Histamine appears to be intimately involved with skeletal muscle during and following exercise. Blocking histamine’s actions during muscle-damaging exercise, via common over-the-counter antihistamines, resulted in increased serum creatine kinase, an indirect marker of muscle damage. Paradoxically, blocking histamine’s actions attenuated muscle strength loss and reduced perceptions of muscle pain for 72 h following muscle-damaging exercise. These results indicate that exercise-induced histamine release may have a broad impact on protecting muscle from exercise-induced damage.
Keywords: inflammation, antihistamines, delayed-onset muscle soreness, muscle damage, recovery from exercise
Abstract
Histamine contributes to elevations in skeletal muscle blood flow following exercise, which raises the possibility that histamine is an important mediator of the inflammatory response to exercise. We examined the influence of antihistamines on postexercise blood flow, inflammation, muscle damage, and delayed-onset muscle soreness (DOMS) in a model of moderate exercise-induced muscle damage. Subjects consumed either a combination of fexofenadine and ranitidine (blockade, n = 12) or nothing (control, n = 12) before 45 min of downhill running (−10% grade). Blood flow to the leg was measured before and throughout 120 min of exercise recovery. Markers of inflammation, muscle damage, and DOMS were obtained before and at 0, 6, 12, 24, 48, and 72 h postexercise. At 60 min postexercise, blood flow was reduced ~29% with blockade compared with control (P < 0.05). Markers of inflammation were elevated after exercise (TNF-ɑ, IL-6), but did not differ between control and blockade. Creatine kinase concentrations peaked 12 h after exercise, and the overall response was greater with blockade (18.3 ± 3.2 kU·l−1·h−1) compared with control (11.6 ± 2.0 kU·l−1·h−1; P < 0.05). Reductions in muscle strength in control (−19.3 ± 4.3% at 24 h) were greater than blockade (−7.8 ± 4.8%; P < 0.05) and corresponded with greater perceptions of pain/discomfort in control compared with blockade. In conclusion, histamine-receptor blockade reduced postexercise blood flow, had no effect on the pattern of inflammatory markers, increased serum creatine kinase concentrations, attenuated muscle strength loss, and reduced pain perception following muscle-damaging exercise.
NEW & NOTEWORTHY Histamine appears to be intimately involved with skeletal muscle during and following exercise. Blocking histamine’s actions during muscle-damaging exercise, via common over-the-counter antihistamines, resulted in increased serum creatine kinase, an indirect marker of muscle damage. Paradoxically, blocking histamine’s actions attenuated muscle strength loss and reduced perceptions of muscle pain for 72 h following muscle-damaging exercise. These results indicate that exercise-induced histamine release may have a broad impact on protecting muscle from exercise-induced damage.
in healthy humans, blood flow to skeletal muscle is matched to metabolism during rest and exercise, but, following aerobic exercise, it exceeds metabolism (14). Increased blood flow following exercise, known as sustained postexercise vasodilation, results primarily from activation of histamine H1 and H2 receptors on the arterial endothelial cells and vascular smooth muscle (25, 27, 28). Sustained postexercise vasodilation is largely restricted to the previously active skeletal muscle vasculature, where it can persist for ≥2 h after exercise cessation (2, 16). The origin of histamine in active skeletal muscle tissue has been hypothesized to be from mast cell degranulation, or it may be created de novo through histidine decarboxylase (15), while the exercise-related stimulus for histamine release or production is unknown.
Histamine is believed to be involved with the initial stages of inflammation following muscle damage (26), initiating margination of capillary endothelial cells and increasing capillary permeability (21) and permitting neutrophil and macrophage infiltration for breakdown and repair of damaged muscle fibers (36, 45). Therefore, it is possible that histamine-mediated postexercise vasodilation may be associated with histamine-induced margination of capillary endothelium, allowing immune cells to participate in muscle repair and remodeling.
In addition to neutrophil and macrophage infiltration, the histamine-related inflammatory response is speculated to increase capillary permeability, leading to increased capillary plasma filtration, muscle volume, and intramuscular pressure (26). Delayed-onset muscle soreness (DOMS), the perception of discomfort and pain that follows muscle-damaging exercise, is thought to be caused by 1) phagocytosis of muscle tissue by neutrophils and macrophages, and/or 2) increased intramuscular pressure-sensitizing perceptions of pain through group III and IV afferent nerve fibers (45). Of note, group III and IV afferent nerves express histamine H1 and H2 receptors, which, when activated, lower the activation threshold of these nerves (11, 30, 35). Thus it is possible that blocking the actions of histamine may be effective in decreasing the perception of DOMS by 1) reducing immune cell and fluid infiltration, and/or 2) blocking group III and IV afferent nerve sensitization by histamine. However, a consequence, if immune cell infiltration is reduced, might be impaired or delayed muscle cell repair.
Despite evidence for a role of histamine in sustained postexercise vasodilation, it is unknown if histamine has an effect on skeletal muscle blood flow during exercise. If histamine-receptor blockade results in a reduction of exercising blood flow without a concomitant increase in extraction, it may increase the metabolic stress of the exercising muscle and potentially result in elevated muscle damage.
These potential interactions between histamine receptors, muscle damage, inflammatory responses, and the accompanying symptoms of muscle damage (DOMS) following exercise have not been systematically examined. Therefore, the purpose of this study was to examine the effect of histamine-receptor blockade on sustained postexercise blood flow, inflammation, muscle damage, and DOMS following an acute bout of muscle-damaging exercise. It was hypothesized that, in comparison to a control condition, histamine H1- and H2-receptor blockade would attenuate sustained postexercise blood flow, reduce inflammation, and result in increased muscle damage and accompanying symptoms of DOMS for up to 72 h following downhill running, a model of aerobic exercise-induced muscle damage.
METHODS
Subjects
This study was approved by the Institutional Review Board of the University of Oregon. Each volunteer gave written and informed consent before participation, and the study conformed to the principles of the Declaration of Helsinki. Twenty healthy (7 women, 13 men), nonsmoking individuals volunteered for the present study. Four male subjects completed the protocol twice, once as a member of the control group (n = 12), and once as a member of the blockade group (n = 12). They were treated as unique and separate individuals for each completion of either the control and blockade trials. All volunteers were sedentary or recreationally active based on exercise habits over the previous 12 mo (22) and maximal oxygen uptake (V̇o2max) measures (8). No subjects were using over-the-counter or prescription medications at the time of the study, with the exception of oral contraceptives. Female participants were studied during the early follicular phase of their menstrual cycle or during the placebo phase of their oral contraceptive to minimize any potential cardiovascular effects of sex-specific hormones.
Screening Visit
Subjects completed an initial screening visit that was intended to familiarize the subjects with all testing procedures and to obtain demographic and anthropometric information (age, height, weight), including measurement of three-site, skin-fold, body-fat measurements (triceps, suprailiac, and midthigh for women; chest, abdominal, midthigh for men), according to the American College of Sports Medicine guidelines (8). In addition, subjects performed an incremental treadmill exercise test composed of 2‐min workload increments (a modified Bruce protocol) to determine V̇o2max. Specifically, after a warm‐up period of easy jogging, subjects self-selected a treadmill speed, which was then held constant as grade was increased by 2% every 2 min up to 10%. Subsequently, speed was increased every 2 min. Selection of the speed increment was based on self‐reported subject activity levels, with the goal of producing exhaustion within 15 min. Whole body oxygen uptake (V̇o2) was measured via a mixing chamber system (Parvomedics, Sandy, UT) integrated with a mass spectrometry system (Marquette MGA 1100, MA Tech Services, St. Louis, MO). Maximal aerobic power was determined when subjects were unable to maintain the speed of the treadmill, had obtained a respiratory exchange ratio of >1.15, and/or had reached subjective exhaustion [rating of perceived exertion on the Borg scale of 19–20 (4)]. The V̇o2max test was performed for estimation of speed for the downhill running protocol.
Study Design
The study consisted of six study visits completed over the course of 72 h, as depicted in Fig. 1. All volunteers were required to abstain from caffeine, alcohol, and exercise for 24 h before and for the duration of the study. Participants reported to the laboratory at 0800 after an overnight fast. After a confirmed negative pregnancy test for women, subjects were randomly assigned to one of two groups: a control group (control), or a histamine-receptor blockade group (blockade). The blockade group consumed 540 mg of fexofenadine (a selective H1-receptor antagonist) and 300 mg ranitidine (a selective H2-receptor antagonist) orally with ~6 oz of water, ~60 min before the start of exercise. The control group consumed only water. Neither the experimenters nor the subjects were blinded to the condition, control or blockade. Next, measurements of quadriceps isometric strength, limb circumference, pressure point algometry, visual analog scale of thigh pain/discomfort, knee and hip range of motion, and a venous blood sample were obtained. Subjects then rested in the supine position, and baseline measurements of femoral blood flow were made 30 min before exercise. Exercise consisted of 45 min of downhill running at a −10% grade. Following exercise, a venous blood sample was obtained. Femoral blood flow was measured every 15 min for 2 h. Then measurements of quadriceps isometric strength, limb circumference, pressure point algometry, visual analog scale of thigh pain/discomfort, as well as knee and hip range of motion were obtained. Follow-up study visits occurred 6, 12, 24, 48, and 72 h after the completion of downhill running. The purposes of the follow-up visits were to assess markers of muscle damage, inflammation, and indexes of DOMS. During the follow-up visits, measurements of quadriceps isometric strength, limb circumference, pressure point algometry, visual analog scale of thigh pain/discomfort, as well as knee and hip range of motion were performed, and a venous blood sample was obtained.
Fig. 1.
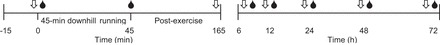
Study time line. The main portion of the study was conducted over 72 h. Each arrow (⇓) represents a time at which quadriceps isometric strength, limb circumference, pressure point algometry, visual analog scale of pain/discomfort, as well as knee and hip flexibility were measured. The blood drop (●) indicates a time point at which venous blood was sampled. During the first 120 min of postexercise recovery (45-165 min on the timeline), blood flow was assessed every 15 min.
Histamine H1- and H2-receptor blockade.
Oral administration of 540 mg of fexofenadine, a selective H1-receptor antagonist, reaches peak plasma concentrations within 1 h and has a 12-h half-life (43). Oral administration of 300 mg ranitidine, a selective H2-receptor antagonist, reaches peak plasma concentration within 2 h and has a 3-h half-life (13). This dosage of histamine-receptor antagonists has demonstrated >90% inhibition of histamine H1 and H2 receptors, lasting for 6 h after administration (13, 44). Fexofenadine and ranitidine are not thought to cross the blood-brain barrier or to have sedative effects (13, 43, 44). Blockade of H1 receptors prevents the formation of the local vasodilators, nitric oxide and prostaglandins, whereas blockade of H2 receptors prevents the decrease in concentration of intracellular calcium within smooth muscle that occurs with the binding of histamine (44). Importantly, it has been shown that histamine H1- and H2-receptor blockade does not alter blood flow, heart rate, blood pressure, or smooth muscle tone at rest (10, 25, 27, 28, 41).
Downhill running.
The initial treadmill speed was based on the screening visit V̇o2max test. A speed was chosen to elicit a heart rate of 150 beats/min, as monitored by a chest strapped heart rate monitor (H1 heart rate sensor, Polar, Lake Success, NY). A heart rate of 150 beats/min was selected for direct comparison to previous downhill running studies (6) that elicited moderate muscle damage, allowed continuous monitoring of effort, and would equate to similar relative exercise intensities between subjects. Once a plateau of heart rate was obtained (within 10 min), the grade of the treadmill was lowered slowly to a −10% grade. Subjects ran at the negative grade for 45 min. Speed was adjusted during the first 15 min to maintain heart rate at ~150 beats/min and was not altered during the remainder of exercise. At 15-min intervals, heart rate and rating of perceived exertion (Borg scale) were recorded, along with a 3-min measure of whole body V̇o2. Subjects exercised in an environmental chamber controlled at 20°C and 50% relative humidity (Tescor, Warminster, PA).
Measurements
All hemodynamic measurements (heart rate, blood pressure, and blood flow) were made pre- and postexercise with subjects in the supine position in a thermoneutral (20–22°C) temperature-controlled environment. Hemodynamic measurements were made before exercise (baseline) and every 15 min throughout 120 min postexercise. Measurements of limb circumference, algometry, muscle strength, muscle soreness, and range of motion were made before exercise, 120 min postexercise, as well as 6, 12, 24, 48, and 72 h postexercise. Venous blood samples were obtained before exercise, immediately postexercise, as well as 6, 12, 24, 48, and 72 h after exercise (Fig. 1).
Heart rate and arterial pressure.
Heart rate was monitored using a three-lead electrocardiogram, and arterial pressure was measured on the right brachial artery using an automated auscultometric sphygmomanometer (Tango+, SunTech Medical, Raleigh, NC). Mean arterial pressure was calculated as 2/3 diastolic pressure plus 1/3 systolic pressure and is reported in millimeters of mercury.
Femoral blood flow.
Femoral artery blood flow was determined via duplex ultrasonography using a linear-array ultrasound transducer (L9-3 probe, Philips iE33, Andover, MA) and standard methods for quantification (5). The entire width of the femoral artery was insonated with an angle of 60°, 2–3 cm proximal to the common femoral artery bifurcation. Velocity measures were made by recording the forward and reverse Doppler frequencies to custom audio recording software on a computer interfaced with the ultrasound system. The recordings were subsequently analyzed using an intensity-weighted algorithm to determine mean blood velocity using custom software. Femoral artery diameter measures were made in triplicate during diastole using digital calipers on the ultrasound system. Mean blood flow velocities from the Doppler ultrasound were corrected for thin beam measurements (5). For this study, velocity measurements were made at an average depth of 1.41 ± 0.02 cm and were thin-beam corrected, based on a known beam width of 1.86 mm, which resulted in an average correction factor of 0.761 ± 0.001 (5). Femoral blood flow was calculated as artery cross-sectional area multiplied by femoral mean blood velocity and reported as milliliters per minute. Femoral blood flow was calculated as:
where femoral blood flow is in ml/min, the mean blood velocity is in cm/s, the femoral diameter is in cm, and 60 was used to convert from ml/s to ml/min. Femoral vascular conductance was calculated as flow/pressure and expressed as ml·min−1·mmHg−1.
Knee extension strength testing.
Maximal voluntary isometric force of the right knee extensors was determined using a custom-built knee-extension apparatus and interfaced with a commercially available strain gauge (DP25-S, Omegadyne, Sunbury, OH). The knee angle was set to 45° of knee extension, and subjects performed two to three maximal knee extension contractions with 60-s rest between attempts. Downhill running can produce muscle damage in many lower body muscle groups, but the quadriceps muscle group was chosen for strength testing due to ease of testing and direct comparison to strength measures from other downhill running protocols (48). The greatest contractile force was accepted as a maximal value using the other trials as verification, as long as they were within 10%; if not, additional trials were performed. Muscle strength 2 h after exercise was variable, and there were no differences in strength loss between groups (P = 0.88), perhaps due to neuromuscular fatigue or substrate depletion (i.e., factors unrelated to the aims of the study), and thus are not reported.
Muscle pain and discomfort.
Subjects’ perceived muscle pain and discomfort were evaluated by means of a visual analog scale questionnaire. Subjects provided a subjective rating of their general muscle soreness by marking a single vertical line along a 100-mm continuous scale with descriptive anchors at 0 mm indicating “no pain/discomfort,” 50 mm “moderate pain/discomfort,” and 100 mm “worst pain/discomfort.” Assessments of pain/discomfort were completed when the quadriceps muscles were in a relaxed state (“passive pain/discomfort”) as well as when the subjects performed a contraction at 50% of their preexercise baseline voluntary contraction (“active pain/discomfort”). The distance from the left end (0 mm) of the scale to the vertical mark allowed for quantification of the pain/discomfort level.
Pain pressure threshold.
The minimum pressure that induces pain or discomfort, pain pressure threshold (12), was evaluated using algometry at three sites on the subject’s right leg: vastus lateralis, rectus femoris, and gastrocnemius. The gastrocnemius pressure threshold was evaluated at the greatest circumference of the calf along the midline of the shank, the rectus femoris was evaluated along the midline of the front of the leg and between 8 and 10 cm above the patella, and the vastus lateralis was evaluated 15-20 cm above the lateral epicondyle of the femur. The sites were marked with a pen for visit-to-visit repeatability. Pain pressure threshold was evaluated with a Wagner digital algometer (model FPX25, Wagner Instruments, Greenwich, CT), according to the methods of Fischer (12) in which the 1-cm2 flat rubber tip is pressed firmly and perpendicularly into the muscle with an increasing force of ~5 N/s. Pressure was stopped when the volunteer indicated the onset of pain/discomfort. Each site was evaluated two times, and values within 5 N were accepted. If not, a third trial was performed, and the two closest values were averaged. The use of algometry does not in itself change perception of pain, as multiple measures made to the same site over a 3-day period do not affect pain scores (34).
Limb circumference.
Circumference measures around the right leg were made at midthigh and around the greatest circumference of the calf while the volunteer was standing. A tape measure equipped with a tension spring (Baseline Evaluation Instruments, Advantage Medical, Carmel, IN) was used for constant and repeatable pressure at each position and time point. Additionally, the sites were marked with a pen for visit-to-visit repeatability. The circumference measures were made around the quadriceps and calf, as these were the two muscle groups expected to experience the most damage and, therefore, edema, and due to ease of measurement.
Knee and hip range of motion.
Range of motion of the knee and hip was assessed using a standard goniometer. The origin of the goniometer was placed at approximately the center of rotation of the knee and hip, while the ends of the goniometer (Baseline Evaluation Instruments, Advantage Medical, Carmel, IN) were aligned with the long axis of the shank and femur and femur and torso, respectively. The goniometer sites were marked with a pen for visit-to-visit repeatability. Subjects decreased their knee and hip angle without the use of their hands. The difference from starting position to end position in degrees was recorded as their range of motion.
Blood sampling.
Venous blood (15 ml) was collected from a superficial vein in the antecubital space into 3 5-ml Vacutainers for collection of serum (no additives) and plasma (lithium heparin anticoagulant) and centrifuged at 1,275 relative centrifugal force. Plasma and serum were aliquoted into cryovials and stored at −80°C until analysis. Serum and plasma were analyzed for creatine kinase using an activity assay (Sigma-Aldrich, St. Louis, MO), tumor necrosis factor α (TNF-α) using a high sensitivity enzyme-linked immunosorbent assay (ELISA; R&D Systems, Abingdon, UK), and interleukin-6 (IL-6) using an ELISA (R&D Systems).
Data Analysis
Subjects were not separated by sex, as previous studies on postexercise blood flow have not demonstrated a sex interaction in recreationally active subjects (41). Additionally, a review of the literature suggests that there are no differences in the magnitude of muscle damage between women and men following similar eccentric exercise protocols (19, 36).
We used four statistical approaches to test our hypotheses. First, changes in blood flow and femoral vascular conductance were analyzed using a stepwise approach that allowed examination of both linear and quadratic relationships across time, and tested whether or not the relationships differ between control and blockade, as used previously (2, 41). Independent variables remained in the model if a minimal P value threshold was met (P < 0.15). Second, heart rate, mean arterial pressure, blood markers, isometric muscle strength, range of motion, and pain pressure threshold were assessed using a between- and within-subjects repeated-measures ANOVA with a priori contrasts when significant effects for drug or time were present. Interactions remained in the model if a minimal P value threshold was met (P < 0.15). Third, differences between groups in age, height, weight, percent body fat, V̇o2max, and area-under-the-curve analysis of blood markers were assessed using unpaired t-tests. Area-under-the-curve analysis was performed for all blood markers per convention, as it is more representative of the magnitude of muscle damage or inflammation than time course data (40). Fourth, a clinically relevant benchmark of a decrease in pain pressure threshold to <84.1% of preexercise was used as a criterion for identifying relevant increases in pain/discomfort (34).
T-tests were run using SigmaPlot (version 12, Systat, San Jose, CA) and ANOVA, and other modeling was run using SAS (SAS Proc GLMSELECT and Proc MIXED, SAS version 9.2; SAS Institute, Cary, NC). For all tests, significance was set at P < 0.05. All data are presented as means ± SE, unless stated otherwise (i.e., Table 1).
Table 1.
Subject characteristics
| Control | Blockade | P Value | |
|---|---|---|---|
| n | 12 (3 F, 9 M) | 12 (4 F, 8 M) | |
| Age, yr | 23 ± 5 | 23 ± 6 | 0.47 |
| Height, cm | 177 ± 11 | 177 ± 12 | 0.80 |
| Weight, kg | 67.9 ± 11.7 | 68.4 ± 10.0 | 0.89 |
| Body fat, % | 9.6 ± 7.3 | 9.8 ± 8.7 | 0.55 |
| V̇o2max, ml·kg−1·min−1 | 47.6 ± 8.7 | 51.0 ± 6.0 | 0.27 |
| Heart rate, beats/min | 61 ± 3 | 59 ± 3 | 0.64 |
| Mean arterial pressure, mmHg | 82 ± 8 | 83 ± 6 | 0.79 |
| Femoral blood flow, ml/min | 217 ± 22 | 200 ± 12 | 0.48 |
| Femoral vascular conductance, ml·min−1·mmHg−1 | 2.66 ± 0.30 | 2.40 ± 0.15 | 0.44 |
| Preexercise isometric force, N | 579 ± 230 | 556 ± 139 | 0.78 |
Values are means ± SD; n, no. of subjects. F, female; M, male. There were no differences between control and blockade groups.
RESULTS
Subject Characteristics
Randomization resulted in control and blockade groups with subjects of similar age, height, weight, body fat percentage, and V̇o2max (Table 1). Four male subjects completed both the blockade and control trials, of which three completed the histamine trial first. There was a 16- to 33-wk break between trials for each repeat subject, well beyond the 9-wk repeated-bout adaptation effect, which was reported by Byrnes et al. (6) using a −10% grade downhill running protocol.
Downhill Running
Heart rate, V̇o2, and rating of perceived exertion during the 45 min of downhill running are shown in Table 2. By design, heart rate was not different between control and blockade groups during the first 15 min and increased similarly from 15 to 30 min of exercise in both groups (P = 0.62, blockade vs. control). V̇o2 was not different between control and blockade groups during the first 15 min and increased similarly from 15 to 30 min of exercise in both groups (P = 0.48, blockade vs. control). V̇o2 during the 45 min of downhill running averaged 57% of V̇o2max measured during the screening visit. Ratings of perceived exertion increased across the exercise session, but were similar between groups (P = 0.28, blockade vs. control; Table 2).
Table 2.
Downhill running measurements
| V̇o2, ml·kg−1·min−1 |
Heart Rate, beats/min |
Rating of Perceived Exertion |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| 15 min | 30 min | 45 min | 15 min | 30 min | 45 min | 15 min | 30 min | 45 min | |
| Control | 25.2 ± 1.1 | 28.1 ± 1.3* | 29.0 ± 1.5* | 147 ± 4 | 159 ± 4* | 161 ± 3* | 12 ± 0 | 13 ± 0* | 14 ± 0*† |
| Blockade | 26.9 ± 1.1 | 29.0 ± 1.3* | 30.3 ± 1.5* | 147 ± 4 | 154 ± 4* | 158 ± 3* | 11 ± 0 | 13 ± 1* | 14 ± 1*† |
Values are means ± SE; n = 12 per group. There were no differences between blockade and control.
P < 0.05 vs. 15 min.
P < 0.05 vs. 30 min.
Femoral Blood Flow and Vascular Conductance
Preexercise femoral blood flow and femoral vascular conductance did not differ between control and blockade (Table 1). As shown in Fig. 2, femoral blood flow and femoral vascular conductance during the recovery from downhill running were increased immediately following exercise in both groups, but returned to baseline preexercise values faster in the blockade group (60 min) compared with the control group (which remained elevated above baseline at 120 min postexercise). At 60 min postexercise, blood flow was ~29% lower with blockade compared with control (P < 0.05).
Fig. 2.
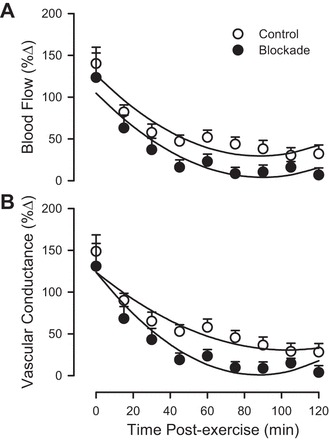
Femoral blood flow (A) and femoral vascular conductance (B) during 120 min of recovery from exercise (downhill running), as a percent change from preexercise. ○, Control; ●, H1/H2 blockade. Values are means ± SE; n = 12 per group. Parallel regression lines for femoral blood flow indicate P < 0.05 blockade vs. control (drug main effect). Nonparallel regression lines for femoral vascular conductance indicate P < 0.05 for blockade vs. control (drug × time interaction).
Heart Rate and Arterial Pressure
Preexercise heart rate and mean arterial pressure did not differ between control and blockade (Table 1). During recovery from exercise, heart rate was elevated over preexercise levels in the control group for the full 120 min following exercise, but was only elevated over preexercise for the first 30 min in the blockade group (P < 0.05). On average, heart rate tended to be lower by 8 ± 1 beats/min with blockade compared with control (P = 0.06, blockade vs. control). Mean arterial pressure decreased by a similar amount following exercise in both the control and blockade group, with a maximal drop in pressure occurring 45 min after exercise (control: −3.8 ± 1.9 mmHg vs. blockade: −3.5 ± 1.9 mmHg; P = 0.69, blockade vs. control).
Muscle Strength
Isometric quadriceps strength could only be assessed in 11 subjects in the blockade group, as one subject developed knee joint pain (which appeared independent of DOMS) that prevented a maximal effort. One control subject had a missing strength measure at 12 h postexercise, and an interpolation of strength at 6 and 24 h was used as a replacement value. Preexercise isometric quadriceps strength did not differ between control and blockade (Table 1). Isometric quadriceps strength was reduced following exercise, and the pattern of this response differed between blockade and control, as shown in Fig. 3. Compared with preexercise, there was a loss of isometric strength from 6 through to 72 h postexercise in control subjects. In contrast, the blockade subjects showed a smaller decrement of strength, which was only present at 12 and 24 h. The greatest decrease in the control subjects’ isometric strength occurred 24 h postexercise, −124 ± 34 N, and represented a −19.3 ± 4.3% change in isometric strength. For comparison, the blockade subjects’ isometric strength at 24 h postexercise was decreased by 40 ± 26 N, a −7.8 ± 4.8% change in strength (P < 0.05 blockade vs. control).
Fig. 3.
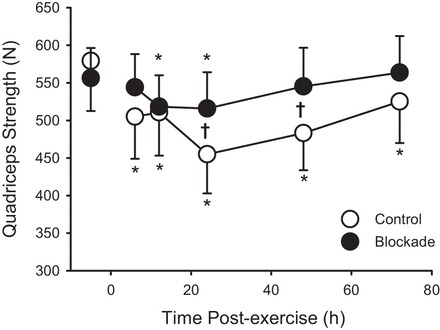
Quadriceps isometric strength during 72 h of recovery from exercise (downhill running). ○, Control; ●, H1/H2 blockade. Values are means ± SE; control n = 12; blockade n = 11. *P < 0.05 vs. preexercise within a group. †P < 0.05, blockade vs. control.
Muscle Pain/Discomfort
Visual analog scale ratings of pain/discomfort were not different between control and blockade preexercise during a passive noncontracted state (P = 0.82) or during a 50% of maximal isometric quadriceps contraction (P = 0.77). The passive pain/discomfort rating was increased following exercise, and the pattern of this response differed between blockade and control, whereas the response for active pain/discomfort rating did not differ between blockade and control, as shown in Fig. 4. For passive ratings, pain/discomfort was elevated across the 72 h for control subjects, but only at 24 and 48 h for blockade. For active ratings, pain/discomfort was elevated through 48 h postexercise with no differences between groups (P = 0.51).
Fig. 4.
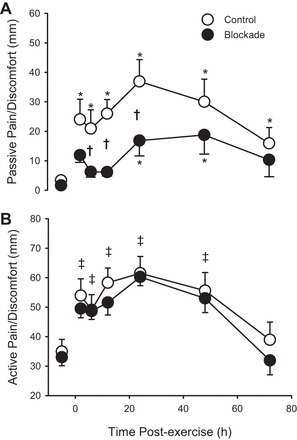
Pain and discomfort rating from visual analog scale under passive conditions (A) and under active conditions (B) (with quadriceps contraction at 50% of maximal voluntary contraction) during 72 h of recovery from exercise (downhill running). ○, Control; ●, H1/H2 blockade. Values are means ± SE; n = 12 per group. *P < 0.05 vs. preexercise within a group. †P < 0.05, blockade vs. control. ‡Where there was no drug effect or drug × time interaction, P < 0.05 vs. preexercise across both groups (main effect of time).
Algometry
One subject from the blockade group did not have data to be analyzed for any muscle group, as their pain pressure threshold exceeded the upper limits of the algometer. Therefore, 12 subjects in the control and 11 subjects in the blockade group comprised the data set. Pain pressure threshold preexercise was not different between control and blockade for the rectus femoris, vastus lateralis, or the gastrocnemius (all P > 0.48). As indicted in Fig. 5, pain pressure thresholds were decreased following exercise (more pain) for all three muscle sites (all P < 0.05) with some variation in the time course of the effect, but there were no detectable differences in pain pressure threshold in the rectus femoris (P = 0.39), vastus lateralis (P = 0.48), or gastrocnemius (P = 0.27). Using an alternative method of analysis, which defines a clinically relevant change in pain as a decrease in pain pressure threshold below 84.1% of baseline (34), which is depicted as dashed reference lines in Fig. 5, a pattern of increased pain sensitivity at all three sites was identified in the control but not in the blockade group. From this analysis, it is evident that the group mean for the pain pressure threshold for the control group in the rectus femoris and vastus lateralis is at or below the threshold values for the first 48 h after exercise, and the gastrocnemius is transiently below this threshold. These results indicate that the majority of the soreness the subjects experienced was in their quadriceps muscles and represent clinically relevant changes in pain sensitivity for the control but not blockade group.
Fig. 5.
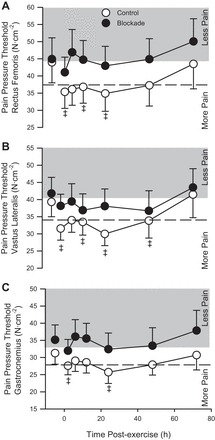
Pain pressure threshold of the rectus femoris (A), vastus lateralis (B), and gastrocnemius (C) during 72 h of recovery from exercise (downhill running). ○, Control; ●, H1/H2 blockade. Values are means ± SE; n = 12 per group. The shaded bands represent an increase in pain pressure threshold in that it required more point pressure to induce a perception of pain. Dashed lines represent a drop in pain pressure threshold of 15.9%, a clinically meaningful change in pain perception. There was no drug effect or drug × time interaction. ‡P < 0.05 vs. preexercise across both groups (main effect of time).
Limb Circumference
Thigh circumference preexercise was not different between control (49.6 ± 1.0 cm) and blockade groups (49.3 ± 1.0 cm; P = 0.80) and did not change over 72 h (P = 0.25). Additionally, there were no differences in preexercise calf circumferences between control and blockade (36.3 ± 0.9 and 36.3 ± 0.6 cm, respectively; P = 0.96). At 2 h postexercise, calf circumference was lower (−0.5 ± 0.2 cm) than preexercise in both control and blockade (P < 0.05), but did not differ between control and blockade (P = 0.59). It returned to baseline circumferences by 6 h postexercise (P = 0.33; data not shown).
Knee and Hip Range of Motion
There were no differences in hip range of motion preexercise between control (123 ± 3°) and blockade (119 ± 3°; P = 0.30), and this measure did not change throughout the 72 h (P = 0.60). Similarly, there were no differences in knee range of motion between the control (118 ± 3°) and blockade (117 ± 2°; P = 0.81), and this measure did not change throughout the 72 h (P = 0.34; data not shown).
Creatine Kinase
Serum creatine kinase concentration was not different preexercise between control (152 ± 31 U/l) and blockade (205 ± 40 U/l), and increased above preexercise concentration after exercise (P < 0.05), as shown in Fig. 6. Creatine kinase values tended to be higher after exercise in the blockade group compared with the control group (P = 0.13). By convention, an area-under-the-curve analysis was performed to represent the extent of muscle damage (and the magnitude of inflammatory responses noted below). The area-under-the-curve analysis indicated that the total load for serum creatine kinase was greater in blockade compared with control (P < 0.05).
Fig. 6.
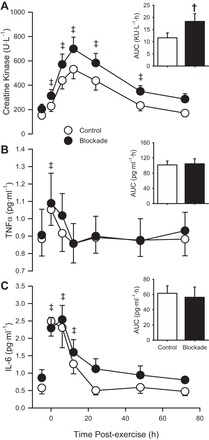
Plasma concentrations of creatine kinase (A), tumor necrosis factor-α (TNF-α; B), and interleukin-6 (IL-6; C) during 72 h of recovery from exercise (downhill running). ○, Control; ●, H1/H2 blockade. Values are means ± SE; n = 12 per group. Areas under the curve (AUC) are presented as inset bar graphs. †P < 0.05, blockade vs. control. ‡Where there was no drug effect or drug × time interaction, P < 0.05 vs. preexercise across both groups (main effect of time).
TNF-α
Plasma TNF-α concentration was not different preexercise between control (0.884 ± 0.098 pg/ml) and blockade (0.906 ± 0.149 pg/ml; P = 0.91) and increased over preexercise concentration immediately postexercise (P < 0.05; Fig. 6). There were no differences in TNF-α between groups in either the pattern across time (P = 0.85) or area under the curve (P = 0.86).
IL-6
Plasma IL-6 concentration was not different preexercise between control (0.575 ± 0.171 pg/ml) and blockade (0.865 ± 0.235 pg/ml; P = 0.33), and increased over preexercise concentration following exercise (P < 0.05; Fig. 6). There were no differences in IL-6 between groups in the pattern across time (P = 0.32) or area under the curve (P = 0.64).
DISCUSSION
Previous research has shown that blood flow to previously active skeletal muscle remains elevated following exercise, primarily from activation of histamine H1 and H2 receptors causing arterial vasodilation (14). The physiological purpose of increased blood flow after exercise is unknown, but may be related to histamine release as part of an inflammatory process to repair skeletal muscle following exercise (36, 37). Additionally, previous reports indicated that blocking histaminergic signaling during exercise may increase the severity of muscle damage (46). Therefore, the purpose of this investigation was to examine the effect of histamine-receptor blockade on 1) postexercise skeletal muscle blood flow, 2) exercise-induced inflammatory responses, 3) severity of muscle damage, and 4) the accompanying symptoms of DOMS following muscle-damaging exercise. It was hypothesized that, in comparison to control, histamine-receptor blockade would decrease postexercise blood flow, the inflammatory response to exercise, and symptoms of DOMS, but increase markers of muscle damage following a bout of moderate muscle-damaging exercise. Important to the interpretation of the findings was that the intensity of exercise during downhill running was similar between control and histamine-receptor blockade groups. All markers of exercise intensity (i.e., heart rate, V̇o2, and perceived exertion) showed that the exercise intervention was equivalent for control and blockade (Table 2). Therefore, as these markers did not differ between groups, differences observed in postexercise blood flow, inflammation, muscle damage, and DOMS are attributable, either directly or indirectly, to blocking the actions of histamine receptors.
Histamine-receptor blockade attenuated sustained postexercise vasodilation following downhill running, a model intended to induce muscle damage and initiate an inflammatory response. This is an important initial finding, as previous research has demonstrated that histamine H1- and H2-receptor blockade diminished sustained postexercise vasodilation following dynamic exercise, such as cycling (10, 25, 27, 28, 38) and single-leg dynamic knee extension (2, 41, 42), models intended to mimic daily exercise routines in muscle groups without overt muscle damage. These data suggest that histamine-receptor activation occurs in response to downhill running and thus may modify a broader range of responses to muscle-damaging aerobic exercise.
In contrast to the effect on vasodilation, histamine-receptor blockade does not appear to affect the inflammatory response associated with muscle damage in this model, as assessed through systemic blood markers of inflammation (TNF-α, IL-6). This was surprising, given that histamine has long been recognized as a chemical mediator of inflammation (7), mast cell degranulation has been associated with models of severe muscle damage (26), and its infusion in skeletal muscle vascular beds increases capillary permeability (21). Histamine increases capillary permeability by inducing margination of endothelial and pericyte cells by activation of H1 receptors, which permits neutrophil and macrophage infiltration for breakdown and repair of damaged muscle fibers (31, 36). Histamine, in addition to increasing capillary permeability, induces endothelial cell production of IL-6, a chemokine involved with the recruitment and differentiation of macrophages (37, 47), and this effect is enhanced in the presence of TNF-α (24). Along these lines, Romero et al. (42) recently demonstrated that histamine-receptor blockade prevents the increased expression of IL-6 mRNA, which occurs postexercise, and broadly affects transcription of multiple chemokine/cytokines. Therefore, it was logical to expect that blocking the action of histamine receptors would blunt a rise in markers of inflammation from the previously active skeletal muscle following downhill running. There was an increase in IL-6 and TNF-ɑ immediately following exercise, and IL-6 remained elevated for 12 h, consistent with findings of elevated TNF-ɑ and IL-6 in biopsy samples from muscles that had undergone muscle-damaging exercise (33). However, these markers of systemic inflammation were not different between control and blockade. It is possible that, despite similar circulating levels, the local expression of these inflammatory signals within skeletal muscle was not reflected systemically (36), i.e., histamine-receptor blockade could have influenced autocrine or paracrine signaling related to inflammatory responses in skeletal muscle, independent of the systemic response. Thus, taken together, it is not clear that histamine-receptor blockade modified the inflammatory response to moderate muscle damage induced by downhill running.
Alternatively, it is possible that an anti-inflammatory effect of histamine-receptor blockade was masked by generation of greater muscle damage with blockade. Several reports suggest that blocking histaminergic signaling may predispose muscle to injury. For example, intentional antihistamine overdose, in the absence of exercise, results in severe muscle damage (9, 20, 39). Additionally, in an analysis of 475 hospital patients with clinical rhabdomyolysis, antihistamine medication was associated with a high proportion of the diagnoses (29). Likewise, antihistamine use immediately preceding exercise was involved in one case report of exertional rhabdomyolysis (46). In the present study, downhill running resulted in increased serum creatine kinase concentrations in the control group, which matched reported values in the literature using the same exercise protocol (6). However, a greater creatine kinase response over the 72-h protocol was noted in the blockade group. It is unknown if histamine-receptor blockade resulted in increased muscle damage, or if blocking histamine receptor’s actions altered the release, metabolism, or filtration of creatine kinase, giving the illusion of increased muscle damage. This is the first known evidence from an interventional study, albeit indirect, of an association between histamine-receptor antagonists and excessive muscle damage that supports descriptive and clinical reports of antihistamine use and muscle damage (9, 20, 39, 46). Studies using other muscle groups, such as the elbow flexors, where more consistent changes in limb circumference and joint angles have been reported, may be needed to provide further supporting evidence of muscle damage. Ultimately, studies using muscle biopsies to visualize muscle damage may prove necessary to corroborate these findings based on circulating creatine kinase.
Although increased muscle damage may have occurred with histamine-receptor blockade, there was an apparently paradoxical protection against strength loss in the blockade group compared with the control group. This is an interesting and unexpected finding, as strength loss has been used as an indirect measure to confirm the presence of muscle damage. The control group experienced an approximate 20% strength loss that is similar to reported strength loss of other downhill-running protocols (6), but the blockade group had a greater preservation of strength. We believe there are two potential explanations for these results. First, although not evident in circulating biomarkers, histamine-receptor blockade may have reduced inflammation, preserving muscle function or reducing discomfort associated with maximal voluntary contractions. Second, histamine-receptor blockade may have reduced the sensitivity of group III/IV afferent neurons. These possibilities are not mutually exclusive explanations.
Of interest to the present study, the skeletal muscle group III/IV afferent fibers that are involved with the sensation of pain/discomfort express histamine H1 and H2 receptors, which, when activated, lower the threshold for stimulation of these nociceptive fibers (11, 30, 35, 45). Therefore, some of the loss of muscle strength experienced during DOMS may be due to direct histaminergic activation of nociceptive afferent fibers that act as a protective mechanism (i.e., by increasing perceptions of pain) to limit further muscle damage, by limiting muscle recruitment and force production. Thus blocking the sensitization of the group III/IV afferent fibers may have resulted in a hypoalgesic state, which permitted greater motor unit recruitment and force generation with histamine-receptor blockade. If this scenario is correct, it sheds light on the “unanswered question of the origin of nociceptor sensitization substances in DOMS” (18). Further evidence that histamine receptors play a role in the sensitization of nociceptors during DOMS comes from measures of decreased pain-pressure thresholds following exercise. When the pain-pressure threshold falls below the criteria of 84.1% of preexercise (34), this indicates a clinically relevant reduction in the pressure it takes to elicit a pain sensation. While the pain pressure threshold decrements were not statistically different between groups, the means for the control group were at or below the 84.1% criterion for 48 h in all three muscles tested. It should be noted that only a single dose of histamine-receptor blockade was given before exercise, and the direct effects of the medication on histamine receptors should have been minimal beyond the 12-h measurements, although profound differences in pain and strength are noted well beyond this time frame. This raises the possibility that histamine’s modulation of group III/IV afferent fibers may involve mechanisms that extend beyond the transient occupation of histamine receptors.
Along these lines, Romero et al. (42) recently published on the effects of histamine on the exercise transcriptome. We remined these data and found that, during recovery from exercise, histamine drives an upregulation of mRNA for two hyperalgesic factors, glial-derived neurotrophic factor and nerve growth factor, as shown in Fig. 7. These are regulators of increased nociceptive function in group III/IV afferent fibers (3, 23).
Fig. 7.
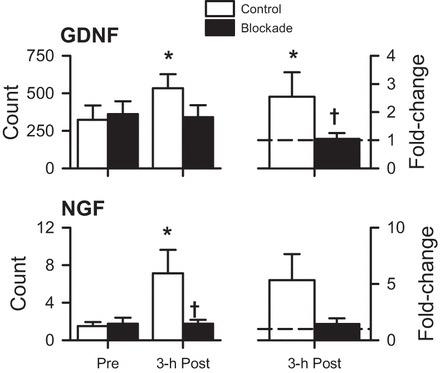
Expression of mRNA for the hyperalgesic factors, glial-derived neurotrophic factor (GDNF) and nerve growth factor (NGF), in samples obtained before exercise (Pre) and at 3-h postexercise (3-h Post), shown as raw counts (left) and fold-change relative to Pre (right) from a prior study using 60-min dynamic one-legged knee-extension exercise (42), and mined from the NCBI Gene Expression Omnibus website (GEO, https://www.ncbi.nlm.nih.gov/geo, accession number GSE71972). Values are means ± SE. Open bars, control; solid bars, H1/H2 blockade. *P < 0.05 vs. preexercise within a group. †P < 0.05 blockade vs. control.
Previous studies have suggested that increased capillary permeability will lead to an increased fluid infiltration of the intramuscular space and increased pressure within muscular compartments. Such an increase in intramuscular pressure, experienced after muscle-damaging exercise, results in increased perceptions of pain via activation of group III and IV afferent fibers (45). It is possible that histamine-receptor blockade prevented fluid infiltration, minimizing the edema and rise in pressure, thus resulting in lower resting pain perceptions with histamine-receptor blockade. However, we were not able to demonstrate any change in edema via limb circumference changes with this model of moderate muscle damage. Nonetheless, it is possible that changes in tissue fluid infiltration within muscle compartments were below the current detection sensitivity and remain clinically relevant and impact pain perceptions.
The histamine receptor antagonists given in the present study, fexofenadine and ranitidine, are highly selective for H1 and H2 receptors, respectively. These two drugs were given as H1 receptor activation on arterial endothelial cells, and H2 receptors on vascular smooth muscle, when stimulated, participate in a sustained postexercise vasodilation (25, 27, 28). H1 and H2 receptors can have opposite effects within a cell type (e.g., activation of H1 receptors on vascular smooth muscle causes contraction, while activation of H2 receptors on vascular smooth muscle causes relaxation), so it is generally the pattern of receptor distribution (e.g., more H2 receptors on vascular smooth muscle, and more H1 receptors on endothelial cells) that determine the net effect of simultaneous activation of receptor subtypes.
The antagonists used in this study have minimal cross-reactivity with H3 and H4 receptors, receptor subtypes commonly associated with immune and central nervous system cells. Furthermore, fexofenadine and ranitidine are not thought to cross the blood-brain barrier and, therefore, have a limited likelihood of affecting centrally located H4 receptors. Thus we acknowledge that the results of the present study do not provide insight into any role that H3 and H4 receptors might play during recovery from exercise.
Perspectives
The results of this experiment have implications for individuals who regularly exercise, those new to exercise (and more prone to DOMS), as well as competitive athletes. Descriptive studies indicate that the incidence of allergies and use of antihistamines in endurance athletes is two times greater than in age-matched controls (1, 17), presumably the result of a switch from nasal to mouth breathing at high ventilation rates (~30 l/min) and its effects on increasing exposure of deep lung tissue to allergens. As a drug category, antihistamines are some of the most widely used medicines, and the amounts administered in this study were within the limits of one-time dosages used for seasonal allergies, allergic reactions, urticaria, or stomach acid reduction. Additionally, many individuals exercise under the direction of personal trainers (or overzealous friends) who may encourage them to exercise to the point that muscle damage, and the resulting DOMS can be severe. Therefore, the combination of antihistamine medication and muscle-damaging exercise may result in situations where more extensive muscle damage may occur, as documented by Springer and Clarkson (46) and supported by the results of the present study. Conversely, athletes competing in multiday events (such as the track and field decathlon, or the multiple qualifying rounds of various athletic competitions) may gain a performance benefit by taking histamine-receptor antagonists. This may allow conservation of strength after strenuous exercise, in conjunction with decreased muscle soreness, across days of competition. Although, if the use of histamine-receptor blockade results in increased muscle damage, the athlete may require an extended period of rest after competition for full muscle recovery.
In conclusion, this study addresses the issue of pharmacotherapy in the interaction between commonly used medications (histamine-receptor antagonists) and exercise-induced muscle damage, and aids in the identification of histamine’s role as a molecular transducer of exercise responses (32). Additionally, the present findings address important issues identified by the National Institutes of Health on the interaction of physical activity, inflammation, and immune function (32). This study demonstrated an attenuation of the sustained postexercise blood flow in response to histamine H1- and H2-receptor blockade in a model that intentionally induced muscle damage. Histamine-receptor blockade did not appear to have an effect on inflammation in the previously active skeletal muscle. However, histamine-receptor blockade increased an indirect blood marker of muscle damage, yet minimized the loss of strength and the perception of pain/discomfort. The findings of this study importantly expand our understanding of the role of histamine during exercise and recovery in healthy individuals and provide insights into the inflammatory response to moderate muscle damage.
GRANTS
This research was supported, in part, by National Heart, Lung, and Blood Institute Grant HL-115027.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
M.R.E. and J.R.H. conception and design of research; M.R.E., S.A.R., D.C.S., J.E.M., and M.J.L. performed experiments; M.R.E. and J.R.H. analyzed data; M.R.E. and J.R.H. interpreted results of experiments; M.R.E. and J.R.H. prepared figures; M.R.E., S.A.R., D.C.S., J.E.M., M.J.L., and J.R.H. drafted manuscript; M.R.E., S.A.R., D.C.S., J.E.M., M.J.L., and J.R.H. edited and revised manuscript; M.R.E., S.A.R., D.C.S., J.E.M., M.J.L., and J.R.H. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the volunteers who were willing to participate in unaccustomed exercise and experience soreness for multiple days. We also thank laboratory coordinators Molly Geiger and Pedro Abdala, as well as undergraduate research assistants Kacy Gilbert-Gard, Mairin Peck, Emily Axelrod, Gabe Lawrence, Laura Preston, Adam Rosencrans, Keeley DeBar, and Marisa Polonsky.
REFERENCES
- 1.Alaranta A, Alaranta H, Heliövaara M, Airaksinen M, Helenius I. Ample use of physician-prescribed medications in Finnish elite athletes. Int J Sports Med 27: 919–925, 2006. doi: 10.1055/s-2006-923811. [DOI] [PubMed] [Google Scholar]
- 2.Barrett-O’Keefe Z, Kaplon RE, Halliwill JR. Sustained postexercise vasodilatation and histamine receptor activation following small muscle-mass exercise in humans. Exp Physiol 98: 268–277, 2013. doi: 10.1113/expphysiol.2012.066605. [DOI] [PubMed] [Google Scholar]
- 3.Bennett DL. Neurotrophic factors: important regulators of nociceptive function. Neuroscientist 7: 13–17, 2001. doi: 10.1177/107385840100700105. [DOI] [PubMed] [Google Scholar]
- 4.Borg G. Perceived exertion as an indicator of somatic stress. Scand J Rehabil Med 2: 92–98, 1970. [PubMed] [Google Scholar]
- 5.Buck TM, Sieck DC, Halliwill JR. Thin-beam ultrasound overestimation of blood flow: how wide is your beam? J Appl Physiol (1985) 116: 1096–1104, 2014. doi: 10.1152/japplphysiol.00027.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Byrnes WC, Clarkson PM, White JS, Hsieh SS, Frykman PN, Maughan RJ. Delayed onset muscle soreness following repeated bouts of downhill running. J Appl Physiol (1985) 59: 710–715, 1985. [DOI] [PubMed] [Google Scholar]
- 7.Dale H. Croonian lectures on some chemical factors in the control of circulation. Lancet 213: 1179–1183, 1929. doi: 10.1016/S0140-6736(00)98917-7. [DOI] [Google Scholar]
- 8.Durstine JL. (Editor). ACSM’s Resource Manual for Guidelines for Exercise Testing and Prescription. Baltimore, MD: Lippincott Williams & Wilkins, 1993. [Google Scholar]
- 9.Emadian SM, Caravati EM, Herr RD. Rhabdomyolysis: a rare adverse effect of diphenhydramine overdose. Am J Emerg Med 14: 574–576, 1996. doi: 10.1016/S0735-6757(96)90103-5. [DOI] [PubMed] [Google Scholar]
- 10.Emhoff CAW, Barrett-O’Keefe Z, Padgett RC, Hawn JA, Halliwill JR. Histamine-receptor blockade reduces blood flow but not muscle glucose uptake during postexercise recovery in humans. Exp Physiol 96: 664–673, 2011. doi: 10.1113/expphysiol.2010.056150. [DOI] [PubMed] [Google Scholar]
- 11.Farzin D, Asghari L, Nowrouzi M. Rodent antinociception following acute treatment with different histamine receptor agonists and antagonists. Pharmacol Biochem Behav 72: 751–760, 2002. doi: 10.1016/S0091-3057(02)00748-7. [DOI] [PubMed] [Google Scholar]
- 12.Fischer AA. Pressure algometry over normal muscles. Standard values, validity and reproducibility of pressure threshold. Pain 30: 115–126, 1987. doi: 10.1016/0304-3959(87)90089-3. [DOI] [PubMed] [Google Scholar]
- 13.Garg DC, Eshelman FN, Weidler DJ. Pharmacokinetics of ranitidine following oral administration with ascending doses and with multiple-fixed doses. J Clin Pharmacol 25: 437–443, 1985. doi: 10.1002/j.1552-4604.1985.tb02873.x. [DOI] [PubMed] [Google Scholar]
- 14.Halliwill JR, Buck TM, Lacewell AN, Romero SA. Postexercise hypotension and sustained postexercise vasodilatation: what happens after we exercise? Exp Physiol 98: 7–18, 2013. doi: 10.1113/expphysiol.2011.058065. [DOI] [PubMed] [Google Scholar]
- 15.Halliwill JR, Sieck DC, Romero SA, Buck TM, Ely MR. Blood pressure regulation X: what happens when the muscle pump is lost? Post-exercise hypotension and syncope. Eur J Appl Physiol 114: 561–578, 2014. doi: 10.1007/s00421-013-2761-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Halliwill JR, Taylor JA, Eckberg DL. Impaired sympathetic vascular regulation in humans after acute dynamic exercise. J Physiol 495: 279–288, 1996. doi: 10.1113/jphysiol.1996.sp021592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang SHS, Johnson K, Pipe AL. The use of dietary supplements and medications by Canadian athletes at the Atlanta and Sydney Olympic Games. Clin J Sport Med 16: 27–33, 2006. doi: 10.1097/01.jsm.0000194766.35443.9c. [DOI] [PubMed] [Google Scholar]
- 18.Hyldahl RD, Hubal MJ. Lengthening our perspective: morphological, cellular, and molecular responses to eccentric exercise. Muscle Nerve 49: 155–170, 2014. doi: 10.1002/mus.24077. [DOI] [PubMed] [Google Scholar]
- 19.Kendall B, Eston R. Exercise-induced muscle damage and the potential protective role of estrogen. Sports Med 32: 103–123, 2002. doi: 10.2165/00007256-200232020-00003. [DOI] [PubMed] [Google Scholar]
- 20.Khosla U, Ruel KS, Hunt DP. Antihistamine-induced rhabdomyolysis. South Med J 96: 1023–1026, 2003. doi: 10.1097/01.SMJ.0000076461.67623.E4. [DOI] [PubMed] [Google Scholar]
- 21.Kjellmer I, Odelram H. The effect of some physiological vasodilators on the vascular bed of skeletal muscle. Acta Physiol Scand 63: 94–102, 1965. doi: 10.1111/j.1748-1716.1965.tb04046.x. [DOI] [PubMed] [Google Scholar]
- 22.Kohl HW, Blair SN, Paffenbarger RS Jr, Macera CA, Kronenfeld JJ. A mail survey of physical activity habits as related to measured physical fitness. Am J Epidemiol 127: 1228–1239, 1988. [DOI] [PubMed] [Google Scholar]
- 23.Lewin GR, Mendell LM. Nerve growth factor and nociception. Trends Neurosci 16: 353–359, 1993. doi: 10.1016/0166-2236(93)90092-Z. [DOI] [PubMed] [Google Scholar]
- 24.Li Y, Chi L, Stechschulte DJ, Dileepan KN. Histamine-induced production of interleukin-6 and interleukin-8 by human coronary artery endothelial cells is enhanced by endotoxin and tumor necrosis factor-α. Microvasc Res 61: 253–262, 2001. doi: 10.1006/mvre.2001.2304. [DOI] [PubMed] [Google Scholar]
- 25.Lockwood JM, Wilkins BW, Halliwill JR. H1 receptor-mediated vasodilatation contributes to postexercise hypotension. J Physiol 563: 633–642, 2005. doi: 10.1113/jphysiol.2004.080325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.MacIntyre DL, Reid WD, McKenzie DC. Delayed muscle soreness. The inflammatory response to muscle injury and its clinical implications. Sports Med 20: 24–40, 1995. doi: 10.2165/00007256-199520010-00003. [DOI] [PubMed] [Google Scholar]
- 27.McCord JL, Beasley JM, Halliwill JR. H2-receptor-mediated vasodilation contributes to postexercise hypotension. J Appl Physiol (1985) 100: 67–75, 2006. doi: 10.1152/japplphysiol.00959.2005. [DOI] [PubMed] [Google Scholar]
- 28.McCord JL, Halliwill JR. H1 and H2 receptors mediate postexercise hyperemia in sedentary and endurance exercise-trained men and women. J Appl Physiol (1985) 101: 1693–1701, 2006. doi: 10.1152/japplphysiol.00441.2006. [DOI] [PubMed] [Google Scholar]
- 29.Melli G, Chaudhry V, Cornblath DR. Rhabdomyolysis: an evaluation of 475 hospitalized patients. Medicine (Baltimore) 84: 377–385, 2005. doi: 10.1097/01.md.0000188565.48918.41. [DOI] [PubMed] [Google Scholar]
- 30.Mobarakeh JI, Sakurada S, Katsuyama S, Kutsuwa M, Kuramasu A, Lin ZY, Watanabe T, Hashimoto Y, Watanabe T, Yanai K. Role of histamine H(1) receptor in pain perception: a study of the receptor gene knockout mice. Eur J Pharmacol 391: 81–89, 2000. doi: 10.1016/S0014-2999(00)00060-1. [DOI] [PubMed] [Google Scholar]
- 31.Murphy DD, Wagner RC. Differential contractile response of cultured microvascular pericytes to vasoactive agents. Microcirculation 1: 121–128, 1994. doi: 10.3109/10739689409148267. [DOI] [PubMed] [Google Scholar]
- 32.Neufer PD, Bamman MM, Muoio DM, Bouchard C, Cooper DM, Goodpaster BH, Booth FW, Kohrt WM, Gerszten RE, Mattson MP, Hepple RT, Kraus WE, Reid MB, Bodine SC, Jakicic JM, Fleg JL, Williams JP, Joseph L, Evans M, Maruvada P, Rodgers M, Roary M, Boyce AT, Drugan JK, Koenig JI, Ingraham RH, Krotoski D, Garcia-Cazarin M, McGowan JA, Laughlin MR. Understanding the cellular and molecular mechanisms of physical activity-induced health benefits. Cell Metab 22: 4–11, 2015. doi: 10.1016/j.cmet.2015.05.011. [DOI] [PubMed] [Google Scholar]
- 33.Nieman DC, Davis JM, Henson DA, Walberg-Rankin J, Shute M, Dumke CL, Utter AC, Vinci DM, Carson JA, Brown A, Lee WJ, McAnulty SR, McAnulty LS. Carbohydrate ingestion influences skeletal muscle cytokine mRNA and plasma cytokine levels after a 3-h run. J Appl Physiol (1985) 94: 1917–1925, 2003. doi: 10.1152/japplphysiol.01130.2002. [DOI] [PubMed] [Google Scholar]
- 34.Nussbaum EL, Downes L. Reliability of clinical pressure-pain algometric measurements obtained on consecutive days. Phys Ther 78: 160–169, 1998. [DOI] [PubMed] [Google Scholar]
- 35.O’Connor PJ, Cook DB. Exercise and pain: the neurobiology, measurement, and laboratory study of pain in relation to exercise in humans. Exerc Sport Sci Rev 27: 119–166, 1999. [PubMed] [Google Scholar]
- 36.Peake J, Nosaka K, Suzuki K. Characterization of inflammatory responses to eccentric exercise in humans. Exerc Immunol Rev 11: 64–85, 2005. [PubMed] [Google Scholar]
- 37.Peake JM, Della Gatta P, Suzuki K, Nieman DC. Cytokine expression and secretion by skeletal muscle cells: regulatory mechanisms and exercise effects. Exerc Immunol Rev 21: 8–25, 2015. [PubMed] [Google Scholar]
- 38.Pellinger TK, Dumke BR, Halliwill JR. Effect of H1- and H2-histamine receptor blockade on postexercise insulin sensitivity. Physiol Rep 1: e00033, 2013. doi: 10.1002/phy2.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pragst F, Herre S, Bakdash A. Poisonings with diphenhydramine--a survey of 68 clinical and 55 death cases. Forensic Sci Int 161: 189–197, 2006. doi: 10.1016/j.forsciint.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 40.Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology 28: 916–931, 2003. doi: 10.1016/S0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- 41.Romero SA, Ely MR, Sieck DC, Luttrell MJ, Buck TM, Kono JM, Branscum AJ, Halliwill JR. Effect of antioxidants on histamine receptor activation and sustained postexercise vasodilatation in humans. Exp Physiol 100: 435–449, 2015. doi: 10.1113/EP085030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Romero SA, Hocker AD, Mangum JE, Luttrell MJ, Turnbull DW, Struck AJ, Ely MR, Sieck DC, Dreyer HC, Halliwill JR. Evidence of a broad histamine footprint on the human exercise transcriptome. J Physiol 594: 5009–5023, 2016. doi: 10.1113/JP272177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Russell T, Stoltz M, Weir S. Pharmacokinetics, pharmacodynamics, and tolerance of single- and multiple-dose fexofenadine hydrochloride in healthy male volunteers. Clin Pharmacol Ther 64: 612–621, 1998. doi: 10.1016/S0009-9236(98)90052-2. [DOI] [PubMed] [Google Scholar]
- 44.Skidgel RA, Erdos EG. Histamine, bradykinin, and their antagonists. In: Goldman & Gilman’s The Pharmacological Basis of Therapeutics (11th Ed.), edited by Brunton LL, Lazo JS, Parker KL. New York: McGrawHill, 2006. [Google Scholar]
- 45.Smith LL. Acute inflammation: the underlying mechanism in delayed onset muscle soreness? Med Sci Sports Exerc 23: 542–551, 1991. doi: 10.1249/00005768-199105000-00006. [DOI] [PubMed] [Google Scholar]
- 46.Springer BL, Clarkson PM. Two cases of exertional rhabdomyolysis precipitated by personal trainers. Med Sci Sports Exerc 35: 1499–1502, 2003. doi: 10.1249/01.MSS.0000084428.51143.8C. [DOI] [PubMed] [Google Scholar]
- 47.Tidball JG, Villalta SA. Regulatory interactions between muscle and the immune system during muscle regeneration. Am J Physiol Regul Integr Comp Physiol 298: R1173–R1187, 2010. doi: 10.1152/ajpregu.00735.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Warren GL, Lowe DA, Armstrong RB. Measurement tools used in the study of eccentric contraction-induced injury. Sports Med 27: 43–59, 1999. doi: 10.2165/00007256-199927010-00004. [DOI] [PubMed] [Google Scholar]


