Abstract
Peptide growth factors stimulate cellular responses through activation of their transmembrane receptors. Multiple intracellular signaling cascades are engaged following growth factor–receptor binding, leading to short- and long-term biological effects. Each receptor-activated signaling pathway does not act in isolation but rather interacts at different levels with other pathways to shape signaling networks that are distinctive for each growth factor. To gain insights into the specifics of growth factor-regulated interactions among different signaling cascades, we developed a HeLa cell line stably expressing fluorescent live-cell imaging reporters that are readouts for two major growth factor-stimulated pathways, Ras–Raf–Mek–ERK and phosphatidylinositol (PI) 3-kinase–Akt. Incubation of cells with epidermal growth factor (EGF) resulted in rapid, robust, and sustained ERK signaling but shorter-term activation of Akt. In contrast, hepatocyte growth factor induced sustained Akt signaling but weak and short-lived ERK activity, and insulin-like growth factor-I stimulated strong long-term Akt responses but negligible ERK signaling. To address potential interactions between signaling pathways, we employed specific small-molecule inhibitors. In cells incubated with EGF or platelet-derived growth factor-AA, Raf activation and the subsequent stimulation of ERK reduced Akt signaling, whereas Mek inhibition, which blocked ERK activation, enhanced Akt and turned transient effects into sustained responses. Our results reveal that individual growth factors initiate signaling cascades that vary markedly in strength and duration and demonstrate in living cells the dramatic effects of cross talk from Raf and Mek to PI 3-kinase and Akt. Our data further indicate how specific growth factors can encode distinct cellular behaviors by promoting complex interactions among signaling pathways.
Keywords: growth factors, cell signaling, signal transduction, protein kinase B, Raf, mitogen/extracellular signal-regulated kinase, extracellular signal-regulated kinase
peptide growth factors mediate the responses of cells to environmental stimuli by regulating the activity of intracellular signaling pathways (11). Growth factor actions begin with receptor binding, which triggers intracellular protein kinase enzymatic activity, leading to the stimulation of multiple downstream signaling networks that typically function through a series of phosphorylation cascades (11, 26). Despite advances in cell biology and biochemistry that have identified and characterized receptors and downstream pathways in great detail, our knowledge of signaling dynamics and how growth factor-initiated inputs are encoded into distinctive cellular outputs remains limited (25, 37). Studying the responses of single cells in a population has been particularly challenging because most experimental methods exhibit inadequate sensitivity and temporal resolution. Moreover, these approaches are unable to account for potential interconnections or feedback and feedforward loops that might modify the duration and magnitude of individual signaling pathways (3, 37, 55, 57).
The Ras–Raf–Mek–ERK cascade has long served as a canonical example for studying signaling outcomes downstream of tyrosine kinase receptors. In seminal work, it was found that different growth factors induced distinct fates in PC-12 cells in a manner related to the duration of ERK activity (29). More recently, progress has been made in understanding mechanisms underlying ERK signaling dynamics. Optogenetic methods have been developed that allow manipulation of the magnitude and duration of ERK activity (60), and fluorescent reporters have been generated that enable the tracking of responses in individual cells (3, 9, 38, 45). Collectively, both older and newer studies have shown that ERK can produce pulsatile or sustained downstream effects, depending on the cell type and the pattern of growth factor exposure (3, 9, 45), and have revealed an important role for negative feedback in modulating signaling outcomes (10, 24, 47, 55, 64).
The phosphatidylinositol (PI) 3-kinase–Akt signaling pathway is also stimulated by growth factors via their tyrosine kinase receptors (28). Active Akt can directly phosphorylate several categories of substrates, including mediators of immediate changes in intermediary metabolism, and cell shape and movement, and can promote long-term effects on cell viability, proliferation, or differentiation (18, 28, 61). Recently, using a live-cell imaging approach with a fluorescent translocation sensor, we characterized Akt signaling responses induced by different growth factors in a fibroblast cell line. Our studies showed that signaling dynamics and outcomes were growth factor specific (15). Insulin-like growth factor-I (IGF-I) and insulin produced sustained concentration-dependent effects, whereas epidermal growth factor (EGF) caused more transient responses. Platelet-derived growth factor (PDGF)-BB and PDGF-AA mediated transient effects at lower growth factor exposures and produced sustained responses at higher concentrations. Moreover, in results analogous to earlier observations on ERK pathway-mediated changes in cell fate, we found that the duration of Akt signaling was related to the ability of individual growth factors to promote cell cycle progression (16).
Our initial observations focusing on a single signaling mediator illustrated the potentially critical roles played by individual growth factor receptors in determining signaling outcomes (15, 16). Each receptor, with its distinct pattern of phosphotyrosine-specific binding domains for signaling intermediates, also may shape the extent of activation of different downstream signaling cascades (35, 41, 62). As a consequence, receptors may be key factors leading to biased stimulation of one pathway vs. another and thus help determine how different growth factors induce distinct biological outcomes (2, 32, 41). This interactive process of cross talk between pathways has many potential ramifications. For instance, in some cells, Ras can stimulate PI 3-kinase, thus leading to Akt activity (2, 22, 32, 43, 63). As a result, a growth factor receptor that directly activates the Ras–Raf–Mek–ERK pathway also may indirectly promote Akt signaling.
In this report, we have evaluated in real time both the Ras–Raf–Mek–ERK and PI 3-kinase–Akt signaling pathways in the same cells using fluorescent translocation sensors. Our results show that different growth factors not only stimulate distinct signaling dynamics but also produce biased activation of one pathway over another. Moreover, we find that cross talk from Ras–Raf–Mek–ERK to PI 3-kinase–Akt has pronounced modulatory effects on Akt signaling activity. Our observations thus provide a quantitative insight into how different growth factors control cellular behaviors and how individual signaling pathways influence each other.
MATERIALS AND METHODS
Materials.
Cell culture media, including Dulbecco’s modified Eagle’s medium (DMEM), phosphate-buffered saline (PBS), FluoroBrite imaging medium, and trypsin/EDTA solution, were purchased from GIBCO-Life Technologies (Carlsbad, CA). FBS was from Hyclone (Logan, UT). Cells were grown and imaged on six-well Bio-One tissue culture plates (Greiner, Monroe, NC). Restriction enzymes, buffers, ligases, and polymerases were obtained from BD Biosciences-Clontech (Palo Alto, CA) and Roche Applied Sciences (Indianapolis, IN). Primary antibodies were purchased from Cell Signaling (Beverly, MA): anti-phospho-PRAS40Thr246 (catalog no. 2997), anti-PRAS40 (no. 2691), anti-phospho-AktThr308 (no. 2965), anti-phospho-AktSer473 (no. 9271), anti-Akt (no. 4691), anti-RskThr359 (no. 8753), anti-Rsk (no. 9355), anti-phospho-ERK1/2Thr202/Tyr204 (no. 4370), and anti-ERK (no. 4695). Secondary antibodies included IR800-conjugated goat anti-rabbit IgG (Rockland, Gilbertsville, PA) and goat anti-rabbit IgG conjugated to Alexa Fluor 680 (Invitrogen, Carlsbad, CA). The IGF-I analog R3-IGF-I was purchased from GroPep (Adelaide, Australia), recombinant human hepatocyte growth factor (HGF) and recombinant human transforming growth factor-α (TGF-α) were from ProSpec (Rehovot, Israel), recombinant human insulin was from Tocris Bioscience (Bristol, UK), mouse EGF was from GIBCO-Life Technologies, and recombinant human PDGF-AA was from Thermo-Fisher Scientific (Rockford, IL). Growth factors were dissolved in 10 mM HCl with 1 mg/ml bovine serum albumin, stored in aliquots at −80°C, and diluted into FluoroBrite imaging medium immediately before use. Small-molecule inhibitors were purchased as follows: PI-103 and U-0126 (Tocris), trametinib (LC Laboratories, Woburn, MA), PLX-4720 and MK-2206 (Selleck, Houston, TX). All inhibitors were solubilized in DMSO and diluted in FluoroBrite imaging medium just before use. The fluorescent signaling protein array (Pathscan Antibody Array Kit no. 9700S) was purchased from Cell Signaling. Other reagents and chemicals were obtained from commercial suppliers.
Production of recombinant lentiviruses and stable cell lines.
The FoxO1-clover recombinant lentivirus has been described (14). To construct the mKate2-ERK2 lentiviral plasmid, tagBFP was replaced with mKate2 in plasmid pHR SFFVp BFP-ERK2 (no. 50848; Addgene, Cambridge, MA); the DNA fragment containing mKate2-ERK2 was then ligated into the pWPXL lentiviral plasmid in place of EGFP (no. 12257; Addgene). Lentiviruses were prepared as outlined previously (13, 59). HeLa cells (ATCC no. CCL-2) were cotransduced with concentrated mKate2-ERK2 and FoxO1-clover lentiviruses in the presence of polybrene (6 μg/ml). Later (7 days), cells were sorted by fluorescence intensity (green, excitation 488 nm, emission 530/40 nm and red, excitation 561 nm, emission 615/24 nm) using a Becton Dickinson Influx cell sorter at the Oregon Health & Science University Flow Cytometry Core Facility. Cell populations were obtained that expressed both FoxO1-clover and mKate2-ERK2. The C3H10T1/2 stable cell line expressing FoxO1-clover was previously described (14).
Live-cell imaging.
Cell imaging was performed using an EVOS FL Auto microscope with a stagetop incubator maintained at 37°C and 5% CO2. Images were collected at ×100 magnification using a ×10 Fluorite objective (numerical aperture: 0.3), with GFP (green: excitation peak, 472/22 nm; emission peak, 510/42 nm) and Texas red LED light cubes (red: excitation peak 585/29 nm; emission peak, 624/40 nm). Data were analyzed with the National Institutes of Health (NIH) ImageJ processing package (Fiji, NIH, Bethesda, MD) using the Subtract Background plug-in to remove background fluorescence, the Stack Reg plug-in (rigid registration) to register images, and the Gaussian Blur plug-in (2 pixels) to reduce pixel-pixel noise (48). To quantify the subcellular translocation of reporter proteins in HeLa cells, nuclei were manually located and labeled using the ROI Manager plug-in following a 2-h incubation in serum-free medium (SFM). This was defined as time 0. HeLa cells underwent minimal migration over a 90-min imaging period such that nuclear x/y coordinates at time 0 could be propagated across the entire image stack using the Multi Measure tool. The location of each nucleus from the green channel image (FoxO1-clover) was used to quantify nuclear intensity of the red channel image (mKate2-ERK2). Nuclear intensities for FoxO1-clover and mKate2-ERK2 in each cell were normalized to the value recorded at time 0 and then scaled based on the average maximal reporter translocation after incubation of cells with EGF (SFM = 0%, peak EGF = 100%). To measure the subcellular distribution of FoxO1-clover in C3H10T1/2 cells the nuclei of individual cells were manually tracked across frames using the mTrackJ plug-in (31). Cells that died, divided, or migrated out of frame were excluded from analysis.
Imaging protocols.
HeLa cells were grown in DMEM containing 10% FBS for 48 h to allow full cell attachment. After two washes with DMEM, cells were incubated in serum-free Fluorobrite imaging medium for 2 h. Growth factors and/or inhibitors were then added, and images were collected every 2.5 min for 90 min. Growth factors included R3-IGF-I (0–250 pM), EGF (0–2.1 nM), HGF (0–1.7 nM), insulin (1 nM), and TGF-α (1.67 nM). For inhibitor studies, HeLa cells were incubated in SFM for 2 h followed by addition of growth factor [EGF (2.1 nM), R3-IGF-I (250 pM), or HGF (1.72 nM)], with or without PI-103 (500 nM), U-0126 (10 μM), trametinib (500 nM), or MK-2206 (1 μM). C3H10T1/2 cells were incubated in SFM for 90 min, followed by the addition of growth factors [EGF (2.1 nM) or PDGF-AA (1.4 nM)] with or without PI-103 (500 nM), U-0126 (10 μM), or PLX-4720 (10 μM). Images from C3H10T1/2 cells were collected every 2 min for 90 min. For all imaging studies a minimum of three independent experiments was performed.
Protein extraction and immunoblotting.
Whole cell protein lysates were collected after washing cells two times with cold PBS followed by the addition of extraction buffer containing protease and phosphatase inhibitors. Protein aliquots (12.5 μg/lane) were separated by SDS-PAGE (12% separating gels) followed by transfer to Immobilon-FL membranes, blocking with 50% AquaBlock solution, and sequential incubation of membranes with primary and secondary antibodies, as described (34). Primary antibodies were added at 1:1,000 dilutions for 16 h at 4°C and secondary antibodies at 1:5,000 dilution for 90 min at 20°C. Images were captured using the LiCoR Odyssey and version 3.0 analysis software (Lincoln, NE). For analysis by protein array, whole cell protein lysates were collected from HeLa cells after addition of the provided cell lysis buffer (Pathscan Antibody Array Kit; Cell Signaling) supplemented with protease inhibitors. Protein aliquots (75 μg/well) from cells incubated with SFM or EGF (2.1 nM) for 15 min ± PI-103 (500 nM) and/or U-0126 (10 μM) were added to each slide well and incubated at 4°C for 16 h. Slides were washed four times with the provided wash buffer and then incubated for 1 h with the detection antibody cocktail at 20°C. Following four additional washes at 20°C, DyLight 680-linked streptavidin from the kit was added to each well and incubated for 30 min at 20°C. After four final washes, slides were dried and visualized with the LiCoR Odyssey.
RESULTS
Growth factor signaling in living cells.
Growth factors typically regulate a broad range of cellular responses by engaging multiple intracellular signaling pathways (11). Although each growth factor binds to its unique receptor, many downstream cascades are shared, leading to the question of how individual growth factors can cause distinct behavioral responses. We recently developed a fluorescent reporter protein designed to measure Akt activity at the single cell level (14, 15). The reporter, which is composed of a modified portion of the well-characterized Akt substrate FoxO1 (6, 39, 40, 66), fused at its COOH-terminus to the green fluorescent protein clover (23), underwent movement from the nucleus to the cytoplasm when phosphorylated by Akt (14, 15) (Fig. 1A, right). We found that different peptide growth factors elicited distinctive dynamic patterns of reporter translocation in living cells that matched their ability to stimulate Akt activity and that signaling strength and duration varied substantially among individual cells in a population (14, 15).
Fig. 1.
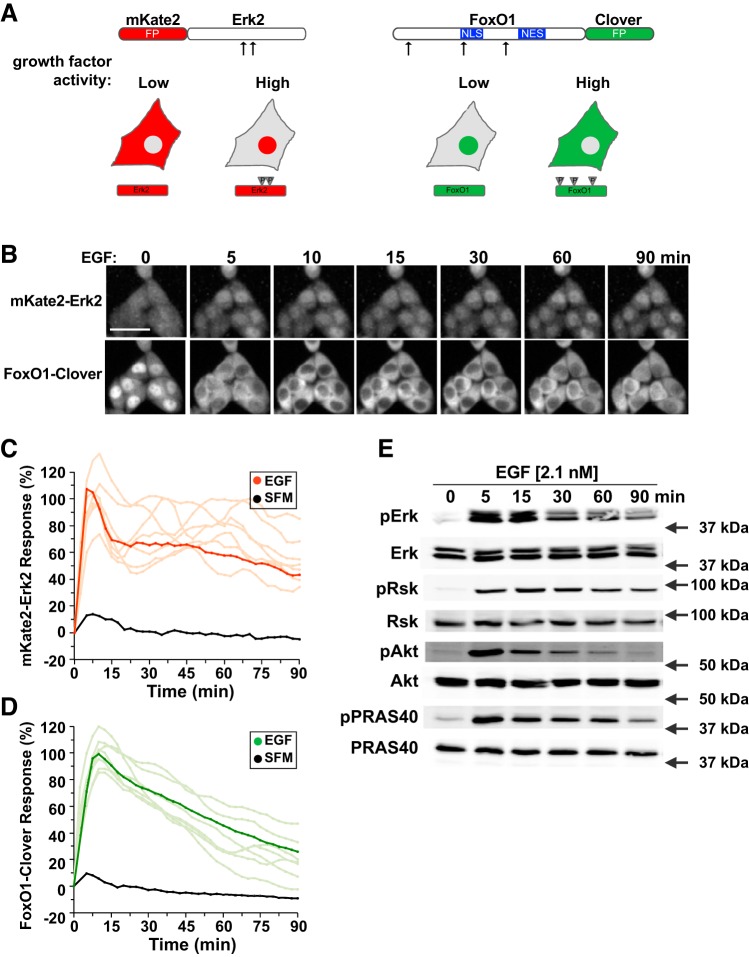
Development of sensors for ERK2 and Akt activity. A: top left, map of mKate2-ERK2 reporter molecule showing the positions of ERK2 phosphorylation sites at T185 and Y187; FP, fluorescent protein; bottom, diagram of the subcellular location of the mKate2-ERK2 reporter in cells with low growth factor signaling, where ERK2 is predominantly cytoplasmic, or high activity, where ERK2 is phosphorylated (P) and can be found in the nucleus; top right, map of FoxO1-clover sensor protein showing the positions of Akt phosphorylation sites at T24, S253, and S316. Also indicated are the nuclear localization sequence (NLS) and nuclear export sequence (NES) of FoxO1; bottom, diagram of location of the FoxO1-clover reporter in cells with low growth factor signaling, where FoxO1 is predominantly nuclear, or high activity, where FoxO1 is highly phosphorylated and is primarily cytoplasmic. B: time-lapse images of a representative experiment showing changes in the subcellular location of both reporter molecules in HeLa cells incubated with epidermal growth factor (EGF, 2.1 nM) for the times indicated. Scale bar = 50 μm. C: time course of the relative nuclear intensity of the mKate2-ERK2 reporter in cells incubated in serum-free medium (SFM) and then exposed to SFM or EGF (2.1 nM) for 90 min. Population averages are presented in red (n = ~140 cells/incubation), with individual traces indicated in salmon (n = 10). The nuclear intensity of the reporter in each cell was normalized to its value at the start of imaging during incubation in SFM and scaled to the average peak EGF response. D: time course of the relative translocation response of the FoxO1-clover reporter in the same cells as in C incubated in SFM and then exposed to SFM or EGF (2.1 nM) for 90 min. Population averages are presented (n = ~140 cells/incubation), with individual traces indicated in lime (n = 10). The nuclear intensity of the reporter in each cell was normalized to its value at the start of imaging during incubation in SFM and scaled to the average peak EGF response. E: expression of phosphorylated ERK1/2 (pERK), total ERK, pRsk1/2, total Rsk, pAktS473 (pAkt), total Akt, pPRAS40, and total PRAS40 by immunoblotting using whole cell protein lysates from HeLa cells after exposure to EGF for up to 90 min. Molecular mass markers are indicated to the right of each immunoblot.
To evaluate a broader template of growth factor-mediated signaling, we generated recombinant HeLa cell lines incorporating two live-cell imaging sensors, FoxO1-clover and mKate2-ERK2 (Fig. 1A, left). The latter fluorescent reporter reads the kinase activity of Mek-1 and -2, upstream stimulators of ERK-1 and -2, and of phosphatases that dephosphorylate the corresponding sites (51). Upon phosphorylation of ERK by Mek, the reporter accumulates in the nucleus (Fig. 1A, left). Incubation of these HeLa cells with a high dose of EGF (2.1 nM) demonstrated nearly coordinate activation of both Akt and Mek. In the population, translocation of mKate2-ERK2 from the cytoplasm to the nucleus was maximal within 5 min of EGF addition, declined to a plateau that was ~70% of maximal by 15 min, and was maintained at this level of activity for at least 90 min, although this varied among individual cells [Fig. 1, B and C and Supplemental Video S1 (Supplemental material for this article is available at the journal website)]. In the same cell population, FoxO1-clover maximally translocated from the nucleus to the cytoplasm within 7.5–10 min after EGF addition. Cytoplasmic localization then declined at a steady rate to ~30% of maximal at 90 min despite the continuing presence of growth factor (Fig. 1, B and D, and Supplemental Video S1), although both maximum cytoplasmic accumulation and the rate of return of the reporter to the nucleus varied among single cells (Fig. 1D). Thus, treatment of HeLa cells with high concentrations of EGF stimulates robust Mek signaling responses, but less sustained Akt activity, and tracking the subcellular distribution of translocation-based sensors by live-cell imaging provides a dynamic picture of signaling with high temporal resolution. Maryu et al. have recently reported on a similar dual-reporter model in which they also have studied the effects of EGF (30).
To determine if reporter behavior in living cells tracked with more traditional measures of growth factor-activated signaling, we performed serial immunoblotting of lysates from HeLa cells exposed to the same concentrations of EGF for variable times. ERK phosphorylation was minimal in cells incubated in SFM and rose to a peak within 5 min of EGF addition. This level of ERK phosphorylation declined by 30 min and was maintained at the lower value for at least 90 min after growth factor addition (Fig. 1E). A relatively similar pattern of phosphorylation was observed for the ERK substrates Rsk1 and -2 (Fig. 1E). Akt phosphorylation also peaked within 5 min of cell exposure to EGF and then rapidly fell such that it was only marginally more than baseline by 90 min (Fig. 1E). The direct Akt substrate PRAS40 showed a similar phosphorylation pattern to Akt (Fig. 1E). Thus, live-cell imaging using translocation sensors that reflect Mek and Akt kinase activity reveals signaling dynamics that are comparable to those seen by immunoblotting.
Variable signaling responses in HeLa cells to different growth factors.
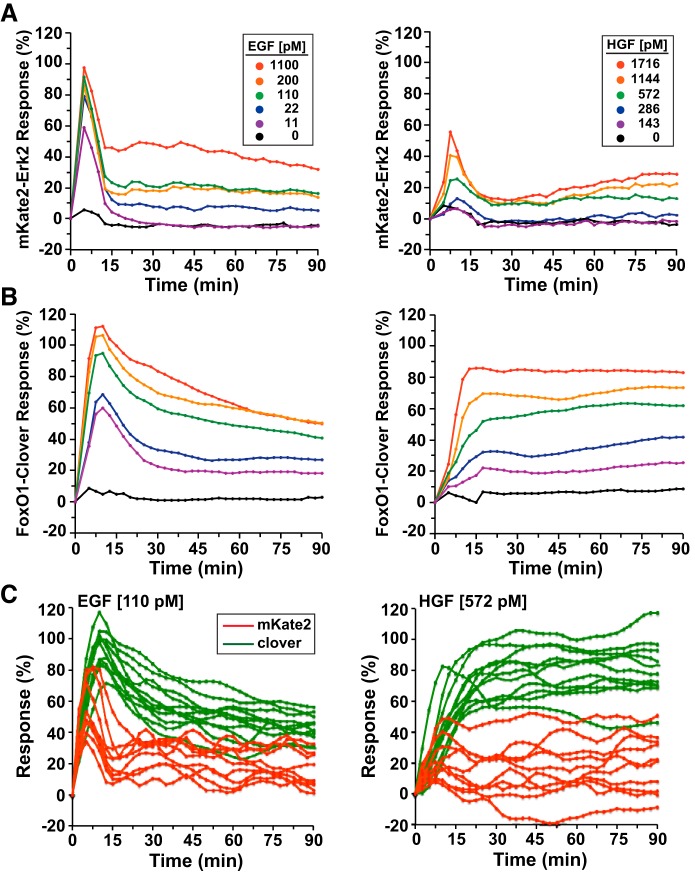
To test the effects of different growth factors on signaling dynamics, we exposed HeLa cells to varying concentrations of EGF, HGF, or IGF-I for up to 90 min (Figs. 2 and 3). Addition of EGF to cells preincubated with SFM caused a rapid dose-dependent peak in nuclear translocation of the mKate2-ERK2 reporter molecule. At the population level, half-maximal accumulation of the reporter in the nucleus was detected by 3–5 min after EGF addition at all growth factor concentrations tested, with maximal values being attained by ~7 min. Signal intensity then rapidly waned by 15 min to a steady-state value that was sustained for up to 90 min at a level ranging from ~0 to 50% of peak value depending on the EGF concentration (Fig. 2A, left).
Fig. 2.
Reporter dynamics after exposure of HeLa cells to different concentrations of EGF or hepatocyte growth factor (HGF). A and B: time course of the relative translocation responses of the mKate2-ERK2 reporter (A) and the FoxO1-clover reporter (B) in cells incubated in SFM and then exposed to different concentrations of EGF (left) or HGF (right) for 90 min. For all experiments illustrated, the relative responsiveness of each reporter protein in each cell was normalized to values at the start of imaging during incubation in SFM and scaled to the average peak response. C: time course of the relative translocation response of the mKate2-ERK2 reporter (red traces) and the FoxO1-clover reporter (green) in individual cells incubated in SFM and then exposed to EGF (110 pM, left) or HGF (572 pM, right) for 90 min. For A–C, population averages are presented (n = ~140 cells/incubation for each treatment group).
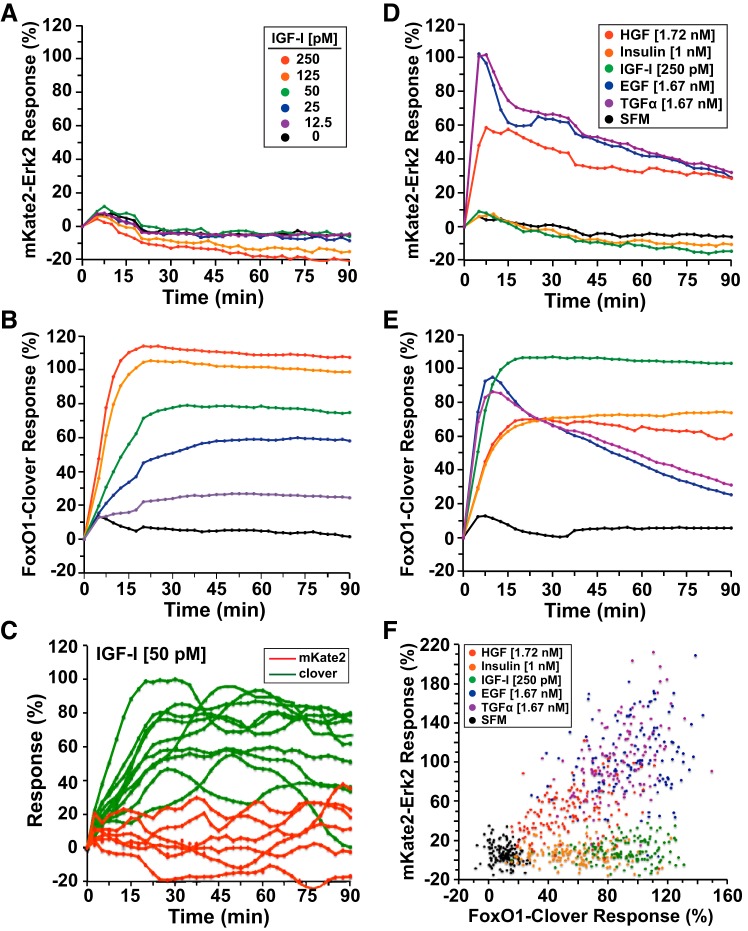
Fig. 3.
Reporter dynamics after exposure of HeLa cells to different concentrations of growth factors. A and B: time course of the relative translocation responses of the mKate2-ERK2 reporter (A) or the FoxO1-clover reporter (B) in cells incubated in SFM and then exposed to several concentrations of insulin-like growth factor-I (IGF-I) for 90 min. Population averages are presented (n = ~140 cells/incubation for each treatment group). For all experiments illustrated, the relative responsiveness of each reporter protein in each cell was normalized to values at the start of imaging during incubation in SFM and scaled to the average peak response. C: time course of the relative translocation response of the mKate2-ERK2 reporter (red traces) and the FoxO1-clover reporter (green) in individual cells incubated in SFM and then exposed to IGF-I (50 pM) for 90 min. D and E: time course of the relative translocation response of the mKate2-ERK2 reporter (D) and the FoxO1-clover reporter (E) in cells incubated in SFM and then exposed to different growth factors for 90 min. Population averages are presented (n = ~140 cells/incubation for each treatment group). For all experiments pictured, the relative responsiveness of each reporter protein in each cell was normalized to values at the start of imaging during incubation in SFM and scaled to the average peak response. F: dot plot of ERK and Akt activity, as indicated by responses of the mKate2-ERK2 reporter at 5 min and the FoxO1- clover reporter at 10 min in individual cells after incubation with SFM (black), transforming growth factor-α (TGF-α, 1.67 nM, purple), EGF (1.67 nM, blue), IGF-I (250 pM, green), insulin (1.0 nM, orange), or HGF (1.72 nM, red) (n = ~150 cells/treatment group).
The signaling response of FoxO1-clover to EGF varied from what was seen with mKate2-ERK2. Half-maximal nuclear-to-cytoplasmic translocation occurred within ~5 min of growth factor addition, and maximal activity was attained by ~10 min. This was followed by a nearly linear fall over the subsequent 20–80 min to a baseline value that was maintained for the duration of each 90-min experiment, with the kinetics and levels of activity depending on EGF concentration (Fig. 2B, left). For both pathways, the lowest EGF concentration tested (11 pM) gave a peak response that was ~50% of the highest dose (1.1 nM) (Fig. 2, A and B, left). Thus, at the population level, EGF caused dose-dependent stimulation of Ras–Raf–Mek–ERK and PI 3-kinase–Akt signaling in HeLa cells.
HGF promoted different signaling dynamics than did EGF. Translocation of the mKate2-ERK2 reporter was transient, peaking within ~8 min and declining to basal values by ~20 min, with peak magnitude varying with HGF concentration (Fig. 2A, right). At the highest levels of HGF exposure (~1.7 nM), the maximal extent of nuclear localization of mKate2-ERK2 was only one-half of what was observed in response to EGF (Fig. 2A, cf. left and right). By contrast, nuclear-to-cytoplasmic translocation of FoxO1-clover in cells incubated with HGF was sustained. Maximal cytoplasmic accumulation of the reporter was observed within ~10–15 min after growth factor addition and reached ~80% of the peak values seen after EGF treatment (Fig. 2B, right). Unlike EGF, after HGF exposure, FoxO1-clover was fully maintained in the cytoplasm in the population for the entire 90-min experimental period (Fig. 2B, cf. left and right).
At the level of individual cells, the effects of EGF and HGF were both heterogeneous. At intermediate concentrations of each growth factor [EGF (110 pM) and HGF (572 pM)], there was significant variability in peak responses and in the duration of signaling (Fig. 2C). For example, Mek activity after HGF ranged from transient to sustained, and maximal FoxO1-clover translocation varied by a factor of two (Fig. 2C, right). Thus, average population responses may mask substantial individual heterogeneity in growth factor-stimulated pathway activity.
IGF-I-activated signaling differed from the responses to EGF or HGF. In the population as a whole, there was minimal subcellular redistribution of mKate2-ERK2 after IGF-I treatment, even at the highest growth factor concentrations (250 pM) (Fig. 3A, left). By contrast, and as shown previously in other cell types (15, 16), IGF-I promoted rapid, robust, dose-dependent, and sustained Akt signaling, as reflected by maximal translocation of FoxO1-clover by ~12–18 min that was maintained for the entire 90-min experimental time course (Fig. 3B). Peak values of reporter translocation in response to IGF-I were indistinguishable from those seen with EGF but were far more sustained (cf. Figs. 3B and 2B, left). As was seen for EGF and HGF, individual cellular responses to IGF-I (50 pM) were highly variable, with mKate2-ERK2 cytoplasmic-to-nuclear translocation ranging from minimal to values as high as seen with EGF (cf. Fig. 3C and 2C, left) and the subcellular redistribution of FoxO1-clover varying over a twofold range, as noted with HGF (cf. Fig. 3C and 2C, right).
Additional experiments showed that overall population signaling responses to TGF-α were nearly identical to those seen with EGF and that responses to insulin mimicked the pattern seen with IGF-I (Fig. 3D). This is not surprising, since TGF-α uses the same receptors as EGF (49) and the insulin and IGF-I receptors employ the same intracellular adaptor molecules to couple with downstream signaling modules (54). Thus, the signaling dynamics that we observed appear to be largely dependent on the specific receptor tyrosine kinases that are activated.
A plot of the maximal responses of mKate2-ERK2 and FoxO1-clover reporters to different growth factors revealed a wide range of signaling patterns in individual cells. ERK and Akt activity was minimal in the vast majority of HeLa cells incubated in SFM (Fig. 3F). By contrast, both insulin and IGF-I led to substantial stimulation of Akt and the consequent nuclear-to-cytoplasmic translocation of FoxO1-clover in nearly all cells, with the IGF-I response being on average two times that of insulin (cf. Fig. 3F, green and orange dots). In these same cells ERK activity was minimal and was similar to what was seen in SFM (cf. Fig. 3F, green or orange vs. black dots). In contrast, signaling by HGF, TGF-α, or EGF led to coordinate activation of both pathways, with the maximal effects of EGF and TGF-α being approximately two times the magnitude of HGF, although there was substantial overlap in single cells (cf. Fig. 3F, blue and red dots). Furthermore, the single-cell peak effects of EGF, TGF-α, and HGF fell along a diagonal, indicating that, in cells treated with these growth factors, high levels of FoxO1-clover translocation tended to be accompanied by high ERK activity. Taken together, the results reveal cell-to-cell heterogeneity in growth factor signaling activity in the population while also demonstrating correlations of pathway-specific signaling responses in individual cells.
Potential signaling cross talk and convergence mechanisms.
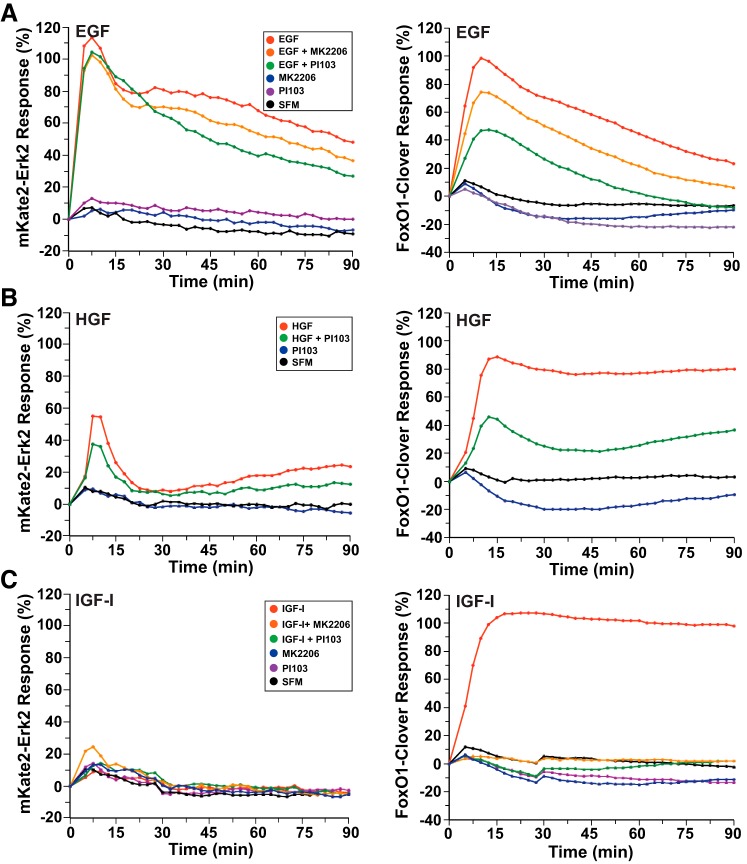
The Ras–Raf–Mek–ERK and PI 3-kinase–Akt signaling pathways have been reported to exhibit cross talk at several levels and to show convergence on similar targets in different cell types and contexts (32). Here we have used small-molecule inhibitors to assess the possible interdependence of these cascades. We find that addition of the dual PI 3-kinase and mammalian target of rapamycin (mTorc) inhibitor PI-103 or the Akt inhibitor MK-2206 had no effect on EGF-stimulated Mek activity but reduced nuclear-to-cytoplasmic translocation of FoxO1-clover by nearly 50 and 25%, respectively (Fig. 4A). Similarly, translocation to the nucleus of mKate2-ERK2 after HGF treatment was not altered by PI-103, but the response of FoxO1-clover was diminished by ~50% (Fig. 4B). In contrast, the same concentrations of PI-103 or MK-2206 that only partially prevented EGF- or HGF-mediated nuclear-to-cytoplasmic translocation of FoxO1-clover were fully effective in blocking the effects of IGF-I in HeLa cells (Fig. 4C). PI-103 also completely inhibited the actions of EGF on nuclear-to-cytoplasmic translocation of FoxO1-clover in C3H10T1/2 cells. Taken together, these results suggest that EGF- and HGF-stimulated signaling may regulate FoxO1-clover differently from IGF-I in HeLa cells, most likely secondary to strong ERK actions.
Fig. 4.
Reporter dynamics after exposure of HeLa cells to growth factors and different signaling inhibitors. A: time course of the relative translocation response of the mKate2-ERK2 reporter (left) and the FoxO1-clover reporter (right) in cells incubated in SFM and then exposed to SFM or EGF (2.1 nM) ± different inhibitors for 90 min. B: time course of the relative translocation response of the mKate2-ERK2 reporter (left) and the FoxO1-clover reporter (right) in cells incubated in SFM and then exposed to SFM or HGF (1.7 nM) ± different inhibitors for 90 min. C: time course of the relative translocation response of the mKate2-ERK2 reporter (left) and the FoxO1-clover reporter (right) in cells incubated in SFM and then exposed to SFM or IGF-I (250 pM) ± different inhibitors for 90 min. For A–C, population averages are presented (n = ~140 cells/incubation for each treatment group). For all experiments depicted, the relative responsiveness of each reporter protein in each cell was normalized to values at the start of imaging during incubation in SFM and scaled to the average peak response.
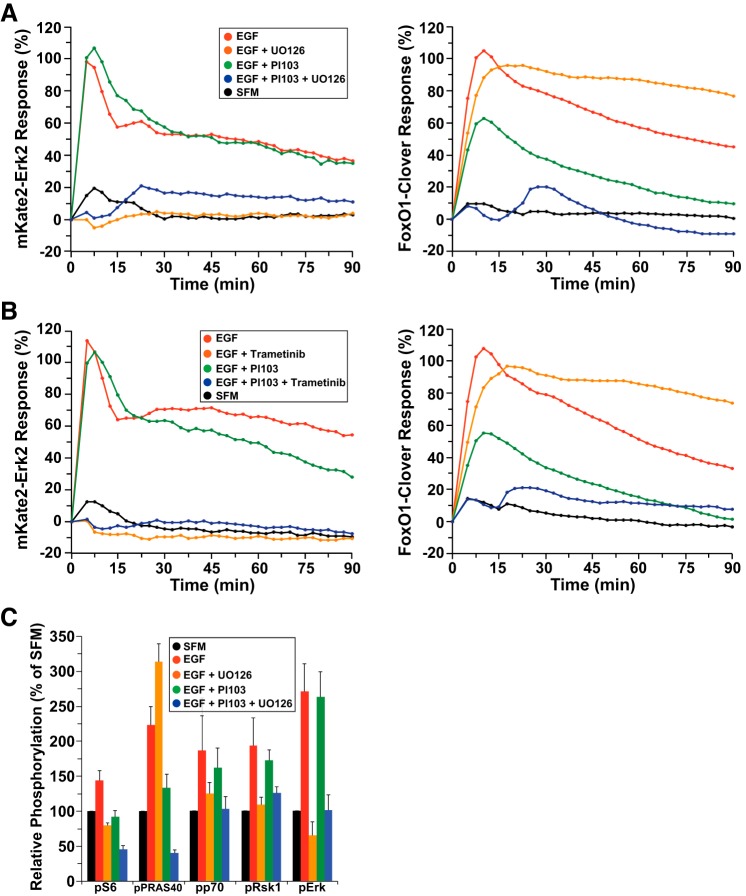
To test this idea of alternative modulation of FoxO1, HeLa cells were incubated with EGF along with the PI-103 and/or Mek inhibitors, U-0126 or trametinib. As expected, each Mek inhibitor completely blocked EGF-stimulated cytoplasmic-to-nuclear movement of the direct Mek substrate mKate2-ERK2 (Fig. 5, A and B, left, and Supplemental Video S2). Surprisingly, each compound also enhanced the duration of translocation of FoxO1-clover in the cytoplasm (Fig. 5, A and B, right, and Supplemental Video S2). In contrast, these small molecules amplified the inhibitory effects of PI-103, leading to nearly complete maintenance of FoxO1-clover in the nucleus after EGF (Fig. 5, A and B, right). Our overall interpretation of these results is that specific loss of Mek-stimulated ERK activity reduces negative feedback on Ras, which leads to sustained stimulation of PI 3-kinase, thus enhancing Akt activity. In contrast, when all paths to ERK and to PI 3-kinase and Akt activation are blocked by a combination of Mek and PI 3-kinase inhibitors, then the FoxO1-clover sensor remains unphosphorylated and nuclear.
Fig. 5.
Reporter dynamics after exposure of HeLa cells to EGF and different signaling inhibitors. A: time course of the relative translocation response of the mKate2-ERK2 reporter (left) and the FoxO1-clover reporter (right) in cells incubated in SFM and then exposed to SFM or EGF (2.1 nM) ± different inhibitors for 90 min. B: time course of the relative translocation response of the mKate2-ERK2 reporter (left) and the FoxO1-clover reporter (right) in cells incubated in SFM and then exposed to SFM or EGF (2.1 nM) ± different inhibitors for 90 min. For A and B, population averages are presented (n = ~140 cells/incubation for each treatment group). For all experiments shown, the relative responsiveness of each reporter protein in each cell was normalized to the values at the start of imaging during incubation in SFM and scaled to the average peak EGF response. C: bar graph showing the average relative change in fluorescence intensity from an antibody array using HeLa cell lysates exposed to SFM or EGF (2.1 nM) ± different inhibitors for 15 min. Results are presented as means ± SE (n = 5 independent experiments). Phosphorylation sites are as follows: S6 ribosomal proteinSer235/236, PRAS40Thr246, p70 S6 kinaseThr421/Ser424, Rsk1Ser380, and ERKThr202/Tyr204.
As a follow up to these studies, we measured the effects of U-0126 on EGF-stimulated phosphorylation of signaling intermediates using a protein array. Incubation of HeLa cells with EGF for 15 min led to increased phosphorylation of Mek and Akt substrates, as well as enhanced phosphorylation of downstream effector molecules. U-0126 blocked EGF-mediated phosphorylation of ERK and Rsk1, and also inhibited phosphorylation of p70 S6 kinase, and S6, but had a stimulatory effect on phosphorylation of the direct Akt substrate PRAS40 (Fig. 5C). In contrast, PI-103 did not alter EGF-stimulated ERK or Rsk phosphorylation but impaired EGF-mediated phosphorylation of PRAS40 and S6 (Fig. 5C). When used together, the two inhibitors (PI-103 and U-0126) reduced PRAS40 and S6 phosphorylation below baseline levels. Thus, in HeLa cells, it appears that inhibition of Mek activity increases EGF-activated PI 3-kinase–Akt signaling and that dual blockade of both pathways reduces putative “Akt” and “ERK” substrates very effectively.
Inhibitory cross talk from ERK to Akt.
To broaden the observations made in Figs. 4 and 5, we examined the effects of small-molecule inhibitors that act at different points upstream of ERK. The Ras–Raf–Mek–ERK pathway contains several redundancies and negative feedback loops. For instance, phosphorylation by ERK can reduce Ras activity, resulting in diminished downstream signaling (24, 51, 64). Thus, Mek inhibitors not only directly block ERK activation but also alter upstream pathway components (24).
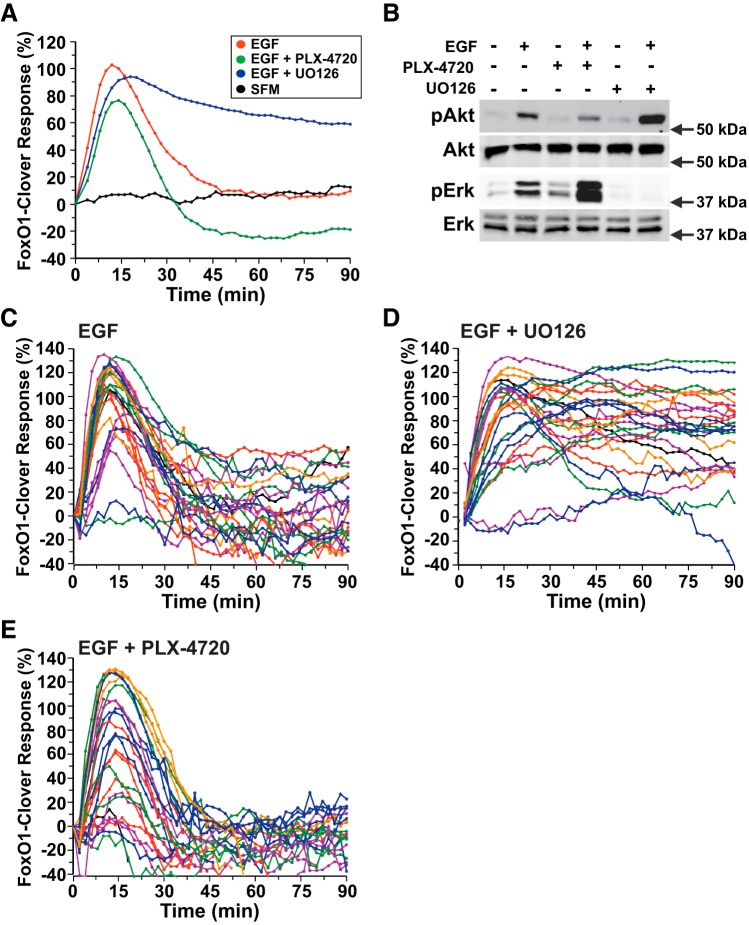
The small molecule PLX-4720 can inhibit a mutant constitutively active B-Raf that contains a valine-to-glutamic acid substitution at amino acid 600 but paradoxically stimulates wild-type Raf1 (36). As a consequence, this drug has been found to increase ERK activity. To assess the effects of PLX-4720 on EGF-mediated signaling, C3H10T1/2 cells, which do not show a strong effect of ERK-mediated signaling on FoxO1-clover translocation (Fig. 6A), were incubated in the presence or absence of PLX-4720 or the Mek inhibitor U-0126, and the subcellular distribution of FoxO1-clover was monitored. In these cells, EGF stimulated transient nuclear-to-cytoplasmic translocation of FoxO1-clover, and this response was potentiated in duration from <45 to >90 min by U-0126 (Fig. 6A). In contrast, reporter translocation was dampened by 20% in magnitude and reduced in duration by PLX-4720 (Fig. 6A). Analogous results were seen after PDGF-AA treatment (Fig. 7A).
Fig. 6.
ERK activity negatively regulates Akt signaling by EGF. A: time course of the relative translocation response of the FoxO1-clover reporter in C3H10T1/2 cells incubated in SFM and then exposed to SFM or EGF (2.1 nM) ± different inhibitors for 90 min. Population averages are presented (n = 50 cells/incubation). The relative responsiveness of the reporter protein in each cell was normalized to the value at the start of imaging during incubation in SFM and scaled to the average peak EGF response. B: expression of pAkt, total Akt, pERK, and total ERK by immunoblotting using whole cell protein lysates from C3H10T1/2 cells after exposure to SFM or EGF plus the indicated inhibitors for 15 min. Molecular mass markers are indicated to the right of each immunoblot. C: time course results for each of 25 individual cells incubated with EGF. D: time course results for each of 25 individual cells incubated with EGF plus U-0126 (10 μM). E: time course results for each of 25 individual cells incubated with EGF and PLX-4720 (10 μM). Cells were imaged every 2 min during each treatment period. The nuclear intensity of the reporter in each cell was normalized to its value at the start of imaging during incubation in SFM and scaled to the average peak EGF response.
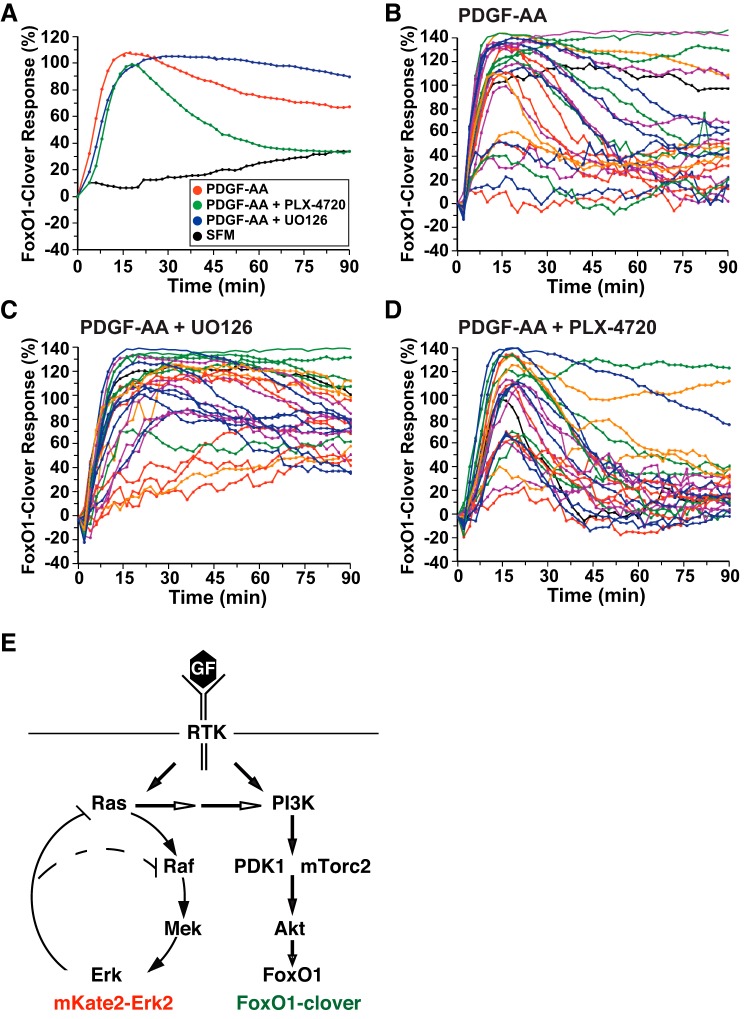
Fig. 7.
ERK activity negatively regulates Akt signaling by platelet-derived growth factor (PDGF)-AA. A: time course of the relative translocation response of the FoxO1-clover reporter in C3H10T1/2 cells incubated in SFM and then exposed to SFM or PDGF-AA (1.4 nM) ± different inhibitors for 90 min. Population averages are presented (n = 50 cells/incubation). The relative responsiveness of the reporter protein in each cell was normalized to the value at the start of imaging during incubation in SFM and scaled to the average peak response. B: time course results for each of 25 individual cells incubated with PDGF-AA. C: time course results for each of 25 individual cells incubated with PDGF-AA plus U-0126 (10 μM). D: time course results for each of 25 individual cells incubated with PDGF-AA and PLX-4720 (10 μM). Cells were imaged every 2 min during each treatment period. The nuclear intensity of the reporter in each cell was normalized to its value at the start of imaging during incubation in SFM and scaled to the average peak response. E: schematic of interrelationships between the Ras–Raf–Mek–ERK and phosphatidylinositol (PI) 3-kinase–Akt signaling pathways. Feedback inhibition by ERK activity on Raf and Ras and activation of PI 3-kinase by Ras are indicated. The locations of mKate2-ERK2 and FoxO1-clover as sensors for these pathways are shown.
Immunoblotting studies revealed similar effects after incubation of cells with EGF for 15 min in the presence or absence of U-0126 or PLX-4720. EGF-mediated Akt phosphorylation was increased by U-0126 and reduced by PLX-4720, whereas ERK phosphorylation was completely blocked by U-0126 and enhanced by PLX-4720 (Fig. 6B).
One key advantage of live-cell imaging with translocation sensors is that all cells in a population may be analyzed individually rather than assessed as a group average (37). Examination of the effects of EGF on the subcellular distribution of FoxO1-clover revealed heterogeneity in both the magnitude and duration of responses in single fibroblasts. Coincubation with U-0126 enhanced the duration of peak signaling after EGF in the majority of cells (cf. Fig. 6, C and D). By contrast, addition of PLX-4720 reduced the time course of FoxO1-clover nuclear-to-cytoplasmic translocation (Fig. 6E). However, in both populations, marked individual cell variability remained. Analogous results were seen after treatment of cells with PDGF-AA (Fig. 7, B–D). Thus, these results show that cross talk occurs between the Ras–Raf–Mek–ERK and PI 3-kinase–Akt cascades in response to EGF and PDGF-AA, demonstrating in C3H10T1/2 and HeLa cells that Mek inhibition potentiates Akt activity (Fig. 7E).
DISCUSSION
Growth factors bind to distinct transmembrane receptors and stimulate multiple intracellular signaling cascades that influence cellular behavior by modifying intermediary metabolism, altering cell shape and movement, changing gene expression, and regulating the rate and extent of cell proliferation or differentiation (26). Each signaling pathway typically consists of a series of interacting proteins that previously were thought to comprise a simple linear pattern and to function in isolation. It is now clear from many cellular and biochemical studies that individual signaling cascades contain multiple feedback and feedforward loops and have extensive interconnections with other pathways (25, 32, 63). Here, we have used fluorescent live-cell imaging sensors to simultaneously track the effects of different growth factors on two major signaling cascades, the Raf–Ras–Mek–ERK and PI 3-kinase–Akt pathways. Our results reveal that individual growth factors initiate signaling responses that vary markedly in strength and duration and demonstrate in living cells the dramatic effects of cross talk from Raf and Mek to PI 3-kinase and Akt, as well as the potential convergence of ERK-mediated signaling on Akt signaling substrates like FoxO1. Our observations illustrate the dynamics of growth factor-initiated signaling cascades in real time and provide insights into how individual growth factors can encode distinct cellular behaviors through the promotion of complex interactions between coregulated pathways.
Discordant signaling pathway dynamics are controlled by cross talk.
Although it has been shown that different growth factors acting through tyrosine kinase receptors stimulate analogous intracellular signaling pathways, overall signaling strength and duration vary considerably among individual growth factors and cell types (5, 7, 14, 20, 35, 63). Here, we find dramatic differences in signaling dynamics in HeLa cells among three different growth factors. We show that EGF stimulated rapid activity of Mek, consisting of a brief peak followed by a sustained plateau. Akt signaling in response to EGF also reached a rapid peak but then fell at a relatively constant rate in the majority of cells studied. HGF promoted similar kinetics of Mek-mediated signaling to ERK but with a magnitude that was less than one-half that of EGF. In contrast, sustained stimulation of Akt activity by HGF resembled the pattern seen with IGF-I, which only minimally activated Mek in most cells (Figs. 2 and 3). Furthermore, pairing the peak mKate2-ERK2 and FoxO1-clover responses revealed cell-to-cell heterogeneity across the population but a correlation within individual cells (Fig. 3F).
Biased signaling responses favoring one cascade over another presumably reflect the nature of the respective growth factor receptors, downstream adaptors, feedback mechanisms, and/or cross talk pathways, all of which potentially vary among EGF, HGF, and IGF-I (5, 25, 26, 32). EGF binds primarily to EGFR (HER1) and through heterodimerization also can activate HER2, HER3, and HER4 (8, 42). HGF binds to the extracellular surface of a homodimeric receptor composed of identical subunits of the tyrosine kinase Met proto-oncogene, which then through intracellular phosphotyrosine-binding sites interacts with a range of signaling modules (12, 46). In contrast, IGF-I binds to the heterotetrameric IGF-I receptor, which primarily engages intracellular signaling pathways through adaptor molecules such as insulin receptor substrate (IRS) 1 and IRS2 (54).
Small-molecule pathway modifiers are useful reagents in live-cell imaging-based signaling experiments, since they allow rapid and fairly specific interference at defined steps in a cascade. Blocking Mek activity with either of two different chemical inhibitors led to sustained EGF-stimulated Akt activity that resembled the effects of IGF-I, since under these conditions ERK was minimally activated (cf. Figs. 4A, 4B, or 5A with Fig. 3A), thus illustrating that cross talk at different levels of the Raf–Ras–Mek–ERK pathway may normally impair PI 3-kinase–Akt signaling. Because similar results were observed after EGF exposure in two different cultured cell types (Figs. 4–6) and also in cells treated with PDGF-AA (Fig. 7), our data demonstrate that this type of inhibitory cross talk may be generalizable and is consistent with previous work using more standard approaches (22, 43, 56). Published studies have found that inhibiting ERK signaling at Mek leads to a block in negative upstream pathway feedback from ERK to Ras (24, 51). As a consequence, Ras activity is not downregulated and can continue to directly stimulate PI 3-kinase and thus Akt (see schematic in Fig. 7E). Although through this mechanism ERK activity can modulate Akt signaling, this type of interaction only seems to occur when a prior signal also stimulates the PI 3-kinase–Akt cascade, since direct induction of Ras using optogenetic methods did not promote PI 3-kinase or Akt activity (60).
Consistent with observations using Mek inhibitors, activation of Raf1 by the small molecule PLX-4720 caused a more rapid decline in Akt signaling in cells incubated with either EGF or PDGF-AA compared with controls (Figs. 6A and 7A). This result provides additional validation that activation of the Raf–Ras–Mek–ERK signaling cascade negatively impacts PI 3-kinase–Akt activity. Of note, however, there were significant differences in overall signaling responsiveness to EGF compared with PDGF-AA. In the absence of chemical inhibitors, PDGF-AA caused more sustained Akt activity, with nuclear-to-cytoplasmic translocation of the FoxO1-clover reporter lasting for >90 vs. 45 min for EGF (cf. Figs. 7A and 6A). In contrast, effects of Raf1 activation by PLX-4720 were more dramatic in cells incubated with EGF, where Akt activity was inhibited far below basal levels, whereas signaling only returned to baseline in cells treated with PDGF-AA (cf. Figs. 6A and 7A). These results probably reflect intrinsically stronger stimulation of the Raf–Ras–Mek–ERK pathway by EGF, and of the PI 3-kinase–Akt cascade by PDGF-AA in C3H10T1/2 cells, and thus demonstrate biased signaling in which one pathway is preferentially activated over another by a given growth factor.
In other cell types, cross talk from the PI 3-kinase–Akt pathway to the Raf–Ras–Mek–ERK cascade via inhibitory phosphorylation of Raf1 by Akt also has been identified (17, 33, 67). In our studies, we only found a small reduction in EGF- or HGF-treated cells in cytoplasmic-to-nuclear translocation of the mKate2-ERK2 reporter by either the Akt inhibitor MK-2206 or the dual PI 3-kinase and mTorc inhibitor PI-103 (Fig. 4, A and B). Thus, in HeLa cells, we observed little reciprocal cross talk from PI 3-kinase or Akt to control Mek activity, potentially illustrating cell-type specificity.
In addition to cross talk, distinct signaling pathways may converge on the same downstream proteins (32). For example, the Akt and Rsk kinases recognize similar amino acid sequences and thus could phosphorylate some of the same substrates, leading to similar biological effects (4, 28, 52). There is also the potential for convergence of two pathways through phosphorylation at different sites in the same target protein (65). These mechanisms could explain why EGF- or HGF-stimulated nuclear-to-cytoplasmic translocation of FoxO1-clover in HeLa cells was not blocked completely either by the Akt inhibitor MK-2206 or by the PI 3-kinase-mTorc inhibitor PI-103 but was fully impaired by combined treatment, including a Mek inhibitor (Fig. 5, A and B).
Limitations and implications of dual-reporter live-cell imaging for cell signaling.
One issue that could limit the impact of our observations is the potential contribution of ERK overexpression on overall cell signaling dynamics, since our reporter molecule contains a functional ERK2. However, unlike previous studies, we did not detect nuclear localization of the reporter protein in the absence of growth factor activity (1, 27). Moreover, it is clear that our live-cell imaging sensors do not occupy parallel positions in the PI 3-kinase–Akt and Raf–Ras–Mek–ERK signaling pathways. FoxO1 is one of a multitude of direct substrates of Akt (28), and thus the FoxO1-clover fusion protein measures the activity of a signaling enzyme with many intracellular targets. In contrast, ERK1 and ERK2 are essentially the only specific substrates for Mek1 and Mek2 (51), and the subcellular translocation of mKate2-ERK2 thus does not give information equivalent to the redistribution of FoxO1-clover on overall signaling dynamics or on the comprehensive nature of cross talk. Future live-cell imaging studies could use an ERK substrate rather than ERK2 to better address these concerns, which would enable a more precise comparison of overall Akt and ERK signaling activity in the same cells.
From a broader perspective, we have attempted to use live-cell imaging to study the dynamics of two major pathways, yet we acknowledge that our results are limited in the context of overall cellular signaling, since additional growth factor-activated cascades also exert significant effects on cellular behaviors (26). For example, readouts from other MAP kinase pathways, Stats, Src kinases, and protein kinase C cascades, as well as micro-RNAs, also play important roles in the short- and longer-term responses to growth factors, and to cytokines and hormones (19, 21, 44, 50, 53, 58). It thus will be critical to develop live-cell imaging sensors for these other pathways to gain a fuller understanding of how growth factors and other agents interactively regulate cell fate and functions under both physiological and pathological conditions. In the future, we envision that live-cell imaging studies with multiple readouts, coupled with other molecular and biochemical methods operating at the single-cell level, should allow better understanding of how a diversity of signaling pathways is integrated in time and space to control specific outcomes in cells, tissues, and organisms.
GRANTS
These studies were supported by National Institutes of Health Research Grant, R01-DK-042748–26 (to P. Rotwein).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.M.G. and P.R. conceived and designed the research; S.M.G. performed experiments; S.M.G. and P.R. analyzed data; S.M.G. and P.R. interpreted results of experiments; S.M.G. and P.R. prepared figures; S.M.G. and P.R. drafted manuscript; S.M.G. and P.R. edited and revised manuscript; S.M.G. and P.R. approved final version of manuscript.
Supplementary Material
ACKNOWLEDGMENTS
We thank the Oregon Health & Science University Flow Cytometry Core Facility for use of flow-sorting equipment.
REFERENCES
- 1.Adachi M, Fukuda M, Nishida E. Two co-existing mechanisms for nuclear import of MAP kinase: passive diffusion of a monomer and active transport of a dimer. EMBO J 18: 5347–5358, 1999. doi: 10.1093/emboj/18.19.5347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aksamitiene E, Kiyatkin A, Kholodenko BN. Cross-talk between mitogenic Ras/MAPK and survival PI3K/Akt pathways: a fine balance. Biochem Soc Trans 40: 139–146, 2012. doi: 10.1042/BST20110609. [DOI] [PubMed] [Google Scholar]
- 3.Albeck JG, Mills GB, Brugge JS. Frequency-modulated pulses of ERK activity transmit quantitative proliferation signals. Mol Cell 49: 249–261, 2013. doi: 10.1016/j.molcel.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alessi DR, Caudwell FB, Andjelkovic M, Hemmings BA, Cohen P. Molecular basis for the substrate specificity of protein kinase B; comparison with MAPKAP kinase-1 and p70 S6 kinase. FEBS Lett 399: 333–338, 1996. doi: 10.1016/S0014-5793(96)01370-1. [DOI] [PubMed] [Google Scholar]
- 5.Borisov N, Aksamitiene E, Kiyatkin A, Legewie S, Berkhout J, Maiwald T, Kaimachnikov NP, Timmer J, Hoek JB, Kholodenko BN. Systems-level interactions between insulin-EGF networks amplify mitogenic signaling. Mol Syst Biol 5: 256, 2009. doi: 10.1038/msb.2009.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 96: 857–868, 1999. doi: 10.1016/S0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 7.Cirit M, Haugh JM. Data-driven modelling of receptor tyrosine kinase signalling networks quantifies receptor-specific potencies of PI3K- and Ras-dependent ERK activation. Biochem J 441: 77–85, 2012. doi: 10.1042/BJ20110833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Citri A, Yarden Y. EGF-ERBB signalling: towards the systems level. Nat Rev Mol Cell Biol 7: 505–516, 2006. doi: 10.1038/nrm1962. [DOI] [PubMed] [Google Scholar]
- 9.Cohen-Saidon C, Cohen AA, Sigal A, Liron Y, Alon U. Dynamics and variability of ERK2 response to EGF in individual living cells. Mol Cell 36: 885–893, 2009. doi: 10.1016/j.molcel.2009.11.025. [DOI] [PubMed] [Google Scholar]
- 10.Dougherty MK, Müller J, Ritt DA, Zhou M, Zhou XZ, Copeland TD, Conrads TP, Veenstra TD, Lu KP, Morrison DK. Regulation of Raf-1 by direct feedback phosphorylation. Mol Cell 17: 215–224, 2005. doi: 10.1016/j.molcel.2004.11.055. [DOI] [PubMed] [Google Scholar]
- 11.Downward J. The ins and outs of signalling. Nature 411: 759–762, 2001. doi: 10.1038/35081138. [DOI] [PubMed] [Google Scholar]
- 12.Fajardo-Puerta AB, Mato Prado M, Frampton AE, Jiao LR. Gene of the month: HGF. J Clin Pathol 69: 575–579, 2016. doi: 10.1136/jclinpath-2015-203575. [DOI] [PubMed] [Google Scholar]
- 13.Gross SM, Rotwein P. Live cell imaging reveals marked variability in myoblast proliferation and fate. Skelet Muscle 3: 10, 2013. doi: 10.1186/2044-5040-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gross SM, Rotwein P. Akt signaling dynamics in individual cells. J Cell Sci 128: 2509–2519, 2015. doi: 10.1242/jcs.168773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gross SM, Rotwein P. Mapping growth-factor-modulated Akt signaling dynamics. J Cell Sci 129: 2052–2063, 2016. doi: 10.1242/jcs.183764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gross SM, Rotwein P. Unraveling growth factor signaling and cell cycle progression in individual fibroblasts. J Biol Chem 291: 14628–14638, 2016. doi: 10.1074/jbc.M116.734194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guan KL, Figueroa C, Brtva TR, Zhu T, Taylor J, Barber TD, Vojtek AB. Negative regulation of the serine/threonine kinase B-Raf by Akt. J Biol Chem 275: 27354–27359, 2000. [DOI] [PubMed] [Google Scholar]
- 18.Hay N. Interplay between FOXO, TOR, and Akt. Biochim Biophys Acta 1813: 1965–1970, 2011. doi: 10.1016/j.bbamcr.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heldin CH, Lu B, Evans R, Gutkind JS. Signals and Receptors. Cold Spring Harb Perspect Biol 8: a005900, 2016. doi: 10.1101/cshperspect.a005900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaushansky A, Gordus A, Chang B, Rush J, MacBeath G. A quantitative study of the recruitment potential of all intracellular tyrosine residues on EGFR, FGFR1 and IGF1R. Mol Biosyst 4: 643–653, 2008. doi: 10.1039/b801018h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kedmi M, Sas-Chen A, Yarden Y. MicroRNAs and Growth Factors: An Alliance Propelling Tumor Progression. J Clin Med 4: 1578–1599, 2015. doi: 10.3390/jcm4081578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kodaki T, Woscholski R, Hallberg B, Rodriguez-Viciana P, Downward J, Parker PJ. The activation of phosphatidylinositol 3-kinase by Ras. Curr Biol 4: 798–806, 1994. doi: 10.1016/S0960-9822(00)00177-9. [DOI] [PubMed] [Google Scholar]
- 23.Lam AJ, St-Pierre F, Gong Y, Marshall JD, Cranfill PJ, Baird MA, McKeown MR, Wiedenmann J, Davidson MW, Schnitzer MJ, Tsien RY, Lin MZ. Improving FRET dynamic range with bright green and red fluorescent proteins. Nat Methods 9: 1005–1012, 2012. doi: 10.1038/nmeth.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Langlois WJ, Sasaoka T, Saltiel AR, Olefsky JM. Negative feedback regulation and desensitization of insulin- and epidermal growth factor-stimulated p21ras activation. J Biol Chem 270: 25320–25323, 1995. doi: 10.1074/jbc.270.43.25320. [DOI] [PubMed] [Google Scholar]
- 25.Lemmon MA, Freed DM, Schlessinger J, Kiyatkin A. The Dark Side of Cell Signaling: Positive Roles for Negative Regulators. Cell 164: 1172–1184, 2016. doi: 10.1016/j.cell.2016.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lemmon MA, Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell 141: 1117–1134, 2010. doi: 10.1016/j.cell.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lenormand P, Sardet C, Pagès G, L’Allemain G, Brunet A, Pouysségur J. Growth factors induce nuclear translocation of MAP kinases (p42mapk and p44mapk) but not of their activator MAP kinase kinase (p45mapkk) in fibroblasts. J Cell Biol 122: 1079–1088, 1993. doi: 10.1083/jcb.122.5.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell 129: 1261–1274, 2007. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marshall CJ. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell 80: 179–185, 1995. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- 30.Maryu G, Matsuda M, Aoki K. Multiplexed fluorescence imaging of Erk and Akt activities and cell-cycle progression. Cell Struct Funct 41: 81–92, 2016. doi: 10.1247/csf.16007. [DOI] [PubMed] [Google Scholar]
- 31.Meijering E, Dzyubachyk O, Smal I. Methods for cell and particle tracking. Methods Enzymol 504: 183–200, 2012. doi: 10.1016/B978-0-12-391857-4.00009-4. [DOI] [PubMed] [Google Scholar]
- 32.Mendoza MC, Er EE, Blenis J. The Ras-ERK and PI3K-mTOR pathways: cross-talk and compensation. Trends Biochem Sci 36: 320–328, 2011. doi: 10.1016/j.tibs.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moelling K, Schad K, Bosse M, Zimmermann S, Schweneker M. Regulation of Raf-Akt cross-talk. J Biol Chem 277: 31099–31106, 2002. doi: 10.1074/jbc.M111974200. [DOI] [PubMed] [Google Scholar]
- 34.Mukherjee A, Rotwein P. Insulin-like growth factor-binding protein-5 inhibits osteoblast differentiation and skeletal growth by blocking insulin-like growth factor actions. Mol Endocrinol 22: 1238–1250, 2008. doi: 10.1210/me.2008-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Niepel M, Hafner M, Pace EA, Chung M, Chai DH, Zhou L, Muhlich JL, Schoeberl B, Sorger PK. Analysis of growth factor signaling in genetically diverse breast cancer lines. BMC Biol 12: 20, 2014. doi: 10.1186/1741-7007-12-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Poulikakos PI, Zhang C, Bollag G, Shokat KM, Rosen N. RAF inhibitors transactivate RAF dimers and ERK signalling in cells with wild-type BRAF. Nature 464: 427–430, 2010. doi: 10.1038/nature08902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Purvis JE, Lahav G. Encoding and decoding cellular information through signaling dynamics. Cell 152: 945–956, 2013. doi: 10.1016/j.cell.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Regot S, Hughey JJ, Bajar BT, Carrasco S, Covert MW. High-sensitivity measurements of multiple kinase activities in live single cells. Cell 157: 1724–1734, 2014. doi: 10.1016/j.cell.2014.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rena G, Guo S, Cichy SC, Unterman TG, Cohen P. Phosphorylation of the transcription factor forkhead family member FKHR by protein kinase B. J Biol Chem 274: 17179–17183, 1999. doi: 10.1074/jbc.274.24.17179. [DOI] [PubMed] [Google Scholar]
- 40.Rena G, Woods YL, Prescott AR, Peggie M, Unterman TG, Williams MR, Cohen P. Two novel phosphorylation sites on FKHR that are critical for its nuclear exclusion. EMBO J 21: 2263–2271, 2002. doi: 10.1093/emboj/21.9.2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Riedel H, Dull TJ, Honegger AM, Schlessinger J, Ullrich A. Cytoplasmic domains determine signal specificity, cellular routing characteristics and influence ligand binding of epidermal growth factor and insulin receptors. EMBO J 8: 2943–2954, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Riese DJ II, Gallo RM, Settleman J. Mutational activation of ErbB family receptor tyrosine kinases: insights into mechanisms of signal transduction and tumorigenesis. BioEssays 29: 558–565, 2007. doi: 10.1002/bies.20582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rodriguez-Viciana P, Warne PH, Dhand R, Vanhaesebroeck B, Gout I, Fry MJ, Waterfield MD, Downward J. Phosphatidylinositol-3-OH kinase as a direct target of Ras. Nature 370: 527–532, 1994. doi: 10.1038/370527a0. [DOI] [PubMed] [Google Scholar]
- 44.Roskoski R., Jr Src protein-tyrosine kinase structure, mechanism, and small molecule inhibitors. Pharmacol Res 94: 9–25, 2015. doi: 10.1016/j.phrs.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 45.Ryu H, Chung M, Dobrzyński M, Fey D, Blum Y, Sik Lee S, Peter M, Kholodenko BN, Li Jeon N, Pertz O. Frequency modulation of ERK activation dynamics rewires cell fate. Mol Syst Biol 12: 866, 2016. doi: 10.15252/msb.20166982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sakai K, Aoki S, Matsumoto K. Hepatocyte growth factor and Met in drug discovery. J Biochem 157: 271–284, 2015. doi: 10.1093/jb/mvv027. [DOI] [PubMed] [Google Scholar]
- 47.Santos SD, Verveer PJ, Bastiaens PI. Growth factor-induced MAPK network topology shapes Erk response determining PC-12 cell fate. Nat Cell Biol 9: 324–330, 2007. doi: 10.1038/ncb1543. [DOI] [PubMed] [Google Scholar]
- 48.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A. Fiji: an open-source platform for biological-image analysis. Nat Methods 9: 676–682, 2012. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schlessinger J. Ligand-induced, receptor-mediated dimerization and activation of EGF receptor. Cell 110: 669–672, 2002. doi: 10.1016/S0092-8674(02)00966-2. [DOI] [PubMed] [Google Scholar]
- 50.Schwartz DM, Bonelli M, Gadina M, O’Shea JJ. Type I/II cytokines, JAKs, and new strategies for treating autoimmune diseases. Nat Rev Rheumatol 12: 25–36, 2016. doi: 10.1038/nrrheum.2015.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shaul YD, Seger R. The MEK/ERK cascade: from signaling specificity to diverse functions. Biochim Biophys Acta 1773: 1213–1226, 2007. doi: 10.1016/j.bbamcr.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 52.Shaw M, Cohen P. Role of protein kinase B and the MAP kinase cascade in mediating the EGF-dependent inhibition of glycogen synthase kinase 3 in Swiss 3T3 cells. FEBS Lett 461: 120–124, 1999. doi: 10.1016/S0014-5793(99)01434-9. [DOI] [PubMed] [Google Scholar]
- 53.Shostak K, Chariot A. EGFR and NF-κB: partners in cancer. Trends Mol Med 21: 385–393, 2015. doi: 10.1016/j.molmed.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 54.Siddle K. Signalling by insulin and IGF receptors: supporting acts and new players. J Mol Endocrinol 47: R1–R10, 2011. doi: 10.1530/JME-11-0022. [DOI] [PubMed] [Google Scholar]
- 55.Sparta B, Pargett M, Minguet M, Distor K, Bell G, Albeck JG. Receptor Level Mechanisms Are Required for Epidermal Growth Factor (EGF)-stimulated Extracellular Signal-regulated Kinase (ERK) Activity Pulses. J Biol Chem 290: 24784–24792, 2015. doi: 10.1074/jbc.M115.662247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Suire S, Hawkins P, Stephens L. Activation of phosphoinositide 3-kinase gamma by Ras. Curr Biol 12: 1068–1075, 2002. doi: 10.1016/S0960-9822(02)00933-8. [DOI] [PubMed] [Google Scholar]
- 57.Tay S, Hughey JJ, Lee TK, Lipniacki T, Quake SR, Covert MW. Single-cell NF-kappaB dynamics reveal digital activation and analogue information processing. Nature 466: 267–271, 2010. doi: 10.1038/nature09145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thomas SJ, Snowden JA, Zeidler MP, Danson SJ. The role of JAK/STAT signalling in the pathogenesis, prognosis and treatment of solid tumours. Br J Cancer 113: 365–371, 2015. doi: 10.1038/bjc.2015.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tiscornia G, Singer O, Verma IM. Production and purification of lentiviral vectors. Nat Protoc 1: 241–245, 2006. doi: 10.1038/nprot.2006.37. [DOI] [PubMed] [Google Scholar]
- 60.Toettcher JE, Weiner OD, Lim WA. Using optogenetics to interrogate the dynamic control of signal transmission by the Ras/Erk module. Cell 155: 1422–1434, 2013. doi: 10.1016/j.cell.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Toker A. Achieving specificity in Akt signaling in cancer. Adv Biol Regul 52: 78–87, 2012. doi: 10.1016/j.advenzreg.2011.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wagner JP, Wolf-Yadlin A, Sevecka M, Grenier JK, Root DE, Lauffenburger DA, MacBeath G. Receptor tyrosine kinases fall into distinct classes based on their inferred signaling networks. Sci Signal 6: ra58, 2013. doi: 10.1126/scisignal.2003994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang CC, Cirit M, Haugh JM. PI3K-dependent cross-talk interactions converge with Ras as quantifiable inputs integrated by Erk. Mol Syst Biol 5: 246, 2009. doi: 10.1038/msb.2009.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Waters SB, Holt KH, Ross SE, Syu LJ, Guan KL, Saltiel AR, Koretzky GA, Pessin JE. Desensitization of Ras activation by a feedback disassociation of the SOS-Grb2 complex. J Biol Chem 270: 20883–20886, 1995. doi: 10.1074/jbc.270.36.20883. [DOI] [PubMed] [Google Scholar]
- 65.Yang JY, Zong CS, Xia W, Yamaguchi H, Ding Q, Xie X, Lang JY, Lai CC, Chang CJ, Huang WC, Huang H, Kuo HP, Lee DF, Li LY, Lien HC, Cheng X, Chang KJ, Hsiao CD, Tsai FJ, Tsai CH, Sahin AA, Muller WJ, Mills GB, Yu D, Hortobagyi GN, Hung MC. ERK promotes tumorigenesis by inhibiting FOXO3a via MDM2-mediated degradation. Nat Cell Biol 10: 138–148, 2008. doi: 10.1038/ncb1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang X, Gan L, Pan H, Guo S, He X, Olson ST, Mesecar A, Adam S, Unterman TG. Phosphorylation of serine 256 suppresses transactivation by FKHR (FOXO1) by multiple mechanisms. Direct and indirect effects on nuclear/cytoplasmic shuttling and DNA binding. J Biol Chem 277: 45276–45284, 2002. doi: 10.1074/jbc.M208063200. [DOI] [PubMed] [Google Scholar]
- 67.Zimmermann S, Moelling K. Phosphorylation and regulation of Raf by Akt (protein kinase B). Science 286: 1741–1744, 1999. doi: 10.1126/science.286.5445.1741. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.