The Global initiative for chronic Obstructive Lung Disease (GOLD) strategy document provides recommendations for the diagnosis, management and prevention of chronic obstructive pulmonary disease (COPD). It is not a guideline but is intended to provide expert opinion based on clinical evidence that can help clinicians in their daily practice. Many respiratory societies have used GOLD as a basis for making their own COPD guidelines 1. The GOLD management strategy undergoes major revision at 5‐year intervals, and the 2017 revision has just been released (available at http://www.goldCOPD.org). There are some substantial changes, reflecting recent evidence from clinical trials and the desire to ensure that treatments are matched to the individual characteristics of patients.
For many years, the treatment of COPD patients was a ‘one size fits all’ approach, with little consideration of personalized management based on individual patient characteristics. GOLD 2012 introduced a combined assessment of COPD, involving the measurement of forced expiratory volume in 1 s (FEV1), symptoms and exacerbation history 2; this was an attempt to modernize COPD treatment, by identifying clinical phenotypes that required different treatment approaches. Previously, COPD was subdivided into severity stages 1–4 based on FEV1 alone. The combined assessment resulted in four groups (A/B/C/D), each with different pharmacological treatment recommendations that aimed to address current symptoms and future risk reduction of events, including exacerbations.
A major practical problem with GOLD 2012 was that groups C and D comprised high‐risk patients based on either FEV1 < 50% predicted, or a history of ≥2 exacerbations requiring antibiotics and/or corticosteroids (or one hospitalization), or both of these criteria 3. Patients with a history of exacerbations are more likely to exacerbate in the future 4. Putting heterogeneous clinical phenotypes together in groups C and D created some confusion.
GOLD 2017 has removed FEV1 from the combined assessment, thus simplifying the categorization of patients for the purpose of pharmacological treatment decisions (see Table 1); C and D patients are frequent exacerbators, while A and B have a low exacerbation frequency. FEV1 is often poorly related to the burden of symptoms on an individual level 5, and there is ample evidence that inhaled bronchodilators and inhaled corticosteroids (ICS) can be used effectively when treating patients on the basis of their symptoms and exacerbation history 3, 6. FEV1 remains a key measurement for COPD diagnosis, and is a prognostic marker 7, but is not required to make pharmacological treatment decisions.
Table 1.
Clinical assessment criteria for GOLD 2017 categories and recommended initial pharmacological treatments
| GOLD Category | Clinical characteristics | Recommended initial pharmacotherapy | |
|---|---|---|---|
| Symptoms | Exacerbation frequency | ||
| A | Low | Low | A bronchodilator |
| B | High | Low | LABA or LAMA |
| C | Low | High | LAMA |
| D | High | High | LAMA & LABA |
Symptoms: High = CAT ≥ 10 or mMRC ≥ 2. Exacerbation frequency: High =2 exacerbations requiring oral corticosteroids and/or antibiotics, or one hospitalization in previous year. CAT, COPD assessment test; GOLD, Global initiative for chronic Obstructive Lung Disease; LABA, long‐acting beta agonist; LAMA, long‐acting muscarinic receptor antagonist; mMRC, modified Medical Research Council grade
GOLD 2017 provides recommendations for initial pharmacotherapy (summarized in Table 1) and stepwise treatment algorithms for each group (A–D), including recommendations for switching treatments and stepping down if there has been no clinical benefit. While many GOLD recommendations for stepping up treatment are based on clinical trial evidence, the recommendations for switching or stepping down are less evidence based, but are an attempt to provide guidance in situations commonly encountered in clinical practice. Stepping down in asthma treatment usually occurs when sustained asthma control has been achieved. GOLD advocates step down mainly for a different reason (treatment failure) that reflects the difficult nature of treating COPD.
Dual bronchodilators containing a long‐acting beta‐agonist and long‐acting muscarinic receptor antagonist (LAMA/LABA) in a single inhaler have been available in recent years, with clinical trials showing beneficial effects on lung function compared with long‐acting bronchodilator monotherapies 8. The benefit on symptoms in the early clinical trials was inconsistent 8. However, subsequent pooled analysis increasing the statistical power, and studies with patient‐reported outcomes as the primary endpoint have clearly demonstrated the clinical benefit of LAMA/LABA combinations 9, 10, 11. The place of these dual bronchodilator combinations as a step up from monotherapy in GOLD 2017 is firmly established, particularly for GOLD B patients. The possibility of starting GOLD B patients immediately on a dual bronchodilator without trying a monotherapy first is mentioned, but the clinical characteristics that predict the success of such an approach remain to be defined.
ICS/LABA combination inhalers are widely prescribed to COPD patients. The evidence for their efficacy comes from many clinical trials conducted in COPD patients with a history of exacerbations 12. There are concerns about the potential for side effects, such as osteoporosis and pneumonia 12. Post hoc clinical trial analyses have demonstrated that higher blood eosinophil counts at the start of the study predict a greater ICS effect on exacerbations, highlighting the potential for this simple blood biomarker to optimize the benefit vs. risk ratio for these drugs 3, 13, 14. GOLD 2017 is positive about the potential of this biomarker, but notes that prospective clinical trial validation is needed, as well as definition of the blood eosinophil cut‐off level to be used in practice.
Group D patients, with frequent exacerbations and a high level of symptoms, are often very difficult to treat. There is evidence that LAMA monotherapy, LAMA/LABA combinations and ICS/LABA combinations all reduce exacerbations and improve symptoms 3, 8, 12. Which one to choose first? GOLD proposes a stepwise approach initially, starting with LAMA or LAMA/LABA (Figure 1 shows the treatment options for GOLD D patients). Dual bronchodilators are preferred over ICS/LABA combinations due to the risk of pneumonia associated with ICS 12 and the findings from a single study in COPD patients with a history of exacerbations that showed fewer exacerbations with LAMA/LABA compared with ICS/LABA treatment 15. However, GOLD recognizes that ICS/LABA could be a better choice in some COPD patients, such as those with higher blood eosinophil counts. I am sure that this debate over the merits of ICS/LABA vs. LAMA/LABA will continue, particularly as the current GOLD recommendation is based largely on the results of a single study. GOLD states that its recommendations for patients in group D (and C) will be re‐evaluated when the results of future clinical trial(s) are available.
Figure 1.
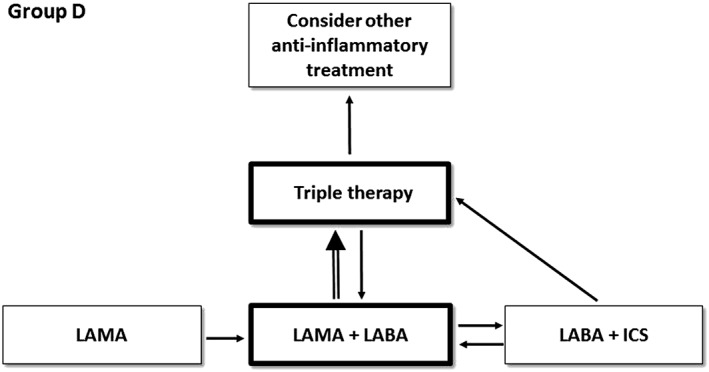
Treatment algorithm recommended by the Global initiative for chronic Obstructive Lung Disease (GOLD) for group D patients. Boxes and arrows that are highlighted are the preferred treatment options. Triple therapy = ICS plus LABA plus LAMA. ICS, inhaled corticosteroids; LABA, long‐acting beta agonist; LAMA, long‐acting muscarinic receptor antagonist
In group D, escalation from two inhaled treatments to ‘triple therapy’ (ICS plus LABA plus LAMA) is recommended for ongoing exacerbations and/or symptoms. Triple therapy is commonly used in clinical practice, although until recently the evidence for its impact on exacerbations has been relatively slim. A large clinical trial has recently reported a 23% reduction in exacerbations requiring antibiotics and/or corticosteroids for patients treated with a single inhaler triple therapy compared with ICS/LABA 16. The results of studies comparing single inhaler triple therapy with LAMA/LABA are keenly awaited, to determine the additional benefit of the ICS in patients being treated with dual bronchodilator therapy.
For patients already on triple therapy and still suffering with exacerbations, GOLD suggests the use of either the phosphodiesterase 4 inhibitor roflumilast or a macrolide. For roflumilast, the clinical phenotype of patients most likely to respond (FEV1 < 50% predicted and chronic bronchitis) has been defined 17, while this information is lacking for macrolides. Furthermore, it is unclear whether the clinical benefit of macrolides is due to anti‐inflammatory or antibacterial activity. The paucity of additional treatment options to add on to triple therapy is a major issue both for prescribers and patients. Over the last decade, pharmaceutical companies have developed a series of combination inhalers containing ICS and/or bronchodilators that have provided small but worthwhile benefits over existing treatments. The high unmet need is for new drugs classes that can be added on to these combination inhalers.
GOLD 2017 has moved firmly towards using symptoms and exacerbations to guide pharmacological treatment decisions, and away from using FEV1 for this purpose. This change of direction has enabled the formulation of a clearer set of recommendations for an individualized approach to treatment, including guidance on a stepwise treatment approach. These recommendations will no doubt be subject to scrutiny and criticism, particularly in areas where there is little or no supporting evidence. The treatment algorithm for GOLD D will probably be the mostly keenly debated, and the results of ongoing clinical trials in this subgroup will no doubt provide fuel for the fire.
Competing Interests
D.S. has received sponsorship to attend international meetings; honoraria for lecturing or attending advisory boards; and research grants from various pharmaceutical companies, including Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, Genentech, GlaxoSmithKline, Glenmark, Merck, NAPP, Novartis, Pfizer, Respivert, SkyPharma, Takeda, Teva, Theravance and Verona. He is a member of the GOLD science committee.
Singh, D. (2017) Pharmacological treatment for COPD; GOLD 2017 changes direction. Br J Clin Pharmacol, 83: 935–937. doi: 10.1111/bcp.13212.
References
- 1. Miravitlles M, Vogelmeier C, Roche N, Halpin D, Cardoso J, Chuchalin AG, et al. A review of national guidelines for management of COPD in Europe. Eur Respir J 2016; 47: 625–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vestbo J, Hurd SS, Agusti AG, Jones PW, Vogelmeier C, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2013; 187: 347–365. [DOI] [PubMed] [Google Scholar]
- 3. Singh D, Roche N, Halpin D, Agusti A, Wedzicha JA, Martinez FJ. Current controversies in the pharmacological treatment of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2016; 194: 541–549. [DOI] [PubMed] [Google Scholar]
- 4. Hurst JR, Vestbo J, Anzueto A, Locantore N, Müllerova H, Tal‐Singer R, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med 2010; 363: 1128–1138. [DOI] [PubMed] [Google Scholar]
- 5. Agusti A, Calverley PM, Celli B, Coxson HO, Edwards LD, Lomas DA, et al. Characterisation of COPD heterogeneity in the ECLIPSE cohort. Respir Res 2010; 11: 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dransfield MT, Bourbeau J, Jones PW, Hanania NA, Mahler DA, Vestbo J, et al. Once‐daily inhaled fluticasone furoate and vilanterol versus vilanterol only for prevention of exacerbations of COPD: two replicate double‐blind, parallel‐group, randomised controlled trials. Lancet Respir Med 2013; 1: 210–223. [DOI] [PubMed] [Google Scholar]
- 7. Soriano JB, Lamprecht B, Ramirez AS, Martinez‐Camblor P, Kaiser B, Alfageme I, et al. Mortality prediction in chronic obstructive pulmonary disease comparing the GOLD 2007 and 2011 staging systems: a pooled analysis of individual patient data. Lancet Respir Med 2015; 3: 443–450. [DOI] [PubMed] [Google Scholar]
- 8. Singh D. New combination bronchodilators for chronic obstructive pulmonary disease: current evidence and future perspectives. Br J Clin Pharmacol 2015; 79: 695–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bateman ED, Chapman KR, Singh D, D'Urzo AD, Molins E, Leselbaum A, et al. Aclidinium bromide and formoterol fumarate as a fixed‐dose combination in COPD: pooled analysis of symptoms and exacerbations from two six‐month, multicentre, randomised studies (ACLIFORM and AUGMENT). Respir Res 2015; 16: 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mahler DA, Decramer M, D'Urzo A, Worth H, White T, Alagappan VK, et al. Dual bronchodilation with QVA149 reduces patient‐reported dyspnoea in COPD: BLAZE study. Eur Respir J 2014; 43: 1599–1609. [DOI] [PubMed] [Google Scholar]
- 11. Singh D, Ferguson GT, Bolitschek J, Gronke L, Hallmann C, Bennett N, et al. Tiotropium + olodaterol shows clinically meaningful improvements in quality of life. Respir Med 2015; 109: 1312–1319. [DOI] [PubMed] [Google Scholar]
- 12. Singh D, Corradi M, Spinola M, Petruzzelli S, Papi A. Extrafine beclometasone diproprionate/formoterol fumarate: a review of its effects in chronic obstructive pulmonary disease. NPJ Prim Care Respir Med 2016; 26: 16030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Siddiqui SH, Guasconi A, Vestbo J, Jones P, Agusti A, Paggiaro P, et al. Blood eosinophils: a biomarker of response to extrafine beclomethasone/formoterol in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2015; 192: 523–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pavord ID, Lettis S, Locantore N, Pascoe S, Jones PW, Wedzicha JA, et al. Blood eosinophils and inhaled corticosteroid/long‐acting beta‐2 agonist efficacy in COPD. Thorax 2016; 71: 118–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wedzicha JA, Banerji D, Chapman KR, Vestbo J, Roche N, Ayers RT, et al. Indacaterol‐glycopyrronium versus salmeterol‐fluticasone for COPD. N Engl J Med 2016; 374: 2222–2234. [DOI] [PubMed] [Google Scholar]
- 16. Singh D, Papi A, Corradi M, Pavlisova I, Montagna I, Francisco C, et al. Single inhaler triple therapy versus inhaled corticosteroid plus long‐acting beta2‐agonist therapy for chronic obstructive pulmonary disease (TRILOGY): a double‐blind, parallel group, randomised controlled trial. Lancet 2016; 388: 963–973. [DOI] [PubMed] [Google Scholar]
- 17. Martinez FJ, Calverley PM, Goehring UM, Brose M, Fabbri LM, Rabe KF. Effect of roflumilast on exacerbations in patients with severe chronic obstructive pulmonary disease uncontrolled by combination therapy (REACT): a multicentre randomised controlled trial. Lancet 2015; 385: 857–866. [DOI] [PubMed] [Google Scholar]


