There are no approved pharmacologic therapies for nonalcoholic steatohepatitis (NASH), the leading cause of chronic liver disease worldwide. This study describes the use of FTY720, a novel small molecule, for the amelioration of NASH in a mouse model. We demonstrate that 2-wk administration of FTY720 to mice with NASH led to a reduction in liver injury, inflammation, and fibrosis. These data provide preclinical rationale for studying this drug in human NASH.
Keywords: fatty liver, FTY720, Kupffer cells, macrophage obesity
Abstract
Nonalcoholic steatohepatitis (NASH) is a lipotoxic disorder, wherein proinflammatory lipids, such as ceramide and its derivative sphingosine 1-phosphate (S1P), contribute to macrophage-associated liver inflammation. For example, we have previously demonstrated a role for S1P in steatotic hepatocyte-derived S1P-enriched extracellular vesicles in macrophage chemotaxis in vitro. Therefore, we hypothesized that FTY720, an S1P antagonist, would ameliorate NASH by inhibiting proinflammatory monocyte chemotaxis. To test our hypothesis, NASH was established in C57BL/6 male mice by feeding a diet high in fructose, saturated fat, and cholesterol for 22 wk. Then mice received daily intraperitoneal injections of FTY720 for 2 wk before analysis of liver injury, inflammation, and fibrosis. FTY720-treated mice with NASH demonstrated improved liver histology with a significant reduction in hepatocyte ballooning and inflammatory foci. Hepatomegaly was reversed, and liver triglycerides were reduced following FTY720 administration to mice with NASH. Correspondingly, serum ALT levels, hepatic inflammatory macrophage accumulation, and the expression of Ly6C in recruited myeloid cells was reduced in FTY720-treated mice. Hepatic collagen accumulation and expression of α-smooth muscle actin were significantly lowered as well. Body composition, energy consumption and utilization, and hepatic sphingolipid composition remained unchanged following FTY720 administration. FTY720 ameliorates murine nonalcoholic steatohepatitis. Reduction in liver injury and inflammation is associated with a reduction in hepatic macrophage accumulation, likely due to dampened recruitment of circulating myeloid cells into the liver. Nonalcoholic steatohepatitis may be a novel indication for the therapeutic use of FTY720.
NEW & NOTEWORTHY There are no approved pharmacologic therapies for nonalcoholic steatohepatitis (NASH), the leading cause of chronic liver disease worldwide. This study describes the use of FTY720, a novel small molecule, for the amelioration of NASH in a mouse model. We demonstrate that 2-wk administration of FTY720 to mice with NASH led to a reduction in liver injury, inflammation, and fibrosis. These data provide a preclinical rationale for studying this drug in human NASH.
nonalcoholic steatohepatitis (NASH) characterized by hepatic inflammation, can result in progressive liver injury with resultant fibrosis, cirrhosis, and hepatocellular carcinoma (33). NASH is viewed under the umbrella of nonalcoholic fatty liver disease (NAFLD), which is the hepatic component of the obesity-associated metabolic syndrome. It is well recognized that inflammation imparts fibrosis risk, and the latter determines patient prognosis in NASH (1, 8). Macrophage-associated sterile innate inflammation is implicated in the progression of NASH (29). The proportion of proinflammatory monocyte-derived macrophages increases significantly in the liver following high-fat feeding (28), whereas, resident hepatic macrophages or Kupffer cells remain unchanged. This supports a role for the active recruitment of bone marrow-derived, circulating proinflammatory monocytes to the liver. We have previously shown that lipid mediators on extracellular vesicles derived from steatotic hepatocytes are chemoattractive to macrophages (13). However, in spite of some understanding of the importance of inflammatory cell recruitment in NASH pathogenesis, there are no regulatory agency-approved pharmacological therapies for NASH patients. Therefore, exploring rational therapeutic avenues is necessary.
NASH is viewed as a lipotoxic disorder, such that alterations in lipid homeostasis generate injurious lipid mediators (13, 24). Palmitic acid, one such lipid mediator, while physiologically abundant, increases in the liver and plasma in NASH patients (30). Palmitate is a building block for sphingolipids, via the formation of C16:0 ceramide in the de novo synthesis pathway (21). Perturbations in sphingolipid levels are associated with human and murine NASH (14, 19, 32, 35). Recent studies have started to elucidate the mechanisms by which elevated sphingolipids, such as C16:0 ceramide, lead to liver damage (32, 35). Ceramides and sphingosine 1-phosphate (S1P) are interlinked via metabolic pathways, such as the formation of sphingosine 1-phosphate from ceramide by the activity of ceramidase and sphingosine kinase, and S1P serving as a substrate for the generation of ceramide by a salvage pathway via the activity of S1P phosphatase and ceramide synthase (5). S1P is a potent signaling lipid, elevated in the circulation in obesity-associated disorders, and is known to have immune-modulatory effects (19, 22, 36). Five known plasma membrane G protein-coupled receptors designated S1P receptors 1–5 (S1P1, S1P2, S1P3, S1P4, and S1P5) mediate S1P signaling. S1P1 is expressed in cells of the immune system and is abundant in macrophages (34). We have previously demonstrated that macrophage chemotaxis in vitro is mediated by S1P1 (13); thus, antagonizing this signaling pathway may be of potential benefit in NASH.
FTY720 (Fingolimod), a derivative of myriocin, is a first-in-class small-molecule inhibitor of sphingosine 1-phosphate signaling (7). It is a substrate for sphingosine kinase, leading to the formation of phosphorylated FTY720, which competitively binds S1P receptors, leading to their internalization, degradation, and eventual downregulation—a process best described for S1P1. Thus, although pharmacologically an agonist, it acts as a functional antagonist by receptor downregulation. It is known to have immune modulatory properties, inhibiting lymphocyte egress from lymph nodes and is currently approved for the treatment of multiple sclerosis. We asked whether antagonism of S1P signaling would be beneficial in NASH? We approached this question by administering this drug to C57BL/6 male mice with established dietary obesity and NASH due to consuming a “Western diet” supplemented with high fructose corn syrup in their drinking water. Herein, we report that FTY720 therapy, in a mouse NASH model, is beneficial in reversing cardinal features of NASH, namely, hepatocyte ballooning and liver inflammation. This is associated with a reduction in macrophage-mediated inflammation and decreased recruitment of proinflammatory monocyte-derived macrophages into the liver.
MATERIALS AND METHODS
Mouse studies.
All animal use was approved by the Institutional Care and Animal Use Committee of the Mayo Clinic and was conducted in accordance with the public health policy on the humane use and care of laboratory animals. Twenty 9-wk-old C56BL/6J male mice were purchased from Jackson Laboratory (Bar Harbor, ME) and were acclimated for 3 wk. Mice were randomized to one of four groups, and mouse cages were set up during the acclimatization period during which all mice received standard rodent chow. One mouse died due to anesthesia during ear marking for identification before the start of the feeding study. The remaining 19 were all included in the study and subsequent analyses. At 12 wk of age, mice in the control groups continued to receive rodent chow (chow diet; CD), whereas the fat, fructose, and cholesterol (FFC) diet groups were switched to a Western diet, which provides 40% kcal from fat and is composed of 34% sucrose, 20% milk fat, and 0.15% cholesterol [AIN-76A Western Diet (originally manufactured as D12079B); TestDiet, St. Louis, MO] for 24 wk. The complete formulation of this diet is available via the manufacturer’s website (http://www.testdiet.com/cs/groups/lolweb/@testdiet/documents/web_content/mdrf/mdux/~edisp/ducm04_051601.pdf). This was supplemented with high fructose corn syrup (23.1 g/l fructose and 18.9 g/l glucose) in their drinking water. We have referred to this diet as the FFC diet and have previously described it to reproduce features of human NASH, such as obesity, insulin resistance, and progressive liver injury and inflammation (12). Mice had unrestricted access to food and water and were housed in standard pathogen-free facilities with 12:12-h day-night circadian cycles. There were three cages of FFC-fed mice housed 3–5 per cage, and three cages of chow-fed mice housed 2–5 per cage. Mice within one cage per diet received either saline or FTY720. Twenty-two weeks into the feeding study, therapy with FTY720 or saline was begun. FTY720 was obtained from Selleckchem (Houston, TX) and dissolved in sterile water at a concentration of 500 μg/ml. Mice received daily intraperitoneal injections of either FTY720 (1 mg/kg) or saline for 14 days, while they continued to be on their respective diets. We administered FTY720 at a dose of 1 mg/kg ip, consistent with the doses used in other studies, which span a wide range from 0.04 to 3 mg/kg (15, 17, 27, 34). As demonstrated using an S1P1 reporter mouse, FTY720 injected at 1 mg/kg ip binds hepatic S1P1 (18). Upon completion of the study, mice were euthanized following a 6-h fast. Platelet-poor plasma was isolated from heparinized blood collected by cardiac puncture by centrifugation at 1,200 g for 20 min at room temperature, followed by 13,000 g at 4°C for 2 min. Plasma was stored at −20°C until further analyses. The liver was excised, weighed, and apportioned for downstream analyses, including RNA extraction, protein extraction, formalin fixation, and paraffin embedding for histology, and cryosectioning.
Measurement of food intake, body composition, physical activity, and indirect calorimetry.
Metabolic parameters in individual mice were measured as previously described (20). Briefly, lean mass and fat mass of individual mice were quantified using computed tomography (EchoMRI; LaTheta, Houston, TX) and were expressed relative to body weight. The comprehensive laboratory animals monitoring system (CLAMS), equipped with an Oxymax Open Circuit Calorimeter System (Columbus Instruments, Columbus, OH), was used to measure oxygen consumption (V̇o2) and carbon dioxide production (V̇co2) of individual mice. These values were used to calculate the respiratory exchange ratio and basal metabolic rate. Resting, activity and total energy expenditure, food intake, and heat production were also assessed as described previously (20). Physical activity of individual mice was measured in the horizontal (ambulation) and vertical (rearing) planes for a 48-h period in the CLAMS using photocells.
Histologic analyses.
Five-micrometer sections of formalin-fixed, paraffin-embedded liver tissues were stained with hematoxylin and eosin using standard techniques and used for histologic grading, according to the NAFLD activity score (16). Fibrosis was assessed by Sirius red staining in 5-µm liver sections, as described by us in detail (25). Immunohistochemistry for Mac-2 (14–5301, 1:200 dilution; eBiosciences, San Diego, CA), Ly6C (ab15627; Abcam, Cambridge, MA), α-smooth muscle actin (αSMA) (ab 124964; 1:100; Abcam), and collagen 1 (ab 34710, 1:50; Abcam) was performed to identify macrophages, proinflammatory monocyte-derived macrophages, activated hepatic stellate cells, and collagen, respectively, using the ABC immunostaining kit (Vector Laboratories, Burlingame, CA), as per the manufacturer’s protocol. Briefly, liver sections were dewaxed in two changes of xylene and rehydrated through graded alcohols. Antigen retrieval was performed by heating slides at 95°C for 30 min in 10 mM sodium citrate, pH 6.0, followed by a peroxidase block, avidin and biotin block, and a block of nonspecific binding sites with rabbit serum diluted in PBS, according to the manufacturer’s instruction. The primary antibody was applied overnight at 4°C in a humidified chamber, followed by biotin-conjugated secondary antibody, and streptavidin-conjugated horseradish peroxidase, according to the manufacturer's instructions (ABC; Vector Laboratories). Avidin-biotin conjugates were visualized using a peroxidase substrate kit (Vector Laboratories). Dehydrated sections were mounted using Permount mounting media (Sigma, St. Louis, MO). The positive areas were captured using the NIS-Elements software (Nikon, Tokyo, Japan) attached to a Nikon microscope mounted with a Nikon DXM 1200F camera (Nikon). Images with uniform settings of magnification, light, and exposure time were used for quantitative image analysis.
Coherent anti-Stokes Raman scattering microscopy.
Coherent anti-Stokes Raman scattering (CARS) microscopy was performed on 5-µm thick label-free frozen liver tissue sections on a two-photon confocal microscope (FluoView FV1000 MPE, Olympus) using the CARS application. Mai Tai DeepSea laser (Spectra-Physics, Santa Clara, CA) was tuned to 800 nm and an XLPlanN 25 × /1.05w MP objective lens was used. For accumulated triglyceride, percent area quantification of the CARS images was performed using National Institutes of Health ImageJ software. Four animals per group and at least 10 pictures for each animal were examined.
Extracellular vesicle isolation.
Plasma extracellular vesicles (EVs) were isolated by differential ultracentrifugation, as previously described in detail (13). Briefly, 200 μl of mouse plasma was diluted with an equal volume of PBS, centrifuged at 2,000 g for 30 min at 4°C. Following this, the supernatant was transferred to new tubes¸ centrifuged at 12,000 g for 30 min, and 110,000 g for 120 min. The pelleted EVs were dissolved in PBS and stored at −80°C until downstream analyses.
Nanoparticle tracking analysis.
The Nanosight nanoparticle tracking analysis NS300 (Malvern Instruments, Malvern, UK) was used to measure the size and concentration of EVs, as previously described (13). EV samples were diluted in PBS and measured within the linear dynamic range of the instrument by recording the light scatter and Brownian motion of each sample of nanoparticles three times, 30 s each, at constant room temperature (22.5°C); particle tracks were analyzed by NanoSight software to measure the concentration of the particles (particles/ml) and size (in nanometers).
Cell isolation and culture.
Primary mouse hepatocytes were isolated from three male mice using a two-step collagenase perfusion method, as previously described (9). Greater than 95% viable isolated hepatocytes were plated in Waymouth’s medium supplemented with 10% FBS with 100 units/ml penicillin and 100 μg/ml streptomycin. Hepatocytes were treated with FTY720, as described below. Murine bone marrow-derived macrophages (BMDM) were isolated and differentiated, as previously described by us (25), and treated with FTY720, as described below. LX-2 cells were maintained in DMEM supplemented with 10% FBS with 100 units/ml penicillin and 100 μg/ml streptomycin. For activation studies, cultured LX-2 cells were washed twice with PBS, placed in serum-free medium for 1 h, following which, cells were activated by treatment with transforming growth factor-β (TGF-β) (R&D Systems, Minneapolis, MN) at a concentration of 10 ng/ml for 1 h. TGF-β-activated cells were treated with FTY720, as described below. BMDM were washed twice with PBS and placed in serum-free medium for 1 h, and then stimulated with LPS at a concentration of 50 ng/ml in RPMI with 2% FBS. One hour after LPS stimulation, cells were treated with FTY720, as described below. An aqueous solution of FTY720 was used at final concentrations of 250 nM before measuring gene expression or apoptosis; sterile water was added to vehicle-treated cells. We used 250 nM FTY720 in our in vitro studies, as we have previously shown that this concentration inhibits macrophage chemotaxis (13). For gene expression analysis, cells were homogenized in TRIzol reagent and RNA extracted using the Quick-RNA micro prep kit (Zymo, Irvine, CA) for LX-2 cells and the Direct-zol RNA kit (Zymo) for BMDM per the manufacturer’s instructions. Quantitative real-time PCR (qPCR) was performed as described below.
Apoptosis assessment.
DAPI was added to cells at a concentration of 2 μg/ml; nuclear morphology was assessed as described by us (23). Three hundred or more total cells were counted per condition, and each experiment was repeated three times.
Quantitative real-time PCR.
Liver tissue preserved in RNA later was recovered and homogenized in TRIzol reagent using a hand-held homogenizer in 1 ml TRIzol per 15–20 mg of liver tissue. The aqueous phase after the addition of 200 μl of chloroform and centrifugation was transferred to new Eppendorf tubes. After the addition of an equal volume of ethanol, the mixture was applied to the RNA isolation column (Zymo), and RNA was extracted according to the manufacturer’s instructions. An in-column DNase digestion step was also performed. Isolated RNA quantity and quality of RNA were assessed with a NanoDrop ND1000 (ThermoScientific, Waltham, MA). Reverse transcription was performed with the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA). Quantitative real-time PCR (qPCR) reactions were run on the LightCycler 480 (Roche, Minneapolis, MN), using the LightCycler 480 SYBR Green 1 Master Mix (Roche). Murine primers are summarized in Table 1, and human primers are summarized in Table 2. 18S and hypoxanthine-guanine (HPRT) were used as murine reference genes, and β-actin was used as a human reference gene to normalize target genes of interest. All data are expressed as fold change relative to chow-fed saline-treated mice or vehicle-treated cells.
Table 1.
Mouse qPCR primer sequences
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| 18S | CGCTTCCTTACCTGGTTGAT | GAGCGACCAAAGGAACCATA |
| HPRT | TCCTCCTCAGACCGCTTTT | TCCTCCTCAGACCGCTTTT |
| αSMA | GTCCCAGACATCAGGGAGTAA | TCGGATACTTCAGCGTCAGGA |
| Col1α1 | CTGGCGGTTCAGGTCCAAT | TTCCAGGCAATCCACGAGC |
| Osteopontin | AGCAAGAAACTCTTCCAAGCAA | GTGAGATTCGTCAGATTCATCCG |
| CTGF | GGGCCTCTTCTGCGATTTC | ATCCAGGCAAGTGCATTGGTA |
| PDGF-BB | AAGTGTGAGACAATAGTGACCCC | CATGGGTGTGCTTAAACTTTCG |
| MMP13 | CTTCTTCTTGTTGAGCTGGACTC | CTTCTTCTTGTTGAGCTGGACTC |
| CCR2 | ATCCACGGCATACTATCAACATC | CAAGGCTCACCATCATCGTAG |
| Ly6C | GCAGTGCTACGAGTGCTATGG | ACTGACGGGTCTTTAGTTTCCTT |
| MAC2 | TGGGCACAGTGAAACCCAAC | TCCTGCTTCGTGTTACACACA |
| Mcp1 | TTAAAAACCTGGATCGGAACCAA | GCATTAGCTTCAGATTTACGGGT |
| Mip1α | TTCTCTGTACCATGACACTCTGC | CGTGGAATCTTCCGGCTGTAG |
| TIMP-1 | AGGTGGTCTCGTTGATTTCT | GTAAGGCCTGTAGCTGTGCC |
| CD1d | GTCCCAGGGCAAGTTGAG | GACTGTCTGCCAGGACGTTC |
| CD11c | CTGGATAGCCTTTCTTCTGCTG | GCACACTGTGTCCGAACTCA |
| CD38 | TCTCTAGGAAAGCCCAGATCG | GTCCACACCAGGAGTGAGC |
| CD69 | TGGTCCTCATCACGTCCTTAATAA | TCCACCTTCTCGTACAAGCCTG |
| CD207 | CCGAAGCGCACTTCACAGT | GCAGATACAGAGAGGTTTCCTCA |
| NKp46 | ATGCTGCCAACACTCACTG | ATGATGGGTTTCGGGAGAGTC |
| CD45R | ACCACCAGGTGAATGTCAATTT | CTTGCTTTCCCTCGGTTCTTT |
| Ly6G | GACTTCCTGCAACACAACTACC | ACAGCATTACCAGTGATCTCAGT |
| CD4 | AGGTGATGGGACCTACCTCTC | GGGGCCACCACTTGAACTAC |
| CD8 | CCGTTGACCCGCTTTCTGT | CGGCGTCCATTTTCTTTGGAA |
| Lass2 | ATGCTCCAGACCTTGTATGACT | CTGAGGCTTTGGCATAGACAC |
| Lass4 | TACCCACATCAGACCCTGAAT | TGAAGTCCTTGCGTTTGACATC |
| Lass6 | GATTCATAGCCAAACCATGTGCC | AATGCTCCGAACATCCCAGTC |
qPCR, quantitative PCR.
Table 2.
Human qPCR primer sequences
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| β-actin | CATGTACGTTGCTATCCAGGC | CTCCTTAATGTCACGCACGAT |
| αSMA | GGAGATCACGGCCCTAGCAC | AGGCCCGGCTTCATCGTAT |
| Col1α1 | TGTGAGGCCACGCATGAG | CAGATCACGTCATCGCACAA |
| CTGF | CAGCATGGACGTTCGTCTG | AACCACGGTTTGGTCCTTGG |
| PDGF-BB | TCCCGAGGAGCTTTATGAGA | ACTGCACGTTGCGGTTGT |
| MCP1 | CAGCCAGATGCAATCAATGCC | TGGAATCCTGAACCCACTTCT |
| IL-1β | ATGATGGCTTATTACAGTGGCAA | GTCGGAGATTCGTAGCTGGA |
Biochemical assays.
Plasma ALT levels were measured using a commercial veterinary chemistry analyzer (VetScan 2; Abaxis, Union City, CA). Triglycerides were measured in liver homogenates prepared in PBS and 0.5% sodium deoxycholate with the Infinity Triglyceride reagent (Fisher Scientific, Hampton, NH) using a protocol adapted from Miao et al. (26).
Lipidomics.
Sphingolipids were extracted from ~5 mg of liver tissue, 25 μl of plasma, or EVs isolated from 200 μl of plasma and measured using tandem mass spectrometry at the Mayo Clinic Metabolomics Core Laboratory, as previously described by us (13). Liver sphingolipids were normalized to protein content. Circulating EV S1P and C16:0 ceramide content were a product of S1P or C16:0 per EV and the number of circulating EVs.
Statistical and data analyses.
Data are presented as means ± SE. Each dot represents data from one mouse for the in vivo data and an independent biologic replicate for the in vitro data presented as dot plots. The Student's two-tailed t-test was used for comparing groups. In instances in which a one-tailed t-test was used, the # sign denotes a P < 0.05. Statistical analyses were performed in GraphPad Prism version 6.00 for Windows (GraphPad Software, La Jolla, CA; www.graphpad.com). A P value of <0.05 was considered significant. Cell culture data are from three distinct biologic replicates.
RESULTS
FTY720 treatment decreased liver injury in FFC-fed mice without altering hepatic sphingolipids or metabolic parameters.
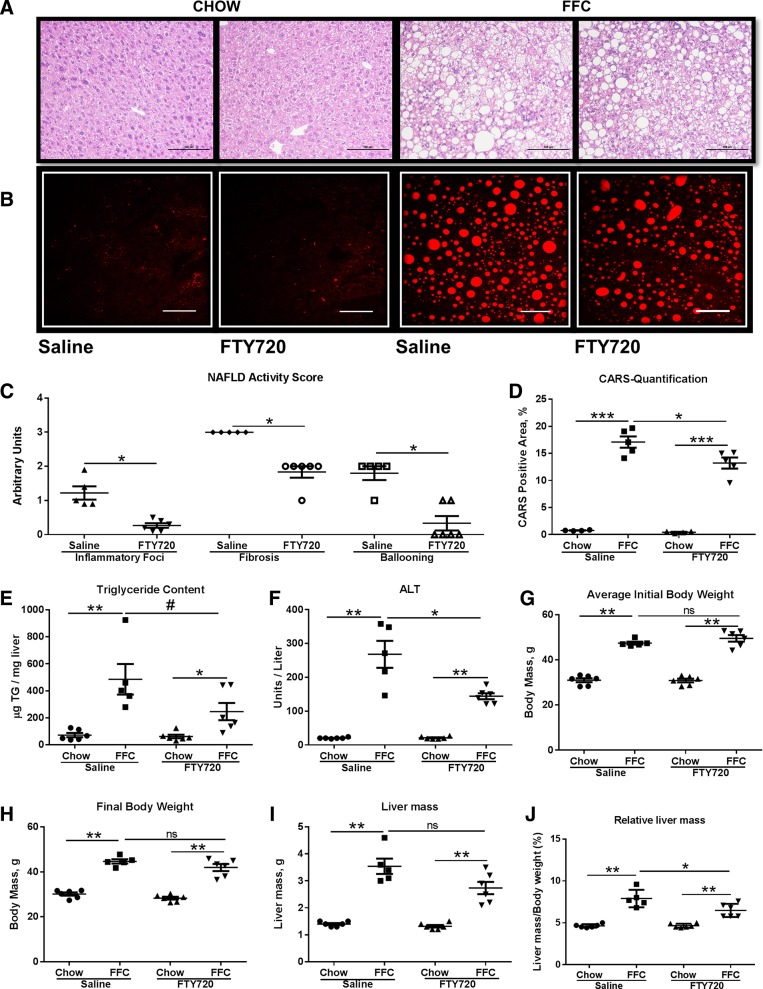
We explored the therapeutic efficacy of FTY720 in a mouse model of NASH generated by feeding mice a FFC diet (high in saturated fat, fructose, and cholesterol) (4). Examination of liver histology of FFC-fed saline-treated mice (control group) demonstrated significant steatosis, hepatocyte ballooning, and parenchymal inflammatory infiltrates, as expected (Fig. 1, A and C). However, FTY720-treated FFC-fed mice (treated group) demonstrated an improvement in hepatocyte ballooning and parenchymal inflammatory infiltrates (Fig. 1A). Histologic parameters were quantified according to the NAFLD activity score (NAS), which showed improved ballooning, inflammation, and fibrosis scores in the treated group, as compared with the control group (Fig. 1C). The NAS scoring system is a categorical staging system, which scores each component as a discontinuous variable and, therefore, lacks discrimination (e.g., 67% steatosis and 97% steatosis, both of which would be scored as stage 3 steatosis). Therefore, to get a quantitative assessment of steatosis, we employed label-free CARS microscopy (Fig. 1, B and D). We noted a reduction in both large and small lipid droplets in the treated mice. There was a 27% reduction in the CARS-positive area in the treated mice (12.8%), as compared with the control mice (17.5%). We also measured liver triglyceride content biochemically across groups. We saw a reduction in hepatic triglyceride content of treated vs. control group livers (Fig. 1E). Histologic improvement in the treated group was associated with a reduction in serum alanine aminotransferase (ALT) (Fig. 1F). The improvement in liver injury was not due to weight loss in the treated group, as both the control group and treated group had comparable initial and final body weights (Fig. 1, G and H). Furthermore, terminal liver mass was comparable between the treated group and control group (Fig. 1I). When normalized to body mass, the relative liver mass was reduced in the treated group (Fig. 1J). Thus, FTY720-treated mice are protected from liver injury and have a reduction in relative liver mass, likely due to a reduction in hepatic triglyceride content.
Fig. 1.
FTY720 treatment decreases liver injury. A: representative hematoxylin-and-eosin-stained liver photomicrographs from chow-fed mice treated with either saline or FTY720 or FFC-fed mice treated with either saline or FTY720 are shown. Scale bar: 100 μm. B: representative CARS photomicrographs from chow-fed mice treated with either saline or FTY720 or FFC-fed mice treated with either saline or FTY720 are shown. Scale bar: 100 μm. C: components of NAFLD activity score consisting of inflammatory foci, fibrosis, and hepatocyte ballooning for FFC-fed saline treated (n = 5) and FFC-fed FTY720-treated mice (n = 6) are shown. *P < 0.05. D: quantification of CARS-positive area from chow-fed mice (n = 4) treated with either saline or FTY720 or FFC-fed mice (n = 5) treated with either saline or FTY720. *P < 0.05, ***P < 0.001. E: liver triglyceride (TG) content measured biochemically in all four groups, *P < 0.05, **P < 0.01, #P < 0.05. Serum ALT (F), initial body weight (G), final body weight (H), liver mass (I), and relative liver mass (J)for the four experimental groups are shown. *P < 0.05, **P < 0.01, ns, not significant.
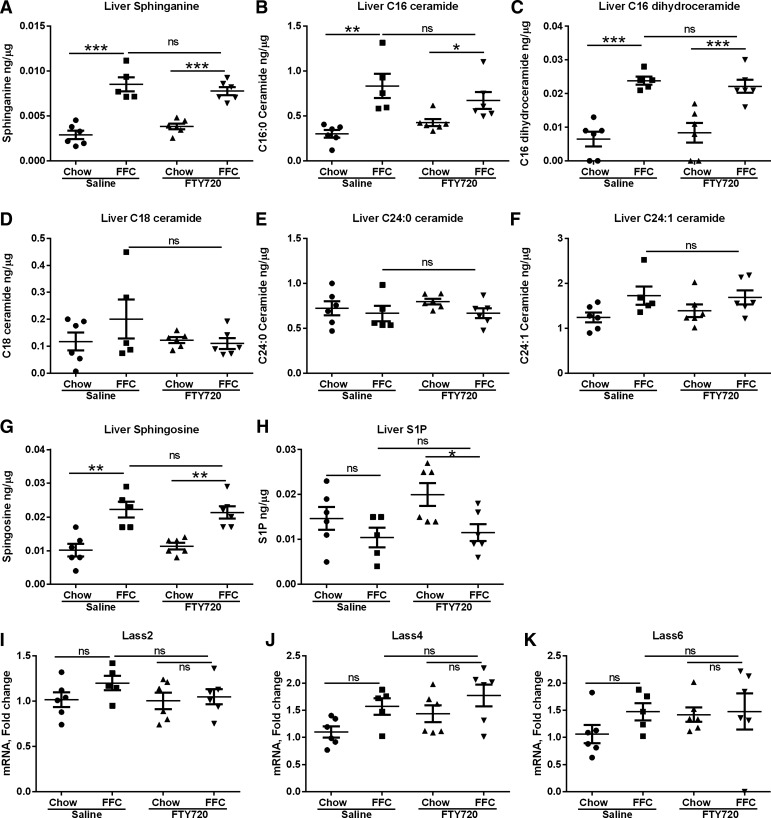
Inhibition of ceramide synthases by FTY720 with resultant tissue depletion of ceramides, dihydroceramides, sphingosine, and S1P has been described previously (2). Given emerging data on the toxicity of accumulated C16:0 ceramide in the liver, we next performed an unbiased tandem mass spectroscopic analysis of the sphingolipidomic content of liver samples from all four groups of mice to determine whether the beneficial effect of FTY720 in mouse NASH was secondary to a reduction in harmful ceramides. When compared with chow-fed mice, FFC feeding led to an increase in sphinganine (Fig. 2A), C16:0 ceramide (Fig. 2B), and C16 dihydroceramide (Fig. 2C); however, there were no differences in these lipids between the control group (FFC-saline) and the treated group (FFC-FTY). We observed no differences between C18:0 ceramide, C24:0 ceramide, and C24:1 ceramide across all four groups (Fig. 2, D–F). There was an increase in liver sphingosine in FFC-fed mice as compared with the chow-fed mice (Fig. 2G), but no changes between the control and treated groups. Liver S1P was depleted in the treated group (FFC-FTY) mice compared with chow-fed FTY720-treated mice (Fig. 2H); however, there was no significant difference between the control group and treated group. The hepatic expression of ceramide synthases that are enriched in the liver (Lass 2, Lass 4, and Lass 6) were unchanged across groups (Fig. 2, I–K) (21, 32, 35). Therefore, the salutary effects of FTY720 on NASH in this study are not secondary to significant changes in hepatic ceramide and sphingolipid content.
Fig. 2.
FFC-feeding increases hepatic sphingolipids, which remain unchanged with FTY720 administration. Liver sphinganine (A), C16:0 ceramide (B), C16 dihydroceramide (C), C18:0 ceramide (D), C24:0 ceramide (E), C24:1 ceramide (F), sphingosine (G), and sphingosine 1-phosphate (S1P; H) were measured by tandem mass spectroscopy and normalized to protein content. Liver expression of ceramide synthases, Lass2 (I), Lass4 (J), and Lass6 (K) was measured by quantitative PCR (qPCR). *P < 0.05, **P < 0.01, ***P < 0.001.
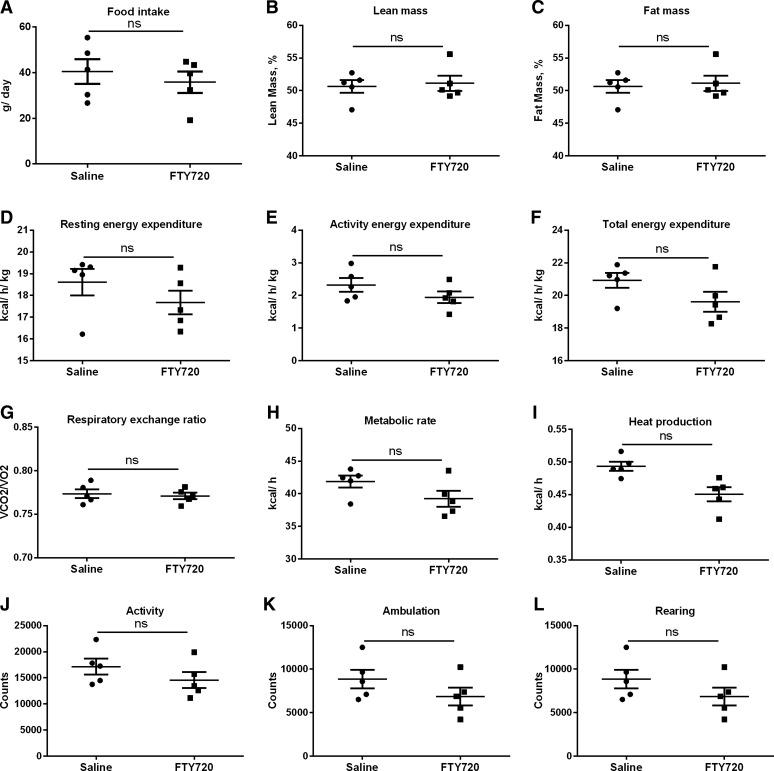
To determine whether changes in food intake and metabolic parameters contributed to the improved liver injury observed in the FTY720-treated mice, a cohort of FFC-fed mice was subjected to comprehensive assessment of metabolic parameters. As demonstrated in Fig. 3, there were no changes in food intake, lean mass percent, fat mass percent, resting energy expenditure, activity energy expenditure, total energy expenditure, respiratory exchange ratio, metabolic rate, heat production, and total activity levels. Therefore, improved liver injury was not secondary to general metabolic improvement in FTY720-treated FFC-fed mice (treated group), compared with the control group.
Fig. 3.
Metabolic parameters remain unchanged in FTY720 treated FFC-fed mice. Food intake (A), lean mass (B), fat mass (C), resting energy expenditure (D), activity energy expenditure (E), total energy expenditure (F) respiratory exchange ratio (G), metabolic rate (H), heat production (I), activity (J), ambulation (H), and rearing (L) were measured in saline-treated FFC-fed and FTY720-treated mice (n = 5 each). Metabolic parameters were measured for 24 h each under fed and fasted conditions. ns, not significant.
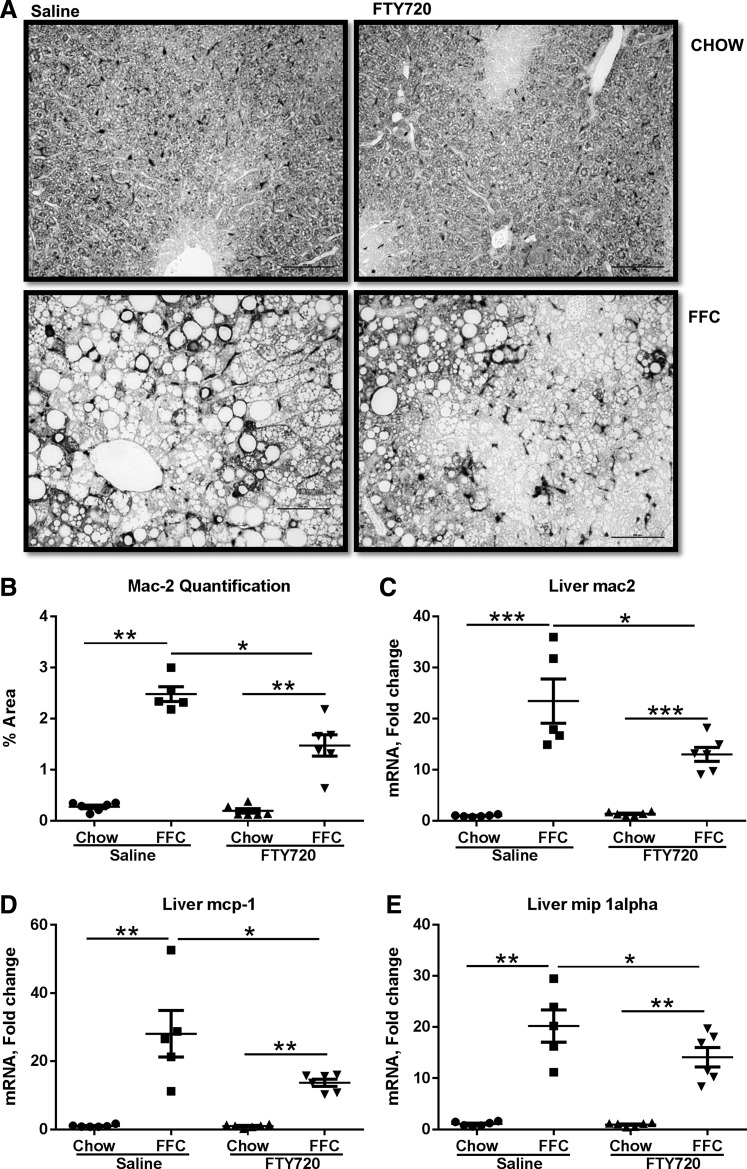
Hepatocyte macrophage accumulation is reduced in FTY720-treated FFC-fed mice.
Proinflammatory macrophage activation is a key contributor to liver injury in NASH (28). Therefore, we next turned our attention to assessment of macrophage-mediated inflammation in FTY720-treated mice. We found an increase in Mac-2 expression in the control group (FFC-saline), indicating a proinflammatory macrophage maturation (6), whereas, there was a reduction in the accumulation of Mac-2-positive macrophages by immunohistochemistry in the treated group (Fig. 4A). This was quantified for all of the mice in the experimental groups and confirmed a significant reduction in macrophage accumulation in the treated group compared with the control group (Fig. 4B) by immunohistochemistry. Consistently, the hepatic mRNA abundance of Mac2 was significantly reduced in the treated group (Fig. 4C). The reduction in hepatic macrophage accumulation was further associated with a reduction in the hepatic expression of two key monocyte-attracting chemokines, monocyte chemotactic protein 1 (MCP1), and macrophage inflammatory protein 1-α (MIP1α) (Fig. 4, D and E) in the treated group compared with the control group. Thus, the FFC-fed mice treated with FTY720 had a reduction in macrophage accumulation in the liver and a reduction in proinflammatory chemokines.
Fig. 4.
Macrophage accumulation in reduced in FTY720-treated FFC-fed mice. A: representative images from Mac-2 immunohistochemistry are shown, scale bar 100 μM. B: quantification of Mac-2-positive area. C: liver mRNA expression of Mac-2 from each of the four groups is shown. *P < 0.05, **P < 0.01, ***P < 0.001. Monocyte chemotactic protein 1 (Mcp-1) (D) and macrophage inflammatory protein 1α (Mip1α) (E) mRNA expression in livers from each of the four experimental groups is shown, *P < 0.05, **P < 0.01, ***P < 0.001.
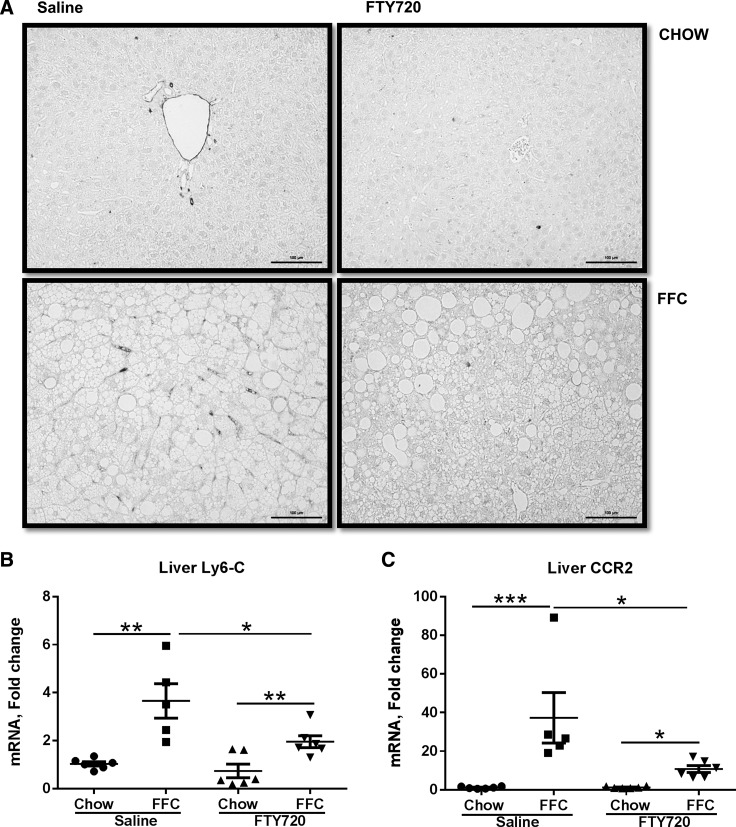
FTY720 impairs recruited myeloid cell accumulation in FFC-fed mice.
Proinflammatory monocytes are recruited to injured tissues in response to recruiting signals such as chemokines. These inflammatory monocyte-derived macrophages express the marker Ly6C, which can be used to identify and quantify monocyte-derived macrophages in the liver (28). We observed scant Ly6C positivity in the chow-fed mice (Fig. 5A). The FFC-fed control group mice had significant Ly6C positivity, which was reduced in the treatment group (Fig. 5A). We quantified this by measuring the liver expression of Ly6C mRNA, and this was correspondingly reduced in the treatment group (Fig. 5B). Ly6C-expressing inflammatory monocyte-derived macrophages also express C-C chemokine receptor type 2 (CCR2), a receptor for the chemokine MCP-1, through which they respond to MCP-1. Therefore, we next measured expression of CCR2, and similar to Ly6C expression, CCR2 was reduced in the treatment group (Fig. 5C). We did not detect any significant differences in dendritic cell markers (CD11c, CD207), NK cell markers (NKp46, CD69, NK1.1), NK T cell marker (CD1d, NK1.1), neutrophil marker (Ly6G), T-cell markers (CD4, CD8, CD69, CD38), and B-cell markers (CD38, CD45R), (data not shown).
Fig. 5.
Proinflammatory monocyte-derived macrophages are reduced in FFC-fed FTY720-treated mice. A: representative images from Ly6C immunohistochemistry are shown (scale bar: 100 μM). B: quantification of liver Ly6C mRNA expression. C: liver C-C chemokine receptor 2 (CCR2) mRNA expression in each of the four experimental groups is shown, *P < 0.05, **P < 0.01, ***P < 0.001.
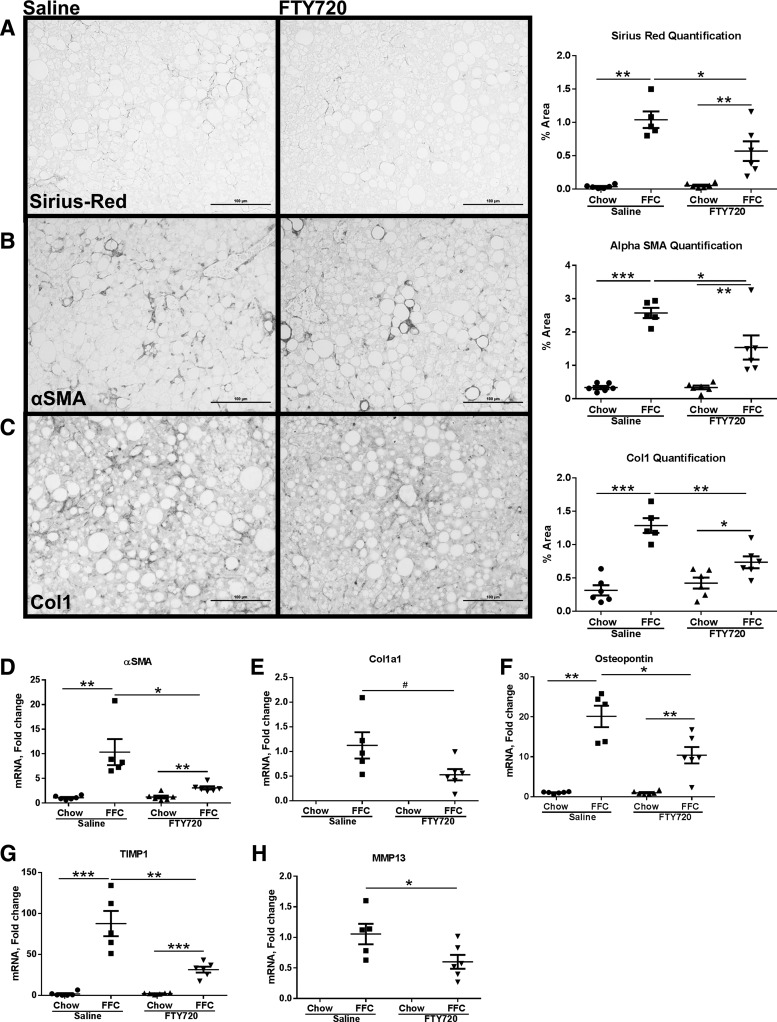
FFC diet induces modest fibrosis, which is reduced by FTY720 treatment.
Hepatic fibrosis is a sequela of chronic liver injury and inflammation; therefore, we asked whether FTY720 treatment reduced fibrosis secondary to the observed reduction in liver injury and inflammation. Fibrosis was assessed by Picrosirius red staining for collagen accumulation in the liver complemented by immunohistochemistry for αSMA and Col1. We observed modest fibrosis at 24 wk of the FFC diet feeding. This was manifest as the typical pericellular (chicken wire) fibrosis observed in murine and human NASH (Fig. 6A) in the control groups (FFC-fed, saline-treated) livers. This was reduced in the treated group, as shown in the representative photomicrographs and quantitative morphometry. Similarly, there was a reduction in αSMA and Col1 expression in the treated mice compared with the control mice (Fig. 6, B and C). Additionally, there was a reduction in the expression of hepatic stellate cell activation and fibrosis genes in the liver. Specifically, the expression of αSMA, a marker of activated hepatic stellate cells, Col1α1, osteopontin, tissue inhibitor of metalloproteinases 1 (TIMP1), and activated stellate cell-derived matrix metalloproteinase 13 (MMP13) were significantly reduced in the treated group compared with the control group (Fig. 6, D–H). However, the data on fibrosis should be interpreted cautiously, as the fibrosis observed in this diet is modest and quantification of small numbers can exaggerate small changes.
Fig. 6.
Liver fibrosis is reduced in FTY720-treated FFC-fed mice. A: representative images from Picrosirius red-stained liver sections from saline-treated and FTY720-treated FFC-fed mice (left; scale bar: 100 μM). Quantification of Picrosirius red-positive area from all four experimental groups is shown on the right. B: representative images from immunohistochemistry for α-smooth muscle actin (αSMA) from saline-treated and FTY720-treated FFC-fed mice liver sections (left; scale bar: 100 μM). Quantification of αSMA-positive area from all four experimental groups is shown on the right. C: representative images from immunohistochemistry for collagen 1(Col1) from saline-treated and FTY720-treated FFC-fed mice liver sections (left; scale bar: 100 μM). Quantification of αSMA-positive area from all four experimental groups is shown on the right. Chow-fed saline-treated and FTY720-treated mice had only minimal normal levels of positivity for Picrosirius red, αSMA, and Col1; therefore, their photomicrographs were omitted. They were included in the quantitate morphometry. Liver mRNA expression of αSMA (D), collagen 1α1 (Col1α1) (E), osteopontin (F), tissue inhibitor of metalloproteinase 1 (Timp1) (G), and matrix metalloproteinase 13 (MMP13) (H) from each of the four groups is shown. Col1α1 and MMP13 mRNAs were not detected in chow-fed mice. #P < 0.05, *P < 0.05, **P < 0.01, ***P < 0.001.
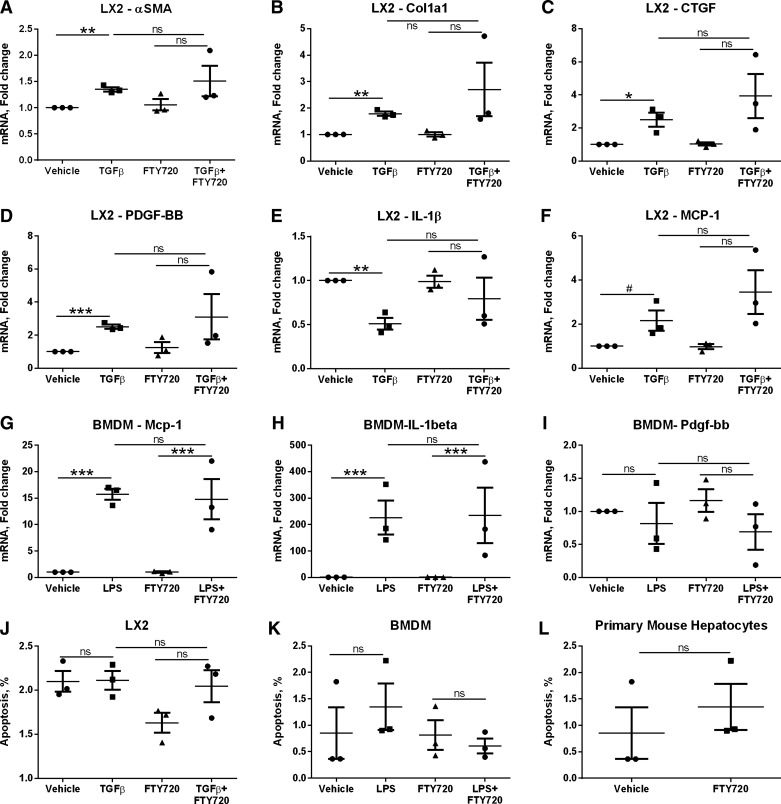
We next asked whether FTY720 directly affected the activation responses of hepatic stellate cells and BMDM (Fig. 7). We found no reduction in TGF-β-stimulated gene expression measured by αSMA, Col1α1, connective tissue growth factor, platelet-derived growth factor BB by FTY720 in LX-2 hepatic stellate cells (Fig. 7, A–D), nor a reduction in the proinflammatory mediators IL-1β and MCP-1) (Fig. 7, E and F). Similarly, we observed no reduction LPS stimulated Mcp-1 and IL-1β expression in BMDM (Fig. 7, G and H), nor any changes in Pdgf-bb expression (Fig. 7I). αSMA, Col 1α1, and Ctgf were not detected in BMDM. Furthermore, there was no FTY720 toxicity observed toward activated hepatic stellate cells (Fig. 7J), activated BMDM (Fig. 7K), and primary mouse hepatocytes (Fig. 7L). Altogether, these data demonstrate a reduction not only in liver injury and inflammation in FFC-FTY720-treated mice and minimal improvement in liver fibrosis, likely secondary to the diminished liver injury and inflammation.
Fig. 7.
FTY720 does not directly alter hepatic stellate cell or macrophage activation. The hepatic stellate cell line (LX-2) was treated with 10 ng/ml TGFβ for 1 h followed by 250 nM FTY720 for 4 h. The expression of markers of hepatic stellate cell activation α-smooth muscle actin (αSMA) (A), Col1α1 (B), connective tissue growth factor (CTGF; C), platelet-derived growth factor-bb (PDGF-BB) (D), and proinflammatory genes interleukin 1 beta (IL1β) (E) and monocyte chemotactic protein 1 (MCP-1; F) was measured by quantitative PCR. BMDMs were treated with 50 ng/ml LPS for 1 h followed by 250 nM FTY720 for 4 h. The expression of proinflammatory genes Mcp-1 (G), and IL-1β (H), and the profibrotic gene platelet-derived growth factor-bb (PDGF-BB) (I) were measured by qPCR. αSMA, Col1α1, and CTGF were not detected in BMDM. J: cell death in LX-2 cells stimulated with TGFβ and treated with FTY720 as above. K: cell death in BMDM stimulated with LPS and treated with FTY720 as above. L: cell death in primary mouse hepatocytes treated with 250 nM FTY720 for 16 h. *P < 0.05.
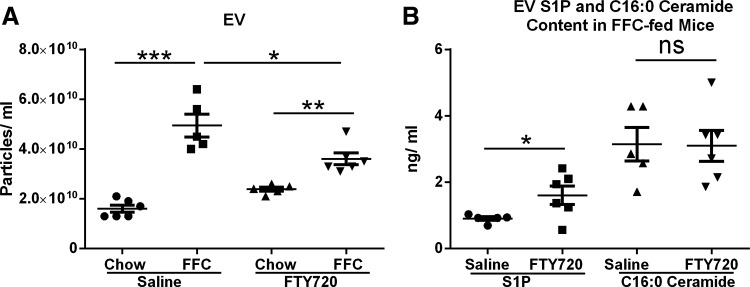
Circulating EVs are lower in FTY72- treated FFC-fed mice, although EV S1P and C16:0 ceramide content is not reduced.
Our previous data suggest that EVs released from stressed steatotic hepatocytes are chemotactic toward macrophages (13); therefore, we next looked at circulating EVs in all four groups of mice. As predicted, there was an increase in circulating EVs in mice fed the FFC diet in comparison to chow-fed mice (Fig. 8A). FFC-fed mice treated with FTY720 (treated group) had lower circulating EVs than the FFC-fed saline injected (control group) mice, although the circulating EVs in this group (FFC-FTY) remained higher than the chow-fed FTY-treated group (Fig. 8A). This reduction in EVs is likely due to a reduction in liver injury in the FFC-fed FTY-treated group, although we cannot exclude a direct effect of FTY720 on release of EVs by steatotic hepatocytes. Furthermore, although EVs were lower in the treated group (FFC-FTY), the EV S1P content and C16:0 ceramide content were not lower (Fig. 8B). This supports our hypothesis that EV S1P acts as a chemoattractant to macrophages via S1P receptor activation on macrophages and that FTY720 administration to mice impairs the recruitment of proinflammatory monocyte-derived macrophages to the liver.
Fig. 8.
FFC-feeding increases circulating extracellular vesicles (EVs) and their sphingolipid content. A: circulating EVs were measured in 200 μl of plasma obtained from each experimental group. EVs were quantified by nanoparticle tracking analysis. *P < 0.05, **P < 0.01, ***P < 0.001. B: circulating EV S1P content and C16:0 ceramide content was calculated as the product of the EV particle concentration and S1P (ng/EV) and C16:0 (ng/EV), respectively. *P < 0.05.
DISCUSSION
In this study, we have demonstrated proof of the therapeutic efficacy of FTY720, an inhibitor of S1P receptors, in reversing established murine NASH. The principal findings of this study are that FTY720-treated mice 1) are protected from liver injury; 2) demonstrate a reduction in hepatic accumulation of activated macrophages; and 3) are associated with a reduction in proinflammatory monocyte recruitment to the liver. These data provide a mechanistic link between sphingolipid signaling and liver inflammation in NASH, as inhibition of S1P receptors with FTY720 was effective in reversing established NASH.
NASH is a complex lipotoxic metabolic disease, wherein sphingolipids are implicated in the pathogenesis of insulin resistance, steatosis, injury, and inflammation. Many independent studies have demonstrated an increase in hepatic ceramides in high-fat-fed murine models of obesity (3, 5, 14, 32, 35); however, their contribution to disease pathogenesis has recently been advanced by the observations that accumulated hepatic C16:0 ceramide is toxic to hepatocytes (32, 35). It has also been demonstrated that ceramides are important in the hepatic-adipose tissue cross talk in obesity, using an inducible ceramidase mouse model (37). In this study, induction of ceramidase (a ceramide breakdown enzyme) in either liver or adipose tissue led to a reduction in hepatic steatosis and improved insulin resistance in high fat-fed mice. Furthermore, S1P, a ceramide derivative is implicated in modulation of immune responses in many diseases, including sterile inflammatory conditions, such as multiple sclerosis (22). It can activate five known G protein-coupled receptors, which vary in their cellular and tissue distribution, thus imparting a variety of S1P target tissues and effects (22, 31, 34). Thus, by extension, the effects of inhibiting S1P signaling are diverse. The therapeutic benefit of the inhibition of S1P signaling in NASH has, heretofore, not been explored and was undertaken in the present study. We found a reduction in liver injury and inflammation with an associated reduction in proinflammatory macrophage accumulation similar to previous reports of FTY720-induced reduction in adipose tissue macrophage accumulation (15, 27). When administered preventively concurrently for the duration of high-fat feeding, or for a longer duration, FTY720 also exerted an anti-obesity effect (27). We treated mice with established NASH for 2 wk to demonstrate the therapeutic efficacy of FTY720 in reversing this disease. In the present study design, we did not observe a significant weight loss in the FTY720-treated mice.
FTY720 is an S1P receptor agonist with activity against four of the five known S1P receptors, S1P1, S1P3, S1P4, and S1P5 (7). Upon binding S1P receptors, it leads to their internalization and downregulation of cell surface receptor availability; thus, FTY720 acts as a functional antagonist. This mechanism is best described for FTY720-mediated downregulation of S1P1 signaling in lymphocytes. S1P1 is abundantly expressed on macrophages (34), and macrophage S1P signaling contributes to sterile inflammatory conditions, such as rheumatoid arthritis, asthma, and atherosclerosis (36). In our study, we noted abundant proinflammatory macrophage accumulation in FFC-fed mice. This was significantly reduced in FTY720-treated mice. We did not observe an increase in other innate immune cell types in FFC-fed mice, nor their reduction in FTY720-treated mice. This could be explained by the predominant contribution of macrophages to NASH pathogenesis. Macrophages are known to express other S1P receptors, including S1P3 and S1P5, both of which can bind FTY720. We plan on examining the specific role of macrophage S1P1 in NASH inflammation in the future by using a conditional macrophage-specific S1P1 knockout mouse model.
Recruitment of circulating proinflammatory monocytes to the liver, wherein, they differentiate into proinflammatory tissue macrophages occurs in the sterile inflammatory response associated with NASH. Liver-derived signals, including chemokines, which can be secreted by both hepatocytes and hepatic macrophages (Kupffer cells) play a role in the recruitment of proinflammatory monocytes to the liver. Increasingly, extracellular vesicles (EVs) have emerged as a key currency for intercellular communication and are implicated in NASH pathogenesis as carrying recruiting and activating signals to macrophages. In our earlier in vitro work, we demonstrated that EVs derived from steatotic hepatocytes are chemoattractive toward macrophages via S1P signaling. We extend our previous observations in the present study and show that inhibition of S1P receptor signaling by FTY720 is, indeed, associated with a reduction in proinflammatory monocyte-derived macrophages in the liver. Furthermore, there is a reduction in the total accumulation of inflammatory macrophages. Although FFC diet induces only modest fibrosis at 24 wk of feeding, we observed a reduction in fibrosis in FTY720-treated FFC-fed mice. This reduction in fibrosis is likely secondary to diminished liver injury and inflammation in the FTY720-treated FFC-fed mice. Though, the antifibrotic effects of FTY720 will need confirmation in additional fibrosis models, similar to our study, it has been reported that hepatic fibrosis can be reduced secondary to improvements in liver injury and inflammation with even 1–2 wk of pharmacologic therapy (10, 11). Furthermore, we did not observe any direct toxicity of FTY720 toward activated hepatic stellate cells or a reduction in the markers of hepatic stellate cell activation and fibrosis, in keeping with our paradigm that the observed changes in fibrosis are secondary to reduction in liver injury and inflammation.
In conclusion, in this study, we demonstrate that a small-molecule inhibitor of S1P signaling ameliorates NASH in a mouse model that recapitulates cardinal features of human NASH. Impaired recruitment of proinflammatory monocyte-derived macrophages is the likely mechanism for the reduction in liver injury and inflammation in FTY720-treated mice. In this proof-of-concept study, we observed a reduction in liver injury and inflammation, not a complete resolution of NASH. Additional studies are needed to confirm the efficacy of this drug in treating murine NASH and to further confirm the mechanism for the observed changes, such as with macrophage-specific S1P1 knockout mice. FTY720 is FDA-approved for the treatment of multiple sclerosis in humans. Our findings in this mouse model of NASH suggest evaluating its use for NASH therapy in humans.
GRANTS
This work was supported by National Institutes of Health (NIH) Grants DK-97178, DK-107402, and DK-111378 (to H. Malhi); the Robert and Elizabeth Strickland Career Development Award from the Division of Endocrinology (to H. Malhi); the Mayo Clinic Center for Cell Signaling (P30DK-084567), AA 21171 (to V. H. Shah), and DK-59615 (to V. H. Shah); the Mayo Clinic Metabolomics Core (U24DK100469, UL1TR000135); and the Mayo Foundation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.S.M., P.H., and H.M. performed experiments; A.S.M., P.H., J.L.M., and H.M. analyzed data; A.S.M., P.H., J.L.M., V.H.S., and H.M. interpreted results of experiments; A.S.M. and H.M. prepared figures; A.S.M. and H.M. drafted manuscript; A.S.M., V.H.S., and H.M. edited and revised manuscript; A.S.M., P.H., J.L.M., V.H.S., and H.M. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors are grateful to Dr. Gregory Gores for critical review of the manuscript, Courtney Hoover for superb administrative assistance, and the generous support of the metabolic phenotyping facility by Robert and Arlene Kogod.
REFERENCES
- 1.Argo CK, Northup PG, Al-Osaimi AM, Caldwell SH. Systematic review of risk factors for fibrosis progression in non-alcoholic steatohepatitis. J Hepatol 51: 371–379, 2009. doi: 10.1016/j.jhep.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 2.Berdyshev EV, Gorshkova I, Skobeleva A, Bittman R, Lu X, Dudek SM, Mirzapoiazova T, Garcia JG, Natarajan V. FTY720 inhibits ceramide synthases and up-regulates dihydrosphingosine 1-phosphate formation in human lung endothelial cells. J Biol Chem 284: 5467–5477, 2009. doi: 10.1074/jbc.M805186200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Błachnio-Zabielska AU, Pułka M, Baranowski M, Nikołajuk A, Zabielski P, Górska M, Górski J. Ceramide metabolism is affected by obesity and diabetes in human adipose tissue. J Cell Physiol 227: 550–557, 2012. doi: 10.1002/jcp.22745. [DOI] [PubMed] [Google Scholar]
- 4.Charlton M, Krishnan A, Viker K, Sanderson S, Cazanave S, McConico A, Masuoko H, Gores G. Fast food diet mouse: novel small animal model of NASH with ballooning, progressive fibrosis, and high physiological fidelity to the human condition. Am J Physiol Gastrointest Liver Physiol 301: G825–G834, 2011. doi: 10.1152/ajpgi.00145.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chaurasia B, Summers SA. Ceramides—lipotoxic inducers of metabolic disorders. Trends Endocrinol Metab 26: 538–550, 2015. doi: 10.1016/j.tem.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 6.Cherayil BJ, Weiner SJ, Pillai S. The Mac-2 antigen is a galactose-specific lectin that binds IgE. J Exp Med 170: 1959–1972, 1989. doi: 10.1084/jem.170.6.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen JA, Chun J. Mechanisms of fingolimod’s efficacy and adverse effects in multiple sclerosis. Ann Neurol 69: 759–777, 2011. doi: 10.1002/ana.22426. [DOI] [PubMed] [Google Scholar]
- 8.Ekstedt M, Hagström H, Nasr P, Fredrikson M, Stål P, Kechagias S, Hultcrantz R. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology 61: 1547–1554, 2015. doi: 10.1002/hep.27368. [DOI] [PubMed] [Google Scholar]
- 9.Faubion WA, Guicciardi ME, Miyoshi H, Bronk SF, Roberts PJ, Svingen PA, Kaufmann SH, Gores GJ. Toxic bile salts induce rodent hepatocyte apoptosis via direct activation of Fas. J Clin Invest 103: 137–145, 1999. doi: 10.1172/JCI4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hirsova P, Ibrahim SH, Bronk SF, Yagita H, Gores GJ. Vismodegib suppresses TRAIL-mediated liver injury in a mouse model of nonalcoholic steatohepatitis. PLoS One 8: e70599, 2013. doi: 10.1371/journal.pone.0070599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hirsova P, Ibrahim SH, Krishnan A, Verma VK, Bronk SF, Werneburg NW, Charlton MR, Shah VH, Malhi H, Gores GJ. Lipid-induced signaling causes release of inflammatory extracellular vesicles from hepatocytes. Gastroenterology 150: 956–967, 2016. doi: 10.1053/j.gastro.2015.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Idrissova L, Malhi H, Werneburg NW, LeBrasseur NK, Bronk SF, Fingas C, Tchkonia T, Pirtskhalava T, White TA, Stout MB, Hirsova P, Krishnan A, Liedtke C, Trautwein C, Finnberg N, El-Deiry WS, Kirkland JL, Gores GJ. TRAIL receptor deletion in mice suppresses the inflammation of nutrient excess. J Hepatol 62: 1156–1163, 2015. doi: 10.1016/j.jhep.2014.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kakazu E, Mauer AS, Yin M, Malhi H. Hepatocytes release ceramide-enriched pro-inflammatory extracellular vesicles in an IRE1α-dependent manner. J Lipid Res 57: 233–245, 2016. doi: 10.1194/jlr.M063412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kasumov T, Li L, Li M, Gulshan K, Kirwan JP, Liu X, Previs S, Willard B, Smith JD, McCullough A. Ceramide as a mediator of non-alcoholic fatty liver disease and associated atherosclerosis. PLoS One 10: e0126910, 2015. doi: 10.1371/journal.pone.0126910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kendall MR, Hupfeld CJ. FTY720, a sphingosine-1-phosphate receptor modulator, reverses high-fat diet-induced weight gain, insulin resistance and adipose tissue inflammation in C57BL/6 mice. Diabetes Obes Metab 10: 802–805, 2008. doi: 10.1111/j.1463-1326.2008.00910.x. [DOI] [PubMed] [Google Scholar]
- 16.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, Yeh M, McCullough AJ, Sanyal AJ, Nonalcoholic Steatohepatitis Clinical Research Network . Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 41: 1313–1321, 2005. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 17.Klingenberg R, Nofer JR, Rudling M, Bea F, Blessing E, Preusch M, Grone HJ, Katus HA, Hansson GK, Dengler TJ. Sphingosine-1-phosphate analogue FTY720 causes lymphocyte redistribution and hypercholesterolemia in ApoE-deficient mice. Arterioscler Thromb Vasc Biol 27: 2392–2399, 2007. doi: 10.1161/ATVBAHA.107.149476. [DOI] [PubMed] [Google Scholar]
- 18.Kono M, Tucker AE, Tran J, Bergner JB, Turner EM, Proia RL. Sphingosine-1-phosphate receptor 1 reporter mice reveal receptor activation sites in vivo. J Clin Invest 124: 2076–2086, 2014. doi: 10.1172/JCI71194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kowalski GM, Carey AL, Selathurai A, Kingwell BA, Bruce CR. Plasma sphingosine-1-phosphate is elevated in obesity. PLoS One 8: e72449, 2013. doi: 10.1371/journal.pone.0072449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.LeBrasseur NK, Schelhorn TM, Bernardo BL, Cosgrove PG, Loria PM, Brown TA. Myostatin inhibition enhances the effects of exercise on performance and metabolic outcomes in aged mice. J Gerontol A Biol Sci Med Sci 64: 940–948, 2009. doi: 10.1093/gerona/glp068. [DOI] [PubMed] [Google Scholar]
- 21.Levy M, Futerman AH. Mammalian ceramide synthases. IUBMB Life 62: 347–356, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maceyka M, Spiegel S. Sphingolipid metabolites in inflammatory disease. Nature 510: 58–67, 2014. doi: 10.1038/nature13475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malhi H, Bronk SF, Werneburg NW, Gores GJ. Free fatty acids induce JNK-dependent hepatocyte lipoapoptosis. J Biol Chem 281: 12,093–12,101, 2006. doi: 10.1074/jbc.M510660200. [DOI] [PubMed] [Google Scholar]
- 24.Malhi H, Gores GJ. Molecular mechanisms of lipotoxicity in nonalcoholic fatty liver disease. Semin Liver Dis 28: 360–369, 2008. doi: 10.1055/s-0028-1091980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Malhi H, Kropp EM, Clavo VF, Kobrossi CR, Han J, Mauer AS, Yong J, Kaufman RJ. C/EBP homologous protein-induced macrophage apoptosis protects mice from steatohepatitis. J Biol Chem 288: 18,624–18,642, 2013. doi: 10.1074/jbc.M112.442954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miao B, Zondlo S, Gibbs S, Cromley D, Hosagrahara VP, Kirchgessner TG, Billheimer J, Mukherjee R. Raising HDL cholesterol without inducing hepatic steatosis and hypertriglyceridemia by a selective LXR modulator. J Lipid Res 45: 1410–1417, 2004. doi: 10.1194/jlr.M300450-JLR200. [DOI] [PubMed] [Google Scholar]
- 27.Moon MH, Jeong JK, Lee JH, Park YG, Lee YJ, Seol JW, Park SY. Antiobesity activity of a sphingosine 1-phosphate analogue FTY720 observed in adipocytes and obese mouse model. Exp Mol Med 44: 603–614, 2012. doi: 10.3858/emm.2012.44.10.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morinaga H, Mayoral R, Heinrichsdorff J, Osborn O, Franck N, Hah N, Walenta E, Bandyopadhyay G, Pessentheiner AR, Chi TJ, Chung H, Bogner-Strauss JG, Evans RM, Olefsky JM, Oh DY. Characterization of distinct subpopulations of hepatic macrophages in HFD/obese mice. Diabetes 64: 1120–1130, 2015. doi: 10.2337/db14-1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nati M, Haddad D, Birkenfeld AL, Koch CA, Chavakis T, Chatzigeorgiou A. The role of immune cells in metabolism-related liver inflammation and development of non-alcoholic steatohepatitis (NASH). Rev Endocr Metab Disord 17: 29–39, 2016. doi: 10.1007/s11154-016-9339-2. [DOI] [PubMed] [Google Scholar]
- 30.Nehra V, Angulo P, Buchman AL, Lindor KD. Nutritional and metabolic considerations in the etiology of nonalcoholic steatohepatitis. Dig Dis Sci 46: 2347–2352, 2001. doi: 10.1023/A:1012338828418. [DOI] [PubMed] [Google Scholar]
- 31.Nixon GF. Sphingolipids in inflammation: pathological implications and potential therapeutic targets. Br J Pharmacol 158: 982–993, 2009. doi: 10.1111/j.1476-5381.2009.00281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raichur S, Wang ST, Chan PW, Li Y, Ching J, Chaurasia B, Dogra S, Öhman MK, Takeda K, Sugii S, Pewzner-Jung Y, Futerman AH, Summers SA. CerS2 haploinsufficiency inhibits β-oxidation and confers susceptibility to diet-induced steatohepatitis and insulin resistance. Cell Metab 20: 687–695, 2014. doi: 10.1016/j.cmet.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 33.Rinella ME. Nonalcoholic fatty liver disease: a systematic review. JAMA 313: 2263–2273, 2015. doi: 10.1001/jama.2015.5370. [DOI] [PubMed] [Google Scholar]
- 34.Singer II, Tian M, Wickham LA, Lin J, Matheravidathu SS, Forrest MJ, Mandala S, Quackenbush EJ. Sphingosine-1-phosphate agonists increase macrophage homing, lymphocyte contacts, and endothelial junctional complex formation in murine lymph nodes. J Immunol 175: 7151–7161, 2005. doi: 10.4049/jimmunol.175.11.7151. [DOI] [PubMed] [Google Scholar]
- 35.Turpin SM, Nicholls HT, Willmes DM, Mourier A, Brodesser S, Wunderlich CM, Mauer J, Xu E, Hammerschmidt P, Brönneke HS, Trifunovic A, LoSasso G, Wunderlich FT, Kornfeld JW, Blüher M, Krönke M, Brüning JC. Obesity-induced CerS6-dependent C16:0 ceramide production promotes weight gain and glucose intolerance. Cell Metab 20: 678–686, 2014. doi: 10.1016/j.cmet.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 36.Weigert A, Weis N, Brüne B. Regulation of macrophage function by sphingosine-1-phosphate. Immunobiology 214: 748–760, 2009. doi: 10.1016/j.imbio.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 37.Xia JY, Holland WL, Kusminski CM, Sun K, Sharma AX, Pearson MJ, Sifuentes AJ, McDonald JG, Gordillo R, Scherer PE. Targeted induction of ceramide degradation leads to improved systemic metabolism and reduced hepatic steatosis. Cell Metab 22: 266–278, 2015. doi: 10.1016/j.cmet.2015.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]