We investigated the influence of nitrate-rich and nitrate-depleted beetroot juice on the muscle metabolic and physiological adaptations to 4 wk of sprint interval training. Compared with placebo, dietary nitrate supplementation reduced the O2 cost of submaximal exercise, resulted in greater improvement in incremental (but not severe-intensity) exercise performance, and augmented some muscle metabolic adaptations to training. Nitrate supplementation may facilitate some of the physiological responses to sprint interval training.
Keywords: beetroot juice supplementation, exercise training, training adaptation, muscle metabolism
Abstract
We hypothesized that 4 wk of dietary nitrate supplementation would enhance exercise performance and muscle metabolic adaptations to sprint interval training (SIT). Thirty-six recreationally active subjects, matched on key variables at baseline, completed a series of exercise tests before and following a 4-wk period in which they were allocated to one of the following groups: 1) SIT and -depleted beetroot juice as a placebo (SIT+PL); 2) SIT and -rich beetroot juice (~13 mmol /day; SIT+BR); or 3) no training and -rich beetroot juice (NT+BR). During moderate-intensity exercise, pulmonary oxygen uptake was reduced by 4% following 4 wk of SIT+BR and NT+BR (P < 0.05) but not SIT+PL. The peak work rate attained during incremental exercise increased more in SIT+BR than in SIT+PL (P < 0.05) or NT+BR (P < 0.001). The reduction in muscle and blood [lactate] and the increase in muscle pH from preintervention to postintervention were greater at 3 min of severe-intensity exercise in SIT+BR compared with SIT+PL and NT+BR (P < 0.05). However, the change in severe-intensity exercise performance was not different between SIT+BR and SIT+PL (P > 0.05). The relative proportion of type IIx muscle fibers in the vastus lateralis muscle was reduced in SIT+BR only (P < 0.05). These findings suggest that BR supplementation may enhance some aspects of the physiological adaptations to SIT.
NEW & NOTEWORTHY We investigated the influence of nitrate-rich and nitrate-depleted beetroot juice on the muscle metabolic and physiological adaptations to 4 wk of sprint interval training. Compared with placebo, dietary nitrate supplementation reduced the O2 cost of submaximal exercise, resulted in greater improvement in incremental (but not severe-intensity) exercise performance, and augmented some muscle metabolic adaptations to training. Nitrate supplementation may facilitate some of the physiological responses to sprint interval training.
the gaseous biological signaling molecule, nitric oxide (NO), is known to modulate several physiological responses to exercise including skeletal muscle perfusion, energy metabolism, and contractile function (41, 69). Nitric oxide synthase enzymes catalyze the oxygen (O2)-dependent production of NO from l-arginine, and it is now known that the products of NO oxidation, nitrate () and nitrite (), can be reduced in vivo to form NO (52, 72). Interestingly, hypoxia and acidosis, physiological environments typical of muscular exercise, facilitate the reduction of to NO (72). Increasing the dietary intake of inorganic to augment circulating and pools may therefore represent a natural means to increase NO bioavailability during exercise.
The physiological effects of ingestion in humans are well documented and may include a reduction in blood pressure (BP) at rest and reduced oxygen uptake (V̇o2) during submaximal exercise (5, 16, 45, 73). Moreover, several studies suggest that supplementation can improve performance in a variety of exercise settings, at least in subelite athletes (2, 14, 71, 73, 78, cf. 40). It has recently been reported that short-term (3–7 days) supplementation may favorably impact the metabolic and contractile properties of skeletal muscle (30, 46, 76). Specifically, the improvements in exercise efficiency and performance that have been observed following dietary supplementation may be related to altered mitochondrial function (46, cf. 76) and to enhanced muscle force or power production (19, 30), which, in turn, might be related to increased perfusion and contractile function (22, 32). It is unclear whether more protracted periods (several weeks) of supplementation may more favorably impact the physiological response to exercise and improve exercise performance. However, given that dietary may specifically enhance the physiological responses of type II muscle fibers to exercise (22, 23, 32, 35) and improve performance during repeated sprint exercise (2, 71, 78), it is possible that supplementation may be of particular value to athletes engaging in high-intensity training.
Sprint interval training (SIT) is known to provide a potent and relatively time-efficient stimulus for enhancing aerobic capacity and endurance exercise performance (11–13, 26). However, the effects of a high- dietary supplement, such as beetroot juice, consumed daily as part of an exercise-training program, on the physiological and muscle metabolic adaptations to training have received limited attention (21, 57). It is possible that the NO-mediated inhibition of O2 consumption at cytochrome c oxidase (10, 17) and resultant local hypoxia may initiate signaling cascades that may be synergistic (or antagonistic) to those generated by SIT (27). Also, similar to the effects of training, elevated NO bioavailability may stimulate angiogenesis (25), mitochondrial biogenesis (58), and the transformation of muscle fiber phenotype (59, 68) through cGMP-dependent gene expression and the activation of regulatory factors, in particular peroxisome proliferator-activated receptor-gamma coactivator-1 alpha (PGC-1α; 37, 43). It could also be anticipated that the lower V̇o2 and reduced adenosine triphosphate (ATP) and phosphocreatine (PCr) cost of muscle force production during high-intensity exercise following supplementation (6, 24) might enable a higher training intensity for the same effort, which, over time, may lead to greater training adaptation (34). An increase in cytosolic calcium concentration ([Ca2+]) and force production during muscle contraction following supplementation (32) may also permit a higher training intensity to be maintained. Given the potentially complementary effects of exercise training and NO bioavailability on metabolic regulation, it is possible that supplementation could augment the physiological adaptations to SIT.
Two recent studies have used different approaches to address this question and have produced somewhat disparate results (21, 57). Muggeridge et al. (57) reported that compared with placebo, supplementation with -containing gels during 3 wk of SIT (4–6 × 15-s sprints, 3 times per week) tended to increase peak work rate during incremental exercise (8.7 vs. 4.7%; P = 0.07) and reduce the fatigue index during repeated sprint exercise (0.5 vs. 7.3%; P = 0.06). De Smet et al. (21) reported that compared with placebo, NaNO3 supplementation during 5 wk of SIT (4–6 × 30-s sprints, 3 times per week), performed in hypoxia, did not improve either incremental exercise or 30-min time trial performance but did result in a significant increase in the proportion of type IIa fibers in the vastus lateralis muscle. Neither study measured potential training-related differences in muscle metabolic responses to exercise with compared with placebo supplementation (for example, [PCr], pH, [lactate], and [glycogen] as determined from muscle biopsy) or compared the effects of training with or placebo with the physiological effects of supplementation alone. It would be of interest to determine whether the intriguing change in muscle fiber type proportions when SIT in hypoxia was performed with supplementation (21) is also evident following SIT in normoxia. Additional studies are required to explore the influence of supplementation on the muscle metabolic adaptations and submaximal and maximal exercise responses to training.
The purpose of this study was, therefore, to evaluate the independent and combined performance and physiological effects of SIT and supplementation during a 4-wk intervention involving the following: SIT with concurrent -depleted beetroot juice supplementation as a placebo (PL); SIT with concurrent -rich beetroot juice supplementation (BR); and -rich beetroot juice supplementation with no training. We tested the hypothesis that 4-wk SIT and 4-wk BR supplementation would independently improve physiological responses and exercise performance but that these effects would be greater when BR supplementation and SIT were combined.
METHODS
Subjects
Eighteen male (age 27 ± 8 yr, height 1.79 ± 0.08 m, body mass 80 ± 13 kg, V̇o2peak 50.4 ± 11.4 ml·kg−1·min−1; means ± SD) and 18 female (age 23 ± 4 yr, height 1.66 ± 0.05 m, body mass 65 ± 9 kg, V̇o2peak 39.8 ± 5.8 ml·kg−1·min−1) participants were recruited. The subjects were recreationally active sportspeople involved in team and/or endurance sports, but they were not highly trained. Following an explanation of the experimental procedures, associated risks, potential benefits, and likely value of the possible findings, subjects gave their written informed consent to participate. The study was approved by the Institutional Research Ethics Committee and conformed to the code of ethics of the Declaration of Helsinki.
Experimental Design
Subjects initially visited the laboratory on three separate occasions over a 5-day period. On visit 1, subjects performed an incremental exercise test on a cycle ergometer for the determination of V̇o2peak and gas exchange threshold (GET). The work rates requiring 80% of the GET (moderate exercise) and 85% Δ (GET plus 85% of the difference between the work rate at GET and V̇o2peak; severe exercise) were calculated and adjusted for mean response time for V̇o2 during incremental exercise (75). Following this, subjects were familiarized to the exercise testing procedures, including completion of a severe-intensity bout of cycle ergometry until exhaustion. On visit 2, subjects completed a 5-min bout of moderate-intensity cycling and an incremental exercise test. On visit 3, subjects completed two bouts of severe-intensity cycling, the first for 3 min and the second until task failure.
In a double-blind, independent-groups design, subjects were then assigned to receive -rich beetroot juice (BR) or -depleted beetroot juice (PL) for 28 days. Three independent groups (n = 12, comprising 6 men and 6 women) were matched at baseline for physical characteristics (i.e., mass, height, and age) as well as physiological and performance variables of interest, principally BP and peak work rate (WR) during incremental exercise and, secondarily, V̇o2peak and GET. Subjects were then either enrolled into a 4-wk supervised SIT program with PL (SIT+PL) or BR (SIT+BR) supplementation or received the -rich beetroot juice for 28 days without undergoing a training intervention (NT+BR).
All groups completed the same exercise tests (at the same absolute work rates) and physiological assessments both before and after the 28-day intervention period. Also, after 14 days, subjects visited the laboratory for an incremental exercise test to assess the short-term changes in aerobic capacity that may be expected following the interventions (11, 65, 73).
Laboratory visits were scheduled at the same time of day (±2 h). Subjects were asked to maintain their normal dietary and exercise behavior throughout the study. However, subjects were instructed to record their diet during the 24 h preceding the first laboratory visit and to repeat this for all subsequent laboratory visits. On days of training, subjects were asked to arrive at the training venue ≥1 h postprandial and to complete a 5-min self-paced warm-up before training commenced. On experimental days, subjects were instructed to arrive at the laboratory ≥3 h postprandial having avoided strenuous exercise and the consumption of alcohol and caffeine in the 12 h preceding each exercise test. For the duration of the study, subjects were asked to refrain from taking other dietary supplements and also to avoid using antibacterial mouthwash as this inhibits the reduction of to in the oral cavity by eliminating commensal bacteria (29).
Supplementation.
Following the preintervention laboratory visits, subjects were allocated to receive concentrated -rich beetroot juice (BR; beetroot juice; ~6.4 mmol of per 70 ml; Beet it; James White Drinks, Ipswich, United Kingdom) or -depleted beetroot juice (PL; placebo beetroot juice; ~0.04 mmol per 70 ml; Beet it; James White Drinks). Subjects consumed 1 × 70 ml of their allocated supplement each morning and evening for the duration of the training or nontraining intervention and recorded their intake in a diary. This approach would be expected to result in elevated plasma [] and [] for each 24-h period (79). Compliance was checked by the return of empty bottles each week and via questionnaire at 2 and 4 wk. BR and PL doses were administered using a double-blind design. On experimental visits at the midintervention point and following the intervention period, subjects consumed 2 × 70 ml of their allocated supplement 2.5 h before the exercise tests.
Incremental exercise tests.
On the first laboratory visit before and following the intervention period as well as at the midintervention point, subjects completed a ramp incremental exercise test on an electronically braked cycle ergometer (Lode Excalibur Sport; Groningen, The Netherlands). The self-selected cadence (75–90 rpm) and saddle and handlebar height and configuration for each subject were recorded on the first visit and reproduced in subsequent visits. Initially, subjects performed 3 min of baseline cycling at 20 W, after which the work rate was increased by 30 W/min until the limit of tolerance. Breath-by-breath pulmonary gas exchange data (Oxycon Pro; Jaeger, Höchberg, Germany) were collected continuously throughout all incremental tests and were averaged over 10-s periods. V̇o2peak and GET were determined as previously described (73).
Step exercise tests.
A 5-min moderate-intensity “step” test was performed on the first laboratory visit before and following the intervention. This was completed 10 min before the ramp incremental test protocol was initiated. On the second laboratory visit before and following the intervention, two severe-intensity step tests were performed, separated by a 20-min period of rest; the first until 3 min, and the second, after 20 min of passive recovery, until task failure. The time to task failure was recorded once the pedal rate fell by >10 rpm below the target cadence. All step tests began with 3 min of pedaling at 20 W before a sudden transition to the target work rate. Muscle biopsies were obtained before and following the 3-min severe-intensity exercise bout and again at task failure in the second bout. Breath-by-breath pulmonary gas exchange data were collected continuously throughout all step tests.
Training intervention.
Following the initial laboratory visits, subjects were allocated to one of the two SIT groups: SIT with PL supplementation (SIT+PL; age 25 ± 7 yr, height 174 ± 10 cm, body mass 73 ± 10 kg); SIT with BR supplementation (SIT+BR; age 24 ± 7 yr, height 174 ± 11 cm, body mass 78 ± 18 kg); or the nontraining group with BR supplementation (NT+BR; age 25 ± 7 yr, height 170 ± 6 cm, body mass 68 ± 9 kg). All three groups consisted of six male and six female subjects. Both SIT groups completed a total of 14 supervised training sessions over a 4-wk period, with at least 24 h separating each training session, while the NT group maintained their habitual exercise patterns. The postintervention laboratory tests were performed at least 48 h following, but within 4 days of, completing the final training session.
During the training sessions, the SIT groups completed a series of 30-s “all-out” sprints (i.e., Wingate test) against a resistance equivalent to 7.5% body mass on a mechanically braked ergometer (model 814E bicycle ergometer; Monark, Stockholm, Sweden; 11–13). Each sprint was separated by a 4-min period of rest in which subjects cycled at a low cadence against a light resistance to reduce venous pooling and sensations of nausea. During weeks 1 and 2 of training, subjects performed 4 × 30-s sprints three times per week, while during weeks 3 and 4, subjects performed 5 × 30-s sprints four times per week. Following a 5-min warm-up of cycling against a light resistance, subjects were given a 10-s countdown and instructed to pedal maximally for 2 s before the appropriate load was applied. Subjects were verbally encouraged to maintain maximal cadence throughout each 30-s sprint.
Measurements
Blood pressure and heart rate.
Before and following the intervention, as well as at the midintervention point, the BP at the brachial artery was measured using an automated sphygmomanometer (Dinamap Pro; GE Medical Systems, Tampa, FL). Following 10-min seated rest in an isolated room, three measurements were recorded. Mean arterial pressure (MAP) was calculated as 1/3 systolic pressure + 2/3 diastolic pressure. The means of the systolic pressure, diastolic pressure, and MAP measurements were used for data analysis.
Blood analysis.
Venous blood was sampled at rest (baseline) before each experimental test. Blood samples were also obtained at 1 min, at 3 min, and at task failure during the severe-intensity exercise bout. The blood samples collected during the severe-intensity exercise bout were drawn from a cannula (Insyte-WTM; Becton Dickinson, Madrid, Spain) inserted into the subject’s antecubital vein and were collected into lithium-heparin vacutainers (Becton Dickinson, Franklin Lakes, NJ). Blood [lactate] and [glucose], as well as plasma [] and [], were analyzed in all samples (square brackets denote concentration). Two hundred microliters of blood were immediately extracted from the lithium-heparin vacutainers and hemolyzed in 200 μl of Triton X-100 solution (Triton X-100; Amresco, Salon, OH) before blood [lactate] and [glucose] were measured (YSI 2300; Yellow Springs Instruments, Yellow Springs, OH). The remaining whole blood from each sample was centrifuged at 4,000 rpm for 8 min at 4°C within 2 min of collection. Plasma was immediately extracted, frozen at −80°C, and subsequently analyzed for [] and [] using chemiluminescence, as described by Wylie et al. (79).
Muscle biopsy.
Muscle samples were obtained from two incisions from the medial region of the vastus lateralis muscle under local anesthesia (1% lidocaine) using the percutaneous Bergström needle biopsy technique (7) with suction. Muscle samples were taken at three different time points before and following the intervention: at rest; following 3 min of severe-intensity exercise; and at task failure from severe-intensity exercise. The postexercise biopsies were taken while subjects remained on the cycle ergometer and were typically collected within 5–10 s of the completion of the exercise bout. Biopsy samples were immediately frozen in liquid nitrogen and stored at −80°C for subsequent analysis.
Muscle metabolites.
Following a freeze-drying process, samples were dissected to remove visible blood, fat, and connective tissue. Approximately 2 mg aliquots of isolated muscle fibers were weighed on fine-balance scales (Mettler Toledo XS105; Leicester, United Kingdom) and stored in 500-µl microcentrifuge tubes at −80°C. Prior to metabolite analysis, 200 µl of 3 M perchloric acid were added to ~2 mg dry wt muscle tissue. Following 3-min centrifugation and 30-min incubation on ice, 170 µl of supernatant were transferred to a fresh microcentrifuge tube, and 255 µl of cooled 2 M potassium bicarbonate (KHCO3) were added. This was centrifuged, and the supernatant was analyzed for [PCr], [ATP], and [lactate] by fluorometric assays as previously described (51).
Muscle glycogen and pH.
Glycogen was extracted from ~1 mg dry wt muscle in 500 μl of 1 M hydrochloric acid (HCl) and hydrolyzed at 100°C for 2 h to glycosyl units, which were measured using an automated glucose analyzer (YSI 2300; Yellow Springs Instruments) to determine muscle [glycogen]. Muscle pH was measured using a micro-pH meter (Sentron SI600; Roden, The Netherlands) following homogenization of ~1 mg dry wt muscle in a nonbuffering solution (145 mM KCl, 10 mM NaCl, and 5 mM NaF).
Muscle fiber type.
Approximately 20 mg of tissue obtained from each resting muscle biopsy sample were embedded in Tissue-Tek optimum cutting temperature compound (Sakura Finetek Europe, Zoeterwoude, The Netherlands), rapidly frozen in liquid nitrogen-cooled isopentane, and stored at −80°C for subsequent histochemical analysis of myocellular characteristics. Serial cross sections (~10 μM thick) were cut in a cryostat (Cryostar NX50; Thermo Scientific) maintained at −16°C. Sections were mounted on three separate slides and preincubated at pH values of 4.3, 4.6, and 10.3. According to the lability to the acid and alkaline preincubation, the fibers were stained for myofibrillar ATPase, identified as type I, IIa, or IIx (9) and counted under an Olympus CKX41 microscope with cellSens Dimension software (Olympus, Tokyo, Japan). For each subject, 214 ± 104 fibers were analyzed, and each fiber type was expressed as a percentage of the total number counted.
Oxygen uptake.
The breath-by-breath V̇o2 data from each step exercise test were initially examined to exclude values lying more than four SDs from the local mean. The filtered data were subsequently linearly interpolated to provide second-by-second values and time aligned to the start of exercise for each individual. The baseline V̇o2 was defined as the mean V̇o2 measured over the final 60 s of the 3-min baseline period. The end-exercise V̇o2 was defined as the mean V̇o2 measured over the final 60 s of exercise.
Statistical Analyses
Differences between groups in preintervention physiological and performance values were tested using a one-way ANOVA. Time-by-group ANOVAs with repeated measures for time were employed to determine the physiological and performance effects consequent to the interventions. In addition, one-way ANOVAs were used to assess differences between groups in the change values for physiological and performance variables preintervention to postintervention. All significant main and interaction effects were followed up by Fisher’s least significant difference post hoc tests. Data that were not normally distributed were log transformed before applying the ANOVA. All values are reported as means ± SD. Statistical significance was accepted at P < 0.05.
RESULTS
Compliance
All subjects within the training groups completed 100% of the training sessions and 100% of the sprints within each training session. All subjects reported that they fully adhered to the supplementation regimen and did not alter dietary and exercise behavior outside of their assigned group-specific intervention.
Plasma [] and []
Preintervention resting plasma [] values were not different between groups (P > 0.05). A significant main effect for time (P < 0.001) and an interaction effect (P < 0.001) were observed for the plasma [] measured at rest. Compared with preintervention, SIT+BR increased resting plasma [] by ~590% at 2 wk and ~960% at 4 wk (both P < 0.001; Fig. 1A), and NT+BR increased resting plasma [] by ~505% at 2 wk and ~1,050% at 4 wk (both P < 0.001; Fig. 1A), but there was no change in resting plasma [] with SIT+PL (P > 0.05; Fig. 1A). Resting plasma [] was also greater at 4 wk compared with 2 wk in both SIT+BR and NT+BR (P < 0.05; Fig. 1A).
Fig. 1.
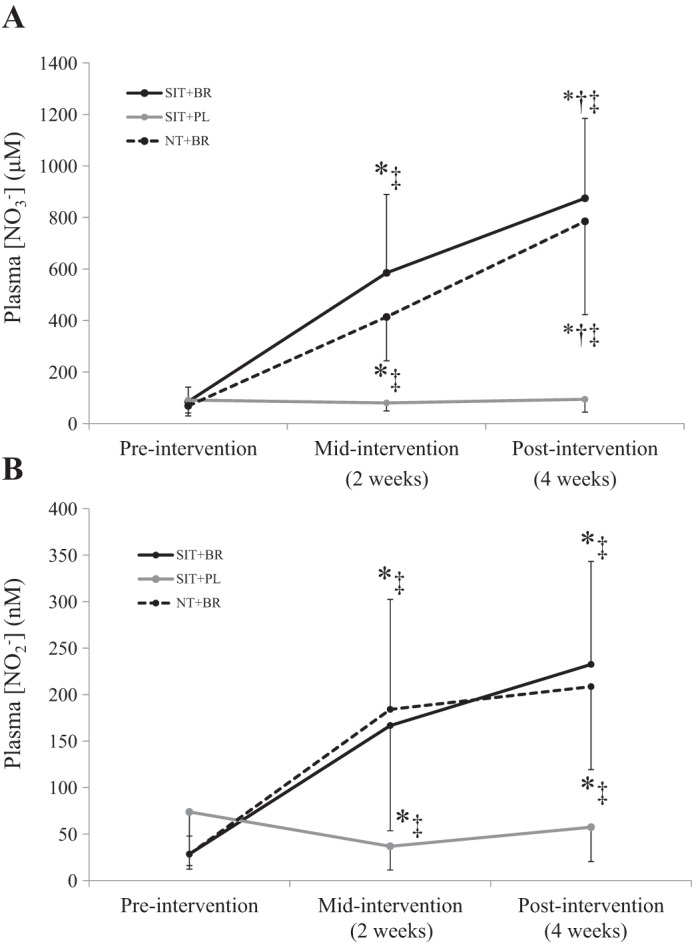
Mean ± SD resting plasma [] (A) and plasma [] (B) responses in SIT+BR (solid black line), SIT+PL (solid grey line), and NT+BR (dashed black line). *Different from preintervention (P < 0.05); †different from midintervention (P < 0.05); ‡different from SIT+PL (P < 0.05).
Preintervention resting plasma [] values were higher in SIT+PL (74 ± 62 nM) compared with SIT+BR (29 ± 19 nM; P < 0.05) and NT+BR (26 ± 13 nM; P < 0.05) but were similar between SIT+BR and NT+BR (P > 0.05). There was a significant main effect for time (P < 0.001) and an interaction effect (P < 0.001) for the plasma [] measured at rest. Compared with preintervention, SIT+BR increased resting plasma [] by ~485% at 2 wk and ~715% at 4 wk (both P < 0.001; Fig. 1B), and NT+BR increased resting plasma [] by ~600% at 2 wk and ~690% at 4 wk (both P < 0.001; Fig. 1B); however, there was no change in resting plasma [] with SIT+PL (P > 0.05; Fig. 1B). There were no differences in the plasma [] measured at rest between 2 and 4 wk in any of the groups (P > 0.05).
Blood Pressure
Systolic BP was not different between the groups before the interventions (P > 0.05; Table 1), but there was a significant main effect for time (P < 0.05) and an interaction effect (P < 0.05). Post hoc tests revealed that compared with preintervention, systolic BP was reduced at 2 and 4 wk (P < 0.05) by 5 ± 6 and 6 ± 4 mmHg, respectively, in SIT+BR (P < 0.05) and by 4 ± 5 and 10 ± 6 mmHg, respectively, in NT+BR (P < 0.05), whereas systolic BP remained unaltered in SIT+PL (P > 0.05; Table 1). Diastolic BP was not different between groups at preintervention (P > 0.05) and remained unaltered at 2 and 4 wk (both P > 0.05) in all interventions (Table 1). The MAP was not different between groups at preintervention but there was a significant main effect for time (P < 0.05) such that MAP was reduced by 3 ± 5 mmHg at 4 wk in both SIT+BR and NT+BR (P < 0.05) but was unchanged with SIT+PL (Table 1). Relative to postintervention resting baseline, plasma [] declined by ~65% at task failure during severe-intensity exercise (P < 0.001) in SIT+BR and NT+BR. The reduction in plasma [] following 3 min of severe-intensity exercise was greater in NT+BR compared with SIT+BR (P < 0.05).
Table 1.
Physiological and performance variables preintervention, midintervention, and postintervention
| SIT+PL |
SIT+BR |
NT+BR |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Pre | Mid | Post | Pre | Mid | Post | Pre | Mid | Post | |
| Blood pressure | |||||||||
| SBP, mmHg | 116 ± 13 | 115 ± 14 | 116 ± 10 | 118 ± 11 | 113 ± 11* | 112 ± 10*‡ | 117 ± 13 | 113 ± 10* | 107 ± 17*‡ |
| DBP, mmHg | 67 ± 8 | 64 ± 7 | 66 ± 4 | 67 ± 9 | 64 ± 9 | 62 ± 7 | 63 ± 5 | 63 ± 7 | 62 ± 8 |
| MAP, mmHg | 83 ± 8 | 81 ± 8 | 83 ± 6 | 84 ± 8 | 80 ± 9 | 79 ± 7* | 82 ± 7 | 80 ± 7 | 77 ± 7* |
| Incremental test | |||||||||
| Peak WR, W | 303 ± 78 | 306 ± 72 | 318 ± 73*† | 298 ± 93 | 305 ± 90* | 321 ± 91*† | 296 ± 66 | 295 ± 67 | 300 ± 67* |
| Δ peak WR, W | — | 4 ± 13 | 16 ± 15*†# | — | 7 ± 10*# | 24 ± 8*†#‡ | — | 0 ± 9 | 4 ± 4* |
| V̇o2peak, l/min | 3.43 ± 0.99 | 3.49 ± 0.97 | 3.50 ± 0.86 | 3.19 ± 1.03 | 3.39 ± 1.06* | 3.47 ± 1.02* | 3.28 ± 1.03 | 3.42 ± 0.99 | 3.42 ± 1.08 |
| V̇o2 at GET, l/min | 1.55 ± 0.49 | 1.49 ± 0.41 | 1.62 ± 0.44 | 1.60 ± 0.37 | 1.58 ± 0.37 | 1.64 ± 0.43 | 1.61 ± 0.46 | 1.62 ± 0.52 | 1.61 ± 0.4 |
| WR at GET, W | 110 ± 32 | 103 ± 34 | 112 ± 27 | 102 ± 30 | 105 ± 32 | 110 ± 27* | 105 ± 34 | 102 ± 29 | 112 ± 27 |
| Moderate-intensity exercise | |||||||||
| End-exercise V̇o2, l/min | 1.57 ± 0.41 | — | 1.67 ± 0.44 | 1.64 ± 0.41 | — | 1.58 ± 0.42*‡ | 1.73 ± 0.32 | — | 1.65 ± 0.34*‡ |
| Severe-intensity exercise | |||||||||
| Time to task failure, s | 297 ± 69 | — | 460 ± 186*# | 248 ± 53 | — | 418 ± 132*# | 266 ± 82 | — | 275 ± 84 |
Values are means ± SD. SIT+PL, high-intensity interval training plus -depleted beetroot juice; SIT+BR, high-intensity interval training plus -rich beetroot juice; NT+BR, no-training plus -rich beetroot juice. Pre, preintervention; mid, midintervention; post, postintervention; SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure; WR, work rate; V̇o2, oxygen uptake; V̇o2peak, peak V̇o2; GET, gas exchange threshold.
Different from preintervention (P < 0.05);
different from midintervention (P < 0.05);
different from NT+BR (P < 0.05);
different from SIT+PL (P < 0.05).
Incremental Exercise Test
Peak WR was not different between the groups at preintervention (P > 0.05; Table 1). There was a significant main effect by time (P < 0.001) and an interaction effect (P < 0.05). Post hoc tests revealed that peak WR was improved at 4 wk compared with preintervention in all groups (P < 0.05; Table 1). However, peak WR increased more from preintervention to postintervention in SIT+BR than in SIT+PL (P < 0.001; Fig. 2). Additionally, peak WR was improved at 2 wk compared with preintervention in SIT+BR only (P < 0.05; Fig. 2).
Fig. 2.
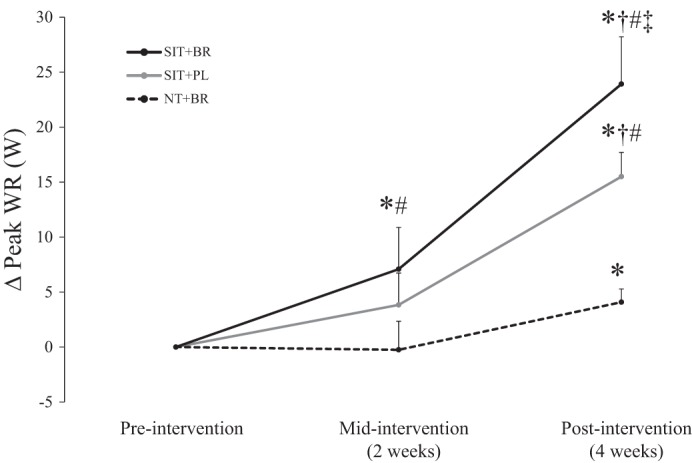
Mean ± SD changes (Δ) in peak WR at midintervention and postintervention in the three groups expressed relative to preintervention baseline. The change in peak WR from preintervention to postintervention was greater in SIT+BR (solid black line) than in SIT+PL (solid grey line) and NT+BR (dashed black line). *Different from preintervention (P < 0.05); †different from midintervention (P < 0.05); #different from NT+BR (P < 0.05); ‡different from SIT+PL (P < 0.05).
V̇o2peak was not different between the groups at preintervention (P > 0.05; Table 1). There was a significant main effect by time on V̇o2peak (P < 0.05). Post hoc analysis revealed that compared with preintervention, V̇o2peak was increased after 2 and 4 wk with SIT+BR (P < 0.05; Table 1) but remained unchanged in SIT+PL and NT+BR (P > 0.05; Table 1). However, there were no differences between the three groups in the change in V̇o2peak from preintervention to postintervention (P > 0.05). There were no significant changes in body mass from preintervention to postintervention in any of the groups.
The V̇o2 at the GET was not different between the groups at preintervention (P > 0.05; Table 1) and was not altered by any intervention (P > 0.05; Table 1). The WR associated with the GET was not different between groups at preintervention (P > 0.05; Table 1). There was a significant main effect for time such that the WR at the GET was increased preintervention to postintervention in SIT+BR only (P < 0.05; Table 1). However, there were no differences between the three groups in the change in the WR at the GET from preintervention to postintervention (P > 0.05).
Step Exercise Tests: Moderate-Intensity Exercise
The V̇o2 measured during baseline cycling at 20 W preceding the transition to moderate-intensity exercise was not different between groups at preintervention (P > 0.05; Table 1) and was not affected by any intervention (P > 0.05; Table 1). The end-exercise V̇o2 during moderate-intensity exercise was not different between groups at preintervention (P > 0.05; Table 1). There was a significant main effect by time (P < 0.05) and an interaction effect (P < 0.05) on end-exercise V̇o2. Post hoc analyses revealed that compared with preintervention, end-exercise V̇o2 was significantly reduced in SIT+BR (P < 0.05) and NT+BR (P < 0.05) but was unaltered in SIT+PL (P > 0.05; Table 1). There was no difference in the change in end-exercise V̇o2 from preintervention to postintervention between the SIT+BR and NT+BR groups (P > 0.05).
Step Exercise Tests: Severe-Intensity Exercise
The time to task failure during severe-intensity exercise was not different between groups at preintervention (P > 0.05; Table 1). There was a significant main effect by time (P < 0.05) and an interaction effect (P < 0.05) such that time to task failure was improved by 163 ± 144 s preintervention to postintervention in SIT+PL (P < 0.05; Table 1) and by 170 ± 90 s preintervention to postintervention in SIT+BR (P < 0.05; Table 1) but was unaltered by NT+BR (P > 0.05; Table 1). There was no difference in the change in the time to task failure from preintervention to postintervention between the SIT+BR and SIT+PL groups (P > 0.05).
Blood [lactate] was not different between groups during severe-intensity exercise at preintervention (P > 0.05). There was a main effect by time on blood [lactate] (P < 0.05). Post hoc analysis revealed that blood [lactate] was lower at 1 min (1.2 ± 1.1 mM decrease from same time point preintervention; P < 0.05; Fig. 3) and at 3 min (1.6 ± 1.5 mM decrease from same time point preintervention; P < 0.05, Fig. 3) during severe-intensity exercise in SIT+BR but not SIT+PL or NT+BR (P > 0.05). Further analyses revealed that the increase in blood [lactate] from rest to 3 min was attenuated postintervention compared with preintervention in SIT+BR (2.7 ± 0.9 vs. 3.9 ± 0.8 mM; P < 0.05). This attenuation was significantly greater than the equivalent change in blood [lactate] from rest to 3 min in SIT+PL (postintervention 4.1 ± 1.9 vs. preintervention 3.7 ± 1.2 mM; P < 0.05).
Fig. 3.
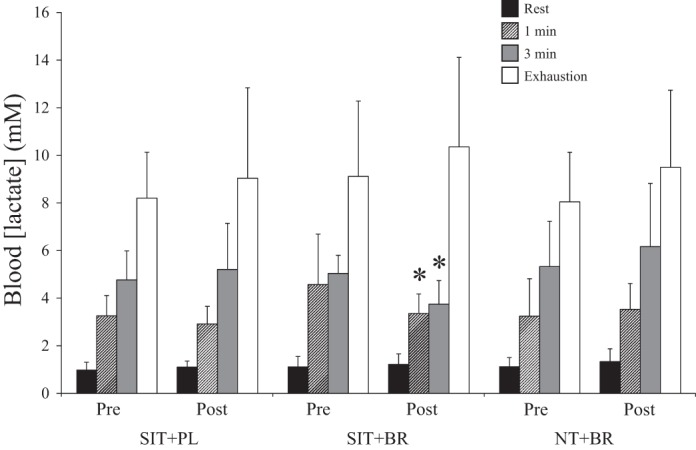
Mean ± SD blood [lactate] at rest (black bars), at 1 min (patterned bars), at 3 min (grey bars), and at task failure (open bars) during severe-intensity exercise. *Different from preintervention (P < 0.05).
Muscle Substrates and Metabolites
Preintervention values for muscle substrates and metabolites during severe-intensity exercise were not different between groups (P > 0.05), and muscle [ATP] and [PCr] were unchanged by the interventions in all groups (P > 0.05).
There were main effects by time on the muscle [lactate] and pH measured at 3 min of severe-intensity exercise (P < 0.05). Post hoc tests revealed that compared with preintervention, muscle [lactate] was lower and pH was higher at 3 min of severe-intensity exercise postintervention in SIT+BR (P < 0.05; Fig. 4). Further analyses revealed that compared with preintervention, the increase in muscle [lactate] and the decrease in muscle pH from rest to 3 min of exercise tended to be attenuated postintervention in SIT+BR (both P = 0.09).
Fig. 4.
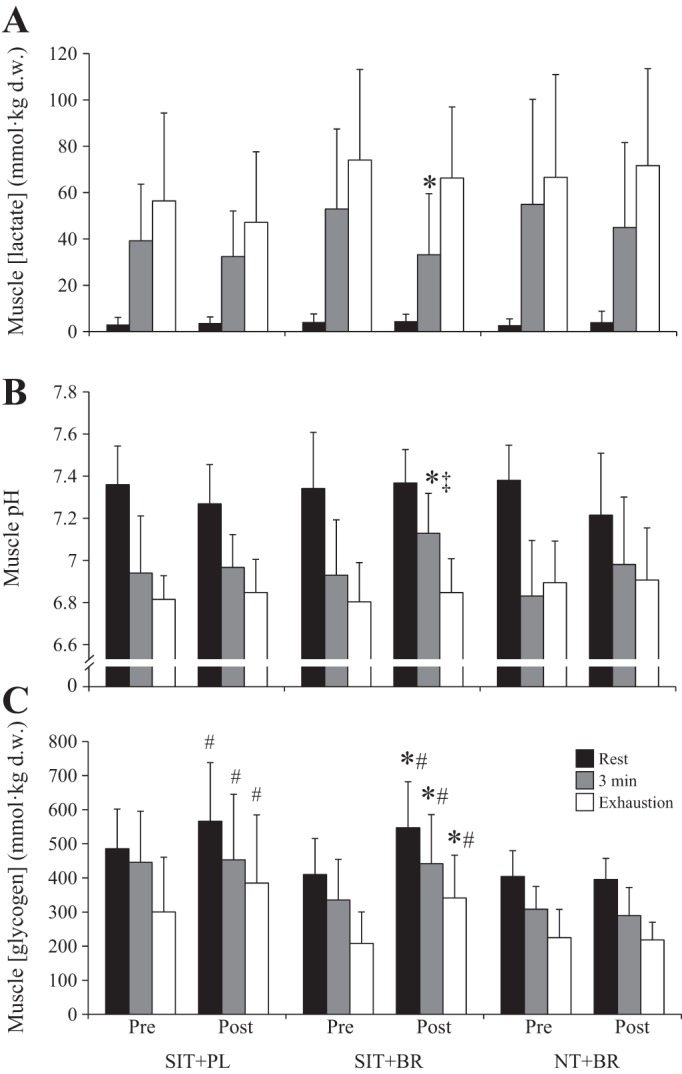
Mean ± SD muscle [lactate] (A), muscle pH (B), and muscle [glycogen] (C) at rest (black bars), at 3 min (grey bars), and at task failure (open bars) during severe-intensity exercise. d.w., Dry weight. *Different from preintervention (P < 0.05); #different from postintervention NT+BR (P < 0.05); ‡different from postintervention SIT+PL (P < 0.05).
There was a main effect by time on muscle [glycogen] measured at rest, at 3 min, and at task failure (all P < 0.05). Post hoc tests revealed that muscle [glycogen] was higher at all three time points postintervention compared with preintervention in SIT+BR (P < 0.05; Fig. 4C). Muscle [glycogen] was also higher at all three time points postintervention in SIT+BR and SIT+PL compared with NT+BR (P < 0.05; Fig. 4). There were no differences between SIT+BR and SIT+PL in the change in muscle [glycogen] from preintervention to postintervention at rest, at 3 min of exercise, or at task failure (P > 0.05).
Muscle Fiber Type
The relative proportion of type I (SIT+BR, 57 ± 16%; SIT+PL, 59 ± 10%; NT+BR, 48 ± 16%), type IIa (SIT+BR, 36 ± 12%; SIT+PL, 36 ± 16%; NT+BR, 44 ± 16%), and type IIx (SIT+BR, 7 ± 8%; SIT+PL, 5 ± 7%; NT+BR, 8 ± 12%) muscle fibers at preintervention was not different between groups (P > 0.05). There was a significant effect of time and an interaction effect on the proportion of type IIx fibers. Post hoc tests revealed that the proportion of type IIx fibers identified in SIT+BR was lower postintervention (4 ± 5%) compared with preintervention (7 ± 8%; P < 0.05). In contrast, the proportion of type IIx fibers identified in SIT+PL tended to be higher postintervention (10 ± 9%) compared with preintervention (5 ± 7%; P = 0.07). The change in type IIx fibers was significantly different in SIT+BR compared with SIT+PL (P < 0.05) but not NT+BR (P > 0.05). There were no differences in the proportion of type I (SIT+BR, 55 ± 12%; SIT+PL, 58 ± 10%; NT+BR, 50 ± 17%) or type IIa (SIT+BR, 41 ± 9%; SIT+PL, 32 ± 16%; NT+BR, 43 ± 14%) muscle fibers following any intervention (P > 0.05). However, there was a significant interaction effect on the proportion of type I and type IIa fibers combined (type I+IIa; P < 0.05). Post hoc tests revealed that the proportion of type I+IIa fibers identified in SIT+BR was higher postintervention (96 ± 6%) compared with preintervention (93 ± 8%; P < 0.05). In contrast, the proportion of type I+IIa fibers identified in SIT+PL tended to be lower postintervention (90 ± 9%) compared with preintervention (95 ± 7%; P = 0.07). The change in type I+IIa fibers was significantly different in SIT+BR and NT+BR compared with SIT+PL (P < 0.05).
DISCUSSION
This is the first study to investigate the combined effect of SIT and supplementation, administered in the form of beetroot juice, on muscle metabolic adaptations and the physiological responses to ramp incremental, moderate-intensity and severe-intensity exercise performance in normoxia. We compared the effects of chronic supplementation alone (NT+BR) with the effects of concurrent -rich (SIT+BR) and -depleted (SIT+PL) beetroot juice supplementation during a SIT intervention. Consistent with our hypotheses, the separate 4-wk interventions of SIT and chronic BR supplementation independently induced several beneficial physiological and/or performance effects. However, the main finding of the present study was that the combination of SIT and BR supplementation provided greater improvements in incremental exercise performance compared with either intervention alone and led to greater improvements in some indexes of muscle metabolic adaptation.
Plasma [] and [] were elevated, and systolic BP was lowered following 2 and 4 wk of BR supplementation, changes which are consistent with elevated systemic NO bioavailability. Interestingly, however, resting plasma [] and [] were not altered following 4 wk of SIT+PL. Previous studies have reported that subjects with higher aerobic fitness and/or training status have higher resting plasma [] and [] compared with less fit and/or sedentary subjects (53, 63). The results of the present study may therefore indicate that short-term SIT, at least when combined with PL supplementation, does not substantially modify nitric oxide synthase activity or protein expression. The reduction in plasma [] from resting baseline to task failure during severe-intensity exercise was similar between NT+BR and SIT+BR (~65% decline). However, the reduction in plasma [] from resting baseline to 3 min of severe-intensity exercise was attenuated in SIT+BR (~25% decline) compared with NT+BR (~45% decline). It is possible that this may be related to differences in training status induced by the separate interventions; for example, less reduction of to NO may have been required following SIT+BR because of training-related improvements in muscle capillarity and oxygenation (18).
The reductions in systolic BP (SIT+BR, −4 and −5%; NT+BR, −6 and −9%; at 2 and 4 wk, respectively) reported in the present study are similar to those previously reported in healthy volunteers following shorter supplementation periods (5, 44, 73, 74). Diastolic BP was unaltered in NT+BR and SIT+PL but was reduced by 7% in SIT+BR. MAP was lowered by ~4% in both -supplemented groups (SIT+BR and NT+BR) but was unaltered in SIT+PL. Collectively, these data indicate that 4 wk of supplementation may result in a greater reduction in BP than 4 wk of SIT alone.
Effect of SIT and BR on Submaximal V̇o2 and V̇o2peak
A high exercise economy, i.e., a low V̇o2 for a given power output, is an important determinant of exercise performance (33). It has been postulated that exercise training can lower the O2 cost of submaximal cycling (55). However, in the present study, the O2 cost of moderate-intensity exercise was only reduced following training in SIT+BR, and the magnitude of the reduction in the O2 cost of exercise was not different from that observed with NT+BR, suggesting that 4 wk of SIT per se has no influence on the O2 cost of submaximal cycling. This finding is consistent with previous work indicating that the O2 cost of exercise may be reduced by dietary supplementation (5, 45, 46, 73). The physiological bases for the improved efficiency following ingestion are likely related to a reduced ATP cost of muscle force production (6) and/or a reduced O2 cost of mitochondrial ATP resynthesis (46, cf. 76).
Despite the low training volume, SIT has emerged as a potent strategy to increase aerobic capacity and endurance exercise performance in as little as 2 wk (11, 65, 70). We found that V̇o2peak was not significantly altered by 4 wk of either NT+BR or SIT+PL. The former result is consistent with the majority of studies that have assessed V̇o2peak following acute or short-term supplementation (5, 6, 39, 45, 78). The lack of effect of SIT on V̇o2peak is also consistent with some (11–13, 26), but not all (65, 70), previous investigations. The physiological and muscle metabolic adaptations to SIT are likely dependent upon the initial training status of the subjects along with the exact nature of the training stimulus, including the frequency and duration of both the sprint and recovery periods (66). In this respect, it is important to highlight that our exercise-training protocol was shorter in duration compared with some studies (13) and the progression in training volume was more gradual than in other studies (4, 65) in which V̇o2peak was increased.
Although the change in V̇o2peak from preintervention to postintervention was not different between the three groups, the increase in V̇o2peak was only greater from preintervention to postintervention in SIT+BR, suggesting that supplementation may enhance the adaptation of V̇o2peak to SIT. Further work is required to confirm this observation and to elucidate the potential cardiovascular and/or metabolic mechanisms which may be responsible.
Effect of SIT and BR on Exercise Performance
The peak WR during incremental exercise at 4 wk was improved in both training groups. It is notable that peak WR was also significantly improved following NT+BR. Although this effect was small, it is consistent with an earlier study which reported a significant increase in peak WR during incremental exercise following 15 days of BR supplementation (73). A greater peak WR at 2 wk of training was only observed with SIT+BR. Moreover, the improvement in peak WR at 4 wk was greater in SIT+BR than in SIT+PL and NT+BR. The greater, and more rapidly attained, improvements in incremental exercise test performance with SIT+BR is presumably a function of the improved exercise economy and/or favorable muscle metabolic profile which would be expected to result in an extended time to reach V̇o2peak. Our results are consistent with a recent study by Muggeridge et al. (57) which reported that 3 wk of SIT (4–6 repeated 15-s sprints) increased peak WR during incremental exercise to a greater extent when subjects were supplemented with compared with placebo.
The time to task failure during severe-intensity exercise was significantly increased after 4 wk of both SIT+BR (group mean change +69%) and SIT+PL (+55%), but not NT+BR (+3%). Despite evidence for an enhanced muscle metabolic response to severe-intensity exercise in SIT+BR compared with SIT+PL (see below), this did not translate into a greater improvement in severe-intensity exercise performance. It is not clear why ramp incremental exercise test performance was improved with SIT+BR when time to task failure during severe-intensity exercise was not, although greater variability in time-to-exhaustion tests may have contributed to the difference (20). Indeed, it is interesting to note that the improvement in time to task failure ranged from 37 to 116% in SIT+BR (with 9/12 subjects improving by >50%) and from 4 to 122% in SIT+PL (with 4/12 subjects improving by >50%). Our results are similar to those of Puype et al. (64), who found that 6 wk of endurance training in normobaric hypoxia with BR supplementation did not improve 30-min time trial performance relative to the placebo condition. However, it remains unclear whether BR supplementation during training could improve performance in other types of exercise. Recent studies indicate that BR may be ergogenic during high-intensity intermittent exercise (2, 71, 78) and that compared with placebo, supplementation during SIT improves fatigue resistance during repeated sprint exercise (57) and may enhance mean power output in a 30-s sprint (21). Further studies are required to investigate whether the subtle enhancements of skeletal muscle adaptation to training with BR might translate into improved performance during these other forms of exercise.
Effect of SIT and BR on the Muscle Metabolic Response to Exercise
Although conflicting data exist, SIT has been implicated in rapid skeletal muscle remodeling (11–13, 26). The extreme perturbations in substrate availability and metabolite accumulation caused by repeated sprint efforts require substantial oxidative energy turnover to restore homeostasis (8). The fluctuations in ATP availability and local O2 tension are potent stimulators of signaling pathways and may induce mitochondrial biogenesis and oxidative enzyme adaptation via the transcription of PGC-1α (28, 31, 47). Recent findings indicate that dietary may favorably affect the contractility (30, 32) and perfusion (22, 23) of type II muscle fibers and reduce the energetic cost of muscle force production during high-intensity exercise (6, 24). Similar to SIT (3, 13, 48–50, 61, 62), elevating and NO bioavailability with chronic -rich BR supplementation may also stimulate the transcription of PGC-1α (43, 54, 58), a key regulator of mitochondrial biogenesis (77) and angiogenesis (1, 15). We therefore determined the effects of 4-wk SIT and 4-wk BR supplementation on the muscle metabolic responses during exercise and tested the hypothesis that these adaptations may be amplified when the interventions were combined.
There were no differences in muscle [ATP], [PCr], [lactate], or pH at rest or at task failure during severe-intensity exercise, postintervention compared with preintervention, in any group. However, at 3 min into severe-intensity exercise, there was evidence of reduced metabolic perturbation, postintervention compared with preintervention, in the SIT+BR group only. Specifically, muscle [lactate] as well as blood [lactate] was lower, and muscle pH was higher, at 3 min of severe-intensity exercise following SIT+BR but not SIT+PL or NT+BR (Figs. 3 and 4), suggesting an enhanced muscle metabolic adaptation to SIT when combined with BR supplementation.
The reason for the small difference in muscle acidosis at the 3-min exercise isotime with SIT+BR compared with SIT+PL is unclear. However, this may be the result of differences in exercise efficiency between the training groups. The lower O2 cost of exercise measured at the same submaximal work rate in SIT+BR would be expected to lower the physiological strain and potentially reduce substrate-level phosphorylation and lactate production during exercise (34). Furthermore, BR supplementation has been shown to elevate microvascular Po2 in type II muscles of exercising rats thus promoting O2 exchange between the capillary and the myocyte and enabling a better preservation of intramuscular homeostasis (22, 23). By better maintaining oxidative function, this mechanism may be important in delaying lactate accumulation during severe-intensity exercise, which is known to mandate an increased recruitment of type II fibers to sustain power output (42). While intake alone would be expected to promote some of these effects (e.g., a lower O2 cost of submaximal exercise in the NT+BR group in the present study), intake combined with training may synergistically improve the muscle metabolic response to severe-intensity exercise. In particular, the SIT+BR group evidenced improved exercise efficiency (which was observed with NT+BR but not SIT+PL) and improved performance and physiological responses/adaptations to maximal exercise (which were observed with SIT+PL but to a much lesser extent with NT+BR).
None of the interventions influenced the proportion of type I muscle fibers identified following training. Interestingly, there was a disparity in the muscle phenotypic response to training between SIT+BR and SIT+PL. Specifically, SIT+BR resulted in a significant reduction in the proportion of type IIx muscle fibers. In contrast, SIT+PL resulted in a trend toward a greater proportion of type IIx fibers following the intervention period. These results suggest that a remodeling of skeletal muscle toward a more oxidative phenotype following SIT (26, 27) may be facilitated by BR supplementation and perhaps hampered by PL supplementation. Our findings are consistent with a recent study which also reported changes in muscle fiber type composition following 5 wk of SIT with ~5-mmol daily supplementation (21). These authors reported that SIT performed in hypoxia resulted in a significant increase in the relative number of type IIa fibers in the vastus lateralis muscle (from ~45 to 56%) when subjects ingested compared with placebo. It is possible that the differences in the muscle metabolic or performance response to exercise following SIT when combined with compared with placebo supplementation (present study; 57) are related to changes in muscle fiber type composition, i.e., a greater reduction in type IIx fibers and/or a greater increase in type IIa fibers (present study; 21).
It is important to highlight that both the BR and PL supplements contain high concentrations of antioxidants including betacyacins and polyphenols (38, 67) which may potentially interfere with skeletal muscle adaptations to training (56, 60). It is possible, therefore, that the adaptations to training in the SIT+PL group were attenuated in the present study because of the simultaneous intake of antioxidants. However, it is also possible that the potential for chronic administration to enhance muscular adaptations and exercise performance with SIT was underestimated in the SIT+BR group for the same reason. On the other hand, it has recently been reported that BR supplementation increases hydrogen peroxide emission from the mitochondria, an effect that could promote redox signaling (76) and enhance training adaptations. Moreover, the combination of with antioxidants might promote the reduction of to NO and facilitate physiological effects (36). Further research should investigate the influence of alone (as NaNO3 or KNO3) and BR on the skeletal muscle adaptations to training. Our study design involved 4 wk of daily BR supplementation with the final dose being consumed on the morning of the postintervention laboratory tests. Our measurements therefore reflect the combined effects of chronic and acute BR (or PL) supplementation superimposed on exercise training. It has been reported recently that 4 wk of BR supplementation continue to exert physiological effects for at least 48 h following the cessation of supplementation (80). Future studies might therefore be designed to partition out the influence of chronic or BR supplementation (without additional acute supplementation) on the adaptations to training.
Conclusions
In the absence of training, chronic BR ingestion resulted in a significant reduction in the O2 cost of moderate-intensity exercise and a small but significant increase in peak WR. SIT+PL resulted in improvements in peak WR during incremental exercise and time to task failure during severe-intensity exercise. Greater changes in peak WR during incremental exercise were found with SIT+BR compared with SIT+PL and NT+BR. In addition, type IIx muscle fiber proportion was reduced, and at the 3-min isotime during severe-intensity exercise, muscle pH was higher and muscle (and blood) [lactate] was lower in SIT+BR only. These findings suggest that the independent physiological and performance effects of SIT and BR supplementation may be enhanced when these interventions are combined. Dietary supplementation in the form of BR may potentiate some exercise performance and muscle metabolic adaptations to SIT.
GRANTS
This study was supported by PepsiCo, Illinois, Grant 2012–1891470. J. Fulford’s salary was supported via a National Institute for Health Research grant.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
C.T., L.J.W., J.R.B., J.F., M.I.B., J.K., S.T.M., S.J.B., A.V., and A.M.J. performed experiments; C.T., L.J.W., J.R.B., J.F., A.V., and A.M.J. analyzed data; C.T., L.J.W., J.F., J.C., S.J.B., A.V., and A.M.J. interpreted results of experiments; C.T., L.J.W., and A.M.J. prepared figures; C.T., L.J.W., J.C., S.J.B., A.V., and A.M.J. drafted manuscript; C.T., L.J.W., J.R.B., J.F., M.I.B., J.K., S.T.M., J.C., S.J.B., A.V., and A.M.J. edited and revised manuscript; C.T., L.J.W., J.R.B., J.F., M.I.B., J.K., S.T.M., J.C., S.J.B., A.V., and A.M.J. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Chiara Gattoni, Tom Male, Charlie Dean, Scott Hobbs, Louis Bowers, and Taro Isidore for assisting during exercise testing and training. We also thank Prof. R. Hugh Morton for statistical advice.
REFERENCES
- 1.Arany Z, Foo SY, Ma Y, Ruas JL, Bommi-Reddy A, Girnun G, Cooper M, Laznik D, Chinsomboon J, Rangwala SM, Baek KH, Rosenzweig A, Spiegelman BM. HIF-independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-1α. Nature 451: 1008–1012, 2008. doi: 10.1038/nature06613. [DOI] [PubMed] [Google Scholar]
- 2.Aucouturier J, Boissière J, Pawlak-Chaouch M, Cuvelier G, Gamelin FX. Effect of dietary nitrate supplementation on tolerance to supramaximal intensity intermittent exercise. Nitric Oxide 49: 16–25, 2015. doi: 10.1016/j.niox.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 3.Baar K, Wende AR, Jones TE, Marison M, Nolte LA, Chen M, Kelly DP, Holloszy JO. Adaptations of skeletal muscle to exercise: rapid increase in the transcriptional coactivator PGC-1. FASEB J 16: 1879–1886, 2002. doi: 10.1096/fj.02-0367com. [DOI] [PubMed] [Google Scholar]
- 4.Bailey SJ, Wilkerson DP, Dimenna FJ, Jones AM. Influence of repeated sprint training on pulmonary O2 uptake and muscle deoxygenation kinetics in humans. J Appl Physiol (1985) 106: 1875–1887, 2009. doi: 10.1152/japplphysiol.00144.2009. [DOI] [PubMed] [Google Scholar]
- 5.Bailey SJ, Winyard P, Vanhatalo A, Blackwell JR, Dimenna FJ, Wilkerson DP, Tarr J, Benjamin N, Jones AM. Dietary nitrate supplementation reduces the O2 cost of low-intensity exercise and enhances tolerance to high-intensity exercise in humans. J Appl Physiol (1985) 107: 1144–1155, 2009. doi: 10.1152/japplphysiol.00722.2009. [DOI] [PubMed] [Google Scholar]
- 6.Bailey SJ, Fulford J, Vanhatalo A, Winyard PG, Blackwell JR, DiMenna FJ, Wilkerson DP, Benjamin N, Jones AM. Dietary nitrate supplementation enhances muscle contractile efficiency during knee-extensor exercise in humans. J Appl Physiol (1985) 109: 135–148, 2010. doi: 10.1152/japplphysiol.00046.2010. [DOI] [PubMed] [Google Scholar]
- 7.Bergström J. Muscle electrolytes in man. Scand J Clin Lab Invest 14: 511–513, 1962. [Google Scholar]
- 8.Børsheim E, Bahr R. Effect of exercise intensity, duration and mode on post-exercise oxygen consumption. Sports Med 33: 1037–1060, 2003. doi: 10.2165/00007256-200333140-00002. [DOI] [PubMed] [Google Scholar]
- 9.Brooke MH, Kaiser KK. Muscle fiber types: how many and what kind? Arch Neurol 23: 369–379, 1970. doi: 10.1001/archneur.1970.00480280083010. [DOI] [PubMed] [Google Scholar]
- 10.Brown GC, Cooper CE. Nanomolar concentrations of nitric oxide reversibly inhibit synaptosomal respiration by competing with oxygen at cytochrome oxidase. FEBS Lett 356: 295–298, 1994. doi: 10.1016/0014-5793(94)01290-3. [DOI] [PubMed] [Google Scholar]
- 11.Burgomaster KA, Hughes SC, Heigenhauser GJF, Bradwell SN, Gibala MJ. Six sessions of sprint interval training increases muscle oxidative potential and cycle endurance capacity in humans. J Appl Physiol (1985) 98: 1985–1990, 2005. doi: 10.1152/japplphysiol.01095.2004. [DOI] [PubMed] [Google Scholar]
- 12.Burgomaster KA, Heigenhauser GJF, Gibala MJ. Effect of short-term sprint interval training on human skeletal muscle carbohydrate metabolism during exercise and time-trial performance. J Appl Physiol (1985) 100: 2041–2047, 2006. doi: 10.1152/japplphysiol.01220.2005. [DOI] [PubMed] [Google Scholar]
- 13.Burgomaster KA, Howarth KR, Phillips SM, Rakobowchuk M, Macdonald MJ, McGee SL, Gibala MJ. Similar metabolic adaptations during exercise after low volume sprint interval and traditional endurance training in humans. J Physiol 586: 151–160, 2008. doi: 10.1113/jphysiol.2007.142109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cermak NM, Gibala MJ, van Loon LJ. Nitrate supplementation’s improvement of 10-km time-trial performance in trained cyclists. Int J Sport Nutr Exerc Metab 22: 64–71, 2012. doi: 10.1123/ijsnem.22.1.64. [DOI] [PubMed] [Google Scholar]
- 15.Chinsomboon J, Ruas J, Gupta RK, Thom R, Shoag J, Rowe GC, Sawada N, Raghuram S, Arany Z. The transcriptional coactivator PGC-1α mediates exercise-induced angiogenesis in skeletal muscle. Proc Natl Acad Sci U S A 106: 21401–21406, 2009. doi: 10.1073/pnas.0909131106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Christensen PM, Nyberg M, Bangsbo J. Influence of nitrate supplementation on VO2 kinetics and endurance of elite cyclists. Scand J Med Sci Sports 23: e21–e31, 2013. doi: 10.1111/sms.12005. [DOI] [PubMed] [Google Scholar]
- 17.Cleeter MW, Cooper JM, Darley-Usmar VM, Moncada S, Schapira AH. Reversible inhibition of cytochrome c oxidase, the terminal enzyme of the mitochondrial respiratory chain, by nitric oxide. Implications for neurodegenerative diseases. FEBS Lett 345: 50–54, 1994. doi: 10.1016/0014-5793(94)00424-2. [DOI] [PubMed] [Google Scholar]
- 18.Cocks M, Shaw CS, Shepherd SO, Fisher JP, Ranasinghe AM, Barker TA, Tipton KD, Wagenmakers AJ. Sprint interval and endurance training are equally effective in increasing muscle microvascular density and eNOS content in sedentary males. J Physiol 591: 641–656, 2013. doi: 10.1113/jphysiol.2012.239566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coggan AR, Leibowitz JL, Kadkhodayan A, Thomas DP, Ramamurthy S, Spearie CA, Waller S, Farmer M, Peterson LR. Effect of acute dietary nitrate intake on maximal knee extensor speed and power in healthy men and women. Nitric Oxide 48: 16–21, 2015. doi: 10.1016/j.niox.2014.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Currell K, Jeukendrup AE. Validity, reliability and sensitivity of measures of sporting performance. Sports Med 38: 297–316, 2008. doi: 10.2165/00007256-200838040-00003. [DOI] [PubMed] [Google Scholar]
- 21.De Smet S, Van Thienen R, Deldicque L, James R, Sale C, Bishop DJ, Hespel P. Nitrate intake promotes shift in muscle fiber type composition during sprint interval training in hypoxia. Front Physiol 7: 233, 2016. doi: 10.3389/fphys.2016.00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferguson SK, Hirai DM, Copp SW, Holdsworth CT, Allen JD, Jones AM, Musch TI, Poole DC. Impact of dietary nitrate supplementation via beetroot juice on exercising muscle vascular control in rats. J Physiol 591: 547–557, 2013. doi: 10.1113/jphysiol.2012.243121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferguson SK, Holdsworth CT, Wright JL, Fees AJ, Allen JD, Jones AM, Musch TI, Poole DC. Microvascular oxygen pressures in muscles comprised of different fiber types: Impact of dietary nitrate supplementation. Nitric Oxide 48: 38–43, 2015. doi: 10.1016/j.niox.2014.09.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fulford J, Winyard PG, Vanhatalo A, Bailey SJ, Blackwell JR, Jones AM. Influence of dietary nitrate supplementation on human skeletal muscle metabolism and force production during maximum voluntary contractions. Pflugers Arch 465: 517–528, 2013. doi: 10.1007/s00424-013-1220-5. [DOI] [PubMed] [Google Scholar]
- 25.Gavin TP, Spector DA, Wagner H, Breen EC, Wagner PD. Nitric oxide synthase inhibition attenuates the skeletal muscle VEGF mRNA response to exercise. J Appl Physiol (1985) 88: 1192–1198, 2000. [DOI] [PubMed] [Google Scholar]
- 26.Gibala MJ, Little JP, van Essen M, Wilkin GP, Burgomaster KA, Safdar A, Raha S, Tarnopolsky MA. Short-term sprint interval versus traditional endurance training: similar initial adaptations in human skeletal muscle and exercise performance. J Physiol 575: 901–911, 2006. doi: 10.1113/jphysiol.2006.112094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gibala MJ, Gillen JB, Percival ME. Physiological and health-related adaptations to low-volume interval training: influences of nutrition and sex. Sports Med 44, Suppl 2: S127–S137, 2014. doi: 10.1007/s40279-014-0259-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gjøvaag TF, Dahl HA. Effect of training with different intensities and volumes on muscle fibre enzyme activity and cross sectional area in the m. triceps brachii. Eur J Appl Physiol 103: 399–409, 2008. doi: 10.1007/s00421-008-0725-7. [DOI] [PubMed] [Google Scholar]
- 29.Govoni M, Jansson EÅ, Weitzberg E, Lundberg JO. The increase in plasma nitrite after a dietary nitrate load is markedly attenuated by an antibacterial mouthwash. Nitric Oxide 19: 333–337, 2008. doi: 10.1016/j.niox.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 30.Haider G, Folland JP. Nitrate supplementation enhances the contractile properties of human skeletal muscle. Med Sci Sports Exerc 46: 2234–2243, 2014. doi: 10.1249/MSS.0000000000000351. [DOI] [PubMed] [Google Scholar]
- 31.Henriksson J, Reitman JS. Quantitative measures of enzyme activities in type I and type II muscle fibres of man after training. Acta Physiol Scand 97: 392–397, 1976. doi: 10.1111/j.1748-1716.1976.tb10279.x. [DOI] [PubMed] [Google Scholar]
- 32.Hernández A, Schiffer TA, Ivarsson N, Cheng AJ, Bruton JD, Lundberg JO, Weitzberg E, Westerblad H. Dietary nitrate increases tetanic [Ca2+]i and contractile force in mouse fast-twitch muscle. J Physiol 590: 3575–3583, 2012. doi: 10.1113/jphysiol.2012.232777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jones AM, Burnley M. Oxygen uptake kinetics: an underappreciated determinant of exercise performance. Int J Sports Physiol Perform 4: 524–532, 2009. doi: 10.1123/ijspp.4.4.524. [DOI] [PubMed] [Google Scholar]
- 34.Jones AM. Influence of dietary nitrate on the physiological determinants of exercise performance: a critical review. Appl Physiol Nutr Metab 39: 1019–1028, 2014. doi: 10.1139/apnm-2014-0036. [DOI] [PubMed] [Google Scholar]
- 35.Jones AM, Ferguson SK, Bailey SJ, Vanhatalo A, Poole DC. Fiber type-specific effects of dietary nitrate. Exerc Sport Sci Rev 44: 53–60, 2016. doi: 10.1249/JES.0000000000000074. [DOI] [PubMed] [Google Scholar]
- 36.Jonvik KL, Nyakayiru J, Pinckaers PJ, Senden JM, van Loon LJ, Verdijk LB. Nitrate-rich vegetables increase plasma nitrate and nitrite concentrations and lower blood pressure in healthy adults. J Nutr 146: 986–993, 2016. doi: 10.3945/jn.116.229807. [DOI] [PubMed] [Google Scholar]
- 37.Kang C, Chung E, Diffee G, Ji LL. Exercise training attenuates aging-associated mitochondrial dysfunction in rat skeletal muscle: role of PGC-1α. Exp Gerontol 48: 1343–1350, 2013. doi: 10.1016/j.exger.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 38.Kazimierczak R, Hallmann E, Lipowski J, Drela N, Kowalik A, Püssa T, Matt D, Luik A, Gozdowski D, Rembiałkowska E. Beetroot (Beta vulgaris L.) and naturally fermented beetroot juices from organic and conventional production: metabolomics, antioxidant levels and anticancer activity. J Sci Food Agric 94: 2618–2629, 2014. doi: 10.1002/jsfa.6722. [DOI] [PubMed] [Google Scholar]
- 39.Kelly J, Fulford J, Vanhatalo A, Blackwell JR, French O, Bailey SJ, Gilchrist M, Winyard PG, Jones AM. Effects of short-term dietary nitrate supplementation on blood pressure, O2 uptake kinetics, and muscle and cognitive function in older adults. Am J Physiol Regul Integr Comp Physiol 304: R73–R83, 2013. doi: 10.1152/ajpregu.00406.2012. [DOI] [PubMed] [Google Scholar]
- 40.Kelly J, Vanhatalo A, Bailey SJ, Wylie LJ, Tucker C, List S, Winyard PG, Jones AM. Dietary nitrate supplementation: effects on plasma nitrite and pulmonary O2 uptake dynamics during exercise in hypoxia and normoxia. Am J Physiol Regul Integr Comp Physiol 307: R920–R930, 2014. doi: 10.1152/ajpregu.00068.2014. [DOI] [PubMed] [Google Scholar]
- 41.Kingwell BA. Nitric oxide-mediated metabolic regulation during exercise: effects of training in health and cardiovascular disease. FASEB J 14: 1685–1696, 2000. doi: 10.1096/fj.99-0896rev. [DOI] [PubMed] [Google Scholar]
- 42.Krustrup P, Söderlund K, Mohr M, Bangsbo J. The slow component of oxygen uptake during intense, sub-maximal exercise in man is associated with additional fibre recruitment. Pflugers Arch 447: 855–866, 2004. doi: 10.1007/s00424-003-1203-z. [DOI] [PubMed] [Google Scholar]
- 43.Lanza IR, Sreekumaran Nair K. Regulation of skeletal muscle mitochondrial function: genes to proteins. Acta Physiol (Oxf) 199: 529–547, 2010. doi: 10.1111/j.1748-1716.2010.02124.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Larsen FJ, Ekblom B, Sahlin K, Lundberg JO, Weitzberg E. Effects of dietary nitrate on blood pressure in healthy volunteers. N Engl J Med 355: 2792–2793, 2006. doi: 10.1056/NEJMc062800. [DOI] [PubMed] [Google Scholar]
- 45.Larsen FJ, Weitzberg E, Lundberg JO, Ekblom B. Effects of dietary nitrate on oxygen cost during exercise. Acta Physiol (Oxf) 191: 59–66, 2007. doi: 10.1111/j.1748-1716.2007.01713.x. [DOI] [PubMed] [Google Scholar]
- 46.Larsen FJ, Schiffer TA, Borniquel S, Sahlin K, Ekblom B, Lundberg JO, Weitzberg E. Dietary inorganic nitrate improves mitochondrial efficiency in humans. Cell Metab 13: 149–159, 2011. doi: 10.1016/j.cmet.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 47.Lin J, Wu H, Tarr PT, Zhang CY, Wu Z, Boss O, Michael LF, Puigserver P, Isotani E, Olson EN, Lowell BB, Bassel-Duby R, Spiegelman BM. Transcriptional co-activator PGC-1 α drives the formation of slow-twitch muscle fibres. Nature 418: 797–801, 2002. doi: 10.1038/nature00904. [DOI] [PubMed] [Google Scholar]
- 48.Little JP, Safdar A, Cermak N, Tarnopolsky MA, Gibala MJ. Acute endurance exercise increases the nuclear abundance of PGC-1α in trained human skeletal muscle. Am J Physiol Regul Integr Comp Physiol 298: R912–R917, 2010. doi: 10.1152/ajpregu.00409.2009. [DOI] [PubMed] [Google Scholar]
- 49.Little JP, Safdar A, Wilkin GP, Tarnopolsky MA, Gibala MJ. A practical model of low-volume high-intensity interval training induces mitochondrial biogenesis in human skeletal muscle: potential mechanisms. J Physiol 588: 1011–1022, 2010. doi: 10.1113/jphysiol.2009.181743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Little JP, Safdar A, Bishop D, Tarnopolsky MA, Gibala MJ. An acute bout of high-intensity interval training increases the nuclear abundance of PGC-1α and activates mitochondrial biogenesis in human skeletal muscle. Am J Physiol Regul Integr Comp Physiol 300: R1303–R1310, 2011. doi: 10.1152/ajpregu.00538.2010. [DOI] [PubMed] [Google Scholar]
- 51.Lowry OH, Passonneau JV. A Flexible System of Enzymatic Analysis. New York: Academic, 1972. [Google Scholar]
- 52.Lundberg JO, Weitzberg E, Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov 7: 156–167, 2008. doi: 10.1038/nrd2466. [DOI] [PubMed] [Google Scholar]
- 53.Maeda S, Miyauchi T, Kakiyama T, Sugawara J, Iemitsu M, Irukayama-Tomobe Y, Murakami H, Kumagai Y, Kuno S, Matsuda M. Effects of exercise training of 8 weeks and detraining on plasma levels of endothelium-derived factors, endothelin-1 and nitric oxide, in healthy young humans. Life Sci 69: 1005–1016, 2001. doi: 10.1016/S0024-3205(01)01192-4. [DOI] [PubMed] [Google Scholar]
- 54.Mo L, Wang Y, Geary L, Corey C, Alef MJ, Beer-Stolz D, Zuckerbraun BS, Shiva S. Nitrite activates AMP kinase to stimulate mitochondrial biogenesis independent of soluble guanylate cyclase. Free Radic Biol Med 53: 1440–1450, 2012. doi: 10.1016/j.freeradbiomed.2012.07.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Montero D, Lundby C. The effect of exercise training on the energetic cost of cycling. Sports Med 45: 1603–1618, 2015. doi: 10.1007/s40279-015-0380-1. [DOI] [PubMed] [Google Scholar]
- 56.Morrison D, Hughes J, Della Gatta PA, Mason S, Lamon S, Russell AP, Wadley GD. Vitamin C and E supplementation prevents some of the cellular adaptations to endurance-training in humans. Free Radic Biol Med 89: 852–862, 2015. doi: 10.1016/j.freeradbiomed.2015.10.412. [DOI] [PubMed] [Google Scholar]
- 57.Muggeridge DJ, Sculthorpe N, James PE, Easton C. The effects of dietary nitrate supplementation on the adaptations to sprint interval training in previously untrained males. J Sci Med Sport 20: 92–97, 2017. doi: 10.1016/j.jsams.2016.04.014. [DOI] [PubMed] [Google Scholar]
- 58.Nisoli E, Clementi E, Paolucci C, Cozzi V, Tonello C, Sciorati C, Bracale R, Valerio A, Francolini M, Moncada S, Carruba MO. Mitochondrial biogenesis in mammals: the role of endogenous nitric oxide. Science 299: 896–899, 2003. doi: 10.1126/science.1079368. [DOI] [PubMed] [Google Scholar]
- 59.Olesen J, Kiilerich K, Pilegaard H. PGC-1α-mediated adaptations in skeletal muscle. Pflugers Arch 460: 153–162, 2010. doi: 10.1007/s00424-010-0834-0. [DOI] [PubMed] [Google Scholar]
- 60.Peternelj TT, Coombes JS. Antioxidant supplementation during exercise training: beneficial or detrimental? Sports Med 41: 1043–1069, 2011. doi: 10.2165/11594400-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 61.Pilegaard H, Ordway GA, Saltin B, Neufer PD. Transcriptional regulation of gene expression in human skeletal muscle during recovery from exercise. Am J Physiol Endocrinol Metab 279: E806–E814, 2000. [DOI] [PubMed] [Google Scholar]
- 62.Pilegaard H, Saltin B, Neufer PD. Exercise induces transient transcriptional activation of the PGC-1alpha gene in human skeletal muscle. J Physiol 546: 851–858, 2003. doi: 10.1113/jphysiol.2002.034850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Poveda JJ, Riestra A, Salas E, Cagigas ML, López-Somoza C, Amado JA, Berrazueta JR. Contribution of nitric oxide to exercise-induced changes in healthy volunteers: effects of acute exercise and long-term physical training. Eur J Clin Invest 27: 967–971, 1997. doi: 10.1046/j.1365-2362.1997.2220763.x. [DOI] [PubMed] [Google Scholar]
- 64.Puype J, Ramaekers M, Van Thienen R, Deldicque L, Hespel P. No effect of dietary nitrate supplementation on endurance training in hypoxia. Scand J Med Sci Sports 25: 234–241, 2015. doi: 10.1111/sms.12199. [DOI] [PubMed] [Google Scholar]
- 65.Rodas G, Ventura JL, Cadefau JA, Cussó R, Parra J. A short training programme for the rapid improvement of both aerobic and anaerobic metabolism. Eur J Appl Physiol 82: 480–486, 2000. doi: 10.1007/s004210000223. [DOI] [PubMed] [Google Scholar]
- 66.Ross A, Leveritt M. Long-term metabolic and skeletal muscle adaptations to short-sprint training: implications for sprint training and tapering. Sports Med 31: 1063–1082, 2001. doi: 10.2165/00007256-200131150-00003. [DOI] [PubMed] [Google Scholar]
- 67.Shepherd AI, Gilchrist M, Winyard PG, Jones AM, Hallmann E, Kazimierczak R, Rembialkowska E, Benjamin N, Shore AC, Wilkerson DP. Effects of dietary nitrate supplementation on the oxygen cost of exercise and walking performance in individuals with type 2 diabetes: a randomized, double-blind, placebo-controlled crossover trial. Free Radic Biol Med 86: 200–208, 2015. doi: 10.1016/j.freeradbiomed.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 68.Smith LW, Smith JD, Criswell DS. Involvement of nitric oxide synthase in skeletal muscle adaptation to chronic overload. J Appl Physiol (1985) 92: 2005–2011, 2002. doi: 10.1152/japplphysiol.00950.2001. [DOI] [PubMed] [Google Scholar]
- 69.Stamler JS, Meissner G. Physiology of nitric oxide in skeletal muscle. Physiol Rev 81: 209–237, 2001. [DOI] [PubMed] [Google Scholar]
- 70.Talanian JL, Galloway SD, Heigenhauser GJ, Bonen A, Spriet LL. Two weeks of high-intensity aerobic interval training increases the capacity for fat oxidation during exercise in women. J Appl Physiol (1985) 102: 1439–1447, 2007. doi: 10.1152/japplphysiol.01098.2006. [DOI] [PubMed] [Google Scholar]
- 71.Thompson C, Wylie LJ, Fulford J, Kelly J, Black MI, McDonagh ST, Jeukendrup AE, Vanhatalo A, Jones AM. Dietary nitrate improves sprint performance and cognitive function during prolonged intermittent exercise. Eur J Appl Physiol 115: 1825–1834, 2015. doi: 10.1007/s00421-015-3166-0. [DOI] [PubMed] [Google Scholar]
- 72.van Faassen EE, Bahrami S, Feelisch M, Hogg N, Kelm M, Kim-Shapiro DB, Kozlov AV, Li H, Lundberg JO, Mason R, Nohl H, Rassaf T, Samouilov A, Slama-Schwok A, Shiva S, Vanin AF, Weitzberg E, Zweier J, Gladwin MT. Nitrite as regulator of hypoxic signaling in mammalian physiology. Med Res Rev 29: 683–741, 2009. doi: 10.1002/med.20151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vanhatalo A, Bailey SJ, Blackwell JR, DiMenna FJ, Pavey TG, Wilkerson DP, Benjamin N, Winyard PG, Jones AM. Acute and chronic effects of dietary nitrate supplementation on blood pressure and the physiological responses to moderate-intensity and incremental exercise. Am J Physiol Regul Integr Comp Physiol 299: R1121–R1131, 2010. doi: 10.1152/ajpregu.00206.2010. [DOI] [PubMed] [Google Scholar]
- 74.Webb AJ, Patel N, Loukogeorgakis S, Okorie M, Aboud Z, Misra S, Rashid R, Miall P, Deanfield J, Benjamin N, MacAllister R, Hobbs AJ, Ahluwalia A. Acute blood pressure lowering, vasoprotective, and antiplatelet properties of dietary nitrate via bioconversion to nitrite. Hypertension 51: 784–790, 2008. doi: 10.1161/HYPERTENSIONAHA.107.103523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Whipp BJ, Davis JA, Torres F, Wasserman K. A test to determine parameters of aerobic function during exercise. J Appl Physiol Respir Environ Exerc Physiol 50: 217–221, 1981. [DOI] [PubMed] [Google Scholar]
- 76.Whitfield J, Ludzki A, Heigenhauser GJ, Senden JM, Verdijk LB, van Loon LJ, Spriet LL, Holloway GP. Beetroot juice supplementation reduces whole body oxygen consumption but does not improve indices of mitochondrial efficiency in human skeletal muscle. J Physiol 594: 421–435, 2016. doi: 10.1113/JP270844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC, Spiegelman BM. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 98: 115–124, 1999. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]
- 78.Wylie LJ, Mohr M, Krustrup P, Jackman SR, Ermιdis G, Kelly J, Black MI, Bailey SJ, Vanhatalo A, Jones AM. Dietary nitrate supplementation improves team sport-specific intense intermittent exercise performance. Eur J Appl Physiol 113: 1673–1684, 2013. doi: 10.1007/s00421-013-2589-8. [DOI] [PubMed] [Google Scholar]
- 79.Wylie LJ, Kelly J, Bailey SJ, Blackwell JR, Skiba PF, Winyard PG, Jeukendrup AE, Vanhatalo A, Jones AM. Beetroot juice and exercise: pharmacodynamic and dose-response relationships. J Appl Physiol (1985) 115: 325–336, 2013. doi: 10.1152/japplphysiol.00372.2013. [DOI] [PubMed] [Google Scholar]
- 80.Wylie LJ, Ortiz de Zevallos J, Isidore T, Nyman L, Vanhatalo A, Bailey SJ, Jones AM. Dose-dependent effects of dietary nitrate on the oxygen cost of moderate-intensity exercise: Acute vs. chronic supplementation. Nitric Oxide 57: 30–39, 2016. doi: 10.1016/j.niox.2016.04.004. [DOI] [PubMed] [Google Scholar]


