Abstract
Aim
The use of selective serotonin reuptake inhibitors (SSRIs) in late pregnancy may be associated with an increased risk of persistent pulmonary hypertension of the newborn (PPHN). Limited data are available on the risk of PPHN associated with serotonin norepinephrine reuptake inhibitors (SNRIs). We aimed to quantify both associations.
Methods
Using data from the Quebec Pregnancy Cohort between 1998 and 2009, we included women covered by the provincial drug plan who had a singleton live birth. Exposure categories were SSRI, SNRI and other antidepressant use; non‐users were considered as the reference category. Generalized estimating equation models were used to obtain risk estimates and 95% confidence intervals (CIs). Confounding by indication was minimized by adjusting for history of maternal depression/anxiety before pregnancy.
Results
Overall, 143 281 pregnancies were included; PPHN was identified in 0.2% of newborns. Adjusting for maternal depression, and other potential confounders, SSRI use during the second half of pregnancy was associated with an increased risk of PPHN [adjusted odds ratio (aOR) 4.29, 95% CI 1.34, 13.77] compared with non‐use of antidepressants; SNRI use during the same time window was not statistically associated with the risk of PPHN (aOR 0.59, 95% CI 0.06, 5.62). Use of SSRIs and SNRIs before the 20th week of gestation was not associated with the risk of PPHN.
Conclusions
Use of SSRIs in the second half of pregnancy was associated with the risk of PPHN. Given our results on SNRIs and the lack of statistical power for these analyses, it is unclear whether SNRI use during pregnancy also increases the risk of PPHN.
Keywords: depression, persistent pulmonary hypertension of the newborn (PPHN), Pregnancy, quebec pregnancy cohort, SSRI, venlafaxine
What is Already Known about this Subject
Persistent pulmonary hypertension of the newborn (PPHN) is a rare but potentially life‐threatening condition that occurs usually within hours after birth.
Use of antidepressants during pregnancy is common, and serotonin reuptake inhibitors (SSRIs) and norepinephrine reuptake inhibitors (SNRIs) are the most commonly prescribed classes of antidepressants during pregnancy.
Use of SSRIs in late pregnancy may be associated with an increased risk of PPHN.
What this Study Adds
Use of SSRIs in the second half of pregnancy was associated with the risk of PPHN.
SNRI use was not statistically significantly associated with an increased risk of PPHN, which could be explained by the small sample size and lack of statistical power.
Given our results, we do not know at this point whether SNRIs are associated with the risk of PPHN.
Introduction
Persistent pulmonary hypertension of the newborn (PPHN) is a rare but potentially life‐threatening condition that occurs usually within hours after birth 1. PPHN occurs when the pulmonary vascular resistance fails to decrease after birth, resulting in decreased pulmonary blood flow and the shunting of deoxygenated blood to the systemic circulation 2. Based on an analysis of its pathophysiology, it was suggested that PPHN might result from constricted pulmonary vasculature or because the vasculature is hypoplastic or remodelled 3. Recent studies indicate that the use of selective serotonin receptor inhibitors (SSRIs) in late pregnancy may be associated with an increased risk of PPHN 4, 5, 6, 7, 8. The mechanism by which SSRIs may affect the pulmonary vasculature and cause PPHN remains unknown. However, serotonin is a potent pulmonary vasoconstrictor 9, mediating the proliferation of pulmonary artery smooth muscle cells through the serotonin transporter 10. In an animal study, in utero SSRI exposure was found to induce pulmonary hypertension in the fetal rat as a result of a developmentally regulated increase in pulmonary vascular smooth muscle proliferation 11.
The use of antidepressants during pregnancy has increased over the last 20 years 12. Although SSRIs are the most commonly prescribed class of antidepressants during pregnancy, an increase in the use of other antidepressants, such as serotonin norepinephrine reuptake inhibitors (SNRIs), has also been observed 13. SNRIs are potent inhibitors of the reuptake of serotonin and norepinephrine, while SSRIs act upon serotonin alone 14.
Confirmatory data on SSRI use during pregnancy and the risk of PPHN are now needed; limited data are available on the risk of PPHN associated with SNRIs 7. This can partly be explained by the fact that PPHN is a rare condition and that large population‐based cohorts are needed. Thus, the purpose of the present study was to quantify the association between SSRI and SNRI use during pregnancy and the risk of PPHN in a large population‐based cohort.
Methods
Data source and study cohort
We conducted a register‐based cohort study using data from the Quebec Pregnancy Cohort (QPC). The QPC is an ongoing population‐based cohort with prospective data collection on all pregnancies that occurred between January 1998 and December 2010 in the province of Quebec. Data on the mothers and children after the end of pregnancy are also collected. Individual‐level information is obtained from province‐wide databases and linked using unique personal identifiers. The QPC was first constructed by identifying all pregnancies in the Régie de l'assurance maladie du Québec (RAMQ) and the Quebec hospitalization archives (MedEcho) databases; subsequently, first day of the last menstrual period (first day of gestation: 1DG) was defined using data on gestational age, which was validated against patients’ charts 15. The research team did not have access to personal identifiers to protect the privacy of the mothers and children included in the QPC. However, the team had access to de‐identified identifiers, to ensure that subjects could be followed up prospectively.
The QPC data sources for the study included the medical service database (RAMQ: diagnoses, medical procedures, socioeconomic status of women and prescribers), the Quebec Public Prescription Drug Insurance database (drug name, start date, dosage, duration), the hospitalization archive database (MedEcho: in‐hospital diagnoses and procedures) and the Quebec Statistics database (ISQ: patient sociodemographics, birth weight). The QPC has been described further by Bérard and Sheehy 16.
Study population
Using data from the Quebec Pregnancy Cohort between 1998 and 2009, we included all pregnancies with continuous prescription drug insurance coverage of at least 12 months before and during pregnancy, and resulting in a singleton live birth. To have a more homogeneous and specific population, we excluded pregnancies exposed to known teratogens during pregnancy (as they are more likely to be diagnosed with adverse pregnancy outcomes, including PPHN) 17, newborns with chromosomal abnormalities (which are unlikely to be due to medication exposure but are increasing the detection of any other adverse outcomes, including PPHN) or with minor congenital malformations alone (which are detected selectively in administrative databases and are therefore increasing the detection of any other adverse outcomes, including PPHN). We did not, however, exclude children born with major malformations because children with PPHN have a high prevalence of cardiac defects. Excluding major malformations would have resulted in a lower‐than‐expected PPHN prevalence. All pregnancies meeting eligibility criteria were analysed. The study was approved by the Quebec Data Access Agency and the CHU Sainte‐Justine Institutional Review Board.
Antidepressant exposure categories
Exposure categories were SSRI (paroxetine, sertraline, citalopram, fluoxetine, fluvoxamine), SNRI (venlafaxine) and other antidepressants (bupropion, mirtazapine, amitriptyline, desipramine, doxepin, imipramine, nortriptyline, trimipramine, clomipramine, L‐tryptophan, trazodone, moclobemide, buspirone) during the relevant time windows. Duloxetine and desvenlafaxine were not included in the SNRI category as they were not on the list of medications reimbursed in Quebec during the calendar years of the study; no pregnant women used these two medications in our cohort. We identified prescription fillings for antidepressants dispensed to women in the cohort from the Quebec Public Prescription Drug Insurance database, with the timing of exposure determined by the dispensed date and duration of prescription. The relevant exposure time window was the second half of pregnancy (21st week of gestation until the end of pregnancy), although we also assessed exposure in the first half of pregnancy (1DG to the 20th week of gestation). Women were considered to have been exposed if they had filled at least one prescription for antidepressants during the time window of interest or if they had filled a prescription for antidepressants before the 20th week of gestation with a duration overlapping with the second half of pregnancy.
Data on prescription fillings were validated and compared with the information in maternal reports, which are more reliable than data on medication prescribing in medical charts; the positive predictive value of prescription drug data in the cohort was found to be at least 87% [95% confidence interval (CI) 70%, 100%] and the negative predictive value was at least 92% (95% CI 86%, 98%) 18.
Outcomes
Cases of PPHN were identified in the RAMQ/MedEcho databases from both maternal and infant files, and defined based on the presence of the International Classification of Diseases, Ninth Revision diagnostic codes (ICD‐9: 416, 747.8) and the International Statistical Classification of Diseases, 10th Revision diagnostic codes (ICD‐10: I27, I521, P293) within the first 6 weeks of life in newborns not transferred to another hospital after birth. According to Palmsten et al. 19, the positive predictive value of these codes is 89.6% (95% CI 77.8%, 95.5%).
Statistical analyses
Crude and adjusted odds ratios (aORs) with 95% CIs were calculated using generalized estimating equations models. For all analyses, the reference category was non‐users of any antidepressants during the specific exposure time window, and both study time window exposures (1DG to the 20th week; 21 weeks to the end of pregnancy) were considered.
Potential confounders were considered for all analyses: (i) sociodemographic variables, including maternal age on the 1DG, maternal marital status (living alone or cohabiting), receipt of social assistance 1 year before or during pregnancy, and area of residence on the 1DG (urban/rural); and (ii) maternal chronic comorbidities during the 12 months prior to and during the first trimester of pregnancy, including depression and anxiety, hypertension (chronic and pregnancy induced), diabetes (mellitus and gestational) and asthma. The previous conditions were identified from either diagnoses or disease‐specific medications. In order to adjust further for potential indication bias, we also considered healthcare utilization during the 12 months prior to the 1DG, including visits to a psychiatrist or obstetrician; hospitalizations or emergency department (ED) visits; the number of medications used other than antidepressants; and the number of different prescribers. All of these variables were either risk factors or determinants for adverse pregnancy outcomes.
Sensitivity analyses were performed, defining exposure as four mutually exclusive categories: exposure only in the first 20 weeks of pregnancy, exposure only between the 21st week until the end of pregnancy, exposure throughout pregnancy and no exposure throughout pregnancy (reference category). Further stratification was carried out on antidepressant classes (SSRI, SNRI, other antidepressants) within the exposure categories. Although the main analysis was more clinically relevant and better reflected use during gestation, this sensitivity analysis was performed to ensure that the severity of depression or modification of dosage during pregnancy were adequately taken into account.
Statistical analyses were performed using SAS (SAS Institute Inc., Version 9.2, Cary, NC, USA).
Results
Overall, 143 281 pregnancies met the inclusion criteria and were considered; PPHN was identified in 0.2% (n = 267) of newborns (Figure 1). Table 1 presents the study characteristics, together with antidepressant class exposures between week 21 to the end of pregnancy, which was our time window of interest. Overall, mothers using antidepressants during pregnancy were somewhat older, and more likely to be on welfare, living alone and have comorbidities than those unexposed during the same time window (≥21 weeks) (Table 1); they had more healthcare utilizations and used more medications overall.
Figure 1.
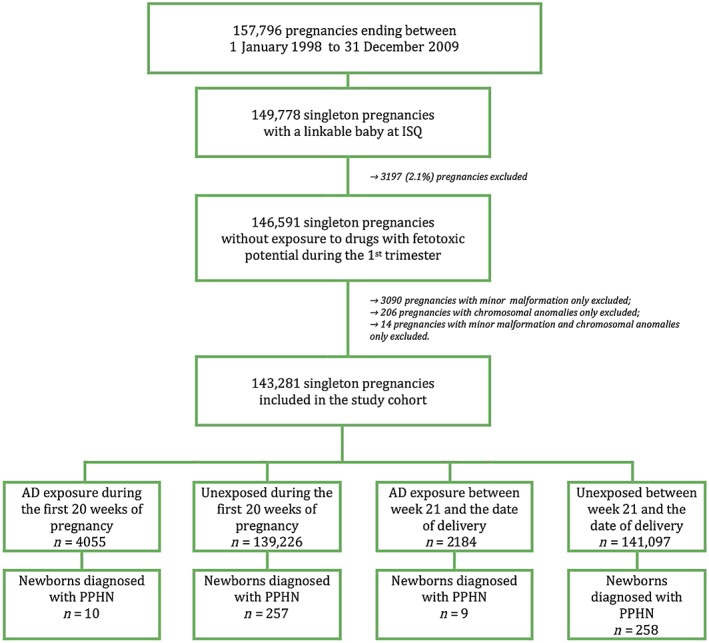
Cohort selection for the analyses of antidepressants and PPHN. AD, antidepressant; ISQ, Quebec Statistics database (patient sociodemographics, birth weight); PPHN, persistent pulmonary hypertension of the newborn
Table 1.
Characteristics of the study cohort
| Unexposed 141 097 (98.5%) | Antidepressant exposure between the 21st week and the end of pregnancya n = 2184 (1.5%) | |||
|---|---|---|---|---|
| SSRI 1537 (70.4%) | SNRI 419 (19.2%) | Other ADs 346 (15.8%) | ||
| Pregnancy‐related | ||||
| Gestational age (weeks), mean (±SD) | 38.8 ± 1.8 | 38.3 ± 1.9 | 38.0 ± 2.0 | 38.2 ± 2.0 |
| Birth weight (g), mean (±SD) | 3359.0 ± 579.1 | 3245.0 ± 607.3 | 3162.1 ± 559.5 | 3188.3 ± 566.5 |
| Newborn gender (male), n (%)b | 71 891 (51.3) | 782 (51.2) | 231 (55.5) | 188 (54.7) |
| PPHN diagnosis, n (%) | 258 (0.2) | 7 (0.5) | 1 (0.2) | 1 (0.3) |
| Antidepressant exposure during the first 20 weeks of pregnancy, n (%) | 2200 (1.6) | 1328 (86.4) | 381 (90.9) | 252 (72.8) |
| Measured on the first day of gestation | ||||
| Maternal age, years, mean (±SD) | 27.7 ± 5.5 | 28.9 ± 5.5 | 28.8 ± 5.5 | 29.4 ± 6.0 |
| Urban dweller, n (%) | 115 768 (82.1) | 1274 (82.9) | 352 (84.0) | 295 (85.3) |
| Social assistance recipient, n (%) | 34 874 (24.7) | 605 (39.4) | 155 (37.0) | 161 (46.5) |
| Living alone, n (%) | 21 207 (15.0) | 371 (24.1) | 116 (27.7) | 110 (31.8) |
| Maternal comorbidities in the year before or during the first trimester: | ||||
| Depression/anxiety, n (%) | 13 561 (9.6) | 992 (64.5) | 280 (66.8) | 174 (50.3) |
| Diabetes, n (%) | 2282 (1.6) | 37 (2.4) | 6 (1.4) | 13 (3.8) |
| Hypertension, n (%) | 3622 (2.6) | 86 (5.6) | 22 (5.3) | 42 (12.1) |
| Asthma, n (%) | 15 784 (11.2) | 365 (23.8) | 109 (26.0) | 102 (29.5) |
| Health services utilization in the year before pregnancy | ||||
| Visit to a general practitioner, mean (±SD) | 3.5 ± 3.8 | 6.8 ± 5.7 | 6.7 ± 5.4 | 7.3 ± 6.4 |
| Visit to specialists other than a psychiatrist or obstetrician, mean (±SD) | 0.9 ± 1.9 | 1.1 ± 1.9 | 0.7 ± 1.5 | 1.5 ± 2.9 |
| At least one of: | ||||
| Psychiatrist visit, n (%) | 2159 (1.5) | 237 (15.4) | 58 (13.8) | 55 (15.9) |
| Obstetrician visit, n (%) | 44 611 (31.6) | 402 (26.2) | 98 (23.4) | 100 (28.9) |
| Emergency department /hospitalization, n (%) | 27 225 (19.3) | 356 (23.2) | 85 (20.3) | 96 (27.8) |
| No. of medications used other than antidepressants, n (%) | ||||
| 0 | 39 477 (28.0) | 106 (6.9) | 22 (5.3) | 25 (7.2) |
| 1–2 | 50 793(36.0) | 369 (24.0) | 88 (21.0) | 71 (20.5) |
| 3–5 | 35 628 (25.3) | 546 (35.2) | 168 (40.1) | 99 (28.6) |
| ≥ 6 | 15 199 (10.8) | 516 (33.6) | 141 (33.7) | 151 (43.6) |
| No. of different prescribers, n (%) | ||||
| 0 | 39 477 (28.0) | 106 (6.9) | 22 (5.3) | 25 (7.2) |
| 1 | 34 527 (24.5) | 243 (15.8) | 61 (14.6) | 49 (14.2) |
| ≥ 2 | 67 093 (47.6) | 1188 (77.3) | 336 (80.2) | 272 (78.6) |
ADs, antidepressants; PPHN, persistent pulmonary hypertension of the newborn; SD, standard deviation; SSRI, selective serotonin reuptake inhibitor; SNRI: serotonin norepinephrine reuptake inhibitor; numbers in parentheses are column percentages unless stated otherwise
The sum of the total number of pregnancies for each type of antidepressant is greater than the total number of exposed pregnancies during the first trimester (n = 4055) as a single pregnancy may have been exposed to more than one antidepressant during the first trimester
Baby gender was missing for 908 pregnancies (893 unexposed pregnancies; 10 SSRI, three SNRI, and two other antidepressant‐exposed pregnancies)
Adjusting for maternal depression, use of antidepressants in the first 20 weeks of pregnancy and other potential confounders, SSRI use during the second half of pregnancy was associated with an increased risk of PPHN (aOR 4.29, 95% CI 1.34, 13.77) compared with non‐use of antidepressants during the same time window; SNRI use during the same time window was not statistically significantly associated with the risk of PPHN (aOR 0.59, 95% CI 0.06, 5.62) (Table 2). Use of SSRIs and SNRIs in the first 20 weeks of gestation was not statistically significantly associated with the risk of PPHN (Table 2). Other independent risk factors for PPHN in multivariate analyses were higher maternal age (aOR 1.04, 95% CI 1.02, 1.07) and maternal diabetes (aOR 2.41, 95% CI 1.27, 4.55) (Table 2).
Table 2.
Risk of PPHN following antidepressant use during pregnancy
| Variables | Crude ORs (95% CI) | Adjusted ORs (95% CI) |
|---|---|---|
| Exposure between week 21 and date of birth: | ||
| Unexposed | 1.0 | 1.0 |
| SSRI | 2.47 (1.14, 5.38) | 4.29 (1.34, 13.77) |
| SNRI | 1.26 (0.17, 9.12) | 0.59 (0.06, 5.62) |
| Other antidepressants | 1.17 (0.15, 9.12) | 1.63 (0.11, 23.97) |
| Exposure during the first 20 weeks of pregnancy: | ||
| Unexposed | 1.0 | 1.0 |
| SSRI | 1.23 (0.54, 2.80) | 0.37 (0.11, 1.22) |
| SNRI | 1.93 (0.61, 6.17) | 1.88 (0.49, 7.14) |
| Other antidepressants | 0.53 (0.07, 3.97) | 0.36 (0.03, 5.22) |
| Maternal age (years) | 1.04 (1.02, 1.06) | 1.04 (1.02, 1.07) |
| Urban dweller (yes/no) | 0.84 (0.62, 1.14) | 0.82 (0.60, 1.11) |
| Social assistance recipient (yes/no) | 1.11 (0.85, 1.46) | 1.04 (0.72, 1.28) |
| Living alone (yes/no) | 1.32 (0.97, 1.79) | 1.36 (0.97, 1.89) |
| In the year prior to the first day of gestation and during the 1st trimester: | ||
| Depression/anxiety | 1.21 (0.84, 1.74) | 1.16 (0.76, 1.78) |
| Diabetes | 2.61 (1.43, 4.78) | 2.41 (1.27, 4.55) |
| Hypertension | 0.99 (0.46, 2.11) | 0.84 (0.38, 1.88) |
| Asthma | 1.06 (0.73, 1.54) | 1.01 (0.67, 1.50) |
| General practitioner visits: | ||
| 0 | 1.0 | 1.0 |
| 1 | 0.79 (0.54, 1.15) | 0.84 (0.58, 1.24) |
| 2–3 | 0.69 (0.49, 0.98) | 0.74 (0.51, 1.08) |
| 4 or more | 0.75 (0.54, 1.02) | 0.75 (0.50, 1.11) |
| Other than psychiatrist specialist visits: | ||
| 0 | 1.0 | 1.0 |
| 1 | 0.92 (0.68, 1.26) | 0.95 (0.68, 1.31) |
| 2 or more | 0.71 (0.51, 1.00) | 0.69 (0.46, 1.01) |
| Psychiatrist visit (yes/no) | 1.53 (0.72, 3.24) | 1.31 (0.59, 2.93) |
| Obstetrician/gynaecologist visit (yes/no) | 0.88 (0.68, 1.15) | 0.89 (0.66, 1.19) |
| Emergency department/hospitalization (yes/no) | 0.98 (0.73, 1.33) | 1.05 (0.75, 1.48) |
| Medications other than antidepressants: | ||
| 0 | 1.0 | 1.0 |
| 1–2 | 0.61 (0.45, 0.84) | 0.69 (0.49, 0.96) |
| 3–5 | 0.85 (0.62, 1.17) | 1.01 (0.71, 1.45) |
| 6 or more | 1.03 (0.70, 1.52) | 1.21 (0.74, 1.98) |
CI, confidence interval; OR, odds ratio; PPHN, persistent pulmonary hypertension of the newborn; SSRI, selective serotonin reuptake inhibitor; SNRI: serotonin norepinephrine reuptake inhibitor
Figure S1 presents the selection of the study cohort when defining mutually exclusive antidepressant exposure categories at cohort entry. Although the number of eligible pregnancies remained the same a priori (n = 143 281), the number of pregnancies analysed decreased because women exposed to multiple antidepressant classes in a different study time window (0–20 weeks, 21 weeks to the end of pregnancy) were excluded (n = 142 697). Nevertheless, this allowed us to use pregnancies with no antidepressant exposure throughout gestation as the reference category. This categorization did not allow us to study the effect of SNRI use during the second half of pregnancy or the effect of the use of other antidepressants (other than SSRIs and SNRIs) throughout pregnancy because no cases of PPHN were identified in these two categories. In this sensitivity analysis, the incidence of PPHN was 1.8/1000 among non‐exposed women; 2.5/1000 among those exposed between the 1st and 20th weeks of gestation, and 3.8/1000 in those exposed in the second half of pregnancy (see Figure S1). Using this exposure categorization, and adjusting for potential confounders, the use of SSRIs in the second half of pregnancy was associated with an increased risk of PPHN (aOR 4.99, 95% CI 1.19, 20.88), which was consistent with our primary analysis; use of other antidepressants (other than SSRIs and SNRIs) during the same time window was associated with a nonstatistically significant association with the risk of PPHN (aOR 5.41, 95% CI 0.76, 38.74) (see Table S1). Given our results on SSRI and SNRI exposure throughout pregnancy (see Table S1), the critical time window seems to be the second half of pregnancy.
Discussion
Our study is one of the few that have looked at the effect of SSRIs during late pregnancy and the risk of PPHN; to our knowledge, this is the only study that was specifically designed to quantify the association between SNRI use during the second half of pregnancy and the risk of PPHN. Although SNRIs and SSRIs might function similarly to treat depressive symptoms, much less is known about their comparative uses during pregnancy. Current recommendations for treatment with SNRIs, such as venlafaxine, during pregnancy are mainly based on the earlier evidence for SSRIs rather than on specific data for these substances.
Our finding of an increased risk of PPHN in association with exposure to SSRIs in late pregnancy was robust to the use of different reference categories, as demonstrated in our sensitivity analysis, and tended to show that the second half of pregnancy is the critical time window for PPHN. It was also consistent with the results of an animal study which showed that fluoxetine (an SSRI) induced fetal pulmonary arterial muscle contractions, significantly increased the pulmonary arterial smooth muscle cell proliferation rate and increased pulmonary arterial medial thickness 11. This was also consistent with the results of other studies 4, 5, 6, 7. In addition, the results from a systematic review and meta‐analysis also suggested an increased risk of PPHN with exposure to SSRI in late pregnancy 8; our finding (aOR 4.29, 95% CI 1.34, 13.77) is consistent with the meta‐analysis result (pooled OR 2.50, 95% CI 1.32, 4.73). However, three other small studies did not find an association between SSRI use in late pregnancy and PPHN; this could partly be explained by their small sample sizes and lack of statistical power 20, 21, 22. In contrast to Reis et al. (OR 2.30, 95% CI 1.29, 3.80) 5 and Kieler (aOR 1.4, 95% CI 1.0, 2.0) 6, we did not find an association between first trimester exposure to SSRI and the risk of PPHN. This could be explained by the fact that PPHN is a rare condition (with a prevalence of 1.8 per 1000 live births in our study), and therefore large population‐based cohorts are needed. Consistent with our findings, data from a meta‐analysis (pooled OR 1.23, 95% CI 0.58, 2.60) 8 and a study by Chambers et al. (aOR 0.3, 95% CI 0.1, 1.2) 4 did not support an association between early pregnancy SSRI exposure and PPHN. The difference in definitions of early exposure among studies may play a role in the discrepancies found; Reis et al. 5 and Kieler et al. 6 only considered first trimester exposure, whereas the current study and that of Chambers et al. 4 considered exposure up to the 20th completed week of gestation.
Our study focused specifically on SNRI exposure and PPHN. Our findings were consistent with those of Huybrechts et al. 7, who did not find a statistically significant association between the use of non‐SSRI antidepressants in late pregnancy and the risk of PPHN (aOR 1.02, 95% CI 0.77–1.35). It remains, however, that our findings on SNRIs were based on small numbers and were thus underpowered.
Strengths and limitations
Study strengths included the use of large registers – administrative and clinical databases that provide population‐based coverage of pregnant women in Quebec, with linkage of data at the individual level; this permitted analysis of a large number of exposed pregnancies, with detailed information regarding exposure, outcomes and potential confounders, limiting selection bias. Exposure data have been validated against maternal report (medication data) 18, 23. Furthermore, although this does not necessarily mean that women actually took their medications, De Jong et al. 24 reported that 94% of all drugs dispensed to pregnant women are taken. Gestational age has been validated 15, decreasing exposure misclassification bias for the studied time window of interest. Data in our study were collected prospectively, limiting recall bias.
Limitations to the study included missing information on potentially important confounders such as smoking, folic acid intake and alcohol intake. Although we could not adjust for these variables specifically, we adjusted for maternal demographic and healthcare utilization variables, which accounted, in part, for lifestyles. Given that it may take many months to obtain an appointment to see a psychiatrist in Quebec, data on psychiatrist visits, rather than the presence of depression, were used as a proxy for severity of disease; diagnoses of depression/anxiety with or without antidepressant use is a better suited definition for the presence of depression, which was used in the present study. Given the number of comparisons made in the present study, we cannot rule out that some of our findings could have occurred by chance alone. Only singletons were considered owing to the fact that singleton and multiple pregnancies have different risk factors for adverse pregnancy outcomes. Only liveborns were considered, as is the case in all such studies. The relevant exposure time window was defined as the second half of pregnancy, recognizing that preterm and term pregnancies might have a different opportunity for the exposure. Nevertheless, it is unlikely to have been an issue in our study because the majority of pregnancies were at term (the mean gestational age was 38.4 weeks), and only singleton live births were included. Finally, as we considered pregnant women insured by the Prescription Drug Insurance programme, the generalizability of the results to those insured by private drug insurance plans could be affected. However, validation studies have shown that pregnant women receiving medication insurance from Quebec's public system have similar characteristics and comorbidities to those who have private medication insurance. Although socioeconomic status might differ between the two groups, this will not have affected the internal validity of the study, given that all such women have been found to have a similar socioeconomic status 25.
Conclusions
The present study suggested that, even if the prevalence of PPHN is low (1.8/1000 children), there is an increased risk associated with exposure to SSRIs in late pregnancy. No statistically significant association was found between exposures to SSRIs from the beginning to the 20th week of gestation or between SNRI use during pregnancy and the risk of PPHN, which could be explained by the small sample size and lack of statistical power. To avoid delays in the diagnosis and treatment of PPHN, healthcare professionals should be made aware of gestational exposure to SSRIs; pregnant women should avoid any unnecessary pregnancy SSRI exposure.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: A.B. is a consultant for plaintiffs in the litigations involving antidepressants and birth defects. All other authors report no financial relationships with commercial interests. A.B. is the holder of a Fonds de la Recherche du Québec‐Santé (FRQ‐S) research chair on Medications and Pregnancy. J.P.Z. is the recipient of a Quebec‐China Post‐doctoral fellowship from the CIHR. S.B. is the recipient of a FRQ‐S Senior career award.
This work was supported by the Canadian Institutes of Health Research (CIHR) – Drug Safety and Effectiveness Network (DSEN) – CAN‐AIM grant.
Contributors
All authors conceived and designed this study. Data were acquired by A.B. A.B. and O.S. carried out the statistical analyses and all authors interpreted the data. The manuscript was drafted by A.B. and J.P.Z., and all authors were involved in the critical revision and approval of the final manuscript.
Supporting information
Table S1 Risk of persistent pulmonary hypertension of the newborn following antidepressant exposure during pregnancy‐using mutually exclusive exposure categories
Figure S1 Selection of pregnancies for the sensitivity analysis on the risk of persistent pulmonary hypertension of the newborn following late pregnancy exposure to antidepressants
Table S1. Supporting info item
Figure S1. Supporting info item
Bérard, A. , Sheehy, O. , Zhao, J.‐P. , Vinet, É. , Bernatsky, S. , and Abrahamowicz, M. (2017) SSRI and SNRI use during pregnancy and the risk of persistent pulmonary hypertension of the newborn. Br J Clin Pharmacol, 83: 1126–1133. doi: 10.1111/bcp.13194.
References
- 1. Hageman JR, Adams MA, Gardner TH. Persistent pulmonary hypertension of the newborn. Trends in incidence, diagnosis, and management. Am J Dis Child 1984; 138: 592–595. [DOI] [PubMed] [Google Scholar]
- 2. Abman SH. New developments in the pathogenesis and treatment of neonatal pulmonary hypertension. Pediatr Pulmonol Suppl 1999; 18: 201–204. [PubMed] [Google Scholar]
- 3. Steinhorn RH. Neonatal pulmonary hypertension. Pediatr Crit Care Med 2010; 11: S79–S84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chambers CD, Hernandez‐Diaz S, Van Marter LJ, Werler MM, Louik C, Jones KL, et al. Selective serotonin‐reuptake inhibitors and risk of persistent pulmonary hypertension of the newborn. N Engl J Med 2006; 354: 579–587. [DOI] [PubMed] [Google Scholar]
- 5. Reis M, Kallen B. Delivery outcome after maternal use of antidepressant drugs in pregnancy: an update using Swedish data. Psychol Med 2010; 40: 1723–1733. [DOI] [PubMed] [Google Scholar]
- 6. Kieler H, Artama M, Engeland A, Ericsson O, Furu K, Gissler M, et al. Selective serotonin reuptake inhibitors during pregnancy and risk of persistent pulmonary hypertension in the newborn: population based cohort study from the five Nordic countries. BMJ 2012; 344: d8012. [DOI] [PubMed] [Google Scholar]
- 7. Huybrechts KF, Bateman BT, Palmsten K, Desai RJ, Patorno E, Gopalakrishnan C, et al. Antidepressant use late in pregnancy and risk of persistent pulmonary hypertension of the newborn. JAMA 2015; 313: 2142–2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Grigoriadis S, Vonderporten EH, Mamisashvili L, Tomlinson G, Dennis CL, Koren G, et al. Prenatal exposure to antidepressants and persistent pulmonary hypertension of the newborn: systematic review and meta‐analysis. BMJ 2014; 348: f6932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McMahon TJ, Hood JS, Nossaman BD, Kadowitz PJ. Analysis of responses to serotonin in the pulmonary vascular bed of the cat. J Appl Physiol (1985) 1993; 75: 93–102. [DOI] [PubMed] [Google Scholar]
- 10. Eddahibi S, Raffestin B, Hamon M, Adnot S. Is the serotonin transporter involved in the pathogenesis of pulmonary hypertension? J Lab Clin Med 2002; 139: 194–201. [DOI] [PubMed] [Google Scholar]
- 11. Fornaro E, Li D, Pan J, Belik J. Prenatal exposure to fluoxetine induces fetal pulmonary hypertension in the rat. Am J Respir Crit Care Med 2007; 176: 1035–1040. [DOI] [PubMed] [Google Scholar]
- 12. Cooper WO, Willy ME, Pont SJ, Ray WA. Increasing use of antidepressants in pregnancy. Am J Obstet Gynecol 2007; 196: 544 e541–544 e545. [DOI] [PubMed] [Google Scholar]
- 13. Jimenez‐Solem E, Andersen JT, Petersen M, Broedbaek K, Andersen NL, Torp‐Pedersen C, et al. Prevalence of antidepressant use during pregnancy in Denmark, a nation‐wide cohort study. PLoS One 2013; 8: e63034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sussman N. SNRIs versus SSRIs: mechanisms of action in treating depression and painful physical symptoms. Primary Care Companion J Clin Psychiatry 2003; 5 (Suppl. 7): 19–26.15156243 [Google Scholar]
- 15. Vilain A, Otis S, Forget A, Blais L. Agreement between administrative databases and medical charts for pregnancy‐related variables among asthmatic women. Pharmacoepidemiol Drug Saf 2008; 17: 345–353. [DOI] [PubMed] [Google Scholar]
- 16. Bérard A, Sheehy O. The Quebec Pregnancy Cohort – prevalence of medication use during gestation and pregnancy outcomes. PLoS One 2014; 9: e93870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chaabane S, Bérard A. Epidemiology of major congenital malformations with specific focus on teratogens. Curr Drug Saf 2013; 8: 128–140. [DOI] [PubMed] [Google Scholar]
- 18. Jobin‐Gervais K, Sheehy O, Bérard A. Can we rely on pharmacy claims databases to ascertain maternal use of medications during pregnancy? Pharmacoepidemiol Drug Saf 2013; S1: 22. [DOI] [PubMed] [Google Scholar]
- 19. Palmsten K, Huybrechts KF, Kowal MK, Mogun H, Hernandez‐Diaz S. Validity of maternal and infant outcomes within nationwide Medicaid data. Pharmacoepidemiol Drug Saf 2014; 23: 646–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Andrade SE, McPhillips H, Loren D, Raebel MA, Lane K, Livingston J, et al. Antidepressant medication use and risk of persistent pulmonary hypertension of the newborn. Pharmacoepidemiol Drug Saf 2009; 18: 246–252. [DOI] [PubMed] [Google Scholar]
- 21. Wilson KL, Zelig CM, Harvey JP, Cunningham BS, Dolinsky BM, Napolitano PG. Persistent pulmonary hypertension of the newborn is associated with mode of delivery and not with maternal use of selective serotonin reuptake inhibitors. Am J Perinatol 2011; 28: 19–24. [DOI] [PubMed] [Google Scholar]
- 22. Wichman CL, Moore KM, Lang TR, St Sauver JL, Heise RH Jr, Watson WJ. Congenital heart disease associated with selective serotonin reuptake inhibitor use during pregnancy. Mayo Clin Proc 2009; 84: 23–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Blais L, Bérard A, Kettani FZ, Forget A. Validity of congenital malformation diagnostic codes recorded in Quebec's administrative databases. Pharmacoepidemiol Drug Saf 2013; 22: 881–889. [DOI] [PubMed] [Google Scholar]
- 24. De Jong van den Berg LT, Feenstra N, Sorensen HT, Cornel MC. Improvement of drug exposure data in a registration of congenital anomalies. Pilot‐study: pharmacist and mother as sources for drug exposure data during pregnancy. EuroMAP Group. Europen Medicine and Pregnancy Group. Teratology 1999; 60: 33–36. [DOI] [PubMed] [Google Scholar]
- 25. Bérard A, Lacasse A. Validity of perinatal pharmacoepidemiologic studies using data from the RAMQ administrative database. Can J Clin Pharmacol 2009; 16: e360–e369. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Risk of persistent pulmonary hypertension of the newborn following antidepressant exposure during pregnancy‐using mutually exclusive exposure categories
Figure S1 Selection of pregnancies for the sensitivity analysis on the risk of persistent pulmonary hypertension of the newborn following late pregnancy exposure to antidepressants
Table S1. Supporting info item
Figure S1. Supporting info item


