Abstract
Parenteral nutrition (PN) is a lifesaving therapy that provides intravenous nutrition support to patients who cannot, or should not, feed via the gastrointestinal (GI) tract. Unfortunately, PN also carries certain risks related to infection and metabolic complications compared with enteral nutrition. In this review, an overview of PN and GI immune and microbiome changes is provided. PN impacts the gut-associated lymphoid tissue functions, especially adaptive immune cells, changes the intestinal epithelium and chemical secretions, and significantly alters the intestinal microbiome. Collectively, these changes functionally result in increased susceptibility to infectious and injurious challenge. Since PN remains necessary in large numbers of patients, the search to improve outcomes by stimulating GI immune function during PN remains of interest. This review closes by describing recent advances in using enteric nervous system neuropeptides or microbially derived products during PN, which may improve GI parameters by maintaining immunity and physiology.
Keywords: parenteral nutrition, enteral nutrition, gastrointestinal immunity, GALT, sIgA, Paneth cells, microbiome, mucosal immunity
parenteral nutrition (PN) provides essential caloric and micronutrient support intravenously in individuals with contraindications to enteral feeding following trauma, elective surgery, or the need for bowel rest due to inflammatory conditions. The term “parenteral” derives from the English para (beside) and ancient Greek enteron (intestine) and is most commonly provided via the central vein (jugular or subclavian vein) as a concentrated hypertonic solution containing elemental nutrients, including dextrose, amino acids, electrolytes, vitamins, and usually emulsified lipids (termed total parenteral nutrition or TPN). PN helps preserve lean body mass, supports immune functions, and reduces metabolic complications and oxidative stress in patients who are otherwise unable to feed (67). Approximately 40,000 individuals remain permanently dependent on TPN and another 350,000 routinely require it for prolonged periods annually to prevent or treat malnutrition in the United States (81). Prior to the clinical implementation of PN, countless numbers of individuals suffered from advanced malnutrition and many others were fated to starve to death.
Despite its clinical importance, the first successful long-term PN administration was not achieved until 1968, highlighting the complexity of this technique (19). The attempts to formulate standardized elemental nutrition solutions by the Mercury space program accelerated the field. Previous attempts at intravenous administration of various solutions, ranging from saline to milk to wine, had been attempted for almost 400 yr (105), but successful PN feeding to a human infant for 6 wk by Dudrick et al. (19) ignited a rapid expansion of use that continued for three decades. During that time, PN was used in both pre- and postoperative patients regardless of nutrient status. Many well-nourished patients or those who could otherwise feed enterally were prophylactically given PN. However, PN inherently carries significant risks related to vascular access, catheter infections, and metabolic complications related to the hypertonic glucose solution, and therefore patients were exposed to these and other risks without any certainty of benefit (79, 109).
The widespread administration of PN was challenged by clinical trials, including one in 400 general surgery patients that preoperatively provided either PN (along with ad libitum oral intake) or a control group provided only ad libitum oral intake (100a). The study found PN increased the risk of major infections and had no impact on noninfectious complication. However, stratification of patients by nutritional status demonstrated PN benefited malnourished patients with improved wound healing compared with controls (100a). These findings highlighted the benefit of PN is greatest in patients with existing nutritional deficiencies. It also illustrated an unnecessary risk in providing PN to well-nourished patients. Identifying malnourished patients remains a challenge, since no universal definition of malnutrition exists and metrics for quantifying nutritional status vary between disease states (64). However, today greater efforts are made to limit PN use to general surgery patients with preexisting malnutrition or patients not expected to tolerate enteral feeding within 7–10 days, since depletion of lean muscle tissue usually occurs within 14 days.
In addition to general surgery, acute infectious and traumatic injuries increase nutritional demands due to hypermetabolism (67). Since humans typically carry 1,200 kcals in glycogen storage, lean muscle mass and peripheral fat are rapidly mobilized to support metabolic requirements in response to injury (102). An increased risk of malnutrition occurs following injury with enhanced immune activation, increased oxygen consumption, and elevated muscle catabolism that result in negative nitrogen balances. Nutrition therapy becomes necessary when patients are unable meet nutritional requirements despite no preexisting deficiencies. While it remains difficult to distinguish the contributions of malnutrition vs. critical illness on clinical outcomes, a greater severity of injury generally correlates with greater risk of malnutrition (4, 70).
It is accepted that enteral nutrition is the preferred route of nutrition therapy in all hemodynamically stable patients who can tolerate oral feeding. Enteral nutrition also has risks, since it is more challenging to obtain gut access, deliver calorie goals, and may induce diarrhea, abdominal distention, and gut upset. However, large numbers of clinical trials now demonstrate a greater benefits with enteral nutrition across many patient populations compared with PN, specifically against the risk of intra-abdominal abscess and pneumonia, length of stay, cost, and mortality (45, 48, 63, 68–70, 79, 94, 100a). Enteral nutrition is even preferred in patients with mild pancreatitis (63). Nevertheless, many patients remain unable to feed enterally and therefore require PN to prevent malnutrition. This review discusses how PN impacts the gut immune system and microbiome, providing a cogent explanation for the greater risk of infection and injury observed during PN.
Early Evidence for the Importance of Enteral Feeding
In the late 1970s and early 1980s, basic science research investigating sepsis models demonstrated malnourished animals survived 10% of the time compared with 70% in well-nourished animals (80). However, if the well-nourished animals were given PN they survived only 10% of the time. While it was thought the PN solution was incomplete, lacking essential nutrients, subsequent work demonstrated animal survival returned to normal when PN was instead given orally, compared with identical volumes provided intravenously (47, 50). These observations highlighted the importance of enteral intake and suggested the gut provides immunological advantages, but mechanisms remained unknown. These observations led to several decades of basic science research into the changes in gut and respiratory immune tract function during PN in murine models, which are summarized in the following sections.
Overview of Gastrointestinal Innervation and Immunity
The gastrointestinal (GI) tract is the largest digestive, endocrine, and immune organ in the body, handling the breakdown and acquisition of nutrients as well as influencing peripheral nutrient handling. The extensive GI neurological networks include the autonomic system and enteric nervous system (ENS) (74), where the autonomic system sympathetic and parasympathetic fibers communicate with the spinal cord and central nervous system (CNS) through the dorsal root ganglia. The ENS, however, is autonomous from the CNS and contains ~108 sensory, motor, and interneurons that release acetylcholine and neuropeptides, including gastrin-releasing peptide (GRP) (74). In response to ingested nutrients and bulk, GRP normally stimulates enteroendocrine cell (EEC) hormone cascades that orchestrate intestinal motility, digestive enzyme production, bile and bicarbonate release, splanchnic bed blood flow, and electrolyte section, which collectively influence the microbial community (16).
ENS fibers are found in close approximation with epithelial cells and immune cells, providing the ENS with the ability to innervate up to 70 or 80% of the total active immune cells in the body (98). Together, these cells defend an enormous interface, approaching 400 m2 in humans, between the host and “external” gut environment. The intestinal mucosa is challenged by continuous interaction with dietary, microbial, and environmental antigens and toxins and must respond accordingly to either defend against injurious/infectious agents or mount tolerance against harmless agents to conserve energy and maintain symbiosis. To achieve this, multiple levels of defense exist, ranging from simple cellular barriers, complex secreted chemical layers, immunological release of specific and nonspecific immunoglobulins (antibodies), and maintenance of a diverse microbial community and their collective genes: the gut microbiome (74). Together, these defenses enable digestion, immune maintenance, and tolerance of commensal microorganisms and the prevention of pathobionts from infecting the host across feeding and fasting cycles.
Changes in Gastrointestinal Immunity Following PN
GALT following PN.
Beneath the epithelial surface a staggering number of adaptive and innate immune cells reside in the GI, termed the gut-associated lymphoid tissues (GALT). Approximately 70% of the total active immune cells in the body normally participate in this compartment (98). A primary GALT function is luminal antigen sampling and release of sIgA on mucosal surfaces, including the GI, but also the respiratory, mammary gland, urogenital tract, salivary glands, and tear ducts. Through antigen-specific and nonspecific binding, sIgA protects mucosal surfaces by pathogen opsonization and exclusion (61). sIgA also mediates immune tolerance when antigens bound by sIgA are phagocytized by antigen-presenting cells, leading to dampened inflammatory immune responses and Treg induction (22). PN induces gut atrophy, characterized by decreased organ wet weight and reduced bowel circumference. Changed in mucosal thickness and total DNA content following PN remains minor, around −10%. Atrophy occurs as a function of decreased GALT cellularity and vascular perfusion, which are related since naive lymphocytes normally circulate systemically and interact with ligands as they enter the splanchnic vasculature (38).
In mice, the total number of Peyer’s patch lymphocytes decreases significantly within 24-48 h after starting PN, reaching a nadir of 50–75% reductions by 3 days, compared with control animals (55). Specialized epithelial cells (M cells) cover the luminal surface of Peyer’s patches and sample luminal antigen for dendritic cells and naive αβ+ T and B lymphocytes within the germinal centers (60). Naive cells express high levels of the integrin L-selectin and moderate levels of the integrin α4β7. L-selectin has high-affinity binding to a modified form of mucosal addressin cellular adhesion molecule-1 (MadCAM-1) found on the high endothelial venules of the Peyer’s patch (98). Following attachment to these ligands, the chemokines, CXCL13, CCL19, and CCL21, direct lymphocytes into the Peyer’s patch via diapedesis (108). Interestingly, no changes occur in the ratios of total T to B lymphocytes and relative population of various subsets. Specifically, the ratio of CD4+ to CD8+ as well as the relative percent of memory cells, activated cells, and naive cells within both the T and B lymphocyte populations remain stable in the Peyer’s patch (36).
MAdCAM-1 expression on the Peyer’s patch high endothelial venules is regulated by activation of at least two pathways, including lymphotoxin β receptor (LTβR) and noncanonical NFκB signaling (13). Active circulating lymphocytes express lymphotoxin α and β on their surface that stimulates MAdCAM-1 and the Th2 cytokine IL-4 through LTβR binding (17). Following PN, the expression of LTβR is significantly decreased within the Peyer’s patch structure as well as the lamina propria compared with enterally fed animals. As a result, MAdCAM-1 levels begin decreasing within 4 h of PN feeding, and expression is maximally reduced after just 48 h (32). Experimentally, blocking LTβR with chimeric Ig fusion proteins in control animals significantly lowers levels of MAdCAM-1 expression. Conversely, providing stimulation of the LTβR during PN, through agonist anti-LTβR monoclonal antibody, results in normalized Peyer’s patch cellularity and higher sIgA levels in the lumen (38). The second pathway that regulates MAdCAM-1 expression is the noncanonical (or alternative) NFκB signaling pathway, which is also stimulated through lymphoid receptors and therefore influenced by LTβR signaling. While the canonical (or classical) NFκB pathway responds to stress or infection and results in proinflammatory cell responses, noncanonical NFκB stimulation induces nuclear P52/RelB dimer formation and expression of MAdCAM-1.
PN feeding results in a decrease in both NFκB pathways, canonical and noncanonical (51). These mechanisms were investigated empirically during PN in mice. In control animals, blocking LTβR signaling results in decreased nuclear P52/RelB and subsequently lower MAdCAM-1, CCL19, CCL20, and CCL25, but does not influence canonical NFκB proteins (32). Reciprocally, agonist stimulation of LTβR during PN feeding elevated levels of MAdCAM-1, P52/RelB, and IL-10, but not Peyer’s patch chemokines. These studies demonstrate the primary outcome of PN on Peyer’s patches is decreased cell recruitment, fewer activated mucosal-specific lymphocytes, and reduced lymphoid signaling. Finally, this hypothesis is supported by blockade experiments of recruitment ligands by monoclonal antibodies for MadCAM-1, L-selectin, or α4β7 in enterally fed control animals (32). Blocking each of these results in decreased Peyer’s patch cellularity that mimics cellularity changes in PN. Furthermore, refeeding enterally after PN reciprocally restores normal Peyer’s patch cellularity within 48 h, showing these changes are sensitive and reversible (33).
After leaving the Peyer’s patch, cells migrate through the mesenteric lymph nodes, exit the thoracic duct, and home back to mucosal effector sites. Following PN, few changes are observed in the mesenteric lymph node lymphocyte populations (55), perhaps because of the limited size and transient nature of this immune compartment. Once in circulation, GALT αβ+ lymphocytes, now expressing lower levels of L-selectin and elevated α4β7, home back to mucosal effector sites throughout the body by binding the unmodified form of MadCAM-1 (23). In the gut, the effector site is the lamina propria and MAdCAM-1 mediates diapedesis into the lamina propria, aided by the chemokines CCL25 and CCL28. During this migration process, B lymphocytes progressively mature into IgA-producing plasma cells, with only 2% of Peyer’s patch B cell population producing IgA, but this increases to 50% in the mesenteric lymph nodes (MLN), 75% in the thoracic duct, and nearly 100% in the lamina propria (77). Within the lamina propria T helper (Th) lymphocyte subpopulations include anti-inflammatory Th2, proinflammatory Th1, Th17, and Th22, and T regulatory (Treg) cells (59). The Th2 cells predominately produce the cytokines IL-4, IL-5, IL-6, IL-9, IL-10, IL-13, and IL-25, which support plasma cell IgA production and epithelial cell IgA transport through the transport protein polymeric immunoglobulin receptor (pIgR) expression (37). pIgR binds to dimeric IgA at the basolateral surface of enterocytes where endocytosis carries the IgA-pIgR complex to the apical surface. There, the IgA-pIgR complex is cleaved and sIgA is released into the lumen. IL-17 cytokine production by Th17 cells also supports IgA functions, while an increase in the Th1 cytokines IL-2, IFN-γ, and TNFα decreases the production and release of IgA at mucosal surfaces, through pIgR expression inhibition (9). The more recently discovered Th22 lymphocytes maintain epithelial defense by stimulating antimicrobial production and release. Additionally, the Treg cells produce the cytokines IL-10 and transforming growth factor-β (TGF-β), which promote plasma cell IgA production (5). Animals lacking TGF-β lack detectable sIgA levels at their intestine and respiratory tract surfaces. TGF-β directly stimulates lymphocyte class switch recombination and plasma cell maturation, required for appropriately directed immune response (24).
PN significantly reduces the total numbers of lymphocytes in the lamina propria and induces major shifts in lymphocyte population subsets. Following PN, a reduced global percentage of activated lymphocytes (CD4+CD25+) express the lamina propria homing ligand, α4β7, indicating reduced recruitment of cells designated for mucosal surfaces from circulation (36). Furthermore, within this activated lymphocyte population, fewer express Treg (CD4+CD25+Foxp3+) and memory (CD44+) markers, leading to reduced capacity for tolerance and attenuated response to previously encountered antigens. Reduced Treg cells is also relevant since TGFβ and IL-10 support plasma cell function and counteract the Th1 cytokine, IFN-γ, that negatively regulates production and secretion of sIgA. Consistent with changes in cell populations, PN significantly decreases lamina propria protein and gene expression of IL-4 and IL-10 levels (55).
In contrast to the lower levels of Th2 cytokines, the Th1-producing cells and cytokines remain stable following PN, leading to a skewed Th1-to-Th2 ratio that favors proinflammatory responses to injury. For example, the normal expression of IL-4 and IL-10 inhibits expression of the endothelial neutrophil recruitment ligand ICAM-1. On the other hand, IFN-γ stimulates ICAM-1 expression (10). Following PN, ICAM-1 expression levels increase on endothelial cells and this results in greater neutrophil entry, measured by myeloperoxidase levels, within the gut submucosal compartment (25). Neutrophils enter the intestine, become primed, and migrate to other organs including the lung and liver. PN induces neutrophil changes following hemorrhagic shock, sepsis, and ischemia. For instance, with gut ischemia-reperfusion, elevated activated neutrophils are found in the portal vein blood, but not peripheral blood, demonstrating a gut origin. No changes are found in circulating levels of neutrophil activation markers CD11a and CD11b between PN and enteral feeding at baseline (25, 26). However, within hours of injury, elevated percentages of neutrophils express CD11b in PN, demonstrating a greater propensity for activation compared with controls. As a result, the hepatic and respiratory organs also become more permeable and leak albumin, and animal mortality increases to 50% with PN compared with 95% survival in controls.
In addition to T lymphocyte and neutrophil changes within the intestine, a significant decrease in memory B cells (CD44+) occurs following PN, resulting in a decreased ability of the mucosa to mount antigen-specific IgA release at baseline and in response to injury (35). The reduction in lamina propria Th2 cytokines following PN has implications for IgA release at the intestinal (and other mucosal) surfaces. First, lower Th2 cytokines, including IL-4, IL-5, IL-10, and IL-13, result in lower plasma cell IgA production in the intestine and lung (49). Second, Th2 cytokines help regulate epithelial cell function, including pIgR expression, which transports IgA to the mucosal surfaces. While PN does not alter airway pIgR expression, it results in significantly lower pIgR expression and protein levels in the intestine, decreasing IgA transport capacity. Accordingly, following PN, reduced levels of pIgR-stimulating Th2 cytokine levels, IL-4, IL-5, IL-10, and IL-13, and stable levels of the pIgR-inhibiting cytokine IFN-γ results in significantly reduced sIgA release in the gut (91). Similar to the Peyer’s patches, these cell populations and cytokine profiles are normalized within 2 days of enteral feeding.
The importance of these mucosal IgA responses is highlighted by mucosal vaccination studies. Mucosal exposure to inactivated poliovirus or enterotoxogenic Escherichia coli results in the release of sIgA specific to these pathogens at mucosal surfaces throughout the body (34, 71). Mothers who are infected with Salmonella produce colostrum containing sIgA specific to this pathogen, while uninfected mothers do not (40). Interestingly, IgA levels do not increase systemically in these and other studies, supporting the concept that the mucosal compartment of the immune system is distinct from the systemic compartment.
Functionally, PN-induced loss of adaptive immunity reduces antigen sequestration and removal of pathogens. This concept was demonstrated with bacterial and viral challenge in rodent models. Providing animals immunization to Pseudomonas (Ps) aeruginosa antigen confers 90% survival when exposed to a subsequent intratracheal Ps challenge, compared with only 10% survival in nonimmunized animals (44). Following immunization with Ps, providing PN or enteral nutrition differentially influences survival. Despite immunization, PN-fed animals survive only 10% of the time compared with 90% survival observed in controls. In the model of intranasal H1N1 virus challenge, animals shed virus for 2 wk following first exposure before increasing H1N1-specific IgA. Mice were then placed on enteral feeding or PN regimens and challenged again. Enterally fed animals cleared the virus within hours; however, PN animals shed H1N1 virus for several days following the second exposure (49). These studies illustrate the impact of PN on adaptive immune memory and function at mucosal surfaces. Importantly, memory is not lost following PN and memory T and B lymphocytes remain viable, since refeeding animals for as little as 48 h after PN is enough to restore rapid adaptive immune response.
These findings indicate that lack of enteral stimulation leads to dramatic changes in the GALT compartment, characterized by lowered lymphocyte cellularity and function in the Peyer’s patch and lamina propria and decreased Th2 cytokines, IgA production, and release on mucosal surfaces. At the same time, innate immune cells such as neutrophils accumulate and become primed, leading to exaggerated proinflammatory responses following injury. Together, these changes fundamentally alter the immunological response of the intestine toward injurious and infectious challenges that are disadvantageous for host homeostasis.
Cellular and chemical barriers following PN.
The most basic line of defense in the gut is the epithelial barrier, which is one of the most actively proliferating cell populations in the body. At the base of the small intestinal crypts, Lgr5+ stem cells rapidly divide and proliferate feeding cells upward toward the villus tips that result in epithelial replacement every 3–5 days (2). Exceptions are small bowel Paneth cells that remain at the crypt base and live between 20 and 30 days. Tight-junction proteins, including zonulins, occludins, and claudins, bind epithelial cells while allowing transient movement of water, electrolytes, and some macromolecules between cells (46). Recent data indicate lamina propria dendritic cells extend dendrites between epithelial cell tight junctions to the lumen for antigen sampling (53). Around 90% of intestinal cells are enterocytes with the remaining 10% comprising specialized endocrine cells (called enteroendocrine cells), mucus-secreting goblet cells, and the specialized microfold cells (M cells) that cover the Peyer’s patch (60). Following PN, epithelial permeability increases significantly (39, 106) and the expression of tight-junctions is decreased. The altered cytokine ratios, especially reduced IL-10 with maintained IFN-γ, contribute to decreased barrier function. Following PN, animals lacking IFN-γ do not display reduced barrier function that occurs in controls. Furthermore, exogenous administration of IL-10 to wild-type animals during PN prevents barrier function loss, albeit not completely, implicating the role of additional cytokines in this regulation (106).
In addition to forming a barrier, the production and release of antimicrobial products that influence microbial composition and function are important contributors of epithelial defense. Enterocytes produce constitutive levels of β-defensins and RegIIIγ that limit microbial growth beneath and within the mucus barrier (100). Since enterocytes comprise 90% of total epithelial cells, this constitutive expression is a major contributor to mucosal microbial defense. Furthermore, Paneth cells are predominately producers of antimicrobial products, including lysozyme, RegIIIγ, sPLA2, angiogenin-4, and α-defensins (termed cryptdins in mice) (90). Paneth cell products regulate microbial growth by targeting various conserved aspects of microbial growth. Estimates suggest the concentration of Paneth cell cryptdins alone may reach 15–100 mg/ml within the crypt space, exceeding levels needed to inhibit microbial growth (1). Following antimicrobial release, these products localize to the anionically charged mucus surface through cationic charge interactions (65). Within the colon, the tight inner layer remains sterile, while microbes colonize a more loosely organized outer layer. In contrast, the small intestine only contains a loose layer of secreted and membrane-bound mucus.
Paneth cell production of antimicrobials is in part regulated by Th2 cytokines, including IL-4, IL-9, and IL-13, the EEC hormone GLP-2, and stimulators of mTORC1, such as insulin (12, 52, 97). The release of intracellular granules containing antimicrobial peptides is stimulated by microbial ligand recognition by Toll-like receptors (TLRs), NOD2, and cholinergic stimulation by parasympathetic nerve innervation (73). Following PN, reduced levels of IL-4 and IL-13 are associated with reduced Paneth cell gene expression of lysozyme, sPLA2, cryptidin-4, and RegIIIγ (8, 29). Enterocyte expression of RegIIIγ is also significantly reduced with PN (54). Luminal levels of these products are also decreased following PN. However, experimental administration of the Th2-stimulating cytokine IL-25 during PN maintained IL-4 and IL-13 cytokine levels and tissue and luminal lysozyme, sPLA2, and RegIIIγ levels, compared with PN alone (30).
Goblet cells produce large mucous glycoproteins, termed mucins, that contain many complex posttranslational modifications including O-glycosylations, N-actyl-galactosamine, galactose, and N-actyl-glucosamine residues (43). Upon release from the goblet cell theca, secreted mucins can expand up to 3,000-fold while absorbing water and subsequently cover the epithelial surface. Mucus functions as a defensive physioelastic layer, allowing passage of ingested contents and limiting microbial access to the surface. In addition to physical barrier functions, mucins contain charged residues, including sialic acid, that localize and concentrate antimicrobial peptides and secreted immunoglobulin-A (sIgA) to the mucus surface (65), providing immunological protection. MUC2 is the most highly expressed mucin in the small intestine (43). Animals lacking the MUC2 gene spontaneously develop enteritis and eventually colonic tumors. In addition to physical and functional properties, data suggest that the glycans contained in the hyperglycosylated MUC2 structure directly inhibit dendritic cell proinflammatory signaling through NFκB, but not tolerogenesis pathways (93).
Following PN, no changes are observed in total goblet cell numbers, but gene expression and luminal levels of MUC2 and trefoil factor 3 (TFF3) are decreased in neonatal piglets and mice (6, 97). MUC2 serves multiple roles in epithelial defense, while TFF3 is thought to promote epithelial repair following injury. Additionally, RELMβ protein and gene expression levels are reduced following PN (6). The function of RELMβ was only recently determined using knockout animals and a model of Citrobacter rodentium enteric infection that induced ulcerative disease of the intestinal mucosa. Unlike TFF3, RELMβ has no direct affect on epithelial proliferation but instead functions as a CD4+ T lymphocyte chemoattractant. The subsequent recruitment of active CD4+ T cells results in IL-22 release that stimulates antimicrobial production by the epithelium (3). The reduction in RELMb following PN suggests this lymphocyte recruitment mechanism is also attenuated. Similar to Paneth cells, goblet cell products are also under the regulation of Th2 cytokines, including IL-4, IL-13, and IL-25 (72). Providing the cytokine IL-25 during PN significantly enhances the total number of goblet cells, elevates tissue IL-4 and IL-13, and increases tissue and luminal MUC2 levels compared with either controls or PN alone (29).
Functionally, these changes hallmarked by decreased Th2 and elevated Th1 cytokines have implications for pathogen susceptibility. Small bowel tissue from PN-fed animals releases lower levels of antimicrobial compounds, including sPLA2, following cholinergic stimulation, compared with enteral controls, which resulted in a reduced capacity to kill P. aeruginosa colony-forming units (CFUs) in vitro (73). This affect was in part due to sPLA2 activity, since adding a catalytic inhibitor for sPLA2 reduced some bactericidal activity of the tissue secretions. Using another technique that examines mucosal susceptibility directly, ex vivo intestinal segment culture, PN administration led to decreased mucosal sPLA2 release and significantly increased enteroinvasive E. coli CFUs in less than 60 min, compared with tissue from control animals (83).
EECs express peptide hormones and mediators that influence gut and peripheral metabolism, physiology, and neurocognition (28). While EECs make up less than 1% of the total number of epithelial cells, they collectively qualify the intestine as the largest endocrine organ in the body. EECs sense luminal nutrients, such as free fatty acids, short-chain fatty acids (SCFAs), glucose, and amino acids, and it was recently recognized that EECs also sense bacterial ligands through high expression of TLRs (75). In response to stimuli, EECs release over 18 peptide hormones, but also the chemokines CXCL-1 and CXCL-3 and the cytokine IL-32, which link them with innate immune regulation (92). Some EEC hormones also appear to directly orchestrate immune function. For instance, mice lacking the GLP-2 receptor have diminished Paneth cell antimicrobial expression and are more susceptible to enteric bacterial infections compared with wild-type counterparts (52). The facet of endocrine-immune cross talk remains largely unexplored in the context of PN but could be fundamentally important for understanding immune function since these interactions represent teleologically ancient signaling that predates the evolution of adaptive immune cells.
The intestinal microbiome following PN.
The vast gut microbial communities (the microbiota) have vital roles in defense and function. The microbiota includes bacteria, archaea, yeasts/fungus, protists, and eukaryotic and prokaryotic viruses that amount to a physical mass of 2–3 kg in the average adult and their collective genes (the microbiome) are estimated to increase the genetic diversity of the mammalian host by 150-fold (95). The bacterial population alone is estimated to contain around 10 trillion organisms from 10 major phyla, including Firmicutes, Bacteriodetes, and Actinobacteria. At the most basic level, microbial organisms assist with nutrient breakdown and synthesis of novel compounds, including SCFAs and vitamins. Commensal organisms also outcompete pathogens for access to the host through competitive exclusion. Commensals feed in part on endogenous secretions from the host, including goblet cell mucin glycoproteins (88), where the presence of extensive O-glycans within the mucin structure serves as a microbial substrate and facilitates epithelial colonization by A. muciniphila, B. thetaiotaomicron, and B. fragilis, among others. Some commensals also produce targeted antimicrobial peptides to limit the growth of other organisms, recently demonstrated by commensals that produce targeted antibiotics against methicillin-resistant Staphylococcus aureus (MRSA) (110).
Except in examples of hibernation or extreme fasting, which are associated with dramatic changes hormone and metabolic set points, the host-microbial relationship is fundamentally influenced by dietary intake and feeding cycles. The most dramatic influencer of microbial community composition is diet. Recent work demonstrated that dietary type (animal or plant based) dramatically shifts the microbiome in as little as 24 h, demonstrating the rapid adaptability of this microbial “virtual organ” (14). During feeding, microbes metabolize otherwise inaccessible dietary nutrients, synthesize de novo compounds including K and B vitamins, and influence intestinal signaling to the peripheral organs (62). Microbes and their small molecules modulate the release of epithelial-derived compounds that target the pancreatic islet and acinar cells (GLP-1, secretin), liver metabolism (FGF15/19), and gall bladder contractility (CCK), among myriad other hormonal, neuronal, immunological, and metabolic functions.
The lack of enteral feeding with PN is associated with dramatic changes in bacterial community structure; changes in nutrient sources within the gut ecological landscape create new environmental pressures for organismal succession patterns. Following PN, the ileal luminal and mucosal associated microbial populations are characterized by decreased relative percentages of the phylum Firmicutes, but increased relative percentages of the phyla Bacteroidetes and Proteobacteria (31, 66). The lumen also demonstrates increased percentages of the phylum Actinobacteria, while the mucosal population displays elevated Verrucomicrobia following PN. The latter phylum is represented by a single mucosal-associated species, Akkermansia muciniphila, which depends on host mucin glycoprotein secretion as a metabolic substrate. In the absence of dietary nutrients, the expansion of A. muciniphila at the mucosal surface represents microbial competitive advantage and succession of the mucous microenvironment. Conversely, the phylum Firmicutes contains many species that prefer dietary carbohydrate metabolism and are therefore less competitive than Proteobacteria during PN (31). Proteobacteria can metabolize broader classes of substrates, including amino acids, and are therefore more starvation resistant than other phyla. Ralls et al. (87) demonstrated labeled TPN amino acid metabolites migrate into the gut lumen and are incorporated into microbial species, including Enterobacteriaceae of the phylum Proteobacteria, fundamentally demonstrating the utilization of host-derived substrates by gut microbial organisms in the absence of enteral feeding. Furthermore, recent work demonstrated the microbial community structure reproducibly oscillates in structure over 24-h circadian rhythms (54). Community structure oscillation patterns occurred both under enteral feeding and PN, eliminating diet as an influence, although different species are present during PN. These findings illustrate the microbial-host interdependence, where microbial communities utilize endogenous host substrates while also synthesizing substrates needed and utilized by the host.
Unfortunately, the changes in microbiome that occur with PN are also characterized by a loss of ecosystem diversity and decreased competitive exclusion of pathogens by the normally robust commensal organisms. For instance, the bloom of Proteobacteria observed following PN includes the opportunistic pathogens E. coli, Salmonella, Yersinia, Helicobacter, and Vibrio, which are commonly associated with infection (31, 66). At the same time certain beneficial commensals, including Bacteroides fragilis, which normally stimulate Treg function and sIgA release by the mucosa decrease with PN (15). These microbial compositional changes occur in concurrence with decreased release of sIgA at the mucosa, as described above. Loss the sIgA enables greater microbial access to the host epithelium. Furthermore, transfer of sterile gut luminal effluent (the metabolome) from PN or enterally fed animals into germ-free hosts demonstrated that the resulting PN metabolome alone induces loss of epithelial barrier and upregulates inflammatory mediators compared with the metabolome from enterally fed animals (87). This suggests the altered microbial community and change in collective small molecules can exacerbate impaired intestinal defense during PN.
In addition to the bacterial pathogens, the yeast/fungal pathogen Candida albicans is a major infectious organism in the ICU, often colonizing catheter surfaces (89). Under normal conditions, C. albicans maintains a lifelong association with human (and many other mammalian) GI tracts and does not normally cause disease. While it remains unclear whether intravenous catheter infections of C. albicans are derived mostly from the gut or skin reservoirs (some certainly come from skin because improved sterile techniques reduce infection rates), experimental intestinal C. albicans exposure during PN leads to increases colonization, mucosal translocation, and disseminated systemic infection of C. albicans compared with enteral feeding (76). These data indicate PN reduces normal GI barrier function against opportunistic pathogens. Since the intestinal microbiota serves as an important priming stimulus for the immune system, promoting various effector and regulatory T lymphocyte populations, it is not surprising that microbial dysbiosis associated with PN can precede aberrant proinflammatory responses to normally harmless stimuli (18, 101).
Surrogates To Stimulate Intestinal Function During PN
PN is necessary in patients who are unable to feed enterally to meet their metabolic demands. Often these individuals are also faced with hypermetabolism following trauma or infection or have elevated systemic inflammation and altered immune status. Since PN is associated with greater risks of infection and the loss of mucosal immune functions in the intestine and respiratory tracts compared with enteral feeding, investigators have searched for PN additives to maintain metabolic and immune function. The use of immune enhancing diets has received attention, including the specific addition of glutamine, arginine, leucine, ω-3 fatty acids, micronutrients (vitamin C, selenium, and zinc) and nucleotides. Of these, glutamine is the most well investigated and empirically supported, being the most abundant free amino acid normally in circulation but becoming conditionally essential during critical illness (104). The addition of nutrients in basic science and clinical studies has been the focus of recent reviews (82). In addition to nutrients, direct targeting of the ENS and the use of microbial metabolites may hold promise in stimulating gut function and immunity during PN (see Table 1).
Table 1.
Summary of known stimulatory functions for the ENS agonist, BBS, and microbial metabolites on aspects of mucosal immunity
| Compounds | GALT Cellularity | Plasma Cell Ig Production | Epithelial Barrier | Paneth Cell Antimicrobials | Goblet Cell Mucus |
|---|---|---|---|---|---|
| BBS | ↑ | ↑IgA | ↑ | ↑ | ↑ |
| AHR ligands | ? | ↑IgA,↓IgM | ? | ↑ | ? |
| SCFAs | ↑ | ↑IgA | ↑ | ? | ↑ |
| Polyamines | ↑ | ↑IgA | ↑ | ? | ↑ |
Arrow indicates direction of mucosal parameter change; ? indicates unknown response.
Enteric nervous system molecules.
Another approach specifically to enhance immune function is the utilization of ENS neuropeptides. The ENS is autonomous from the CNS and contains 108 neurons that release neuropeptides, including GRP, substance P, and vasoactive intestinal peptide, which influence many aspects of GI physiology. Over 2 meters of nerves innervate each cubic centimeter of intestinal mucosa. In response to feeding cycles, GRP release by the ENS regulates numerous aspects of GI function by propagating release of numerous downstream effector molecules. During PN, significant bowel wall contraction also occurs, but the percentage of nNOS-expressing ENS cells remains unchanged, suggesting the ENS remains in tact during PN (21). The GRP analog bombesin (BBS) shares seven amino acid residues and binds GRP receptors. BBS has been extensively studied in rodents to maintain mucosal immune function during PN. Several EEC compounds have also been investigated during PN in rodents, including neurotensin and CCK but are not as effective as BBS (28a). It should be noted that the use of stimulatory compounds may not be safe in patients, especially the critically ill, but the following are a summary of results from animal studies demonstrating proof of concept.
Within the GALT, BBS restores murine cellularity of the Peyer’s patch and lamina propria compartments during PN (36). While BBS administration has little effect on MAdCAM-1 expression, it increased blood flow to the splanchnic bed. Within the lamina propria, BBS administration during PN results in restoration of cells displaying the mucosal homing ligand (α4β7) as well as an increased relative percentage of activated (CD25+), memory (CD44+), and Tregs (FOXp3+) lymphocytes (36). These changes prevent the decreased CD4-to-CD8 ratio and loss of Th2 cytokines that occurs with PN alone. BBS also elevates B cells within the lamina propria compared with PN alone. Increased IL-10 levels following BBS support plasma cell function and tissue pIgR levels and maintain sIgA release at mucosal surfaces, both at baseline and in response to injury (35, 84, 107). Accordingly, the loss of adaptive immunity to H1N1 and P. aeruginosa following PN, previously described, is also maintained with BBS administration in animals (28a). Immunized animals provided only PN survive infectious challenge to P. aeruginosa and shed H1N1 virus following exposure similar to nonimmunization animals. The mechanism likely involves the restoration of IgA producing plasma cells that aid in pathogen clearance with BBS stimulation. In addition to immunological affects, BBS normalizes bowel wall constriction and elevates fecal output compared with PN alone (21). Interestingly, BBS also maintains pancreatic function during PN, influencing metabolic parameters (85).
These data demonstrate that GI stimulation with ENS neuropeptides, including BBS, during PN maintains GALT function and mucosal adaptive immune responses to pathogens in rodents despite an absence of enteral intake and stimulation. Additionally, BBS influences the epithelial barrier, including Paneth antimicrobial levels (7). During PN, BBS maintains expression of sPLA2 and lysozyme, but not luminal levels. While BBS stimulates gene and protein expression, parasympathetic stimuli are still required for degranulation of antimicrobials. BBS also prevents the increased susceptibility of ex vivo ileal explants to enteroinvasive E. coli observed in PN alone (7). The utilization of ENS neuropeptides works in animals and could hold promise for the stimulation of normal immunological function in the absence of the feeding signals that initiate GALT function, adaptive immunity, and mucosal barrier function including antimicrobial peptide production.
AHR molecules.
Since the intestinal microbiome produces many known molecules that modulate host physiology and immune function, many that are reduced under PN, exploring the use of these compounds offers the potential to discover surrogates for enteral stimulation. One class of compounds is the aryl hydrocarbon receptor (Ahr) ligands that, in the gut, influence IL-22 production and have the potential to influence epithelial defense (86). IL-22 stimulates antimicrobial production by the intestinal epithelium. Animals deficient in Ahr demonstrate increased susceptibility to C. rodentium and greater mortality. While Ahr is not involved in plasma cell IgE production, and possibly inhibits IgM production, Ahr stimulation elevates B cell maturation in bone marrow and has not been explored in the stimulation of IgA in the gut (58, 99).
SCFAs.
Another class of microbial metabolites are the SCFAs, which are produced in abundant quantities under normal feeding, reaching −130 mmol/kg in colonic contents and normally found in circulation. Through G protein-coupled receptors, including GRP41, GPR42, GPR109a, and others, SCFAs serve as ligands for epithelial and immune function, as well as metabolic substrates when absorbed by the epithelium. In animals, SCFA administration increases the numbers of IgA+ B cells in the gut, as well as IgG and IgA levels in serum (42). SCFA administration also enhances IgA production in the gut of mice during infection with C. rodentium and enhances IgA levels specific to the pathogen. SCFAs downregulate inflammatory responses by monocytes and induce differentiation of Treg and IL-10-producing T cells (96). In human plasma cells, SCFAs have been shown to enhance IgG and IgA production in vitro (42). Furthermore, SCFAs stimulate goblet cell mucin gene expression and improve barrier function (27, 103).
Polyamines.
Polyamines are polycationic molecules derived from host and microbial cells and are found in high levels in the GI tract. Feeding polyamine-deficient diets leads to mucosal hypoplasia and loss of development (57). Consistently, polyamines also drive epithelial barrier function and tight-junction proteins, enhancing occluding and E-cadherin expression, and improve mucus production (11, 56). Within the GALT compartment, polyamines have been shown to drive IgA production and enhance maturation of lamina propria CD4+ T cells (20, 78). The use of these and other microbial metabolites that have evolved with the mammalian gut offer conserved mechanisms for the stimulation of gut function in the absence of normal dietary intake.
Conclusions
PN provides critical nutrition support in many patients who are unable to feed adequately via their GI tract (see Fig. 1). The development and use of PN has saved countless numbers of lives, and clinical refinement of this complicated technique continues to improve. Unfortunately, PN is also associated with greater risk of infection in the abdomen and respiratory tract compared with enteral feeding. Despite the fact that many patients who require PN are inherently more critically ill and sometimes malnourished than those who can tolerate enteral feeding, decades of basic science and clinical research support the hypothesis that dramatic changes within the GI tract also influence clinical outcomes by impairing immune function and susceptibility to infection. The findings that PN negatively alters GALT-adaptive and innate immune cells with the loss of mucosal sIgA release, impairs epithelial barrier and chemical secretions, and disrupts the normal microbiome provide cogent explanations for increased infectious susceptibility and exaggerated inflammatory responses during PN. Fortunately, many of these GI functions are also under the regulation of ENS innervation. Since enteral nutrients normally stimulate the ENS, using strategies targeting ENS innervation of GI immunity and physiology may maintain mucosal immune functions. Exogenous administration of the GRP neuropeptide analog BBS improves immune function and reduces infectious susceptibility in rodent models. Explorations of administering microbial metabolites, such as Ahr ligands, SCFAs, and polyamines during PN are open areas of investigation that may also hold promise in maintaining GI function.
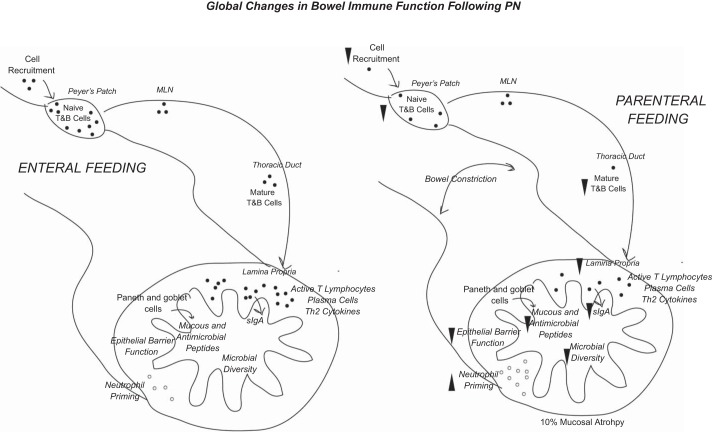
Fig. 1.
Overview of the global changes that occur in the intestine during PN compared with enteral feeding. Following PN, reduced cell recruitment leads to fewer lymphocytes in the GALT, resulting in less IgA production and release at mucosal surfaces. These changes are associated with reduced tight-junction protein expression, antimicrobial production, and mucus release. The altered mucosal secretions and absence of enteral nutrients results in microbial community dysbiosis, which may independently lead to further barrier disruption.
GRANTS
This work was funded by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK105728-01A1.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
J.F.P. drafted manuscript; edited and revised manuscript; approved final version of manuscript.
REFERENCES
- 1.Ayabe T, Satchell DP, Wilson CL, Parks WC, Selsted ME, Ouellette AJ. Secretion of microbicidal alpha-defensins by intestinal Paneth cells in response to bacteria. Nat Immunol 1: 113–118, 2000. doi: 10.1038/77783. [DOI] [PubMed] [Google Scholar]
- 2.Barker N. Adult intestinal stem cells: critical drivers of epithelial homeostasis and regeneration. Nat Rev Mol Cell Biol 15: 19–33, 2014. doi: 10.1038/nrm3721. [DOI] [PubMed] [Google Scholar]
- 3.Bergstrom KSB, Morampudi V, Chan JM, Bhinder G, Lau J, Yang H, Ma C, Huang T, Ryz N, Sham HP, Zarepour M, Zaph C, Artis D, Nair M, Vallance BA. Goblet cell derived RELM-β recruits CD4+ T cells during infectious colitis to promote protective intestinal epithelial cell proliferation. PLoS Pathog 11: e1005108, 2015. doi: 10.1371/journal.ppat.1005108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borlase BC, Moore EE, Moore FA. The abdominal trauma index—a critical reassessment and validation. J Trauma 30: 1340–1344, 1990. doi: 10.1097/00005373-199011000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Bowcutt R, Malter LB, Chen LA, Wolff MJ, Robertson I, Rifkin DB, Poles M, Cho I, Loke P. Isolation and cytokine analysis of lamina propria lymphocytes from mucosal biopsies of the human colon. J Immunol Methods 421: 27–35, 2015. doi: 10.1016/j.jim.2015.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Busch RA, Heneghan AF, Pierre JF, Neuman JC, Reimer CA, Wang X, Kimple ME, Kudsk KA. Bombesin preserves goblet cell resistin-like molecule β during parenteral nutrition but not other goblet cell products. JPEN J Parenter Enteral Nutr 40: 1042–1049, 2016. doi: 10.1177/0148607115585353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Busch RA, Heneghan AF, Pierre JF, Wang X, Kudsk KA. The enteric nervous system neuropeptide, bombesin, reverses innate immune impairments during parenteral nutrition. Ann Surg 260: 432–44r, 2014. doi: 10.1097/SLA.0000000000000871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Busch RA, Jonker MA, Pierre JF, Heneghan AF, Kudsk KA. Innate mucosal immune system response of balb/c vs c57bl/6 mice to injury in the setting of enteral and parenteral feeding. JPEN J Parenter Enteral Nutr 40: 256–263, 2016. doi: 10.1177/0148607114558489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cao AT, Yao S, Gong B, Elson CO, Cong Y. Th17 cells upregulate polymeric Ig receptor and intestinal IgA and contribute to intestinal homeostasis. J Immunol 189: 4666–4673, 2012. doi: 10.4049/jimmunol.1200955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang Y-J, Holtzman MJ, Chen C-C. Interferon-gamma-induced epithelial ICAM-1 expression and monocyte adhesion. Involvement of protein kinase C-dependent c-Src tyrosine kinase activation pathway. J Biol Chem 277: 7118–7126, 2002. doi: 10.1074/jbc.M109924200. [DOI] [PubMed] [Google Scholar]
- 11.Chen J, Rao JN, Zou T, Liu L, Marasa BS, Xiao L, Zeng X, Turner DJ, Wang J-Y. Polyamines are required for expression of Toll-like receptor 2 modulating intestinal epithelial barrier integrity. Am J Physiol Gastrointest Liver Physiol 293: G568–G576, 2007. doi: 10.1152/ajpgi.00201.2007. [DOI] [PubMed] [Google Scholar]
- 12.Clevers HC, Bevins CL. Paneth cells: maestros of the small intestinal crypts. Annu Rev Physiol 75: 289–311, 2013. doi: 10.1146/annurev-physiol-030212-183744. [DOI] [PubMed] [Google Scholar]
- 13.Connor EM, Eppihimer MJ, Morise Z, Granger DN, Grisham MB. Expression of mucosal addressin cell adhesion molecule-1 (MAdCAM-1) in acute and chronic inflammation. J Leukoc Biol 65: 349–355, 1999. [DOI] [PubMed] [Google Scholar]
- 14.David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA, Biddinger SB, Dutton RJ, Turnbaugh PJ. Diet rapidly and reproducibly alters the human gut microbiome. Nature 505: 559–563, 2014. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.David R. Regulatory T cells: A helping hand from Bacteroides fragilis. Nat Rev Immunol 10: 539, 2010. doi: 10.1038/nri2827. [DOI] [PubMed] [Google Scholar]
- 16.Debas HT, Mulvihill SJ. Neuroendocrine design of the gut. Am J Surg 161: 243–249, 1991. doi: 10.1016/0002-9610(91)91139-A. [DOI] [PubMed] [Google Scholar]
- 17.Dejardin E, Droin NM, Delhase M, Haas E, Cao Y, Makris C, Li Z-W, Karin M, Ware CF, Green DR. The lymphotoxin-beta receptor induces different patterns of gene expression via two NF-kappaB pathways. Immunity 17: 525–535, 2002. doi: 10.1016/S1074-7613(02)00423-5. [DOI] [PubMed] [Google Scholar]
- 18.Devkota S, Wang Y, Musch MW, Leone V, Fehlner-Peach H, Nadimpalli A, Antonopoulos DA, Jabri B, Chang EB. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10−/− mice. Nature 487: 104–108, 2012. doi: 10.1038/nature11225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dudrick SJ, Wilmore DW, Vars HM, Rhoads JE. Long-term total parenteral nutrition with growth, development, and positive nitrogen balance. Surgery 64: 134–142, 1968. [PubMed] [Google Scholar]
- 20.Dufour C, Dandrifosse G, Forget P, Vermesse F, Romain N, Lepoint P. Spermine and spermidine induce intestinal maturation in the rat. Gastroenterology 95: 112–116, 1988. doi: 10.1016/0016-5085(88)90298-3. [DOI] [PubMed] [Google Scholar]
- 21.Erickson CS, Barlow AJ, Pierre JF, Heneghan AF, Epstein ML, Kudsk KA, Gosain A. Colonic enteric nervous system analysis during parenteral nutrition. J Surg Res 184: 132–137, 2013. doi: 10.1016/j.jss.2013.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fagarasan S. Evolution, development, mechanism and function of IgA in the gut. Curr Opin Immunol 20: 170–177, 2008. doi: 10.1016/j.coi.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 23.Fagarasan S, Kawamoto S, Kanagawa O, Suzuki K. Adaptive immune regulation in the gut: T cell-dependent and T cell-independent IgA synthesis. Annu Rev Immunol 28: 243–273, 2010. doi: 10.1146/annurev-immunol-030409-101314. [DOI] [PubMed] [Google Scholar]
- 24.Feng T, Elson CO, Cong Y. Treg cell-IgA axis in maintenance of host immune homeostasis with microbiota. Int Immunopharmacol 11: 589–592, 2011. doi: 10.1016/j.intimp.2010.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fukatsu K, Kudsk KA, Zarzaur BL, Sabek O, Wilcox HG, Johnson CD. Increased ICAM-1 and beta2 integrin expression in parenterally fed mice after a gut ischemic insult. Shock 18: 119–124, 2002. doi: 10.1097/00024382-200208000-00005. [DOI] [PubMed] [Google Scholar]
- 26.Fukatsu K, Zarzaur BL, Johnson CD, Lundberg AH, Wilcox HG, Kudsk KA. Enteral nutrition prevents remote organ injury and death after a gut ischemic insult. Ann Surg 233: 660–668, 2001. doi: 10.1097/00000658-200105000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gaudier E, Jarry A, Blottière HM, de Coppet P, Buisine MP, Aubert JP, Laboisse C, Cherbut C, Hoebler C. Butyrate specifically modulates MUC gene expression in intestinal epithelial goblet cells deprived of glucose. Am J Physiol Gastrointest Liver Physiol 287: G1168–G1174, 2004. doi: 10.1152/ajpgi.00219.2004. [DOI] [PubMed] [Google Scholar]
- 28.Gunawardene AR, Corfe BM, Staton CA. Classification and functions of enteroendocrine cells of the lower gastrointestinal tract. Int J Exp Pathol 92: 219–231, 2011. doi: 10.1111/j.1365-2613.2011.00767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28a.Hanna KM, Zarzaur BL, Fukatsu K, DeWitt RC, Renegar KB, Sherrell C, Wu Y, Kudsk KA. Individual neuropeptides regulate gut-associated lymphoid tissue integrity, intestinal immunoglobulin A levels, and respiratory antibacterial immunity. JPEN J Parenter Enteral Nutr 24: 261–269, 2000. [DOI] [PubMed] [Google Scholar]
- 29.Heneghan AF, Pierre JF, Gosain A, Kudsk KA. IL-25 improves luminal innate immunity and barrier function during parenteral nutrition. Ann Surg 259: 394–400, 2014. doi: 10.1097/SLA.0b013e318284f510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heneghan AF, Pierre JF, Kudsk KA. IL-25 improves IgA levels during parenteral nutrition through the JAK-STAT pathway. Ann Surg 258: 1065–1071, 2013. doi: 10.1097/SLA.0b013e318277ea9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heneghan AF, Pierre JF, Tandee K, Shanmuganayagam D, Wang X, Reed JD, Steele JL, Kudsk KA. Parenteral nutrition decreases paneth cell function and intestinal bactericidal activity while increasing susceptibility to bacterial enteroinvasion. JPEN J Parenter Enteral Nutr 38: 817–824, 2014. doi: 10.1177/0148607113497514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ikeda S, Kudsk KA, Fukatsu K, Johnson CD, Le T, Reese S, Zarzaur BL. Enteral feeding preserves mucosal immunity despite in vivo MAdCAM-1 blockade of lymphocyte homing. Ann Surg 237: 677–685, 2003. doi: 10.1097/01.SLA.0000064364.40406.EA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Janu P, Li J, Renegar KB, Kudsk KA. Recovery of gut-associated lymphoid tissue and upper respiratory tract immunity after parenteral nutrition. Ann Surg 225: 707–717, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jertborn M, Svennerholm AM, Holmgren J. Saliva, breast milk, and serum antibody responses as indirect measures of intestinal immunity after oral cholera vaccination or natural disease. J Clin Microbiol 24: 203–209, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jonker MA, Hermsen JL, Sano Y, Heneghan AF, Lan J, Kudsk KA. Small intestine mucosal immune system response to injury and the impact of parenteral nutrition. Surgery 151: 278–286, 2012. doi: 10.1016/j.surg.2010.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jonker MA, Heneghan AF, Fechner JH, Pierre JF, Sano Y, Lan J, Kudsk KA. gut lymphocyte phenotype changes after parenteral nutrition and neuropeptide administration. Ann Surg 262: 194–201, 2015. doi: 10.1097/SLA.0000000000000878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaetzel CS. The polymeric immunoglobulin receptor: bridging innate and adaptive immune responses at mucosal surfaces. Immunol Rev 206: 83–99, 2005. doi: 10.1111/j.0105-2896.2005.00278.x. [DOI] [PubMed] [Google Scholar]
- 38.Kang W, Gomez FE, Lan J, Sano Y, Ueno C, Kudsk KA. Parenteral nutrition impairs gut-associated lymphoid tissue and mucosal immunity by reducing lymphotoxin Beta receptor expression. Ann Surg 244: 392–399, 2006. doi: 10.1097/01.sla.0000234797.42935.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kansagra K, Stoll B, Rognerud C, Niinikoski H, Ou C-N, Harvey R, Burrin D. Total parenteral nutrition adversely affects gut barrier function in neonatal piglets. Am J Physiol Gastrointest Liver Physiol 285: G1162–G1170, 2003. doi: 10.1152/ajpgi.00243.2003. [DOI] [PubMed] [Google Scholar]
- 40.Kantele A, Häkkinen M, Moldoveanu Z, Lu A, Savilahti E, Alvarez RD, Michalek S, Mestecky J. Differences in immune responses induced by oral and rectal immunizations with Salmonella typhi Ty21a: evidence for compartmentalization within the common mucosal immune system in humans. Infect Immun 66: 5630–5635, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim M, Qie Y, Park J, Kim CH. Gut microbial metabolites fuel host antibody responses. Cell Host Microbe 20: 202–214, 2016. doi: 10.1016/j.chom.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim YS, Ho SB. Intestinal goblet cells and mucins in health and disease: recent insights and progress. Curr Gastroenterol Rep 12: 319–330, 2010. doi: 10.1007/s11894-010-0131-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.King BK, Kudsk KA, Li J, Wu Y, Renegar KB. Route and type of nutrition influence mucosal immunity to bacterial pneumonia. Ann Surg 229: 272–278, 1999. doi: 10.1097/00000658-199902000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kondrup J, Rasmussen HH, Hamberg O, Stanga Z; Ad Hoc ESPEN Working Group . Nutritional risk screening (NRS 2002): a new method based on an analysis of controlled clinical trials. Clin Nutr 22: 321–336, 2003. doi: 10.1016/S0261-5614(02)00214-5. [DOI] [PubMed] [Google Scholar]
- 46.Krug SM, Schulzke JD, Fromm M. Tight junction, selective permeability, and related diseases. Semin Cell Dev Biol 36: 166–176, 2014. doi: 10.1016/j.semcdb.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 47.Kudsk KA, Carpenter G, Petersen S, Sheldon GF. Effect of enteral and parenteral feeding in malnourished rats with E. coli-hemoglobin adjuvant peritonitis [Online]. [29 Aug. 2015]. J Surg Res 31: 105–110, 1981. doi: 10.1016/0022-4804(81)90037-8. [DOI] [PubMed] [Google Scholar]
- 48.Kudsk KA, Croce MA, Fabian TC, Minard G, Tolley EA, Poret HA, Kuhl MR, Brown RO. Enteral versus parenteral feeding. Effects on septic morbidity after blunt and penetrating abdominal trauma. Ann Surg 215: 503–513, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kudsk KA, Li J, Renegar KB. Loss of upper respiratory tract immunity with parenteral feeding. Ann Surg 223: 629–538, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kudsk KA, Stone JM, Carpenter G, Sheldon GF. Enteral and parenteral feeding influences mortality after hemoglobin-E. coli peritonitis in normal rats. J Trauma 23: 605–609, 1983. doi: 10.1097/00005373-198307000-00010. [DOI] [PubMed] [Google Scholar]
- 51.Lan J, Heneghan AF, Sano Y, Jonker MA, Omata J, Xu W, Pierre JF, Kudsk KA. Parenteral nutrition impairs lymphotoxin β receptor signaling via NF-κB. Ann Surg 253: 996–1003, 2011. doi: 10.1097/SLA.0b013e31821224eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee S-J, Lee J, Li KK, Holland D, Maughan H, Guttman DS, Yusta B, Drucker DJ. Disruption of the murine Glp2r impairs Paneth cell function and increases susceptibility to small bowel enteritis. Endocrinology 153: 1141–1151, 2012. doi: 10.1210/en.2011-1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lelouard H, Fallet M, de Bovis B, Méresse S, Gorvel J-P. Peyer’s patch dendritic cells sample antigens by extending dendrites through M cell-specific transcellular pores. Gastroenterology 142: 592–601.e3, 2012. doi: 10.1053/j.gastro.2011.11.039. [DOI] [PubMed] [Google Scholar]
- 54.Leone V, Gibbons SM, Martinez K, Hutchison AL, Huang EY, Cham CM, Pierre JF, Heneghan AF, Nadimpalli A, Hubert N, Zale E, Wang Y, Huang Y, Theriault B, Dinner AR, Musch MW, Kudsk KA, Prendergast BJ, Gilbert JA, Chang EB. Effects of diurnal variation of gut microbes and high-fat feeding on host circadian clock function and metabolism. Cell Host Microbe 17: 681–689, 2015. doi: 10.1016/j.chom.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li J, Kudsk KA, Gocinski B, Dent D, Glezer J, Langkamp-Henken B. Effects of parenteral and enteral nutrition on gut-associated lymphoid tissue. J Trauma 39: 44–52, 1995. [DOI] [PubMed] [Google Scholar]
- 56.Liu L, Guo X, Rao JN, Zou T, Xiao L, Yu T, Timmons JA, Turner DJ, Wang J-Y. Polyamines regulate E-cadherin transcription through c-Myc modulating intestinal epithelial barrier function. Am J Physiol Cell Physiol 296: C801–C810, 2009. doi: 10.1152/ajpcell.00620.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Löser C, Eisel A, Harms D, Fölsch UR. Dietary polyamines are essential luminal growth factors for small intestinal and colonic mucosal growth and development. Gut 44: 12–16, 1999. doi: 10.1136/gut.44.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lu H, Crawford RB, Suarez-Martinez JE, Kaplan BLF, Kaminski NE. Induction of the aryl hydrocarbon receptor-responsive genes and modulation of the immunoglobulin M response by 2,3,7,8-tetrachlorodibenzo-p-dioxin in primary human B cells. Toxicol Sci 118: 86–97, 2010. doi: 10.1093/toxsci/kfq234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Luckheeram RV, Zhou R, Verma AD, Xia B. CD4+T cells: differentiation and functions. Clin Dev Immunol 2012: 925135, 2012. doi: 10.1155/2012/925135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mabbott NA, Donaldson DS, Ohno H, Williams IR, Mahajan A. Microfold (M) cells: important immunosurveillance posts in the intestinal epithelium. Mucosal Immunol 6: 666–677, 2013. doi: 10.1038/mi.2013.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Macpherson AJ, McCoy KD, Johansen F-E, Brandtzaeg P. The immune geography of IgA induction and function. Mucosal Immunol 1: 11–22, 2008. doi: 10.1038/mi.2007.6. [DOI] [PubMed] [Google Scholar]
- 62.Magnúsdóttir S, Ravcheev D, de Crécy-Lagard V, Thiele I. Systematic genome assessment of B-vitamin biosynthesis suggests co-operation among gut microbes. Front Genet 6: 148, 2015. doi: 10.3389/fgene.2015.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Marik PE, Zaloga GP. Meta-analysis of parenteral nutrition versus enteral nutrition in patients with acute pancreatitis. BMJ 328: 1407, 2004. doi: 10.1136/bmj.38118.593900.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Meijers JMM, van Bokhorst-de van der Schueren MA, Schols JM, Soeters PB, Halfens RJ. Defining malnutrition: mission or mission impossible? Nutrition 26: 432–440, 2010. doi: 10.1016/j.nut.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 65.Meyer-Hoffert U, Hornef MW, Henriques-Normark B, Axelsson L-G, Midtvedt T, Pütsep K, Andersson M. Secreted enteric antimicrobial activity localises to the mucus surface layer. Gut 57: 764–771, 2008. doi: 10.1136/gut.2007.141481. [DOI] [PubMed] [Google Scholar]
- 66.Miyasaka EA, Feng Y, Poroyko V, Falkowski NR, Erb-Downward J, Gillilland MG III, Mason KL, Huffnagle GB, Teitelbaum DH. Total parenteral nutrition-associated lamina propria inflammation in mice is mediated by a MyD88-dependent mechanism. J Immunol 190: 6607–6615, 2013. doi: 10.4049/jimmunol.1201746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mizock BA. Immunonutrition and critical illness: an update. Nutrition 26: 701–707, 2010. doi: 10.1016/j.nut.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 68.Moore EE, Jones TN. Benefits of immediate jejunostomy feeding after major abdominal trauma—a prospective, randomized study. J Trauma 26: 874–881, 1986. doi: 10.1097/00005373-198610000-00003. [DOI] [PubMed] [Google Scholar]
- 69.Moore FA, Feliciano DV, Andrassy RJ, McArdle AH, Booth FV, Morgenstein-Wagner TB, Kellum JM Jr, Welling RE, Moore EE. Early enteral feeding, compared with parenteral, reduces postoperative septic complications. The results of a meta-analysis. Ann Surg 216: 172–183, 1992. doi: 10.1097/00000658-199208000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Moore FA, Moore EE, Jones TN, McCroskey BL, Peterson VM. TEN versus TPN following major abdominal trauma—reduced septic morbidity. J Trauma 29: 916–923, 1989. [DOI] [PubMed] [Google Scholar]
- 71.Nathavitharana KA, Catty D, Raykundalia C, McNeish AS. Presence of secretory IgA antibodies to an enteric bacterial pathogen in human milk and saliva. Arch Dis Child Fetal Neonatal Ed 72: F102–F106, 1995. doi: 10.1136/fn.72.2.F102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Oeser K, Schwartz C, Voehringer D. Conditional IL-4/IL-13-deficient mice reveal a critical role of innate immune cells for protective immunity against gastrointestinal helminths. Mucosal Immunol 8: 672–682, 2015. doi: 10.1038/mi.2014.101. [DOI] [PubMed] [Google Scholar]
- 73.Omata J, Pierre JF, Heneghan AF, Tsao FHC, Sano Y, Jonker MA, Kudsk KA. Parenteral nutrition suppresses the bactericidal response of the small intestine. Surgery 153: 17–24, 2013. doi: 10.1016/j.surg.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ottaway CA. Neuroimmunomodulation in the intestinal mucosa. Gastroenterol Clin North Am 20: 511–529, 1991. [PubMed] [Google Scholar]
- 75.Palazzo M, Balsari A, Rossini A, Selleri S, Calcaterra C, Gariboldi S, Zanobbio L, Arnaboldi F, Shirai YF, Serrao G, Rumio C. Activation of enteroendocrine cells via TLRs induces hormone, chemokine, and defensin secretion. J Immunol 178: 4296–4303, 2007. doi: 10.4049/jimmunol.178.7.4296. [DOI] [PubMed] [Google Scholar]
- 76.Pappo I, Polacheck I, Zmora O, Feigin E, Freund HR. Altered gut barrier function to Candida during parenteral nutrition. Nutrition 10: 151–154, 1994. [PubMed] [Google Scholar]
- 77.Parrott DM. The gut as a lymphoid organ. Clin Gastroenterol 5: 211–228, 1976. [PubMed] [Google Scholar]
- 78.Pérez-Cano FJ, González-Castro A, Castellote C, Franch A, Castell M. Influence of breast milk polyamines on suckling rat immune system maturation. Dev Comp Immunol 34: 210–218, 2010. doi: 10.1016/j.dci.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 79.Peter JV, Moran JL, Phillips-Hughes J. A metaanalysis of treatment outcomes of early enteral versus early parenteral nutrition in hospitalized patients. Crit Care Med 33: 213–220, 2005. doi: 10.1097/01.CCM.0000150960.36228.C0. [DOI] [PubMed] [Google Scholar]
- 80.Petersen SR, Kudsk KA, Carpenter G, Sheldon GE. Malnutrition and immunocompetence: increased mortality following an infectious challenge during hyperalimentation. J Trauma 21: 528–533, 1981. doi: 10.1097/00005373-198107000-00004. [DOI] [PubMed] [Google Scholar]
- 81.Pfuntner A, Wier LM, Elixhauser A. . Overview of Hospital Stays in the United States, 2011: Statistical Brief #166. Agency for Healthcare Research and Quality (US), 2013. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb166.pdf. [PubMed] [Google Scholar]
- 82.Pierre JF, Heneghan AF, Lawson CM, Wischmeyer PE, Kozar RA, Kudsk KA. Pharmaconutrition review: physiological mechanisms. JPEN J Parenter Enteral Nutr 37, Suppl 51S–65S, 2013. doi: 10.1177/0148607113493326. [DOI] [PubMed] [Google Scholar]
- 83.Pierre JF, Heneghan AF, Meudt JM, Shea MP, Krueger CG, Reed JD, Kudsk KA, Shanmuganayagam D. Parenteral nutrition increases susceptibility of ileum to invasion by E coli. J Surg Res 183: 583–591, 2013. doi: 10.1016/j.jss.2013.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pierre JF, Heneghan AF, Wang X, Roenneburg DA, Groblewski GE, Kudsk KA. Bombesin improves adaptive immunity of the salivary gland during parenteral nutrition. JPEN J Parenter Enteral Nutr 39: 190–199, 2015. doi: 10.1177/0148607113507080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pierre JF, Neuman JC, Brill AL, Brar HK, Thompson MF, Cadena MT, Connors KM, Busch RA, Heneghan AF, Cham CM, Jones EK, Kibbe CR, Davis DB, Groblewski GE, Kudsk KA, Kimple ME. The gastrin-releasing peptide analog bombesin preserves exocrine and endocrine pancreas morphology and function during parenteral nutrition. Am J Physiol Gastrointest Liver Physiol 309: G431–G442, 2015. doi: 10.1152/ajpgi.00072.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Qiu J, Heller JJ, Guo X, Chen ZM, Fish K, Fu Y-X, Zhou L. The aryl hydrocarbon receptor regulates gut immunity through modulation of innate lymphoid cells. Immunity 36: 92–104, 2012. doi: 10.1016/j.immuni.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ralls MW, Demehri FR, Feng Y, Raskind S, Ruan C, Schintlmeister A, Loy A, Hanson B, Berry D, Burant CF, Teitelbaum DH. Bacterial nutrient foraging in a mouse model of enteral nutrient deprivation: insight into the gut origin of sepsis. Am J Physiol Gastrointest Liver Physiol 311: G734–G743, 2016. doi: 10.1152/ajpgi.00088.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Reunanen J, Kainulainen V, Huuskonen L, Ottman N, Belzer C, Huhtinen H, de Vos WM, Satokari R. Akkermansia muciniphila adheres to enterocytes and strengthens the integrity of the epithelial cell layer. Appl Environ Microbiol 81: 3655–3662, 2015. doi: 10.1128/AEM.04050-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Romanowski K, Zaborin A, Valuckaite V, Rolfes RJ, Babrowski T, Bethel C, Olivas A, Zaborina O, Alverdy JC. Candida albicans isolates from the gut of critically ill patients respond to phosphate limitation by expressing filaments and a lethal phenotype. PLoS One 7: e30119, 2012. doi: 10.1371/journal.pone.0030119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Salzman NH, Bevins CL. Dysbiosis—a consequence of Paneth cell dysfunction. Semin Immunol 25: 334–341, 2013. doi: 10.1016/j.smim.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 91.Sano Y, Hermsen JL, Kang W, Gomez FE, Lan J, Maeshima Y, Kudsk KA. Parenteral nutrition maintains pulmonary IgA antibody transport capacity, but not active transport, following injury. Am J Surg 198: 105–109, 2009. doi: 10.1016/j.amjsurg.2008.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Selleri S, Palazzo M, Deola S, Wang E, Balsari A, Marincola FM, Rumio C. Induction of pro-inflammatory programs in enteroendocrine cells by the Toll-like receptor agonists flagellin and bacterial LPS. Int Immunol 20: 961–970, 2008. doi: 10.1093/intimm/dxn055. [DOI] [PubMed] [Google Scholar]
- 93.Shan M, Gentile M, Yeiser JR, Walland AC, Bornstein VU, Chen K, He B, Cassis L, Bigas A, Cols M, Comerma L, Huang B, Blander JM, Xiong H, Mayer L, Berin C, Augenlicht LH, Velcich A, Cerutti A. Mucus enhances gut homeostasis and oral tolerance by delivering immunoregulatory signals. Science 342: 447–453, 2013. doi: 10.1126/science.1237910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sherrington A, Newham JJ, Bell R, Adamson A, McColl E, Araujo-Soares V. Systematic review and meta-analysis of internet-delivered interventions providing personalized feedback for weight loss in overweight and obese adults. Obes Rev 17: 541–551, 2016. doi: 10.1111/obr.12396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shreiner AB, Kao JY, Young VB. The gut microbiome in health and in disease. Curr Opin Gastroenterol 31: 69–75, 2015. doi: 10.1097/MOG.0000000000000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Singh N, Gurav A, Sivaprakasam S, Brady E, Padia R, Shi H, Thangaraju M, Prasad PD, Manicassamy S, Munn DH, Lee JR, Offermanns S, Ganapathy V. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity 40: 128–139, 2014. doi: 10.1016/j.immuni.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Steenwinckel V, Louahed J, Lemaire MM, Sommereyns C, Warnier G, McKenzie A, Brombacher F, Van Snick J, Renauld J-C. IL-9 promotes IL-13-dependent paneth cell hyperplasia and up-regulation of innate immunity mediators in intestinal mucosa. J Immunol 182: 4737–4743, 2009. doi: 10.4049/jimmunol.0801941. [DOI] [PubMed] [Google Scholar]
- 98.Suzuki K, Kawamoto S, Maruya M, Fagarasan S. GALT: organization and dynamics leading to IgA synthesis. Adv Immunol 107: 153–185, 2010. doi: 10.1016/B978-0-12-381300-8.00006-X. [DOI] [PubMed] [Google Scholar]
- 99.Tanaka G, Kanaji S, Hirano A, Arima K, Shinagawa A, Goda C, Yasunaga S, Ikizawa K, Yanagihara Y, Kubo M, Kuriyama-Fujii Y, Sugita Y, Inokuchi A, Izuhara K. Induction and activation of the aryl hydrocarbon receptor by IL-4 in B cells. Int Immunol 17: 797–805, 2005. doi: 10.1093/intimm/dxh260. [DOI] [PubMed] [Google Scholar]
- 100.Vaishnava S, Yamamoto M, Severson KM, Ruhn KA, Yu X, Koren O, Ley R, Wakeland EK, Hooper LV. The antibacterial lectin RegIIIgamma promotes the spatial segregation of microbiota and host in the intestine. Science 334: 255–258, 2011. doi: 10.1126/science.1209791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100a.Veterans Affairs Total Parenteral Nutrition Cooperative Study Group. Perioperative total parenteral nutrition in surgical patients. N Engl J Med 325: 525–532, 1991. doi: 10.1056/NEJM199108223250801. [DOI] [PubMed] [Google Scholar]
- 101.Ward MA, Pierre JF, Leal RF, Huang Y, Shogan B, Dalal SR, Weber CR, Leone VA, Musch MW, An GC, Rao MC, Rubin DT, Raffals LE, Antonopoulos DA, Sogin ML, Hyman NH, Alverdy JC, Chang EB. Insights into the pathogenesis of ulcerative colitis from a murine model of stasis-induced dysbiosis, colonic metaplasia, and genetic susceptibility. Am J Physiol Gastrointest Liver Physiol 310: G973–G988, 2016. doi: 10.1152/ajpgi.00017.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wernerman J, Hammarqvist F, Gamrin L, Essén P. Protein metabolism in critical illness. Baillieres Clin Endocrinol Metab 10: 603–615, 1996. doi: 10.1016/S0950-351X(96)80756-7. [DOI] [PubMed] [Google Scholar]
- 103.Willemsen LEM, Koetsier MA, van Deventer SJH, van Tol EAF. Short chain fatty acids stimulate epithelial mucin 2 expression through differential effects on prostaglandin E(1) and E(2) production by intestinal myofibroblasts. Gut 52: 1442–1447, 2003. doi: 10.1136/gut.52.10.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wischmeyer PE. Glutamine: role in critical illness and ongoing clinical trials. Curr Opin Gastroenterol 24: 190–197, 2008. doi: 10.1097/MOG.0b013e3282f4db94. [DOI] [PubMed] [Google Scholar]
- 105.Wretlind A, Szczygieł B [Total parenteral nutrition. History. Present time. Future]. Pol Merkuriusz Lek 4: 181–185, 1998. [PubMed] [Google Scholar]
- 106.Yang H, Feng Y, Sun X, Teitelbaum DH. Enteral versus parenteral nutrition: effect on intestinal barrier function. Ann N Y Acad Sci 1165: 338–346, 2009. doi: 10.1111/j.1749-6632.2009.04026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zarzaur BL, Wu Y, Fukatsu K, Johnson CD, Kudsk KA. The neuropeptide bombesin improves IgA-mediated mucosal immunity with preservation of gut interleukin-4 in total parenteral nutrition-fed mice. Surgery 131: 59–65, 2002. doi: 10.1067/msy.2002.118319. [DOI] [PubMed] [Google Scholar]
- 108.Zhai S-K, Volgina VV, Sethupathi P, Knight KL, Lanning DK. Chemokine-mediated B cell trafficking during early rabbit GALT development. J Immunol 193: 5951–5959, 2014. doi: 10.4049/jimmunol.1302575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhan L, Yang I, Kong B, Shen J, Gorczyca L, Memon N, Buckley BT, Guo GL. Dysregulation of bile acid homeostasis in parenteral nutrition mouse model. Am J Physiol Gastrointest Liver Physiol 310: G93–G102, 2016. doi: 10.1152/ajpgi.00252.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zipperer A, Konnerth MC, Laux C, Berscheid A, Janek D, Weidenmaier C, Burian M, Schilling NA, Slavetinsky C, Marschal M, Willmann M, Kalbacher H, Schittek B, Brötz-Oesterhelt H, Grond S, Peschel A, Krismer B. Human commensals producing a novel antibiotic impair pathogen colonization. Nature 535: 511–516, 2016. doi: 10.1038/nature18634. [DOI] [PubMed] [Google Scholar]