A, Na/K-ATPase activity and ouabain sensitivity in crude membrane preparations from AAC-19 and LM-α3-1 cells were comparable. In contrast, ouabain-sensitive 86Rb+ influx was significantly lower in LM-α3-1 cells than in AAC-19 cells under normal culture conditions (P < 0.05, Fig. 4A), suggesting that the number of catalytically competent units that were not transporting ions was higher in LM-α3-1 than in AAC-19 cells. To understand the origin of this difference, we next tested whether Na/K-ATPase from these cells had different apparent affinities for Na+ and K+. As revealed by the dose-response curves shown in Fig. 3, the Km for K+ of Na/K-ATPase α3 was 2.3 ± 0.6 mM, comparable (P = 0.4) to that of α1 (1.8 ± 0.5 mM). Consistent with reports in other tissues (4, 47), the Km for Na+ of Na/K-ATPase α3 was 18.41 ± 3.1 mM and significantly higher than that of α1 (11.7 ± 2.2 P < 0.05). Consequently, the most straightforward explanation for the lower influx observed in Fig. 4A is that, because of their lower K0.5 for Na+, Na/K-ATPase α3 units were transporting ions in LM-α3-1 at a submaximal rate that was lower than that of Na/K-ATPase α1 units in AAC-19. This was tested explicitly by increasing the rate of passive Na+ transport by using monensin to reach maximal and sustained (“clamped”) intracellular Na+ levels in AAC-19 and LM-α3-1 cells (18). As expected, Na/K-ATPase-mediated 86Rb+ influx substantially increased in both cell lines (Fig. 4B), reaching a comparable maximal value of ~10 nmol·min−1·mg−1 protein that was not significantly different between AAC-19 and LM-α3-1 cells in the presence of monensin. Consistent with a role for the lower Km for Na+ of α3 in the observed lower rate of influx in basal conditions, clamping intracellular Na+ increased the influx in AAC-19 by 1.83 ± 0.15-fold, which was significantly less than the 2.89 ± 0.15-fold increase observed in LM-α3-1 (P < 0.01).
Fig. 2.
Na/K-ATPase and dose-effect of ouabain in rescued cells. Ouabain-sensitive ATPase activity was obtained as described in materials and methods. Data are shown as means ± SE from at least three separate experiments.
Fig. 4.
Na/K-ATPase-mediated 86Rb+ influx. Basal level (A) and basal and monensin-treated (B) ouabain-sensitive 86Rb+ influx was measured as described in materials and methods. Values were normalized per protein amount. Data are shown as means ± SE and from at least three separate experiments. *P < 0.05.
Fig. 3.
Substrate-dependent ATPase activity. Crude membranes were prepared from AAC-19 and LM-α3-1 cells, and ATPase activity was measured under varying concentrations of potassium chloride (KCl) (A) and sodium chloride (NaCl) (B). Data are shown as means ± SE from at least three separate experiments.
Signaling properties of Na/K-ATPase α3 in LM-α3-1 cells.
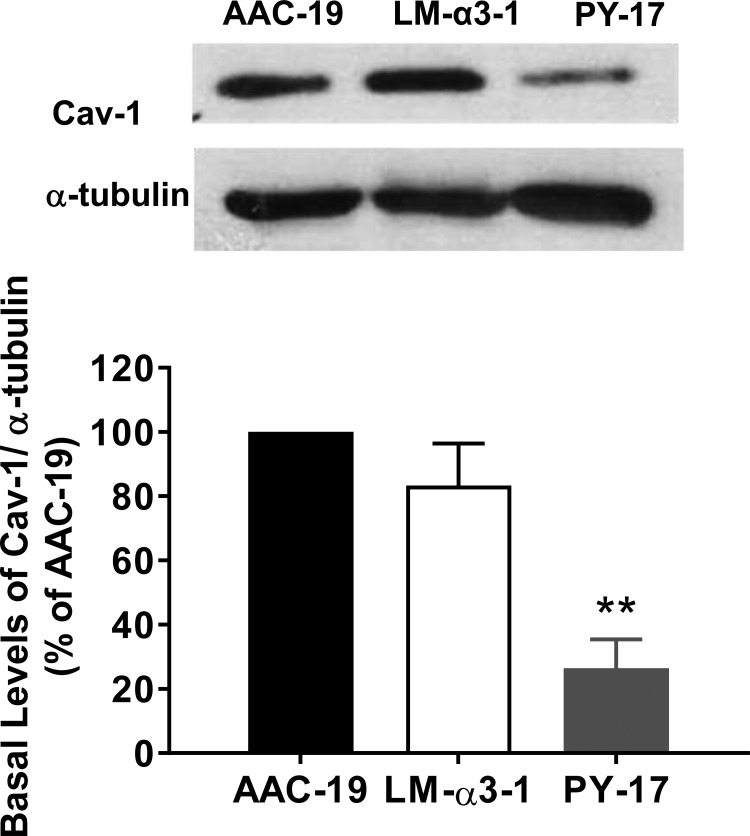
Caveolin-1 expression is critical to caveolae structure and influences cell signal transduction (6, 40). We have shown that Na/K-ATPase α1 associates with caveolin-1 in caveolae and that caveolin-1 is critical for ouabain signaling (45, 52). Knockdown of Na/K-ATPase α1 increases endocytosis and degradation of caveolin-1 and thereby decreases caveolin-1 content in PY-17 cells, and we have also shown that caveolin 1 expression can be rescued by rat Na/K-ATPase α1 expression in AAC-19 (10). As shown in Fig. 5, caveolin-1 protein content was comparable in AAC-19 and LM-α3-1, and significantly higher than that in PY-17. This is in sharp contrast to that in α2 expressing cells (57).
Fig. 5.
Caveolin expression in LM-α3-1. Whole cell lysates from AAC-19, LM-α3-1, and PY-17 cells were separated by SDS-PAGE and analyzed by Western blotting for the expression of caveolin-1 (Cav-1). A representative Western blot is shown. Quantification of data is shown as means ± SE from at least three separate experiments. **P < 0.01 compared with AAC-19.
We next assessed whether Na/K-ATPase α3 could regulate basal Src activity. We have previously reported that knockdown of Na/K-ATPase α1 results in increased basal Src activity in PY-17 cells, and that rescuing the knockdown cells with rat α1 was sufficient to reduce the elevated basal Src activity in AAC-19 cells (30). As shown in Fig. 6A, LM-α3-1 cells had increased basal levels of phosphorylation of Src at pY418 in comparison to AAC-19, suggesting that α3 was not regulating basal Src activity in a α1-like manner. Similar to Src, basal ERK phosphorylation was increased in LM-α3-1 compared with AAC-19. In contrast, there was no statistically significant change in Akt phosphorylation (Fig. 6C). Accordingly, to the proposed model for Na/K-ATPase-mediated regulation of Src via protein-protein interaction (29), the apparent lack of basal Src regulation in LM-α3-1 compared with AAC-19 cells could originate from a difference in the critical NaKtide sequence between α1 and α3 polypeptides. As shown in the sequence alignment (Fig. 7A), ~20% of amino acids differ between rat α1 and α3 NaKtides. Moreover, this difference is conserved among different species (Fig. 7A). To explicitly test the hypothesis that, unlike its α1 counterpart, α3 NaKtide sequence does not regulate basal Src kinase activity, the effect of a synthetic peptide was tested in vitro on purified Src. In support of this hypothesis, the results presented in Fig. 7B indicated that α3 NaKtide, like α2 NaKtide (57), does not effectively inhibit Src kinase activity compared with (α1-based) NaKtide.
Fig. 6.
Basal phosphorylation levels of protein kinases. Total cell lysates collected from AAC-19 and LM-a3-1 cells were separated by SDS-PAGE and analyzed by Western blotting for pScr (Src pY418) (A), pERK (B), and pAkt (C). A representative Western blot is shown, and the quantitative data are means ± SE from at least three independent experiments. *P < 0.05 compared with AAC-19.
Fig. 7.
Effect of Na/K-ATPase peptides on Src by in vitro kinase assay. A: amino acid sequence of α1 NaKtide and α3 NaKtide among different species. The amino acids of α3 NaKtide that are different from those of α1 NaKtide are in bold and underlined. B: Src (4.5 units) was incubated with different concentrations of NaKtide and α3 NaKtide for 15 min and then assayed for pY418 Src. Quantitative data from at least three independent experiments are shown as means ± SE. *P < 0.05 compared with control.
Binding of ouabain causes conformational changes in Na/K-ATPase α1, resulting in the activation of Src and consequently the transactivation of EGFR and the stimulation of Ras/Raf/ERK cascade (17). This activation is blocked in α1 knockdown PY-17 cells and restored when PY-17 cells are rescued by the expression of rat Na/K-ATPase α1 (AAC-19 cells). The next series of studies aimed at comparing the effects of ouabain on signaling pathways in LM-α3-1 cells and AAC-19 cells. Since Na/K-ATPase inhibition by either 10 or 100 µM ouabain is comparable for wild-type rodent Na/K-ATPase α1 and clonally selected rat α3 Na/K-ATPase (data not shown), these concentrations were applied to both AAC-19 and LM-α3-1 cells for 10 min. We observed that 10 and 100 μM ouabain stimulated Src phosphorylation within minutes in AAC-19 cells (Fig. 8A, left). Maximal activation at 10 min was apparently reached with 10 µM, with no further increase and even a trend toward a decrease with 100 µM, and a similar trend was observed for ERK (Fig. 8B). The precise mechanism underlying the temporal and spatial regulation of transient Src and ERK phosphorylation by ouabain is largely unknown, but it is possible that Src and ERK phosphorylations were submaximal at 10 min for a concentration of 100 µM. In LM-α3-1 cells, ouabain failed to stimulate Src (Fig. 8A, right). At the highest concentration of 100 μM, a statistically significant inhibition was actually detected. While the biological significance and consequences of this observation remain to be explicitly assessed, a possible mechanisms is a modulation of Src phosphotyrosine phosphatase. Somewhat surprisingly, when LM-α3-1 cells were exposed to different concentrations of ouabain and assayed for ERK activation, we found that ouabain was able to activate ERK in LM-α3-1 cells as in AAC-19 cells (Fig. 8B). These findings suggest that α3 may wire signaling partners differently than α1 by using a Src-independent signaling process. Ouabain binding to Na/K-ATPase also regulates the PI3K/Akt pathway in many types of cells, including LLC-PK1 cells (49). As shown in Fig. 8C, ouabain treatment did stimulate Akt phosphorylation in LM-α3-1 cells, comparable to the effect observed in the control AAC-19 cells.
Fig. 8.
Effect of ouabain on cell signaling. Cells were serum starved, treated with 10 and 100 µM ouabain for 10 min, and harvested. Total cell lysates collected from AAC-19 and LM-α3-1 cells were separated by SDS-PAGE and analyzed by Western blotting for Src pY418 and c-Src (A), pERK and ERK (B), and pAkt and Akt (C). A representative Western blot is shown, and the quantitative data are means ± SE from at least three independent experiments. *P < 0.05 and **P < 0.01 compared with control.
Since ouabain stimulated ERK but not Src in LM-α3-1 cells, we further interrogated the pathway leading to ERK activation by using selected pathway-specific inhibitors before treatment with ouabain 10 μM for 10 min. As shown in Fig. 9B, ouabain stimulated ERK phosphorylation. Addition of 5 µM of the Src inhibitor PP2 did not modify basal ERK phosphorylation (not shown) and did not block ouabain-induced ERK phosphorylation in LM-α3-1 cells (Fig. 9B). This is in contrast to what we and others have reported in α1-expressing tissue and cells, and is consistent with a Src-independent mechanism. The EGFR inhibitor PD153035 (PD) also failed to block ouabain-induced activation of ERK in LM-α3-1 cells (Fig. 9C), consistent with the proposed model of interreceptor cross-talk between Na/K-ATPase α1, Src, and EGFR to transduce the signal from ouabain to the Ras/MAPK cascade. In addition to Src and EGFR, ERK can be activated through PI3K and PKC pathways (55, 63). To test whether PI3K is involved in ouabain-induced ERK activation in LM-α3-1, cells were pretreated with the PI3K LY 29400 (LY). This resulted in an increased in basal ERK phosphorylation (not shown), but ouabain-induced activation was blunted (Fig. 9D). Similarly, PKC inhibiton by GO6983 (GO) before ouabain exposure effectively blocked ouabain-induced ERK activation. To assess the role of Ca2+ in ouabain-induced activation of ERK in LM-α3-1 cells, we treated the cells in the presence or absence of extracellular Ca2+ in the culture media as detailed in materials and methods. As shown in Fig. 10, Ca2+ did not seem to affect ouabain-induced ERK activation in those cells.
Fig. 9.
Effect of inhibitors on ouabain-stimulated ERK activation. LM-α3-1 cells were treated with 10 µM ouabain for 10 min without pretreatment (A) or following preincubation with selected inhibitors as follows: 5 µM Src inhibitor PP2 for 30 min (B), 2 µM EGFR inhibitor PD153035 (PD) for 60 min (C), 50 µM PI3K inhibitor LY 29400 (LY) for 60 min (D), or 1 µM PKC inhibitor GO6983 (GO) for 60 min (E). A representative Western blot is shown, and the quantitative data are means ± SE from at least three independent experiments. *P < 0.05 compared with control.
Fig. 10.

Effect of extracellular calcium (Ca2+). LM-α3-1 cells were preincubated in Krebs-Henseleit (KH) buffer with (1.8 mM) or without Ca2+ for 30 min and then treated with 10 µM ouabain for 10 min and harvested. Total cell lysates were collected and separated by SDS-PAGE and analyzed by Western blotting for pERK and ERK. A representative Western blot is shown, and the quantitative data are means ± SE from at least three independent experiments. *P < 0.05 compared with control (no ouabain).
Cell growth of LM-α3-1.
Na/K-ATPase α1 is important for cell proliferation. Knockdown of Na/K-ATPase α1 inhibited cell growth in PY-17 cells. Restoration of α1 expression rescued the growth defect in AAC19 cells (49). To assess the role of α3, we measured cell proliferation in LM-α3-1 cells. After 120 h, the number of LM-α3-1 cells was increased by 22 ± 0.8-fold, slightly less than α1-expressing AAC 19 (27 ± 0.9-fold, P < 0.05) but clearly higher than PY-17 cells (10 ± 0.04-fold, P < 0.01). Hence, Na/K-ATPase α3 is capable of supporting cell growth.
DISCUSSION
There is increased experimental and clinical evidence that Na/K-ATPase α3 serves an important and isoform-specific function in neurons. Many aspects of this specific role have remained unclear, partly because the ubiquitous presence of α1 has hindered efforts to characterize α3-specific functions in mammalian cell systems. We here report the generation and characterization of LM-α3-1, the first stable mammalian cell line expressing Na/K-ATPase α3 in the absence of detectable α1. We observe that in these cells, the α3 enzymes have lower basal Na+ pump activity and lower Na+ affinity than α1-containing enzymes in AAC-19 cells, but showed a greater capacity to be activated when intracellular Na+ was increased. The data presented in Fig. 1E suggest that α3 expression in α1 knockdown PY-17 cells rescued the expression of Na/K-ATPase β1 and caveolin-1 to levels comparable with those observed in PY-17 cells rescued with Na/K-ATPase α1 in AAC-19 cells. However, in contrast to Na/K-ATPase α1, α3 could not regulate Src. Upon exposure to ouabain, Src activation did not occur, yet ERK and Akt were activated. Inhibitor studies revealed that ERK activation was through a Src-independent pathways involving PI3K and PKC. Hence, α3 expression conferred signaling properties that were clearly distinct from that of cells expressing Na/K-ATPase α1.
In our opinion, the most salient characteristics of LM-α3-1 are reduced cellular Na/K-ATPase-mediated ion transport in physiological conditions and Src-independent signaling activity.
It should be noted that the difference between Na/K-ATPase-mediated transport in LM-α3-1 and AAC-19 in physiological conditions was no longer observed when intracellular Na+ was clamped with monensin (Fig. 4), suggesting that α3-containing enzymes had a lower Na+ affinity. Arguably, a lower intracellular Na+ content could have also explained the reduced Na/K-ATPase-dependent transport, but the kinetic analyses presented in Figs. 3 and 4 showed otherwise. Indeed, Na/K-ATPase-dependent transport measured in each cell line (86Rb+ influx in Fig. 4: ~54% in AAC-19 and 35% in LM-α3-1) was exactly as predicted by Na/K-ATPase activity for a physiological Na+ concentration of ~10 mM for the corresponding isoform (i.e., ~56% for α1 and ~38% for α3; Fig. 3), strongly suggesting that intracellular Na+ was comparable between the two cell lines.
From a non-ion-pumping standpoint, multiple lines of evidence suggest that Na/K-ATPase α1 interacts with Src kinase and regulates its basal activity through two pairs of direct protein-protein interactions. Support for this model comes from experimental evidence that include coimmunoprecipitation, fluorescence resonance energy transfer, in vitro pulldown assays, peptides derived from Na/K-ATPase α1, and gain-of-function/loss-of-function in targeted mutants (3, 27, 29–31, 48, 60, 61). Also, increasing number of animal studies are reinforcing the model (28, 46). As reiterated by Askari et al. (16) and Koendrink et al. (54), studies in cell-free systems have shown that modulation of Src phosphorylation can also occur through simple change of substrate availability when the two proteins are close and may contribute to some of the effects we have observed in cells or animal studies. The concept of direct Na/K-ATPase/Src interaction itself has been challenged, and future studies may reveal whether methodological considerations (e.g., use of preformed complexes, selection of antibodies, and the phosphorylation status of Src) could help clarify some of the apparent discrepancies (50, 62).
In contrast to this regulatory role on Src, evidence presented herein and in Xie et al. (57) do not support such a regulatory role for Na/K-ATPase α2 or α3. This results in elevated levels of phosphorylated Src in LX-α2-4 and LM-α3-1 cells compared with AAC-1 cells, which most likely causes the increased levels of basal ERK phosphorylation also observed in those two cell lines. Upon addition of a Na/K-ATPase ligand such as ouabain, an important distinction between Na/K-ATPase α2 and α3 is revealed, as further phosphorylation of ERK occurs in a Src-independent manner in LM-α3-1 but not in LX-α2-4 cells. Based on the pharmacological studies presented in Fig. 9, PKC and/or PI3K pathways are involved in Src-independent α3-mediated ERK phosphorylation in response to ouabain.
The impact of this emerging concept of isoform-specific signaling is yet to be explored, but a simple comparison of PY-17, AAC-19, LX-α2-4, and LM-α3-1 growth rates points to profound effects on cell physiology. Indeed, Na/K-ATPase α1 knockdown cells (PY-17) have a drastically reduced cell growth rate that is about a third of that of α1-rescued AAC-19 [10- vs. 27-fold increase in cell number at day 5 (30)]. Rescue with Na/K-ATPase α2 or α3 did not restore AAC-19-like cell growth, but revealed a major difference between the two cell lines, with α3 conferring a clear growth advantage compared with α2 (22- vs. 8-fold increase in cell number at day 5) (57). Sustained and unregulated activation of ERK is known to prevent cell cycle progression (11) and is likely to play a role in the growth defect observed in PY-17, LX-α2-4, and LM-α3-1. Although it remains to be determined, the lack of caveolin-1 rescue by α2 might be an important factor leading to the more severe PY-17-like growth defect observed in LX-α2-4 compared with LM-α3-1, consistent with the role of caveolin-1 in growth factor signaling (36).
Although the role of Src-independent ERK and Akt signaling through α3 in mature neurons that do not proliferate remains to be investigated using a different readout, restricted expression of Na/K-ATPase α3 and abundance in neurons has long pointed to an essential and isoform-specific function for this isoform in the brain. Studies in genetically engineered mouse models with altered expression of α3 have shown a correlative link to cognitive deficits (33, 38) and mood disturbances (25, 26). Perhaps even more critically, the identification of mutations of α3 in patients with rapid-onset dystonia Parkinsonism (13) and alternating hemiplegia of childhood (20), combined with recent findings in persons suffering from Alzheimer’s disease (39, 65), have underlined the clinical importance of Na/K-ATPase α3-specific function. Mechanistically, studies in heterologous expression systems have revealed the impact of these mutations on the ion-pumping function of α3-containing enzymes (13, 20, 53), but also pointed to additional levels of complexity to explain how various mutations on α3 result in so clearly distinct diseases (19) that could be further investigated using the LM-α3-1 system. Finally, LM-α3-1 may help explain the restricted pattern of expression of α3. For example, based on the “pump-leak” hypothesis (51) and the kinetic results presented herein, abundance of high Na+ affinity Na/K-ATPase α1 rather than α3 in absorptive epithelia may not come as a surprise, as α1 is more active and consequently able to more dynamically orchestrate ion-driven transport of fluid and nutrients at lower Na+ concentrations.
Overall, we anticipate that newly generated mammalian cell lines including LM-α3-1 will be useful in future studies as manageable and reliable cell systems in which α-isoform-specific mechanisms of the ion-pumping and non-ion-pumping functions of the Na/K-ATPase protein complex can be extensively explored.
GRANTS
This work was supported, in part, by National Heart, Lung, and Blood Institute Grant HL-109015.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
N.M., Y.X., Q.D., M.B., and I.L. performed experiments; N.M. and Q.D. analyzed data; N.M. interpreted results of experiments; N.M. prepared figures; N.M., S.V.P., and Z.X. drafted manuscript; S.V.P. and Z.X. edited and revised manuscript; S.V.P. and Z.X. approved final version of manuscript; Z.X. conceived and designed research.
ACKNOWLEDGMENTS
We thank Carla Cook for proofreading the manuscript and for technical support.
REFERENCES
- 1.Aperia A, Akkuratov EE, Fontana JM, Brismar H. Na+-K+-ATPase, a new class of plasma membrane receptors. Am J Physiol Cell Physiol 310: C491–C495, 2016. doi: 10.1152/ajpcell.00359.2015. [DOI] [PubMed] [Google Scholar]
- 2.Azarias G, Kruusmägi M, Connor S, Akkuratov EE, Liu XL, Lyons D, Brismar H, Broberger C, Aperia A. A specific and essential role for Na,K-ATPase α3 in neurons co-expressing α1 and α3. J Biol Chem 288: 2734–2743, 2013. doi: 10.1074/jbc.M112.425785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banerjee M, Duan Q, Xie Z. SH2 ligand-like effects of second cytosolic domain of Na/K-ATPase α1 subunit on Src kinase. PLoS One 10: e0142119, 2015. doi: 10.1371/journal.pone.0142119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blanco G. Na,K-ATPase subunit heterogeneity as a mechanism for tissue-specific ion regulation. Semin Nephrol 25: 292–303, 2005. doi: 10.1016/j.semnephrol.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 5.Blanco G, Mercer RW. Isozymes of the Na-K-ATPase: heterogeneity in structure, diversity in function. Am J Physiol Renal Physiol 275: F633–F650, 1998. [DOI] [PubMed] [Google Scholar]
- 6.Boscher C, Nabi IR. Caveolin-1: role in cell signaling. Adv Exp Med Biol 729: 29–50, 2012. doi: 10.1007/978-1-4614-1222-9_3. [DOI] [PubMed] [Google Scholar]
- 7.Broude NE, Modyanov NN, Monastyrskaya GS, Sverdlov ED. Advances in Na+,K+-ATPase studies: from protein to gene and back to protein. FEBS Lett 257: 1–9, 1989. doi: 10.1016/0014-5793(89)81773-9. [DOI] [PubMed] [Google Scholar]
- 8.Burkard C, Verheije MH, Haagmans BL, van Kuppeveld FJ, Rottier PJ, Bosch BJ, de Haan CA. ATP1A1-mediated Src signaling inhibits coronavirus entry into host cells. J Virol 89: 4434–4448, 2015. doi: 10.1128/JVI.03274-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burlaka I, Liu XL, Rebetz J, Arvidsson I, Yang L, Brismar H, Karpman D, Aperia A. Ouabain protects against Shiga toxin-triggered apoptosis by reversing the imbalance between Bax and Bcl-xL. J Am Soc Nephrol 24: 1413–1423, 2013. doi: 10.1681/ASN.2012101044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cai T, Wang H, Chen Y, Liu L, Gunning WT, Quintas LE, Xie ZJ. Regulation of caveolin-1 membrane trafficking by the Na/K-ATPase. J Cell Biol 182: 1153–1169, 2008. doi: 10.1083/jcb.200712022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chambard JC, Lefloch R, Pouysségur J, Lenormand P. ERK implication in cell cycle regulation. Biochim Biophys Acta 1773: 1299–1310, 2007. doi: 10.1016/j.bbamcr.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 12.Chen Y, Kennedy DJ, Ramakrishnan DP, Yang M, Huang W, Li Z, Xie Z, Chadwick AC, Sahoo D, Silverstein RL. Oxidized LDL-bound CD36 recruits an Na+/K+-ATPase-Lyn complex in macrophages that promotes atherosclerosis. Sci Signal 8: ra91, 2015. doi: 10.1126/scisignal.aaa9623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Carvalho Aguiar P, Sweadner KJ, Penniston JT, Zaremba J, Liu L, Caton M, Linazasoro G, Borg M, Tijssen MAJ, Bressman SB, Dobyns WB, Brashear A, Ozelius LJ. Mutations in the Na+/K+-ATPase α3 gene ATP1A3 are associated with rapid-onset dystonia parkinsonism. Neuron 43: 169–175, 2004. doi: 10.1016/j.neuron.2004.06.028. [DOI] [PubMed] [Google Scholar]
- 14.Drummond CA, Hill MC, Shi H, Fan X, Xie JX, Haller ST, Kennedy DJ, Liu J, Garrett MR, Xie Z, Cooper CJ, Shapiro JI, Tian J. Na/K-ATPase signaling regulates collagen synthesis through microRNA-29b-3p in cardiac fibroblasts. Physiol Genomics 48: 220–229, 2016. doi: 10.1152/physiolgenomics.00116.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fontana JM, Burlaka I, Khodus G, Brismar H, Aperia A. Calcium oscillations triggered by cardiotonic steroids. FEBS J 280: 5450–5455, 2013. doi: 10.1111/febs.12448. [DOI] [PubMed] [Google Scholar]
- 16.Gable ME, Abdallah SL, Najjar SM, Liu L, Askari A. Digitalis-induced cell signaling by the sodium pump: on the relation of Src to Na+/K+-ATPase. Biochem Biophys Res Commun 446: 1151–1154, 2014. doi: 10.1016/j.bbrc.2014.03.071. [DOI] [PubMed] [Google Scholar]
- 17.Haas M, Askari A, Xie Z. Involvement of Src and epidermal growth factor receptor in the signal-transducing function of Na+/K+-ATPase. J Biol Chem 275: 27832–27837, 2000. [DOI] [PubMed] [Google Scholar]
- 18.Haber RS, Pressley TA, Loeb JN, Ismail-Beigi F. Ionic dependence of active Na-K transport: “clamping” of cellular Na+ with monensin. Am J Physiol 253: F26–F33, 1987. [DOI] [PubMed] [Google Scholar]
- 19.Heinzen EL, Arzimanoglou A, Brashear A, Clapcote SJ, Gurrieri F, Goldstein DB, Jóhannesson SH, Mikati MA, Neville B, Nicole S, Ozelius LJ, Poulsen H, Schyns T, Sweadner KJ, van den Maagdenberg A, Vilsen B; ATP1A3 Working Group . Distinct neurological disorders with ATP1A3 mutations. Lancet Neurol 13: 503–514, 2014. doi: 10.1016/S1474-4422(14)70011-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heinzen EL, Swoboda KJ, Hitomi Y, Gurrieri F, Nicole S, de Vries B, Tiziano FD, Fontaine B, Walley NM, Heavin S, Panagiotakaki E; European Alternating Hemiplegia of Childhood (AHC) Genetics ConsortiumBiobanca e Registro Clinico per l’Emiplegia Alternante (I.B.AHC) Consortium; European Network for Research on Alternating Hemiplegia (ENRAH) for Small and Medium-sized Enterpriese (SMEs) Consortium; Fiori S, Abiusi E, Di Pietro L, Sweney MT, Newcomb TM, Viollet L, Huff C, Jorde LB, Reyna SP, Murphy KJ, Shianna KV, Gumbs CE, Little L, Silver K, Ptáček LJ, Haan J, Ferrari MD, Bye AM, Herkes GK, Whitelaw CM, Webb D, Lynch BJ, Uldall P, King MD, Scheffer IE, Neri G, Arzimanoglou A, van den Maagdenberg AM, Sisodiya SM, Mikati MA, Goldstein DB. De novo mutations in ATP1A3 cause alternating hemiplegia of childhood. Nat Genet 44: 1030–1034, 2012. doi: 10.1038/ng.2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herrera VL, Emanuel JR, Ruiz-Opazo N, Levenson R, Nadal-Ginard B. Three differentially expressed Na,K-ATPase α subunit isoforms: structural and functional implications. J Cell Biol 105: 1855–1865, 1987. doi: 10.1083/jcb.105.4.1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karpova L, Eva A, Kirch U, Boldyrev A, Scheiner-Bobis G. Sodium pump α1 and α3 subunit isoforms mediate distinct responses to ouabain and are both essential for survival of human neuroblastoma. FEBS J 277: 1853–1860, 2010. doi: 10.1111/j.1742-4658.2010.07602.x. [DOI] [PubMed] [Google Scholar]
- 23.Karpova LV, Bulygina ER, Boldyrev AA. Different neuronal Na+/K+-ATPase isoforms are involved in diverse signaling pathways. Cell Biochem Funct 28: 135–141, 2010. doi: 10.1002/cbf.1632. [DOI] [PubMed] [Google Scholar]
- 24.Kinoshita PF, Yshii LM, Vasconcelos AR, Orellana AM, Lima LS, Davel AP, Rossoni LV, Kawamoto EM, Scavone C. Signaling function of Na,K-ATPase induced by ouabain against LPS as an inflammation model in hippocampus. J Neuroinflammation 11: 218, 2014. doi: 10.1186/s12974-014-0218-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kirshenbaum GS, Clapcote SJ, Duffy S, Burgess CR, Petersen J, Jarowek KJ, Yücel YH, Cortez MA, Snead OC III, Vilsen B, Peever JH, Ralph MR, Roder JC. Mania-like behavior induced by genetic dysfunction of the neuron-specific Na+,K+-ATPase α3 sodium pump. Proc Natl Acad Sci USA 108: 18144–18149, 2011. doi: 10.1073/pnas.1108416108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kirshenbaum GS, Saltzman K, Rose B, Petersen J, Vilsen B, Roder JC. Decreased neuronal Na+, K+-ATPase activity in Atp1a3 heterozygous mice increases susceptibility to depression-like endophenotypes by chronic variable stress. Genes Brain Behav 10: 542–550, 2011. doi: 10.1111/j.1601-183X.2011.00691.x. [DOI] [PubMed] [Google Scholar]
- 27.Lai F, Madan N, Ye Q, Duan Q, Li Z, Wang S, Si S, Xie Z. Identification of a mutant α1 Na/K-ATPase that pumps but is defective in signal transduction. J Biol Chem 288: 13295–13304, 2013. doi: 10.1074/jbc.M113.467381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Z, Zhang Z, Xie JX, Li X, Tian J, Cai T, Cui H, Ding H, Shapiro JI, Xie Z. Na/K-ATPase mimetic pNaKtide peptide inhibits the growth of human cancer cells. J Biol Chem 286: 32394–32403, 2011. doi: 10.1074/jbc.M110.207597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Z, Cai T, Tian J, Xie JX, Zhao X, Liu L, Shapiro JI, Xie Z. NaKtide, a Na/K-ATPase-derived peptide Src inhibitor, antagonizes ouabain-activated signal transduction in cultured cells. J Biol Chem 284: 21066–21076, 2009. doi: 10.1074/jbc.M109.013821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liang M, Cai T, Tian J, Qu W, Xie ZJ. Functional characterization of Src-interacting Na/K-ATPase using RNA interference assay. J Biol Chem 281: 19709–19719, 2006. doi: 10.1074/jbc.M512240200. [DOI] [PubMed] [Google Scholar]
- 31.Liang M, Tian J, Liu L, Pierre S, Liu J, Shapiro J, Xie ZJ. Identification of a pool of non-pumping Na/K-ATPase. J Biol Chem 282: 10585–10593, 2007. doi: 10.1074/jbc.M609181200. [DOI] [PubMed] [Google Scholar]
- 32.Lingrel JB, Kuntzweiler T. Na+,K+-ATPase. J Biol Chem 269: 19659–19662, 1994. [PubMed] [Google Scholar]
- 33.Lingrel JB, Williams MT, Vorhees CV, Moseley AE. Na,K-ATPase and the role of α isoforms in behavior. J Bioenerg Biomembr 39: 385–389, 2007. doi: 10.1007/s10863-007-9107-9. [DOI] [PubMed] [Google Scholar]
- 34.Liu J, Periyasamy SM, Gunning W, Fedorova OV, Bagrov AY, Malhotra D, Xie Z, Shapiro JI. Effects of cardiac glycosides on sodium pump expression and function in LLC-PK1 and MDCK cells. Kidney Int 62: 2118–2125, 2002. doi: 10.1046/j.1523-1755.2002.00672.x. [DOI] [PubMed] [Google Scholar]
- 35.Liu L, Mohammadi K, Aynafshar B, Wang H, Li D, Liu J, Ivanov AV, Xie Z, Askari A. Role of caveolae in signal-transducing function of cardiac Na+/K+-ATPase. Am J Physiol Cell Physiol 284: C1550–C1560, 2003. doi: 10.1152/ajpcell.00555.2002. [DOI] [PubMed] [Google Scholar]
- 36.Liu P, Rudick M, Anderson RG. Multiple functions of caveolin-1. J Biol Chem 277: 41295–41298, 2002. doi: 10.1074/jbc.R200020200. [DOI] [PubMed] [Google Scholar]
- 37.Lucchesi PA, Sweadner KJ. Postnatal changes in Na,K-ATPase isoform expression in rat cardiac ventricle. Conservation of biphasic ouabain affinity. J Biol Chem 266: 9327–9331, 1991. [PubMed] [Google Scholar]
- 38.Moseley AE, Williams MT, Schaefer TL, Bohanan CS, Neumann JC, Behbehani MM, Vorhees CV, Lingrel JB. Deficiency in Na,K-ATPase α isoform genes alters spatial learning, motor activity, and anxiety in mice. J Neurosci 27: 616–626, 2007. doi: 10.1523/JNEUROSCI.4464-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ohnishi T, Yanazawa M, Sasahara T, Kitamura Y, Hiroaki H, Fukazawa Y, Kii I, Nishiyama T, Kakita A, Takeda H, Takeuchi A, Arai Y, Ito A, Komura H, Hirao H, Satomura K, Inoue M, Muramatsu S, Matsui K, Tada M, Sato M, Saijo E, Shigemitsu Y, Sakai S, Umetsu Y, Goda N, Takino N, Takahashi H, Hagiwara M, Sawasaki T, Iwasaki G, Nakamura Y, Nabeshima Y, Teplow DB, Hoshi M. Na, K-ATPase α3 is a death target of Alzheimer patient amyloid-β assembly. Proc Natl Acad Sci USA 112: E4465–E4474, 2015. doi: 10.1073/pnas.1421182112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parton RG, Simons K. The multiple faces of caveolae. Nat Rev Mol Cell Biol 8: 185–194, 2007. doi: 10.1038/nrm2122. [DOI] [PubMed] [Google Scholar]
- 41.Pierre SV, Sottejeau Y, Gourbeau JM, Sánchez G, Shidyak A, Blanco G. Isoform specificity of Na-K-ATPase-mediated ouabain signaling. Am J Physiol Renal Physiol 294: F859–F866, 2008. doi: 10.1152/ajprenal.00089.2007. [DOI] [PubMed] [Google Scholar]
- 42.Pierre SV, Xie Z. The Na,K-ATPase receptor complex: its organization and membership. Cell Biochem Biophys 46: 303–316, 2006. doi: 10.1385/CBB:46:3:303. [DOI] [PubMed] [Google Scholar]
- 43.Pressley TA, Duran MJ, Pierre SV. Regions conferring isoform-specific function in the catalytic subunit of the Na,K-pump. Front Biosci 10: 2018–2026, 2005. doi: 10.2741/1677. [DOI] [PubMed] [Google Scholar]
- 44.Price EM, Lingrel JB. Structure-function relationships in the Na,K-ATPase α subunit: site-directed mutagenesis of glutamine-111 to arginine and asparagine-122 to aspartic acid generates a ouabain-resistant enzyme. Biochemistry 27: 8400–8408, 1988. doi: 10.1021/bi00422a016. [DOI] [PubMed] [Google Scholar]
- 45.Quintas LEM, Pierre SV, Liu L, Bai Y, Liu X, Xie Z-J. Alterations of Na+/K+-ATPase function in caveolin-1 knockout cardiac fibroblasts. J Mol Cell Cardiol 49: 525–531, 2010. doi: 10.1016/j.yjmcc.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sodhi K, Maxwell K, Yan Y, Liu J, Chaudhry MA, Getty M, Xie Z, Abraham NG, Shapiro JI. pNaKtide inhibits Na/K-ATPase reactive oxygen species amplification and attenuates adipogenesis. Sci Adv 1: e1500781, 2015. doi: 10.1126/sciadv.1500781. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 47.Sweadner KJ. Enzymatic properties of separated isozymes of the Na,K-ATPase. Substrate affinities, kinetic cooperativity, and ion transport stoichiometry. J Biol Chem 260: 11508–11513, 1985. [PubMed] [Google Scholar]
- 48.Tian J, Cai T, Yuan Z, Wang H, Liu L, Haas M, Maksimova E, Huang XY, Xie ZJ. Binding of Src to Na+/K+-ATPase forms a functional signaling complex. Mol Biol Cell 17: 317–326, 2006. doi: 10.1091/mbc.E05-08-0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tian J, Li X, Liang M, Liu L, Xie JX, Ye Q, Kometiani P, Tillekeratne M, Jin R, Xie Z. Changes in sodium pump expression dictate the effects of ouabain on cell growth. J Biol Chem 284: 14921–14929, 2009. doi: 10.1074/jbc.M808355200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tomasiak M, Stelmach H, Rusak T, Ciborowski M, Radziwon P. The involvement of Na+/K+-ATPase in the development of platelet procoagulant response. Acta Biochim Pol 54: 625–639, 2007. [PubMed] [Google Scholar]
- 51.Tosteson DC, Hoffman JF. Regulation of cell volume by active cation transport in high and low potassium sheep red cells. J Gen Physiol 44: 169–194, 1960. doi: 10.1085/jgp.44.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang H, Haas M, Liang M, Cai T, Tian J, Li S, Xie Z. Ouabain assembles signaling cascades through the caveolar Na+/K+-ATPase. J Biol Chem 279: 17250–17259, 2004. doi: 10.1074/jbc.M313239200. [DOI] [PubMed] [Google Scholar]
- 53.Weigand KM, Messchaert M, Swarts HGP, Russel FGM, Koenderink JB. Alternating Hemiplegia of Childhood mutations have a differential effect on Na+,K+-ATPase activity and ouabain binding. Biochim Biophys Acta 1842: 1010–1016, 2014. doi: 10.1016/j.bbadis.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 54.Weigand KM, Swarts HG, Fedosova NU, Russel FG, Koenderink JB. Na,K-ATPase activity modulates Src activation: a role for ATP/ADP ratio. Biochim Biophys Acta 1818: 1269–1273, 2012. doi: 10.1016/j.bbamem.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 55.Wu J, Akkuratov EE, Bai Y, Gaskill CM, Askari A, Liu L. Cell signaling associated with Na+/K+-ATPase: activation of phosphatidylinositide 3-kinase IA/Akt by ouabain is independent of Src. Biochemistry 52: 9059–9067, 2013. doi: 10.1021/bi4011804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wu J, Li D, Du L, Baldawi M, Gable ME, Askari A, Liu L. Ouabain prevents pathological cardiac hypertrophy and heart failure through activation of phosphoinositide 3-kinase α in mouse. Cell Biosci 5: 64, 2015. doi: 10.1186/s13578-015-0053-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xie J, Ye Q, Cui X, Madan N, Yi Q, Pierre SV, Xie Z. Expression of rat Na-K-ATPase α2 enables ion pumping but not ouabain-induced signaling in α1-deficient porcine renal epithelial cells. Am J Physiol Cell Physiol 309: C373–C382, 2015. doi: 10.1152/ajpcell.00103.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xie Z, Cai T. Na+-K+-ATPase-mediated signal transduction: from protein interaction to cellular function. Mol Interv 3: 157–168, 2003. doi: 10.1124/mi.3.3.157. [DOI] [PubMed] [Google Scholar]
- 59.Xie ZJ. Molecular mechanisms of Na/K-ATPase-mediated signal transduction, in Na,K-Atpase and Related Cation Pumps: Structure, Function, and Regulatory Mechanisms (Jorgensen PL, Karlish SJD, Maunsbach AB, editors). New York: New York Acad. Sci, 2003, p. 497–503. [DOI] [PubMed] [Google Scholar]
- 60.Ye Q, Lai F, Banerjee M, Duan Q, Li Z, Si S, Xie Z. Expression of mutant α1 Na/K-ATPase defective in conformational transition attenuates Src-mediated signal transduction. J Biol Chem 288: 5803–5814, 2013. doi: 10.1074/jbc.M112.442608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ye Q, Li Z, Tian J, Xie JX, Liu L, Xie Z. Identification of a potential receptor that couples ion transport to protein kinase activity. J Biol Chem 286: 6225–6232, 2011. doi: 10.1074/jbc.M110.202051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yosef E, Katz A, Peleg Y, Mehlman T, Karlish SJ. Do Src kinase and Caveolin interact directly with Na,K-ATPase? J Biol Chem 291: 11736–11750, 2016. doi: 10.1074/jbc.M116.721084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yuan Z, Cai T, Tian J, Ivanov AV, Giovannucci DR, Xie Z. Na/K-ATPase tethers phospholipase C and IP3 receptor into a calcium-regulatory complex. Mol Biol Cell 16: 4034–4045, 2005. doi: 10.1091/mbc.E05-04-0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yudowski GA, Efendiev R, Pedemonte CH, Katz AI, Berggren PO, Bertorello AM. Phosphoinositide-3 kinase binds to a proline-rich motif in the Na+, K+-ATPase α subunit and regulates its trafficking. Proc Natl Acad Sci USA 97: 6556–6561, 2000. doi: 10.1073/pnas.100128297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang LN, Sun YJ, Pan S, Li JX, Qu YE, Li Y, Wang YL, Gao ZB. Na+-K+-ATPase, a potent neuroprotective modulator against Alzheimer disease. Fundam Clin Pharmacol 27: 96–103, 2013. doi: 10.1111/fcp.12000. [DOI] [PubMed] [Google Scholar]