Abstract
Aims
Intravenous (IV) iron infusions have been associated with hypophosphataemia (HP) and hypersensitivity reactions (HSRs). No studies have compared the side effects of ferric carboxymaltose (FCM) with those of isomaltoside 1000 (ISM). This study aimed to describe the occurrence of HP and HSRs following the administration of either FCM or ISM.
Methods
Data on 231 outpatients treated with IV iron infusions, between November 2011 and April 2014, were collected. During that period, the department made a switch from FCM to ISM and then back to FCM. Of the 231 patients, 39 received both FCM and ISM during the period. The prevalences of HP and HSRs were compared between the two drugs.
Results
We found more HP events when FCM was given (64 vs. 9; P < 0.01). In contrast, more patients had mild HSRs when ISM was given (2.5% vs. 10.7%; P < 0.01). A comparison of the two drugs in the subpopulation who received both drug types (n = 39) revealed a difference in phosphate decrease (P < 0.01), with the most marked decrease occurring with FCM. Nine patients who had HSRs were exposed to both drugs. No potential HSR crossover between the two drugs was found.
Conclusion
We found a higher risk of HP with FCM administration when compared to ISM administration. Conversely, we found a higher risk of mild HSRs with ISM administration when compared to FCM administration. The impacts of the two types of side effects should be considered when choosing an IV iron drug.
Keywords: anaemia, inflammatory bowel disease, intravenous iron, iron deficiency
What is Already Known about this Subject
Hypophosphataemia and drop in phosphate levels following intravenous iron infusions have been reported.
Hypersensitivity reactions (HSRs) following intravenous iron infusions have been reported.
Current recommendations suggest that all intravenous iron drugs should be contraindicated in a patient with a history of HSRs.
What this Study Adds
Two intravenous iron drugs have been compared in the same population in a clinical setting.
The occurrence of hypophosphataemia was most frequent for ferric carboxymaltose and HSRs was most frequent for isomaltoside 1000.
No hypersensitivity crossover between the two drugs were found.
The study uncovers dilemmas when choosing the best intravenous iron drug in clinical practice.
Table of Links
This Table lists key ligands in this article that are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY 1.
Introduction
Intravenous (IV) iron supplementation is increasingly used to treat iron deficiency and anaemia. In patients with chronic inflammatory bowel disease (IBD), clinical guidelines suggest that iron deficiency should be supplemented intravenously, and IV iron treatment has become the standard of care in many outpatient gastroenterology settings 2. Intravenous administration is generally considered safe, but iron infusions have been associated with both hypophosphataemia (HP) and hypersensitivity reactions (HSRs) 3, 4, 5, 6, 7, 8, 9, 10.
HP may cause symptoms similar to those of refeeding syndrome, i.e., a broad spectrum including fatigue and cardiac arrhythmia, but low plasma phosphate levels may also occur without any symptoms experienced by the patient 11, 12, 13, 14, 15. The mechanism for the development of HP with the administration of different IV iron drugs is unknown, but because fibroblast growth factor 23 (FGF23) is critically involved in phosphate homeostasis, changes in intact FGF23 levels may contribute to the development of HP 6, 16.
Compared with the first generation of IV iron drugs that use mainly iron‐dextran complexes, new IV iron formulations, including ferric carboxymaltose (FCM) and isomaltoside 1000 (ISM), may cause fewer HSRs, although post‐marketing data for FCM and ISM are sparse when compared to the first generation of IV iron drugs 3. Adverse drug reactions to all marketed IV drugs were investigated in a post‐marketing assessment from the European Medicines Agency, Committee for Medicinal Products for Human Use (EMA‐CHMP) 3. In a population of 393 160 patient–years, hypersensitivity to FCM was reported in 236 cases (0.06%). According to the Ring and Messmer classification, the vast majority (200 cases) were grade I or grade II reactions, which are mild to moderate reactions 3, 17. Adverse drug reactions to ISM occurred in 26 cases (0.02%). Of the 26 cases, 25 were grade I or grade II reactions. The authors of the assessment report recommended that for patients with a history of HSRs to any IV iron containing products, any IV iron products should be contraindicated.
In clinical practice, the choice of drug is influenced by efficacy, cost, and potential side effects and complications. With regard to IV iron, these considerations imply a balance between the risk of HP and the risk of an HSR. In the Department of Hepatology and Gastroenterology, Aarhus University Hospital, Denmark, we have used IV iron for more than a decade. We have clinical experience with three drugs: iron sucrose, FCM and ISM. When FCM was commercially marketed in 2009, we changed all of our iron infusions from iron sucrose to FCM due to its easier administration and lower overall cost 18. In August 2012, FCM was replaced by ISM due to a significantly lower drug cost in Denmark. At any time point, the patients were given our first‐choice IV iron drug (FCM or ISM), unless they had a history of either severe HP or HSRs to our first‐choice drug. When using ISM, we observed a relatively high number of HSRs, and for safety reasons, we switched back to FCM in October 2013. A drawback when using FCM was a higher rate of HP compared with ISM. Consequently, we have experience with both FCM and ISM as the first drug of choice in the same patient population. Based on our clinical experience, we were able to compare the two drugs.
The aim of this study was to describe the occurrence of the two most common complications associated with IV iron administration – HP and HSR – following the administration of either FCM or ISM.
Materials and methods
Study design
This study was a single‐centre, retrospective analysis performed with a stable, unselected patient cohort in an outpatient gastroenterology setting.
Subjects and setting
All patients who received IV iron infusion at the Department of Hepatology and Gastroenterology, Aarhus University Hospital, Denmark, between November 2011 and April 2014 were identified in the hospital's electronic medical registry and included in this study. Because public reimbursement requires electronic coding of each individual iron infusion, all infusions could be identified through this registry. Data were collected from the patients' electronic medical records and included the following information: sex, age, diagnosis, dose and type of IV iron given. Data on side effects and complications were documented during follow‐up clinical visits. Consequently, data on HSRs were obtained from each patient's medical record. Many iron treatments were given as several infusions over two to three weeks. Therefore, iron infusions are regarded as an ‘infusion‐series.’ A majority of patients received 1–3 infusion series. For the patients who received more than two IV iron infusion series in the study period, data from the first two series were used in the analysis.
Biochemical measurements
The patients had blood samples drawn prior to the administration of IV iron infusions and according to a scheduled follow‐up program after the first infusion. Routine biochemical follow‐up included the measurement of plasma phosphate at predetermined time points. The blood sample results were categorised into four time points: baseline (covering day −7 to day 0), week 2 (day 4 to day 21), week 5 (day 22 to day 49) and week 10 (day 50 to day 90). If several samples were drawn in a given time period, the lowest level of phosphate was used for analysis. HP was defined as a phosphate concentration < 2.0 mg dl−1 and severe HP as a phosphate concentration < 1.0 mg dl−1.
HSRs
The Ring and Messmer Severity Scale Quantification of Intensity of Anaphylactoid Reaction was used to classify the HSRs to IV iron infusions 17. In brief, the reactions are classified into four categories. Grade I is skin symptoms and mild fever reactions, grade II is measurable but not life‐threatening symptoms, grade III is shock, and grade IV is cardiac and/or respiratory arrest.
Statistical analyses
Data are presented as medians, ranges, and interquartile ranges (IQRs) or as numbers and percentages with 95% confidence intervals (CIs). Differences between data were determined using Student t test, the χ2 test, Fisher's exact test or Spearman's rank correlations. Logistic regression analysis was applied to compute the crude prevalence odds ratios as estimates of relative risk with associated 95% CIs. All statistical tests were two‐sided with a statistical significance level of 5%. All analyses were carried out using the software program Stata 13 (StataCorp. 2013. Stata Statistical Software: Release 13. College Station, TX, USA: StataCorp LP).
Ethical considerations
According to Danish law, approval from the Danish Ethical Committee was not needed.
Results
Patients and iron infusions
Between November 2011 and April 2014, a total of 308 IV iron infusion series were administered to 231 patients. Out of all infusion series, 192 (62%) were FCM and 116 (38%) were ISM. A total of 125 patients received FCM only, 67 patients received ISM only, and 39 patients received both types of iron drugs. The median dose administered was 1000 mg (range, 500–2500), with no difference between the two drugs. Patient characteristics are presented in Table 1. Most patients (144, 62%), had IBD. Of these, 101 had Crohn's disease and 43 had ulcerative colitis. The median age of all patients was 41 years (range, 18–87), and a majority (n = 156) were female.
Table 1.
Characteistics of 231 patients who received intravenous iron drug
| Age, years, median (range) | 41 (18–87) | |
| Sex, male, n (%) | 74 (32.0) | |
| Diagnosis | ||
| Crohn's disease, n (%) | 101 (43.7) | |
| Ulcerative colitis, n (%) | 43 (18.6) | |
| Coeliac disease, n (%) | 9 (3.9) | |
| Iron‐deficiency anaemia, n (%) | 17 (7.4) | |
| Anaemia – other, n (%) | 23 (10.0) | |
| Other diagnosis, n (%) | 38 (16.4) | |
| Intravenous iron treatment | ||
| ISM only, n (%) | 67 (29.0) | |
| FCM only, n (%) | 125 (54.1) | |
| Both iron treatments, n (%) | 39 (16.9) | |
| Total dose, mg, median (range) | 1000 (500–2500) | |
| Baseline phosphate, mg dl −1 , median (IQR) [RI] | 3.2 (2.9–3.6) | [2.2–4.5] |
| Baseline ferritin, μg l −1 , median (IQR) [RI] | 12 (8–21) | [15–300] |
| Baseline haemoglobin, g dl −1 , median (IQR) [RI] | 11.5 (10.3–12.7) | [> 13 men] [> 12 women] |
| Baseline CRP, mg l −1 , median (IQR) [RI] | 1.7 (0.6–5.5) | [< 8] |
CRP, C‐reactive protein; FCM, ferric carboxymaltose; IQR, interquartile range; ISM, isomaltoside 1000; RI, reference interval
As shown in Figure 1A, the increase in haemoglobin levels following iron infusion was similar with the two drugs analysed.
Figure 1.
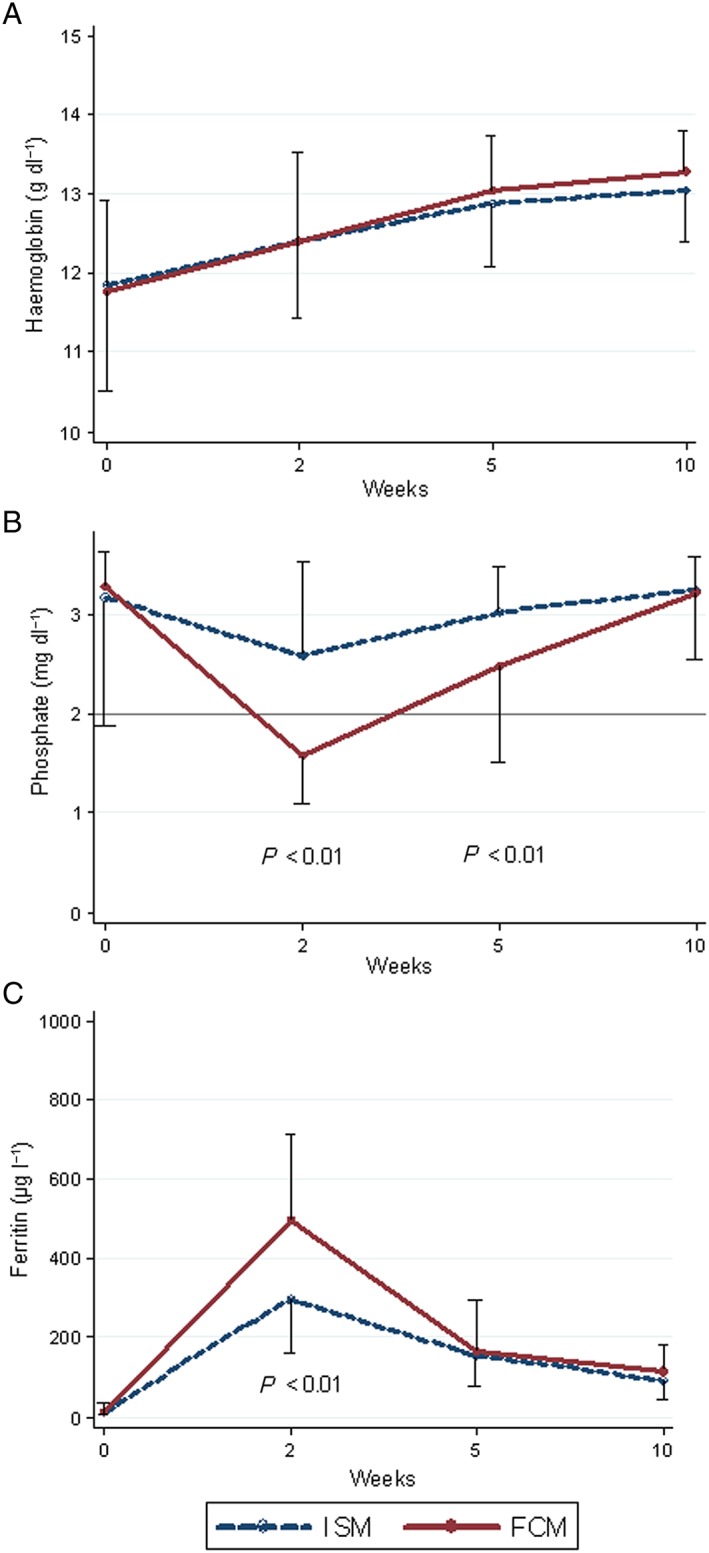
Median levels and interquatile ranges of (A)haemoglobin, (B) phosphate and (C) ferritin after IV iron infusions, stratified by drug type (n = 117). ISM, isomaltoside 1000; FCM, ferric carboxymaltose
HP
Of the 308 infusion series administered, baseline and follow‐up measurements of plasma phosphate were available in 223 (72%). We observed no statistically significant differences in sex, age, or the type of IV iron given between the patients for whom phosphate data were available and for those without complete biochemical monitoring. Significantly more patients who had received FCM experienced HP during follow‐up compared with those who had received ISM (Table 2). Out of five patients with a low baseline phosphate, two also had HP at week 2. None of these five patients developed severe HP. We only observed severe HP after the administration of FCM. We also observed a significantly more pronounced drop in plasma phosphate from baseline in patients who had received FCM than following ISM (Figure 1B). In addition, the increase in ferritin was more pronounced with FCM than with ISM (Figure 1C). In both drug groups, we observed a modest but significant association between the rise in the ferritin levels and the drop in the phosphate levels from baseline to week 2 (Figure 2). Among 39 patients who had received both FCM and ISM, follow‐up data were available for 32 (82%). The dose of IV iron given was the same for both drugs. Paired analysis of the changes in plasma phosphate revealed a significantly more pronounced drop following FCM compared with ISM, and this difference was sustained at week 5 (Figure 3A). Although not statistically significant, the initial rise in plasma ferritin was more pronounced with FCM than with ISM (Figure 3B).
Table 2.
Proportions of cases with low phosphate levels after IV infusions of different forms of iron. Exact values, relative drops and prevalence odds ratios (PORs) are shown
| Baseline | Week 2 | Week 5 | Week 10 | |
|---|---|---|---|---|
| Days after baseline, median (IQR) | 10 (7–16) | 28 (28–35) | 60 (60–74) | |
| FCM/ISM, n | 141/82 | 84/33 | 114/59 | 106/59 |
| Cases included, n | 223 | 117 | 173 | 165 |
| Phosphate < 2 mg dl −1 , n | 5 | 73 | 43 | 7 |
| FCM/ISM, n | 64/9 a | 37/6 a | 6/1 | |
| FCM, POR (95% Cl) | 6.7 (3.1–14.6) | 4.5 (1.8–11.2) | 3.6 (0.4–30.4) | |
| Phosphate < 1 mg dl −1 , n | 0 | 13 | 4 | 1 |
| FCM/ISM b | 13/0 | 4/0 | 1/0 | |
| Phosphate drop c > 25%, n | 81 | 65 | 21 | |
| FCM/ISM | 70/11 a | 60/5 a | 14/7 | |
| FCM, POR (95% CI) | 10.0 (4.0–25.2) | 12.2 (4.6–32.8) | 1.1 (0.4–3.0) | |
| Phosphate drop c > 50%, n | 52 | 31 | 1 | |
| FCM/ISM | 50/2 a | 29/2 a | 1/0 b | |
| FCM, POR (95% Cl) | 22.8 (5.1–101.6) | 9.8 (2.3–42.9) | – |
CI, confidence interval; FCM, ferric carboxymaltose; IQR, interquartile range; ISM, isomaltoside 1000
P < 0.01
No POR, as all cases were exposed to FCM
From baseline
Figure 2.
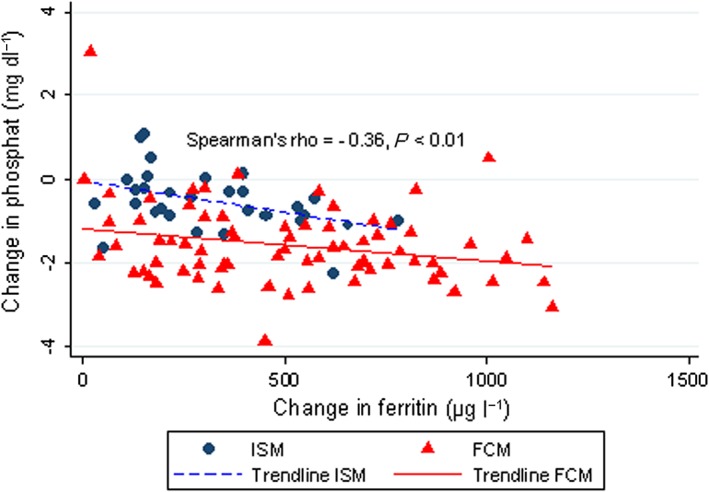
Correlation between changes in phosphate and ferritin from baseline to week 2. ISM, isomaltoside 1000; FCM, ferric carboxymaltose
Figure 3.
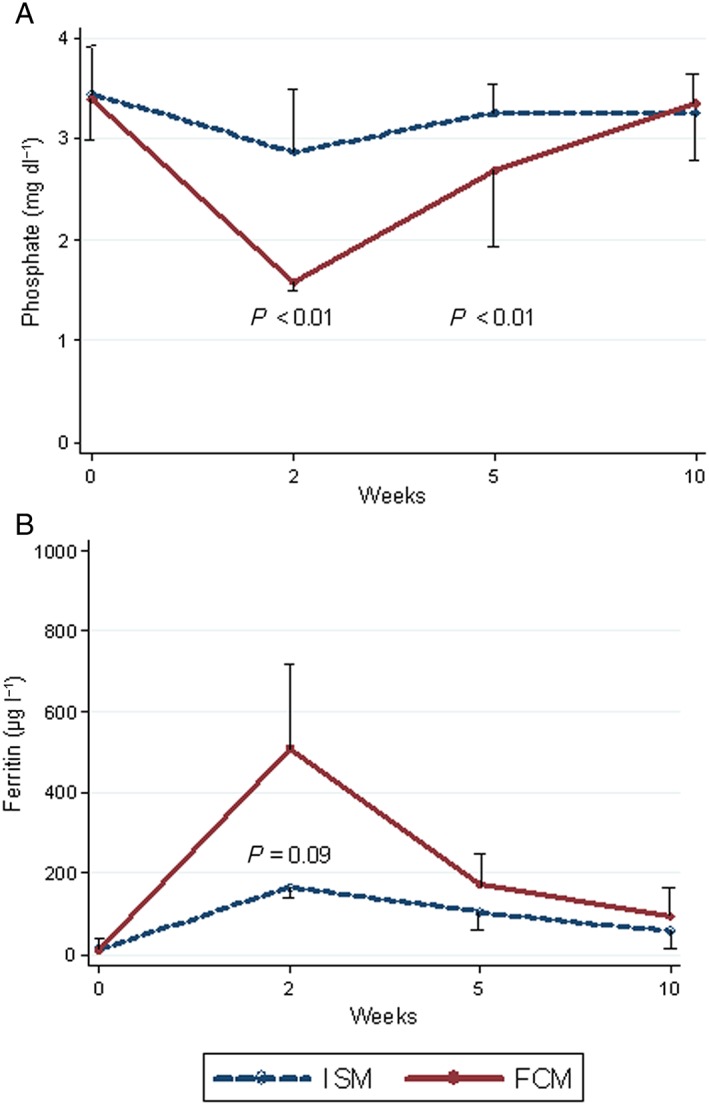
Median levels and interquatile ranges of (A) phosphate and (B) ferritin after IV iron infusions for 32 patients who received both drugs, stratified by drug type. ISM, isomaltoside 1000; FCM, ferric carboxymaltose
We did not observe any specific pattern between the patients' intake of concomitant medication and the development of HP.
Hypersensitivity reactions
Of 231 patients who received IV iron, an HSR was documented in the patient records in 14 cases (6.1%). Two of the 14 patients experienced an HSR more than once, resulting in a total of 16 HSR events (6.4%, CI: 3.7–9.9%). Six (5.9%, CI: 1.4–10.6%) of 101 patients with Crohn's disease experienced HSRs. In ulcerative colitis, the HSR frequency was four (9.3%, CI: 2.6–22.1%) of 43 patients. For other diagnoses, the frequency was four (4.6%, CI: 1.3–11.4%) of 87 patients. We observed no significant associations between other patient characteristics and the occurrence of an HSR (Table 3).
Table 3.
Characteristics of patients who experienced hypersensitivity reactions following intravenous iron infusion
| Patients with HSRs (n = 14) | Patients without HSRs (n = 217) | P value | |
|---|---|---|---|
| Age, years, median (range) | 34 (18–86) | 42 (18–87) | 0.07 |
| Sex, male, n (%) | 3 (21.4) | 71 (32.7) | 0.56 |
| Diagnosis | |||
| Crohn's disease, n (%) | 6 (42.8) | 95 (43.8) | 0.95 |
| Ulcerative colitis, n (%) | 4 (28.6) | 39 (18.0) | 0.32 |
| Other, n (%) | 4 (28.6) | 83 (38.2) | 0.47 |
| Intravenous iron treatment | |||
| ISM only, n (%) | 2 (14.3) | 65 (30.0) | 0.21 |
| FCM only, n (%) | 3 (21.4) | 122 (56.2) | 0.01 |
| Both, n (%) | 9 a (64.3) | 30 (13.8) | < 0.01 |
| Total dose planned, mg, median (range) b | 1000 (500–1000) | 1000 (500–2500) | 0.01 |
| Known hypersensitivity, n (%) c | 6 (42.9) | 75 (34.6) | 0.53 |
FCM, ferric carboxymaltose; HSR, hypersensitive reaction; ISM, isomaltoside 1000
Only one patient had HSRs to both drugs
Per treatment cycle. Patients with HSRs did not always receive the entire planned dose
To compounds other than intravenous iron
All HSRs were evaluated to be grade I (n = 9) or grade II (n = 7) on the Ring and Messmer Scale, and all patients recovered within a few days. All HSRs were reported to the relevant public health authorities in Denmark.
When stratified by the type of IV iron, four (2.5%, CI: 0.7–6.2%) of 162 patients had an HSR to FCM and 11 (10.7%, CI: 5.5–18.3%) of 103 patients had an HSR to ISM. Thus, four‐fold more patients experienced an HSR to ISM than to FCM (P < 0.01, Fisher's exact test).
Nine of the 14 patients who experienced an HSR were exposed to both FCM and ISM. Six patients had been exposed to FCM first without any HSRs and then exposed to ISM leading to an HSR in all six cases. Five of these were again exposed to FCM without any signs of HSRs. One of the six patients was not re‐exposed to IV iron. Two patients received ISM first and developed HSRs. None of them showed any signs of an HSR when subsequently exposed to FCM. One patient first developed an HSR to FCM, then to ISM. We did not find any pattern between the patients' intake of concomitant medication and the development of HSRs. Further details are shown in the supplementary material (Table S1).
Discussion
This study reveals clinically relevant differences in the occurrence of HP and HSRs between two commercially available IV iron drugs, FCM and ISM. While hypophosphatemia occurred more frequently with FCM, ISM was associated with more HSRs. To our knowledge, our study is the first to compare these complications to FCM and ISM in an unselected outpatient population. The amount of iron provided in our study was the same for both drug types; therefore, the focus must be on the different wrapping and release mechanisms of the iron. For FCM, iron is covered in a round shell of carboxymaltose, whereas for ISM, a matrix‐like structure of isomaltoside is used to bind the iron.
HP occurred in up to 50% of patients who received FCM. This finding is in accordance with other findings, as was our finding of HP in <10% of patients who had received ISM 19, 20, 21. A recent review revealed HP frequencies for ISM to be between 0 and 7%, with the highest levels in patients with IBD 22, 23. No studies have compared the frequency of HP between the two drugs in the same population, and no head‐to‐head comparisons have been conducted. The drop in plasma phosphate levels following FCM administration occurred concomitantly with a marked increase in plasma ferritin. This observation was consistent both in the unpaired analyses of patients who only received one drug and in the paired analyses of the subgroup of patients who received both drug types.
The observed frequency of HSRs was markedly higher than that reported by the EMA‐CHMP report in general, and we found a four‐fold higher occurrence of HSRs with ISM than with FCM 3. The latter is in contrast to the EMA‐CHMP report. Based on the 14 patients who experienced HSRs in our study, we observed no potential HSR crossover between the two drugs. This finding may strengthen the theory that HSRs to IV iron infusions are probably due to the presence of nanoparticles than to the iron itself 24. A recent review did not identify any significant differences between HSRs to IV iron drugs and HSRs to other IV drugs, suggesting that a major mechanism is complement activation‐related pseudoallergy 24. Furthermore, the authors suggested a revision and extension of the EMA‐CHMP guidance. Our results do not challenge the quality of the EMA‐CHMP report, but they may call into question the completeness of the source data. A systematic review and meta‐analysis of published trials showed an increased relative risk (2.7) of HSRs to IV iron infusions 25. The trials were published between 1965 and 2013. The risk of HSRs to FCM was 3.4 and thus slightly higher than the overall risk. Only one trial using ISM was included. The authors did not report any HSRs to ISM, although four of 225 patients reported HSRs (e.g., flushing, itching, rashes and breathlessness) in the primary trial. The findings on HSRs in our study emphasize the differences between the results found when a drug is tested in clinical trials and the results of clinical experience with the same drug post‐marketing.
Phosphate plays a key role in many biological processes. The phosphate balance is regulated by factors such as parathyroid hormone and vitamin D. However, new insights suggest that other factors, such as FGF23, are involved in the regulation. Wolf et al. 26 found that FGF23 is increased when iron deficiency is present. Furthermore, a model for different FGF23 production related to the type of IV iron given (FCM and iron dextran) has been presented 6. The model explains how FCM tends to trigger the production of intact FGF23 (iFGF23), which will lead to a drop in phosphate levels. Low levels of phosphate can aid physicians in recognising other symptoms. Many patients have symptoms such as increased fatigue or muscle pain. The patients in our study were not systematically questioned about this issue, but another study found that fatigue worsened in 30% of patients after treatment with FCM 20. Furthermore, severe HP (< 1 mg dl−1) can lead to serious events such as cardiac arrest or metabolic encephalopathy 13, 14, 27, 28.
A strength of our study is that both types of drugs were used in the same population but at different times. We had patients who were treated with both drugs and could be used as their own controls and thereby eliminate biological variation. Another strength is that the population was large and unselected. The risk of selection bias was minimized by using inclusion criteria linked to reimbursement. We therefore believe that our data are representative for clinical practice.
Our study has some limitations. In 28% of patients, phosphate values had not been measured at all predetermined times. If the likelihood of obtaining complete biochemical follow‐up is associated with the occurrence of symptoms, this would lead us to overestimate an association between IV iron treatment and HP. Likewise, the high percentage of patients with IBD would tend to include more patients with active inflammation and hence prone to HP from other causes. However, this potential bias would probably not be associated with the choice of drug, as this was determined by regulation secondary to the pricing of the drug. The study was conducted at a single centre, which may have biased our results in terms of patient selection. However, the single‐centre study design may improve the consistency of the data. We analysed data on HP infusion wise and data on HSR patient wise. We regarded HP to be most related to the infusion itself while HSRs were regarded to be most related to the individual. An episode with HP does not necessarily contraindicate further treatment, while HSRs contradict further treatment with the specific drug.
The clinical efficacies of FCM and ISM seem to be similar, as shown in Figure 1A. The choice of drug therefore depends on other factors. Currently, the drug costs of FCM and ISM are the same in Denmark. Importantly, the indirect cost of extra visits and blood samples related to HP or HSRs may influence the choice. The potential side effects may also influence the drug used, as some side effects can be prevented. The risk of developing HP following the administration of FCM may be prevented if phosphate supplementation is provided as a prophylactic to all patients. However, according to the iFGF23 theory, such supplementation would not increase the plasma phosphate levels, because increased iFGF23 levels would cause an increased urinary loss of phosphate 6. We found a correlation between a rise in plasma ferritin levels and a reduction in the phosphate level. This finding could illustrate some kind of refeeding effect when giving large doses of iron. A lower initial iron dose may have some preventive effect on the development of HP. Instead of giving a 1000 mg dose followed by a 500 mg dose a few weeks later, giving a 500 mg dose first followed by a 1000 mg dose might prevent the development of HP. Lowering the initial dose of IV iron or prolonging infusion time may minimize the risk of the development of HSRs, which is a particular concern with ISM. Because the majority of HSRs from IV iron drugs are assumed to be complement activation‐related pseudo‐allergy related, it has been suggested that slowing the infusion rate of the different IV iron supplementations might be most effective in reducing the prevalence of HSRs 24, 29. Although these suggestions are straightforward consequences of the present study, which was conducted in a clinical setting, rigorous clinical trials that test the hypotheses would add important evidence.
In conclusion, not all forms of IV iron are equal. We found a significantly higher risk of HP when giving FCM when compared to ISM. Conversely, we found a significantly higher risk of mild HSRs when giving ISM when compared to FCM. The overall risk of HSRs was much higher than the risk presented in the EMA‐CHMP report.
Competing Interests
There are no competing interests to declare.
Supporting information
Table S1 Fourteen patients with 17 hypersensitivity reactions to intravenous iron infusions sorted by series. Hypersensitivity reactions are marked in bold
Supporting info item
Bager, P. , Hvas, C. L. , and Dahlerup, J. F. (2017) Drug‐specific hypophosphatemia and hypersensitivity reactions following different intravenous iron infusions. Br J Clin Pharmacol, 83: 1118–1125. doi: 10.1111/bcp.13189.
References
- 1. Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SP, et al. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucl Acids Res 2016; 44: D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dignass AU, Gasche C, Bettenworth D, Birgegård G, Danese S, Gisbert JP, et al. European consensus on the diagnosis and management of iron deficiency and anaemia in inflammatory bowel diseases. J Crohns Colitis 2015; 9: 211–222. [DOI] [PubMed] [Google Scholar]
- 3. EMA‐CHMP (2013). Assessment report for: Iron containing intravenous (IV) medicinal products EMA/549569/2013. Available at http://www.ema.europa.eu/ema/index.jsp?curl=pages/includes/document/document_detail.jsp?webContentId=WC500150771&mid=WC0b01ac058009a3dc (last accessed 27 June 2016).
- 4. Hussain I, Bhoyroo J, Butcher A, Koch TA, He A, Bregman DB. Direct comparison of the safety and efficacy of ferric carboxymaltose versus iron dextran in patients with iron deficiency anemia. Anemia 2013; 2013: 169107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Van Wyck DB, Mangione A, Morrison J, Hadley PE, Jehle JA, Goodnough LT. Large‐dose intravenous ferric carboxymaltose injection for iron deficiency anemia in heavy uterine bleeding: a randomized, controlled trial. Transfusion 2009; 49: 2719–2728. [DOI] [PubMed] [Google Scholar]
- 6. Wolf M, Koch TA, Bregman DB. Effects of iron deficiency anemia and its treatment on fibroblast growth factor 23 and phosphate homeostasis in women. J Bone Miner Res 2013; 28: 1793–1803. [DOI] [PubMed] [Google Scholar]
- 7. Schouten BJ, Hunt PJ, Livesey JH, Frampton CM, Soule SG. FGF23 elevation and hypophosphatemia after intravenous iron polymaltose: a prospective study. J Clin Endocrinol Metab 2009; 94: 2332–2337. [DOI] [PubMed] [Google Scholar]
- 8. Sanchez González R, Ternavasio‐de la Vega HG, Moralejo Alonso L, Inés Revuelta S, Fuertes Martín A. Intravenous ferric carboxymaltose‐associated hypophosphatemia in patients with iron deficiency anemia. A common side effect. Med Clin (Barc) 2015; 145: 108–111. [DOI] [PubMed] [Google Scholar]
- 9. Blazevic A, Hunze J, Boots JM. Severe hypophosphataemia after intravenous iron administration. Neth J Med 2014; 72: 49–53. [PubMed] [Google Scholar]
- 10. Fierz YC, Kenmeni R, Gonthier A, Lier F, Pralong F, Coti BP. Severe and prolonged hypophosphatemia after intravenous iron administration in a malnourished patient. Eur J Clin Nutr 2014; 68: 531–533. [DOI] [PubMed] [Google Scholar]
- 11. Subramanian R, Khardori R. Severe hypophosphatemia: pathophysiologic implications, clinical presentations, and treatment. Medicine (Baltimore) 2000; 79: 1–8. [DOI] [PubMed] [Google Scholar]
- 12. Liu PY, Jeng CY. Severe hypophosphatemia in a patient with diabetic ketoacidosis and acute respiratory failure. J Chin Med Assoc 2004; 67: 355–359. [PubMed] [Google Scholar]
- 13. O'Connor LR, Wheeler WS, Bethune JE. Effect of hypophosphatemia on myocardial performance in man. N Engl J Med 1977; 297: 901–903. [DOI] [PubMed] [Google Scholar]
- 14. Ognibene A, Ciniglio R, Greifenstein A, Jarjoura D, Cugino A, Blend D, et al. Ventricular tachycardia in acute myocardial infarction: the role of hypophosphatemia. South Med J 1994; 87: 65–69. [DOI] [PubMed] [Google Scholar]
- 15. Crook MA. Refeeding syndrome: problems with definition and management. Nutrition 2014; 30: 1448–1455. [DOI] [PubMed] [Google Scholar]
- 16. Bergwitz C, Jüppner H. Regulation of phosphate homeostasis by PTH, vitamin D, and FGF23. Annu Rev Med 2010; 61: 91–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ring J, Messmer K. Incidence and severity of anaphylactoid reactions to colloid volume substitutes. Lancet 1977; 1: 466–469. [DOI] [PubMed] [Google Scholar]
- 18. Bager P, Dahlerup JF. The health care cost of intravenous iron treatment in IBD patients depends on the economic evaluation perspective. J Crohns Colitis 2010; 4: 427–430. [DOI] [PubMed] [Google Scholar]
- 19. Barish CF, Koch T, Butcher A, Morris D, Bregman DB. Safety and efficacy of intravenous ferric carboxymaltose (750 mg) in the treatment of iron deficiency anemia: two randomized, controlled trials. Anemia 2012; 2012: 172104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hardy S, Vandemergel X. Intravenous iron administration and hypophosphatemia in clinical practice. Int J Rheumatol 2015; 2015: 468675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Favrat B, Balck K, Breymann C, Hedenus M, Keller T, Mezzacasa A. Evaluation of a single dose of ferric carboxymaltose in fatigued, iron‐deficient women − PREFER a randomized, placebo‐controlled study. PLoS One 2014; 9: e94217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kalra PA, Bhandari S. Efficacy and safety of iron isomaltoside (Monofer) in the management of patients with iron deficiency anemia. Int J Nephrol Renovasc Dis 2016; 9: 53–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Reinisch W, Altorjay I, Zsigmond F, Primas C, Vogelsang H, Novacek G, et al. A 1‐year trial of repeated high‐dose intravenous iron isomaltoside 1000 to maintain stable hemoglobin levels in inflammatory bowel disease. Scand J Gastroenterol 2015; 50: 1226–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Szebeni J, Fishbane S, Hedenus M, Howaldt S, Locatelli F, Patni S, et al. Hypersensitivity to intravenous iron: classification, terminology, mechanisms and management. Br J Pharmacol 2015; 172: 5025–5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Avni T, Bieber A, Grossman A, Green H, Leibovici L, Gafter‐Gvili A. The safety of intravenous iron preparations: systematic review and meta‐analysis. Mayo Clin Proc 2015; 90: 12–23. [DOI] [PubMed] [Google Scholar]
- 26. Wolf M, White KE. Coupling fibroblast growth factor 23 production and cleavage: iron deficiency, rickets, and kidney disease. Curr Opin Nephrol Hypertens 2014; 23: 411–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Becker S, Dam G, Hvas CL. Refeeding encephalopathy in a patient with severe hypophosphataemia and hyperammonaemia. Eur J Clin Nutr 2015; 69: 279–281. [DOI] [PubMed] [Google Scholar]
- 28. Turnbull J, Lumsden D, Siddiqui A, Lin J, Lim M. Osmotic demyelination syndrome associated with hypophosphataemia: 2 cases and a review of literature. Acta Paediatr 2013; 102: e164–e168. [DOI] [PubMed] [Google Scholar]
- 29. Rampton D, Folkersen J, Fishbane S, Hedenius M, Howaldt S, Locatelli F, et al. Hypersensitivity reactions to intravenous iron: guidance for risk minimization and management. Haematologica 2014; 99: 1671–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Fourteen patients with 17 hypersensitivity reactions to intravenous iron infusions sorted by series. Hypersensitivity reactions are marked in bold
Supporting info item


