Abstract
Arguments exist as to the cause of chronic fatigue syndrome (CFS). Some think that it is an example of symptom amplification indicative of functional or psychogenic illness, while our group thinks that some CFS patients may have brain dysfunction. To further pursue our encephalopathy hypothesis, we did spinal taps on 31 women and 13 men fulfilling the 1994 case definition for CFS and on 8 women and 5 men serving as healthy controls. Our outcome measures were white blood cell count, protein concentration in spinal fluid, and cytokines detectable in spinal fluid. We found that significantly more CFS patients had elevations in either protein levels or number of cells than healthy controls (30 versus 0%), and 13 CFS patients had protein levels and cell numbers that were higher than laboratory norms; patients with abnormal fluid had a lower rate of having comorbid depression than those with normal fluid. In addition, of the 11 cytokines detectable in spinal fluid, (i) levels of granulocyte-macrophage colony-stimulating factor were lower in patients than controls, (ii) levels of interleukin-8 (IL-8) were higher in patients with sudden, influenza-like onset than in patients with gradual onset or in controls, and (iii) IL-10 levels were higher in the patients with abnormal spinal fluids than in those with normal fluid or controls. The results support two hypotheses: that some CFS patients have a neurological abnormality that may contribute to the clinical picture of the illness and that immune dysregulation within the central nervous system may be involved in this process.
Chronic fatigue syndrome (CFS) is a medically unexplained illness characterized by severe and often disabling fatigue accompanied by infectious, rheumatological, and neuropsychiatric symptoms. One hypothesis driving a good deal of CFS research is that the illness stems from immunological dysfunction. The logic for this hypothesis is twofold: first, a substantial proportion of CFS patients have a sudden, influenza-like illness onset, and second, the symptoms of CFS are produced in healthy people given cytokines (7, 18). Unfortunately, there are few carefully controlled data to support this hypothesis. In two plasma cytokine studies using classical statistical methods, we found no differences in immune variables between CFS patients and sedentary, age- and sex-matched controls (10, 17). In fact, a recent comprehensive analysis of all controlled studies on immune function in CFS found little evidence to support the immune dysfunction hypothesis (16).
In parallel work, we have reported data suggesting that some CFS patients have neurological dysfunction. Specifically, we reported that patients with a sudden illness onset (4) as well as those with no history of psychiatric diagnosis (3) had more evidence of cognitive dysfunction than other CFS groups. Moreover, we showed that CFS patients with no history of psychiatric diagnosis had more abnormalities on brain magnetic resonance imaging (MRI) (11) and that those patients with MRI abnormalities had poorer physical function than those without these abnormalities (2). These results suggested that the abnormalities were not merely epiphenomena of CFS but instead contributed to the patients' symptom burden.
We reasoned that if some CFS patients had their illness on a neurological basis, we would find abnormalities either in spinal fluid and/or in spinal fluid cytokines. This report provides some evidence for both of these hypotheses.
MATERIALS AND METHODS
Subjects were 31 women and 13 men who fulfilled the 1994 case definition for CFS (5) and 8 female and 5 male healthy controls. All subjects signed informed consent forms approved by the New Jersey Medical School's Institutional Review Board. The patients were all self- or physician-referred to our center, and control subjects responded to media advertisements or community-wide postings. All subjects completed the multidimensional fatigue inventory (20) and then underwent a careful history and physical examination by a licensed health care professional expert in evaluating patients with medically unexplained fatigue and pain. A note was made of whether the illness onset was sudden (i.e., flu-like) or gradual. Patients had a set of blood tests done to rule out common causes of severe fatigue, such as anemia, liver disease, hypothyroidism, human immunodeficiency virus, lupus, etc. All subjects then underwent a psychiatric diagnostic interview designed to identify major psychiatric diagnoses (15). None of the healthy controls was positive for psychiatric disease, and none took medicines other than birth control pills. Eleven of the patients were not taking medicines. No subject in either group had a history of loss of consciousness.
Subjects then underwent lumbar puncture. Fluid was sent to the laboratory for white blood cell (WBC) counts and quantification of protein concentration. Aliquots were frozen for later analysis of the following cytokines: interleukin-1α (IL-1α), IL-1β, IL-3, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12p40, IL-12p70, IL-13, IL-15, tumor necrosis factor alpha, gamma interferon (IFN-γ), granulocyte-macrophage colony-stimulating factor (GM-CSF), RANTES, and monocyte chemoattractant protein 1. We chose to survey a broad range of cytokines here because of the dearth of data on their levels in spinal fluid for both CFS patients as well as for healthy controls. We were able to measure so many cytokines in only 50 μl of spinal fluid because of the recent development of the Luminex multiplex bead technology and a reagent set (Beadlyte 48-011 human 22-plex cytokine detection system) available from Upstate USA, Inc. (Charlottesville, Va.). We validated this kit for measuring cytokines in spinal fluid by spiking spinal fluid with known amounts of each of the cytokines and showing good recoveries and parallelism with the standard curves.
Data analysis and statistics.
The first study outcome was the frequency of abnormal spinal fluid. We defined “abnormal” based on laboratory norms (i.e., WBC counts greater than 4 cells/μl or protein values greater than 45 mg/dl). The second study outcome was the identification of cytokines detectable in spinal fluid. IL-2, IL-3, IL-4, IL-12p70, IL-13, IFN-γ, and tumor necrosis factor alpha were undetectable in spinal fluid. The following cytokines were above the minimal detectable levels in only some subjects: IL-1α (26%), IL-1β (2%), IL-7 (57%), IL-12p40 (52%), and RANTES (63%). Whether these cytokines were detectable or not was randomly distributed across patients and controls. IL-5, IL-6, IL-8, IL-10, IL-15, GM-CSF, and monocyte chemoattractant protein 1 were present in the spinal fluid of every subject. Statistical analysis of cytokine levels was confined to those that were detected in at least 26% of the samples.
In looking for cytokine differences between CFS patients and controls, we began with several a priori rules. We required data, corrected for age and sex, to differ significantly at the 0.05 level between controls and CFS patients or to differ among controls and CFS subgroups stratified by mode of illness onset, presence or absence of Axis I psychopathology, or presence or absence of spinal fluid abnormality.
General linear regression models were used in these analyses. Variables were checked for normality and homoscedasticity; some variables were log transformed when necessary. We also performed other simple statistical tests: chi-square tests were used for the comparison of rates between groups of interest, t tests or analyses of variance were used to compare means between groups of interest, and Pearson product moment correlation coefficients were computed to determine the relation between two continuous variables.
RESULTS
Except for two patients with unsteadiness on closed-eye, tandem Romberg, neurological examination was normal for all subjects. Healthy controls were significantly younger than CFS patients (33.0 ± 10.5 years versus 41.4 ± 8.0 years; P < 0.01). None of the taps was traumatic. No healthy volunteer had more than 3 WBCs/μl, and protein levels were 40 mg/dl or below. Of the 44 CFS patients, 4 patients had normal protein levels with elevated numbers of WBCs (6, 7, 9, and 20 WBCs/mm); 1 patient had an elevated protein level of 67 mg/dl and 5 WBCs/μl; and 8 patients had elevated protein levels alone (range, 46 to 93 mg/dl; median level from all 8 patients with elevations = 51 mg/dl). Thus, 13 of 44 patients (30%) had spinal fluid cell counts or protein values outside of our laboratory's normal range. None of the controls showed any abnormalities (Fisher's test comparing rates in patients to those in controls, one-tailed, P = 0.02). Six subjects, all in the CFS group, developed post-spinal tap headaches; only one of these had an abnormal spinal fluid result.
Age correlated significantly with protein level for the controls (r = 0.55; P = 0.05) but not for the entire group of CFS patients (r = 0.08) nor for the subgroups with normal or abnormal spinal fluid protein levels (r = −0.17 and 0.11, respectively). CFS patients had higher levels of spinal fluid protein than healthy controls (36.8 ± 14.1 versus 26.4 ± 7.5 mg/dl; P < 0.01). This difference remained significant (P = 0.05) after adjusting for age. There was no significant correlation between age and white blood cell count for the healthy and/or CFS patients.
There was no significant difference in age between CFS patients with normal and abnormal spinal fluids. There were no significant differences between patients with normal and abnormal spinal fluids in the rate of sudden illness onset (32.3 versus 23.1%) or in the magnitude of general fatigue as assessed by the multidimensional fatigue inventory (18.8 ± 1.6 versus 18.8 ± 1.5). Rates of current major depression were lower in those patients with abnormal spinal fluids than in those with normal spinal fluids (0 versus 27%; one-tailed, P < 0.04), but rates of lifetime depression or overall Axis I diagnoses did not differ between the groups (46.2 versus 48.4% and 69.2 versus 51.6%, respectively).
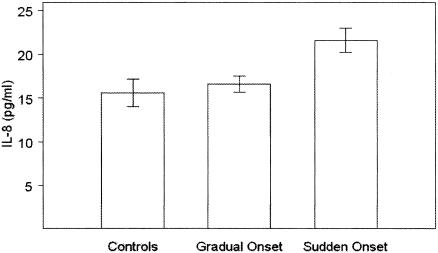
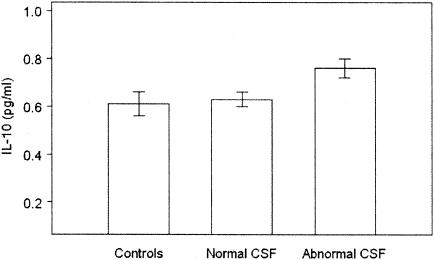
After adjustment for age and gender, none of the cytokines except for GM-CSF, IL-8, and IL-10 differed significantly between controls and CFS patients or among controls and CFS subgroups stratified by mode of illness onset, presence or absence of Axis I psychopathology, or presence or absence of spinal fluid abnormality. Levels of GM-CSF were significantly lower in CFS patients than in controls (3.2 ± 0.5 [standard error] versus 5.7 ± 1.0 pg/ml; P < 0.03). Levels of IL-8 were significantly higher in CFS patients with sudden onset than in patients with gradual onset or in controls (P < 0.007 for both comparisons) (Fig. 1). Levels of IL-10 were significantly higher in CFS patients with abnormal spinal fluid than in those with normal spinal fluid or in controls (P < 0.025 for both comparisons) (Fig. 2).
FIG. 1.
IL-8 levels in controls and CFS patients with gradual and sudden illness onset.
FIG. 2.
The level of IL-10 was significantly higher in CFS patients with abnormal spinal fluid than in those with normal spinal fluid or in controls (P < 0.025 for both comparisons).
After adjustment for age, spinal fluid protein level correlated significantly with IL-10 level (r = 0.38; P = 0.002) but not with levels of IL-8 or GM-CSF. White blood cell count was significantly associated with IL-10 level (r = 0.30, P = 0.05) for patients only but not with IL-8 or GM-CSF levels for either patients or controls. The correlations of IL-10 with spinal fluid protein and cell count are consistent with the elevated levels of this cytokine in CFS patients with abnormal spinal fluid compared to those with normal spinal fluid.
DISCUSSION
Two diametrically opposite explanations exist for illnesses such as CFS. One takes the position that these illnesses are “functional somatic syndromes” occurring in people with no disease but with heightened concerns about otherwise normal symptoms (1). Another position, which we hold, is that CFS is a heterogeneous illness, with some patients having a neurological cause to their symptoms. The data presented here support our position. Thirty percent of CFS patients undergoing lumbar puncture had elevations in protein levels and/or in white blood cell count relative to laboratory norms; in contrast, no control had such elevations.
Our prior work suggested that stratifying based on a history of psychiatric diagnosis was useful in identifying CFS patients with neurological abnormalities. Specifically, the subgroup of patients with no history of having had a psychiatric disorder was the group with the most brain MRI abnormalities (11) and the most abnormalities on neuropsychological testing (3). We interpreted those data as suggesting the presence of encephalopathy in this subgroup of patients. The work reported here provides some additional support for that stratification strategy in that the group of patients with abnormalities in their spinal fluid had a lower rate of having current depression than those with normal spinal fluids.
Because patients with CFS have many of the same symptoms as healthy people infused with cytokines (7, 18), one hypothesis for the cause of CFS is the existence of immune dysfunction. However, two groups have reviewed the literature and have concluded that insufficient evidence exists to support that hypothesis (14, 16). If some CFS patients had an unrecognized encephalopathy, we reasoned that we might find evidence for immune dysfunction in spinal fluid. An earlier study comparing CFS patients to others undergoing myelography did report elevations in levels of IFN-α (13).
Extending the results of that earlier study, we did find evidence for cytokine differences between our patient and healthy control groups. First, we found that CFS patients as a group had lower levels of GM-CSF than controls. Next, the subgroup with sudden illness onset, as would occur following a viral infection, had increases in IL-8, a cytokine previously reported to be present at high levels in the plasma of patients with fibromyalgia, an illness which overlaps with CFS (6, 21); however, for the patients whose results are reported here, the presence or absence of comorbid fibromyalgia did not alter the outcome. In addition, the patient subgroup with abnormal spinal fluid had elevated levels of IL-10 in spinal fluid. Finding elevations in cytokines in patients with viral-type illness onset and in others with abnormal spinal fluid supports our working hypothesis that some CFS patients have their illness due to a neurological problem.
It is possible that these cytokine abnormalities could interact in a way that could lead to brain dysfunction. GM-CSF is a major hematopoietic regulator governing the functions of granulocyte and macrophage lineage populations at all stages of maturation, as well as playing an important role in maintaining chronic inflammation (8). Low levels of this important growth factor in the presence of IL-8 and IL-10 can lead to a downregulated immune response to certain kinds of bacterial infections (12). Although speculative, it is possible that the elevated levels of IL-10 found here are responsible for downregulation of GM-CSF in these patients. Supporting this hypothesis is a report showing that IL-10 inhibits GM-CSF-dependent monocyte survival and macrophage generation (9). The role of IL-8 is less clear but may be contributory.
How these alterations in cytokine levels lead to the severe fatigue and widespread pain consistent with the diagnosis of CFS requires further research. However, the finding of reduced levels of GM-CSF with increases in IL-8 in some patients and in IL-10 in others does support the hypothesis that some of the symptoms of CFS may be due to immune dysfunction within the central nervous system. A recent study showing elevations of IL-8 and IL-10 levels during chemotherapy-induced symptoms resembling some of those seen in CFS provides additional evidence for this hypothesis (19).
Acknowledgments
This work was supported by NIH Center grant AI-32247.
We acknowledge the thoughtful comments of Neil Cherniack in writing this paper.
REFERENCES
- 1.Barsky, A. J., and J. F. Borus. 1999. Functional somatic syndromes. Ann. Intern. Med. 130:910-921. [DOI] [PubMed] [Google Scholar]
- 2.Cook, D. B., G. Lange, J. DeLuca, and B. H. Natelson. 2001. Relationship of brain MRI abnormalities and physical functional status in CFS. Int. J. Neurosci. 107:1-6. [DOI] [PubMed] [Google Scholar]
- 3.DeLuca, J., S. K. Johnson, S. P. Ellis, and B. H. Natelson. 1997. Cognitive functioning is impaired in chronic fatigue syndrome patients devoid of psychiatric disease. J. Neurol. Neurosurg. Psychiatry 62:151-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DeLuca, J., S. K. Johnson, S. P. Ellis, and B. H. Natelson. 1997. Sudden vs gradual onset of chronic fatigue syndrome differentiates individuals on cognitive and psychiatric measures. J. Psychiatr. Res. 31:83-90. [DOI] [PubMed] [Google Scholar]
- 5.Fukuda, K., S. E. Straus, I. Hickie, M. C. Sharpe, A. Komaroff, A. Schluederberg, J. F. Jones, A. R. Lloyd, S. Wessely, N. G. Gantz, G. P. Holmes, L. Steele, M. Reyes, S. Abbey, J. Rest, H. Jolson, D. L. Peterson, J. H. M. M. Vercoulen, U. Tirelli, B. Evengard, B. H. Natelson, and W. C. Reeves. 1994. The chronic fatigue syndrome: a comprehensive approach to its definition and study. Ann. Intern. Med. 121:953-959. [DOI] [PubMed] [Google Scholar]
- 6.Gür, A., M. Karakoç, K. Nas, R. Çevik, A. Denli, and J. Saraç. 2002. Cytokines and depression in cases with fibromyalgia. J. Rheumatol. 29:358-361. [PubMed] [Google Scholar]
- 7.Hamblin, T. J. 1990. Interleukin 2: side effects are acceptable. BMJ 300:275-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamilton, J. A. 2002. GM-CSF in inflammation and autoimmunity. Trends Immunol. 23:403-408. [DOI] [PubMed] [Google Scholar]
- 9.Hashimoto, S. I., I. Komuro, M. Yamada, and K. S. Akagawa. 2001. IL-10 inhibits granulocyte-macrophage colony-stimulating factor-dependent human monocyte survival at the early stage of the culture and inhibits the generation of macrophages. J. Immunol. 167:3619-3625. [DOI] [PubMed] [Google Scholar]
- 10.LaManca, J. J., S. Sisto, J. E. Ottenweller, S. Cook, A. Peckerman, Q. Zhang, T. N. Denny, W. C. Gause, and B. H. Natelson. 1999. Immunological response in chronic fatigue syndrome following a graded exercise test to exhaustion. J. Clin. Immunol. 19:135-142. [DOI] [PubMed] [Google Scholar]
- 11.Lange, G., J. DeLuca, J. A. Maldjian, H. J. Lee, L. A. Tiersky, and B. H. Natelson. 1999. Brain MRI abnormalities exist in a subset of patients with chronic fatigue syndrome. J. Neurol. Sci. 171:3-7. [DOI] [PubMed] [Google Scholar]
- 12.Lee, E. H., and Y. Rikihisa. 1996. Absence of tumor necrosis factor alpha, interleukin-6 (IL-6), and granulocyte-macrophage colony-stimulating factor expression but presence of IL-1β, IL-8, and IL-10 expression in human monocytes exposed to viable or killed Ehrlichia chaffeensis. Infect. Immun. 64:4211-4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lloyd, A., I. Hickie, A. Brockman, J. Dwyer, and D. Wakefield. 1991. Cytokine levels in serum and cerebrospinal fluid in patients with chronic fatigue syndrome and control subjects. J. Infect. Dis. 164:1023-1024. [DOI] [PubMed] [Google Scholar]
- 14.Lyall, M., M. Peakman, and S. Wessely. 2003. A systematic review and critical evaluation of the immunology of chronic fatigue syndrome. J. Psychosom. Res. 55:79-90. [DOI] [PubMed] [Google Scholar]
- 15.Marcus, S., L. N. Robins, and K. Bucholz. 1990. Quick diagnostic interview schedule 3R version 1. Washington University School of Medicine, St. Louis, Mo.
- 16.Natelson, B. H., M. H. Haghighi, and N. M. Ponzio. 2002. Evidence for the presence of immune dysfunction in chronic fatigue syndrome. Clin. Diagn. Lab. Immunol. 9:747-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Natelson, B. H., J. J. LaManca, T. N. Denny, A. Vladutiu, J. Oleske, N. Hill, M. T. Bergen, L. Korn, and J. Hay. 1998. Immunologic parameters in chronic fatigue syndrome, major depression, and multiple sclerosis. Am. J. Med. 105:43S-49S. [DOI] [PubMed] [Google Scholar]
- 18.Pavol, M. A., C. A. Meyers, J. L. Rexer, A. D. Valentine, P. J. Mattis, and M. Talpaz. 1995. Pattern of neurobehavioral deficits associated with interferon alfa therapy for leukemia. Neurology 45:947-950. [DOI] [PubMed] [Google Scholar]
- 19.Pusztai, L., T. R. Mendoza, J. M. Reuben, M. M. Martinez, J. S. Willey, J. Lara, A. Syed, H. A. Fritsche, E. Bruera, D. Booser, V. Valero, B. Arun, N. Ibrahim, E. Rivera, M. Royce, C. S. Cleeland, and G. N. Hortobagyi. 2004. Changes in plasma levels of inflammatory cytokines in response to paclitaxel chemotherapy. Cytokine 25:94-102. [DOI] [PubMed] [Google Scholar]
- 20.Smets, E. M. A., B. Garssen, B. Bonke, and J. C. J. M. De Haes. 1995. The Multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. J. Psychosom. Res. 39:315-325. [DOI] [PubMed] [Google Scholar]
- 21.Wallace, D. J., M. Linker-Israeli, D. Hallegue, S. Silverman, D. Silver, and M. H. Weisman. 2001. Cytokines play an aetiopathogenetic role in fibromyalgia: a hypothesis and pilot study. Rheumatology 40:743-749. [DOI] [PubMed] [Google Scholar]