Abstract
Antibodies to malondialdehyde (MDA)-modified low-density lipoprotein (LDL), copper-oxidized LDL (oxLDL), Nɛ(carboxymethyl) lysine (CML)-modified LDL, and advanced glycosylation end product (AGE)-modified LDL were obtained by immunization of rabbits with in vitro-modified human LDL preparations. After absorption of apolipoprotein B (ApoB) antibodies, we obtained antibodies specific for each modified lipoprotein with unique patterns of reactivity. MDA-LDL antibodies reacted strongly with MDA-LDL and also with oxLDL. CML-LDL antibodies reacted strongly with CML-LDL and also AGE-LDL. oxLDL antibodies reacted with oxLDL but not with MDA-LDL, and AGE-LDL antibodies reacted with AGE-LDL but not with CML-LDL. Capture assays were set with each antiserum, and we tested their ability to capture ApoB-containing lipoproteins isolated from precipitated immune complexes (IC) and from the supernatants remaining after IC precipitation (free lipoproteins). All antibodies captured lipoproteins contained in IC more effectively than free lipoproteins. Analysis of lipoproteins in IC by gas chromatography-mass spectrometry showed that they contained MDA-LDL and CML-LDL in significantly higher concentrations than free lipoproteins. A significant correlation (r = 0.706, P < 0.019) was obtained between the MDA concentrations determined by chemical analysis and by the capture assay of lipoproteins present in IC. In conclusion, we have developed capture assays for different LDL modifications in human ApoB/E lipoprotein-rich fractions isolated from precipitated IC. This approach obviates the interference of IC in previously reported modified LDL assays and allows determination of the degree of modification of LDL with greater accuracy.
Human lipoproteins are known to undergo spontaneous modification in humans. Several chemical processes are involved in this modification. Oxidation affects both the lipid and protein components of low-density lipoprotein (LDL). Reactive aldehyde products formed during the oxidation of polyunsaturated fatty acids, such as malondialdehyde (MDA) and 4-hydroxynonenal (HNE), are capable of attaching covalently to the ɛ-amino groups of lysine residues of apolipoprotein B (ApoB) (21, 22, 33). Advanced glycosylation also affects LDL (19). This process involves a chain of chemical reactions that starts with the covalent, nonenzymatic addition of reducing sugars to protein amino groups (Schiff base and Amadori adducts). Additional reactions take place leading to the formation of a heterogeneous family of sugar-amino acid adducts collectively known as “advanced glycosylation end products” (AGE) (31), including AGE-modified LDL (AGE-LDL).
Both oxidized LDL (oxLDL) and AGE-LDL have been shown to have proatherogenic and proinflammatory properties (11). This has led to a burst of interest in the development of techniques for their assay in human sera. The immunogenicity of modified lipoproteins first reported by Steinbrecher et al. has been well documented in studies involving immunization of laboratory animals with in vitro-modified lipoproteins (23). The immunogenicity of these modifications in experimental animals allowed the production of monoclonal antibodies specific for MDA and HNE-lysine which reacted with oxLDL prepared in vitro, as well as with LDL isolated from atherosclerotic plaques (16, 33). AGE-modified proteins including LDL (AGE-LDL) have also been shown to be immunogenic (6). Antibodies raised in laboratory animals have been used for the detection of AGE-modified proteins in serum (15) and tissues (14, 15). Human autoantibodies to modified LDL have also been extensively characterized (28) and shown to recognize primarily MDA-modified LDL and Nɛ(carboxymethyl) lysine (CML)-modified LDL (29). Some groups have proposed the assay of such autoantibodies as a surrogate measurement for modified LDL, but this approach is riddled with inaccuracies because of the interference caused by the formation of antigen-antibody complexes involving modified forms of LDL and the corresponding antibodies (28). We have shown in previous studies that LDL-containing immune complexes (LDL-IC) can be precipitated with 4% polyethylene glycol (PEG), and that while modified forms of LDL are easily measurable in PEG precipitates, very little modified LDL remains soluble after IC precipitation. The enzymoimmunoassay techniques developed by different groups for the detection of modified forms of LDL and AGE-modified proteins (7, 9, 15, 24, 25) attempt to avoid interference of IC and other confounding factors by a variety of means. While most groups will trust the ability of immobilized antibodies to preferentially capture and retain modified LDL at high sample dilutions, this may not be necessarily the case when monoclonal antibodies are used for capture of modified LDL. Other groups have proposed different approaches to this issue, such as using competition between bound modified LDL and the unknown LDL in test samples to which antibody is added (5), using purified LDL instead of whole serum or plasma (8) or adding sodium dodecyl sulfate (32) or 4% PEG (26) to the samples. However, none of the groups provided evidence for the effective dissociation of IC as a consequence of the assay conditions.
We have demonstrated that rabbits immunized with MDA-LDL, copper-oxidized LDL (oxLDL), AGE-LDL, and CML-LDL produce antibodies that recognize each one of these modifications individually. For example, rabbit oxLDL antibodies show only a marginal degree of reactivity with MDA-LDL, rabbit MDA-LDL antibodies react only weakly with oxLDL, and rabbit AGE-LDL antibodies do not react with CML-LDL (29). Using these antibodies, we have developed capture assays whose specificity and ability to capture in vivo-modified human LDL, isolated from circulating IC, have been demonstrated.
MATERIALS AND METHODS
Isolation of human LDL.
Blood for lipoprotein isolation was collected from four healthy volunteers in EDTA at 0.4 mmol/liter after 12 h of fasting. The donors used for this purpose were normolipemic healthy volunteers not receiving prescription medications for any acute or chronic condition and without family history of coronary artery disease, peripheral vascular disease, or stroke. None of the volunteers was receiving antioxidant therapy. All volunteers gave permission with informed consent for blood collection. LDL was isolated from pooled plasma, after density adjustment (1.019 < density < 1.063 g/ml) with potassium bromide, by preparative ultracentrifugation at 50,000 rpm for 17 h on a Beckman L-80 ultracentrifuge with a type 70 Ti rotor (19). After isolation, LDL was washed by ultracentrifugation, dialyzed against a 0.15-mol/liter sodium chloride solution containing 300 μmol of EDTA (pH 8.0) per liter, passed through an Acrodisc filter (0.22-μm pore size) in order to sterilize and remove aggregates, and stored under nitrogen in the dark at 4°C.
Preparation of modified proteins.
oxLDL was prepared according to our modification (10) of the protocol described by Steinbrecher (22). LDL was diluted in phosphate-buffered saline (PBS) to a protein concentration of 1.5 mg/ml and incubated with 40 μmol of copper chloride (CuCl2) per liter. The degree of oxidation was monitored continuously by fluorescence emission with a luminescence spectrophotometer (SLM-AMINCO Series 2; Spectronic Instruments, Rochester, N.Y.). LDL oxidation was stopped 4 to 6 h after the fluorescence values reached the peak (≥1.1 fluorescence units). On average, this corresponds to the following degree of modification: 4 to 7 mmol of MDA per mol of lysine (0.4 to 0.7% modification of lysine residues), 0.8 mmol of CML per mol of lysine (0.08% modification of lysine residues), and 0.25 mmol of Nɛ(carboxyethyl) lysine (CEL) per ml of lysine (0.025% modification of lysine residues).
AGE-LDL was prepared by a modification of the method described by Schmidt et al. (20). Freshly isolated LDL (1.5 mg/ml) was sterilized by passage through a 0.2-μm-pore filter, added to 150 mM glucose-6-phosphate in 200 mM phosphate buffer, pH 8.0, containing 40 μM butylated hydroxytoluene and 400 μM EDTA, filter sterilized a second time, and incubated for 8 weeks at 37°C. At the end of the incubation, LDL was dialyzed for 24 h against three changes of 4 liters of 0.15 M NaCl-0.3 mM EDTA, pH 8.0. Our AGE-LDL modifications contained 4.6 mmol of CML per mol of lysine (0.5% modification of lysine residues) and 0.45 mmol of CEL per mol of lysine, corresponding to 0.05% modification of lysine residues). The MDA content of AGE-LDL was usually below the detection limit, but in some preparations MDA was detectable in small amounts, similar to those measured for CEL.
MDA modification of proteins was performed according to Haberland et al. (4) by incubating equal volumes of freshly isolated LDL and 0.2 M MDA for 3 h at 37°C, followed by extensive dialysis against 0.15 M NaCl with 0.3 mM EDTA, pH 8.0. The degree of modification of the MDA-LDL preparations used to immunize rabbits was 67 mmol of MDA per mol of lysine, corresponding to the modification of 6.7% of lysine residues.
CML-modified proteins were prepared by incubation of the protein with glyoxylic acid and NaBH3CN in phosphate buffer at 37°C, as described previously for the preparation of CML in bovine serum albumin (17). The degree of modification of the CML-LDL used in our studies was113 mmol of CML per mol of lysine (11.3% modification of lysine residues).
The characteristics of the different modified lipoproteins used in this study are summarized in Table 1.
TABLE 1.
Characteristics of the different types of modified LDL used in this study
| Form of LDL | LDL modificationa
|
||
|---|---|---|---|
| mmol of MDA/ mol of lysine | mmol of CEL/ mol of lysine | mmol of CML/ mol of lysine | |
| oxLDL | 4-7 | 0.25 | 0.8 |
| MDA-LDL | 67 | NDb | ND |
| AGE-LDL | ND | 0.45 | 4.6 |
| CML-LDL | ND | ND | 113 |
Data obtained by SIM-GC/MS.
ND, not detectable (below the detection limit of SIM-GC/MS [0.005 mmol/mol of lysine]).
Isolation of ApoB-containing lipoproteins from circulating IC.
Human sera obtained as part of the ongoing Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) study aimed at assessing the development of macrovascular disease in type 1 diabetes (1, 30) were used in this part of the study. Sera were kept at −70°C until our studies were performed. Isolation of soluble IC by precipitation with 4% PEG was performed as previously described (30). ApoB-rich lipoproteins (LDL and other ApoB-containing lipoproteins) were isolated from the supernatant and from the resuspended PEG-precipitated IC. The supernatants were split: one-half was fractionated on an immobilized oxLDL column, and the other half was fractionated on an immobilized AGE-LDL column. The washout fractions from both oxLDL and AGE-LDL columns were combined and then chromatographed on heparin-agarose columns (Sigma-Aldrich Corp., St. Louis, Mo.) that retain ApoB/E-containing lipoproteins. The PEG-precipitated IC were first submitted to affinity chromatography on protein G-Sepharose, and the washout fractions, containing all precipitated proteins other than immunoglobulin G (IgG), were later fractionated on heparin-agarose columns. The lipoprotein-containing samples obtained from the heparin-agarose columns were dialyzed against saline containing 0.3 mM EDTA, pH 8.0, and stored at 4°C until tested in the capture assays.
Analysis of LDL modifications.
Analysis of modified lipoproteins for their content of CML, CEL, and the advanced lipoxidation end products MDA-lysine and HNE-lysine was carried out by selected ion monitoring-gas chromatography-mass spectrometry (SIM-GC/MS) as described by Requena et al. (18). The degree of modification of MDA-LDL was also estimated by the thiobarbituric acid reactive substances assay (13), using MDA as a standard.
Rabbit antibodies.
Antibodies to modified LDL were obtained by immunization of New Zealand White female rabbits with different modifications of human LDL (AGE-LDL, CML-LDL, oxLDL, and MDA-LDL) as previously described (29). The resulting rabbit antiserum was first fractionated by affinity chromatography with immobilized protein G, and the IgG fractions were absorbed in a column of immobilized native LDL. Depending on the protocol, the washout from the column of immobilized native LDL, containing antibodies to modified LDL and irrelevant IgG, was used as purified to capture modified LDL or as a peroxidase conjugate, prepared in our laboratory with the reagents and protocol obtained from Roche Diagnostics, Manheim, Germany, to detect captured LDL. The eluate, containing purified IgG antibodies to native LDL, predominantly reactive with ApoB, was used either to capture LDL or as a peroxidase conjugate to detect captured LDL.
Capture assays.
Capture assays were carried out in two basic protocols. To capture MDA-LDL, oxLDL, and AGE-LDL, purified rabbit antibodies of the different specificities were absorbed to the plates at a protein concentration of approximately 35 μg/ml, chosen as optimal based on the signal/noise ratio, from three different concentrations (87.5, 35, and 17.5 μg/ml). After washing off the unabsorbed antibodies, the plates were blocked with 5% bovine serum albumin. Serial dilutions of modified LDL preparations were added to the plates, and rabbit peroxidase-conjugated anti-human ApoB, at a concentration of 0.67 μg/ml (chosen as optimal between two concentrations, 0.22 and 0.67 μg/ml), was used to detect bound LDL. To capture CML-LDL, we immobilized purified rabbit anti-human ApoB at a protein concentration of 22 μg/ml to capture LDL from the patient's samples and used rabbit peroxidase-conjugated anti-CML-LDL at a concentration of 0.5 μg/ml to detect bound CML-LDL. The capture assays for MDA-LDL and CML-LDL were calibrated with reference preparations of oxLDL and CML-LDL with known concentrations of MDA and CML, determined by SIM-GC/MS. The capture assay for oxLDL was calibrated in micrograms per milliliter, taking advantage of the fact that we have determined a reproducible method for the preparation of oxLDL with a consistent degree of modification (10). The assay of AGE-LDL could not be calibrated by any of the methods described above because AGE-LDL antibodies do not react with CML-LDL and because the degree of modification of different AGE-LDL preparations is variable. Therefore, we calibrated the assay in arbitrary units, defined as the reciprocal of the dilution of the reference AGE-LDL preparation that results in an optical density (OD) lower than 0.5 with a standard time for the reaction of the conjugate antibody with its substrate. In all assays, the concentration of LDL added to the antibody-containing wells was kept constant (5 μg/ml) and the final values were expressed as millimoles of MDA or CML per mole of lysine, micrograms of oxLDL per microgram of protein, or units of AGE-LDL per microgram of protein.
Technical validation and recovery studies.
Intra-assay reproducibility was determined from six replicates of one in vitro-modified LDL preparation and from six replicates of an unknown LDL isolated from a PEG precipitate, both adjusted at a protein concentration of 5 μg/ml. Interassay reproducibility was determined by assaying the same samples in sextuplicate in six different plates.
Recovery experiments were performed by mixing equal volumes of native and modified LDL at 5 μg/ml and determining the total amounts measured by the capture assay by the calibration methods described above.
PEG precipitation of IC and isolation of free and IC-bound ApoB-rich lipoproteins.
Fractionation of human sera with 4% PEG was performed as previously described (30). ApoB/E-containing lipoproteins (native and modified) were isolated from the supernatant and from the resuspended PEG precipitates. The supernatants were fractionated directly on heparin-agarose columns (Sigma-Aldrich Corp., St. Louis, Mo.) that retain ApoB/E-containing lipoproteins (2). The PEG precipitates were first submitted to affinity chromatography on protein G-Sepharose and the washout, containing all precipitated proteins other than IgG, was later fractionated on heparin-agarose columns. The lipoprotein-containing samples were pooled and dialyzed against saline containing 0.3 mM EDTA, pH 8.0, and tested by SIM-GC/MS and by the capture assays.
RESULTS
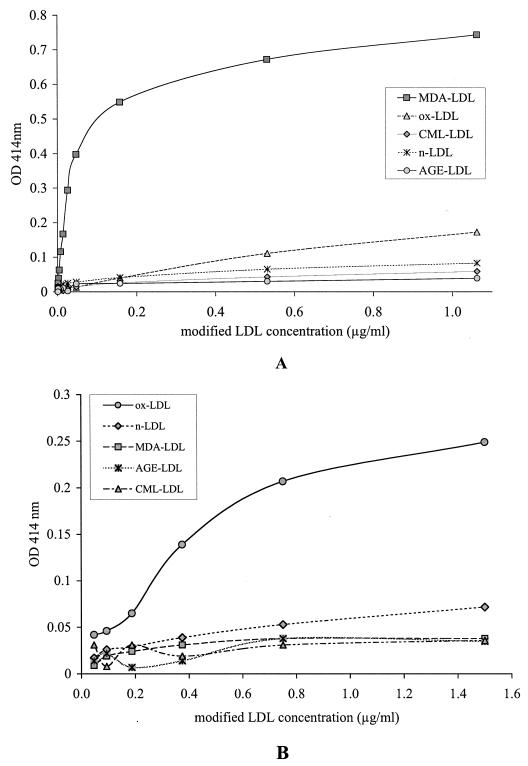
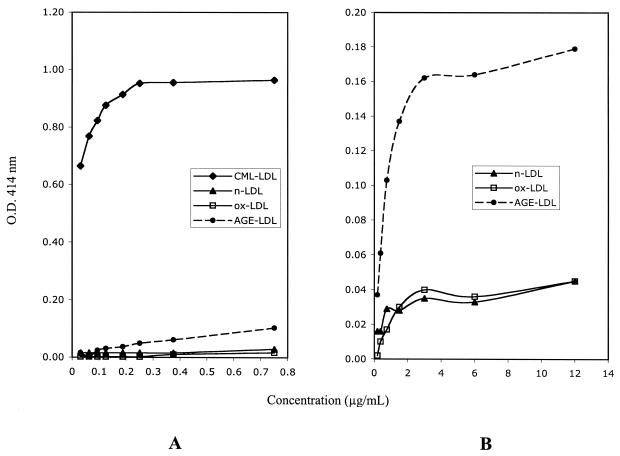
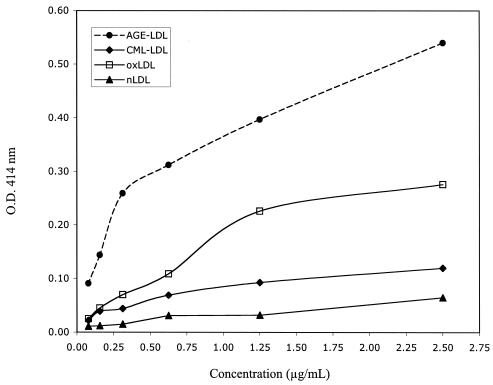
The capture assays for MDA-LDL and oxLDL (Fig. 1) showed specificity for the corresponding modification. However, while MDA-LDL antibodies captured oxLDL with low efficiency (Fig. 1A), oxLDL antibodies did not capture MDA-LDL (Fig. 1B). The capture assay for CML-LDL (Fig. 2) showed great specificity and efficiency for CML-LDL (Fig. 2A). At high protein concentrations, AGE-LDL was captured with greater efficiency than oxLDL and native LDL (Fig. 2B). Trying different conditions, we determined that the best discrimination between the capture of AGE-LDL versus the capture of oxLDL and native LDL with CML-LDL antibody was obtained when ApoB antibody was used for capture and peroxidase-labeled CML-LDL antibody was used for detection. The capture assay with AGE-LDL antibodies captured AGE-LDL with high efficiency and oxLDL with low efficiency (Fig. 3). The capture of CML-LDL was only marginally higher than the capture of native LDL.
FIG. 1.
Diagrammatic representation of the results of specificity testing for the capture assays for MDA-LDL (A) and oxLDL (B). n-LDL, native LDL.
FIG. 2.
Diagrammatic representation of the results of specificity testing for the capture assay for CML-LDL. Panel A illustrates the preferential capture of CM-LDL at low protein concentrations. Panel B illustrates the preferential capture of AGE-LDL relatively to oxLDL and native LDL (nLDL) at high protein concentrations.
FIG. 3.
Diagrammatic representation of the results of specificity testing for the capture assay for AGE-LDL. nLDL, native LDL.
The sensitivity, reproducibility, and recovery data for the four capture assays are summarized in Table 2. The intra-assay coefficients of variation (CV) were under 10%, and the interassay CV were under 11%. The recovery data were equally excellent, ranging from 94% (AGE-LDL assay) to 103% (MDA-LDL assay).
TABLE 2.
Sensitivity, reproducibility, and recovery data for the capture assays of MDA-LDL, oxLDL, CML-LDL, and AGE-LDL
| Sensitivity | MDA-LDL (0.042 mmol/mol of lysine) | oxLDL (0.03 μg/μg of protein) | CML-LDL (0.086 mmol/mol of lysine) | AGE-LDL (0.037 U/μg of protein) |
|---|---|---|---|---|
| Intra-assay CV (%)a | ||||
| In vitro-modified LDL | 5.96 | 6.76 | 5.03 | 5.30 |
| LDL from IC | 9.69 | 8.56 | 5.29 | 5.47 |
| Interassay CV (%)b | ||||
| In vitro-modified LDL | 7.94 | 9.48 | 6.53 | 5.61 |
| LDL from IC | 10.17 | 10.55 | 8.86 | 6.18 |
| % Recoveryc | 103 ± 2 | 100 ± 3 | 98 ± 3 | 94 ± 1 |
The intra-assay coefficient of variation was determined using six replicates of both an in vitro-modified LDL and an LDL sample purified from LDL-IC on one plate. Data are expressed as the mean CV obtained in six separate assays of both samples.
The interassay CV was determined over six separate assays, using six replicates of both an in vitro-modified LDL and an LDL sample purified from LDL-IC complex. The CV for each sample was calculated from a total of 36 determinations in six different runs.
Recovery experiments were performed by adding a known amount of in vitro-modified LDL specific for the given assay into native LDL and measuring the concentration of modified LDL in the supplemented sample. Values are expressed as mean ± 1 standard deviation.
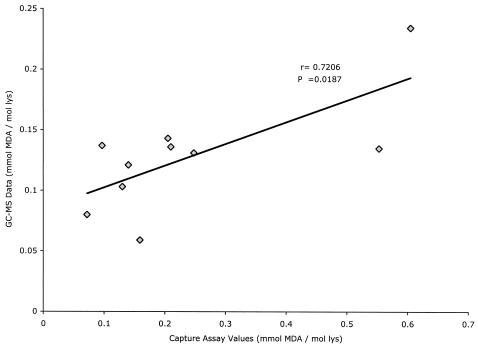
Previous observations have demonstrated that ApoB/E-rich lipoproteins isolated from PEG-precipitated IC are enriched in MDA-LDL and CML-LDL, using isotope dilution SIM-GC/MS for their measurement (30). We compared the capture assay's ability to distinguish the LDL isolated from PEG-precipitated IC from that remaining free in the supernatants and compared the concentrations of MDA-LDL determined by GC/MS and by capture assay. The ApoB-rich lipoproteins isolated from precipitated IC were enriched in all the different LDL modifications tested by our capture assays relative to ApoB-rich lipoproteins isolated from the supernatants after centrifugation of precipitated IC (Table 3). The comparison of MDA-LDL levels assayed by SIM-GC/MS and the MDA-LDL capture assay in 10 different ApoB-rich lipoprotein preparations isolated from precipitated IC showed a good correlation between both assays (r = 0.706, P = 0.0187), although, in general, the absolute values calculated from the capture assay were greater than those obtained by GC/MS (Fig. 4).
TABLE 3.
Comparison of capture values obtained with equal concentrations of ApoB/E-rich lipoproteins purified from PEG-precipitated IC and the corresponding supernatants from sera collected from 12 patients with type 1 diabetes
| Capture assay | Value for:
|
||
|---|---|---|---|
| Supernatant | PEG- precipitated IC (mean ± SD) | Pa | |
| MDA-LDL (mmol of MDA/ mol of lysine) | 0.031 ± 0.013b | 0.502 ± 0.278 | <0.0001 |
| oxLDL (μg/μg of protein) | 0.001 ± 0.005 | 0.648 ± 0.361 | <0.0001 |
| CML-LDL (mmol of CML/ mol of lysine) | 0.259 ± 0.140 | 0.576 ± 0.134 | <0.0001 |
| AGE-LDL (U/μg of protein) | 0.008 ± 0.015 | 0.112 ± 0.067 | 0.0003 |
Statistical analysis was performed with the two-tailed paired t test.
Mean ± 1 standard deviation (SD).
FIG. 4.
Linear regression analysis of the correlation between assays for MDA-LDL in LDL isolated from PEG precipitates by the capture assay and by chemical analysis by GC/MS. lys, lysine.
DISCUSSION
There is great interest in the assay of modified lipoproteins in serum or plasma samples because of their potential pathogenic role in atherosclerosis (11). Given the immunogenicity of modified lipoproteins, several groups have developed immunoassays, particularly for MDA-LDL and AGE-LDL (7, 9, 15, 24, 25). Previous studies conducted in our laboratory showed that rabbits immunized with MDA-LDL, oxLDL, AGE-LDL, and CML-LDL produced antibodies that recognized epitopes unique to those different in vitro modifications of LDL (29). We have now demonstrated that the same antibodies are able to capture modified LDL isolated from human sera and that they can be used to develop capture assays for different modifications of LDL with excellent accuracy and reproducibility. The recovery rates were close to 100%, except in the case of the MDA-LDL assay, in which it exceeded 100%. At 103%, the recovery value for MDA-LDL is within the range of variation of the assay but could also reflect the recognition of spontaneously modified LDL in the native LDL preparation (27).
The specificity of our rabbit MDA-LDL antibodies could be verified by comparison with the results of GC/MS assays of MDA in ApoB-rich lipoproteins obtained from PEG precipitates. A significant correlation existed between the two assays. Similar validations were not possible for the other assays. In the case of CML-LDL, the chemical assay of CML appeared less sensitive than the capture assay and we did not obtain sufficient data to compare the two assays. In the cases of the oxLDL and AGE-LDL capture assays, the unknown nature of the epitopes recognized by the rabbit antibodies makes any such comparative analysis impossible.
The four antibodies used in the assay recognize different epitopes of modified LDL. Although human antibodies to oxLDL react primarily with MDA epitopes, rabbit antibodies to oxLDL recognize a different epitope, also present in spontaneously modified human LDL (29). Similarly, human antibodies to AGE-LDL react primarily with CML-LDL (30), but rabbit antibodies to AGE-LDL recognize epitope(s) unrelated to CML, which have been previously described by Ikeda et al. (6). Our data suggest that these epitopes are expressed, at lower levels, by oxLDL. As such, our rabbit AGE-LDL antibody does not differentiate well between oxLDL and AGE-LDL.
All our antibodies captured significantly higher levels of modified LDL in the ApoB/E-rich lipoproteins isolated from IC. The highest level of discrimination between LDL isolated from IC (apparently more modified) and LDL that remains soluble after IC precipitation was obtained with the antibodies to oxLDL and CML-LDL. The results obtained with MDA-LDL and oxLDL antibodies suggest that the majority of oxLDL molecules in circulation are part of circulating IC. Although we have previously proven that IC are precipitated with PEG and native LDL is not (1, 12, 30), we cannot state that all modified LDL is precipitated because of its involvement in IC formation. Other possible causes for precipitation in the presence of low concentrations of PEG, such as aggregation, cannot be easily ruled out, although the presence of LDL aggregates in circulation has never been proven. On the other hand, there was a significant difference in the data obtained with CML-LDL and AGE-LDL antibodies, because while the AGE-LDL antibodies did not react with free LDL, CML-LDL antibodies did. Therefore, CML-LDL seems to exist in circulation in both IC-bound and free forms.
The formation of circulating IC containing modified LDL and the corresponding antibodies has significant implications for the assay of modified LDL in whole serum. During the development of the capture assays, we observed that, when we tried to perform them with whole serum samples, the OD versus dilution curves were rather complex and the ranking of different samples by their apparent contents of modified LDL changed at different dilutions. This could be a consequence of the interaction of modified lipoproteins with autoantibodies of different affinities, leading to complex dissociation curves that interfere with their capture. The interference of IC in the modified LDL assays has been recognized by Ehara et al. (3), who used isolated LDL in their capture assay. However, it is highly unlikely that sequential ultracentrifugation will result in dissociation of IC, and the IC themselves are likely to sediment at a different rate from native LDL. The addition of 4% PEG to the samples (26) is likely to enhance IC formation, as this is the concentration that we use to precipitate IC from serum samples. Addition of sodium dodecyl sulfate to the samples (32) may help dissociate IC but may also negatively affect the antigen-antibody reaction. Our protocol, involving the isolation of antibody-free LDL from serum fractions, can certainly avoid the interference of IC in the assay but has as its main drawback the addition of several preparative steps that significantly complicate the screening of large numbers of samples. Future work will focus on two areas: the clinical significance of the detection of different types of modified LDL in PEG precipitates and the simplification of our assay protocol to allow clinical studies in large groups of patients.
Acknowledgments
The research reported in this publication was supported by a program project grant funded by the National Institutes of Health/NHLBI (PO1-HL55782) (M.L.V. and G.V.), the Juvenile Diabetes Research Foundation International (1-20002-812) (M.L.-V. and G.V.), the Research Service of the Ralph H. Johnson Department of Veteran Affairs Medical Center (M.L.-V.), and by grants from the USPHS DK19971 and the Juvenile Diabetes Research Foundation (JDRF-1-2000-663) (S.R.T.). We also want to acknowledge the support of the DCCT/EDIC investigators sponsored through research contracts from the Division of Diabetes, Endocrinology and Metabolic Diseases of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institutes of Health.
REFERENCES
- 1.Atchley, D. H., M. F. Lopes-Virella, D. Zheng, and G. Virella. 2002. Oxidized LDL-anti-oxidized LDL immune complexes and diabetic nephropathy. Diabetologia 45:1562-1571. [DOI] [PubMed] [Google Scholar]
- 2.Bentzen, C. L., K. J. Acuff, B. Marechal, M. A. Rosenthal, and M. E. Volk. 1982. Direct determination of lipoprotein cholesterol distribution with micro-scale affinity chromatography columns. Clin. Chem. 28:1451-1456. [PubMed] [Google Scholar]
- 3.Ehara, S., M. Ueda, T. Naruko, K. Haze, A. Itoh, M. Otsuka, R. Komatsu, T. Matsuo, H. Itabe, T. Takano, Y. Tsukamoto, M. Yoshiyama, K. Takeuchi, J. Yoshikawa, and A. E. Becker. 2001. Elevated levels of oxidized low density lipoprotein show a positive relationship with the severity of acute coronary syndromes. Circulation 103:1955-1960. [DOI] [PubMed] [Google Scholar]
- 4.Haberland, M. E., A. M. Fogelman, and P. A. Edwards. 1982. Specificity of receptor-mediated recognition of malondialdehyde-modified low density lipoproteins. Proc. Natl. Acad. Sci. USA 79:1712-1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holvoet, P., J. M. Stassen, J. Van Cleemput, D. Collen, and J. Vanhaecke. 1998. Oxidized low density lipoproteins in patients with transplant-associated coronary artery disease. Arterioscler. Thromb. Vasc. Biol. 18:100-107. [DOI] [PubMed] [Google Scholar]
- 6.Ikeda, K., R. Nagai, T. Sakamoto, H. Sano, T. Araki, N. Sakata, H. Nakayama, M. Yoshida, S. Ueda, and S. Horiuchi. 1998. Immunochemical approaches to AGE-structures: characterization of anti-AGE antibodies. J. Immunol. Methods 215:95-104. [DOI] [PubMed] [Google Scholar]
- 7.Itabe, H., E. Takeshima, H. Iwasaki, J. Kimura, Y. Yoshida, T. Imanaka, and T. Takano. 1994. A monoclonal antibody against oxidized lipoprotein recognizes foam cells in atherosclerotic lesions. Complex formation of oxidized phosphatidylcholines and polypeptides. J. Biol. Chem. 269:15274-15279. [PubMed] [Google Scholar]
- 8.Itabe, H., H. Yamamoto, T. Imanaka, K. Shimamura, H. Uchiyama, J. Kimura, T. Sanaka, Y. Hata, and T. Takano. 1996. Sensitive detection of oxidatively modified low density lipoprotein using a monoclonal antibody. J. Lipid Res. 37:45-53. [PubMed] [Google Scholar]
- 9.Kotani, K., M. Maekawa, T. Kanno, A. Kondo, N. Toda, and M. Manabe. 1994. Distribution of immunoreactive malondialdehyde-modified low-density lipoprotein in human serum. Biochim. Biophys. Acta 1215:121-125. [DOI] [PubMed] [Google Scholar]
- 10.Lopes-Virella, M. F., S. Koskinen, M. Mironova, D. Horne, R. Klein, C. Chasssereau, C. Enockson, and G. Virella. 2000. The preparation of copper-oxidized LDL for the measurement of oxidized LDL antibodies by EIA. Atherosclerosis 152:105-113. [DOI] [PubMed] [Google Scholar]
- 11.Lopes-Virella, M. F., and G. Virella. 2003. The role of immune and inflammatory processes in the development of macrovascular disease in diabetes. Front. Biosci. 8:s750-s768. [DOI] [PubMed] [Google Scholar]
- 12.Mironova, M., G. Virella, I. Virella-Lowell, and M. F. Lopes-Virella. 1997. Anti-modified LDL antibodies and LDL-containing immune complexes in IDDM patients and healthy controls. Clin. Immunol. Immunopathol. 85:73-82. [DOI] [PubMed] [Google Scholar]
- 13.Morel, D. W., J. R. Hessler, and G. M. Chisolm. 1983. Low density lipoprotein cytotoxicity induced by free-radical peroxidation of lipid. J. Lipid Res. 24:1070. [PubMed] [Google Scholar]
- 14.Nakamura, Y., Y. Horii, T. Nishino, H. Shiiki, Y. Sakaguchi, T. Kagoshima, K. Dohi, Z. Makita, H. Vlassara, and R. Bucala. 1993. Immunochemical localization of advanced glycosylation end products in coronary atheroma and cardiac tissue in diabetes mellitus. Am. J. Pathol. 143:1649-1656. [PMC free article] [PubMed] [Google Scholar]
- 15.Onorato, J. M., S. R. Thorpe, and J. W. Baynes. 1998. Immunohistochemical and ELISA assays for biomarkers of oxidative stress in aging and disease. Ann. N. Y. Acad. Sci. 854:277-290. [DOI] [PubMed] [Google Scholar]
- 16.Palinski, W., M. E. Rosenfeld, S. Ylä-Herttuala, G. C. Gurtner, S. S. Socher, S. W. Butler, S. Parthasarathy, T. E. Carew, D. Steinbergand, and J. L. Witztum. 1989. Low density lipoprotein undergoes oxidative modification in vivo. Proc. Natl. Acad. Sci. USA 86:1372-1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reddy, S., J. Bichler, K. J. Wells-Knecht, S. R. Thorpe, and J. W. Baynes. 1995. N epsilon-(carboxymethyl)lysine is a dominant advanced glycation end product (AGE) antigen in tissue proteins. Biochemistry 34:10872-10878. [DOI] [PubMed] [Google Scholar]
- 18.Requena, J. R., M. X. Fu, M. U. Ahmed, A. J. Jenkins, T. J. Lyons, J. W. Baynes, and S. R. Thorpe. 1997. Quantification of malondialdehyde and 4-hydroxynonenal adducts to lysine residues in native and oxidized human low-density lipoproteins. Biochem. J. 322:317-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmidt, A. M., O. Hori, J. X. Chen, J. F. Li, J. Crandall, J. Zhang, R. Cao, S. D. Yan, J. Brett, and D. Stern. 1995. Advanced glycation endproducts interacting with their endothelial receptor induce expression of vascular cell adhesion molecule-1 (VCAM-1) in cultured human endothelial cells and in mice. A potential mechanism for the accelerated vasculopathy of diabetes. J. Clin. Investig. 96:1395-1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmidt, A. M., S. D. Yan, J. Brett, R. Mora, R. Nowygrod, and D. Stern. 1993. Regulation of human mononuclear phagocyte migration by cell surface-binding proteins for advanced glycation end products. J. Clin. Investig. 91:2155-2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steinberg, D. 1988. Metabolism of lipoproteins and their role in pathogenesis of atherosclerosis. Atheroscler. Rev. 18:1-23. [Google Scholar]
- 22.Steinbrecher, U. P. 1987. Oxidation of human low density lipoprotein results in derivatization of lysine residues of apolipoprotein B by lipid peroxide decomposition products. J. Biol. Chem. 262:3603-3608. [PubMed] [Google Scholar]
- 23.Steinbrecher, U. P., M. Eisher, J. L. Witztum, and L. K. Curtiss. 1984. Immunogenicity of homologous low density lipoprotein after methylation, ethylation, acetylation, or carbamylation: generation of antibodies specific for derivatized lysine. J. Lipid Res. 25:1109-1116. [PubMed] [Google Scholar]
- 24.Takeuchi, M., Z. Makita, K. Yanagisawa, Y. Kameda, and T. Koike. 1999. Detection of noncarboxymethyllysine and carboxymethyllysine advanced glycation end products (AGE) in serum of diabetic patients. Mol. Med. 5:393-405. [PMC free article] [PubMed] [Google Scholar]
- 25.Takeuchi, M., Y. Yanase, N. Matsuura, S. Yamagishi Si, Y. Kameda, R. Bucala, and Z. Makita. 2001. Immunological detection of a novel advanced glycation end-product. Mol. Med. 7:783-791. [PMC free article] [PubMed] [Google Scholar]
- 26.Toshima, S., A. Hasegawa, M. Kurabayashi, H. Itabe, T. Takano, J. Sugano, K. Shimamura, J. Kimura, I. Michishita, T. Suzuki, and R. Nagai. 2000. Circulating oxidized low density lipoprotein levels. A biochemical risk marker for coronary heart disease. Arterioscler. Thromb. Vasc. Biol. 20:2243-2247. [DOI] [PubMed] [Google Scholar]
- 27.Virella, G., S. Koskinen, G. Krings, J. M. Onorato, S. R. Thorpe, and M. Lopes-Virella. 2000. Immunochemical characterization of purified human oxidized low-density lipoprotein antibodies. Clin. Immunol. 95:135-144. [DOI] [PubMed] [Google Scholar]
- 28.Virella, G., and M. F. Lopes-Virella. 2003. Lipoprotein autoantibodies: measurement and significance. Clin. Diagn. Lab. Immunol. 10:499-505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Virella, G., S. Thorpe, N. L. Alderson, M. B. Derrick, C. Chassereau, J. M. Rhett, and M. F. Lopes-Virella. 2004. Definition of the immunogenic forms of modified human LDL recognized by human autoantibodies and by rabbit hyperimmune antibodies. J. Lipid Res. 45:1859-1867. [DOI] [PubMed] [Google Scholar]
- 30.Virella, G., S. R. Thorpe, N. L. Alderson, E. M. Stephan, D. H. Atchley, F. Wagner, M. F. Lopes-Virella et al. 2003. Autoimmune response to advanced glycosylation end-products of human low density lipoprotein. J. Lipid Res. 443:487-493. [DOI] [PubMed] [Google Scholar]
- 31.Vlassara, H., R. Bucala, and L. Striker. 1994. Pathogenic effects of advanced glycosylation: biochemical, biologic, and clinical implications for diabetes and aging. Lab. Investig. 70:138-151. [PubMed] [Google Scholar]
- 32.Yamazaki, K., H. Bujo, K. Taira, N. Itou, M. Shibasaki, K. Takahashi, and Y. Saito. 2004. Increased circulating malondialdehyde-modified LDL in the patients with familial combined hyperlipidemia and its relation with the hepatic lipase activity. Atherosclerosis 172:181-187. [DOI] [PubMed] [Google Scholar]
- 33.Yla-Herttuala, S., W. Palinski, M. E. Rosenfeld, S. Parthasarathy, T. E. Carew, S. Butler, J. L. Witztum, and D. Steinberg. 1989. Evidence for the presence of oxidatively modified low density lipoprotein in atherosclerotic lesions of rabbit and man. J. Clin. Investig. 84:1086-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]