To the best of our knowledge, this is the first time the volatile molecule content of macaque breath has been comprehensively sampled and analyzed. We do so here in a Biosafety Level 3 setting in the context of M. tuberculosis lung infection. The breath of nonhuman primates represents a novel fluid that could provide insight into disease pathogenesis.
Keywords: breath, tuberculosis, GC×GC-TOFMS, macaque
Abstract
Breath is hypothesized to contain clinically relevant information, useful for the diagnosis and monitoring of disease, as well as understanding underlying pathogenesis. Nonhuman primates, such as the cynomolgus macaque, serve as an important model for the study of human disease, including over 70 different human infections. In this feasibility study, exhaled breath was successfully collected in less than 5 min under Biosafety Level 3 conditions from five anesthetized, intubated cynomolgus and rhesus macaques, before and after lung infection with M. tuberculosis. The breath was subsequently analyzed using comprehensive two-dimensional gas chromatography coupled to time-of-flight mass spectrometry. A total of 384 macaque breath features were detected, with hydrocarbons being the most abundant. We provide putative identification for 19 breath molecules and report on overlap between the identified macaque breath compounds and those identified in previous human studies.
NEW & NOTEWORTHY To the best of our knowledge, this is the first time the volatile molecule content of macaque breath has been comprehensively sampled and analyzed. We do so here in a Biosafety Level 3 setting in the context of M. tuberculosis lung infection. The breath of nonhuman primates represents a novel fluid that could provide insight into disease pathogenesis.
the analysis of exhaled breath holds great potential for medical diagnostics and monitoring. Breath is produced in ample supply and its capture is noninvasive (2). Molecules detected in breath may originate from normal metabolism or from disease-specific processes (5, 31). A number of breath tests with clinical application have been approved by the FDA, including carbon dioxide test for capnography, carbon monoxide test for neonatal jaundice (46), hydrogen and methane tests for gastrointestinal diagnosis (10, 16), nitric oxide test for monitoring asthma therapy (41, 47), the detection of heart transplant rejection (38), and the 13C-urea test for Helicobacter pylori infection (21). Other applications that show promise include the diagnosis of lung cancer (15), chronic obstructive pulmonary disease (11, 17, 48), infections associated with cystic fibrosis (42, 43), and bacterial pneumonias (50, 51). Biomarkers detectable in breath may be produced by a pathogen, the host, and/or from the host-pathogen interaction. Therefore, animal models most closely representing the human disease, and especially the host-pathogen immune interface, are essential to identify and validate potential biomarker compounds and signatures for human use.
The macaque serves as an important model for the study of human disease, including over 70 different human infections (20). Similar to humans in almost every aspect of their anatomy and physiology (1, 7, 34), macaques have been shown to respond as humans do to a wide variety of human immunological, pathological and drug agents, a quality not always shared by other animal models (6, 19). For diseases such as tuberculosis, macaques have been shown to share the complete spectrum of clinical and pathological characteristics of human infection, allowing the disease progression to be monitored through measures similar to those used for human Mycobacterium tuberculosis infection (35). The use of breath analysis can provide new insights into the processes that occur during this infection. Studies in macaques with M. tuberculosis infection, where the infection timing and dose can be controlled, and the severity of disease assessed by imaging and other measures, will be an important bridge to the use of breath analysis in humans.
Two-dimensional gas chromatography time-of-flight mass spectrometry (GC×GC-TOFMS) is the ideal platform for use in untargeted analysis of complex mixtures such as breath. Phillips and colleagues (39) showed that GC×GC-TOFMS analysis of breath could lead to the detection of as many as 2,000 compounds in a single sample, a 10-fold increase over traditional GC-MS. The additional resolving power of GC×GC-TOFMS comes from the second chromatography column, allowing separation of compounds that would normally co-elute using a conventional single column setup (14).
In the present pilot and feasibility study, comprehensive breath analysis using GC×GC-TOFMS has been introduced to the macaque model for the first time, and the reproducibility of the method is evaluated. We do so in a Biosafety Level 3 (BSL-3) setting, enabling the study of breath in conjunction with microbes that can cause serious or potentially lethal disease through the inhalation route of exposure (47a). Information gained from this technique can be used for biomarker discovery with applications ranging from disease diagnosis to disease state progression monitoring.
MATERIALS AND METHODS
Animals.
Three adult cynomolgus macaques (Macaca fascicularis) (8–9 yr old, 6.5–7.87 kg) and two adult rhesus macaques (Macaca mulatta) (6–8 yr old, 5.5 kg) were purchased from Valley Biosystems (West Sacramento, CA) and housed at the University of Pittsburgh in the Regional Biocontainment Laboratory, a BSL-3 primate facility. All animal protocols and procedures were approved by the University of Pittsburgh’s Institutional Animal Care and Use Committee. These animals were enrolled in separate studies, but samples were obtained for the purpose of this study before and during infection. Prior to admission to the study, each animal underwent rigorous testing, including body weight monitoring, complete blood count, chemistry panel, urinalysis, and tuberculin skin test to verify they were free of any preexisting diseases.
Breath collection and concentration.
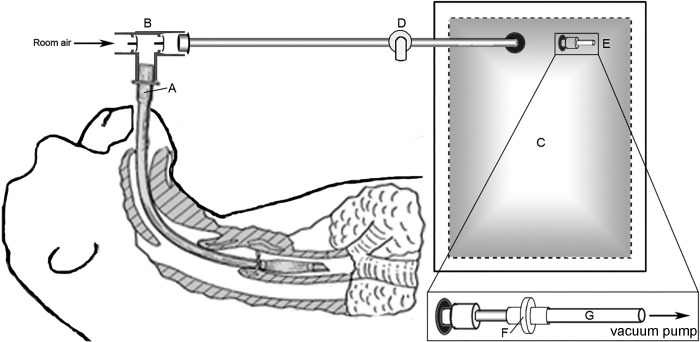
For breath collection, the animals were sedated with an intramuscular injection of ketamine (10–20 mg/kg, Butler Schein Animal Health Dublin, OH) and intubated with a low-pressure cuffed endotracheal (ET) tube. With the animal freely breathing through the ET tube, a 5-liter Tedlar bag (SKC, Eighty Four, PA) was attached via non-rebreathing T-valve (Instrumentation industries, Bethel Park, PA), and over the course of 3–5 min, 3.5–4 liters of breath was collected (Fig. 1, A–E). As breath molecules are often in low abundance and samples cannot always be analyzed immediately, we concentrated and stored the breath molecules onto a three-bed thermal desorption tube containing Carbopack Y, Carbopack X, and Carboxen 1000 (Supelco, Bellefonte, PA), a sorbent combination that has been previously optimized for the collection of a wide range of breath molecules (26). To facilitate concentration, a vacuum pump was used to draw 1.5 liters of breath from the Tedlar bag through a 0.22-µm filter (for the removal of potential pathogens), and onto the thermal desorption tube at a rate of 150 ml/min. By drawing filter-sterilized room air via vacuum pump onto a sorbent trap of the same design, 1.5 liters of room air samples were collected. Samples were stored at 4°C and analyzed within 1 mo of breath collection. A diagram of the breath collection system is shown in Fig. 1 (patent pending).
Fig. 1.
Schematic of breath collection system. A sedated, freely breathing macaque is intubated with cuffed endotracheal (ET) tube (A). A nonrebreathing valve (B) is attached to ET tube, and breath is collected into a 5-liter Tedlar bag (C). After 3–5min of breath collection, 3.5–4 liters of breath is collected. After breath collection is complete, the Tedlar bag is disconnected from the nonrebreathing valve, and the shutoff valve (D) to the bag is closed. Within 30 min of breath collection, the push-pull valve (E) is opened. Breath is filtered via 0.22-μm filter (F) and drawn onto a three-bed sorbent tube (G) containing Carbopack Y, Carbopack X, and Carboxen 1000 via vacuum pump.
M. tuberculosis infection and confirmation.
M. tuberculosis strain Erdman was used for infection. The Erdman strain is highly virulent and widely used in laboratory studies involving pathogenesis and immunization (32). A frozen aliquot was diluted in sterile saline, sonicated for 15 s, and diluted to the appropriate concentration for infection. An aliquot of the dilution used for infection was plated on 7H10 agar plates to determine colony forming units (CFUs) in the inoculant. Macaques were anesthetized with ketamine (10–20 mg/kg, intramuscularly) and infected via intrabronchial instillation as previously described (6). The cynomolgus macaques received 4 CFUs Erdman strain M. tuberculosis, while the rhesus were infected with 7 or 9 CFUs. Animals were infected on 2 days, 2 mo apart. Infection was confirmed by tuberculin skin test conversion of negative to positive and by PET/CT scan (28, 29).
Sample collection time points.
Breath was collected from each of the five animals once before infection (over 2 independent sample collection days) and once 1–2 mo postinfection with M. tuberculosis Erdman strain (over 4 independent sample collection days). Two technical replicates (1.5 liters) were collected from the same breath collection bag at each sample collection time point. A total of 14 room air samples were collected throughout the breath collection time course, including one to two samples each day of breath collection.
Analytical experimental conditions (GC×GC-TOFMS).
A Pegasus 4D (LECO, St. Joseph, MI) GC×GC-TOFMS instrument was equipped with a rail autosampler (MPS, Gerstel, Linthicum Heights, MD). The experimental conditions for sample loading and injection for the GC×GC-TOFMS instrument setup are summarized in Table 1. Data acquisition and analysis was performed with ChromaTOF software, version 4.50 (LECO).
Table 1.
Summary of sample concentration and GC×GC-TOFMS instrument parameters for the analysis of macaque breath in this study
| Sample preconcentration | |
|---|---|
| Sorbent trap packing (sample volume; adsorption flow rate) | Carbopack Y, Carbopack X, Carboxen 1000 (1.5 liters; 150 ml/min) |
| Sample injection | |
| Solvent venting time (temperature; flow rate) | 10 min (50°C; 75 ml/min) |
| Thermal desorption time (temperature; flow rate) | 5 min (330°C; 75 ml/min) |
| Cryofocusing time (temperature) | 5 min (−120°C) |
| Injection time (temperature) | 3 min (275°C) |
| Chromatography | |
| Column no. 1; (length × internal diameter × film thickness) | Rxi-624Sil (Restek, Bellefonte, PA); (60 m × 250 μm × 1.4 μm) |
| Column no. 2; (length × internal diameter × film thickness) | Stabilwax (Restek); (1.5 m × 250 μm × 0.5 μm) |
| Modulation period (hot/cold) | 2 s total (alternating 0.5 s/0.5 s hot/cold) |
| Carrier gas (flow rate) | helium (2 ml/min) |
| Time-of-flight mass spectrometry | |
| Acquisition range | 30–500 m/z |
| Acquisition rate | 200 spectra/s |
| Ion source temperature | 200°C |
Chromatographic Alignment and feature identification.
Chromatographic data were processed and aligned with ChromaTOF software. For peak identification, a signal-to-noise (S/N) cutoff was set at 50, and resulting peaks were identified by a forward search of the National Institute of Standards and Technology 2011 Library. For the alignment of peaks across chromatograms, maximum first and second-dimension retention time deviations were set at 6 and 0.1 s, respectively, and the interchromatogram spectral match threshold was set at 600. A S/N ratio of 50:1 was required in at least one chromatogram and a minimum of 10:1 in all others. For putative peak identification, a forward match score of at least 850 was required. A forward match score of at least 600 was required for compound chemical classifications to be assigned. The International Union of Pure and Applied Chemistry functional group priorities were used to classify molecules that contained multiple functional groups.
Statistical analysis.
All statistical analyses were performed with Python version 2.7.1. Prior to statistical analysis, breath and room air samples were normalized by probabilistic quotient normalization (13). For the identification of discriminatory features between preinfection, postinfection, and room air samples, random forest classification algorithm was used. Features were selected as discriminatory if they were ranked in the top 100 in 95/100 random forest iterations of 1,000 trees, based on their mean decrease in accuracy. Principal components analysis (22) was used to visualize the variance between samples in the data set. Compound significance was tested using the Mann-Whitney U-test (30), with Benjamini-Hochberg correction (4). A significance level of P < 0.05 was selected.
RESULTS
The use of breath for the evaluation of animal health requires a simple, fast, and reliable breath collection system. We developed a method where breath can be collected from an anesthetized animal into a Tedlar bag in 3–5 min and subsequently concentrated onto a thermal desorption tube for storage (Fig. 1). Figure 2 is composed of representative GC×GC-TOFMS chromatograms of preinfection, postinfection, and room air samples. Breath and room air chromatograms were aligned by using the measured first and second-dimension retention times and the mass spectral patterns of each chromatographic peak.
Fig. 2.
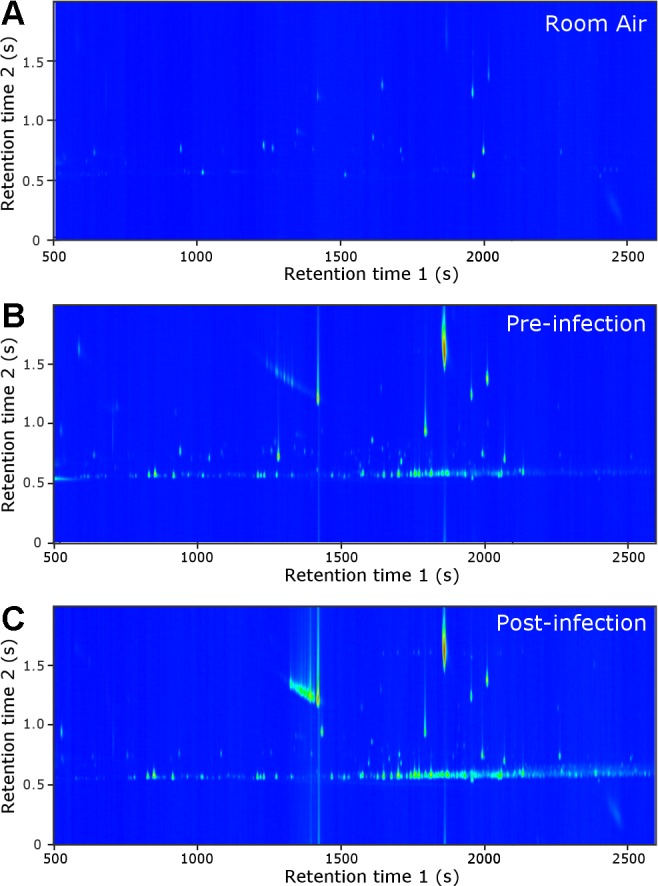
Representative two-dimensional gas chromatograms of room air (A) as well as preinfection (B) and postinfection macaque breath (C).
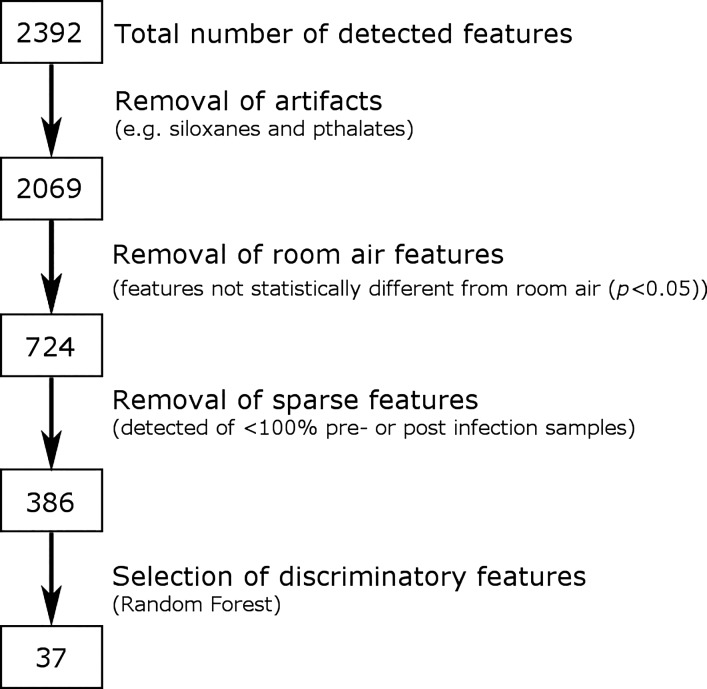
Across the 20 breath samples analyzed, a total of 2,392 total features were detected. Features were reduced by the removal of artifacts (e.g., siloxanes and phthalates) (3) and by the removal of features not statistically different from room air (P > 0.05), resulting in a total of 724 breath features. Out of these remaining features, 384 peaks were found to be present in 100% of preinfection samples or 100% of postinfection samples, representing the subset of features most reliably present in this data set. Figure 3 displays a flow chart of these data reduction steps. The profile of 384 features was found to be highly reproducible among the analyzed breath samples, with an average Spearman’s rank correlation (Spearman’s ρ) of 0.83 for technical replicates, 0.71 among pre- or postinfection samples, and 0.67 between pre- and postinfection samples.
Fig. 3.
Data reduction steps. A total of 2,392 features were detected in at least one of the 20 macaque breath samples analyzed. Features were reduced by the removal of artifacts (e.g., siloxanes and phthalates), the removal of features not statistically different between breath and room air (P < 0.05), the removal of sparsely present features (not present in 100% of preinfection samples or 100% of postinfection samples), and by selection of discriminatory features through random forest classification.
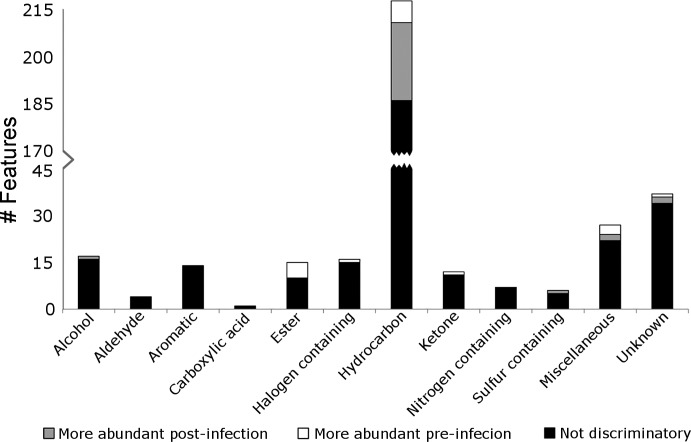
The compound classifications of the 384 breath features, as dictated by their mass spectral matches to the National Institute of Standards and Technology 2011 Library are shown in Fig. 4. Compound classes included alcohols, aldehydes, aromatics, carboxylic acids, esters, hydrocarbons, ketones, and halogen, nitrogen and sulfur-containing molecules. There were 27 compounds that did not fit into any of these classifications and were categorized as miscellaneous. Thirty-seven compounds were designated as unknowns. The majority of identified chromatographic features were classified as hydrocarbons, making up 56% of the 384 features found to be reliably present in the breath samples. All other compound classes were composed of less than 10% of the total number of features. To assess the difference between preinfection macaque breath, postinfection macaque breath, and room air samples, random forest classification algorithm was used (see materials and methods). Out of the 384 reliably present features, 49 were selected as discriminatory through random forest classification. Figure 4 shows the number of discriminatory features in each class greater in preinfection samples (white), the number of discriminatory features greater in postinfection samples (gray), and the number of features not selected as discriminatory (black). The majority of discriminatory features were also hydrocarbons, accounting for 65% of the 49 features. Discriminatory features were identified in all compound classes except the aldehydes, aromatic, carboxylic acids, and nitrogen-containing molecules.
Fig. 4.
Compound classification for macaque breath molecules from preinfection and postinfection animals. Black indicates compounds not selected as discriminatory, gray represents discriminatory compounds higher expressed in postinfection samples, and white represents discriminatory compounds that were higher expressed in preinfection samples.
To further illustrate qualitative and quantitative differences between preinfection, postinfection, and room air samples, when looking at the 49 selected discriminatory features, principal components analysis was used (Fig. 5). Each sample type forms a distinct cluster, indicating that the majority of variance explained is between these sample types. Room air samples are shown to cluster together, demonstrating that there were no major environmental changes between the breath collection time points. The profile of 49 features was found to most highly correlate between technical replicates (Spearman’s ρ = 0.93), followed by within class samples (Spearman’s ρ = 0.83), and finally between-class samples (Spearman’s ρ = 0.61). On average, all 49 features are more abundant in breath as compared with room air samples. Thirty-two out of the 49 features are more abundant in postinfection samples as compared with preinfection samples, with three being statistically different (P < 0.05). Of the 17 features more abundant on average in preinfection samples as compared with postinfection samples, 11 were found to be statistically different (P < 0.05).
Fig. 5.
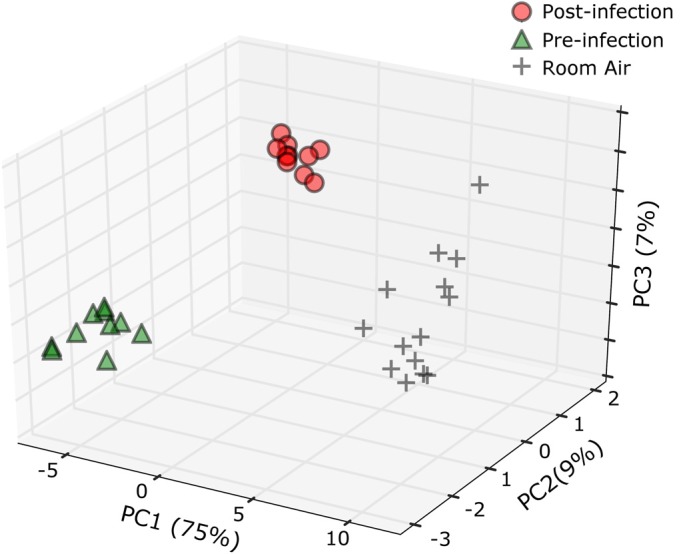
Principal component analysis of pre- and postinfection macaque breath as well as room air samples. Peaks included are the 49 identified as discriminatory through random forest classification.
Out of the 49 breath features selected as discriminatory, 19 were assigned putative identifications (see materials and methods) (Table 2).
Table 2.
Table of putatively identified macaque breath molecules that discriminate pre- and postinfection macaque breath samples
| Putative Peak Identification | Formula | Chemical Abstracts Service (CAS) number | Reported in Healthy Human Breath | Reported as M. tuberculosis Breath Biomarker |
|---|---|---|---|---|
| Higher expressed in postinfection samples | ||||
| (Z)-3-Tetradecene | C14H28 | 41,446–67–7 | ||
| 1,1′-Bicyclohexyl* | C12H22 | 92–51–3 | ||
| 2,2-Dimethylheptane* | C9H20 | 1,071–26–7 | (9) | |
| 2,6,11-trimethyldodecane | C15H32 | 31,295–56–4 | ||
| 2-Ethylhexyl isohexyl ester sulfurous acid | C14H30O3 | 959,067–41–5 | ||
| 3-Methyl-dodecane | C13H28 | 17,312–57–1 | ||
| Dodecane | C12H26 | 112–40–3 | (9) | (24) |
| Hexadecane | C16H34 | 544–76–3 | (9) | |
| Hexylcyclohexane | C12H24 | 4,292–75–5 | (37) | |
| Octylcyclohexane | C14H28 | 1,795–15–9 | ||
| Tridecane* | C13H28 | 629–50–5 | (9) | (36, 37, 40) |
| Higher expressed in preinfection samples | ||||
| 2-Heptanone* | C7H14O | 110–43–0 | (9) | |
| Acetic acid, phenyl ester | C8H8O2 | 122–79–2 | ||
| Allyl heptanoate* | C10H18O2 | 142–19–8 | ||
| 4-Methylene-1-(1-methylethyl)- bicyclo[3.1.0]hexane* | C10H16 | 3,387–41–5 | ||
| 2-Methylbutyl ester butanoic acid* | C9H18O2 | 51,115–64–1 | ||
| n-Amyl isovalerate* | C10H20O2 | 25,415–62–7 | ||
| o-Cymene* | C10H14 | 527–84–4 | (9) | |
| Trans-á-Ocimene* | C10H16 | 502–99–8 | ||
Indicates features statistically different between preinfection and postinfection samples (P < 0.05).
DISCUSSION
Here, we collected breath from freely breathing anesthetized animals in less than 5 min, analyzed the samples using GC×GC-TOFMS, and identified 384 macaque breath features from five animals sampled before and after infection with M. tuberculosis. To the best of our knowledge, this is the first time the volatile molecule content of macaque breath has been comprehensively sampled and analyzed. To date, only two studies have applied GC×GC-TOFMS for the analysis of breath, both from healthy human subjects. Phillips and colleagues (39) reported that of 2,000 breath features they detected from 34 participants, 95 were in common to 90% of participants. Das and colleagues (12) reported that of the 1,379 features detected among 47 participants, 41 were found in the breath of all subjects. Differences in the number of features identified in these human studies compared with the 384 features detected in macaque breath could be due to the study population composition and characteristics, method of breath collection (intubation vs. mouthpiece), differences in analytical methods used for sample analysis, impact of anesthesia on the host, et cetera.
The breath of healthy humans has been characterized by GC-MS in seven studies (18, 23, 27, 33, 44, 45, 49), resulting in a cumulative total of 872 putatively identified breath compounds (9). Of the 19 discriminatory macaque breath features given putative identifications, 6 have been reported in the breath of healthy humans (Table 2). Furthermore, 56% of the 384-detected macaque breath features are classified as hydrocarbons, which is similar in proportion as among the 872 previously reported human breath compounds (46%). The proportion of compounds within the remaining classes were relatively similar for both healthy human and macaque breath; for example, each remaining class contained less than 10% of the total number of detected compounds, with the exception of nitrogenous molecules, which made up 11% of human-detected breath features and only 2% of macaque breath features. The true extent of similarity and differences between the exhaled breath molecules of macaques in comparison to humans remains unknown.
After determining that breath sampling from the macaques was reproducible, we explored the application of breath analysis in the macaque model of M. tuberculosis infection, where we tentatively identified breath features that discriminate among preinfection, postinfection, and room air samples. Although never conducted before in macaques, there have been three published humans studies that investigated the use of breath biomarkers for the diagnosis of M. tuberculosis disease using GC-MS (25, 37, 40). Among these studies, a total of 45 breath biomarkers have been putatively identified. Our data from nonhuman primates confirmed three previously identified human breath biomarkers for M. tuberculosis disease: dodecane (24), hexylcyclohexane (37), and tridecane. Tridecane is the only compound to have been previously reported in multiple human M. tuberculosis disease breath studies (36, 37, 40) (Table 2). Further insight into shared features that are putative biomarkers is not possible because of our sample size and other variables as noted above. Extending these studies to larger cohorts of animals is underway.
Conclusions and Outlook
Nonhuman primates, such as cynomolgus and rhesus macaques, serve as one of the closest analogs to humans for the study of disease (6, 19, 20, 35). Breath is a relatively noninvasive sample hypothesized to contain clinically relevant information for diagnosis and comprehensive disease monitoring. In this study, exhaled breath was successfully collected in less than 5 min under BSL-3 conditions from anesthetized, intubated cynomolgus and rhesus macaques, before and after infection with M. tuberculosis. The breath was subsequently analyzed by GC×GC-TOFMS. We report on the reliability of breath collection as well as an overlap between the identified macaque breath compounds and those previously reported in human studies involving healthy participants as well as individuals with M. tuberculosis disease. An effective evaluation of the similarities and differences between macaque and human breath studies, however, is not possible because of the low population size employed. Future studies could apply this breath collection and analysis approach for the identification and validation of healthy and disease state biomarkers.
GRANTS
This study was supported by National Institutes of Health Grants HL-110811 (to J. Flynn) and AI-114674 (to J. Flynn) and the Bill and Melinda Gates Foundation (to J. Flynn, P. Lin, and J. Hill).
DISCLOSURES
The authors have pursued patent protection for the device described in the manuscript.
AUTHOR CONTRIBUTIONS
T.R.M. and L.B. analyzed data; T.R.M., L.B., and J.E.H. interpreted results of experiments; T.R.M. prepared figures; T.R.M. drafted manuscript; T.R.M., L.B., J.L.F., C.A.S., P.L.L., and J.E.H. edited and revised manuscript; T.R.M., L.B., J.L.F., C.A.S., P.L.L., and J.E.H. approved final version of manuscript; J.T. and M.O. performed experiments; J.E.H. conceived and designed research.
ACKNOWLEDGMENTS
We would like to thank Mavra Nasir, Christiaan Rees, and Flavio Franchina for their help in editing this manuscript.
REFERENCES
- 1.Ackermann RR, Baliff J, Foxman S, Helbig J, Lesser J, Mosbacher M, Senturia S, Sklar DP. Long-tailed or Crab-eating Macaque and Rhesus Macaque. A Comparative Primate Anatomy: Dissection Manual. St. Louis, MO: Washington Univ. in St Louis, 2003, p. 18–24. [Google Scholar]
- 2.Amann A, Miekisch W, Schubert J, Buszewski B, Ligor T, Jezierski T, Pleil J, Risby T. Analysis of exhaled breath for disease detection. Annu Rev Anal Chem (Palo Alto, Calif) 7: 455–482, 2014. doi: 10.1146/annurev-anchem-071213-020043. [DOI] [PubMed] [Google Scholar]
- 3.Bean HD, Dimandja J-MD, Hill JE. Bacterial volatile discovery using solid phase microextraction and comprehensive two-dimensional gas chromatography-time-of-flight mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 901: 41–46, 2012. doi: 10.1016/j.jchromb.2012.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B 57: 289–300, 1995. [Google Scholar]
- 5.Buszewski B, Kęsy M, Ligor T, Amann A. Human exhaled air analytics: biomarkers of diseases. Biomed Chromatogr 21: 553–566, 2007. doi: 10.1002/bmc.835. [DOI] [PubMed] [Google Scholar]
- 6.Capuano SV III, Croix DA, Pawar S, Zinovik A, Myers A, Lin PL, Bissel S, Fuhrman C, Klein E, Flynn JL. Experimental Mycobacterium tuberculosis infection of cynomolgus macaques closely resembles the various manifestations of human M. tuberculosis infection. Infect Immun 71: 5831–5844, 2003. doi: 10.1128/IAI.71.10.5831-5844.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carlsson H-E, Schapiro SJ, Farah I, Hau J. Use of primates in research: a global overview. Am J Primatol 63: 225–237, 2004. doi: 10.1002/ajp.20054. [DOI] [PubMed] [Google Scholar]
- 9.de Lacy Costello B, Amann A, Al-Kateb H, Flynn C, Filipiak W, Khalid T, Osborne D, Ratcliffe NM. A review of the volatiles from the healthy human body. J Breath Res 8: 014001, 2014. doi: 10.1088/1752-7155/8/1/014001. [DOI] [PubMed] [Google Scholar]
- 10.de Lacy Costello BP, Ledochowski M, Ratcliffe NM. The importance of methane breath testing: a review. J Breath Res 7: 024001, 2013. doi: 10.1088/1752-7155/7/2/024001. [DOI] [PubMed] [Google Scholar]
- 11.Cristescu SM, Gietema HA, Blanchet L, Kruitwagen CLJJ, Munnik P, van Klaveren RJ, Lammers JW, Buydens L, Harren FJ, Zanen P. Screening for emphysema via exhaled volatile organic compounds. J Breath Res 5: 046009, 2011. doi: 10.1088/1752-7155/5/4/046009. [DOI] [PubMed] [Google Scholar]
- 12.Das MK, Bishwal SC, Das A, Dabral D, Varshney A, Badireddy VK, Nanda R. Investigation of gender-specific exhaled breath volatome in humans by GCxGC-TOF-MS. Anal Chem 86: 1229–1237, 2014. doi: 10.1021/ac403541a. [DOI] [PubMed] [Google Scholar]
- 13.Dieterle F, Ross A, Schlotterbeck G, Senn H. Probabilistic quotient normalization as robust method to account for dilution of complex biological mixtures. Application in 1H NMR metabonomics. Anal Chem 78: 4281–4290, 2006. doi: 10.1021/ac051632c. [DOI] [PubMed] [Google Scholar]
- 14.Dimandja J-MD. GCXGC: Comprehensive 2-D GC provides high-performance separations in terms of selectivity, sensitivity, speed, and structure. Anal Chem 76: 167A–174A, 2004. [Google Scholar]
- 15.Dragonieri S, Annema JT, Schot R, van der Schee MPC, Spanevello A, Carratú P, Resta O, Rabe KF, Sterk PJ. An electronic nose in the discrimination of patients with non-small cell lung cancer and COPD. Lung Cancer 64: 166–170, 2009. doi: 10.1016/j.lungcan.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 16.Eisenmann A, Amann A, Said M, Datta B, Ledochowski M. Implementation and interpretation of hydrogen breath tests. J Breath Res 2: 046002, 2008. doi: 10.1088/1752-7155/2/4/046002. [DOI] [PubMed] [Google Scholar]
- 17.Fens N, Zwinderman AH, van der Schee MP, de Nijs SB, Dijkers E, Roldaan AC, Cheung D, Bel EH, Sterk PJ. Exhaled breath profiling enables discrimination of chronic obstructive pulmonary disease and asthma. Am J Respir Crit Care Med 180: 1076–1082, 2009. doi: 10.1164/rccm.200906-0939OC. [DOI] [PubMed] [Google Scholar]
- 18.Filipiak W, Ruzsanyi V, Mochalski P, Filipiak A, Bajtarevic A, Ager C, Denz H, Hilbe W, Jamnig H, Hackl M, Dzien A, Amann A. Dependence of exhaled breath composition on exogenous factors, smoking habits and exposure to air pollutants. J Breath Res 6: 036008, 2012. doi: 10.1088/1752-7155/6/3/036008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flynn JL. Lessons from experimental Mycobacterium tuberculosis infections. Microbes Infect 8: 1179–1188, 2006. doi: 10.1016/j.micinf.2005.10.033. [DOI] [PubMed] [Google Scholar]
- 20.Gardner MB, Luciw PA. Macaque models of human infectious disease. ILAR J 49: 220–255, 2008. doi: 10.1093/ilar.49.2.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gisbert JP, Pajares JM. Review article: 13C-urea breath test in the diagnosis of Helicobacter pylori infection—a critical review. Aliment Pharmacol Ther 20: 1001–1017, 2004. doi: 10.1111/j.1365-2036.2004.02203.x. [DOI] [PubMed] [Google Scholar]
- 22.Hastie TJ, Tibshirani RJ, Friedman J. The Elements of Statistical Learning. Data Mining, Inference and Prediction. New York: Springer, 2001. [Google Scholar]
- 23.Kischkel S, Miekisch W, Sawacki A, Straker EM, Trefz P, Amann A, Schubert JK. Breath biomarkers for lung cancer detection and assessment of smoking related effects—confounding variables, influence of normalization and statistical algorithms. Clin Chim Acta 411: 1637–1644, 2010. doi: 10.1016/j.cca.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 24.Kolk AHJ, van Berkel JJBN, Claassens MM, Walters E, Kuijper S, Dallinga JW, van Schooten F-J. Breath-based biomarkers for tuberculosis. SPIE Proc 8371: 83710A, 2012. [DOI] [PubMed] [Google Scholar]
- 25.Kolk AHJ, van Berkel JJBN, Claassens MM, Walters E, Kuijper S, Dallinga JW, van Schooten FJ. Breath analysis as a potential diagnostic tool for tuberculosis. Int J Tuberc Lung Dis 16: 777–782, 2012. [DOI] [PubMed] [Google Scholar]
- 26.Libardoni M, Stevens PT, Waite JH, Sacks R. Analysis of human breath samples with a multi-bed sorption trap and comprehensive two-dimensional gas chromatography (GCxGC). J Chromatogr B Analyt Technol Biomed Life Sci 842: 13–21, 2006. doi: 10.1016/j.jchromb.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 27.Ligor T, Ligo r M, Amann A, Ager C, Bachler M, Dzien A, Buszewski B. The analysis of healthy volunteers’ exhaled breath by the use of solid-phase microextraction and GC-MS. J Breath Res 2: 046006, 2008. doi: 10.1088/1752-7155/2/4/046006. [DOI] [PubMed] [Google Scholar]
- 28.Lin PL, Rodgers M, Smith L, Bigbee M, Myers A, Bigbee C, Chiosea I, Capuano SV, Fuhrman C, Klein E, Flynn JL. Quantitative comparison of active and latent tuberculosis in the Cynomolgus Macaque model. Infect Immun 77: 4631–4642, 2009. doi: 10.1128/IAI.00592-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin PL, Coleman T, Carney JP, Lopresti BJ, Tomko J, Fillmore D, Dartois V, Scanga C, Frye LJ, Janssen C, Klein E, Barry CE, Flynn JL. Radiologic responses in cynomolgus macaques for assessing tuberculosis chemotherapy regimens. Antimicrob Agents Chemother 57: 4237–4244, 2013. doi: 10.1128/AAC.00277-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mann HB, Whitney DR. On a test of whether one of two random variables is stochastically larger than the other. Ann Math Stat 18: 50–60, 1947. doi: 10.1214/aoms/1177730491. [DOI] [Google Scholar]
- 31.Miekisch W, Schubert JK, Noeldge-Schomburg GFE. Diagnostic potential of breath analysis—focus on volatile organic compounds. Clin Chim Acta 347: 25–39, 2004. doi: 10.1016/j.cccn.2004.04.023. [DOI] [PubMed] [Google Scholar]
- 32.Miyoshi-Akiyama T, Matsumura K, Iwai H, Funatogawa K, Kirikae T. Complete annotated genome sequence of Mycobacterium tuberculosis Erdman. J Bacteriol 194: 2770, 2012. doi: 10.1128/JB.00353-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mochalski P, King J, Klieber M, Unterkofler K, Hinterhuber H, Baumann M, Amann A. Blood and breath levels of selected volatile organic compounds in healthy volunteers. Analyst (Lond) 138: 2134–2145, 2013. doi: 10.1039/c3an36756h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O’Neil RM, Ashack RJ, Goodman FR. A comparative study of the respiratory responses to bronchoactive agents in rhesus and cynomolgus monkeys. J Pharmacol Methods 5: 267–273, 1981. doi: 10.1016/0160-5402(81)90094-2. [DOI] [PubMed] [Google Scholar]
- 35.Peña JC, Ho W-Z. Monkey models of tuberculosis: lessons learned. Infect Immun 83: 852–862, 2015. doi: 10.1128/IAI.02850-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Phillips M, Basa-Dalay V, Blais J, Bothamley G, Chaturvedi A, Modi KD, Pandya M, Natividad MPR, Patel U, Ramraje NN, Schmitt P, Udwadia ZF. Point-of-care breath test for biomarkers of active pulmonary tuberculosis. Tuberculosis (Edinb) 92: 314–320, 2012. doi: 10.1016/j.tube.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 37.Phillips M, Basa-Dalay V, Bothamley G, Cataneo RN, Lam PK, Natividad MPR, Schmitt P, Wai J. Breath biomarkers of active pulmonary tuberculosis. Tuberculosis (Edinb) 90: 145–151, 2010. doi: 10.1016/j.tube.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 38.Phillips M, Boehmer JP, Cataneo RN, Cheema T, Eisen HJ, Fallon JT, Fisher PE, Gass A, Greenberg J, Kobashigawa J, Mancini D, Rayburn B, Zucker MJ. Prediction of heart transplant rejection with a breath test for markers of oxidative stress. Am J Cardiol 94: 1593–1594, 2004. doi: 10.1016/j.amjcard.2004.08.052. [DOI] [PubMed] [Google Scholar]
- 39.Phillips M, Cataneo RN, Chaturvedi A, Kaplan PD, Libardoni M, Mundada M, Patel U, Zhang X. Detection of an extended human volatome with comprehensive two-dimensional gas chromatography time-of-flight mass spectrometry. PLoS One 8: e75274, 2013. doi: 10.1371/journal.pone.0075274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Phillips M, Cataneo RN, Condos R, Ring Erickson GA, Greenberg J, La Bombardi V, Munawar MI, Tietje O. Volatile biomarkers of pulmonary tuberculosis in the breath. Tuberculosis (Edinb) 87: 44–52, 2007. doi: 10.1016/j.tube.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 41.Pike K, Selby A, Price S, Warner J, Connett G, Legg J, Lucas JSA, Peters S, Buckley H, Magier K, Foote K, Drew K, Morris R, Lancaster N, Roberts G. Exhaled nitric oxide monitoring does not reduce exacerbation frequency or inhaled corticosteroid dose in paediatric asthma: a randomised controlled trial. Clin Respir J 7: 204–213, 2013. doi: 10.1111/j.1752-699X.2012.00306.x. [DOI] [PubMed] [Google Scholar]
- 42.Robroeks CMHHT, van Berkel JJBN, Dallinga JW, Jöbsis Q, Zimmermann LJI, Hendriks HJE, Wouters MFM, van der Grinten CPM, van de Kant KDG, van Schooten F-J, Dompeling E. Metabolomics of volatile organic compounds in cystic fibrosis patients and controls. Pediatr Res 68: 75–80, 2010. doi: 10.1203/PDR.0b013e3181df4ea0. [DOI] [PubMed] [Google Scholar]
- 43.Romoli R, Papaleo MC, de Pascale D, Tutino ML, Michaud L, LoGiudice A, Fani R, Bartolucci G. Characterization of the volatile profile of Antarctic bacteria by using solid-phase microextraction-gas chromatography-mass spectrometry. J Mass Spectrom 46: 1051–1059, 2011. doi: 10.1002/jms.1987. [DOI] [PubMed] [Google Scholar]
- 44.Rudnicka J, Kowalkowski T, Ligor T, Buszewski B. Determination of volatile organic compounds as biomarkers of lung cancer by SPME-GC-TOF/MS and chemometrics. J Chromatogr B Analyt Technol Biomed Life Sci 879: 3360–3366, 2011. doi: 10.1016/j.jchromb.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 45.Statheropoulos M, Sianos E, Agapiou A, Georgiadou A, Pappa A, Tzamtzis N, Giotaki H, Papageorgiou C, Kolostoumbis D. Preliminary investigation of using volatile organic compounds from human expired air, blood and urine for locating entrapped people in earthquakes. J Chromatogr B Analyt Technol Biomed Life Sci 822: 112–117, 2005. doi: 10.1016/j.jchromb.2005.05.028. [DOI] [PubMed] [Google Scholar]
- 46.Tidmarsh GF, Wong RJ, Stevenson DK. End-tidal carbon monoxide and hemolysis. J Perinatol 34: 577–581, 2014. doi: 10.1038/jp.2014.66. [DOI] [PubMed] [Google Scholar]
- 47.Tomikawa M, Ogura K, Iikura K, Yanagida N, Sato S, Komata T, Shukuya A, Koike Y, Ebisawa M. Asthma diagnosis and treatment - 1005. Optimization for the withdrawal of inhaled corticosteroid treatment by monitoring fractional exhaled nitric oxide (feno) and lung functions. World Allergy Organ J 6, Suppl 1: P5, 2013. doi: 10.1186/1939-4551-6-S1-P5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47a.US Department of Health and Human Services, Centers for Disease Control and Prevention, National Institutes of Health. Biosafety in microbiological and biomedical laboratories. US Government Printing Office, Washington, 2007. [Google Scholar]
- 48.Van Berkel JJBN, Dallinga JW, Möller GM, Godschalk RWL, Moonen E, Wouters EFM, Van Schooten FJ. Development of accurate classification method based on the analysis of volatile organic compounds from human exhaled air. J Chromatogr B Analyt Technol Biomed Life Sci 861: 101–107, 2008. doi: 10.1016/j.jchromb.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 49.Van Berkel JJBN, Dallinga JW, Möller GM, Godschalk RWL, Moonen EJ, Wouters EFM, Van Schooten FJ. A profile of volatile organic compounds in breath discriminates COPD patients from controls. Respir Med 104: 557–563, 2010. doi: 10.1016/j.rmed.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 50.Filipiak W, Beer R, Sponring A, Filipiak A, Ager C, Schiefecker A, Lanthaler S, Helbok R, Nagl M, Troppmair J, Amann A. Breath analysis for in vivo detection of pathogens related to ventilator-associated pneumonia in intensive care patients: a prospective pilot study. J Breath Res 9: 016004, 2015. doi: 10.1088/1752-7155/9/1/016004. [DOI] [PubMed] [Google Scholar]
- 51.Zhu J, Bean HD, Jiménez-Díaz J, Hill JE. Secondary electrospray ionization-mass spectrometry (SESI-MS) breathprinting of multiple bacterial lung pathogens, a mouse model study. J Appl Physiol 114: 1544–1549, 2013. doi: 10.1152/japplphysiol.00099.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]