Abstract
Aims
A recombinant human serum albumin‐interferon alpha2a fusion protein (rHSA/IFNα2a) is expected to extend the half‐life of IFNα2a. This study aims to evaluate the tolerability, safety and efficacy of rHSA/IFNα2a.
Methods
This is an open, randomized, positive control, multiple‐dose ascending Phase Ib study. A panel of 32 treatment naïve and non‐cirrhotic chronic hepatitis B patients were divided into four cohorts, and each received 600, 750 or 900 μg of rHSA/IFNα2a or 180 μg of PEG‐IFNα2a for 3 months. Tolerability, pharmacokinetics and antiviral responses were assessed.
Results
Thirty‐one of 32 enrolled patients completed the treatment study. The rHSA/IFNα2a treatment was better tolerated than the PEG‐IFNα2a 180 μg treatment, as evidenced by blood cell counts and higher serum albumin levels. Half‐life (t 1/2) of rHSA/IFNα2a was estimated to be 120–140 h, and is potentially suitable for a dosing interval of 2 weeks or longer. Pharmacokinetics of the last dose between rHSA/IFNα2a 750 μg and PEG‐IFNα2a 180 μg, with the exception of t 1/2, was comparable, and a similar kinetics of inhibiting HBV DNA replication was observed in both groups. Mean reductions in serum HBV DNA levels after treatment were −1.32, −2.13, −1.10 and −2.48 log10 IU/ml in the 600, 750 and 900 μg rHSA/IFNα2a groups and PEG‐IFNα2a group, respectively.
Conclusions
The rHSA/IFNα2a treatment was well tolerated and can be administered biweekly. Similar efficacy in inhibiting HBV replication was observed in both PEG‐IFNα2a and rHSA/IFNα2a 750 μg groups.
Keywords: albumin‐interferon, antiviral responses, HBV, pharmacokinetics
What is Already Known about this Subject
A recombinant human serum albumin‐interferon alpha2a fusion protein (rHSA/IFNα2a) was expressed in Pichia pastoris in fusion with albumin, which is expected to extend the half‐life of IFNα2a.
Little is known about the tolerability, pharmacokinetics and antiviral activity of rHSA/IFNα2a treatment for HBV.
What this Study Adds
rHSA/IFNα2a was well tolerated and effective at inhibiting HBV DNA.
The rHSA/IFNα2a treatment was well tolerated and can be administered biweekly.
Introduction
Chronic hepatitis B (CHB) affects 240 million people worldwide, and the disease burden is enormous in endemic regions 1, 2. Persistent viral replication is independently linked to dismal outcomes of chronic HBV infection, including cirrhosis, hepatocellular carcinoma and severe complication‐related mortality 3, 4. Although effective antiviral therapies exist, all have specific limitations from the emergence of drug resistance to certain safety concerns associated with long‐term use. To date, the recommended therapy for chronic HBV infection includes the use of either α‐interferon or nucleoside analogues (i.e. lamivudine, adefovir, entecavir, telbivudine or tenofovir). The α‐interferon therapy is only partially effective, is frequently limited by adverse effects, such as fatigue/asthenia, pyrexia, myalgia and headache, and is also expensive. Although HBV replication can be efficiently inhibited by nucleoside analogues, it rebounds after withdrawal. The development of drug‐resistant mutants is frequently detected with early generation of nucleotide analogues 5, 6. Furthermore, lifelong antiviral treatment is necessary for most patients, as fewer than 10% of treated patients experience clearance of chronic HBV infection, which is marked by the seroconversion of positive hepatitis B surface antigen (HBsAg) to positive anti‐HBs antibody 5, 6.
IFN‐α is one of the approved antivirals for treating chronic HBV infection, and the advantages of IFN‐α treatment include the lack of drug resistance and a definite treatment course that usually takes 48 weeks. Nucleos(t)ide analogues (NAs) suppress viral replication, improve liver injury, reverse a certain degree of fibrosis and block the progression of chronic liver disease. However, indefinite treatment is required and chronic HBV infection rarely cured.
Different treatment strategies for using long acting immunomodulation, RNA interference and viral entry inhibition are being explored and likely advance the treatment of hepatitis B 7, 8. rHSA/IFNα2a is a novel fusion protein translated from genes encoding human IFN‐α and albumin, which is expressed in Pichia pastoris. The resultant 85.7‐kDa molecule is a single polypeptide that combines the antiviral property of IFN‐α with the long serum half‐life of albumin 9. The fusion with albumin can delay the degradation of interferon, which is functionally similar to the pegging of IFNα2a.
We conducted a Phase 1b trial with rHSA/IFNα2a. The objectives of this study were to present the characteristics, pharmacokinetics (PK), pharmacodynamics and clinical and virologic outcomes of rHSA/IFNα2a treatment, a novel therapeutic for treating chronic HBV infection.
Patients and methods
Subjects
The trial took place in our hospital, located in northeast China. Naïve chronic hepatitis B patients undergoing treatment were enrolled into this study. Inclusion criteria were: male or female subjects, aged 18–65 years; subjects with chronic HBV infection (serum HBsAg detectable for >6 months); subjects who are serum HBeAg positive with HBV DNA >20 000 IU ml−1 or serum HBeAg negative with HBV DNA >2000 IU ml−1; the subject's serum alanine aminotransferase (ALT) had to be >2× ULN, but below 10× ULN.
Exclusion criteria were: subjects who received steroid treatment or immunosuppression 3 months prior to entry; subjects who received interferon therapy or nucleotide analogue therapy 6 months prior to this study; subjects with existing active lung disease or history of interstitial lung disease; subjects with Hb < lower normal limits and/or absolute neutrophil count (ANC) <1.5 × 109 l−1 and/or platelet count <90 × 109 l−1 and/or white blood cell count (WBC) <3 × 109 l−1; subjects with other concurrent severe chronic medical conditions; subjects with evidence of hepatic decompensation; subjects seropositive for HIV and HCV; subjects with past or current thyroid disease under treatment.
The study protocol and informed consent form were in conformance with the principles embodied in the Declaration of Helsinki, and this clinical trial (ClinicalTrials.gov number: NCT01671787) was approved by the Ethics Committee of the First Hospital of Jilin University. All subjects signed an informed consent prior to enrolment.
Study design
This was an open, positive control, multiple‐dose ascending, Phase Ib clinical trial of rHSA/IFNα2a. A total of 32 subjects were enrolled, and were divided into four cohorts. These cohorts received 600, 750 or 900 μg of rHSA/IFNα2a or 180 μg of PEG‐IFNα2a (positive control); in which tolerability, antiviral response and PK were assessed. rHSA/IFNα2a was administered in three escalating doses (600, 750 or 900 μg) biweekly, and PEG‐IFNα2a was administered at 180 μg weekly, subcutaneously. The treatment was paused for 4 weeks (washout) after the first administration to collect samples for PK analysis, and each group resumed treatment for an additional 12 weeks. Safety and antiviral responses were assessed at weeks 5, 9, 13 and 17. Study drugs were uniformly administered in the clinical research centre to assure the quality. After completion of the dosing period, subjects were required to undergo follow‐up visits at weeks 15, 16 or 17 for off‐treatment safety, antiviral response and PK evaluations. Subjects treated with rHSA/IFNα2a 600 μg were first evaluated and observed for 8 weeks. If the subjects tolerated the initial dose, the next two groups started receiving 750 μg of rHSA/IFNα2a or Peg‐IFNα‐2a 180 μg. If subjects in the 750 μg group, who were also observed for 8 weeks, tolerated the dose, the last groups commenced receiving 900 μg of rHSA/IFNα2a. The study design is presented schematically in Figure 1.
Figure 1.
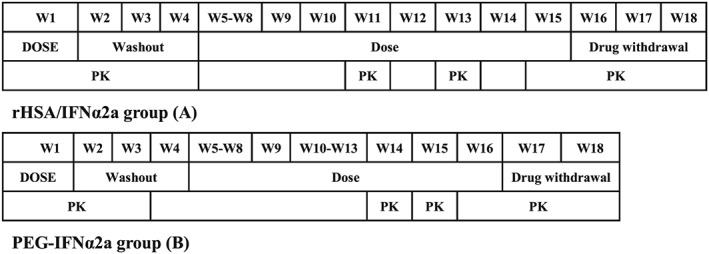
Flow chart of the study
Assessment of adverse events and tolerability
Adverse events were regularly assessed by clinical laboratory tests, vital signs, 12‐lead electrocardiograms (ECGs), abdominal ultrasound, chest X‐ray, physical examination, and concomitant medications throughout the study using the baseline data as control. Adverse events were coded using the Medical Dictionary for Regulatory Activities (MedDRA, version 16; MedDRA MSSO, McLean, VA). The severity of adverse events and laboratory abnormalities were graded according to the protocol of Common Terminology Criteria for Adverse Events (CTCAE) 4.0 that defines toxicity criteria.
Tolerability was assessed by physicians who participated in this trial at week 9, after each treatment group had been observed for 8 weeks. If more than 50% of the subjects had any of the following adverse events, the dose escalation was terminated: ANC <0.5 × 109 l−1, platelet (PLT) count <30 × 109 l−1, ALT >10 ULN, persistently elevated ALT or accompanied by bilirubin elevation after dose titration, serum total bilirubin >51.3 μmol l−1, development of ascites, hepatic encephalopathy, or psychiatric disorders, allergic reactions, uncontrolled thyroid disease, diabetes mellitus, as well as serious damage to the heart, kidneys, brain and lungs. The process for dose reduction consisted of two steps. If ANC or PLT count was between 0.5 × 109 l−1 and 0.75 × 109 l−1 or between 30 × 109 l−1 and 50 × 109 l−1, respectively, the drug dose was reduced. If ANC or PLT count fell below 0.5 × 109 l−1 or 30 × 109 l−1, respectively, the drug was discontinued. If ALT levels increased to >10 ULN, the drug was also discontinued. Ascending of drug dose resumed once cytopenia was recovered (i.e. ANC were ≥0.75 × 109 l−1 or PLT count was ≥50 × 109 l−1).
Study drugs and administration method
Test drugs
rHSA/IFNα2a was expressed in Pichia pastoris in fusion with albumin by Beijing Bio‐Fortune Ltd. rHSA/IFNα2a is 750 amino acids long, with a molecular weight of 85 694.50 and an isoelectric point of 5.845. The tested batch number was 92 13/20121001. This plasmid was created by genetic engineering technology, in which human serum albumin and IFN‐α genes were seamlessly fused. Then the plasmid was integrated into the chromosome of P. pastoris. The P. pastoris can secrete the fusion protein (rHSA/IFNα2a) into inorganic salt medium. The secreted fusion protein was purified by highly effective separation and purification technology and lyophilized. The lyophilized proteins are reconstituted in 1 ml physiological saline and administrated by subcutaneous injection. Pegasys (PEG‐IFNα2a 180 μg) was purchased from Roche Pharmaceuticals Ltd (batch number is B1318//201302–201601). Both were stored at 4°C until use.
rHSA/IFNα2a and PEG‐IFNα2a were subcutaneously injected at a 5‐cm periumbilical area biweekly for seven doses and weekly for 13 doses, respectively. IFNα2a concentration in 750 μg of rHSA/IFNα2a was equal to that in PEG‐IFNα2a 180 μg.
Efficacy
The primary antiviral endpoint was the log change in serum HBV DNA from baseline (day 1) to week 17. Other end points included HBeAg serum conversion rate, the reduction in HBeAg level, and the normalization rate of ALT and AST. Blood samples were collected at weeks 5, 9, 13 and 17.
Blood collection for pharmacokinetic, neopterin kinetic and IFN antibody analysis
Blood samples for rHSA/IFNα2a PK and neopterin kinetic analyses were collected pre‐dose and at 2, 6, 12, 24, 48, 60, 72, 84, 96, 120, 168, 240, 336, 504 and 672 h post‐dose at weeks 1 and 15 (first dose and last dose), and pre‐dose at weeks 11 and 13 (the fifth and sixth dose). Blood samples for PEG‐IFNα2a PK analyses were collected at pre‐dose and at 2, 6, 12, 24, 48, 60, 72, 84, 96, 120, 168, 240, 336 and 504 h post‐dose at weeks 1 and 16 (first and last dose), and pre‐dose at weeks 14 and 15 (the eleventh and twelfth dose). Blood samples for IFN antibody analyses were collected pre‐dose on weeks 1, 5, 9, 13 and 17. Blood samples were collected and placed into a vacutainer without an anti‐coagulant, clotted for 30 min, and centrifuged at 3724 × g for 10 min at 4°C.
Detection methods
HBV DNA level was tested using Roche's COBAS TaqMan kit. The undetectable level was 15 IU ml−1. Tests were conducted at the Hepatology Department of the First Hospital of Jilin University, Changchun, China. Liver and kidney function, as well as biochemical parameters, were tested by an automatic biochemical instrument. Blood cell counts and routine urine were also tested by an automatic detection instrument. These were all performed at the Clinical Laboratory of the First Hospital of Jilin University. Concentrations of IFN alpha2a, neopterin and conjugated antibody in serum were determined and validated by enzyme‐linked immunosorbent assay (ELISA) at our hospital laboratories. Serum IFN neutralizing antibodies were tested using the vesicular stomatitis virus and ELISA method at the same laboratory.
Statistical methods
Serum PK parameters including maximum observed serum concentration (C max), time to maximum observed serum concentration (T max), area under the concentration–time curve from time of dosing (zero hours) to the last time point with measurable serum concentration (AUC0–t) prior to next dose, AUC from time of dosing (zero hours) extrapolated to infinity (AUC0–∞), as well as terminal elimination half‐life of the drug in serum (t 1/2), clearance (CLz/F), MRT0–∞, volume (Vz/F) at first dose and C ss max, AUCss0–t, AUCss0–∞, t ss 1/2, T ss max, CLz/Fss, MRTss0–∞, Vz/Fss, accumulation rate (R), degree of fluctuation (Df) and C avg at last dose, were estimated based on the observed concentration–time data by the noncompartmental PK approach using WinNonlin version 6.4 (Pharsight Corporation, Mountain View, CA).
Variables analysed using Student's t‐test or Kruskal–Wallis test or regression or correlation analysis were established using the SAS 9.1 software (USA). Results are presented as mean ± standard deviation. P‐values < 0.05 in two‐sided tests were considered statistically significant.
Results
A total of 91 subjects were screened initially, and 32 of them were enrolled and treated in the trial. Thirty‐one subjects completed all safe and anti‐virus efficacy studies, and 28 of them completed the PK study. In general, demographics and disease characteristics in each treatment group were well‐matched, except for age. Subjects were younger in the Peg‐IFNα2a 180 μg group than in the rHSA/IFNα2a group. However, the difference was not statistically significant (P > 0.05, Table 1). The majority of subjects were males, 30/32 (93%) of subjects were Han Chinese, and 4/32 (12.5%) were HBeAg‐negative.
Table 1.
Demographics and disease characteristics of the four cohorts at baseline
| Baseline parameter |
rHSA/IFNα2a
600 μg
(n = 8) |
rHSA/IFNα2a
750 μg
(n = 8) |
rHSA/IFNα2a
900 μg
(n = 8) |
Peg‐IFNα2a
180 μg
(n = 8) |
P |
|---|---|---|---|---|---|
| Gender (male/female) | 6/2 | 5/3 | 6/2 | 4/4 | >0.05 |
| Ethnic (Han/other) | 7/1 | 7/1 | 8/0 | 8/0 | >0.05 |
| Age, mean (SD) years | 39.88 ± 9.43 | 30.88 ± 7.75 | 36.50 ± 10.98 | 25.00 ± 4.14 | >0.05 |
| Smoker (yes/no) | 1/7 | 3/5 | 1/7 | 3/5 | >0.05 |
| Drinker (yes/no) | 2/6 | 0/8 | 0/8 | 1/7 | >0.05 |
| BMI, mean (SD), kg m −2 | 22.83 ± 2.41 | 22.86 ± 2.70 | 23.31 ± 2.68 | 21.55 ± 2.72 | >0.05 |
| HBV DNA, mean (SD) log 10 IU/ml | 7.93 ± 0.63 | 7.64 ± 0.79 | 7.28 ± 1.14 | 7.33 ± 0.92 | >0.05 |
| HBsAg, IU ml −1 | >250 | >250 | >250 | >250 | – |
| HBeAg, IU ml −1 | 755.67 ± 505.23 | 509.81 ± 477.99 | 464.41 ± 564.22 | 805.19 ± 524.76 | >0.05 |
| ALT, mean (SD) U l −1 | 189.13 ± 71.27 | 199.50 ± 99.80 | 141.63 ± 51.65 | 139.63 ± 63.86 | >0.05 |
| HBeAg negative, n (%) | 1 (12.5) | 2 (25) | 0 (0) | >0.05 |
Tolerability
rHSA/IFNα2a was well‐tolerated after over seven injection treatments, and tolerability was better than for PEG‐IFNα2a. During the study period, body weight fluctuated once, but no significant change was found. Physical examination, abdominal colour Doppler ultrasound, chest X‐ray and ECG analysis did not reveal any significant change before and after treatment. There were no significant changes in urine and blood coagulation routines.
Red blood cell count, haemoglobin, WBC count, neutrophil cell count and lymphocyte absolute value, and PLT counts decreased to different extents in each treatment group after dosing. The extent of reduction was larger in the PEG‐IFNα2a group than in the rHSA/IFNα2a treatment groups. The time for gradually stabilizing the decreased blood cell count was shorter in the rHSA/IFNα2a groups (approximately 4–7 weeks) than that in the PEG‐IFNα2a group (approximately 7–9 weeks), and decreased levels of blood cell count between different rHSA/IFNα2a groups were similar. There were no significant dose‐related reductions among the rHSA/IFNα2a groups (Figure 2 and Table 2).
Figure 2.
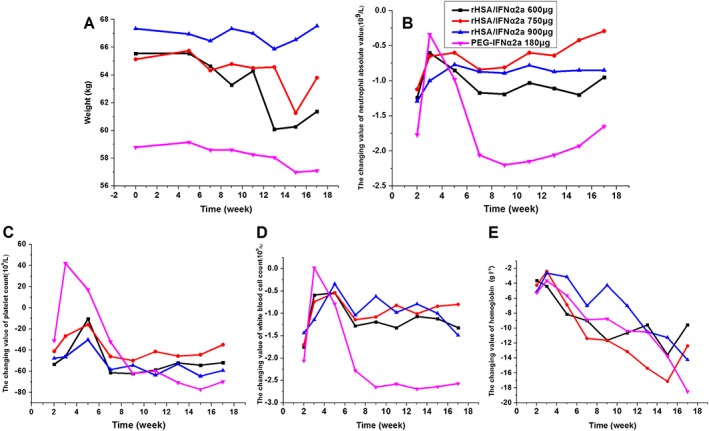
Reduced value of safety factors at different time points after treatment in each group (all compared with the baseline values). Body weight (A); neutrophil absolute value (B); platelet counts (C); WBC counts (D); haemoglobin absolute value (E)
Table 2.
Stabilization time and lowest value of decreased blood cell counts in each treatment group
| Factor | rHSA/IFNα2a group | PEG‐IFNα2a 180 μg group | ||||
|---|---|---|---|---|---|---|
| Maximal reduction in parameter (Week 17) | Stabilization time | Lowest value (time) | Reduction in parameter (Week 17) | Stabilization time | Lowest value (time) | |
| Neutrophil absolute value | −0.95 × 109 l−1 | Week 4 | 0.69 × 109 l−1 (Week 2) | −1.65 × 109 l−1 | Week 7 | 0.7 × 109 l−1 (Week 9) |
| WBC count | −1.49 × 109 l−1 | Week 7 | 1.38 × 109 l−1 (Week 2) | −2.57 × 109 l−1 | Week 9 | 2.15 × 109 l−1 (Week 15) |
| Platelet count | −59.25 × 109 l−1 | Week 7 | 65 × 109 l−1 (Week 2) | −69.88 × 109 l−1 | Week 9 | 77 × 109 l−1 (Week 17) |
| Haemoglobin Absolute value | −14.25 g l−1 | Week 7 or persistently decreaseda | 110 g l−1 (Week 5) | −18.5 g l−1 | Persistently decreased | 93 g l−1 (Week 11) |
Week 7 (rHSA/IFNα2a 600 μg and 750 μg) or persistently decreased (rHSA/IFNα2a 900 μg); WBC, white blood cell
The severity of adverse events was evaluated according to CTCAE version 4.0. There were 76, 96, 110 and 126 adverse event occurrences in the 600, 750 or 900 μg rHSA/IFNα2a or PEG‐IFNα2a 180 μg groups, respectively. The frequency of adverse events did not significantly increase with the increased rHSA/IFNα2a dosages. The majority of these adverse events were reductions in neutrophil absolute value, WBC counts, albumin, PLT count and haemoglobin absolute value, which were all evaluated to be drug‐related.
The severity of the majority of the adverse events was grade 1–2. Adverse events at grade 3 occurred in 21 subjects, in which three, eight, two and eight of these subjects, respectively, appeared in the 600, 750, 900 μg rHSA/IFNα2a groups and the PEG‐IFNα2a 180 μg group, indicating no dose‐dependence. Grade 3 adverse events mainly involved the reduction in neutrophil absolute value and WBC count, as well as elevated ALT (Table 3).
Table 3.
Treatment‐related adverse events in each treatment group (times)
| Group | Grade 1 | Grade 2 | Grade 3 | Grade 4/5 | Total |
|---|---|---|---|---|---|
| rHSA/IFNα2a 600 μg | 52 (68.42) | 21 (27.63) | 3 (3.95) | 0 (0.00) | 76 |
| rHSA/IFNα2a 750 μg | 64 (66.67) | 24 (25.00) | 8 (8.33) | 0 (0.00) | 96 |
| rHSA/IFNα2a 900 μg | 85 (77.27) | 23 (20.91) | 2 (1.82) | 0 (0.00) | 110 |
| Peg‐IFNα2a 180 μg | 87 (69.05) | 31 (24.60) | 8 (6.35) | 0 (0.00) | 126 |
At the early stage of treatment, most subjects experienced fever and headache. With the treatment time extended, most subjects appeared to adapt to interferon therapy. Furthermore, the adaptation period was shorter in the rHSA/IFNα2a groups (approximately 6–10 weeks) than in the PEG‐IFNα2a group (approximately 8–13 weeks). Occurrence rates of fever, body aches and headache were higher in the PEG‐IFNα2a 180 μg group than in the rHSA/IFNα2a 750 μg groups (Table 4). Three subjects were administered 0.5 g of acetaminophen once for fever, and two subjects were given 4 mg of chlorpheniramine once for allergic reactions. All subjects recovered well from these adverse events.
Table 4.
Frequency of common adverse events in each treatment group (number of subjects)
| Event, n (%) | rHSA/IFNα2a 600 μg (n = 8) | rHSA/IFNα2a 750 μg (n = 8) | rHSA/IFNα2a 900 μg (n = 8) | Peg‐IFNα2a 180 μg (n = 8) |
|---|---|---|---|---|
| Albumin decreased | 5 | 4 | 3 | 6 |
| White blood cell count decreased | 5 | 7 | 3 | 6 |
| Haemoglobin decreased | 1 | 3 | 2 | 5 |
| Platelet count decreased | 4 | 3 | 5 | 6 |
| Neutrophil absolute value decreased | 6 | 8 | 6 | 7 |
| Vomit | 0 | 0 | 2 | 0 |
| Back pain | 0 | 0 | 1 | 1 |
| Fever | 1 | 2 | 4 | 3 |
| Fatigue | 0 | 2 | 5 | 1 |
| Ache all over | 0 | 2 | 0 | 3 |
| Headache | 0 | 4 | 1 | 5 |
| Dizziness | 0 | 0 | 3 | 1 |
| Knee‐joint pain | 0 | 1 | 1 | 0 |
| Drowsiness | 0 | 0 | 3 | 0 |
ALT level in one subject, who received 600 μg of rHSA/IFNα2a, was elevated to 620 U l−1 (>10 ULN); and the drug was discontinued after 8 weeks of treatment. A 25% reduction of dosage was applied at the sixth and eighth dosing in one subject, and at the sixth dosing in the second patient in PEG‐IFNα2a group, due to ANC reduction. All the remaining subjects completed their designated regimens. Prior to the last dosing, one subject in the PEG‐IFNα2a 180 μg group experienced erythema nodosum, which was PEG‐IFNα2a‐related and recorded as a serious adverse event (SAE) by the principal investigator. After 4 months of treatment with hydroxychloroquine, the erythema nodosum was cured without adjusting the PEG‐IFNα2a dosage. No patient death occurred during the study period.
Immunogenicity test
There was no detectable neutralizing antibody to IFNα2a and rHSA after rHSA/IFNα2a treatment (Table 5), and some of the subjects appeared to have detectable activity of binding antibody. However, there was no observed effect on the efficacy of interferon in vivo.
Table 5.
Detection of rHSA/IFNα2a and Peg‐IFNα2a antibodies
| Group | IFN alpha2a binding antibody | Recombinant human serum albumin binding antibody | Neutralizing antibody |
|---|---|---|---|
| rHSA/IFNα2a 600 μg | 2 | 2 | 0 |
| rHSA/IFNα2a 750 μg | 1 | 1 | 0 |
| rHSA/IFNα2a 900 μg | 2 | 2 | 0 |
| Peg‐IFNα2a 180 μg | 1 | – | 0 |
Pharmacokinetic analysis of IFNα2a
Serum IFNα2a concentration–time profiles of the different treatment groups following the first and last dose of rHSA/IFNα2a and PEG‐IFNα2a are shown in Figure 3. IFNα2a PK parameters are shown in Table 6. The end‐stage elimination of the IFNα2a was estimated in accordance with the one compartment model, using mean serum IFNα2a concentration–time profiles. Serum IFNα2a concentration appeared to be maintained at a steady state after multiple doses, as extracted from the concentration–time profiles (Figure 4).
Figure 3.
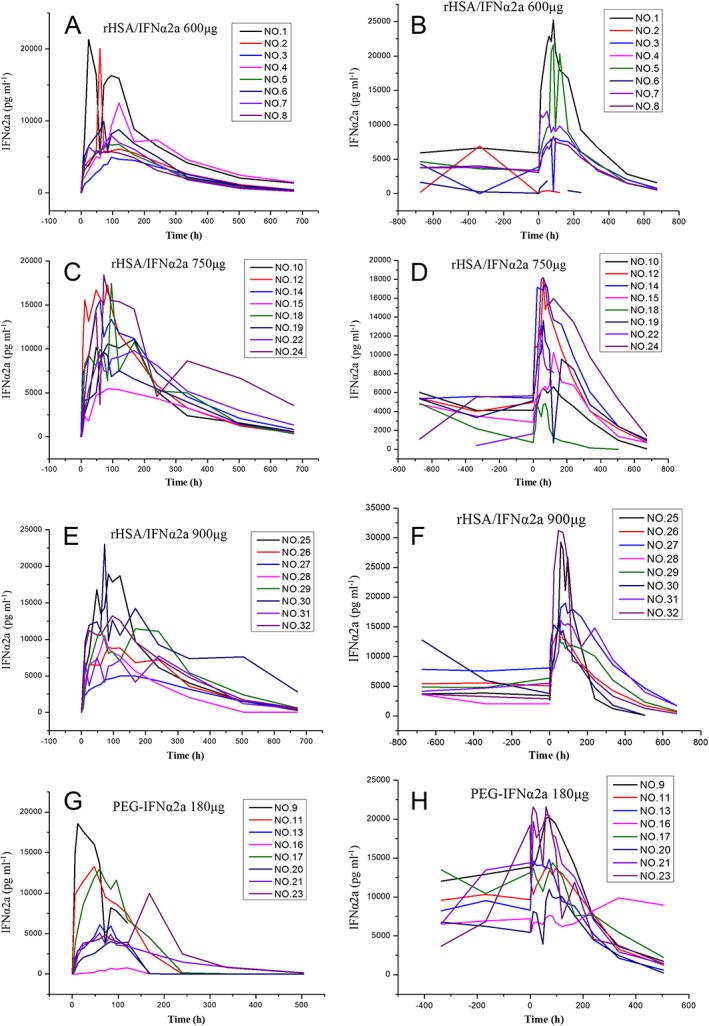
IFNα2a plasma concentration–time profiles in the treatment groups, following the first and last dose of rHSA/IFNα2a 600, 750 and 900 μg and PEG‐IFNα2a 180 μg. First dose of rHSA/IFNα2a 600 μg (A); last dose of rHSA/IFNα2a 600 μg (B); first dose of rHSA/IFNα2a 750 μg (C); last dose of rHSA/IFNα2a 750 μg (D); first dose of rHSA/IFNα2a 900 μg (E); last dose of rHSA/IFNα2a 900 μg (F); first dose of PEG‐IFNα2a 180 μg (G); last dose of PEG‐IFNα2a 180 μg (H)
Table 6.
Comparison of pharmacokinetic parameters of IFNα2a between the first and last dosing in each treatment group
| t 1/2 | T max | C max | AUC 0–t | AUC 0–∞ | ||
|---|---|---|---|---|---|---|
| Group | h | h | pg ml −1 | pg ml −1 *h | pg ml −1 *h | |
| rHSA/IFNα2a 600 μg | First dose | 135.01 ± 36.47 | 75.00 ± 30.59 | 11 167.54 ± 6341.74 | 2 381 878.01 ± 879 806.14 | 2 518 504.60 ± 1 022 285.89 |
| Last dose | 121.61 ± 46.76 | 74.00 ± 20.67 | 12 602.02 ± 9248.22 | 3 094 334.86 ± 1 948 097.91 | 3 241 543.80 ± 2 060 188.09 | |
| P | 0.92 | 0.72 | 0.35 | 0.35 | 0.35 | |
| rHSA/IFNα2a 750 μg | First dose | 146.81 ± 53.16 | 102.00 ± 42.55 | 13 087.84 ± 4721.09 | 3 469 923.88 ± 1 077 195.14 | 3 758 873.40 ± 1 460 815.12 |
| Last dose | 114.02 ± 38.72 | 80.57 ± 27.46 | 12 723.33 ± 5572.80 | 3 525 403.11 ± 1 927 750.91 | 3 666 225.14 ± 2 027 391.45 | |
| P | 0.31 | 0.23 | 0.87 | 0.4 | 0.74 | |
| rHSA/IFNα2a 900 μg | First dose | 122.80 ± 58.90 | 117.00 ± 59.05 | 12 346.84 ± 5962.98 | 3 360 387.88 ± 1 277 834.78 | 3 552 902.90 ± 1567 601.55 |
| Last dose | 107.76 ± 41.63 | 56.57 ± 13.35 | 20 285.66 ± 6933.92 | 4 540 507.10 ± 1 288 646.44 | 4 694 539.83 ± 1432 681.48 | |
| P | 0.87 | 0.02 | 0.06 | 0.18 | 0.24 | |
| PEG‐IFNα2a 180 μg | First dose | 58.49 ± 41.67 | 76.50 ± 47.97 | 8844.80 ± 5896.85 | 997 666.67 ± 582 275.47 | 1 096 047.58 ± 510 189.16 |
| Last dose | 112.40 ± 29.14 | 69.00 ± 8.49 | 15 510.89 ± 4961.44 | 3 761 834.45 ± 707 272.76 | 3 938 956.18 ± 856 911.91 | |
| P | 0.06 | 1 | 0.01 | 0.01 | 0.03 |
| Group | CLz/F | MRT 0–∞ | Vz/F | Df | R | Cavg | |
|---|---|---|---|---|---|---|---|
| L h −1 | h | L | % | – | pg ml −1 | ||
| rHSA/IFNα2a 600 μg | First dose | 0.35 ± 0.10 | 255.44 ± 55.19 | 64.22 ± 14.26 | |||
| Last dose | 2.68 ± 6.00 | 229.04 ± 71.46 | 161.24 ± 285.76 | 166.97 ± 96.49 | 1.19 ± 0.11 | 7331.42 ± 4725.62 | |
| P | 0.35 | 0.92 | 0.35 | ||||
| rHSA/IFNα2a 750 μg | First dose | 0.31 ± 0.10 | 294.83 ± 94.74 | 63.26 ± 21.08 | |||
| Last dose | 0.43 ± 0.43 | 243.34 ± 70.08 | 52.93 ± 19.92 | 74.14 ± 125.49 | 0.1 ± 1.16 | 4156.16 ± 6831.17 | |
| P | 0.74 | 0.31 | 0.31 | ||||
| rHSA/IFNα2a 900 μg | First dose | 0.39 ± 0.14 | 279.55 ± 85.73 | 65.53 ± 30.69 | |||
| Last dose | 0.25 ± 0.05 | 218.84 ± 78.44 | 37.17 ± 12.06 | 73.08 ± 135.66 | 0.11 ± 1.14 | 2267.22 ± 10 869.59 | |
| P | 0.09 | 0.04 | 0.02 | ||||
| PEG‐IFNα2a 180 μg | First dose | 2.35 ± 3.92 | 130.80 ± 67.30 | 93.75 ± 98.69 | |||
| Last dose | 0.39 ± 0.14 | 207.54 ± 32.82 | 54.98 ± 13.46 | 55.9 ± 22.61 | 1.55 ± 0.23 | 12 573.85 ± 3706.2 | |
| P | 0.01 | 0.06 | 0.5 |
Last dose: IFNα2a serum concentration achieved a steady state after multiple doses, and pharmacokinetic parameters were C ssmax, AUCss0–t, AUCss0–∞, t ss 1/2, T ssmax, CLz/Fss, MRTss0–∞, and Vz/Fss, respectively.
Figure 4.
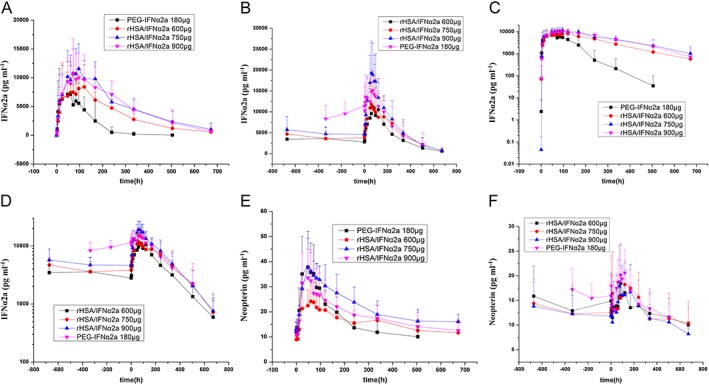
Mean (± SD) IFNα2a or neopterin plasma concentration–time profiles in the treatment group, following the first and last dose of rHSA/IFNα2a 600, 750 and 900 μg, and PEG‐IFNα2a 180 μg. Vertical error bars represent the standard deviation of the mean. IFNα2a plasma line concentration–time profiles of the first dose (A); IFNα2aplasma line concentration–time profiles of the last dose (B); IFNα2a plasma log concentration–time profiles of the first dose (C); IFNα2a plasma log concentration–time profiles of the last dose (D); neopterin plasma line concentration–time profiles of the first dose (E); neopterin plasma line concentration–time profiles of the last dose (F)
Following the single dose administration of rHSA/IFNα2a on day 1, serum IFNα2a concentrations were increased with increased doses, reaching a maximum level within 75–117 h. Consistent with the longer half‐life of IFNα2a (122.8–146.81 h), serum IFNα2a concentrations in the rHSA/IFNα2a groups were higher than in the PEG‐IFNα2a group at 168 h after dosing (the next administration time of PEG‐IFNα2a) (Figure 3 and Table 6).
Following the last dosing of rHSA/IFNα2a, serum IFNα2a concentrations increased with increased doses, reaching a maximum level within 56.57–80.57 h. The half‐life of IFNα2a (>100 h) was not changed after the last dose of rHSA/IFNα2a. However, the half‐life of PEG‐IFNα2a 180 μg was extended to 100 h or longer. Serum IFNα2a concentrations were similar among the rHSA/IFNα2a 750 and 900 μg, and PEG‐IFNα2a 180 μg groups at 168 h following the last dose (the next administration time of PEG‐IFNα2a). Long half‐life, together with the features of PK parameters and serum concentration–time profiles, are shown in Figures 3 and 4.
Analysis of the extent of fluctuation
The extent of fluctuation was similar among the rHSA/IFNα2a 600, 750 and 900 μg groups (1.30–1.70), which was higher than in the PEG‐IFNα2a group (0.559) (Table 6).
Analysis of the accumulation rate
The accumulation rate was similar among the rHSA/IFNα2a 600, 750 and 900 μg groups (1.1–1.2), but was lower than in the PEG‐IFNα2a group (1.55) (Table 6).
Comparison of PK parameters between the first and last dose
IFNα2a AUC and C max increased while CLz/F decreased after the last dose, compared to the first dosing in the PEG‐IFNα2a 180 μg group (P < 0.05). IFNα2a PK parameters did not change after the last dose in the rHSA/IFNα2a 600 and 750 μg groups, compared to the first dosing (P < 0.05). Furthermore, T max, MRT0–∞ and Vz/F of IFNα2a decreased after the last dose in the rHSA/IFNα2a 900 μg group, compared to the first dosing (P < 0.05) (Table 6).
Comparison of PK parameters between the rHSA/IFNα2a 750 μg and PEG‐IFNα2a 180 μg groups
The t 1/2, AUC and MRT0–∞ were higher in the rHSA/IFNα2a 750 μg group than in the PEG‐IFNα2a 180 μg group, and CLz/F was lower in the rHSA/IFNα2a 750 μg group than in the PEG‐IFNα2a group at first dosing (P < 0.05). The t 1/2, C max, AUC, MRT and Vz/F were similar between the rHSA/IFNα2a 750 μg and PEG‐IFNα2a 180 μg groups (P < 0.05). CLz/F of the last dose was lower in the rHSA/IFNα2a 750 μg group than in the PEG‐IFNα2a 180 μg group (P < 0.05) (Table 7).
Table 7.
Comparison of pharmacokinetic parameters between rHSA/IFNα2a 750 μg and PEG‐IFNα2a 180 μg
| Group | t 1/2 | T max | C max | AUC 0–t | AUC 0–∞ |
|---|---|---|---|---|---|
| h | h | pg ml −1 | pg ml −1 *h | pg ml −1 *h | |
| First dose of IFNα2a | |||||
| rHSA/IFNα2a 600 μg | 135.01 ± 36.47 | 75.00 ± 30.59 | 11 167.54 ± 6341.74 | 2 381 878.01 ± 879 806.14 | 2 518 504.60 ± 1 022 285.89 |
| PEG‐IFNα2a 180 μg | 58.49 ± 41.67 | 76.50 ± 47.97 | 8844.80 ± 5896.85 | 997 666.67 ± 582 275.47 | 1 096 047.58 ± 510 189.16 |
| P‐value | <0.0001 | 0.16 | 0.23 | <0.0001 | <0.0001 |
| Last dose of IFNα2a | |||||
| rHSA/IFNα2a 600 μg | 121.61 ± 46.76 | 74.00 ± 20.67 | 12 602.02 ± 9248.22 | 3 094 334.86 ± 1948 097.91 | 3 241 543.80 ± 2 060 188.09 |
| PEG‐IFNα2a 180 μg | 112.40 ± 29.14 | 69.00 ± 8.49 | 15 510.89 ± 4961.44 | 3 761 834.45 ± 707 272.76 | 3 938 956.18 ± 856 911.91 |
| P‐value | 0.96 | 0.9 | 0.78 | 0.28 | 0.78 |
| First dose of neopterin | |||||
| rHSA/IFNα2a 600 μg | 78.00 ± 45.36 | 29.24 ± 7.37 | 10 607.02 ± 2893.85 | ||
| PEG‐IFNα2a 180 μg | 51.00 ± 22.90 | 40.70 ± 14.29 | 8939.34 ± 2368.70 | ||
| P‐value | 0.19 | 0.96 | <0.0001 | ||
| Last dose of neopterin | |||||
| rHSA/IFNα2a 600 μg | 68.33 ± 54.59 | 19.71 ± 6.71 | 8759.53 ± 3026.83 | ||
| PEG‐IFNα2a 180 μg | 87.00 ± 27.77 | 28.36 ± 3.96 | 10 348.77 ± 2107.19 | ||
| P‐value | 0.96 | 0.61 | 0.19 | ||
| Group | CLz/F | MRT 0‐∞ | Vz/F | R | Cavg | Df |
|---|---|---|---|---|---|---|
| L h −1 | h | L | – | pg ml −1 | % | |
| First dose of IFNα2a | ||||||
| rHSA/IFNα2a 600 μg | 0.35 ± 0.10 | 255.44 ± 55.19 | 64.22 ± 14.26 | |||
| PEG‐IFNα2a 180 μg | 2.35 ± 3.92 | 130.80 ± 67.30 | 93.75 ± 98.69 | |||
| P‐value | <0.0001 | 0.01 | 0.87 | |||
| Last dose of IFNα2a | ||||||
| rHSA/IFNα2a 600 μg | 2.68 ± 6.00 | 229.04 ± 71.46 | 161.24 ± 285.76 | 1.19 ± 0.11 | 7331.42 ± 4725.62 | 166.97 ± 96.49 |
| PEG‐IFNα2a 180 μg | 0.39 ± 0.14 | 207.54 ± 32.82 | 54.98 ± 13.46 | 1.55 ± 0.23 | 12 573.85 ± 3706.2 | 55.9 ± 22.61 |
| P‐value | <0.0001 | 0.34 | 0.07 | 0.71 | 0.71 | 0.07 |
Last dose: IFNα2a serum concentration achieved a steady state after multiple doses, and the parameters were C ssmax, AUCss0–t, AUCss0–∞, t ss 1/2, T ssmax, CLz/Fss, MRTss0–∞, and Vz/Fss, respectively.
Analysis of linear correlations between exposure and rHSA/IFNα2a dosage
Over the range of IFNα2a exposures (C max and AUC) for each of the rHSA/IFNα2a dose groups on day 1, when the slope (90% confidence interval) in the power model was less than 1, this indicated the saturation of absorption PKs at the first dose. In addition, when the slope in the power model was more than 1, this indicated the saturation of elimination PKs at the last dose (Table 8).
Table 8.
Linear regression analysis between exposure of rHSA/IFNα2a and its dosage
| PK parameter | Dosing | Regression coefficient | Lower 90% CI | Upper 90% CI |
|---|---|---|---|---|
| C max | First dose | 0.35 | −0.67 | 1.37 |
| C max | Last dose | 2.33 | 0.27 | 4.38 |
| AUC 0–t | First dose | 0.86 | 0.13 | 1.59 |
| AUC 0–t | Last dose | 2.41 | −0.23 | 5.06 |
| AUC 0–∞ | First dose | 0.85 | 0.04 | 1.66 |
| AUC 0–∞ | Last dose | 2.31 | −0.28 | 4.91 |
Last dose: IFNα2a serum concentration achieved a steady state after multiple doses, and pharmacokinetic parameters were C ssmax, AUCss0–t and AUCss0–∞, respectively. PK, pharmacokinetic.
Kinetic analysis of neopterin
Serum neopterin concentration–time profiles in different treatment groups following the first and last dose of rHSA/IFNα2a and PEG‐IFNα2a are shown in Figure 5. Neopterin kinetic parameters are shown in Table 9. The end‐stage elimination of neopterin deviated from the mean serum neopterin concentration–time profiles (Figure 5).
Figure 5.
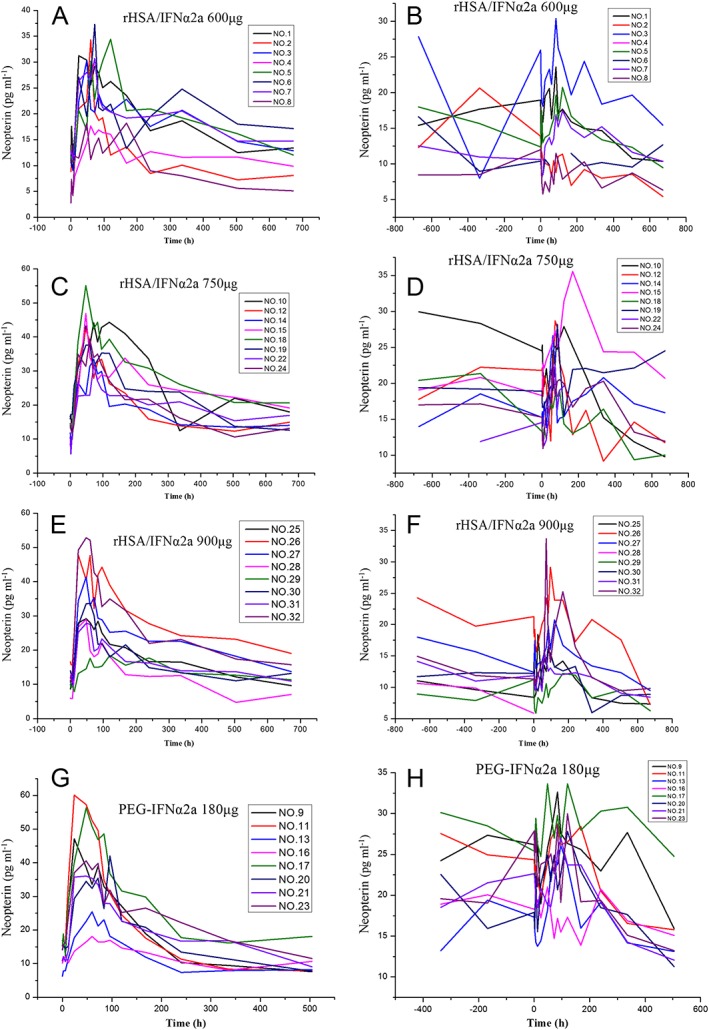
Neopterin plasma concentration–time profiles in treatment groups following the first and last doses of rHSA/IFNα2a 600, 750 and 900 μg, and PEG‐IFNα2a 180 μg. First dose of rHSA/IFNα2a 600 μg (A); last dose of rHSA/IFNα2a 600 μg (B); first dose of rHSA/IFNα2a 750 μg (C); last dose of rHSA/IFNα2a 750 μg (D); first dose of rHSA/IFNα2a 900 μg (E); last dose of rHSA/IFNα2a 900 μg (F); first dose of PEG‐IFNα2a 180 μg (G); last dose of PEG‐IFNα2a 180 μg (H)
Table 9.
Comparison of kinetics parameters of neopterin between the first and last dosing in each group
| Group | T max | C max | AUC 0–t | |
|---|---|---|---|---|
| h | Nmol l −1 ml −1 | Nmol l −1 ml −1 *h | ||
| rHSA/IFNα2a 600 μg | First dose | 78.00 ± 45.36 | 29.24 ± 7.37 | 10 607.02 ± 2893.85 |
| Last dose | 68.33 ± 54.59 | 19.71 ± 6.71 | 8759.53 ± 3026.83 | |
| P | 0.79 | 0.05 | 0.07 | |
| rHSA/IFNα2a750 μg | First dose | 66.00 ± 24.00 | 41.32 ± 7.59 | 14 677.07 ± 2778.31 |
| Last dose | 94.29 ± 38.84 | 26.98 ± 5.13 | 12 232.28 ± 2969.15 | |
| P | 0.08 | 0.02 | 0.13 | |
| rHSA/IFNα2a 900 μg | First dose | 55.50 ± 27.91 | 35.00 ± 11.45 | 12 464.27 ± 3781.08 |
| Last dose | 89.14 ± 45.88 | 20.93 ± 7.72 | 8268.78 ± 2181.01 | |
| P | 0.06 | 0.02 | 0.02 | |
| PEG‐IFNα2a 180 μg | First dose | 51.00 ± 22.90 | 40.70 ± 14.29 | 8939.34 ± 2368.70 |
| Last dose | 87.00 ± 27.77 | 28.36 ± 3.96 | 10 348.77 ± 2107.19 | |
| P | 0.03 | 0.04 | 0.07 |
Last dose: IFNα2a serum concentration achieved a steady state after multiple doses, and pharmacokinetic parameters were C ssmax, AUCss0–t, AUCss0–∞, t ss 1/2, T ssmax, CLz/Fss, MRTss0–∞, and Vz/Fss, respectively.
Comparison of neopterin kinetic parameters between the first and last dose
Neopterin AUC and C max decreased and T max increased at the last dose in the rHSA/IFNα2a group, compared to the first dosing, except for T max in the rHSA/IFNα2a 600 μg group. Neopterin C max decreased and AUC and T max increased at the last dose in the PEG‐IFNα2a group, compared to the first dosing. The P‐values are listed in Table 9.
Comparison of neopterin kinetic parameters between the rHSA/IFNα2a 750 μg and PEG‐IFNα2a groups
Neopterin AUC0–t of the first dose was higher in the rHSA/IFNα2a 750 μg group than in the PEG‐IFNα2a group (P < 0.05). Other PK parameters were similar between these two groups (Table 7).
Antiviral efficacy
HBV DNA level decreased after treatment, and mean changes in serum HBV DNA were −1.32, −2.13, −1.10 and −2.48 log10 IU/ml for the 600, 750 and 900 μg groups and the PEG‐IFNα2a group, respectively. The decreased level between the rHSA/IFNα2a 750 μg and PEG‐IFNα2a groups were similar. At the end of treatment, there was no significant difference in reduced HBV DNA levels among the treated groups (P = 0.35). Almost all of the subjects in the rHSA/IFNα2a 750 μg group had HBV DNA that decreased for more than 2 log10 compared to baseline after the first dosing, and in the PEG‐IFNα2a group after the fifth dosing (week 9) (Figure 6). There was no difference in the percentage with more than 2 log10 reduction between groups (P = 0.25).
Figure 6.
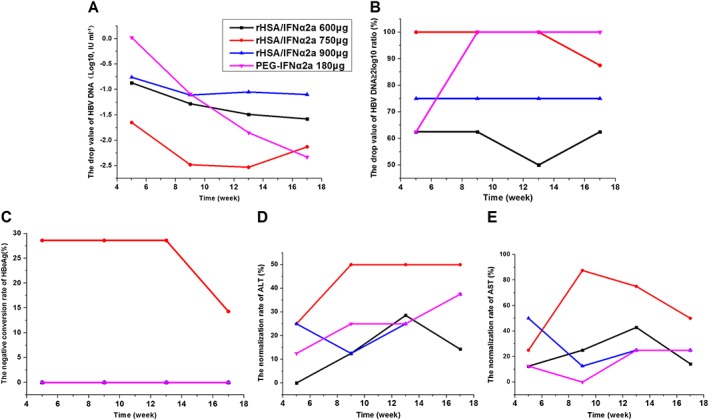
Reduced levels of HBV markers at different time points after treatment in each group (all compared with the baseline values). HBV DNA (A); rate of HBV DNA reducing value ≥2 log10 (B); HBeAg seroconversion (C); ALT normalization rate (D); AST normalization rate (E)
Serum HBeAg level decreased after treatment. HBeAg‐negative conversion rates were 0% (0/6), 14.28% (1/7), 0% (0/6) and 0% (0/8), respectively; and HBeAg levels decreased by −488.8 ± 493.23, −183.7 ± 292.35, −118.2 ± 404.09 and −556.4 ± 434.87 IU ml−1 (P = 0.10) in the 600, 750 and 900 μg groups and the PEG‐IFNα2a group, respectively. The HBeAg‐negative conversion rate and normalization rate of ALT and AST were higher in the rHSA/IFNα2a 750 μg groups than in the PEG‐IFNα2a group (Figure 6). None of treated patients had HBsAg‐negative conversion, HBeAg serum conversion, or HBsAg serum conversion.
ALT and AST levels decreased in all groups. ALT normalization rates were 14.28%, 50%, 37.5% and 37.5% (P = 0.54), while AST normalization rates were 14.28%, 50%, 25% and 25% (P = 0.46) in the 600, 750 and 900 μg groups and in the PEG‐IFNα2a group, respectively.
Because this was a Phase Ib study, the sample size was small, resulting in insufficient power in statistics. However, the antiviral efficacy can be observed through the changed trend.
Analysis of linear correlations between the anti‐viral effect and exposures of IFNα2a and the production of neopterin in the rHSA/IFNα2a group
First, decreased levels in serum HBV DNA and HBeAg from week 17 were determined and compared with baseline values; and AUC0–t (IFNα2a), C max (IFNα2a), AUC0–t (neopterin) and C max (neopterin) were computed after the last dose of rHSA/IFNα2a. Then, a linear correlation analysis was carried out between the decreased levels of HBeAg and HBV DNA and exposures to IFNα2a and neopterin at the last dose of rHSA/IFNα2a. All correlation coefficients were negative. However, there were no significant differences, and the correlation was very weak, if at all (Table 10).
Table 10.
Possible correlations between decreased levels of HBeAg and HBV DNA, and exposures of IFNα2a and neopterin at last dosing of rHSA/IFNα2a
| Anti‐viral factors | Parameter | AUC ss0–t (IFNα2a) | C ssmax (IFNα2a) | AUC ss0–t (Neopterin) | C ssmax (Neopterin) |
|---|---|---|---|---|---|
| Decreased levels of HBV DNA | r | −0.07716 | 0.02092 | −0.27961 | −0.19993 |
| P value | 0.7465 | 0.9302 | 0.2325 | 0.398 | |
| N | 20 | 20 | 20 | 20 | |
| Decreased levels of HBeAg | r | −0.11988 | −0.13495 | −0.4296 | −0.28997 |
| P value | 0.6147 | 0.5705 | 0.0587 | 0.2149 | |
| N | 20 | 20 | 20 | 20 |
Discussion
In this Phase 1b study, we evaluated the safety and efficacy profiles of 600, 750 and 900 μg of rHSA/IFNα2a in the treatment of chronic hepatitis B patients for 17 weeks. We found that rHSA/IFNα2a was better tolerated while delivering comparable efficacy with the 750 μg dose in inhibiting HBV replication and normalizing ALT, compared to the 180 μg PEG‐IFNα2a treatment.
Interferon treatment is often accompanied by adverse reactions that range from flu‐like symptoms, bone marrow suppression and nervous system symptoms to gastrointestinal discomfort. The frequency and severity of these adverse reactions are related to the size and frequency of interferon dosage, as well as individual factors 10. Fever and headache symptoms occurred more often and the reduction of blood cell count was more severe in the PEG‐IFNα2a 180 μg group, compared with the rHSA/IFNα2a 750 μg group in this study. The adaptation time to IFN‐related side effects was shorter in the rHSA/IFNα2a groups than in the PEG‐IFNα2a group. Polyethylene glycol may have immunogenicity to incite immune response, while albumin is a component of human serum proteins. Different properties in carrier molecules may produce different extents of adverse events 11.
The half‐life of rHSA/IFNα2a determined in this study was approximately 140 h. A longer half‐life was a result of the slow progress at the elimination phase and flat accumulation over time. The accumulation rate over time was approximately 1.1–1.2, which indicates that the elimination and accumulation of IFN were at an equilibrium state during rHSA/IFNα2a treatment. Therefore, the rHSA/IFNα2a was successfully constructed to improve PK profile and enable the reduction of dosing 12.
It is worth noting that the IFNα2a level in the rHSA/IFNα2a 750 μg dose was equal to the PEG‐IFNα2a 180 μg dose. Systemic exposures were similar between rHSA/IFNα2a 750 μg and PEG‐IFNα2a 180 μg doses after multiple dosing. These results support the conclusion that serum IFNα2a concentration can be effectively maintained for a longer period through fusion with rHSA, which reduces dosing frequency from weekly to biweekly. Pegylation prolongs the IFN's half‐life to approximately 40–80 h, and increases sustained virological response (SVR) rate among the treated chronic hepatitis C patients 13. Since human serum albumin is a carrier protein with a half‐life of 14–20 days, the fusion of IFNα2a to albumin extends the half‐life of the recombinant polypeptide to approximately 150 h, while it can maintain biological activity for 2–4 weeks, as tested in treated chronic hepatitis C patients 12, 14, 15.
There was no detectable neutralizing antibody to rHSA/IFNα2a after rHSA/IFNα2a treatment, which indicates that no new antigenic epitope was introduced in the fused rHSA/IFNα2a, and the recombinant molecule would not generate immune response to compromise antiviral function 16.
Albumin‐IFN alpha was originally developed to treat chronic hepatitis C 17. In order to investigate clinical applications for treating chronic hepatitis B patients, the antiviral efficacy of rHSA/IFNα2a was evaluated in this study. After seven doses of rHSA/IFNα2a or 13 doses of PEG‐IFNα2a, similar patterns of reducing serum HBV DNA were observed between rHSA/IFNα2a 750 μg and PEG‐IFNα2a 180 μg treatments, which revealed that the efficacy of rHSA/IFNα2a in suppressing HBV DNA replication was comparable to PEG‐IFNα2a.
Factors for predicting response to the antiviral treatment of chronic hepatitis B include high ALT level at baseline, HBV DNA < 107 IU ml−1, female and good treatment compliance 18, 19, 20, 21. A significant reduction in serum HBV DNA levels was detected in each of the rHSA/IFNα2a treatment groups (Figure 6). At week 17, mean change in HBV DNA were approximately −1.32, −2.13 and −1.10 log10 IU/ml in rHSA/IFNα2a 600, 750 and 900 μg, respectively. The potency of inhibiting HBV DNA replication was comparable between rHSA/IFNα2a 750 μg and PEG‐IFNα2a 180 μg, and both resulted in a more than 2 log10 reduction in HBV DNA level. HBeAg‐negative conversion rate and ALT and AST normalization rates were higher in the rHSA/IFNα2a 750 μg group than in the PEG‐IFNα2a 180 μg group, which may have resulted from the prolonged half‐life of IFNα2a that helped maintain effective drug concentration 22.
It was not clear why antiviral efficacy in the rHSA/IFNα2a 900 μg group was inferior to that in the rHSA/IFNα2a 750 μg group. Subjects in the rHSA/IFNα2a 900 μg group were older than subjects in the rHSA/IFNα2a 750 μg group, although other parameters were similar between these two groups, indicating that older age may be a factor for poor response in the rHSA/IFNα2a 900 μg group 23. The saturation production of neopterin at the first and last dose indicated that rHSA/IFNα2a 750 μg was an effective dose, and was also better tolerated 19.
Since patient compliance to the treatment regimen is important in maximizing SVR rates, longer‐acting rHSA/IFNα2a treatments can reduce dosing frequency, which also contributes to better tolerability 24, 25, 26. Albumin can prevent the peak concentration of interferon by biweekly doses, but the long‐term steady‐state of IFN level kept stimulating antiviral function, which decreases adverse effects and facilitates compliance. Unpegged IFN‐α was administered three times a week, and serum IFN concentrations rapidly increased and decreased, which may affect the maintenance of constant antiviral efficacy.
The inconvenient administration that requires multi‐injection and significant adverse effects limits the clinical application of interferon. Many patients select nucleos(t)ide analogues (NAs) as the first line of therapy because they are convenient to use and well‐tolerated despite infinite treatment course. However, a finite duration of pegylated interferon is still an attractive alternative treatment because it provides higher rates of suppression of HBV replication off‐therapy compared with NAs. In addition, the rates of HBeAg/HBsAg loss or seroconversion are increased over time among patients who respond to PEG‐IFN therapy 27. Here, we demonstrate that the rHSA/IFNα2a treatment was well‐tolerated and can be administered biweekly, only requiring 24 injections over a 48‐week course. In addition, this new product will likely break the monopoly market of pegylated interferon and introduce the competition that will cut the price of the drug. Convenient administration, lower cost and a similar anti‐HBV efficacy are the advantages of rHSA/IFNα2a.
In conclusion, rHSA/IFNα2a, a new long‐acting IFNα2a, was found to be better tolerated by chronic hepatitis B patients compared to PEG‐IFNα2a. The rHSA/IFNα2a delivers a significantly longer half‐life of IFNα2a and increases IFNα2a exposure compared to PEG‐IFNα2a. The antiviral efficacy of rHSA/IFNα2a 750 μg was similar to PEG‐IFNα2a 180 μg. On the basis of these results, rHSA/IFNα2a 750 μg dose was selected for further evaluation in Phase II trials for treating chronic hepatitis B patients.
The study does have some limitations. The ratio of male to female patients was 21:11, so the majority of subjects were males. When the tolerability and pharmacokinetic characteristics of a drug are analysed, an ideal ratio of males to females is 1:1. However, the incidence of HBV infection is higher in the male population, which impacts the availability of female subjects.
Competing Interests
There are no competing interests to declare.
This work was supported by the National Natural Science Foundation of China (grant numbers: 30872174, 81473037, 81300313, 81602897) and National Key S&T Special Projects (2014ZX09303303).
Ding, Y. , Lou, J. , Chen, H. , Li, X. , Wu, M. , Li, C. , Liu, J. , Liu, C. , Li, Q. , Zhang, H. , and Niu, J. (2017) Tolerability, pharmacokinetics and antiviral activity of rHSA/IFNα2a for the treatment of chronic hepatitis B infection. Br J Clin Pharmacol, 83: 1056–1071. doi: 10.1111/bcp.13184.
Contributor Information
Hong Zhang, Email: jhongzhang@qq.com.
Junqi Niu, Email: junqinu@126.com.
References
- 1. Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet 2015; 386: 1546–1555. [DOI] [PubMed] [Google Scholar]
- 2. Ott JJ, Stevens GA, Groeger J, Wiersma ST. Global epidemiology of hepatitis B virus infection: new estimates of age‐specific HBsAg seroprevalence and endemicity. Vaccine 2012; 30: 2212–2219. [DOI] [PubMed] [Google Scholar]
- 3. Iloeje UH, Yang HI, Chen CJ. Natural history of chronic hepatitis B: what exactly has REVEAL revealed? Liver Int 2012; 32: 1333–1341. [DOI] [PubMed] [Google Scholar]
- 4. Chen CJ, Yang HI, Su J, Jen CL, You SL, Lu SN, et al. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA 2006; 295: 65–73. [DOI] [PubMed] [Google Scholar]
- 5. Baran B. Nucleos(t)ide analogs in the prevention of hepatitis B virus related hepatocellular carcinoma. World J Hepatol 2015; 7: 1742–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. European Association for the Study of the Liver . EASL clinical practice guidelines: management of chronic hepatitis B virus infection. J Hepatol 2012; 57: 167–185. [DOI] [PubMed] [Google Scholar]
- 7. Lampertico P, Maini M, Papatheodoridis G. Optimal management of hepatitis B virus infection – EASL Special Conference. J Hepatol 2015; 63: 1238–1253. [DOI] [PubMed] [Google Scholar]
- 8. Nelson DR, Benhamou Y, Chuang WL, Lawitz EJ, Rodriguez‐Torres M, Flisiak R, et al. Albinterferon Alfa‐2b was not inferior to pegylated interferon‐α in a randomized trial of patients with chronic hepatitis C virus genotype 2 or 3. Gastroenterology 2010; 139: 1267–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pianko S, Zeuzem S, Chuang WL, Foster GR, Sarin SK, Flisiak R, et al. Randomized trial of albinterferon alfa‐2b every 4 weeks for chronic hepatitis C virus genotype 2/3. J Viral Hepat 2012; 19: 623–634. [DOI] [PubMed] [Google Scholar]
- 10. Kirkwood JM, Bender C, Agarwala S, Tarhini A, Shipe‐Spotloe J, Smelko B, et al. Mechanisms and management of toxicities associated with high‐dose interferon alfa‐2b therapy. J Clin Oncol 2002; 20: 3703–3718. [DOI] [PubMed] [Google Scholar]
- 11. Merlot AM, Kalinowski DS, Kovacevic Z, Jansson PJ, Lane DJ, Huang ML, et al. Making a case for albumin – a highly promising drug‐delivery system. Future Med Chem 2015; 7: 553–556. [DOI] [PubMed] [Google Scholar]
- 12. Zeuzem S, Yoshida EM, Benhamou Y, Pianko S, Bain VG, Shouval D, et al. Albinterferon alfa‐2b dosed every two or four weeks in interferon‐naïve patients with genotype 1 chronic hepatitis C. Hepatology 2008; 48: 407–417. [DOI] [PubMed] [Google Scholar]
- 13. Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Gonçales FL Jr, et al. Peginterferon alfa‐2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med 2002; 347: 975–982. [DOI] [PubMed] [Google Scholar]
- 14. Chuang VT, Kragh‐Hansen U, Otagiri M. Pharmaceutical strategies utilizing recombinant human serum albumin. Pharm Res 2002; 19: 569–577. [DOI] [PubMed] [Google Scholar]
- 15. Bain VG, Kaita KD, Yoshida EM, Swain MG, Heathcote EJ, Neumann AU, et al. A phase 2 study to evaluate the antiviral activity, safety, and pharmacokinetics of recombinant humanalbumin‐interferon alfa fusion protein in genotype 1 chronic hepatitis C patients. J Hepatol 2006; 44: 671–678. [DOI] [PubMed] [Google Scholar]
- 16. Halfon P, Pérusat S, Bourlière M, Bronowicki JP, Trimoulet P, Benhamou Y, et al. Neutralizing antibodies to interferon‐α and circulating interferon in patients with chronic hepatitis C non‐responding to pegylated interferon plus ribavirin re‐treated by pegylated interferon‐α‐2a and ribavirin (ANRS HC16 GAMMATRI substudy). J Med Virol 2010; 82: 2027–2031. [DOI] [PubMed] [Google Scholar]
- 17. Rustgi VK. Albinterferon alfa‐2b, a novel fusion protein of human albumin and human interferon alfa‐2b, for chronic hepatitis C. Curr Med Res Opin 2009; 25: 991–1002. [DOI] [PubMed] [Google Scholar]
- 18. Bingfa X, Qinglin F, Hui H, Canjun W, Wei W, Lihua S. Anti‐hepatitis B virus activity and mechanisms of recombinant human serum albumin‐interferon‐alpha‐2b fusion protein in vitro and in vivo . Pharmacology 2009; 83: 323–332. [DOI] [PubMed] [Google Scholar]
- 19. Huber C, Batchelor JR, Fuchs D, Hausen A, Lang A, Niederwieser D, et al. Immune response‐associated production of neopterin. Release from macrophages primarily under control of interferon‐gamma. J Exp Med 1984; 160: 310–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. de Veer MJ, Holko M, Frevel M, Walker E, Der S, Paranjape JM, et al. Functional classification of interferon‐stimulated genes identified using microarrays. J Leukoc Biol 2001; 69: 912–920. [PubMed] [Google Scholar]
- 21. Zoulim F. Assessment of treatment efficacy in HBV infection and disease. J Hepatol 2006; 44 (1 Suppl): S95–S99. [DOI] [PubMed] [Google Scholar]
- 22. Buster EH, Hansen BE, Lau GK, Piratvisuth T, Zeuzem S, Steyerberg EW, et al. Factors that predict response of patients with hepatitis B e antigen‐positive chronic hepatitis B to peginterferon‐alfa. Gastroenterology 2009; 137: 2002–2009. [DOI] [PubMed] [Google Scholar]
- 23. Bonino F, Marcellin P, Lau GK, Hadziyannis S, Jin R, Piratvisuth T, et al. Predicting response to peginterferon alpha‐2a, lamivudine and the two combined for HBeAg‐negative chronic hepatitis B. Gut 2007; 56: 699–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, et al. Peginterferon alfa‐2b plus ribavirin compared with interferon alfa‐2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet 2001; 358: 958–965. [DOI] [PubMed] [Google Scholar]
- 25. McHutchison JG, Manns M, Patel K, Poynard T, Lindsay KL, Trepo C, et al. Adherence to combination therapy enhances sustained response in genotype‐1‐infected patients with chronic hepatitis C. Gastroenterology 2002; 123: 1061–1069. [DOI] [PubMed] [Google Scholar]
- 26. Kubo S, Takemura S, Tanaka S, Shinkawa H, Nishioka T, Nozawa A, et al. Management of hepatitis B virus infection during treatment for hepatitis B virus‐related hepatocellular carcinoma. World J Gastroenterol 2015; 21: 8249–8255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kao JH. HBeAg‐positive chronic hepatitis B: why do I treat my patients with pegylated interferon? Liver Int 2014; 34: 112–119. [DOI] [PubMed] [Google Scholar]


