It is currently well established that poorly controlled diabetes reduces both skeletal muscle mass and muscle capillarization. These muscle-specific features of diabetes may, in turn, compromise insulin sensitivity and glucose control. Using a model of streptozotocin-induced diabetes, we show the vascular complications linked with disease and how chronic exposure to exercise and prazosin (an α1-adrenergic antagonist) can reduce these complications and improve glycemic control.
Keywords: diabetes, exercise, capillaries, muscle, prazosin
Abstract
Type-1 diabetes mellitus (T1D) causes impairments within the skeletal muscle microvasculature. Both regular exercise and prazosin have been shown to improve skeletal muscle capillarization and metabolism in healthy rats through distinct angiogenic mechanisms. The aim of this study was to evaluate the independent and additive effects of voluntary exercise and prazosin treatment on capillary-to-fiber ratio (C:F) in streptozotocin (STZ)-treated diabetic rats. STZ (65 mg/kg) was intraperitoneally administered to male Sprague-Dawley rats (n = 36) to induce diabetes, with healthy, nondiabetic, sedentary rats (n = 10) as controls. The STZ-treated rats were then divided into sedentary (SED) or exercising (EX; 24-h access to running wheels) groups and then further subdivided into prazosin (Praz) or water (H2O) treatment groups: nondiabetic-SED-H2O, STZ-SED-H2O, STZ-EX-H2O, STZ-SED-Praz, and STZ-EX-Praz. After 3 wk, untreated diabetes significantly reduced the C:F in tibialis anterior (TA) and soleus muscles in the STZ-SED-H2O animals (both P < 0.05). Voluntary exercise and prazosin treatment independently resulted in a normalization of C:F within the TA (1.86 ± 0.12 and 2.04 ± 0.03 vs 1.71 ± 0.09, P < 0.05) and the soleus (2.36 ± 0.07 and 2.68 ± 0.14 vs 2.13 ± 0.12, P < 0.05). The combined STZ-EX-Praz group resulted in the highest C:F within the TA (2.26 ± 0.07, P < 0.05). Voluntary exercise volume was negatively correlated with fed blood glucose levels (r2 = −0.7015, P < 0.01) and, when combined with prazosin, caused further enhanced nonfasted glucose (P < 0.01). Exercise and prazosin reduced circulating nonesterified fatty acids more than either stimulus alone (P < 0.05). These results suggest that the distinct stimulation of angiogenesis, with both regular exercise and prazosin treatment, causes a cooperative improvement in the microvascular complications associated with T1D.
NEW & NOTEWORTHY It is currently well established that poorly controlled diabetes reduces both skeletal muscle mass and muscle capillarization. These muscle-specific features of diabetes may, in turn, compromise insulin sensitivity and glucose control. Using a model of streptozotocin-induced diabetes, we show the vascular complications linked with disease and how chronic exposure to exercise and prazosin (an α1-adrenergic antagonist) can reduce these complications and improve glycemic control.
type-1 diabetes mellitus (T1D) is a chronic autoimmune disease targeting the pancreatic β-cells resulting in little to no insulin production and hyperglycemia (8). Despite exogenous insulin therapy, individuals with diabetes have an increased risk for long-term microvascular and macrovascular complications, which can significantly impact their morbidity and mortality (7, 10). T1D is also associated with impaired angiogenesis in skeletal muscle (1, 38). It is generally held that hyperglycemia itself, or some metabolic byproduct of hyperglycemia, induces remodeling of capillaries within the skeletal muscle, resulting in a lower capillary-to-fiber ratio (C:F) and ultimately affecting regional hemodynamic regulation (26, 41).
New capillary growth within the skeletal muscle occurs via existing capillaries through two morphologically different, and separately inducible, forms of physiological angiogenesis termed sprouting or nonsprouting angiogenesis (13). The growth of new capillaries in response to exercising muscle is a highly regulated process (16), which is stimulated by increased functional hyperemia and shear stress (19), mechanical stretch of the tissue in addition to increases in metabolic demand, or reduced oxygen delivery (12). These signals modulate the expression and activity of proangiogenic and angiostatic factors, which act together to regulate sprouting angiogenesis (13).
In addition to insulin therapy, regular exercise is an established management strategy for T1D, improving glucose uptake and insulin sensitivity within skeletal muscle (15). Additional health benefits include increased cardiorespiratory fitness, improved endothelial function, increased vascular health, and quality of life (6, 15). Regular endurance exercise increases skeletal muscle capillarization in healthy individuals (22). However, the effects of endurance training on changes in capillarization in diabetic animals and patients are contradictory (20, 27, 29, 44). Vascular function has been shown to improve with physical activity in both animal models of diabetes (27) and in patients with T1D (6), but these alterations were unable to fully restore the diabetes-induced defects (14, 33).
Prazosin, an α1-adrenergic antagonist, increases skeletal muscle capillarization via a vascular endothelial growth factor (VEGF)- and endothelial nitric oxide synthase-dependent pathway (eNOS) (5, 45). These two mechanisms of action for prazosin ultimately cause longitudinal splitting of existing capillaries (46), which is distinct from the angiogenic, or sprouting, mechanisms that are observed with exercise. Prazosin has been used in many rodent studies (3, 9, 47) to selectively increase skeletal muscle C:F, but it has yet to be used as an agent to stimulate angiogenesis in an animal model of T1D. Chronic treatment with prazosin in streptozotocin (STZ)-induced diabetic rats reduces elevations in blood pressure and cholesterol levels and improves cardiac function (18), but its effect in muscle is unknown in the STZ model. In humans with type 2 diabetes, prazosin enhances hepatic function (11, 34), improves insulin sensitivity, and lowers lipid levels (21, 32), in addition to decreasing blood pressure (11, 21, 32, 34).
The aim of this study was to evaluate the independent and combined effects of exercise and prazosin administration on skeletal muscle capillary content in the STZ-induced rodent model of T1D.
METHODS
This study was carried out in accordance with the recommendations of the Canadian Council for Animal Care guidelines and was approved by the York University Animal Care Committee (2013–5). The Guide for the Care and Use of Laboratory Animals (8th ed., 2011) was followed.
Animals.
Adult, male Sprague-Dawley rats (Charles River Laboratories; initial mass of 225–250 g, n = 46) were individually housed (lights on 12-h cycle: lights off 12-h cycle) after 1 wk of acclimatization to room temperature (22–23°C)- and humidity (50–60%)-controlled facilities.
Experimental design.
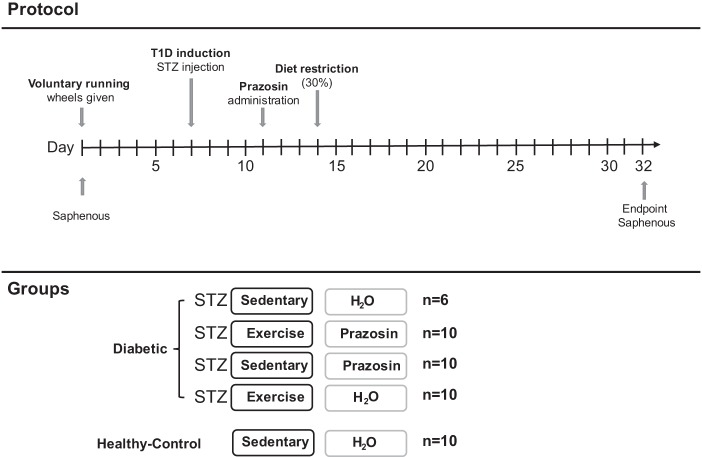
A timeline of the experimental protocol is shown in Fig. 1. All animals had access to voluntary running wheels for 1 wk. Each exercising animal was placed into rodent cages with 24-h access to a running wheel (Harvard Apparatus), while sedentary animals were housed in standard cages. Running distance was assessed daily. Seven days after wheel assignment, a single injection of STZ (65 mg/kg) was administered to induce diabetes. Animals were also provided with sugar water (20% sucrose) to assist with diabetes development, and any exercising animals had their wheels removed overnight to further promote diabetes induction. Two days after diabetes inducement, animals were divided into one of five groups: nondiabetic-SED-H2O (control), STZ-SED-H2O, and STZ-EX-H2O, all given regular drinking water; and STZ-SED-Praz and STZ-EX-Praz, all given drinking water containing prazosin hydrochloride (5 mg/kg; P7791; Sigma-Aldrich). Five days after diabetes induction, all animals had their food reduced to 30% of their total body weight to improve glycemia in the diabetic animals. Body mass, fed blood glucose, food intake, and fluid consumption were measured daily for each rodent and any changes in the rodents' health were noted and monitored.
Fig. 1.
Schematic of the experimental design. Basal saphenous vein measurement for corticosterone and nonesterified fatty acid (NEFA) concentrations occurred on day 1 before the provision of voluntary running wheels. Streptozotocin (STZ) was administered on day 7, prazosin on day 11 and on day 14 animals had their food reduced to 30% of their total body weight to improve glycemia in the diabetic animals. Animals were euthanized on day 32 (end point) with a final saphenous vein sample. T1D, type 1 diabetes.
Blood glucose, nonesterified fatty acid, and corticosterone sampling.
Blood glucose values were measured with a glucometer (AlphaTRAK; Abbott Laboratories) and 5 μl of blood from the tip of the tail. Blood samples were collected from each animal via saphenous vein for nonesterified fatty acid (NEFA kit, HR Series NEFA-HR; Wako Chemicals) and corticosterone (MP Biomedical) concentration measurements on days 1 (basal) and 32. Glucose area under the curve (AUC) was measured relative to each animal’s individual day 11 glucose value and the net area was used to account for the lowering in blood glucose observed in both exercising groups.
Capillary-to fiber analysis.
Skeletal muscle, tibialis anterior (TA), and soleus, from euthanized animals, was embedded in tissue freezing medium, frozen in liquid nitrogen, and cryosectioned (10-µm thick). TA and soleus sections (10-µm thick) were fixed with 3.7% paraformaldehyde before being stained with fluorescein isothiocyanate-conjugated Griffonia simplicifolia isolectin B4 (1:100; Vector Laboratories). Sections were viewed using a Zeiss M200 inverted microscope with a ×20 objective and images were captured using Metamorph imaging software. Capillary-to-fiber counts were averaged from five to seven independent fields of view per animal by a blinded observer, and ~45 fibers/image were counted.
Western blotting.
Immunoblotting was carried out on protein extracts from rat soleus or TA muscles as previously described (2). Frozen muscle (20-40 mg) was mixed at 4°C with RIPA buffer. For each sample, protein extracts were prepared using two stainless carbide beads (Retsch, Fisher Scientific, Montreal, Canada) in the Retsch MM400 tissue lyser (30 pulses/s; Retsch, Haan, Germany). Denatured samples (30 µg/well) were subjected to SDS-PAGE and blotted onto nitrocellulose membranes. After blocking with 5% fat-free milk at room temperature for 45 min, the blots were probed overnight at 4°C with primary antibodies against the following proteins: β-actin (sc-47778; Santa Cruz Biotechnology, Santa Cruz, CA), murine double minute-2 (Mdm2; noncommercial clone 2A10 (2, 39, 40), VEGF (clone VG-1; 05–1117; Millipore, Etobicoke, ON, Canada), or TSP-1 (clone A6.1; MS-421-P0; Invitrogen, Burlington, ON, Canada). After incubation with secondary antibody (cat. no. P0260; Dako, Carpinteria, CA), proteins were visualized with chemiluminescence (Millipore) on Imaging Station 4000MM Pro (Carestream Health, Rochester, NY) or on X-ray film (CL-XPosure Film; prod. no. 34090; Thermo Scientific, Rockford, IL). Blots were analyzed with Carestream software.
Statistical analysis.
All data are represented as means ± SE, with a criterion of P < 0.05 and P < 0.01 and were assessed as stated using two-way ANOVAs as a means of statistical significance. Individual differences were calculated using Bonferroni’s post hoc test (Statistica, StatSoft). A t-test was also used to compare values for nondiabetic and STZ-treated rats.
RESULTS
Capillary-to-fiber content.
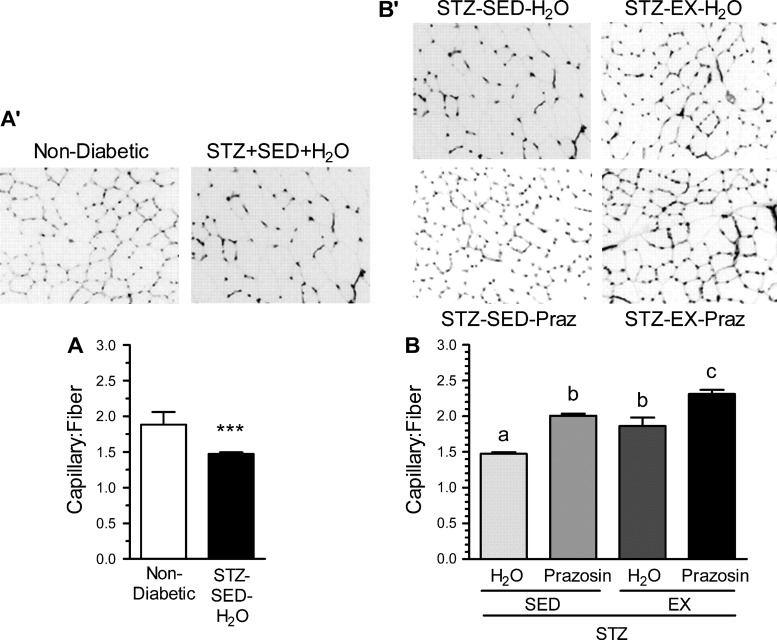
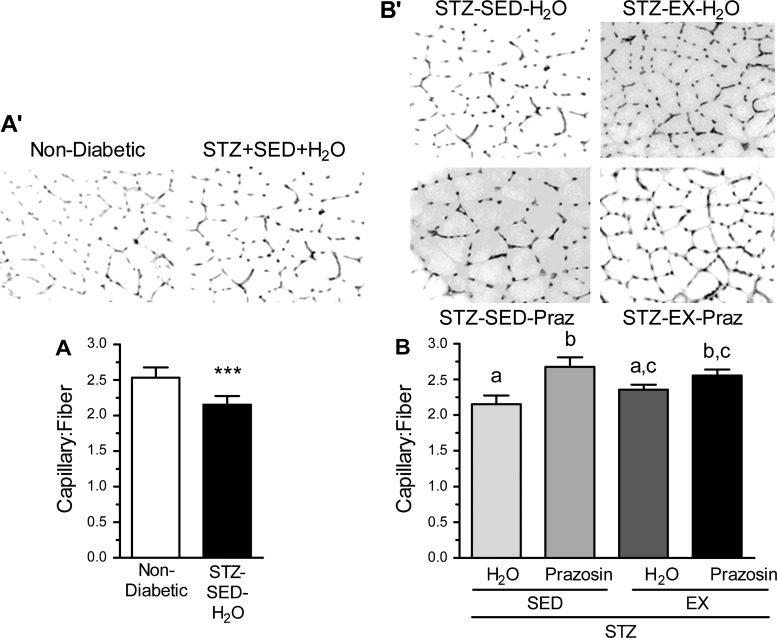
The effect of STZ-induced diabetes on the skeletal muscle microvasculature was evaluated using the C:F, expressed relative to fiber number to account for diabetes-related muscle atrophy. There was obvious capillary rarefaction in the TA of the STZ-SED-H2O group when compared with the nondiabetic, control group (Fig. 2, A and A′, P < 0.001), which was paralleled within the soleus of the STZ-SED-H2O animals (Fig. 3, A and A′, P < 0.001).
Fig. 2.
Capillary-to-fiber ratio (C:F) in the tibialis anterior in nondiabetic and STZ-treated rats (A and A′) and after 20 days of voluntary exercise and prazosin cotreatment (B and B′). All values are means ± SE (n = 3–5 per group). SED, sedentary; EX, exercise; Praz, prazosin. ***Significantly different from nondiabetic group (P < 0.05). Different letters (a, b, and c) indicate a significant difference (main effect treatment and activity) between groups (P < 0.001), following two-way ANOVA (post hoc test).
Fig. 3.
C:F in the soleus of nondiabetic and STZ-treated rats (A and A′) and after 20 days of voluntary exercise and prazosin cotreatment (B and B′). All values are means ± SE (n = 5 per group). ***Significantly different from nondiabetic group (P < 0.001). Different letters (a, b, and c) indicate a significant difference (main effect of treatment) between groups (P < 0.05), using a two-way ANOVA (post hoc test).
Within the TA, C:F for both the STZ-SED-Praz and the STZ-EX-H2O groups were significantly higher from the STZ-SED-H2O group (Fig. 2, B and B′, P < 0.001), although they were not significantly different from each other. This suggests that individually, both prazosin and exercise were capable of improving the diabetes-induced loss of capillaries toward that observed in nondiabetic control rats. The STZ-EX-Praz animals had significantly higher C:F in comparison with the STZ-EX-H2O and STZ-SED-Praz animals (P < 0.001), signifying that cotreatment exerted an additive effect on angiogenesis. We also observed an increase in C:F within the soleus of the STZ-SED-Praz animals (P < 0.01) and the STZ-EX-Praz animals (Fig. 3, B and B′, P < 0.05); however, exercise was unable to improve the diabetes-induced rarefaction independently.
Circulating glucose concentrations.
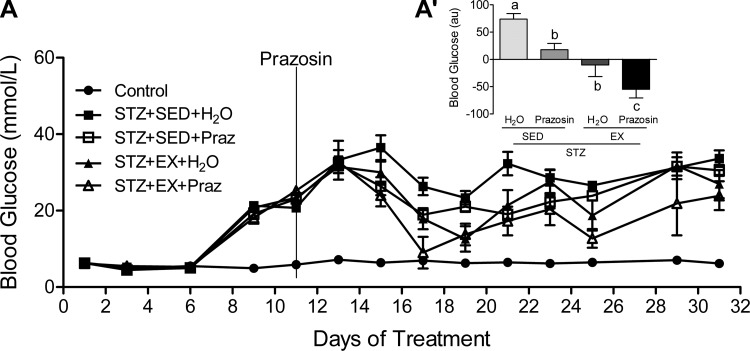
Hyperglycemia was evident in all STZ-treated animals (Fig. 4A). When analyzed as an AUC from day 11 onward (the date of prazosin initiation), there was a significant main effect of both exercise and prazosin to improve daily fed glucose concentrations, with the most favorable response occurring in the STZ-EX-Praz group (Fig. 4A′, P < 0.01), suggesting that the cotreatment produced the most beneficial result.
Fig. 4.
Blood glucose values across the experimental timeline (A) and expressed as area under the curve (AUC; A′). AUC values are calculated from day 11 (prazosin administration) until day 32 (end point). A main effect of both prazosin (P < 0.01) and exercise (P < 0.01) to improve daily glucose concentrations was found, with the cotreatment (STZ-EX-Praz) producing the most significant improvement. All values are means ± SE (n = 6–10 per group). Different letters (a, b, and c) indicate a significant difference (main effect treatment and activity) between groups (P < 0.05), following two-way ANOVA (post hoc test).
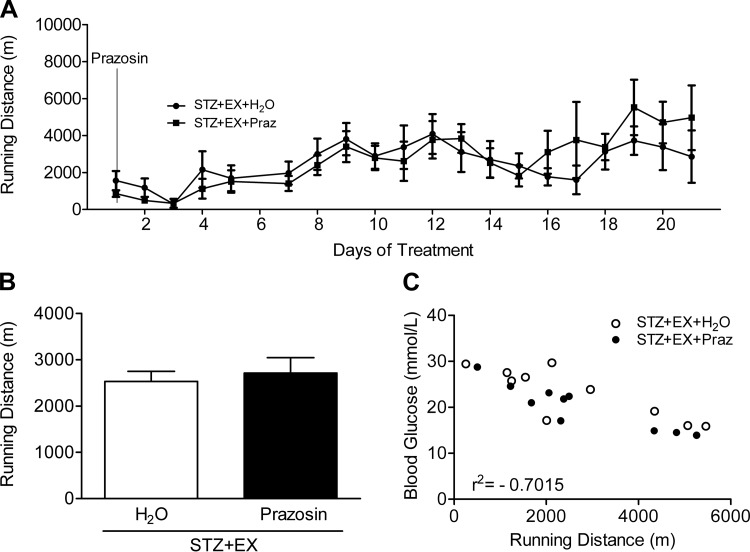
Running distance.
Daily running distance was graphed in relation to prazosin administration (Fig. 5A) and no difference was found between the average running distance in the two exercising groups (Fig. 5B). A significant negative correlation was observed between individual mean blood glucose concentrations and cumulative running distances (r2 = −0.70; Fig. 5C, P < 0.01).
Fig. 5.
Voluntary running distances over time (A) and the total mean values (B) were graphed from the initiation of the cotreatment (prazosin administration). Running distance was negatively correlated to mean blood glucose concentration (C) (r2 = −0.7015, P < 0.01). All values are means ± SE (n = 6–10).
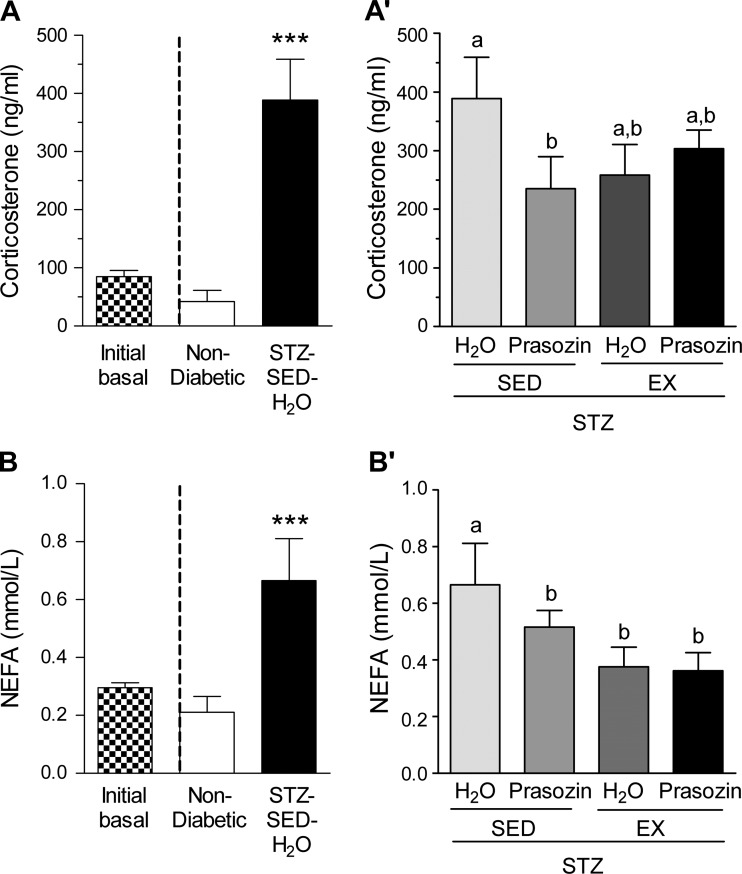
Corticosterone and NEFAs.
STZ-induced diabetes affected the concentrations of circulating corticosterone and NEFAs. Before prazosin administration, corticosterone and NEFA concentrations were not different across the STZ-treatment group, so these data were pooled (initial basal, Fig. 6, A and B). All STZ-treated animals developed significantly elevated corticosterone concentrations (Fig. 6A, P < 0.001) at the end of the treatment period, and only the prazosin group showed significantly decreased values (Fig. 6A′, P < 0.05). There was a significant effect of voluntary exercise, prazosin and their combination, to improve NEFA values (Fig. 6B′, P < 0.05).
Fig. 6.
Corticosterone and NEFA in nondiabetic and STZ-treated rats (A and B) and after 20 days of voluntary exercise and prazosin cotreatment (A′) and (B′). Black and white bar represents combined basal value before STZ injection. All values are means ± SE (n = 6–10 per group). ***Significantly different from nondiabetic group (P < 0.001). Different letters (a and b) indicate a significant difference (main effect treatment/activity for corticosterone and activity for NEFA) between groups (all P < 0.05), following two-way ANOVA (post hoc test).
Mdm2, VEGF-A, and TSP-1.
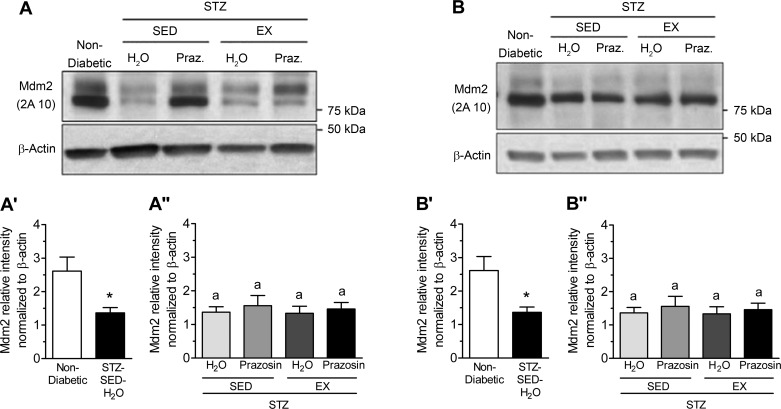
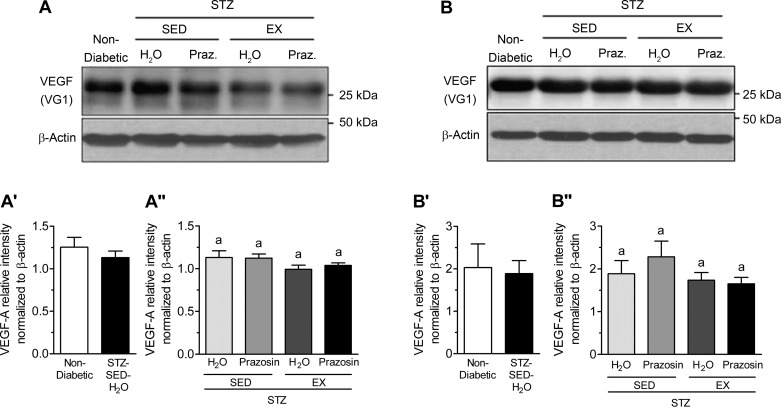
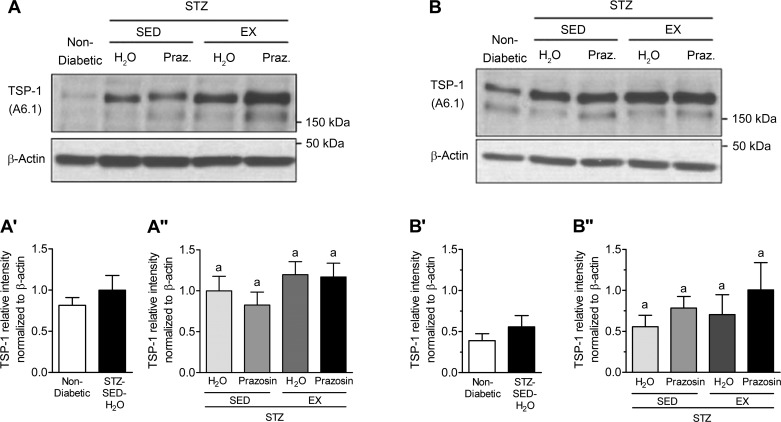
VEGF-A and TSP-1, key pro- and antiangiogenic molecules in skeletal muscle, are influenced by Mdm2, and these three markers have been used to understand the changes observed in skeletal muscle C:F. In both the TA and soleus muscles, STZ-treated rats had significantly decreased Mdm2 protein content vs. nondiabetic rats (Fig. 7, A, A′, B, and B′, P < 0.05). Within the TA, Mdm2 protein content was lowest in the STZ-SED-H2O group but was only significantly lower than the STZ-SED-Praz group (Fig. 7A″, P > 0.05). Soleus Mdm2 protein content was not significantly modified in the four different treatment groups (Fig. 7B″). There was no significant effect of STZ treatment, prazosin, or exercise on VEGF-A protein content within either the TA or soleus muscles (Fig. 8, A, A′, A″, B, B′, and B″, P > 0.05). There was also no significant effect of STZ treatment, prazosin, or exercise on TSP-1 protein content within either the TA or soleus muscles (Fig. 9, A, A′, A″, B, B′, and B″, P > 0.05).
Fig. 7.
Skeletal muscle murine double minute-2 (Mdm2) protein levels are decreased in STZ-induced T1D. A: murine double minute-2 (Mdm2) protein in control (nondiabetic) and STZ-treated rat tibialis anterior (TA) muscles. A′: Mdm2 protein in nondiabetic and STZ-treated rat TA muscle. *P ≤ 0.01 following unpaired Student’s t-test analysis. A″: Mdm2 protein in the TA muscles of sedentary, prazosin-treated sedentary, exercised, or exercised and prazosin-treated STZ-treated rats. Different letters indicate significant difference, P ≤ 0.05 following two-way ANOVA. B: Mdm2 protein in nondiabetic and STZ-treated rat soleus muscles. B′: Mdm2 protein in nondiabetic and STZ-treated rat soleus muscle. *P ≤ 0.05 following unpaired Student’s t-test analysis. B″: Mdm2 protein in the soleus muscles of sedentary, prazosin-treated sedentary, exercised, or exercised and prazosin-treated STZ-treated rats. All data are means ± SE (n = 6 per group). Same letter (a) indicates no significant differences were found between groups following two-way ANOVA.
Fig. 8.
STZ-induced diabetes does not alter skeletal muscle vascular endothelial growth factor (VEGF) protein levels. A: VEGF protein in control (nondiabetic) and STZ-treated rat TA muscles. A′: VEGF protein in nondiabetic and STZ-treated rat TA muscle. No significant difference following unpaired Student’s t-test analysis. A″: VEGF protein in the TA muscles of sedentary, prazosin-treated sedentary, exercised, or exercised and prazosin-treated STZ-treated rats. B: VEGF protein in nondiabetic and STZ-treated rat soleus muscles. B′: VEGF protein in nondiabetic and STZ-treated rat soleus muscle. No significant difference following unpaired Student’s t-test analysis. B″: VEGF protein in the soleus muscles of sedentary, prazosin-treated sedentary, exercised, or exercised and prazosin-treated STZ-treated rats. All data are means ± SE (n = 6 per group). Same letters indicate no significant differences between groups following two-way ANOVA.
Fig. 9.
STZ-induced diabetes does not alter skeletal muscle thrombospondin-1 (TSP-1) protein levels. A: TSP-1 protein in control (nondiabetic) and STZ-treated TA muscles. A′: TSP-1 protein in nondiabetic and STZ-treated rat TA muscle. No significant difference following unpaired Student’s t-test analysis. A″: TSP-1 protein in the TA muscles of sedentary, prazosin-treated sedentary, exercised, or exercised and prazosin-treated STZ-treated rats. B: TSP-1 protein in nondiabetic and STZ-treated rat soleus muscles. B′: TSP-1 protein in nondiabetic and STZ-treated rat soleus muscle. No significant difference following unpaired Student’s t-test analysis. B″: TSP-1 protein in the soleus muscles of sedentary, prazosin-treated sedentary, exercised, or exercised and prazosin-treated STZ-treated rats. All data are means ± SE (n = 6 per group). Same letters indicate no significant differences between groups following two-way ANOVA.
DISCUSSION
Using a combined therapeutic approach, we have shown that exposure to 19 days of combined voluntary exercise and prazosin treatment caused improved skeletal muscle vascularization, through enhanced C:F within the TA muscle and a normalization of the C:F within the soleus muscle of STZ-treated rats. Acutely, prazosin administration is not known to improve glycemia in diabetes; however, we found that prazosin-induced skeletal muscle capillarization, which was independent of voluntary exercise, was associated with improvements in nonfasted (fed) glucose concentrations. When combined with voluntary exercise, prazosin treatment caused additional improvements in fed glucose concentrations and circulating lipid levels, thereby suggesting that the two treatments can act in an additive fashion to improve muscle capillarization and metabolism in this animal model of T1D. To our knowledge, this is the first time that these two therapeutic modalities have been combined in an animal model (health or disease) and suggests that there is likely an additive effect of both prazosin and exercise on angiogenesis and metabolic rescue in diabetes.
It is well established that endurance exercise promotes new capillary growth (22) in both animals and healthy humans (35); however, literature regarding the effect of exercise on the pathological effects of T1D within the skeletal muscle vasculature is inconclusive (20, 44). In healthy rats, exercise training causes increased VEGF mRNA and protein content, which stimulates further enhancements in muscle capillarization (16). When examined in STZ-induced diabetic mice, treadmill endurance training was unable to improve quadriceps femoris cross-sectional fiber area or C:F after 5 wk of exercise training (27). In our study, uncontrolled STZ-induced diabetes caused significant capillary rarefaction, a decrease in the number of capillaries in an area of tissue, in both the TA (Fig. 2, A and A′) and soleus muscles (Fig. 3, A and A′), which is consistent with previous studies (28, 41). When given access to voluntary exercise wheels, STZ-induced diabetic rats showed a significant increase in C:F in the TA (Fig. 2, B and B′) and a slight, but not significant, tendency to increase C:F in the soleus (Fig. 3, B and B′) when compared with sedentary STZ-treated rats. The exercise modality in our study was voluntary wheel running and this might have elicited different muscle recruitment between the TA vs. soleus muscles (25), although there was no difference in volume of exercise between treatment groups (Fig. 5, A and B). Additionally, angiogenic potential appears to be inversely proportional to the original capillarity, i.e., it is easier to induce in fast muscle than slow or cardiac muscle (12), another possible explanation for the muscle type-specific differences observed in capillarity.
The enhancement of skeletal muscle capillarization, through nonsprouting angiogenesis with prazosin treatment, results in an increase in capillarity in glycolytic and oxidative skeletal muscles in healthy rats (3, 9, 47). However, to our knowledge, this study is the first to illustrate the beneficial effects of prazosin on skeletal muscle capillary rarefaction in STZ-induced diabetic rats. We observed a normalization of C:F in both TA and soleus muscles of sedentary, STZ-treated rats as their C:F values were equivalent to those measured in the control group. When coupled with wheel running, there was an additive effect on the angiogenic response, specifically in the TA. It is worth noting that the skeletal muscle angiogenesis observed within both the TA and soleus (the normalization) occurred amidst significantly elevated circulating corticosterone levels (Fig. 6, A and A′), a hormone that has been linked to capillary rarefaction (42). In addition to the fact that both treatment modalities result in distinct forms of angiogenesis, there are data suggesting that the combination of high-volume, low-intensity exercise, like voluntary wheel running, actually elicits a more rapid angioadaptive response than what is observed after treadmill exercise training (35).
Exercise is a widely recognized strategy for improving glycemia in diabetes (6), as regular exercise leads to the improvement of whole body glucose and lipid metabolism and enhanced skeletal muscle glucose disposal (17) and insulin sensitivity (23, 24). We observed a significant effect of voluntary exercise to improve fed glucose concentrations in the STZ-treated rats, and this result was amplified when the animals were coadministered prazosin (Fig. 4, A and A′). These improvements in blood glucose, regardless of prazosin administration, were also correlated to running distance (Fig. 5C), suggesting that higher running volumes caused better fed glucose concentrations. In our study, sedentary STZ-induced diabetic rats treated with prazosin also showed improved fed glucose values throughout the experimental protocol, an unexpected but interesting observation, given the inconsistent findings on prazosin treatment in various models of hyperglycemia (4, 18, 31). As prazosin is not known to acutely lower blood glucose levels, our results highlight the importance of skeletal muscle capillary content on glucose homeostasis and demonstrate that increased capillarization likely enhances insulin sensitivity and glucose disposal, an observation recently seen in healthy rats (3).
The angiogenic response is controlled by a dynamic balance between anti- and proangiogenic factors (36). VEGF-A and TSP-1 are key pro- and antiangiogenic molecules (30, 37). Interestingly, we have recently brought evidence that the E3 ubiquitin ligase Mdm2 could be a central regulator of skeletal muscle angiogenesis, partly by regulating VEGF-A and TSP-1 expression (2, 40).
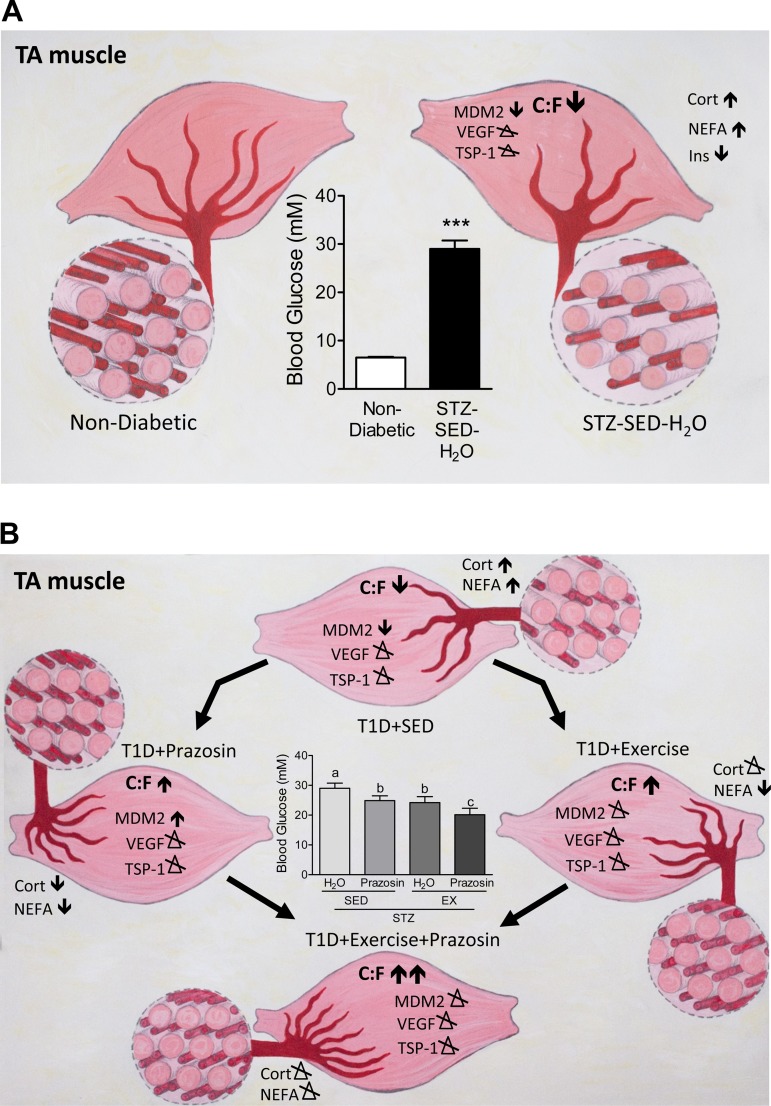
Our current results show, for the first time, that STZ-treated rats had a significant decrease in Mdm2 protein levels in both the TA and soleus muscles, as summarized in Fig. 10A. This reduction in Mdm2 could explain the lowered skeletal muscle capillarization in both muscles in sedentary diabetic rats, as Mdm2 protein levels have been shown to be closely related to endothelial content within the skeletal muscle (40). It was previously observed that both capillarization and Mdm2 protein levels were significantly lower in a model of type-2 diabetes and in skeletal muscles from Zucker diabetic fatty (ZDF) rats (40). Further results demonstrated that voluntary running efficiently restored both the impaired skeletal muscle capillarization and Mdm2 protein levels within the ZDF rats. In contrast to these findings, the voluntary exercise stimulus applied within our current study did not rescue the observed decrease in Mdm2 protein in the diabetic animals (Fig. 10B) and suggests that the physiological response of Mdm2 to exercise could be lost in uncontrolled, T1D muscle.
Fig. 10.
Summary of the effects of prazosin and exercise within the TA skeletal muscle of STZ-treated rats. A: STZ treatment causes increased fed blood glucose levels, elevations in corticosterone (Cort), NEFA, and decreased insulin (Ins) content. Within the TA, STZ treatment decreased C:F and Mdm2 protein content while both VEGF and TSP-1 were unaffected. B: STZ-treated rats were exposed to either prazosin treatment, voluntary exercise, or a combination of both. Individually, prazosin and voluntary exercise increased C:F and decreased both NEFA and fed blood glucose concentrations. Prazosin treatment increased Mdm2 protein content while VEGF and TSP-1 were unaffected. Voluntary exercise caused no alterations to angiogenic proteins. The combination of prazosin with voluntary exercise resulted in the most improved fed blood glucose concentrations and C:F. Both Cort and NEFA concentrations were unaffected and there were no alterations to angiogenic proteins.
No significant alterations in TSP-1 or VEGF-A protein levels in response to diabetes induction, prazosin treatment, or voluntary exercise were observed in either the TA or soleus muscles. Moreover, VEGF receptor expression in soleus and TA muscle were unchanged by STZ treatment nor by exercise or prazosin treatment (data not shown). Kivelä et al. (27) have shown that STZ-induced diabetic mice had lower VEGF-A protein levels at 3 and 5 wk postdiabetes induction and a concomitant increase in TSP-1 expression. In that study, while exercise served to delay the reduction in VEGF-A levels, it was not sufficient to attenuate the elevation in TSP-1 mRNA. The C:F is increased as early as 14 days of treadmill running in rodent skeletal muscle (43); therefore, we hypothesize that alterations in VEGF-A and TSP-1 protein levels could have occurred at an earlier time point to stimulate the growth of capillaries. While VEGF-A protein could have been elevated before the measured time point to induce the angiogenic process, the trend for TSP-1 protein levels could indicate the stopping or slowing down of capillary growth, as the balance between oxygen and nutrient supply and demand had been achieved in the skeletal muscle.
In summary, the significant improvement to fed glucose concentration observed after cotreatment with voluntary exercise and prazosin administration could be the result of enhancements in insulin sensitivity and lipid metabolism, increased skeletal muscle glucose disposal, and possibly improved diffusion conditions for glucose in the muscle as the result of heightened skeletal muscle angiogenesis. These results suggest that the combination of both voluntary exercise and prazosin administration could lead to a cooperative improvement in peripheral vascular complications linked to T1D and may perhaps prevent future complications through the augmentation of skeletal muscle capillarization and glycemia status.
GRANTS
This work was funded by the Natural Science and Engineering Research Council of Canada Discovery Grant (to T. L. Haas and M. C. Riddell). E. C. Dunford is a recipient of the Natural Science and Engineering Research Council of Canada Doctoral Scholarship.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
E.C.D., E.L., J.A., and E.R.M. performed experiments; E.C.D., E.L., J.A., E.R.M., and M.C.R. analyzed data; E.C.D., E.L., J.A., E.R.M., T.L.H., O.B., and M.C.R. interpreted results of experiments; E.C.D., E.L., J.A., and E.R.M. prepared figures; E.C.D., E.L., J.A., E.R.M., and O.B. drafted manuscript; E.C.D., E.L., J.A., E.R.M., T.L.H., O.B., and M.C.R. edited and revised manuscript; E.C.D., E.L., J.A., E.R.M., T.L.H., O.B., and M.C.R. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge Dr. Ruth Brown for help with statistics. Additional thanks to Alison Joy Webster-Dunford for the artwork in Fig. 10, A and B.
REFERENCES
- 1.Abaci A, Oğuzhan A, Kahraman S, Eryol NK, Unal S, Arinç H, Ergin A. Effect of diabetes mellitus on formation of coronary collateral vessels. Circulation 99: 2239–2242, 1999. doi: 10.1161/01.CIR.99.17.2239. [DOI] [PubMed] [Google Scholar]
- 2.Aiken J, Roudier E, Ciccone J, Drouin G, Stromberg A, Vojnovic J, Olfert IM, Haas T, Gustafsson T, Grenier G, Birot O. Phosphorylation of murine double minute-2 on Ser166 is downstream of VEGF-A in exercised skeletal muscle and regulates primary endothelial cell migration and FoxO gene expression. FASEB J 30: 1120–1134, 2016. doi: 10.1096/fj.15-276964. [DOI] [PubMed] [Google Scholar]
- 3.Akerstrom T, Laub L, Vedel K, Brand CL, Pedersen BK, Lindqvist AK, Wojtaszewski JF, Hellsten Y. Increased skeletal muscle capillarization enhances insulin sensitivity. Am J Physiol Endocrinol Metab 307: E1105–E1116, 2014. doi: 10.1152/ajpendo.00020.2014. [DOI] [PubMed] [Google Scholar]
- 4.Barbieri C, Caldara R, Ferrari C, Dal Bo GA, Paracchi A, Romussi M, Curtarelli G. Metabolic effects of prazosin. Clin Pharmacol Ther 27: 313–316, 1980. doi: 10.1038/clpt.1980.41. [DOI] [PubMed] [Google Scholar]
- 5.Baum O, Da Silva-Azevedo L, Willerding G, Wöckel A, Planitzer G, Gossrau R, Pries AR, Zakrzewicz A. Endothelial NOS is main mediator for shear stress-dependent angiogenesis in skeletal muscle after prazosin administration. Am J Physiol Heart Circ Physiol 287: H2300–H2308, 2004. doi: 10.1152/ajpheart.00065.2004. [DOI] [PubMed] [Google Scholar]
- 6.Chimen M, Kennedy A, Nirantharakumar K, Pang TT, Andrews R, Narendran P. What are the health benefits of physical activity in type 1 diabetes mellitus? A literature review. Diabetologia 55: 542–551, 2012. doi: 10.1007/s00125-011-2403-2. [DOI] [PubMed] [Google Scholar]
- 7.Coleman SK, Rebalka IA, D’Souza DM, Hawke TJ. Skeletal muscle as a therapeutic target for delaying type 1 diabetic complications. World J Diabetes 6: 1323–1336, 2015. doi: 10.4239/wjd.v6.i17.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cryer PE. Minireview: glucagon in the pathogenesis of hypoglycemia and hyperglycemia in diabetes. Endocrinology 153: 1039–1048, 2012. doi: 10.1210/en.2011-1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dawson JM, Hudlická O. The effects of long term administration of prazosin on the microcirculation in skeletal muscles. Cardiovasc Res 23: 913–920, 1989. doi: 10.1093/cvr/23.11.913. [DOI] [PubMed] [Google Scholar]
- 10.Dawson SI, Willis J, Florkowski CM, Scott RS. All-cause mortality in insulin-treated diabetic patients: a 20-year follow-up. Diabetes Res Clin Pract 80: e6–e9, 2008. doi: 10.1016/j.diabres.2007.10.033. [DOI] [PubMed] [Google Scholar]
- 11.Dubuisson L, Desmoulière A, Decourt B, Evadé L, Bedin C, Boussarie L, Barrier L, Vidaud M, Rosenbaum J. Inhibition of rat liver fibrogenesis through noradrenergic antagonism. Hepatology 35: 325–331, 2002. doi: 10.1053/jhep.2002.31166. [DOI] [PubMed] [Google Scholar]
- 12.Egginton S. Invited review: activity-induced angiogenesis. Pflugers Arch 457: 963–977, 2009. doi: 10.1007/s00424-008-0563-9. [DOI] [PubMed] [Google Scholar]
- 13.Egginton S. Physiological factors influencing capillary growth. Acta Physiol (Oxf) 202: 225–239, 2011. doi: 10.1111/j.1748-1716.2010.02194.x. [DOI] [PubMed] [Google Scholar]
- 14.Fuchsjäger-Mayrl G, Pleiner J, Wiesinger GF, Sieder AE, Quittan M, Nuhr MJ, Francesconi C, Seit HP, Francesconi M, Schmetterer L, Wolzt M. Exercise training improves vascular endothelial function in patients with type 1 diabetes. Diabetes Care 25: 1795–1801, 2002. doi: 10.2337/diacare.25.10.1795. [DOI] [PubMed] [Google Scholar]
- 15.Galassetti P, Riddell MC. Exercise and type 1 diabetes (T1DM). Compr Physiol 3: 1309–1336, 2013. doi: 10.1002/cphy.c110040. [DOI] [PubMed] [Google Scholar]
- 16.Gavin TP. Basal and exercise-induced regulation of skeletal muscle capillarization. Exerc Sport Sci Rev 37: 86–92, 2009. doi: 10.1097/JES.0b013e31819c2e9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goodyear LJ, Kahn BB. Exercise, glucose transport, and insulin sensitivity. Annu Rev Med 49: 235–261, 1998. doi: 10.1146/annurev.med.49.1.235. [DOI] [PubMed] [Google Scholar]
- 18.Goyal RK, Bangaru RA, Lakkad NB, Rao MV. Effect of chronic treatment with atenolol and prazosin in streptozotocin induced diabetic rats. Indian J Physiol Pharmacol 40: 220–224, 1996. [PubMed] [Google Scholar]
- 19.Hellsten Y, Hoier B. Capillary growth in human skeletal muscle: physiological factors and the balance between pro-angiogenic and angiostatic factors. Biochem Soc Trans 42: 1616–1622, 2014. doi: 10.1042/BST20140197. [DOI] [PubMed] [Google Scholar]
- 20.Henriksson J. Effects of physical training on the metabolism of skeletal muscle. Diabetes Care 15: 1701–1711, 1992. doi: 10.2337/diacare.15.11.1701. [DOI] [PubMed] [Google Scholar]
- 21.Hirano T, Yoshino G, Kashiwazaki K, Adachi M. Doxazosin reduces prevalence of small dense low density lipoprotein and remnant-like particle cholesterol levels in nondiabetic and diabetic hypertensive patients. Am J Hypertens 14: 908–913, 2001. doi: 10.1016/S0895-7061(01)02141-0. [DOI] [PubMed] [Google Scholar]
- 22.Hudlicka O, Brown M, Egginton S. Angiogenesis in skeletal and cardiac muscle. Physiol Rev 72: 369–417, 1992. [DOI] [PubMed] [Google Scholar]
- 23.James DE, Burleigh KM, Kraegen EW, Chisholm DJ. Effect of acute exercise and prolonged training on insulin response to intravenous glucose in vivo in rat. J Appl Physiol Respir Environ Exerc Physiol 55: 1660–1664, 1983. [DOI] [PubMed] [Google Scholar]
- 24.James DE, Kraegen EW, Chisholm DJ. Effects of exercise training on in vivo insulin action in individual tissues of the rat. J Clin Invest 76: 657–666, 1985. doi: 10.1172/JCI112019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kano Y, Shimegi S, Masuda K, Sakato H, Ohmori H, Katsuta S. Effects of different intensity endurance training on the capillary network in rat skeletal muscle. Int J Microcirc Clin Exp 17: 93–96, 1997. doi: 10.1159/000179213. [DOI] [PubMed] [Google Scholar]
- 26.Kindig CA, Sexton WL, Fedde MR, Poole DC. Skeletal muscle microcirculatory structure and hemodynamics in diabetes. Respir Physiol 111: 163–175, 1998. doi: 10.1016/S0034-5687(97)00122-9. [DOI] [PubMed] [Google Scholar]
- 27.Kivelä R, Silvennoinen M, Touvra AM, Lehti TM, Kainulainen H, Vihko V. Effects of experimental type 1 diabetes and exercise training on angiogenic gene expression and capillarization in skeletal muscle. FASEB J 20: 1570–1572, 2006. doi: 10.1096/fj.05-4780fje. [DOI] [PubMed] [Google Scholar]
- 28.Krause MP, Riddell MC, Gordon CS, Imam SA, Cafarelli E, Hawke TJ. Diabetic myopathy differs between Ins2Akita+/− and streptozotocin-induced type 1 diabetic models. J Appl Physiol (1985) 106: 1650–1659, 2009. doi: 10.1152/japplphysiol.91565.2008. [DOI] [PubMed] [Google Scholar]
- 29.Leinonen H, Matikainen E, Juntunen J. Permeability and morphology of skeletal muscle capillaries in type 1 (insulin-dependent) diabetes mellitus. Diabetologia 22: 158–162, 1982. doi: 10.1007/BF00283744. [DOI] [PubMed] [Google Scholar]
- 30.Malek MH, Olfert IM. Global deletion of thrombospondin-1 increases cardiac and skeletal muscle capillarity and exercise capacity in mice. Exp Physiol 94: 749–760, 2009. doi: 10.1113/expphysiol.2008.045989. [DOI] [PubMed] [Google Scholar]
- 31.Martin S, Kolb-Bachofen V, Kiesel U, Kolb H. Pathogenesis of low dose streptozotocin induced diabetes in mice: requirement for alpha 1-adrenoceptor activation and vasoactive amine release. Diabetologia 32: 140–142, 1989. doi: 10.1007/BF00505187. [DOI] [PubMed] [Google Scholar]
- 32.Maruyama H, Saruta T, Itoh H, Koyama K, Kido K, Itoh K, Takei I, Kataoka K. Effect of alpha-adrenergic blockade on blood pressure, glucose, and lipid metabolism in hypertensive patients with non-insulin-dependent diabetes mellitus. Am Heart J 121: 1302–1306, 1991. doi: 10.1016/0002-8703(91)90437-M. [DOI] [PubMed] [Google Scholar]
- 33.Mason NJ, Jenkins AJ, Best JD, Rowley KG. Exercise frequency and arterial compliance in non-diabetic and type 1 diabetic individuals. Eur J Cardiovasc Prev Rehabil 13: 598–603, 2006. doi: 10.1097/01.hjr.0000216546.07432.b2. [DOI] [PubMed] [Google Scholar]
- 34.Oben JA, Roskams T, Yang S, Lin H, Sinelli N, Li Z, Torbenson M, Huang J, Guarino P, Kafrouni M, Diehl AM. Sympathetic nervous system inhibition increases hepatic progenitors and reduces liver injury. Hepatology 38: 664–673, 2003. doi: 10.1053/jhep.2003.50371. [DOI] [PubMed] [Google Scholar]
- 35.Olfert IM, Baum O, Hellsten Y, Egginton S. Advances and challenges in skeletal muscle angiogenesis. Am J Physiol Heart Circ Physiol 310: H326–H336, 2016. doi: 10.1152/ajpheart.00635.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Olfert IM, Birot O. Importance of anti-angiogenic factors in the regulation of skeletal muscle angiogenesis. Microcirculation 18: 316–330, 2011. doi: 10.1111/j.1549-8719.2011.00092.x. [DOI] [PubMed] [Google Scholar]
- 37.Olfert IM, Howlett RA, Tang K, Dalton ND, Gu Y, Peterson KL, Wagner PD, Breen EC. Muscle-specific VEGF deficiency greatly reduces exercise endurance in mice. J Physiol 587: 1755–1767, 2009. doi: 10.1113/jphysiol.2008.164384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rivard A, Silver M, Chen D, Kearney M, Magner M, Annex B, Peters K, Isner JM. Rescue of diabetes-related impairment of angiogenesis by intramuscular gene therapy with adeno-VEGF. Am J Pathol 154: 355–363, 1999. doi: 10.1016/S0002-9440(10)65282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roudier E, Aiken J, Slopack D, Gouzi F, Mercier J, Haas TL, Gustafsson T, Hayot M, Birot O. Novel perspective: exercise training stimulus triggers the expression of the oncoprotein human double minute-2 in human skeletal muscle. Physiol Rep 1: e00028, 2013. doi: 10.1002/phy2.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roudier E, Forn P, Perry ME, Birot O. Murine double minute-2 expression is required for capillary maintenance and exercise-induced angiogenesis in skeletal muscle. FASEB J 26: 4530–4539, 2012. doi: 10.1096/fj.12-212720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sexton WL, Poole DC, Mathieu-Costello O. Microcirculatory structure-function relationships in skeletal muscle of diabetic rats. Am J Physiol Heart Circ Physiol 266: H1502–H1511, 1994. [DOI] [PubMed] [Google Scholar]
- 42.Shikatani EA, Trifonova A, Mandel ER, Liu ST, Roudier E, Krylova A, Szigiato A, Beaudry J, Riddell MC, Haas TL. Inhibition of proliferation, migration and proteolysis contribute to corticosterone-mediated inhibition of angiogenesis. PLoS One 7: e46625, 2012. doi: 10.1371/journal.pone.0046625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Slopack D, Roudier E, Liu ST, Nwadozi E, Birot O, Haas TL. Forkhead BoxO transcription factors restrain exercise-induced angiogenesis. J Physiol 592: 4069–4082, 2014. doi: 10.1113/jphysiol.2014.275867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wallberg-Henriksson H, Gunnarsson R, Henriksson J, Ostman J, Wahren J. Influence of physical training on formation of muscle capillaries in type I diabetes. Diabetes 33: 851–857, 1984. doi: 10.2337/diab.33.9.851. [DOI] [PubMed] [Google Scholar]
- 45.Williams JL, Cartland D, Hussain A, Egginton S. A differential role for nitric oxide in two forms of physiological angiogenesis in mouse. J Physiol 570: 445–454, 2006. doi: 10.1113/jphysiol.2005.095596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou A, Egginton S, Hudlická O, Brown MD. Internal division of capillaries in rat skeletal muscle in response to chronic vasodilator treatment with alpha1-antagonist prazosin. Cell Tissue Res 293: 293–303, 1998. doi: 10.1007/s004410051121. [DOI] [PubMed] [Google Scholar]
- 47.Ziada A, Hudlicka O, Tyler KR. The effect of long-term administration of alpha 1-blocker prazosin on capillary density in cardiac and skeletal muscle. Pflugers Arch 415: 355–360, 1989. doi: 10.1007/BF00370888. [DOI] [PubMed] [Google Scholar]