Abstract
Extracellular vesicles (EVs) are membrane-bound vesicles that are released by cells into their extracellular environment, have selective enrichment of specific proteins and RNA, and can mediate intercellular communication. In this review we highlight recent observations of the role of EVs in liver injury, viral hepatitis, alcoholic or nonalcoholic liver disease, biliary tract disease, and liver cancers. Potential applications as markers of diseases or for therapeutic applications are outlined to emphasize the new opportunities that are arising from the study of EVs.
Keywords: extracellular vesicles, liver diseases, cancer, microRNA
cell-to-cell communication is a central component of normal physiology by enabling the exchange of information that is essential for maintaining tissue homeostasis. Several mechanisms by which intercellular communication can occur have been identified and include both the release of secreted proteins such as growth factors and cytokines and direct physical interactions such as nanotubes. Extracellular vesicles (EVs) are membrane-bound vesicles that are released by cells into their environment (Fig. 1). Selective enrichment of proteins, lipids, DNA, and RNA has been detected within EVs, and extensive catalogs are available (24–26). The recognition that EVs released from a cell can be taken up by another and transfer their contents has led to the postulate that transfer of EV cargo could represent a mechanism of intercellular communication (1, 36, 43). EVs have been implicated in many physiological and pathophysiological processes, such as cancer progression and metastasis (10, 22), immune modulation (60), angiogenesis (14, 35), tissue regeneration (18, 29), and neurodegenerative diseases (44). In addition to information exchange, EVs have the potential for both disease diagnosis and therapeutic applications. Demonstration of a role for EVs or their selective cargos in disease pathogenesis will support their potential use as disease biomarkers. The ability to selectively manipulate EV content through either exogenous or endogenous approaches offers further opportunities for their use in disease therapeutics.
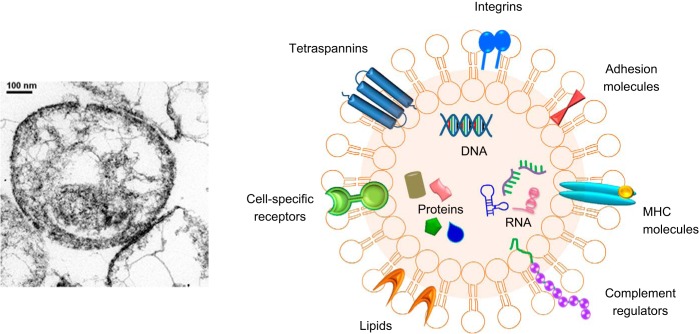
Fig. 1.
Extracellular vesicles. Transmission electron micrograph of extracellular vesicles isolated from liver cancer cells in culture (left) and schematic to illustrate typical contents (right). EVs contain several types of molecules, proteins, DNA, mRNA, lncRNA, and miRNA, some of which are selectively enriched and specific to cell of origin. MHC, major histocompatibility complex.
Extracellular Vesicles or Exosomes?
There are many different types of secreted EVs, varying by their size, cell of origin, biogenesis, content, and physiological setting for their release. These include, but are not limited to, exosomes, microvesicles, and apoptotic bodies (36, 45). The nomenclature used for EVs is complicated by the inconsistent use of terms that hold different meanings for different groups. Indeed, in our initial studies of EVs in liver cancers, we were admonished by peer reviewers to use the term “exosome” instead of “nanovesicle” (28). Albert Claude first noted the release of vesicles containing RNA in 1938 (9). Other reports of EVs were described by Wolf as platelet “dust” in 1967 (59) or calcifying matrix vesicles by Anderson in 1969 (3). In the first use of the term “exosome” by Trams et al. in 1981, vesicles were not limited or defined by size (55). However, subsequent usage was more restricted to vesicles released from multivesicular bodies, popularized by the studies of Harding in 1983 and others (19). Subsequently, size restriction and biogenesis have been commonly used to differentiate exosomes from other EVs. Adding to the confusion is that many isolation approaches result in heterogeneous preparations of EVs that cannot be resolved by size alone and, moreover, vesicles other than biogenetically defined exosomes may have functional effects. While sucrose density gradient ultracentrifugation may be appropriate for exosome isolations, the use of resin-based separation protocols will not separate out protein complexes from other EVs. Preparations of “microvesicles” obtained by centrifugation may contain smaller particles such as exosomes of similar density. In this context, there is a lack of data published to date that unambiguously link a specific functional role with a defined type of vesicle. Furthermore, the term “exosome” is also used for an RNA-degrading complex. The lack of standard nomenclature results in ambiguous and confusing descriptions. Efforts to standardize terminology are underway (8). We recommend a critical approach to terminology that is consistent in description, with validated approaches for their identification and characterization. We note that many reported studies have not been done with pure isolations of vesicles and caution readers about the fallacy of making assumptions about physiological functions of a given type of vesicle unless the homogeneity and purity of the vesicle preparation have been demonstrated.
In this mini-review, we will highlight recent studies that illustrate selected roles of EVs, including studies in exosomes, in liver diseases and settings such as liver injury, viral hepatitis, alcoholic or nonalcoholic liver disease, biliary tract disease, and liver cancers. We further highlight potential roles of EV in diagnosis or therapy of liver diseases such as nonalcoholic fatty liver disease (7) and hepatocellular carcinoma (HCC) (6).
EVs in Liver Disease Pathophysiology
Liver injury.
Liver injury can arise as a consequence of direct toxicity, ischemia, or metabolic perturbations. As vectorial mediators of intercellular communication, EVs may contribute to local environmental responses to cellular or tissue injury and potentially also participate in repair or restoration. These concepts have been evaluated in the context of liver tumor microenvironment but have been largely unexplored in many parenchymal liver diseases. It has been proposed that stressed hepatocytes may release EVs to mediate early immune responses during drug-induced liver injury (21). Indeed, exposure to acetaminophen, a prototypical hepatotoxicant, can alter the content of liver-specific RNA within hepatocyte-derived EVs, notably at subtoxic levels. Hepatic ischemia-reperfusion injury is a major cause of liver damage during hepatic resection and transplantation and a major cause of graft dysfunction posttransplantation. EVs derived from hepatocytes can contribute to liver repair and regeneration after injury and can induce hepatocyte proliferation in a dose-dependent manner both in vitro and in vivo. These EVs can deliver the synthetic machinery necessary to form sphingosine-1-phosphate in target hepatocytes and thereby promote liver regeneration after hepatic injury following ischemia/reperfusion or partial hepatectomy (41).
Viral hepatitis.
The functional involvement of cell-derived EVs in hepatitis is highlighted by studies showing a functional role of hepatocyte-derived exosomes in carrying viral RNA as well as infectious hepatitis virus particles (12), as well as by the observation that exosome-mediated transmission of hepatitis C virus (HCV) can establish productive infection in hepatocytes (46). With the former, transport is dependent on endosomal sorting complexes required for transport III-associated proteins, apoptosis-linked gene 2 (ALG-2)-interacting protein X (Alix), and vacuolar protein sorting-associated protein 4B (Vps4B; 39). A contributing role of EVs in transmission of viral hepatitis is further supported by studies in which knockdown of autophagy in HCV-infected hepatocytes can reduce HCV release within EVs, likely resulting from increased intracellular HCV RNA and accumulation of infectious virus particles in cells (48). In this setting, the innate immune response in HCV-infected hepatocytes is further enhanced (49). The contribution of EVs in intercellular signaling has been highlighted in studies showing that HCV infection can induce human liver endothelial cells to release EVs that inhibit viral replication autocrine interferon signaling (16) (Fig. 2). Determining specific contributions of EVs to viral infection and transmission will enhance our understanding of the mechanisms of chronic viral hepatitis.
Fig. 2.
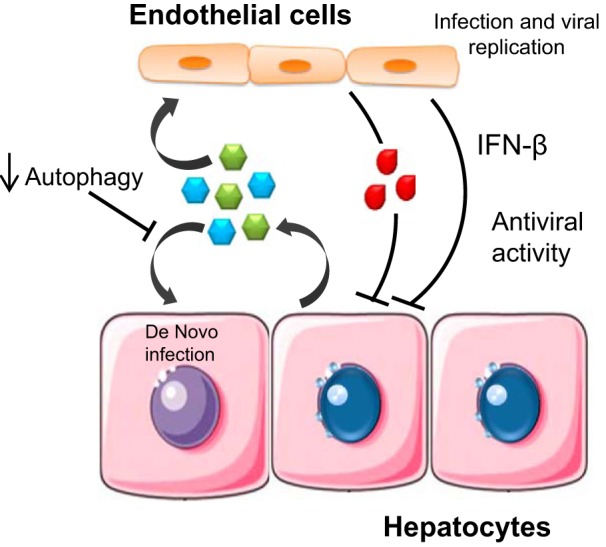
Examples of diverse roles of EVs in viral hepatitis. EVs from infected hepatocytes can transfer virions and viral RNA to adjacent cells promoting de novo infection. In contrast, EVs from hepatic endothelial cells that have been exposed to viral particles can promote antiviral activity.
Alcoholic liver disease.
The role of alcohol and its metabolites in inducing liver injury and inflammation is well established (40), and the contributions of EVs to pathogenesis of alcoholic liver disease are now being evaluated. Ethanol exposure increases the amount of EVs released by hepatocytes. In addition, alcohol-induced EVs can lead to CD40 ligand (CD40L)-mediated activation and infiltration of macrophages and concomitant activation of caspase-3. Furthermore, higher levels of CD40L-enriched EVs were noted in sera from patients with alcoholic hepatitis than controls. Thus EV release from alcohol-exposed hepatocytes and specific EV contents such as CD40L may contribute to inflammation and subsequent liver disease (56).
Nonalcoholic fatty liver disease.
Lipid-mediated hepatocyte dysfunction and macrophage-associated inflammation are reported hallmarks of nonalcoholic steatohepatitis (NASH). Insights into the contribution of EVs to pathogenesis of NASH were provided by the observation that lipid-induced death receptor-5 (DR5) signaling releases EVs from hepatocytes and can induce an inflammatory macrophage phenotype via tumor necrosis factor-related apoptosis-inducing ligand-mediated activation of interleukin (IL)-1β and IL-6 mRNAs (20). The therapeutic implications were emphasized by the reduction of EV-mediated liver injury, inflammation, and fibrosis noted with administration of rho-associated, coiled-coil-containing protein kinase 1 inhibitor fasudil in a murine model of NASH (20). Selective alterations in EV proteome and microRNA (miRNA) have been reported in studies using choline-deficient l-amino acid (CDAA)-fed mice, an animal model of NASH (45). Studies to establish the mechanistic contribution of these observations to the pathogenesis of NASH in humans are awaited.
Biliary tract disease.
A role of EVs in biliary epithelial cell biology and signaling has been recognized for many years, following observations that interactions between EVs and cholangiocyte cilia can modulate cell signaling and proliferation through activation of ERK1/2 signaling and involving miR-15A (34). EVs have also been implicated in biliary infection with Cryptosporidium parvum, in which Toll-like receptor 4 (TLR4)/IKK2 mediates luminal release of EVs from the biliary and intestinal epithelium (23). A role for EVs in promoting mucosal immunity was suggested by the presence of antimicrobial peptides cathelicidin-37 and beta-defensin 2 in these studies. EVs have also been implicated in primary biliary cirrhosis (PBC). There are significant differences in EV miRNA expression between PBC compared with control groups (54). Circulating EVs in PBC can alter expression of costimulatory molecules on antigen-presenting cells ex vivo and thereby could contribute to regulation of T cell activation. Thus EVs may play several distinct roles in biliary epithelial cell physiology as well as during infection or autoimmune disease in the biliary tract.
Liver cancers.
EV-mediated intercellular communication between tumor and other cells within the tumor microenvironment can support the development, spread, and acquired resistance to drugs in liver and other cancers. Tumor-supporting roles of EVs have been reported in studies in other cancers such as pancreatic cancer and glioma (2, 10). Some studies have reported antitumor effects, for example, endothelial cell vesicles contribute to an antitumor response via modulating miRNA expression (5). Specific EV components have been implicated in phenotypic changes in recipient cells following their uptake. Therefore the specific context, content, and cell type of origin of EVs are likely to be important determinants of their functional contribution to the formation or growth of tumors.
hepatocellular cancers.
In a seminal observation, uptake of HCC-derived EVs by recipient cells resulted in EV miRNA-dependent modulation of transforming growth factor-β-activated kinase 1 (TAK1)-associated intercellular signaling (28). EV-mediated miRNA transfer has also been implicated in effects mediated by HCC tumor suppressor VPS4A and promoter IGF1 (4, 58). In addition to transfer of miRNA, the transfer of long noncoding RNA (lncRNA) such as linc-VLDLR and linc-ROR within EV promotes chemoresistance and responses to hypoxia in HCC (51, 52). H19 within EVs released by CD90 + HCC cells can contribute to endothelial cell changes resulting in an angiogenic phenotype.
The contributions of EVs to tumor-related immune responses are being recognized. Natural killer (NK) cell-derived EVs, isolated from either cell culture supernatants or from plasma, can lyse human tumor cells in vitro (33). Furthermore, immune cell-derived EVs containing integrin-αMβ2 or CD147 can facilitate HCC metastasis. An intriguing report showed that HCC development could be inhibited by mesenchymal stem cell-derived EVs (27). Exposure to HCC cell-derived EVs can enhance antitumor effects of NK cells or suppressive effects of dendritic cells and offer the potential for subsequent use in immunotherapy. Indeed, a phase I clinical trial of EV-based antitumor immunotherapy for colorectal cancer combined granulocyte-macrophage colony-stimulating factor with isolated EVs and reported benefit from the combination, although not from EV therapy alone (11).
biliary tract cancers.
Cholangiocarcinoma (CCA), tumors arising from the biliary tract, are highly desmoplastic tumors and are typically associated with dense fibrous stroma. The interrelationship of tumor and stromal cells is highlighted by studies in which CCA cell-derived EVs supported fibroblastic differentiation of mesenchymal stem cells (MSCs), resulting in upregulation of chemokine (C-X-C motif) ligand 1 (CXCL-1), chemokine (C-C motif) ligand 2 (CCL2), and IL-6. These differentiated MSCs could, in turn, alter tumor cell proliferation revealing a feedback loop between MSCs and tumor cells (17). CCA cell-derived EVs can modulate β-catenin, reduce E-cadherin expression, and induce migration and invasion of H69 cells (13). Thus CCA-derived EVs can play a critical role in modulating local environmental changes that can support tumor progression.
hepatic metastases.
EVs released from tumor cells at distant sites such as the pancreas or colon can travel to the liver, establish a premetastatic niche, and thus contribute to hepatic metastases. Similar effects can occur in other sites; thus EV from mouse and human lung-, liver-, and brain-tropic tumor cells can travel to their homing organs. These observations directly support Stephen Paget’s hypothesis on metastatic organotropism from over a century ago. Organ-specific metastases can be predicted by EV integrin expression (22). EVs from liver cancer cells can preferentially home into the liver Kupffer cells (22). Indeed, targeting αvβ5 decreases vesicle uptake as well as organ-specific metastasis.
EVs as Disease Biomarkers
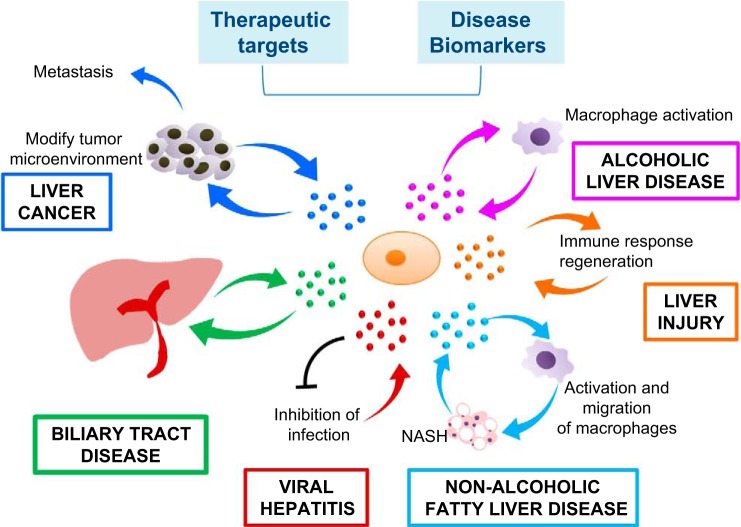
The association of specific EV constituents with disease offers potential for the use of EVs as biomarkers to diagnose and predict behavior or treatment response in many different types of liver diseases (Fig. 3).
Fig. 3.
EVs in liver diseases. EVs from hepatocytes can mediate early immune responses in drug-induced liver injury, promote viral hepatitis, promote liver regeneration in ischemia-reperfusion injury and hepatectomy, and promote alcoholic and nonalcoholic steatohepatitis through activation of macrophages. EVs from hepatic endothelial cells can inhibit viral progression, whereas EVs from stem cells can reduce liver injury. Liver cancer cell EVs can create a premetastatic niche, educate the tumor stroma, and promote metastasis. EVs can be exploited for therapeutic intervention. Selective EV contents that are specific to cell of origin such as miRNA are potential biomarkers for disease.
The utility of EV-associated miRNA as potential biomarkers of alcoholic injury has been explored. An increase in the number of circulating vesicles as well as the presence of differentially expressed miRNAs were noted in sera of alcohol-fed mice or from plasma of alcoholic hepatitis patients or healthy controls. Among the several EV-associated miRNAs that were upregulated in the sera of chronic alcohol-fed mice compared with control animals, miRNA-192, miRNA-122, and miRNA-30a had value for diagnosis of alcohol-induced liver injury. Moreover, miRNA-192 and miRNA-30a were significantly upregulated in the plasma of alcoholic hepatitis patients compared with healthy controls suggesting a potential role as biomarkers for this condition (38).
An analysis of EV contents from CDAA-fed mice identified several proteins present only in EVs and enrichment of NASH-associated proteins such as those involved in cell stress, cell death, and angiogenesis compared with controls. Compared with control diet-fed animals, an enrichment of circulating miR-122 and miR-192 and a decreased hepatic expression of these miRNAs was noted in CDAA-fed animals (45). Further studies to establish the sensitivity and specificity of these EV contents as biomarkers in NASH are necessary.
Studies to evaluate circulating miRNA in HCC cohorts using PCR have identified EV miRNA such as miR-939, miR-595, and miR-519d as candidate biomarkers (15). Serum levels of EV miR-18a, miR-221, miR-222, and miR-224 were significantly higher in patients with chronic hepatitis B virus (HBV)-related HCC than in those with either HBV alone or liver cirrhosis, whereas serum levels of EV miR-101, miR-106b, miR-122, and miR-195 were lower in patients with HCC than in patients with HBV (50). These studies await further validation in larger patient cohorts and in other conditions such as HCV or nonalcoholic fatty liver disease. A study of biliary miRNAs presumed to be from EVs from patients with benign or malignant biliary obstruction described a miRNA-based panel for diagnosis of cholangiocarcinoma with a sensitivity of 67% and specificity of 96% (30, 47). These studies were not based on absolute quantitation, and demonstration of reproducibility of the algorithm used and subsequent clinical utility is awaited. The potential for using tumor-associated and tumor cell-specific changes in EVs as markers to diagnose liver cancers is being evaluated.
Therapeutic Applications of EVs
The potential for therapeutic use of EVs for liver diseases is exemplified by the multiple applications that have been described, such as to correct metabolic deficiencies, promote liver regeneration, impede cancer progression, and improve liver function and graft vs. host rejection in liver transplantation, to name a few. The cellular source of EVs in these studies has been diverse, with examples of applications of EVs derived from adipose or bone marrow-derived MSCs, epithelial cells, dendritic or B cells, and plant-derived EVs. MSC EVs have been shown to alleviate liver fibrosis (31), suppress tumor growth (27), and promote hepatic regeneration in drug-induced liver injury models (53). Similarly, murine bone marrow stromal cells pulsed with homologous tumor-derived EVs can lead to inhibition of tumor cell growth. Beneficial effects of orally administered plant-derived EVs such as ginger-derived nanoparticles have been reported in alcoholic liver injury (61). An active ingredient, shogaol, in these EVs can activate nuclear factor erythroid 2-related factor 2 in a TLR4/Toll/IL-1 receptor (TIR) domain-containing adaptor protein inducing interferon-β (TRIF)-dependent manner and, in turn, induce liver-detoxifying/antioxidant genes that protect from oxidative stress (61).
On a different note, EVs can be used to deliver exogenous RNA molecules to liver cells in vitro and in vivo. In one study, B cell EVs were used to deliver exogenous miRNA-155 mimic and could modulate miR-155 expression in hepatocytes, in vitro and in vivo (37). Cells can transfer endogenous miRNA and deliver small interfering RNA targeting HCV replication or CD81 via EVs (42). Similarly, drug (cisplatin)-loaded EVs can inhibit HCC progression and prolong survival (57). Packaging of miR-122 within EVs was noted in adipose tissue-derived mesenchymal stem cells transfected with miR-122 expression plasmids and, subsequently, was capable of modulating therapeutic sensitivity of HCC in preclinical models (32).
Challenges and Future Perspectives
In recent years, we have seen significant progress in our understanding of the biological relevance of EVs, with comprehensive analyses of their protein, DNA, RNA, and lipid content, and well-defined examples of specific roles in disease pathobiology. There is clear potential for the use of EVs for disease diagnosis, as well as the application of EVs for targeted delivery and therapeutic intervention in liver and other diseases, but much work is needed. As with any emerging field, there are many questions to be answered, and several challenges exist. There is a paucity of knowledge about their biogenesis, uptake, and cellular functions. Emerging data indicate that EV contents can vary with cell type, environmental influences, and context of their release. Likewise, cell-type specificity of effects following uptake is possible. The ability to isolate pure populations of different functional populations of EVs is limited.
The use of EVs as drug delivery vehicles is appealing as they can potentially encompass biologically active mediators such as protein or RNA, can survive in the circulation, and can effectively transfer their contents into cells. Apart from demonstrating proof of efficacy and potency of any EV-based therapies, in vivo analyses of pharmacokinetics, biodistribution, dose escalation, toxicity, and immunogenicity will be needed. Therapeutic application will likely depend on the ability to produce EVs on a large scale, with minimal immunogenicity, with capability to engineer their contents.
The study of EVs has been plagued with a lack of consistent nomenclature, but recent consensus efforts to standardize the nomenclature and ontology will be helpful. The inability to distinguish between EV contents that are biologically insignificant, physiologically relevant, or pathologically associated with disease is a major roadblock. Overcoming this will require careful and detailed analyses of EV contents and their functional roles in carefully defined models. Other major unanswered questions relate to mechanisms by which selected EV contents are processed and incorporated within EVs, how these are released following their uptake, and proof of retained functionality. In particular, the contribution of EVs to disease pathogenesis needs to be carefully defined. The delineation of the roles and context-dependent involvement of EVs as vectors for intercellular communication between cells, and even tissues, offers several exciting possibilities for new areas of investigation and research that will surely further our knowledge of physiological processes.
GRANTS
This work was supported in part by National Institutes of Health Grants R01-DK-063970 and UH3-TR-000884.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.M., A.M., I.K.Y., M.P., and T.P. prepared figures; S.M., I.K.Y., and T.P. drafted manuscript; S.M., I.K.Y., M.P., and T.P. edited and revised manuscript; A.M. and T.P. approved final version of manuscript; T.P. conceived and designed research.
REFERENCES
- 1.Alenquer M, Amorim MJ. Exosome biogenesis, regulation, and function in viral infection. Viruses 7: 5066–5083, 2015. doi: 10.3390/v7092862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Nedawi K, Meehan B, Micallef J, Lhotak V, May L, Guha A, Rak J. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat Cell Biol 10: 619–624, 2008. doi: 10.1038/ncb1725. [DOI] [PubMed] [Google Scholar]
- 3.Anderson HC. Vesicles associated with calcification in the matrix of epiphyseal cartilage. J Cell Biol 41: 59–72, 1969. doi: 10.1083/jcb.41.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Basu S, Bhattacharyya SN. Insulin-like growth factor-1 prevents miR-122 production in neighbouring cells to curtail its intercellular transfer to ensure proliferation of human hepatoma cells. Nucleic Acids Res 42: 7170–7185, 2014. doi: 10.1093/nar/gku346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bovy N, Blomme B, Frères P, Dederen S, Nivelles O, Lion M, Carnet O, Martial JA, Noël A, Thiry M, Jérusalem G, Josse C, Bours V, Tabruyn SP, Struman I. Endothelial exosomes contribute to the antitumor response during breast cancer neoadjuvant chemotherapy via microRNA transfer. Oncotarget 6: 10253–10266, 2015. doi: 10.18632/oncotarget.3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bupathi M, Kaseb A, Meric-Bernstam F, Naing A. Hepatocellular carcinoma: where there is unmet need. Mol Oncol 9: 1501–1509, 2015. doi: 10.1016/j.molonc.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chawla KS, Talwalkar JA, Keach JC, Malinchoc M, Lindor KD, Jorgensen R. Reliability and validity of the chronic liver disease questionnaire (CLDQ) in adults with non-alcoholic steatohepatitis (NASH). BMJ Open Gastroenterol 3: e000069, 2016. doi: 10.1136/bmjgast-2015-000069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheung KH, Keerthikumar S, Roncaglia P, Subramanian SL, Roth ME, Samuel M, Anand S, Gangoda L, Gould S, Alexander R, Galas D, Gerstein MB, Hill AF, Kitchen RR, Lötvall J, Patel T, Procaccini DC, Quesenberry P, Rozowsky J, Raffai RL, Shypitsyna A, Su AI, Théry C, Vickers K, Wauben MH, Mathivanan S, Milosavljevic A, Laurent LC. Extending gene ontology in the context of extracellular RNA and vesicle communication. J Biomed Semantics 7: 19, 2016. doi: 10.1186/s13326-016-0061-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Claude A. Concentration and purification of chicken tumor I agent. Science 87: 467–468, 1938. doi: 10.1126/science.87.2264.467. [DOI] [PubMed] [Google Scholar]
- 10.Costa-Silva B, Aiello NM, Ocean AJ, Singh S, Zhang H, Thakur BK, Becker A, Hoshino A, Mark MT, Molina H, Xiang J, Zhang T, Theilen TM, García-Santos G, Williams C, Ararso Y, Huang Y, Rodrigues G, Shen TL, Labori KJ, Lothe IM, Kure EH, Hernandez J, Doussot A, Ebbesen SH, Grandgenett PM, Hollingsworth MA, Jain M, Mallya K, Batra SK, Jarnagin WR, Schwartz RE, Matei I, Peinado H, Stanger BZ, Bromberg J, Lyden D. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat Cell Biol 17: 816–826, 2015. doi: 10.1038/ncb3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dai S, Wei D, Wu Z, Zhou X, Wei X, Huang H, Li G. Phase I clinical trial of autologous ascites-derived exosomes combined with GM-CSF for colorectal cancer. Mol Ther 16: 782–790, 2008. doi: 10.1038/mt.2008.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dreux M, Garaigorta U, Boyd B, Décembre E, Chung J, Whitten-Bauer C, Wieland S, Chisari FV. Short-range exosomal transfer of viral RNA from infected cells to plasmacytoid dendritic cells triggers innate immunity. Cell Host Microbe 12: 558–570, 2012. doi: 10.1016/j.chom.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dutta S, Reamtong O, Panvongsa W, Kitdumrongthum S, Janpipatkul K, Sangvanich P, Piyachaturawat P, Chairoungdua A. Proteomics profiling of cholangiocarcinoma exosomes: a potential role of oncogenic protein transferring in cancer progression. Biochim Biophys Acta 1852: 1989–1999, 2015. doi: 10.1016/j.bbadis.2015.06.024. [DOI] [PubMed] [Google Scholar]
- 14.Escudero CA, Herlitz K, Troncoso F, Acurio J, Aguayo C, Roberts JM, Truong G, Duncombe G, Rice G, Salomon C. Role of extracellular vesicles and microRNAs on dysfunctional angiogenesis during preeclamptic pregnancies. Front Physiol 7: 98, 2016. doi: 10.3389/fphys.2016.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fornari F, Ferracin M, Trerè D, Milazzo M, Marinelli S, Galassi M, Venerandi L, Pollutri D, Patrizi C, Borghi A, Foschi FG, Stefanini GF, Negrini M, Bolondi L, Gramantieri L. Circulating microRNAs, miR-939, miR-595, miR-519d and miR-494, identify cirrhotic patients with HCC. PLoS One 10: e0141448, 2015. doi: 10.1371/journal.pone.0141448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giugliano S, Kriss M, Golden-Mason L, Dobrinskikh E, Stone AE, Soto-Gutierrez A, Mitchell A, Khetani SR, Yamane D, Stoddard M, Li H, Shaw GM, Edwards MG, Lemon SM, Gale M Jr, Shah VH, Rosen HR. Hepatitis C virus infection induces autocrine interferon signaling by human liver endothelial cells and release of exosomes, which inhibits viral replication. Gastroenterology 148: 392–402.e13, 2015. doi: 10.1053/j.gastro.2014.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haga H, Yan IK, Takahashi K, Wood J, Zubair A, Patel T. Tumour cell-derived extracellular vesicles interact with mesenchymal stem cells to modulate the microenvironment and enhance cholangiocarcinoma growth. J Extracell Vesicles 4: 24900, 2015. doi: 10.3402/jev.v4.24900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han C, Sun X, Liu L, Jiang H, Shen Y, Xu X, Li J, Zhang G, Huang J, Lin Z, Xiong N, Wang T. Exosomes and their therapeutic potentials of stem cells. Stem Cells Int 2016: 7653489, 2016. doi: 10.1155/2016/7653489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harding CV, Heuser JE, Stahl PD. Exosomes: looking back three decades and into the future. J Cell Biol 200: 367–371, 2013. doi: 10.1083/jcb.201212113. [Erratum. J Cell Biol 201: 485, 2013. doi:] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hirsova P, Ibrahim SH, Krishnan A, Verma VK, Bronk SF, Werneburg NW, Charlton MR, Shah VH, Malhi H, Gores GJ. Lipid-induced signaling causes release of inflammatory extracellular vesicles from hepatocytes. Gastroenterology 150: 956–967, 2016. doi: 10.1053/j.gastro.2015.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holman NS, Mosedale M, Wolf KK, LeCluyse EL, Watkins PB. Subtoxic alterations in hepatocyte-derived exosomes: an early step in drug-induced liver injury? Toxicol Sci 151: 365–375, 2016. doi: 10.1093/toxsci/kfw047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A, Tesic Mark M, Molina H, Kohsaka S, Di Giannatale A, Ceder S, Singh S, Williams C, Soplop N, Uryu K, Pharmer L, King T, Bojmar L, Davies AE, Ararso Y, Zhang T, Zhang H, Hernandez J, Weiss JM, Dumont-Cole VD, Kramer K, Wexler LH, Narendran A, Schwartz GK, Healey JH, Sandstrom P, Labori KJ, Kure EH, Grandgenett PM, Hollingsworth MA, de Sousa M, Kaur S, Jain M, Mallya K, Batra SK, Jarnagin WR, Brady MS, Fodstad O, Muller V, Pantel K, Minn AJ, Bissell MJ, Garcia BA, Kang Y, Rajasekhar VK, Ghajar CM, Matei I, Peinado H, Bromberg J, Lyden D. Tumour exosome integrins determine organotropic metastasis. Nature 527: 329–335, 2015. doi: 10.1038/nature15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu G, Gong AY, Roth AL, Huang BQ, Ward HD, Zhu G, Larusso NF, Hanson ND, Chen XM. Release of luminal exosomes contributes to TLR4-mediated epithelial antimicrobial defense. PLoS Pathog 9: e1003261, 2013. doi: 10.1371/journal.ppat.1003261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kalra H, Simpson RJ, Ji H, Aikawa E, Altevogt P, Askenase P, Bond VC, Borràs FE, Breakefield X, Budnik V, Buzas E, Camussi G, Clayton A, Cocucci E, Falcon-Perez JM, Gabrielsson S, Gho YS, Gupta D, Harsha HC, Hendrix A, Hill AF, Inal JM, Jenster G, Krämer-Albers EM, Lim SK, Llorente A, Lötvall J, Marcilla A, Mincheva-Nilsson L, Nazarenko I, Nieuwland R, Nolte-’t Hoen EN, Pandey A, Patel T, Piper MG, Pluchino S, Prasad TS, Rajendran L, Raposo G, Record M, Reid GE, Sánchez-Madrid F, Schiffelers RM, Siljander P, Stensballe A, Stoorvogel W, Taylor D, Thery C, Valadi H, van Balkom BW, Vázquez J, Vidal M, Wauben MH, Yáñez-Mó M, Zoeller M, Mathivanan S. Vesiclepedia: a compendium for extracellular vesicles with continuous community annotation. PLoS Biol 10: e1001450, 2012. doi: 10.1371/journal.pbio.1001450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keerthikumar S, Chisanga D, Ariyaratne D, Al Saffar H, Anand S, Zhao K, Samuel M, Pathan M, Jois M, Chilamkurti N, Gangoda L, Mathivanan S. ExoCarta: A web-based compendium of exosomal cargo. J Mol Biol 428: 688–692, 2016. doi: 10.1016/j.jmb.2015.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim DK, Lee J, Kim SR, Choi DS, Yoon YJ, Kim JH, Go G, Nhung D, Hong K, Jang SC, Kim SH, Park KS, Kim OY, Park HT, Seo JH, Aikawa E, Baj-Krzyworzeka M, van Balkom BW, Belting M, Blanc L, Bond V, Bongiovanni A, Borràs FE, Buée L, Buzás EI, Cheng L, Clayton A, Cocucci E, Dela Cruz CS, Desiderio DM, Di Vizio D, Ekström K, Falcon-Perez JM, Gardiner C, Giebel B, Greening DW, Gross JC, Gupta D, Hendrix A, Hill AF, Hill MM, Nolte-’t Hoen E, Hwang DW, Inal J, Jagannadham MV, Jayachandran M, Jee YK, Jørgensen M, Kim KP, Kim YK, Kislinger T, Lässer C, Lee DS, Lee H, van Leeuwen J, Lener T, Liu ML, Lötvall J, Marcilla A, Mathivanan S, Möller A, Morhayim J, Mullier F, Nazarenko I, Nieuwland R, Nunes DN, Pang K, Park J, Patel T, Pocsfalvi G, Del Portillo H, Putz U, Ramirez MI, Rodrigues ML, Roh TY, Royo F, Sahoo S, Schiffelers R, Sharma S, Siljander P, Simpson RJ, Soekmadji C, Stahl P, Stensballe A, Stępień E, Tahara H, Trummer A, Valadi H, Vella LJ, Wai SN, Witwer K, Yáñez-Mó M, Youn H, Zeidler R, Gho YS. EVpedia: a community web portal for extracellular vesicles research. Bioinformatics 31: 933–939, 2015. doi: 10.1093/bioinformatics/btu741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ko SF, Yip HK, Zhen YY, Lee CC, Lee CC, Huang CC, Ng SH, Lin JW. Adipose-derived mesenchymal stem cell exosomes suppress hepatocellular carcinoma growth in a rat model: apparent diffusion coefficient, natural killer T-cell responses, and histopathological features. Stem Cells Int 2015: 853506, 2015. doi: 10.1155/2015/853506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kogure T, Lin WL, Yan IK, Braconi C, Patel T. Intercellular nanovesicle-mediated microRNA transfer: a mechanism of environmental modulation of hepatocellular cancer cell growth. Hepatology 54: 1237–1248, 2011. doi: 10.1002/hep.24504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lener T, Gimona M, Aigner L, Börger V, Buzas E, Camussi G, Chaput N, Chatterjee D, Court FA, Del Portillo HA, O’Driscoll L, Fais S, Falcon-Perez JM, Felderhoff-Mueser U, Fraile L, Gho YS, Görgens A, Gupta RC, Hendrix A, Hermann DM, Hill AF, Hochberg F, Horn PA, de Kleijn D, Kordelas L, Kramer BW, Krämer-Albers EM, Laner-Plamberger S, Laitinen S, Leonardi T, Lorenowicz MJ, Lim SK, Lötvall J, Maguire CA, Marcilla A, Nazarenko I, Ochiya T, Patel T, Pedersen S, Pocsfalvi G, Pluchino S, Quesenberry P, Reischl IG, Rivera FJ, Sanzenbacher R, Schallmoser K, Slaper-Cortenbach I, Strunk D, Tonn T, Vader P, van Balkom BW, Wauben M, Andaloussi SE, Théry C, Rohde E, Giebel B. Applying extracellular vesicles based therapeutics in clinical trials: an ISEV position paper. J Extracell Vesicles 4: 30087, 2015. doi: 10.3402/jev.v4.30087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li L, Masica D, Ishida M, Tomuleasa C, Umegaki S, Kalloo AN, Georgiades C, Singh VK, Khashab M, Amateau S, Li Z, Okolo P, Lennon AM, Saxena P, Geschwind JF, Schlachter T, Hong K, Pawlik TM, Canto M, Law J, Sharaiha R, Weiss CR, Thuluvath P, Goggins M, Shin EJ, Peng H, Kumbhari V, Hutfless S, Zhou L, Mezey E, Meltzer SJ, Karchin R, Selaru FM. Human bile contains microRNA-laden extracellular vesicles that can be used for cholangiocarcinoma diagnosis. Hepatology 60: 896–907, 2014. doi: 10.1002/hep.27050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li T, Yan Y, Wang B, Qian H, Zhang X, Shen L, Wang M, Zhou Y, Zhu W, Li W, Xu W. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate liver fibrosis. Stem Cells Dev 22: 845–854, 2013. doi: 10.1089/scd.2012.0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lou G, Song X, Yang F, Wu S, Wang J, Chen Z, Liu Y. Exosomes derived from miR-122-modified adipose tissue-derived MSCs increase chemosensitivity of hepatocellular carcinoma. J Hematol Oncol 8: 122, 2015. doi: 10.1186/s13045-015-0220-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lugini L, Cecchetti S, Huber V, Luciani F, Macchia G, Spadaro F, Paris L, Abalsamo L, Colone M, Molinari A, Podo F, Rivoltini L, Ramoni C, Fais S. Immune surveillance properties of human NK cell-derived exosomes. J Immunol 189: 2833–2842, 2012. doi: 10.4049/jimmunol.1101988. [DOI] [PubMed] [Google Scholar]
- 34.Masyuk AI, Huang BQ, Ward CJ, Gradilone SA, Banales JM, Masyuk TV, Radtke B, Splinter PL, LaRusso NF. Biliary exosomes influence cholangiocyte regulatory mechanisms and proliferation through interaction with primary cilia. Am J Physiol Gastrointest Liver Physiol 299: G990–G999, 2010. doi: 10.1152/ajpgi.00093.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Merino-González C, Zuñiga FA, Escudero C, Ormazabal V, Reyes C, Nova-Lamperti E, Salomón C, Aguayo C. Mesenchymal stem cell-derived extracellular vesicles promote angiogenesis: potencial clinical application. Front Physiol 7: 24, 2016. doi: 10.3389/fphys.2016.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mohankumar S, Patel T. Extracellular vesicle long noncoding RNA as potential biomarkers of liver cancer. Brief Funct Genomics, 15: 249–256, 2016. doi: 10.1093/bfgp/elv058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Momen-Heravi F, Bala S, Bukong T, Szabo G. Exosome-mediated delivery of functionally active miRNA-155 inhibitor to macrophages. Nanomedicine 10: 1517–1527, 2014. doi: 10.1016/j.nano.2014.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Momen-Heravi F, Saha B, Kodys K, Catalano D, Satishchandran A, Szabo G. Increased number of circulating exosomes and their microRNA cargos are potential novel biomarkers in alcoholic hepatitis. J Transl Med 13: 261, 2015. doi: 10.1186/s12967-015-0623-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morita E, Sandrin V, McCullough J, Katsuyama A, Baci Hamilton I, Sundquist WI. ESCRT-III protein requirements for HIV-1 budding. Cell Host Microbe 9: 235–242, 2011. doi: 10.1016/j.chom.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nagy LE, Ding WX, Cresci G, Saikia P, Shah VH. Linking pathogenic mechanisms of alcoholic liver disease with clinical phenotypes. Gastroenterology 150: 1756–1768, 2016. doi: 10.1053/j.gastro.2016.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nojima H, Freeman CM, Schuster RM, Japtok L, Kleuser B, Edwards MJ, Gulbins E, Lentsch AB. Hepatocyte exosomes mediate liver repair and regeneration via sphingosine-1-phosphate. J Hepatol 64: 60–68, 2016. doi: 10.1016/j.jhep.2015.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pan Q, Ramakrishnaiah V, Henry S, Fouraschen S, de Ruiter PE, Kwekkeboom J, Tilanus HW, Janssen HL, van der Laan LJ. Hepatic cell-to-cell transmission of small silencing RNA can extend the therapeutic reach of RNA interference (RNAi). Gut 61: 1330–1339, 2012. doi: 10.1136/gutjnl-2011-300449. [DOI] [PubMed] [Google Scholar]
- 43.Parasramka MA, Maji S, Matsuda A, Yan IK, Patel T. Long non-coding RNAs as novel targets for therapy in hepatocellular carcinoma. Pharmacol Ther 161: 67–78, 2016. doi: 10.1016/j.pharmthera.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Polanco JC, Scicluna BJ, Hill AF, Götz J. Extracellular vesicles isolated from the brains of rTg4510 mice seed tau protein aggregation in a threshold-dependent manner. J Biol Chem 291: 12445–12466, 2016. doi: 10.1074/jbc.M115.709485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Povero D, Eguchi A, Li H, Johnson CD, Papouchado BG, Wree A, Messer K, Feldstein AE. Circulating extracellular vesicles with specific proteome and liver microRNAs are potential biomarkers for liver injury in experimental fatty liver disease. PLoS One 9: e113651, 2014. doi: 10.1371/journal.pone.0113651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ramakrishnaiah V, Thumann C, Fofana I, Habersetzer F, Pan Q, de Ruiter PE, Willemsen R, Demmers JA, Stalin Raj V, Jenster G, Kwekkeboom J, Tilanus HW, Haagmans BL, Baumert TF, van der Laan LJ. Exosome-mediated transmission of hepatitis C virus between human hepatoma Huh7.5 cells. Proc Natl Acad Sci U S A 110: 13109–13113, 2013. doi: 10.1073/pnas.1221899110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shigehara K, Yokomuro S, Ishibashi O, Mizuguchi Y, Arima Y, Kawahigashi Y, Kanda T, Akagi I, Tajiri T, Yoshida H, Takizawa T, Uchida E. Real-time PCR-based analysis of the human bile microRNAome identifies miR-9 as a potential diagnostic biomarker for biliary tract cancer. PLoS One 6: e23584, 2011. doi: 10.1371/journal.pone.0023584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shrivastava S, Devhare P, Sujijantarat N, Steele R, Kwon YC, Ray R, Ray RB. Knockdown of autophagy inhibits infectious hepatitis C virus release by the exosomal pathway. J Virol 90: 1387–1396, 2015. doi: 10.1128/JVI.02383-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shrivastava S, Raychoudhuri A, Steele R, Ray R, Ray RB. Knockdown of autophagy enhances the innate immune response in hepatitis C virus-infected hepatocytes. Hepatology 53: 406–414, 2011. doi: 10.1002/hep.24073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sohn W, Kim J, Kang SH, Yang SR, Cho JY, Cho HC, Shim SG, Paik YH. Serum exosomal microRNAs as novel biomarkers for hepatocellular carcinoma. Exp Mol Med 47: e184, 2015. doi: 10.1038/emm.2015.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Takahashi K, Yan IK, Haga H, Patel T. Modulation of hypoxia-signaling pathways by extracellular linc-RoR. J Cell Sci 127: 1585–1594, 2014. doi: 10.1242/jcs.141069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Takahashi K, Yan IK, Wood J, Haga H, Patel T. Involvement of extracellular vesicle long noncoding RNA (linc-VLDLR) in tumor cell responses to chemotherapy. Mol Cancer Res 12: 1377–1387, 2014. doi: 10.1158/1541-7786.MCR-13-0636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tan CY, Lai RC, Wong W, Dan YY, Lim SK, Ho HK. Mesenchymal stem cell-derived exosomes promote hepatic regeneration in drug-induced liver injury models. Stem Cell Res Ther 5: 76, 2014. doi: 10.1186/scrt465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tomiyama T, Yang GX, Zhao M, Zhang W, Tanaka H, Wang J, Leung PS, Okazaki K, He XS, Lu Q, Coppel RL, Bowlus CL, Gershwin ME. The modulation of co-stimulatory molecules by circulating exosomes in primary biliary cirrhosis. Cell Mol Immunol (September 21, 2015). doi: 10.1038/cmi.2015.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Trams EG, Lauter CJ, Salem N Jr, Heine U. Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. Biochim Biophys Acta 645: 63–70, 1981. doi: 10.1016/0005-2736(81)90512-5. [DOI] [PubMed] [Google Scholar]
- 56.Verma VK, Li H, Wang R, Hirsova P, Mushref M, Liu Y, Cao S, Contreras PC, Malhi H, Kamath PS, Gores GJ, Shah VH. Alcohol stimulates macrophage activation through caspase-dependent hepatocyte derived release of CD40L containing extracellular vesicles. J Hepatol 64: 651–660, 2016. doi: 10.1016/j.jhep.2015.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang SH, Shen Y, Li J, Xiang ZW, Fan WK, Chen L. [Experimental studies on anti-mouse hepatocellular carcinoma effects of cisplatin combined with exosomes]. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 25: 49–52, 2009. [PubMed] [Google Scholar]
- 58.Wei JX, Lv LH, Wan YL, Cao Y, Li GL, Lin HM, Zhou R, Shang CZ, Cao J, He H, Han QF, Liu PQ, Zhou G, Min J. Vps4A functions as a tumor suppressor by regulating the secretion and uptake of exosomal microRNAs in human hepatoma cells. Hepatology 61: 1284–1294, 2015. doi: 10.1002/hep.27660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wolf P. The nature and significance of platelet products in human plasma. Br J Haematol 13: 269–288, 1967. doi: 10.1111/j.1365-2141.1967.tb08741.x. [DOI] [PubMed] [Google Scholar]
- 60.Wong WY, Lee MM, Chan BD, Kam RK, Zhang G, Lu AP, Tai WC. Proteomic profiling of dextran sulfate sodium induced acute ulcerative colitis mice serum exosomes and their immunomodulatory impact on macrophages. Proteomics 16: 1131–1145, 2016. doi: 10.1002/pmic.201500174. [DOI] [PubMed] [Google Scholar]
- 61.Zhuang X, Deng ZB, Mu J, Zhang L, Yan J, Miller D, Feng W, McClain CJ, Zhang HG. Ginger-derived nanoparticles protect against alcohol-induced liver damage. J Extracell Vesicles 4: 28713, 2015. doi: 10.3402/jev.v4.28713 . [DOI] [PMC free article] [PubMed] [Google Scholar]