Abstract
Recent studies have implicated the Hippo pathway and its transcriptional effectors YAP and TAZ as necessary for fibroblast activation and tissue fibrosis. To test the specific and sufficient roles for TAZ in driving autonomous fibroblast activation, we cultured NIH3T3 fibroblasts expressing a doxycycline-inducible nuclear-localized mutant of TAZ (TAZ4SA) in scaffold-free 3D hanging drop spheroids, or on matrices of specified mechanical rigidity. Control NIH3T3 fibroblasts formed spheroids in hanging drop culture that remained stable and neither increased nor decreased in size significantly over 15 days. In contrast, TAZ4SA-transduced fibroblasts grew robustly in spheroid culture, and expressed enhanced levels of genes encoding profibrotic soluble factors connective tissue growth factor (CTGF), endothelin-1 (Et-1), and plasminogen activator inhibitor 1 (PAI-1). However, TAZ4SA expression was unable to enhance expression of extracellular matrix (ECM)-encoding genes Col1a1, Col1a2, Col3a1, or Fn1 in spheroid culture. Micromechanical testing indicated that spheroids composed of either control or TAZ4SA-expressing cells were highly compliant and indistinguishable in mechanical properties. In fibroblasts cultured on 2D matrices of compliance similar to spheroids, TAZ4SA expression was able to enhance contractile force generation, but was unable to enhance ECM gene expression. In contrast, culture on stiff hydrogels potentiated TAZ4SA enhancement of ECM expression. TAZ4SA enhancement of Col1a1 expression on soft matrices was potentiated by TGF-β1, while on stiff matrices it was abrogated by inhibition of myocardin-related transcription factor, demonstrating context-dependent crosstalk of TAZ with these pathways. These findings demonstrate sufficiency of TAZ activation for driving fibroblast proliferation, contraction, and soluble profibrotic factor expression, and mechanical context-dependent crosstalk of TAZ with other pathways in regulating Col1a1 expression.
Keywords: extracellular matrix, fibrosis, Hippo, stiffness, collagen
fibrosis is characterized by aberrant proliferation and matrix deposition by fibroblasts, leading to destruction of normal tissue architecture and replacement with fibrotic scar tissue (22). Recent work in a variety of tissues has implicated the Hippo pathway transcriptional effectors YAP and TAZ in the process of fibroblast activation and fibrosis (1, 2, 10, 13, 21, 28). We recently observed that fibroblasts expressing TAZ4SA, a mutant form of TAZ with four serine to alanine substitutions that prevent TAZ cytoplasmic sequestration, rapidly colonize the lung after intravenous injection, replacing normal tissue architecture with increased cellularity and a collagen-rich extracellular matrix (10). The Hippo pathway itself is sensitive to the stiffness of the mechanical environment (17), and YAP and TAZ crosstalk with other mechanosensitive pathways (20, 31), thus it is increasingly appreciated that this pathway plays a leading role in orchestrating cellular responses to an altered mechanical environment (19), including fibroblast activation in stiffened fibrotic tissues (1, 2, 10, 13, 21, 28). However, YAP and TAZ also interact with and integrate a wide variety of other upstream inputs and downstream signaling cascades (19). The potential role for TAZ as an essential and sufficient initiator of fibroblast activation per se within the compliant physical environment that typifies normal soft tissues remains relatively unexplored.
Here we developed a scaffold-free culture system to explore whether fibroblasts spontaneously, or under the control of TAZ4SA, possess the capacity for growth and activation in free-floating spheroids. We observe that fibroblast growth is normally limited under such conditions, but that expression of TAZ4SA overcomes this limit to drive sustained spheroid growth. Interestingly, we do not observe enhanced ECM gene expression (Col1a1, Col1a2, Col3a1, and FN1) in spheroid culture under the influence of TAZ4SA, although expression of genes encoding profibrotic mediators connective tissue growth factor (CTGF), Et-1, and plasminogen activator inhibitor 1 (PAI-1) are all significantly enhanced. We hypothesized that the absence of a stiff mechanical environment and preexisting matrix scaffold within spheroids may limit TAZ4SA-mediated matrix synthesis. In agreement with this hypothesis, we observed that both control and TAZ4SA spheroids exhibit very low elastic moduli, and that TAZ4SA-induced extracellular matrix gene expression is suppressed when cells are cultured on highly compliant 2D matrices. In contrast, TAZ4SA was sufficient, even on such compliant matrices, to promote traction force generation. Together our results demonstrate a central role for TAZ activation in driving proliferative, contractile, and profibrotic signaling programs in fibroblasts independent of matrix stiffness, but a requirement for a stiffened mechanical environment for TAZ to enhance Col1a1 expression. Interestingly, TGF-β1 stimulation on soft matrices enabled TAZ4SA-mediated Col1a1 expression, while inhibition of myocardin-related transcription factor (MRTF) on stiff matrices abrogated TAZ4SA-mediated Col1a1 expression, demonstrating crosstalk between TAZ and other mechanosensitive pathways that regulate fibroblast activation. Delineation of mechanical context-dependent and independent roles for TAZ provides new insight into the autonomous and cooperative profibrotic functions of this effector in initiating fibroblast activation and tissue fibrosis.1
METHODS
Cell culture.
Doxycycline-inducible Tet-On NIH3T3 cells were generated as previously described (10). Briefly, the Lenti-X HTX packaging system (Clontech) along with the pLVX-Tet-On Advanced plasmid (no. 632162, Clontech) were used to generate lentivirus that was then used to transduce the tetracycline-controlled transactivator, rtTA-Advanced, into NIH3T3 cells. Stable pools of Tet-On-NIH3T3 were selected with 1 μg/ml G-418 sulfate, and these cells were then infected with lentivirus transducing 3xFLAG-tagged human TAZ-4SA [S66A, S89A, S117A, and S311A (7)] from the pLVX-Tight-Puro plasmid (no. 632162, Clontech). Stable dox-inducible TAZ4SA-expressing and control cells were grown in DMEM plus 10% FBS, 10,000 U/ml of penicillin, 10,000 μg/ml of streptomycin, and 25 μg/ml of amphotericin B, along with continuous exposure to selection reagents 1 μg/ml G-418 sulfate and 2 μg/ml puromycin. Cells were grown in monolayer culture in 75 cm2 cell culture flasks in a humidified environment containing 5% CO2 at 37°C. Cells were harvested at 70–80% confluence, using 0.25% Trypsin/EDTA. Cells were counted using a hemocytometer and centrifuged in 15 ml conical tubes for 5 min at 1,000 rpm. Cells were used at passages 8–16.
Spheroid culture.
Spheroids were initiated at 500 cells per well (40 μl) in 96-well hanging drop plates (3D Biomatrix). Each well gave rise to one uniform spheroid by day 3 of the experiment. Medium was exchanged on all plates beginning on day 1 following spheroid formation and every other day thereafter until the completion of the experiments (day 7 or day 15). Media exchange was carried out by removing 14 µl from each well and replacing with 20 µl (accounting for evaporative losses) of fresh media containing 0, 1, 10, or 100 ng/ml of doxycycline. Light micrographs (2D) were obtained for viable spheroids every other day beginning on day 3 of spheroid culture (Nikon TMS). Spheroid diameter in light micrographs was measured using either the oval or the freehand function found in ImageJ software (National Institutes of Health, Bethesda, MD). To measure changes in spheroid growth, a total of six independent replicate experiments were performed for TAZ4SA cells, while three independent replicate experiments were performed with control cells. In each experiment, six spheroids were analyzed for each doxycycline treatment group, with the area at each time point averaged across the six spheroids to generate a single experimental value for each replicate experiment.
For end point atomic force microscopy (AFM) analysis, RNA isolation or confocal imaging, spheroids were harvested into glass-bottom, 96-well, black plates (receiving plate) containing 50 μl of cell culture media in each well. The hanging drop plate was stacked on top of the receiving plate, and 100 µl of culture medium was slowly dispensed through the access holes to transfer the hanging drops to the plate below. For AFM and RNA analysis, spheroids were collected and processed as described below. For confocal imaging, medium was removed from the transfer plate containing the spheroids using a 1.5 ml plastic pipette and rinsed once with PBS. Spheroids were covered with 100 µl of 4% paraformaldehyde and incubated overnight at 4°C. Spheroids were then incubated with 0.3% Triton X-100 in PBS for 5 min. After rinsing in PBS, nuclei of the cells in spheroid were stained by incubating the spheroid for 30 min at 4°C with 3 μM 4′,6-diamidino-2-phenylindole, dihydrochloride (DAPI, Thermo Scientific) in PBS. Confocal spheroid images were acquired with a multiphoton laser scanning microscope using a ×10 (numerical aperture 0.40) objective in a FV1000MPE (Olympus) with a field size of 1,024 μm × 1,024 μm.
AFM micromechanical characterization.
AFM force measurements on TAZ4SA spheroids were performed using a Bioscope Catalyst microscope (Bruker). Spheroids were transferred to 35 mm glass-bottom dishes filled with culture media. Five spheroids each were prepared from TAZ4SA-transduced fibroblasts grown in 0 or 100 ng/ml doxycycline for 7 days. For each spheroid, five force curves were collected from the spheroid surface using a sphere-tipped probe (Novascan, Ames, IA) with a diameter of 5 μm and a spring constant of ≈100 pN/nm (measured by thermal fluctuation method). In accordance with methods described previously (12), force-indentation profiles were acquired with a maximum deflection of 250 nm and a rate of 20 μm/s. The elastic modulus (Young’s modulus) was calculated from fitting force-indentation data using a Hertz sphere model assuming a Poisson’s ratio of 0.5.
Compliant 2D culture.
Polyacrylamide hydrogels were prepared as described previously (15, 18) with minor modifications. Briefly, 15-mm no. 1 glass coverslips (Fisher Scientific) were treated with a 0.4% solution of 3-methacryloxypropyltrimethoxysilane (Sigma Aldrich) in acetone for 10 min, rinsed once with fresh acetone, and air-dried. Two prepolymerization solutions of variable ratios of acrylamide:bis-acrylamide (Bio-Rad) were prepared as described previously (18)(%acrylamide:%bis-acrylamide [Young’s Modulus], 3:0.11 [1.0 kPa], and 12:0.24 [75 kPa]). Thirty microliters of each prepolymerization mixture were delivered and sandwiched between the above silanized coverslip and a SurfaSil (Thermo Scientific)-treated, hydrophobic glass slide for 0.5–1.0 min. After polymerization, the silanized coverslips with hydrogel attached were carefully peeled off from the hydrophobic slide, covered with water, and then sterilized by heating the plate in a microwave, stopping immediately before the water reached the boiling point. The hydrogels were incubated in 1 mg/ml sterile dopamine hydrochloride solution in 50 mM HEPES (pH 8.5) for 15 min to coat the gel surface with polymerized dopamine (10). The gels were rinsed three times with 50 mM HEPES (pH 8.5) to remove residual dopamine and functionalized by incubation for 30 min with 0.05 mg/ml sterile collagen I (PureCol, Advanced BioMatrix) in PBS. Control or TAZ4SA-transduced fibroblasts were plated at 35,000 cells per well onto polyacrylamide hydrogels in 24-well plates. For some experiments, control and TAZ4SA-transduced fibroblasts cultured on polyacrylamide hydrogels were treated for 24 h with TGF-β1 (2 ng/ml), MRTF activator ISX-9 (20 μM), MRTF inhibitor CCG-203971 (10 μM), or Rho-associated protein kinase (ROCK) inhibitor Y-27632 (10 μM).
RNA isolation and quantitative PCR.
Total RNA from the cells grown on hydrogels was isolated with RNeasy Plus mini kit (QIAGEN) according to the manufacturer’s instruction. RNA from spheroids was isolated with NucleoSpin RNA XS kit (Macherey-Nagel) using poly(-A) RNA as carrier RNA that allows elution of RNA in as little as 5 µl with a funnel-shaped thrust ring and a small amount of silica membrane area. Two hundred fifty nanograms of extracted RNA were used to synthesize cDNA using SuperScript VILO DNA Synthesis Kit (Invitrogen). Real-time quantitative PCR (qPCR) was performed using FastStart Essential DNA Green Master (Roche, Indianapolis, IN) and analyzed using a LightCycler 96 (Roche). Data are expressed as a fold change by ΔΔCt relative to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) housekeeping gene. Primers used for qPCR are shown in Table 1.
Table 1.
Primer sequences used for qPCR
| Gene | Forward Primer Sequence | Reverse Primer Sequence |
|---|---|---|
| TAZ (WWTR1)* | TTCCTAGGGTCTTGCCATGT | AGTCCTACGACGTGACCGAC |
| Taz ± (Wwtr1) | CTTGCTGGTGTTGGTGATTC | ATCAGCCTCTGAATCATGTGAA |
| Ctgf | CCTGCGACCCACACAAG | GACCCACCGAAGACACAG |
| Edn1 | ACATCATCTGGGTCAACACTC | TGAGCGCACTGACATCTAAC |
| Serpine1 | CAAGATGCTATGGGATTCAAAGTC | GTACTGATCTCATTCTTGTTCCAC |
| Col1a1 | ATCATAGCCATAGGACATCTGG | CTGGACAGCCTGGACTTC |
| Col1a2 | CCAGCGAAGAACTCATACAGC | GGACACCCCTTCTACGTTGT |
| Col3a1 | TCTCTAGACTCATAGGACTGACC | TTCTTCTCACCCTTCTTCATCC |
| Fn1 | GTTTCCTCGGTTGTCCTTCT | GACTGTACTTGTCTAGGCGAAG |
| GAPDH* | TGTAGTTGAGGTCAATGAAGGG | ACATCGCTCAGACACCATG |
| Gapdh | GTGGAGTCATACTGGAACATGTAG | AATGGTGAAGGTCGGTGTG |
Primers to human sequence. All others are primers to mouse sequence.
Traction force microscopy.
Contractile forces exerted by cells on different stiffness matrices were assessed by traction force microscopy as described previously (15). Briefly, polyacrylamide substrates with Young’s moduli of 1 and 75 kPa were prepared, and fluorescent sulfate-modified latex microspheres (0.2 μm, 505/515 ex/em, Fluo- Spheres, Life Technologies) were conjugated to the gel surfaces after treatment with 1 mg/ml of 3-hydroxytyramine hydrochloride (Sigma-Aldrich) in 50 mM HEPES solution (pH 8.5) for 5 min. Cells were plated on the gels overnight and then treated with 0 or 100 ng/ml doxycycline and incubated for 24 h before traction force measurements. Phase contract images of cells and fluorescent images of gel surface-conjugated fluorescent beads were acquired for each cell before and after trypsinization using a ×20 magnification objective. Tractions forces were estimated by measuring bead displacement fields and computing corresponding traction fields using TractionsForAll (http://www.mayo.edu/research/labs/tissue-repair-mechanobiology/software).
Statistical analysis.
Data are presented as means ± SE, and values were compared using unpaired t-tests or analysis of variance (ANOVA) with Bonferroni or Tukey’s post-test for multiple comparisons. P < 0.05 was considered significant.
RESULTS
To generate 3D fibroblast cultures in a scaffold-free environment, we used commercially available hanging drop plates that allowed us to reliably generate a single fibroblast spheroid per well. Spheroids were initiated by seeding 500 cells per each hanging drop, and control and TAZ4SA-transduced fibroblasts were subsequently fed growth media supplemented with varying doses of doxycycline every other day for up to 15 days. Spheroids formed within 3 days and were imaged at regular intervals thereafter by light microscopy to record changes in their two-dimensional projected area as a proxy for spheroid growth (Fig. 1). Control fibroblast spheroids remained stable in size and shape over time, and this behavior was not influenced by regular feeding with doxycycline. In contrast, TAZ4SA-transduced fibroblast spheroids dramatically increased in size, with the largest change in area observed with the highest concentration of doxycycline (Fig. 1), consistent with dose-dependent effects of doxycycline on TAZ4SA expression (10). Confocal imaging of cell nuclei within control and TAZ4SA spheroids confirmed that changes in spheroid size corresponded with increased cell number, indicating that net proliferation played a dominant role in the increase in spheroid size (Fig. 1).
Fig. 1.
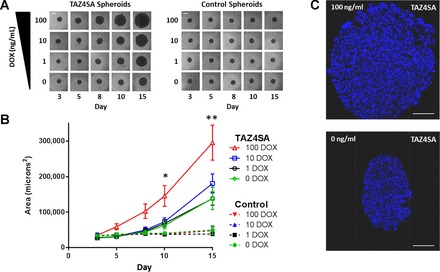
TAZ4SA promotes growth of fibroblast spheroids in scaffold-free culture. A: spheroid growth was tracked by repeated light microscopy imaging over days 3–15 after seeding. Spheroids composed of control cells were stable in shape and size over this time period and were insensitive to doxycycline treatment (DOX). In contrast, spheroids composed of TAZ4SA-transduced fibroblasts increased in size over time while maintaining roughly spherical shape. Spheroid size changes in TAZ4SA spheroids were proportional to doxycycline dose. Scale bars (white lines) are 200 μM. B: quantitative assessment of spheroid growth by measuring 2D projected area of spheroids in light micrographs. A total of 6 independent replicate experiments were performed for TAZ4SA cells, while 3 independent replicate experiments were performed with control cells. Data shown are means ± SE. Data were analyzed by two-way ANOVA and Tukey’s test for multiple comparisons. *P < 0.05 comparing TAZ4SA 100 to all control spheroid groups and to 0, 1, or 10 ng/ml DOX; **P < 0.05 comparing TAZ4SA 100 to 0, 1, or 10 ng/ml DOX, and each TAZ4SA group to its respective control group (e.g., TAZ4SA 10 ng/ml DOX vs. CTRL 10 ng/ml DOX). C: representative confocal images of cell nuclei (DAPI labeled) within TAZ4SA spheroids incubated with 0 or 100 ng/ml DOX for 15 days demonstrating increasing cell numbers that accompany spheroid growth, indicative of net fibroblast proliferation. Scale bars (white lines) are 100 μm.
Interestingly, the TAZ4SA spheroids not treated with doxycycline demonstrated enhanced growth relative to spheroids composed of control cells (Fig. 1). To investigate the cause for this, we developed primers specific to endogenous mouse Taz, as well as the human TAZ4SA sequence. Analysis of transcripts levels from TAZ4SA spheroids by qPCR indicated that human TAZ4SA mRNA was expressed at levels two times higher than endogenous mouse Taz, even in the absence of doxycycline, consistent with some “leak” in this inducible expression system (Fig. 2); as expected, human TAZ was not detectable in control spheroids. TAZ4SA-transduced fibroblasts also expressed higher levels of mouse Taz than control cells, suggesting a possible positive feedback role of TAZ4SA on expression of endogenous Taz itself. Exposure of TAZ4SA-transduced cells to doxycycline dramatically enhanced TAZ4SA expression levels, as expected for this inducible expression system (Fig. 2). Together these results confirm that the growth advantage of TAZ4SA transduced cells at baseline and in response to doxycycline correspond with graduated increases in expression of endogenous Taz as well as TAZ4SA.
Fig. 2.
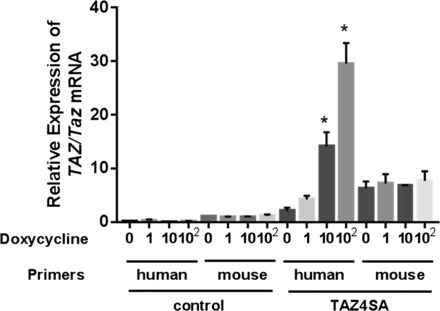
Both endogenous Taz and human TAZ transcripts are increased in doxycycline-inducible TAZ4SA-transduced cells. Quantitative PCR measurements were used to assay transcript levels for endogenous mouse Taz and human TAZ in spheroids composed of control and TAZ4SA-transduced fibroblasts. Human and mouse specific primers were used to measure transcript abundance in the presence of 0, 1, 10, or 100 ng/ml doxycycline. Relative expression levels were normalized to transcript levels of mouse Taz in control cells in the absence of doxycycline. Data shown are means ± SE; n = 3. *P < 0.05, one-way analysis of variance with Bonferroni multiple comparisons test between absence and each concentration of doxycycline.
Because TAZ has previously been implicated in fibrogenic activation of fibroblasts (10, 27, 28), we expected that TAZ4SA would prompt extracellular matrix deposition within fibroblast spheroids. However, routine histological examination of TAZ4SA and control spheroids did not indicate the presence of detectable extracellular matrix within the spheroids. To gain insight into possible profibrotic effects of TAZ expression in spheroids, TAZ4SA spheroids were collected and pooled from 0 and 100 ng/ml doxycycline exposure conditions and mRNA was analyzed using the Fibrosis RT2 Profiler PCR Array (Qiagen). No differences in expression of fibrillar collagen-encoding genes were observed, and replicate analysis of Col1a1, Col1a2, Col3a1, and FN1 by qPCR in independent experiments confirmed this observation (Fig. 3A). However, significant TAZ4SA-mediated increases in expression of pro-fibrotic genes Ctgf (encoding connective tissue growth factor [CTGF]), Edn1 (encoding endothelin [Et]-1) and Serpine1 (encoding plasminogen activator inhibitor [PAI]-1) were observed and confirmed by replicate independent qPCR analyses (Fig. 3A). In contrast, doxycycline treatment of control spheroids did not significantly alter transcript levels for any of the above genes (Fig. 3B). These findings demonstrate that TAZ activation within spheroid culture increases expression of genes that encode soluble profibrotic signals, but is not sufficient per se to drive expression of fibrillar collagens and fibronectin.
Fig. 3.
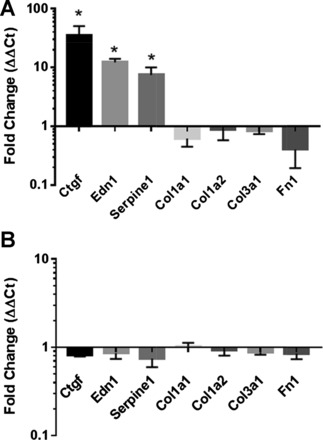
Enhanced expression of genes encoding profibrotic mediators, but not ECM genes, in TAZ4SA-transduced fibroblast spheroids. Quantitative PCR measurements were used to assay transcript levels for the extracellular matrix encoding genes Col1a1, Col1a2, Col3a1, and Fn1, as well as genes encoding paracrine profibrotic factors (Ctgf, Edn1, and Serpine1). A: RNA was harvested and pooled from 10 TAZ4SA-transduced fibroblast spheroids exposed to 0 or 100 ng/ml doxycycline, and the experiment was repeated 4 times. Fold change in transcript levels normalized to 0 ng/ml doxycycline controls are shown for each gene. B: for comparison, RNA was harvested and pooled from 10 control spheroids exposed to 0 or 100 ng/ml doxycycline. Data shown are means ± SE; n = 4. *P < 0.05, unpaired t-test relative to control (0 ng/ml doxycycline).
Recent work has demonstrated that Hippo pathway signaling interacts with other mechanosensitive pathways to shape transcriptional programs within cells (10, 20, 23, 27, 28, 31, 35). Therefore we hypothesized that the mechanical environment within spheroids was too compliant to engage these cooperating transcriptional pathways, thereby preventing the full complement of transcriptional effects of TAZ4SA, including ECM expression and deposition, from being observed. Intact spheroids composed of TAZ4SA-transduced fibroblasts were analyzed by atomic force microscopy microindentation to characterize their mechanical properties. Comparison of untreated and doxycycline-treated (100 ng/ml) spheroids demonstrated expected changes in spheroid size, but no significant difference in Young’s modulus (Fig. 4). Both untreated and doxycycline-treated spheroids were highly compliant, with Young’s modulus in the range of 0.5–3.5 kPa, similar in nature to normal lung and other soft tissues (10, 11, 33).
Fig. 4.
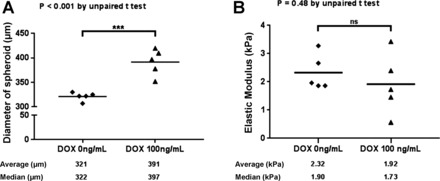
Fibroblast spheroids are highly compliant and insensitive to enhanced TAZ4SA expression. TAZ4SA-transduced fibroblast spheroids were cultured for 7 days in the presence of 0 or 100 ng/ml doxycycline. A: at this time point the expected differential effect of TAZ4SA on spheroid size was observed, as measured by spheroid diameter (n = 5). ***P < 0.05, unpaired t-test. B: atomic force microscopy microindentation was used to compare the mechanical properties of the same TAZ4SA spheroids as in A. Five spheroids per condition were analyzed and five force curves were collected from each spheroid and averaged to generate a single Young’s modulus value per spheroid. The elastic modulus of spheroids from 0 and 100 ng/ml doxycycline conditions were statistically indistinguishable (ns, not significant).
To investigate whether the compliant nature of the spheroids may have influenced TAZ effects on transcript expression, we cultured TAZ4SA-transduced fibroblasts on highly compliant polyacrylamide hydrogels (1.0 kPa) within the range we observed for spheroid stiffness. For comparison, we also cultured TAZ4SA-transduced fibroblasts on stiff polyacrylamide hydrogels (75 kPa) approximating fibrotic tissue Young’s modulus (10, 11, 33). We previously observed that TAZ4SA expression was sufficient to enhance Serpine1/PAI-1 expression on compliant hydrogels (10), and confirmed here that doxycycline-induced TAZ4SA enhanced Serpine1 expression on both soft and stiff matrices (Fig. 5E). However, while TAZ4SA expression on stiff matrices significantly enhanced Col1a1, Col1a2, Col3a1, and FN1 transcript levels (Fig. 5, A–D) in agreement with our prior observations (10), it was not sufficient to enhance expression of these transcripts on compliant hydrogels; rather, TAZ4SA expression actually reduced transcript levels for all three collagen-encoding genes under such conditions (Fig. 5, A–D). These results indicate that the transcriptional effects of TAZ are complex and in some cases dependent on mechanical context. Thus, interactions between TAZ and other mechanosensitive pathways likely influence aspects of the transcriptional program driven by TAZ4SA expression. Moreover, our results on compliant hydrogels are consistent with our earlier observation that TAZ4SA expression within the highly compliant mechanical environment observed in scaffold-free spheroids is not sufficient to promote Col1a1 gene expression (Fig. 3A).
Fig. 5.
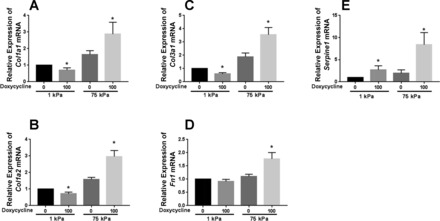
TAZ4SA effects on ECM gene expression depend on matrix stiffness. Quantitative PCR measurements were used to assay transcript levels for the ECM-encoding gene Col1a1 (A), Col1a2 (B), Col3a1 (C), and Fn1 (D), as well as PAI-1 encoding gene Serpine1 (E). RNA was harvested from cells grown on polyacrylamide hydrogels of 1 and 75 kPa Young’s modulus, with control and TAZ4SA-transduced cells exposed to 0 or 100 ng/ml doxycycline. The experiment was repeated 3 times. Data shown are means ± SE; n = 3. *P < 0.05, unpaired t-test between 0 and 100 ng/ml doxycycline for each stiffness.
To directly test whether specific mechanosensitive and profibrotic pathways may engage with TAZ to regulate gene expression on compliant matrices, we stimulated control and TAZ4SA-transduced fibroblasts with TGF-β1 or a small-molecule activator of MRTF, ISX-9 (32). Neither was able to significantly enhance Col1a1 transcript levels on compliant hydrogels in control cells, demonstrating that these stimuli, like TAZ itself, were insufficient to overcome the mechanical environment (Fig. 6, A and B). However, the combination of TAZ4SA expression and TGF-β1 stimulation did significantly enhance Col1a1 expression, with the combination of TAZ4SA and ISX-9 showing more modest effects. To further evaluate crosstalk between TAZ and MRTF, we treated TAZ4SA-transduced fibroblasts on stiff matrices with the small molecular MRTF inhibitor CCG-203971. This compound significantly reduced baseline Col1a1 expression in the absence of doxycycline, and it completely reversed TAZ4SA-mediated increases in Col1a1 expression, demonstrating an essential role for MRTF in TAZ-mediated Col1a1 expression (Fig. 6C). Finally, both TAZ and MRTF activation have been shown to depend on ROCK-mediated actin dynamics and contraction, with TGF-β also activating ROCK; hence we tested whether the ROCK inhibitor Y-27632 would attenuate TAZ4SA-mediated Col1a1 expression on a stiff matrix. Interestingly, Y-27632 did not alter baseline Col1a1 transcript levels in the absence of doxycycline, but it completely reversed the TAZ4SA-mediated increase in Col1a1 expression (Fig. 6D), consistent with a pivotal role for ROCK in this response.
Fig. 6.
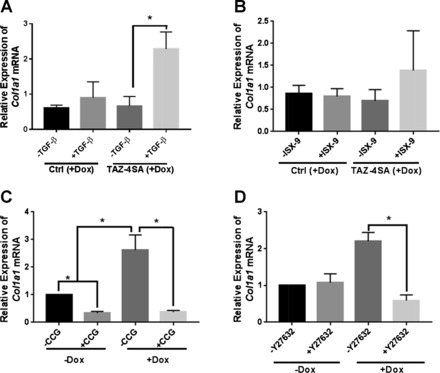
Context-dependent crosstalk between TAZ, TGF-β1, MRTF, and ROCK mediate Col1a1 expression. Col1a1 transcript levels were evaluated by qPCR. A and B: control and TAZ-4SA-transduced fibroblasts were cultured on 1 kPa compliant hydrogels with 100 ng/ml doxycycline for 72 h to maximally induce TAZ4SA expression and were stimulated in the final 24 h with 0 or 2 ng/ml TGF-β1 (A), or 0 or 20 μM MRTF activator ISX-9 (B). Col1a1 transcript levels were normalized to each cell type not treated with doxycycline. C and D: TAZ4SA-transduced fibroblasts were cultured on 75 kPa hydrogels with 100 ng/ml doxycycline for 72 h to maximally induce TAZ4SA expression or without doxycycline as controls and were treated for the final 24 h with 10 μM MRTF inhibitor CCG-203971 (C) or 10 μM ROCK inhibitor Y-27632 (D). Col1a1 transcript levels were normalized to TAZ4SA cells not treated with doxycycline. Data shown are means ± SE; n = 3. *P < 0.05, unpaired t-test.
The observation of mechanical context-dependent effects of TAZ4SA on collagen expression raises a question as to how ECM deposition in the highly compliant lung environment could be accomplished by TAZ4SA-expressing cells, as previously observed (10). One possibility is that cells in this prior study engrafted in locally stiff regions of the lung, or that the process of cell invasion locally altered the local mechanical or biochemical environment, potentiating TAZ4SA effects. Another possibility is that cells could rapidly modify extracellular matrix mechanical properties through contractile force-mediated reorganization of fibrillar matrices (5). Local changes in ECM density, along with the nonlinear mechanical properties of biological polymers, result in local stiffening when cells actively contract within matrices (8, 34). To test whether TAZ4SA expression confers enhanced contractile activity even on soft 2D synthetic matrices, we compared traction force generation in the presence and absence of doxycycline in TAZ4SA-transduced and control cells. While traction forces generated on soft matrices were dramatically lower than on stiff matrices, in agreement with previous observations (14, 15), we noted that TAZ4SA expression was sufficient to confer enhanced contractile function even on very compliant hydrogels (Fig. 7). Taken together, our findings demonstrate that TAZ activation alone supports a variety of profibrotic effects, with proliferation, tractional forces, and profibrotic paracrine signaling emerging even within compliant spheroid or matrix conditions, whereas TAZ-mediated collagen gene expression requires a stiffer environment or crosstalk with other profibrotic signals such as TGF-β1 or MRTF.
Fig. 7.
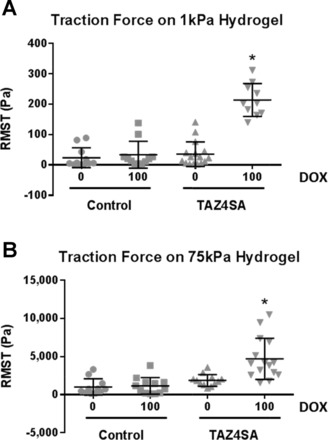
TAZ4SA expression is sufficient to enhance traction forces on both compliant and rigid matrices. Traction force microscopy was used to assay fibroblast contractile function on matrices of defined stiffness. Control and TAZ4SA-transduced fibroblasts were grown on polyacrylamide hydrogels of highly compliant (1 kPa, A) or relatively rigid (75 kPa, B) Young’s modulus and exposed to 0 or 100 ng/ml doxycycline. Individual cells were analyzed and the root mean square traction (RMST) was computed, with results displayed as a single data point on the plot representing each cell. Data shown are from n = 10–16 cells per condition, with bars showing population mean ± SE *P < 0.05, one-way ANOVA with Bonferroni multiple comparisons test between absence and presence of doxycycline.
DISCUSSION
Fibrosis is a pathologic process driven by aberrant accumulation of contractile fibroblasts and production of excess extracellular matrix (22). Unraveling the full complement of signals that initiate and sustain fibroblast activation remains a major barrier to our understanding of the pathogenesis of fibrosis. Recent work has demonstrated that fibroblasts are exquisitely sensitive to the mechanical properties of their microenvironment (30, 31), and that pathological changes in mechanical environment likely play an important role in sustaining fibroblast activation and fibrotic progression (30, 33). Here we developed a scaffold-free spheroid culture system to study how fibroblasts grow and remodel in the absence of an externally imposed mechanical environment. This work adapts well-developed techniques for spheroid culture which have been predominantly applied to the study of tumor cell biology (16). Prior reports of scaffold-free fibroblast cultures have demonstrated that fibroblasts exhibit limited capacity to grow and deposit matrix in spheroids (24–26), though some disease-derived cells have been reported to differ in these capacities (6, 29), raising the tantalizing possibility that such culture systems could be used to better understand the mechanisms that lead to aberrant cellular activation and matrix deposition in fibrosis.
Our results demonstrate that control fibroblasts, as in prior publications (24–26), display a limited capacity to grow in spheroid culture. In contrast, TAZ4SA-transduced fibroblasts overcome inherent growth limitations to proliferate in scaffold-free spheroids. Interestingly, TAZ and YAP are known to contribute to cell cycle progression, and their sequestration in the cytoplasm contributes to cell cycle arrest (19). Control of TAZ and YAP cytoplasmic sequestration occurs via Hippo pathway-mediated serine phosphorylation and is regulated by both cell contact and absence of a sufficiently rigid mechanical environment (17). Our results show that an ectopic increase in nuclear TAZ activity by expression of TAZ4SA, a mutant TAZ with alanine substitutions for Hippo pathway-regulated serine residues, overrides the conditions within spheroid cultures that block fibroblast proliferation. It will be interesting in future studies to better understand whether lack of fibroblast proliferation in spheroid culture arises from mechanical compliance and lack of a suitably rigid mechanical environment, cell contact-mediated growth inhibition, or both. The latter of these two mechanisms is well studied in epithelial cells, where specific cell-cell adhesion-based signals have been delineated (3); in fibroblasts the presence of such cell-contact based mechanisms of Hippo pathway signaling have not been as well studied.
While our results indicate that TAZ4SA expression overcomes growth limitations in spheroid culture, it failed to overcome apparent limitations on fibrillar collagen and fibronectin gene expression, suggesting the need for additional cofactors. Moreover, TAZ4SA expression on soft hydrogels modestly, but significantly, reduced transcript levels for Col1a1, Col1a2, and Col3a1, demonstrating that in certain contexts nuclear active TAZ may serve to repress fibrotic gene transcripts (Fig. 5), an unexpected finding deserving of further attention. Recent work has expanded the already sizable list of proteins with which TAZ (and YAP) interact beyond the canonical TEAD family of transcription factors (19) to include the mechanoresponsive myocardin-related transcription factor (MRTF) (9, 27, 35), as well as β-catenin and SMADs (20, 23, 28, 31). Interactions with these (and/or other) factors may be necessary to allow TAZ-related enhancement of ECM gene expression and matrix deposition, as evidenced by the potentiating effect of TGF-β1 stimulation, and inhibitory effect of MRTF inhibition, on Col1a1 expression in TAZ4SA-expressing cells. In contrast to the inability of TAZ4SA expression alone to induce ECM production, it did strongly enhance expression of multiple genes that encode paracrine factors supportive of fibroblast activation and fibrosis, including CTGF, endothelin-1, and PAI-1. Thus it is apparent from our current results, and recent published work (10, 13, 21, 28), that TAZ nuclear function is essential for multiple key profibrotic functions, and that TAZ activation itself is sufficient to drive a subset of key transcriptional and functional fibroblast behaviors.
Interestingly, one of the functions for which TAZ is necessary and sufficient is contraction of both soft and stiff matrices spanning the range of matrix compliance observed in normal and fibrotic soft tissues (10, 11, 33). Biological matrices display nonlinear mechanical behaviors including strain-stiffening (34); that is, they become less compliant as they are deformed. The capacity of TAZ4SA expression to promote fibroblast force generation, which correspondingly would lead to strain and strain-stiffening in the fibroblast’s surrounding extracellular matrix (4), raises the intriguing possibility that TAZ activation plays an essential role in initiation of fibroblast matrix remodeling. A wide variety of upstream signals, including several associated with injury and inflammation, are known to activate TAZ (17, 19). Our results suggest that TAZ activation in fibroblasts residing within a compliant matrix would generate contractile forces that might locally stiffen the extracellular matrix; such changes in local matrix stiffness, accompanied by enhanced production of autocrine/paracrine signals would potentially engage additional mechanoresponsive and profibrotic pathways, leading to sustained fibroblast activation and the capacity for matrix deposition. Our results thus not only reinforce prior findings that TAZ activation is an essential contributor to the sustained mechanical activation of fibroblasts in fibrotic tissues (10), but introduce the concept that TAZ activation may play a central role as an early initiator of fibroblast activation via proliferation, contractile loading of the ECM, and expression of profibrotic paracrine signals. Further elucidation of the functional and transcriptional effects of TAZ activation, as well as the likely complex and context-dependent interactions of TAZ with other transcriptionally active proteins, will further our understanding of the pivotal roles TAZ plays in initiation and amplification of fibroblast activation and tissue fibrosis.
GRANTS
This work was supported by National Institutes of Health National Heart, Lung, and Blood Institute Grants HL-092961 (to D. J. Tschumperlin) and HL-124392 (to X. Varelas).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
A.J.J., K.M.C., D.S., and K.M.J.S. performed experiments; A.J.J., K.M.C., D.S., K.M.J.S., and D.J.T. analyzed data; A.J.J., K.M.C., D.S., K.M.J.S., and D.J.T. interpreted results of experiments; A.J.J., K.M.C., and D.S. prepared figures; A.J.J. and D.J.T. drafted manuscript; A.J.J., K.M.C., D.S., K.M.J.S., S.E.H., X.V., and D.J.T. edited and revised manuscript; A.J.J., K.M.C., D.S., K.M.J.S., S.E.H., X.V., and D.J.T. approved final version of manuscript; D.J.T. conceived and designed the research.
Footnotes
This article is the topic of an Editorial Focus by Bram Piersma and Ruud A. Bank (19a).
REFERENCES
- 1.Bertero T, Cottrill KA, Annis S, Bhat B, Gochuico BR, Osorio JC, Rosas I, Haley KJ, Corey KE, Chung RT, Nelson Chau B, Chan SYA. A YAP/TAZ-miR-130/301 molecular circuit exerts systems-level control of fibrosis in a network of human diseases and physiologic conditions. Sci Rep 5: 18277, 2015. doi: 10.1038/srep18277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caliari SR, Perepelyuk M, Cosgrove BD, Tsai SJ, Lee GY, Mauck RL, Wells RG, Burdick JA. Stiffening hydrogels for investigating the dynamics of hepatic stellate cell mechanotransduction during myofibroblast activation. Sci Rep 6: 21387, 2016. doi: 10.1038/srep21387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gumbiner BM, Kim NG. The Hippo-YAP signaling pathway and contact inhibition of growth. J Cell Sci 127: 709–717, 2014. doi: 10.1242/jcs.140103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jansen KA, Bacabac RG, Piechocka IK, Koenderink GH. Cells actively stiffen fibrin networks by generating contractile stress. Biophys J 105: 2240–2251, 2013. doi: 10.1016/j.bpj.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kopanska KS, Bussonnier M, Geraldo S, Simon A, Vignjevic D, Betz T. Quantification of collagen contraction in three-dimensional cell culture. Methods Cell Biol 125: 353–372, 2015. doi: 10.1016/bs.mcb.2014.10.017. [DOI] [PubMed] [Google Scholar]
- 6.Lee WJ, Choi IK, Lee JH, Kim YO, Yun CO. A novel three-dimensional model system for keloid study: organotypic multicellular scar model. Wound Repair Regen 21: 155–165, 2013. doi: 10.1111/j.1524-475X.2012.00869.x. [DOI] [PubMed] [Google Scholar]
- 7.Lei QY, Zhang H, Zhao B, Zha ZY, Bai F, Pei XH, Zhao S, Xiong Y, Guan KL. TAZ promotes cell proliferation and epithelial-mesenchymal transition and is inhibited by the hippo pathway. Mol Cell Biol 28: 2426–2436, 2008. doi: 10.1128/MCB.01874-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leung LY, Tian D, Brangwynne CP, Weitz DA, Tschumperlin DJ. A new microrheometric approach reveals individual and cooperative roles for TGF-β1 and IL-1β in fibroblast-mediated stiffening of collagen gels. FASEB J 21: 2064–2073, 2007. doi: 10.1096/fj.06-7510com. [DOI] [PubMed] [Google Scholar]
- 9.Liu CY, Chan SW, Guo F, Toloczko A, Cui L, Hong W. MRTF/SRF dependent transcriptional regulation of TAZ in breast cancer cells. Oncotarget 7: 13706–13716, 2016. doi: 10.18632/oncotarget.7333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu F, Lagares D, Choi KM, Stopfer L, Marinković A, Vrbanac V, Probst CK, Hiemer SE, Sisson TH, Horowitz JC, Rosas IO, Fredenburgh LE, Feghali-Bostwick C, Varelas X, Tager AM, Tschumperlin DJ. Mechanosignaling through YAP and TAZ drives fibroblast activation and fibrosis. Am J Physiol Lung Cell Mol Physiol 308: L344–L357, 2015. doi: 10.1152/ajplung.00300.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu F, Mih JD, Shea BS, Kho AT, Sharif AS, Tager AM, Tschumperlin DJ. Feedback amplification of fibrosis through matrix stiffening and COX-2 suppression. J Cell Biol 190: 693–706, 2010. doi: 10.1083/jcb.201004082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu F, Tschumperlin DJ. Micro-mechanical characterization of lung tissue using atomic force microscopy. J Vis Exp 54: e2911, 2011. doi: 10.3791/2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mannaerts I, Leite SB, Verhulst S, Claerhout S, Eysackers N, Thoen LF, Hoorens A, Reynaert H, Halder G, van Grunsven LA. The Hippo pathway effector YAP controls mouse hepatic stellate cell activation. J Hepatol 63: 679–688, 2015. doi: 10.1016/j.jhep.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 14.Marinković A, Liu F, Tschumperlin DJ. Matrices of physiologic stiffness potently inactivate idiopathic pulmonary fibrosis fibroblasts. Am J Respir Cell Mol Biol 48: 422–430, 2013. doi: 10.1165/rcmb.2012-0335OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marinković A, Mih JD, Park JA, Liu F, Tschumperlin DJ. Improved throughput traction microscopy reveals pivotal role for matrix stiffness in fibroblast contractility and TGF-β responsiveness. Am J Physiol Lung Cell Mol Physiol 303: L169–L180, 2012. doi: 10.1152/ajplung.00108.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mehta G, Hsiao AY, Ingram M, Luker GD, Takayama S. Opportunities and challenges for use of tumor spheroids as models to test drug delivery and efficacy. J Control Release 164: 192–204, 2012. doi: 10.1016/j.jconrel.2012.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meng Z, Moroishi T, Guan KL. Mechanisms of Hippo pathway regulation. Genes Dev 30: 1–17, 2016. doi: 10.1101/gad.274027.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mih JD, Sharif AS, Liu F, Marinkovic A, Symer MM, Tschumperlin DJ. A multiwell platform for studying stiffness-dependent cell biology. PLoS One 6: e19929, 2011. doi: 10.1371/journal.pone.0019929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Piccolo S, Dupont S, Cordenonsi M. The biology of YAP/TAZ: hippo signaling and beyond. Physiol Rev 94: 1287–1312, 2014. doi: 10.1152/physrev.00005.2014. [DOI] [PubMed] [Google Scholar]
- 19a.Piersma B, Bank RA. Keeping fibroblasts in suspense: TAZ-mediated signaling activates a context-dependent profibrotic phenotype. Focus on “TAZ activation drives fibroblast spheroid growth, expression of profibrotic paracrine signals, and context-dependent ECM gene expression.” Am J Physiol Cell Physiol, 2017. doi: 10.1152/ajpcell.00362.2016. [DOI] [PubMed] [Google Scholar]
- 20.Piersma B, Bank RA, Boersema M. Signaling in fibrosis: TGF-β, WNT, and YAP/TAZ converge. Front Med (Lausanne) 2: 59, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Piersma B, de Rond S, Werker PM, Boo S, Hinz B, van Beuge MM, Bank RA. YAP1 is a driver of myofibroblast differentiation in normal and diseased fibroblasts. Am J Pathol 185: 3326–3337, 2015. doi: 10.1016/j.ajpath.2015.08.011. [DOI] [PubMed] [Google Scholar]
- 22.Rockey DC, Bell PD, Hill JA. Fibrosis–a common pathway to organ injury and failure. N Engl J Med 372: 1138–1149, 2015. doi: 10.1056/NEJMra1300575. [DOI] [PubMed] [Google Scholar]
- 23.Saito A, Nagase T. Hippo and TGF-β interplay in the lung field. Am J Physiol Lung Cell Mol Physiol 309: L756–L767, 2015. doi: 10.1152/ajplung.00238.2015. [DOI] [PubMed] [Google Scholar]
- 24.Salmenperä P, Kankuri E, Bizik J, Sirén V, Virtanen I, Takahashi S, Leiss M, Fässler R, Vaheri A. Formation and activation of fibroblast spheroids depend on fibronectin-integrin interaction. Exp Cell Res 314: 3444–3452, 2008. doi: 10.1016/j.yexcr.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 25.Salmenperä P, Karhemo PR, Räsänen K, Laakkonen P, Vaheri A. Fibroblast spheroids as a model to study sustained fibroblast quiescence and their crosstalk with tumor cells. Exp Cell Res 345: 17–24, 2016. doi: 10.1016/j.yexcr.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 26.Sirén V, Salmenperä P, Kankuri E, Bizik J, Sorsa T, Tervahartiala T, Vaheri A. Cell-cell contact activation of fibroblasts increases the expression of matrix metalloproteinases. Ann Med 38: 212–220, 2006. doi: 10.1080/07853890500494999. [DOI] [PubMed] [Google Scholar]
- 27.Speight P, Kofler M, Szászi K, Kapus A. Context-dependent switch in chemo/mechanotransduction via multilevel crosstalk among cytoskeleton-regulated MRTF and TAZ and TGFβ-regulated Smad3. Nat Commun 7: 11642, 2016. doi: 10.1038/ncomms11642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Szeto SG, Narimatsu M, Lu M, He X, Sidiqi AM, Tolosa MF, Chan L, De Freitas K, Bialik JF, Majumder S, Boo S, Hinz B, Dan Q, Advani A, John R, Wrana JL, Kapus A, Yuen DA. YAP/TAZ are mechanoregulators of TGF-β-Smad signaling and renal fibrogenesis. J Am Soc Nephrol. 2016. doi: 10.1681/ASN.2015050499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Torry DJ, Richards CD, Podor TJ, Gauldie J. Anchorage-independent colony growth of pulmonary fibroblasts derived from fibrotic human lung tissue. J Clin Invest 93: 1525–1532, 1994. doi: 10.1172/JCI117131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tschumperlin DJ. Matrix, mesenchyme, and mechanotransduction. Ann Am Thorac Soc 12, Suppl 1: S24–S29, 2015. doi: 10.1513/AnnalsATS.201407-320MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tschumperlin DJ, Liu F, Tager AM. Biomechanical regulation of mesenchymal cell function. Curr Opin Rheumatol 25: 92–100, 2013. doi: 10.1097/BOR.0b013e32835b13cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Velasquez LS, Sutherland LB, Liu Z, Grinnell F, Kamm KE, Schneider JW, Olson EN, Small EM. Activation of MRTF-A-dependent gene expression with a small molecule promotes myofibroblast differentiation and wound healing. Proc Natl Acad Sci USA 110: 16850–16855, 2013. doi: 10.1073/pnas.1316764110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wells RG. The role of matrix stiffness in regulating cell behavior. Hepatology 47: 1394–1400, 2008. doi: 10.1002/hep.22193. [DOI] [PubMed] [Google Scholar]
- 34.Wen Q, Janmey PA. Effects of non-linearity on cell-ECM interactions. Exp Cell Res 319: 2481–2489, 2013. doi: 10.1016/j.yexcr.2013.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu OM, Miyamoto S, Brown JH. Myocardin-related transcription Factor A and yes-associated protein exert dual control in G protein-coupled receptor- and RhoA-mediated transcriptional regulation and cell proliferation. Mol Cell Biol 36: 39–49, 2015. doi: 10.1128/MCB.00772-15. [DOI] [PMC free article] [PubMed] [Google Scholar]


