Abstract
Nucleosomes are the dominant autoantigens in patients with systemic lupus erythematosus (SLE), and immune complexes involving nucleosomes are the major cause of tissue damage. The activity of DNase I, the enzyme responsible for nucleosome degradation, has been found to be decreased in patients with SLE. However, it is not known whether DNase activity is a clinically useful parameter. The aim of our study was to assess DNase activity in a prospective study of 113 patients with SLE in relation to disease activity and organ involvement. We included two control groups: 9 patients with undifferentiated connective tissue disease (UCTD) and 14 healthy individuals. DNase activity was found to be lower in patients with SLE (63.75% ± 12.1%) than in the controls (81.3% ± 9.25%) (P < 0.001). DNase activity in patients with UCTD (64.9% ± 18.2%; P = 0.854) did not differ from that in patients with SLE. Patients with SLE had higher antinucleosome antibody titers (356.3 ± 851) than the controls (1.44 ± 2.77; P < 0.01) or UCTD patients (39.9 ± 57.7; P < 0.01). In addition, samples positive for antinucleosome antibodies displayed low levels of DNase activity. Within the SLE group, the presence of renal disease had no impact on DNase activity or antinucleosome antibody titers. Also, the SLE disease activity index showed no correlation with DNase activity. In a longitudinal study of six SLE patients, DNase activity did not follow disease activity or autoantibody titers. Our results confirm that serum DNase activity is decreased in patients with SLE, but we conclude that it is not a clinically useful parameter for the prediction of flare-ups of disease or renal involvement.
Systemic lupus erythematosus (SLE) is an autoimmune disease characterized by the production of a wide range of pathological autoantibodies. Those directed against chromatin components, e.g., double-stranded DNA (dsDNA), histones, and the nucleosome, are of paramount pathological importance (6, 8, 20).
Recent studies of patients with SLE suggest the increasing diagnostic importance of antinucleosome antibodies, in addition to antibodies directed against dsDNA (1, 17). These circulating antibodies may form immune complexes with their target antigens, the glomerular deposition of which will lead to the development of renal damage (12, 14).
The incidence of immune complex-mediated glomerulonephritis (GN) among SLE patients varies from 30 to 60%. Several studies have confirmed that autoantibodies are produced through an antigen-driven T-cell-dependent mechanism (13, 23, 27). According to this model, the defective clearance of apoptotic cell debris predisposes individuals to SLE through the accumulation of the chromatin components arising from the dying cells (5, 28).
DNase I (pancreatic DNase) and DNase II (spleen acid DNase) cleave nucleosomal DNA, which promotes the disposal of circulating nuclear material. DNase I, a glycoprotein with a molecular mass of 30,400 Da, is a cation-binding secretory endonuclease that digests dsDNA in a sequence-dependent manner (24). DNase II, a glycoprotein with a molecular mass of 45 kDa, is an endonuclease with an acidic pH optimum and no requirement for bivalent cations. It is present in lysosomes, nuclei, and some secretions (16).
For a long time it has been suspected that defects in DNase I may play a role in the development of SLE and lupus nephritis (11). Studies of SLE-prone or DNase I-deficient mouse strains have confirmed this model (15, 19). We set out to test the hypothesis that SLE patients have decreased serum DNase activity compared to those of healthy controls and patients with undifferentiated connective tissue disease (UTCD), a condition related to SLE.
We also investigated the differences in DNase activities and serum antinucleosome levels between two subgroups of SLE patients, those with and without renal involvement. Finally, the relation between DNase activity and SLE disease activity was also studied.
MATERIALS AND METHODS
A total of 113 SLE patients (33 with active GN and 18 with a history of GN) were enrolled in the study, after they provided informed consent. Of these 113 patients, 105 were females and 8 were males (mean ± standard deviation [SD] age, 38.3 ± 14.1 years; age range, 13 to 79 years).
A total of 185 serum samples were obtained from these patients. Patients were monitored at three outpatient clinics of Semmelweis Medical University, Budapest, Hungary, and all fulfilled the revised criteria for SLE of the American College of Rheumatology (25). The sera from nine patients with UCTD (18) (7 females and 2 males; mean ± SD age, 45.8 ± 11.9 years) and from 14 healthy individuals (11 females and 3 males; mean ± SD age, 43 ± 22.1 years) were used as controls. Venous blood samples were taken without anticoagulation; sera were stored at −20°C for up to 1 month. Long-term storage was performed at −80°C. If more than one serum sample was available, the patients in the cross-sectional studies were characterized by their mean DNase and antibody levels. Individual data were used for longitudinal studies.
Antinucleosome and anti-dsDNA antibody levels were measured by an enzyme-linked immunosorbent assay (ELISA; Orgentec GmbH, Mainz, Germany), according to the instructions of the manufacturer. Serum DNase activity was also measured by an ELISA (Orgentec), according to the instructions of the manufacturer. Briefly, DNase enzyme from patients' sera was allowed to react with the specific substrate coated onto the plate during incubation at 37°C for an hour, followed by the addition of horseradish peroxidase-conjugated antibodies to the residual DNase substrate. The developing color of the tetramethylbenzidine substrate is in negative correlation with the amount of active DNase present in the serum sample tested. A standard series was included with the kit.
All determinations were done in two replicates. Samples for longitudinal studies were measured together to preclude the errors derived from interassay variability.
SLE disease activity was expressed by the SLE disease activity index (SLEDAI) (7). The data were first analyzed by descriptive statistics. Student's t test was used for comparison of the mean DNase activities, as this parameter was found to have a normal distribution in the groups investigated. Continuous but not normally distributed variables, such as the antibody levels, were compared by the Mann-Whitney U test. The Spearman (nonparametric) rank-order correlation coefficient (rS) was determined to characterize the correlations between variables.
RESULTS
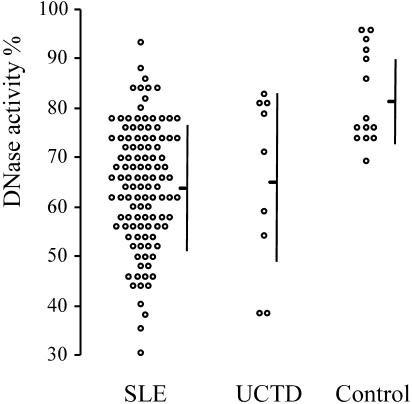
The DNase activity in the sera of SLE patients was found to be lower than that in the sera of the controls (means ± SDs, 63.75% ± 12.1% and 81.3% ± 9.25%, respectively; P < 0.001) (Fig. 1). The normal range of DNase activity is 70 to 100%, according to the kit manufacturer.
FIG. 1.
Serum DNase activity in three groups of patients. Mean ± SD DNase activities are shown. The 113 SLE patients had significantly decreased DNase activity (63.75% ± 12.1%) compared with that for the 14 controls (81.3% ± 9.25%) (P < 0.001). The sera of the nine UCTD patients also presented decreased DNase activity (64.9% ± 18.2%), similar to the values for the SLE patients but not significantly different from those for the controls (P = 0.0295).
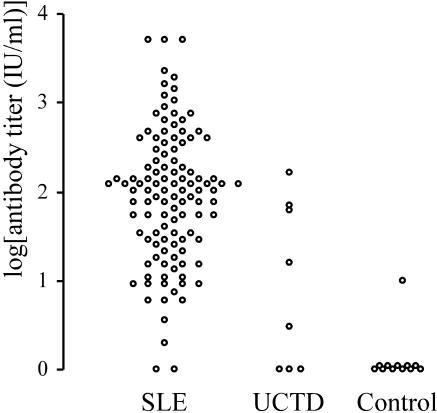
SLE patients had significantly higher antinucleosome antibody titers (356.3 ± 851 IU/ml) than the control group (1.44 ± 2.77 IU/ml) (P < 0.01) (Fig. 2).
FIG. 2.
Serum antinucleosome antibody titers in three group of patients. The serum antinucleosome antibody levels in 136 individuals are shown. The patients with SLE had significantly higher titers (356.3 ± 851 IU/ml; P < 0.01) than the UCTD patients (39.9 ± 57.7 IU/ml) and the controls (1.44 ± 2.77 IU/ml). No significant difference between the UCTD group and the nonsystemic autoimmune control group was detected (P = 0.167).
The DNase activity in the group of SLE patients without renal involvement (65.48% ± 13.04%) did not differ significantly from those in individuals with active GN (60.95% ± 11.23%; P = 0.082) or those with a history of GN (63.5% ± 10.32%; P = 0.505). The difference in the DNase activities between the two groups of patients with present and former renal involvement was not significant (P = 0.420).
The antinucleosome antibody levels were almost equally high in the following three groups of SLE patients: patients without GN (459 ± 1,120 IU/ml), patients with active GN (247 ± 344 IU/ml), and patients with a history of GN (171 ± 183 IU/ml). No statistically significant difference was found between these values (P > 0.05 for all comparisons).
The UCTD patients had decreased serum DNase activity, similar to the SLE patients (64.9% ± 18.2% and 63.75% ± 12.1%, respectively; P = 0.854), but their serum DNase activity was not significantly different from that of the control group (P = 0. 295) (Fig. 1).
The antinucleosome antibody titers in the sera of the UCTD patients were significantly lower than those in the sera of the SLE patients (39.9 ± 57.7 and 356.3 ± 851 IU/ml, respectively; P < 0.01) and were significantly higher than those in the sera of the controls (39.9 ± 57.7 and 1.44 ± 2.77 IU/ml, respectively; P < 0.01) (Fig. 2).
Serum DNase activity negatively correlated with the logarithmic value of the serum antinucleosome antibody concentration (rS = −0.256). This correlation was found to be statistically significant (P < 0.01). In support of an association between antinucleosome antibody production and DNase levels, we found that SLE patients with positive antinucleosome antibody test results (defined as a titer ≥20 IU/ml), as a group, had significantly lower DNase activities than their antinucleosome-negative counterparts (63.3% ± 13.3% and 71.7% ± 13.0%, respectively; P < 0.001).
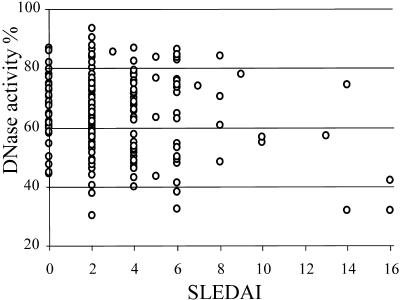
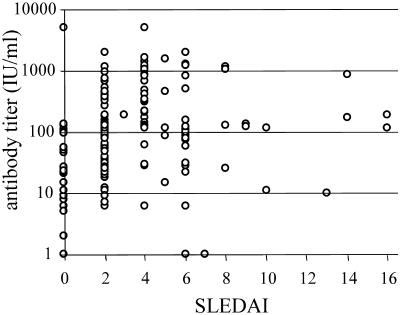
No correlation between DNase activity and SLEDAI (rS = −0.073; Fig. 3) was found within the SLE group. Not surprisingly, log antinucleosome antibody levels correlated significantly with SLEDAIs (rS = 0.369; P < 0.01) (Fig. 4), as did the log anti-dsDNS levels (rS = 0.494; P < 0.01).
FIG. 3.
Relation of serum DNase activity to SLEDAI. The DNase activities of the 208 samples studied according to the SLEDAI scores (presented on the x axis) for the patients at the time of blood taking are shown. No significant correlation between the two parameters was observed, although a slight tendency was observed.
FIG. 4.
Relation of serum antinucleosome antibody titer to SLEDAI. The serum antinucleosome antibody titers of the 208 samples studied are shown according to the SLEDAI scores (presented on the x axis) for the patients at the time of blood taking. A significant correlation (rS = 0.369; P < 0.01) can be observed between the two parameters.
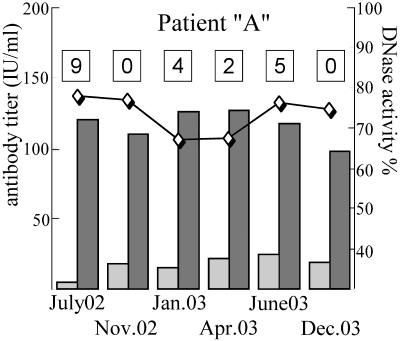
During a longitudinal study, we found that DNase activity in the sera of six patients did not follow the SLEDAI score or the antinucleosome or anti-dsDNA levels (Fig. 5).
FIG. 5.
Follow-up of an SLE patient. The parameters for six samples from an SLE patient are shown. The dates of blood taking are shown under the bars. Bars show anti-DNA and antinucleosome antibody levels (scale on the left); the line with rhombuses shows the relative DNase activity (scale on the right). The SLEDAI scores for the patient at the time of blood taking are shown in the boxes at the top.
DISCUSSION
Our observations confirm previous reports (11, 26) that DNase enzyme activity is significantly decreased in the sera of SLE patients compared to those in the sera of healthy individuals.
The sera from the small group of patients with UCTD showed similarly decreased DNase activity. Monitoring of these patients should clarify whether UCTD patients with reduced DNase activity are more prone to the development of SLE than those with nearly normal DNase activity.
The reasons for the reduced enzyme activity have not been determined. A mutation in the second exon of the DNASE1 gene has been reported (29), but its low prevalence does not explain the decreased activity of the enzyme in most SLE patients (3, 10, 21, 26).
There are conflicting reports on an inhibitor responsible for the reduced DNase activity. Some suggest the presence (30) and others suggest the absence (11, 26) of such a factor, thought to be an antibody (30).
One might expect lower DNase activity in lupus patients with GN. However, we did not find a significant difference between the DNase activity of our patient groups with and without lupus nephritis. This indicates that reduced DNase activity is but one factor involved in the development of immune complex-mediated GN.
In this study, antibodies against nucleosomes were found in 79.7% of SLE patients. Other groups report prevalences ranging from 37.5 to 100% (4, 9, 17, 22). Although antinucleosome antibody titers show a correlation with SLEDAI, the association is probably a consequence of the strong correlation between antinucleosome and anti-dsDNA antibody titers, with the latter being an important component of SLEDAI. Similar results have been published previously (17, 22), but not all investigators have found the same correlation (2), perhaps due to the characterization of disease activity by the use of scoring systems other than SLEDAI (European Consensus Lupus Activity Measurement [ECLAM] or Systemic Lupus Activity Measure [SLAM]) (4, 9). It is of interest that one of the studies with negative results used the same kit used in the present work to measure antinucleosome antibody titers (9).
The balance between the levels of circulating nucleosomes, DNase, and antinucleosome antibodies is likely dynamic. Therefore, it is difficult to prove a causative role of decreased DNase levels and, consequently, elevated nucleosome antigen levels in any cross-sectional study. For example, Amoura et al. (2) found that SLE patients with increased circulating nucleosome antigen levels have low antinucleosome antibody levels, suggesting that the formation of immune complexes is a process involved in the clearance of intact nucleosomes, while the activity of DNase, another potential clearance mechanism, was not investigated. These data by Amoura et al. (2) do not contradict the pathogenic role of low DNase levels in SLE. Clear proof of the possible association would require simultaneous investigation of DNase activity and circulating nucleosome and antinucleosome antibody levels.
In summary, our results indicate that reduced serum DNase activity is characteristic of SLE but is not a clinically useful parameter for the prediction of disease activity or renal involvement.
Acknowledgments
This work was supported by a grant from the Hungarian National Science Foundation (grant T037646).
REFERENCES
- 1.Amoura, Z., S. Koutouzov, and J. C. Piette. 2000. The role of nucleosomes in lupus. Curr. Opin. Rheumatol. 12:369-373. [DOI] [PubMed] [Google Scholar]
- 2.Amoura, Z., J. C. Piette, H. Chabre, P. Cacoub, T. Papo, B. Wechsler, J. F. Bach, and S. Koutouzov. 1997. Circulating plasma levels of nucleosomes in patients with systemic lupus erythematosus. Arthritis Rheum. 40:2217-2225. [DOI] [PubMed] [Google Scholar]
- 3.Balada, E., J. Ordi-Ros, S. Hernanz, J. Villareal, F. Cortes, M. Vilardell-Tarres, and M. Labrador. 2002. DNASE I mutation and systemic lupus erythematosus in a Spanish population: comment on the article by Tew et al. Arthritis Rheum. 46:1974-1976. [DOI] [PubMed] [Google Scholar]
- 4.Benucci, M., F. L. Gobbi, A. Del Rosso, S. Cesaretti, L. Niccoli, and F. Cantini. 2003. Disease activity and antinucleosome antibodies in systemic lupus erythematosus. Scand. J. Rheumatol. 32:42-45. [DOI] [PubMed] [Google Scholar]
- 5.Berden, J. H., C. Grootscholten, W. C. Jurgen, and J. van der Vlag. 2002. Lupus nephritis: a nucleosome waste disposal defect? J. Nephrol. 15(Suppl. 6):s1-s10. [PubMed] [Google Scholar]
- 6.Blatt, N. B., and G. D. Glick. 1999. Anti-DNA autoantibodies and systemic lupus erythematosus. Pharmacol. Ther. 83:125-139. [DOI] [PubMed] [Google Scholar]
- 7.Bombardier, C., D. D. Gladman, M. B. Urowitz, D. Caron, C. H. Chang, et al. 1992. Derivation of the SLEDAI: a disease activity index for lupus patients. Arthritis Rheum. 35:630-640. [DOI] [PubMed] [Google Scholar]
- 8.Burlingame, R. W., and R. Cervera. 2002. Anti-chromatin (anti-nucleosome) autoantibodies. Autoimmun. Rev. 1:321-328. [DOI] [PubMed] [Google Scholar]
- 9.Cairns, A. P., S. A. McMillan, A. D. Crockard, G. K. Meenagh, E. M. Duffy, D. J. Armstrong, and A. L. Bell. 2003. Antinucleosome antibodies in the diagnosis of systemic lupus erythematosus. Ann. Rheum. Dis. 62:272-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chakraborty, P., H. H. Kacem, K. Makni-Karray, F. Jarraya, J. Hachicha, and H. Ayadi. 2003. The A/T mutation in exon 2 of the DNASE1 gene is not present in Tunisian patients with systemic lupus erythematosus or in healthy subjects. Arthritis Rheum. 48:3297-3298. [DOI] [PubMed] [Google Scholar]
- 11.Chitrabamrung, S., R. L. Rubin, and E. M. Tan. 1981. Serum deoxyribonuclease I and clinical activity in systemic lupus erythematosus. Rheumatol. Int. 1:55-60. [DOI] [PubMed] [Google Scholar]
- 12.Cortes-Hernandez, J., J. Ordi-Ros, M. Labrador, S. Bujan, E. Balada, A. Segarra, and M. Vilardell-Tarres. 2004. Antihistone and anti-double-stranded deoxyribonucleic acid antibodies are associated with renal disease in systemic lupus erythematosus. Am. J. Med. 116:165-173. [DOI] [PubMed] [Google Scholar]
- 13.Herrmann, M., O. M. Zoller, M. Hagenhofer, R. Voll, and J. R. Kalden. 1996. What triggers anti-dsDNA antibodies? Mol. Biol. Rep. 23:265-267. [DOI] [PubMed] [Google Scholar]
- 14.Koffler, D., V. Agnello, R. Thoburn, and H. G. Kunkel. 1971. Systemic lupus erythematosus: prototype of immune complex nephritis in man. J. Exp. Med. 134(Suppl.):169s. [PubMed] [Google Scholar]
- 15.Macanovic, M., and P. J. Lachmann. 1997. Measurement of deoxyribonuclease I (DNase) in the serum and urine of systemic lupus erythematosus (SLE)-prone NZB/NZW mice by a new radial enzyme diffusion assay. Clin. Exp. Immunol. 108:220-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.MacLea, K. S., R. J. Krieser, and A. Eastman. 2002. Revised structure of the active form of human deoxyribonuclease IIα. Biochem. Biophys. Res. Commun. 292:415-421. [DOI] [PubMed] [Google Scholar]
- 17.Min, D. J., S. J. Kim, S. H. Park, Y. I. Seo, H. J. Kang, W. U. Kim, C. S. Cho, and H. Y. Kim. 2002. Anti-nucleosome antibody: significance in lupus patients lacking anti-double-stranded DNA antibody. Clin. Exp. Rheumatol. 20:13-18. [PubMed] [Google Scholar]
- 18.Mosca, M., R. Neri, and S. Bombardieri. 1999. Undifferentiated connective tissue diseases (UCTD): a review of the literature and a proposal for preliminary classification criteria. Clin. Exp. Rheumatol. 17:615-620. [PubMed] [Google Scholar]
- 19.Napirei, M., H. Karsunky, B. Zeynik, H. Stephan, H. G. Mannherz, and T. Moroy. 2000. Features of systemic lupus erythematosus in Dnase1-deficient mice. Nat. Genet. 25:177-181. [DOI] [PubMed] [Google Scholar]
- 20.Pisetsky, D. S. 1998. Antibody responses to DNA in normal immunity and aberrant immunity. Clin. Diagn. Lab. Immunol. 5:1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simmonds, M. J., J. M. Heward, M. A. Kelly, A. Allahabadia, H. Foxall, C. Gordon, J. A. Franklyn, and S. C. Gough. 2002. A nonsense mutation in exon 2 of the DNase I gene is not present in UK subjects with systemic lupus erythematosus and Graves' disease: comment on the article by Rood et al. Arthritis Rheum. 46:3109-3110. [DOI] [PubMed] [Google Scholar]
- 22.Simon, J. A., J. Cabiedes, E. Oritz, J. Alcocer-Varela, and J. Sanchez-Guerrero. 2004. Anti-nucleosome antibodies in patients with systemic lupus erythematosus of recent onset. Potential utility as a diagnostic tool and disease activity marker. Rheumatology (Oxford) 43:220-224. [DOI] [PubMed] [Google Scholar]
- 23.Spronk, P. E., G. Horst, B. T. van der Gun, P. C. Limburg, and C. G. Kallenberg. 1996. Anti-dsDNA production coincides with concurrent B and T cell activation during development of active disease in systemic lupus erythematosus (SLE). Clin. Exp. Immunol. 104:446-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suck, D., and C. Oefner. 1986. Structure of DNase I at 2.0 Å resolution suggests a mechanism for binding to and cutting DNA. Nature 321:620-625. [DOI] [PubMed] [Google Scholar]
- 25.Tan, E. M., A. S. Cohen, J. F. Fries, A. T. Masi, D. J. McShane, N. F. Rothfield, J. G. Schaller, N. Talal, and R. J. Winchester. 1982. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 25:1271-1277. [DOI] [PubMed] [Google Scholar]
- 26.Tew, M. B., R. W. Johnson, J. D. Reveille, and F. K. Tan. 2001. A molecular analysis of the low serum deoxyribonuclease activity in lupus patients. Arthritis Rheum. 44:2446-2447. [DOI] [PubMed] [Google Scholar]
- 27.Van Bruggen, M. C., C. Kramers, and J. H. Berden. 1996. Autoimmunity against nucleosomes and lupus nephritis. Ann. Med. Interne (Paris) 147:485-489. [PubMed] [Google Scholar]
- 28.Walport, M. J. 2000. Lupus, DNase and defective disposal of cellular debris. Nat. Genet. 25:135-136. [DOI] [PubMed] [Google Scholar]
- 29.Yasumoto, K., T. Horiuchi, S. Kagami, H. Tsukamoto, C. Hashimura, M. Urushihara, and Y. Kuroda. 2001. Mutation of DNASE1 in people with systemic lupus erythematosus. Nat. Genet. 28:313-314. [DOI] [PubMed] [Google Scholar]
- 30.Yeh, T. M., H. C. Chang, C. C. Liang, J. J. Wu, and M. F. Liu. 2003. Deoxyribonuclease-inhibitory antibodies in systemic lupus erythematosus. J. Biomed. Sci. 10:544-551. [DOI] [PubMed] [Google Scholar]